Abstract
One of the biggest problems in the treatment of idiopathic Parkinson’s disease is the lack of new drugs that slow its progression. L-Dopa remains the star drug in the treatment of this disease, although it induces severe side effects. The failure of clinical studies with new drugs depends on the use of preclinical models based on neurotoxins that do not represent what happens in the disease since they induce rapid and expansive neurodegeneration. We have recently proposed a single-neuron degeneration model for idiopathic Parkinson’s disease that requires years to accumulate enough lost neurons for the onset of motor symptoms. This single-neuron degeneration model is based on the excessive formation of aminochrome during neuromelanin synthesis that surpass the neuroprotective action of the enzymes DT-diaphorase and glutathione transferase M2-2, which prevent the neurotoxic effects of aminochrome. Although the neurotoxic effects of aminochrome do not have an expansive effect, a stereotaxic injection of this endogenous neurotoxin cannot be used to generate a preclinical model in an animal. Therefore, the aim of this review is to evaluate the strategies for pharmacologically increasing the expression of DT diaphorase and GSTM2-2 and molecules that induce the expression of vesicular monoamine transporter 2, such as pramipexole.
Keywords: dopamine, VMAT2, neuromelanin, neurodegeneration, neuroprotection, Parkinson’s disease, pramipexole, nicotine, KEAP1/NRF2
1. Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disease that affects the control of the motor system. Although intensive research has been carried out for decades to decipher the molecular mechanism that triggers this disease, it is still not clear what triggers the degeneration of the neuromelanin-containing dopaminergic neurons of the nigrostriatal system.
The discovery in 1960 that low dopamine levels in Parkinson’s disease are a product of the loss of dopaminergic neurons that contain neuromelanin has been one of the most important discoveries in understanding the molecular mechanisms of this disease. However, although several mechanisms related to the neurodegeneration of the nigrostriatal system have been identified, such as mitochondrial dysfunction, oxidative stress, dysfunction of both proteasomal and lysosomal protein degradation systems, endoplasmic reticulum stress, neuroinflammation, and the formation of neurotoxic oligomers of alpha-synuclein, it is not yet known what triggers these mechanisms [1,2,3,4,5,6,7].
A great advance in the research into molecular mechanisms has been the discovery of several mutations associated with familial Parkinson’s disease, since it has made it possible to reveal certain proteins that play a role in the development of Parkinson’s disease symptoms. The first of these proteins was a mutation in the alpha-synuclein gene that induces the formation of neurotoxic oligomers. Other mutations in genes that are involved in mitochondrial dysfunction include parkin, PTEN-induced putative kinase 1, and Protein/nucleic acid deglycase 1 [8]. Mutations in the leucine-rich repeat kinase 2 gene that would be involved in the control of membrane trafficking and lysosomal function have been reported [9]. Mutations in the ubiquitin carboxy-terminal hydrolase-L1, ATPase Cation Transporting 13A2, and other genes have also been reported to be associated with familial Parkinson’s disease [10]. The discovery of these genes associated with familial Parkinson’s disease has been a great contribution to molecular studies of the disease. The mutation of alpha-synuclein that induces its aggregation into neurotoxic oligomers resulting in lysosomal, mitochondrial, and endosomal dysfunction has had an enormous impact on basic research. The discovery of the ability of alpha-synuclein aggregates to spread between the gut, brainstem, and higher brain regions has allowed some researchers to suggest the stage hypothesis of Parkinson’s disease [11]. However, it must be remembered that familial Parkinson’s disease only represents 5–10% of the cases of this disease and it is a mistake to think that preclinical models with mutations of these genes will represent what happens in patients with idiopathic Parkinson’s disease. However, several preclinical models have been developed with animals that express mutations of some genes associated with familial Parkinson’s disease [12,13,14]. Overexpression of the human alpha-synuclein gene in rats induces an alteration in the gut microbiota [15].
There are many researchers who consider that environmental exposure plays an important role in the degenerative process of the nigrostriatal system that induces the loss of neuromelanin-containing dopaminergic neurons. Others think that exposure to heavy metals, solvents, pesticides, and environmental toxins could be partly responsible for the rapid growth in Parkinson’s disease [16]. A study on the possible role of exposure to local traffic-related air pollution in central California, USA, which included 761 patients with Parkinson’s disease and 910 healthy controls, concluded that exposure to local traffic-related air pollution is associated with an increased risks of developing Parkinson’s disease [17]. Exposure of workers in manganese mines was reported more than 60 years ago. Exposure to manganese is not only limited to manganese mines but also to workers who work in welding where they are exposed to the fumes that develop during this activity [18]. However, in subjects with Parkinsonism induced by metals such as manganese, copper, or pesticides such as paraquat, atypical Parkinsonism with early onset is induced in young workers [19,20]. This group of people exposed to pollutants who develop Parkinsonism cannot be included in the group of idiopathic Parkinson’s disease patients and constitute a special group that constitute approximately 20% of the total Parkinsonian individuals.
The possibility that environmental factors may play a role in triggering the mechanisms involved in the degenerative process and loss of neuromelanin-containing dopaminergic neurons of the nigrostriatal system in idiopathic Parkinson’s disease is questionable. The best evidence that exogenous factors or neurotoxins do not play a role in idiopathic Parkinson’s disease is from drug addicts exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Subjects who consumed drugs contaminated with MPTP developed severe Parkinsonism in just three days [21] which contrasts with the extremely slow generative and propagation process of idiopathic Parkinson’s disease, which takes many years.
The existence of premotor symptoms has been reported as olfactory dysfunctions, depression, insomnia, anxiety, rapid eye movement sleep behavior, constipation, and cognitive decline [22]. It has been proposed that Lewy bodies and Lewy neurites with alpha-synuclein immunoreactive deposits expand from regions such as the anterior olfactory nucleus to other regions, until they affect the substantia nigra where motor symptoms are generated [23]. However, this hypothesis of stages of the disease that progress from region to region of the brain of the patient with Parkinson’s disease is controversial since it would be valid for patients with an early onset of the disease but not for patients with a late onset such as the idiopathic form of the disease [24]. Although there may be premotor symptoms, they ultimately all come together in the loss of dopaminergic neurons that contain neuromelanin.
The extremely rapid effect of MPTP-Induced Parkinsonism in individuals exposed to this drug suggests that the neurotoxin that triggers neurodegeneration of the nigrostriatal system in idiopathic Parkinson’s disease cannot be of exogenous origin since it will have a massive, expansive, and rapid neurodegeneration [21].
Exposure to manganese, copper, and 3,4-methylenedioxymethamphetamine also leads to the development of early-onset Parkinsonism [20,25,26]. On the contrary, to trigger an extremely slow degenerative process that takes years, such as in idiopathic Parkinson disease, the neurotoxin that triggers this neurodegeneration is presumably of endogenous origin and does not have an expansive character. This endogenous neurotoxin is probably formed inside the dopaminergic neurons that contain neuromelanin. Possible neurotoxins generated within dopaminergic neurons are neurotoxic oligomers of alpha-synuclein, 3,4-dihydroxyphenylacetaldehyde (DOPAL) and aminochrome that are formed during neuromelanin synthesis.
Alpha-synuclein is normally found in its monomeric state but under certain circumstances it can be aggregated to fibrils that accumulate in Lewy bodies and Lewy neurites, which are not exclusive to the nigrostriatal system, but this aggregation occurs in other brain regions that are expanding from region to region [27,28]. Alpha-synuclein has the ability to spread from neuron to neuron through its secretion and subsequent uptake of the receiving neuron [29,30]. Seed spread of alpha-synuclein fibrils has been observed in different brain regions and serum of patients [31,32,33]. The internalization of alpha-synuclein released is an endosome-lysosome mechanism [34]. A stage of different levels of development of Parkinson’s disease based on the spread of Lewy bodies has been proposed. This disease stage helps explain the appearance of premotor symptoms [23]. However, the role of alpha-synuclein aggregation to fibrils and its formation of deposits in Lewis bodies that spread across brain regions has been questioned. It has been suggested that the Braak stages are not valid for patients with late onset such as patients with idiopathic Parkinson’s disease, but are valid for patients with early onset of the disease and those with long-lasting motor symptoms [24].
Another argument against the role of Lewy bodies in Parkinson’s disease pathology is the absence of Lewy bodies in patients with familial Parkinson’s disease associated with mutations in the Parkin and leucine-rich repeat kinase 2 genes [35,36,37,38]. Lewy bodies loaded with alpha-synuclein are observed in postmortem tissues from patients with Parkinson’s disease, which correspond to tissues that have survived the degenerative process for years. If alpha-synuclein deposits in Lewy bodies were neurotoxic, they could not be observed in postmortem tissue from patients with Parkinson’s disease [39]. Another point against the neurotoxic role of Lewy bodies loaded with alpha-synuclein deposits is the propagative nature of these Lewy bodies. The spread of alpha-synuclein fibrils deposited in Lewy bodies through exosomes [40] occurs intracellularly to neighboring neurons and subsequently to other regions. This propagative mode of alpha-synuclein fibril transfer to other neurons should imply a rapid progression of the neurodegenerative process and disease progression because the propagation of these fibrils does not involve a single fibril but a large number. In addition, it has been proposed that the accumulation of alpha-synuclein fibrils in Lewy bodies could actually be a neuroprotective mechanism [41].
Monomeric alpha-synuclein is also aggregated to oligomers that are considered the species responsible for the neurotoxic action of this protein [42,43]. Alpha-synuclein oligomers induce synaptic impairment, endoplasmic reticulum stress, mitochondrial dysfunction, loss of regulation of proteostasis, neuroinflammation, cell apoptosis, lysosomal dysfunction, oxidative stress, and autophagy impairment [27,44,45,46,47,48]. However, the propagative nature of alpha-synuclein [49] will imply rapid neurodegeneration of the nigrostriatal system when neurotoxic oligomers are formed, which is the opposite of what occurs in the disease. Mutations in the alpha-synuclein gene induce the formation of neurotoxic alpha-synuclein oligomers in familial Parkinson’s disease that are transmitted to neighboring neurons through exosomes [49,50,51,52]. Alpha-synuclein alone does not aggregate into neurotoxic oligomers and the question is what induces the aggregation of alpha-synuclein to neurotoxic oligomers in neuromelanin-containing dopaminergic neurons in the nigrostriatal system in idiopathic Parkinson’s disease. In in vitro experiments the formation of oligomers was reported in the presence of iron, copper, manganese, DOPAL, or rotenone [53,54,55,56,57]. However, the massive degeneration that these metals can generate that induces early-onset Parkinsonism in young workers [20,58] does not coincide with the extremely slow degenerative process that occurs in idiopathic Parkinson’s disease, which takes years. An experiment in mice revealed an increase in alpha-synuclein oligomers and neurodegeneration by increasing dopamine and alpha-synuclein levels [59]. Alpha-synuclein aggregates were observed during dopamine oxidation catalyzed by aminochrome and 5,6-indolequinone [60,61]. Aminochrome forms neurotoxic oligomers in cell culture when the enzyme DT-diaphorase is silenced with siRNA [62].
Aminochrome is an endogenous neurotoxin that is an intermediate formed in the synthesis of neuromelanin inside of the neurons lost in Parkinson’s disease. Neuromelanin is synthesized from the oxidation of the catechol dopamine group to three ortho-quinones (dopamine --> dopamine ortho-quinone --> aminochrome --> 5,6-indolequinone --> neuromelanin) [63,64]. These ortho-quinones are potentially neurotoxic but aminochrome is the most stable and neurotoxic [65,66]. Aminochrome induces oxidative stress, mitochondrial dysfunction, formation of neurotoxic alpha-synuclein oligomers, dysfunction of both lysosomal and proteasomal protein degradation systems, neuroinflammation, and endoplasmic reticulum stress [67,68,69,70,71,72].
Another neurotoxin that can be formed in dopaminergic neurons is DOPAL, which is the product of the oxidative deamination of dopamine catalyzed by monoamine oxidase [73]. DOPAL is converted to 3,4-dihydroxyphenylacetic acid catalyzed by the enzyme aldehyde dehydrogenase-1. DOPAL can form adducts with alpha-synuclein, generating the formation of oligomers and their accumulation that induce neurodegeneration [55]. The addition of DOPAL and A53T alpha-synuclein to glial cells demonstrated that glia cells can take up DOPAL and increase alpha-synuclein oligomerization intracellularly [74]. DOPAL-induced alpha-synuclein oligomerization increases in the presence of divalent metals such as Cu2+, Fe2+, and Mn2+ [75]. A study with astrocytes showed that DOPAL induces apoptosis and oxidative and nitrative stress, and lowers mitochondrial function. An experiment performed with postmortem tissue from patients with Parkinson’s disease revealed a low expression of the enzyme aldehyde dehydrogenase-1 [76]. A low expression of this enzyme would imply an accumulation of DOPAL that can be oxidized to ortho-quinones and have neurotoxic effects [73,76,77]. However, this low expression was observed in postmortem tissue from patients with late-onset Parkinson’s disease who survived the neurodegenerative process, which raises questions as to its role as an endogenous neurotoxin that triggers the neurodegenerative process.
The aim of this review is to propose a different point of view on how to approach the search for new drugs to halt or reduce the progression of idiopathic Parkinson’s disease, based on a new concept to interpret the degenerative process of neuromelanin-containing dopaminergic neurons as a single-neuron degeneration model [78] (Table 1).
Table 1.
Summary.
|
|
|
|
|
|
2. Dopamine Metabolism
Dopamine is synthesized in the cytosol of dopaminergic neurons from the amino acid L-tyrosine that is converted into L-3,4-dihydroxyphenylalanine (L-Dopa) in a reaction catalyzed by the enzyme tyrosine hydroxylase where a hydroxyl group is introduced into position 3 of tyrosine forming a catechol structure [79]. L-Dopa is subsequently decarboxylated forming dopamine in a reaction catalyzed by the enzyme aromatic L-amino acid decarboxylase [80]. The objective of dopamine synthesis is its accumulation in monoaminergic neurotransmission vesicles through vesicular monoamine transporter-2 (VMAT2), which catalyzes its uptake from the cytosol into the interior of these vesicles [81].
Free dopamine in the cytosol can be degraded through its oxidative deamination catalyzed by the enzyme monoamine oxidase. Alternatively, free dopamine in the cytosol can be oxidized to neuromelanin. The catechol group of dopamine is oxidized to form three ortho-quinones sequentially, namely dopamine ortho-quinone, aminochrome, and 5,6-indolequinone, which finally polymerize to form neuromelanin [65,79].
The synthesis of neuromelanin is a normal and harmless pathway since, in healthy older adults, the neuromelanin-containing dopaminergic neurons are intact at the time of death [82]. However, in older adults with Parkinson’s disease, the majority of neuromelanin-containing dopaminergic neurons have been lost [83,84]. The reason that neuromelanin-containing dopaminergic neurons are lost in the substantia nigra of patients with Parkinson’s disease depends on the neurotoxic action of transient ortho-quinones that are formed during neuromelanin synthesis. Aminochrome is the most stable and neurotoxic transient ortho-quinone since: (i) it can be reduced with one electron by flavoenzymes that transfer an electron to a leukoaminochrome o-semiquinone radical, which is extremely reactive with oxygen [85]. Autoxidation of the leukoaminochrome o-semiquinone radical generates redox cycling between aminochrome and leukoaminochrome o-semiquinone that reduces dioxygen to superoxide. This redox cycling implies that the dioxygen that is needed to complete the mitochondrial electron transfer that is ultimately required for oxidative phosphorylation of ADP to ATP is depleted. Additionally, this redox cycling also depletes NADH which is used in the mitochondrial electron transport chain. Finally, this redox cycling generates oxidative stress and ATP depletion that is required, among other things, for the neurotransmission of dopamine from monoaminergic vesicles; and (ii) aminochrome is also capable of forming adducts with proteins such as alpha-synuclein, actin, α and β-tubulin, mitochondrial complex 1, ATP13A, and other proteins [62,86,87]. The neurotoxic effects of aminochrome induce oxidative stress, neuroinflammation, formation of neurotoxic alpha-synuclein oligomers, mitochondrial dysfunction, endoplasmic reticulum stress, and dysfunction of both lysosomal and proteasomal protein degradation systems [67,68,69,70,71,72].
Aminochrome is a transient metabolite that in in vitro experiments monitored with NMR has been determined to be stable 40 min before beginning its conversion to 5,6-indolequinone, which polymerizes to neuromelanin [66]. However, in the cytosol of a neuron that is full of proteins, enzymes, lipids, and other biomolecules, the stability of aminochrome is much lower since it is either reduced by flavoenzymes or forms adducts with proteins, which prevents this endogenous neurotoxin from having an expansion that affects neighboring neurons. This implies that the neurotoxic effects of aminochrome only affect a single neuron. This single-neuron degeneration model could explain why the loss of neuromelanin-containing dopaminergic neurons in a patient with idiopathic Parkinson’s disease is extremely slow, taking years before the onset of motor symptoms and also later during the progression of the disease [78] (Table 2).
Table 2.
Summary.
|
|
|
3. Neuroprotection against Aminochrome Neurotoxicity
The question is how neuromelanin synthesis can be a normal and harmless process if it requires the formation of the endogenous neurotoxin aminochrome. This can be explained by the existence of the enzyme DT-diaphorase that reduces two-electron aminochrome to leukoaminochrome, preventing the reduction of one-electron aminochrome to a leukoaminochrome o-semiquinone radical catalyzed by flavoenzymes that reduce one-electron quinones [88,89]. DT-diaphorase prevents the aminochrome-induced death of dopaminergic neurons, mitochondrial dysfunction, oxidative stress, lysosomal dysfunction, disruption of cytoskeletal architecture, dysfunction of protein degradation of the proteasomal system, and autophagy [65,68,90].
In 1997 we began a scientific collaboration with Professor Bengt Mannervik to study the ability of glutathione transferases to conjugate aminochrome. Interestingly, human glutathione transferase M2-2 was the most active isoenzyme within these isoenzymes and its conjugate 4-S-glutathionyl-5,6-dihydroxyindoline is resistant to biological oxidative agents such as dioxygen, superoxide, and hydrogen peroxide [91]. Glutathione transferase M2-2 conjugates not only aminochrome but also its precursor dopamine ortho-quinone to 5-glutathioneyldopamine, which is degraded to 5-cysteinyldopamine [92]. Interestingly, 5-cysteinyldopamine has been detected in neuromelanin and human cerebrospinal fluid, suggesting that it is a final reaction in which a stable product is produced that is eliminated from neuromelanin-containing dopaminergic neurons into the cerebrospinal fluid and accumulated in neuromelanin [93,94]. Glutathione transferase M2-2 is not expressed in neuromelanin-containing dopaminergic neurons where the aminochrome triggers the degeneration of these neurons. Astrocytes can take up dopamine released during neurotransmission. Dopamine within astrocytes can be oxidized to aminochrome, where glutathione transferase M2-2 can conjugate both aminochrome and its precursor dopamine ortho-quinone. However, it has been reported that astrocytes secrete exosomes loaded with glutathione transferase M2-2 that penetrate dopaminergic neurons, discharging this enzyme into their cytosol to increase the protection of these neurons against the neurotoxic effects of aminochrome [90,95,96,97] (Table 3).
Table 3.
Summary.
|
|
4. Prevention of Dopamine Oxidation-Dependent Neurotoxicity
One of the fundamental events in the progression of idiopathic Parkinson’s disease is the appearance of motor symptoms when 60% of neuromelanin-containing dopaminergic neurons are lost [98]. However, it has been proposed that the onset of the disease is observed when 50–60% of the dopaminergic terminals of the striatum have been lost, which would correspond to a 30% loss of dopaminergic neurons of the substantia nigra [99]. The speed of the degenerative process of neuromelanin-containing dopaminergic neurons in the nigrostriatal system is extremely slow and lasts for many years [78]. This suggests that this degenerative process is not expansive in nature and that the neurotoxin that triggers it seems to be of endogenous origin. Therefore, the prevention of dopamine oxidation to aminochrome is a potential way to inhibit the loss of neuromelanin-containing dopaminergic neurons in idiopathic Parkinson’s disease, if we consider that the oxidation of dopamine to aminochrome plays an essential role in the degenerative process of neuromelanin-containing dopaminergic neurons [100].
The oxidation of dopamine to neuromelanin depends on the existence of free dopamine in the cytosol and the presence of metals or enzymes with peroxidase activity. One of the possible sources of free dopamine is its synthesis from the amino acid L-tyrosine, which requires the action of two enzymes (tyrosine hydrolase and aromatic enzyme L-amino acid decarboxylase). Subsequently, vesicular monoamine transporter 2 (VMAT-2), which is expressed in monoaminergic neurotransmission vesicles, transports the newly synthesized dopamine into the vesicles. Inside the monoaminergic neurotransmission vesicles, dopamine can accumulate in high concentrations without the risk of oxidation since these vesicles have a slightly acidic pH inside [101]. These vesicles have an H+-ATPase that pumps protons into these vesicles, generating a slightly acidic pH inside them [81].
Interestingly, the enzymes tyrosine hydrolase and aromatic enzyme L-amino acid decarboxylase form a type of complex with VMAT-2 that prevents the existence of free dopamine since the newly synthesized L-dopa is immediately converted into dopamine that is transported to the monoaminergic vesicles of neurotransmission catalyzed by VMAT-2 [102]. The other source of free dopamine in the cytosol of neuromelanin-containing dopaminergic neurons is the reuptake of dopamine released during neurotransmission via dopamine transporters. However, the dopamine transporter, VMAT-2 and synaptogyrin-3 also form a type of complex that prevents the dopamine reuptake by the dopamine transporter from being released directly into the cytosol [103]. Therefore VMAT-2 plays a key role in preventing the oxidation of dopamine to neuromelanin in the cytosol of dopaminergic neurons.
The level of VMAT-2 expression may play a fundamental role in preventing the oxidation of dopamine to neuromelanin that generates three potentially neurotoxic ortho-quinones. There is an inverse relationship between neuromelanin levels and VMAT-2 expression which is based on the fact that the major accumulation of neuromelanin occurs in the substantia nigra, which has less VMAT-2 expression compared to the midbrain dopaminergic neurons of VTA that have less neuromelanin formation despite producing more dopamine and a higher expression of VMAT-2 [104,105]. The possibility that the degeneration of axons [99] may depend on the oxidation of dopamine to aminochrome due to the leak of dopamine from the monoaminergic vesicles located in the dopaminergic terminals located in the striatum does not seem to be feasible because the presence of neuromelanin has not been observed in the striatum (Table 4).
Table 4.
Summary.
|
|
|
5. Clinical Studies in Parkinson’s Disease
One of the biggest concerns in Parkinson’s disease research is the failure of all clinical studies of drugs that aimed to modify the course of the disease (isradipine, coenzyme Q10, TCH346, mitoquinone, nilotinib, zonisamide, deferiprone, prasinezumab, and cinpanemab) or regenerate dopaminergic neurons (neurturin analogue of GDNF) [106]. All these clinical studies were based on successful preclinical studies that used exogenous neurotoxins such as MPTP or 6-hydroxydopamine, which induce a rapid, massive, and propagative degenerative process [107]. Preclinical studies with coenzyme Q10 were successful [108,109,110] but clinical studies did not show a benefit for patients with Parkinson’s disease [111]. Mito-Q(10), a modified coenzyme Q10 analogue, showed a clear neuroprotective effect in MPTP and a 6-hydroxydopamine preclinical model [112,113,114] but in clinical studies they did not show neuroprotective effects in patients with Parkinson’s disease [115]. Neuroprotective effects of urate were demonstrated in preclinical models based on 6-hydroxydopamine [116,117,118]. However, a clinical study failed to show benefits in patients with Parkinson’s disease [119]. One of the possible explanations for the failure of these clinical studies is that the degenerative process of idiopathic Parkinson’s disease is extremely slow. The evaluation of patients with MDS-UPDRS is unable to detect progress as a result of the therapeutic action of the drugs used in these clinical studies because the progress of the neurogenerative process is so slow. Recently, it has been published that the number of dopaminergic neurons of the substantia nigra considering both hemispheres varies between 800,000 and 1,000,000 dopaminergic neurons, which implies that when the motor symptoms appear in the disease, only between 320,000 to 400,000 dopaminergic neurons remain, after 60% of those neurons have disappeared [120]. As the degenerative process of the nigrostriatal system in idiopathic Parkinson’s disease is extremely slow, it is possible that the positive therapeutic effect observed in these preclinical studies with exogenous neurotoxins is impossible to determine in clinical studies because the speed of the degenerative process in the disease is extremely slow. Recently, the single-neuron degeneration model has been proposed, where the degenerative process is induced by the endogenous neurotoxin aminochrome that induces non-propagative neurodegeneration. The loss of a single neuromelanin-containing dopaminergic neuron accumulates over years until reaching a 60% loss when motor symptoms appear [78] (Table 5).
Table 5.
Summary.
|
|
|
6. VMAT-2 as a Target to Develop New Drugs for Parkinson’s Disease
It is urgent to search for new therapeutic targets for Parkinson’s disease therapy, but the choice of the preclinical model is key to success. Based on the extreme slowness of the degenerative process and the progress of Parkinson’s disease, which takes years, we consider that the search for new targets with therapeutic effects on the disease should be based on a single-neuron degeneration model. The oxidation of dopamine to aminochrome during the synthesis of neuromelanin plays an essential role in the degenerative process of dopaminergic neurons containing neuromelanin in the nigrostriatal system. Therefore, the prevention of the oxidation of dopamine to aminochrome may be the most neuroprotective action to protect these neurons that are lost in Parkinson’s disease in the substantia nigra.
The role of VMAT-2 in preventing the oxidation of dopamine to neuromelanin has been described for many years [104,105]. However, the inhibition of VMAT-2 expression in the single-neuron degeneration model plays a key role since inhibiting the oxidation of dopamine to aminochrome does not require the overexpression of the neuroprotective enzymes DT-diaphorase and glutathione transferase M2-2 to prevent the neurotoxic effects of aminochrome [90]. The essential role of VMAT2 in preventing neurodegeneration of dopaminergic neurons containing neuromelanin-dependent dopamine oxidation has been demonstrated with the use of viral-mediated small-hairpin RNA interference of VMAT2. Loss of VMAT2 expression resulted in increased cytosolic dopamine concentration and subsequent degeneration of the nigrostriatal dopaminergic system. The addition of exogenous VMAT2 prevents the neurotoxic effects created by silencing the expression of this transporter [121].
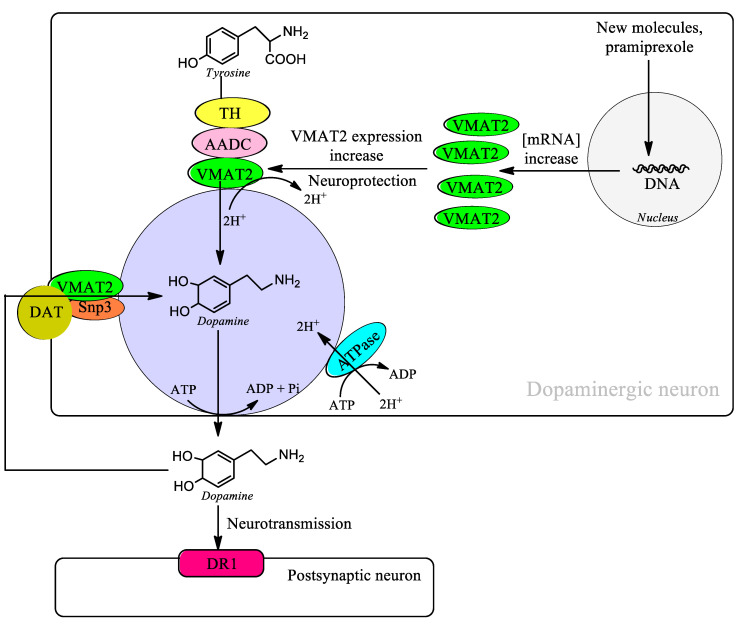
Pramipexole is a dopamine agonist used in the therapy of Parkinson’s disease, and SPECT studies have demonstrated a neuroprotective effect in patients with Parkinson’s disease. Patients treated with the agonist ropinirole also showed a significant neuroprotective effect on nigrostriatal neurons [122]. The neuroprotective effects of pramipexole in patients with Parkinson’s disease are controversial since in a study with patients diagnosed within two years from an age range of 30 to 79 years, no significant differences were observed at 15 months [123]. However, this study included patients with early and late onset and did not focus solely on patients with idiopathic Parkinson’s disease. In a study carried out in the human neuroblastoma cell line SH-SY5Y, it has been shown that pramipexole induces the expression of VMAT2 mRNA levels, which suggests the neuroprotective effect observed in SPECT studies [124]. This suggests that the search for molecules that induce the expression of VMAT2 may be a target for the search for new neuroprotective molecules in the treatment of Parkinson’s disease that can change the course of the disease. The increase in the expression of VMAT2 implies a risk of the existence of free dopamine in the cytosol that can be oxidized to neuromelanin that requires the formation of aminochrome that can potentially be neurotoxic decreases. (Figure 1, Table 6).
Figure 1.
Increased expression of VMAT2 prevents the existence of free dopamine in the cytosol and the synthesis of aminochrome during neuromelanin synthesis.
Table 6.
Summary.
|
|
|
VMAT2 is in the membrane of monoaminergic vesicles and catalyzes the transport of dopamine into the interior of monoaminergic vesicles where it is completely stable thanks to a slightly acidic environment. VMAT2 plays an essential role in preventing the existence of free dopamine in the cytosol and its oxidation to neuromelanin. Free dopamine in the cytosol can exist thanks to the synthesis of dopamine from the amino acid tyrosine and the reuptake of dopamine released during neurotransmission through the dopamine transporter (DAT). However, during dopamine synthesis VMAT forms a kind of complex with the enzymes tyrosine hydroxylase (TH) and aromatic L-amino acid decarboxylase (AADC) that prevents the existence of free dopamine in the cytosol. During the reuptake of dopamine through DAT after neurotransmission VMAT2 also forms a kind of complex with DAT and synaptogyrin-3 (Snp3) that prevents the existence of free dopamine in the cytosol.
7. Kelch-like ECH-Associated Protein 1/Nuclear Factor E2-Related Factor 2 (KEAP1/NRF2) Activation as a Target to Develop New Drugs for Parkinson’s Disease
The expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione transferase, catalase, heme oxygenase, and DT-diaphorase play an important role in neutralizing the effects of oxidative stress. Activation of the KEAP1/NRF2 signaling pathway allows NRF2 to activate the expression of antioxidant enzyme genes by binding to the antioxidant responsive element [125]. However, the activation of the KEAP2/NRF2 pathway in cancer cells can help develop resistance to antineoplastic drugs in which its mechanism of action is related to the generation of oxidative stress in cancer cells such as ovarian cancer or cervical and endometrial cancer [126,127]. In patients with preeclampsia, the activation of the KEAP1/NRF2 pathway has a protective effect to help neutralize oxidative stress and inflammation [128]. In other pathologies, the activation of the KEAP1/NRF2 pathway can have a protective effect, such as in celiac disease [129], ischemia/reperfusion [130], traumatic lung injury [131], nephrolithiasis [132], cardiovascular disease [133], and renal injury [134].
In the case of Parkinson’s disease, oxidative stress is one of the mechanisms involved in the degenerative process of neuromelanin-containing dopaminergic neurons [78]. Furthermore, the KEAP1/NRF2 signaling pathway induces the enzymes DT-diaphorase and glutathione transferase M2-2 [135,136,137], which prevent the neurotoxic effects of aminochrome that is formed during the synthesis of neuromelanin [78].
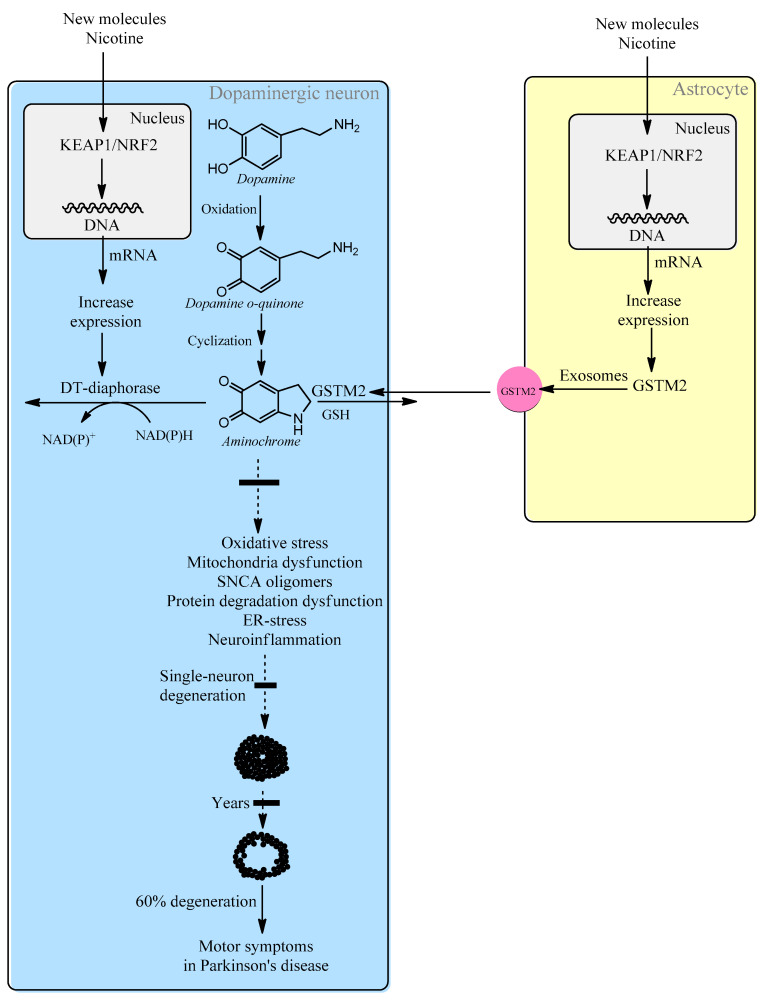
In the single-neuron degeneration model, the endogenous neurotoxin aminochrome is the molecule that triggers the degenerative process that leads to the loss of neuromelanin-containing dopaminergic neurons in an individual neuron. However, aminochrome cannot be used in a preclinical animal model because it is technically impossible to inject a single neuron with aminochrome. An intracerebral injection will have a massive effect on all the neurons as far as the aminochrome injection reaches. Therefore, it is technically impossible to test new molecules for the treatment of Parkinson’s disease in a single-neuron degeneration model. For this reason, a new strategy and target for the treatment of idiopathic Parkinson’s disease is to search for molecules that activate the KEAP1/NRF2 signaling pathway that leads to the induction of increased expression of DT-diaphorase and glutathione transferase M2-2 (Figure 2). Molecules such as nicotine that activate this pathway and also inhibit the neurotoxic effects of aminochrome in cell cultures may be potential new drugs for the treatment of idiopathic Parkinson’s disease [138,139] (Table 7).
Figure 2.
Intracellular increased expression of DT-diaphorase and glutathione transferase M2-2 through activation of the KEAP1/NRF2 signaling pathway will prevent single-neuron degeneration in idiopathic Parkinson’s disease.
Table 7.
Summary.
|
|
|
|
8. Conclusions
The absence of drugs that can halt or significantly slow the progression of idiopathic Parkinson’s disease requires the scientific community to explore new ideas such as the single-neuron degeneration model. This model of single-neuron neurodegeneration is based on the fact that the synthesis of neuromelanin can generate the endogenous neurotoxin aminochrome under certain circumstances. Neuromelanin synthesis is a normal and harmless process since healthy elderly people have neuromelanin-containing dopaminergic neurons intact in the substantia nigra at the time of death. However, the excessive production of aminochrome overcomes the neuroprotective capacity of the enzymes DT-diaphorase and glutathione transferase M2-2 that finally generates aminochrome neurotoxicity. The chemical characteristics of aminochrome, such as short stability time in the cytosol that depends on the presence of flavoenzymes that can reduce it or proteins with which it forms adducts, prevent it from having an expansive character that affects neighboring neurons [62,66,88,89]. This implies that aminochrome-induced neurotoxicity affects individual dopaminergic neurons, explaining the extremely slow rate of the degenerative process and progression of idiopathic Parkinson’s disease, which takes years.
If we agree that the oxidation of dopamine to aminochrome plays an essential role in the loss of neuromelanin-containing dopaminergic neurons in idiopathic Parkinson’s disease, we have to look for molecules that increase the expression of the enzymes DT-diaphorase and glutathione transferase M2-2 that prevent the neurotoxic effects of aminochrome, or molecules that inhibit the oxidation of dopamine to neuromelanin. The targets that our research should aim at in the search for new drugs for the treatment of idiopathic Parkinson’s disease include: (i) searching for molecules such as pramipexole that increase the expression of the VMAT2 transporter that prevents the existence of free dopamine that can oxidize the endogenous neurotoxin aminochrome during neuromelanin synthesis. Neuromelanin synthesis is inversely proportional to the level of VMAT2 expression. A higher expression of VMAT2 results in lower neuromelanin synthesis [106,107]; and (ii) searching for molecules that activate the KEAP1/NRF2 pathway to induce the expression of the neuroprotective enzymes DT-diaphorase and glutathione transferase M2-2 that prevent the neurotoxic effects of aminochrome during the synthesis of neuromelanin in dopaminergic neurons of the nigrostriatal system [78].
Author Contributions
Conceptualization, S.H. and J.S.-A.; writing—original draft preparation, J.S.-A.; writing—review and editing, S.H.; literature revision, S.H. and J.S.-A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lees A.J., Tolosa E., Olanow C.W. Four pioneers of L-dopa treatment: Arvid Carlsson, Oleh Hornykiewicz, George Cotzias, and Melvin Yahr. Mov. Disord. 2015;30:19–36. doi: 10.1002/mds.26120. [DOI] [PubMed] [Google Scholar]
- 2.Henrich M.T., Oertel W.H., Surmeier D.J., Geibl F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023;18:83. doi: 10.1186/s13024-023-00676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Qu S., Zhang Z., Tan L., Chen X., Zhong H.J., Chong C.M. Strategies targeting endoplasmic reticulum stress to improve Parkinson’s disease. Front. Pharmacol. 2023;14:1288894. doi: 10.3389/fphar.2023.1288894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Y., Zhong G., Ren M., Sun T., Li Y., Ye M., Ma C., Guo Y., Liu C. The Role of Ubiquitin-Proteasome System and Mitophagy in the Pathogenesis of Parkinson’s Disease. Neuromol. Med. 2023;25:471–488. doi: 10.1007/s12017-023-08755-0. [DOI] [PubMed] [Google Scholar]
- 5.Ko T.K., Tan D.J.Y. Is Disrupted Mitophagy a Central Player to Parkinson’s Disease Pathology? Cureus. 2023;15:e35458. doi: 10.7759/cureus.35458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nechushtai L., Frenkel D., Pinkas-Kramarski R. Autophagy in Parkinson’s Disease. Biomolecules. 2023;13:1435. doi: 10.3390/biom13101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Q., Yang P., Liu Y., Ding J., Lu M., Hu G. The interplay between α-Synuclein and NLRP3 inflammasome in Parkinson’s disease. Biomed. Pharmacother. 2023;168:115735. doi: 10.1016/j.biopha.2023.115735. [DOI] [PubMed] [Google Scholar]
- 8.Choong C.J., Mochizuki H. Involvement of Mitochondria in Parkinson’s Disease. Int. J. Mol. Sci. 2023;24:17027. doi: 10.3390/ijms242317027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baidya A.T., Deshwal S., Das B., Mathew A.T., Devi B., Sandhir R., Kumar R. Catalyzing a Cure: Discovery and development of LRRK2 inhibitors for the treatment of Parkinson’s disease. Bioorg. Chem. 2023;143:106972. doi: 10.1016/j.bioorg.2023.106972. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniyan S., Kuriakose B.B., Mushfiq S., Prabhu N.M., Muthusamy K. Gene Signals and SNPs Associated with Parkinson’s Disease: A Nutrigenomics and Computational Prospective Insights. Neuroscience. 2023;533:77–95. doi: 10.1016/j.neuroscience.2023.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Morris H.R., Spillantini M.G., Sue C.M., Williams-Gray C.H. The pathogenesis of Parkinson’s disease. Lancet. 2024;403:293–304. doi: 10.1016/S0140-6736(23)01478-2. [DOI] [PubMed] [Google Scholar]
- 12.Karikari A.A., McFleder R.L., Ribechini E., Blum R., Bruttel V., Knorr S., Gehmeyr M., Volkmann J., Brotchie J.M., Ahsan F., et al. Neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson’s disease mice. Brain Behav. Immun. 2022;101:194–210. doi: 10.1016/j.bbi.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.M., Anwar S., Kim Y., Brown J., Comte I., Cai H., Cai N.N., Wade-Martins R., Szele F.G. The A30P α-synuclein mutation decreases subventricular zone proliferation. Hum. Mol. Genet. 2019;28:2283–2294. doi: 10.1093/hmg/ddz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuber S., Rajsombath M., Minakaki G., Winkler J., Müller C.P., Ericsson M., Caldarone B., Dettmer U., Selkoe D.J. Abrogating Native α-Synuclein Tetramers in Mice Causes a L-DOPA-Responsive Motor Syndrome Closely Resembling Parkinson’s Disease. Neuron. 2018;100:75–90.e5. doi: 10.1016/j.neuron.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh Y., Trautwein C., Romani J., Salker M.S., Neckel P.H., Fraccaroli I., Abeditashi M., Woerner N., Admard J., Dhariwal A., et al. Overexpression of human alpha-Synuclein leads to dysregulated microbiome/metabolites with ageing in a rat model of Parkinson disease. Mol. Neurodegener. 2023;18:44. doi: 10.1186/s13024-023-00628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan L., Heusinkveld H.J., Paul K.C., Hughes S., Darweesh S.K.L., Bloem B.R., Homberg J.R. Towards improved screening of toxins for Parkinson’s risk. NPJ Park. Dis. 2023;9:169. doi: 10.1038/s41531-023-00615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon D., Paul K.C., Yu Y., Zhang K., Folle A.D., Wu J., Bronstein J.M., Ritz B. Traffic-related air pollution and Parkinson’s disease in central California. Environ. Res. 2024;240:117434. doi: 10.1016/j.envres.2023.117434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirandeh E., Palizgir A., Kassiri N. An Overview of the Relationship Between Occupational Manganese Exposure and Parkinsonism. Cureus. 2022;14:e32161. doi: 10.7759/cureus.32161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racette B.A. Manganism in the 21st century: The Hanninen lecture. Neurotoxicology. 2014;45:201–207. doi: 10.1016/j.neuro.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caviedes P., Segura-Aguilar J. The price of development in Chile: Overcoming environmental hazards produced by heavy industrial exploitation. Neuroreport. 2001;12:A25. doi: 10.1097/00001756-200103260-00004. [DOI] [Google Scholar]
- 21.Williams A. MPTP parkinsonism. Br. Med. J. 1984;289:1401–1402. doi: 10.1136/bmj.289.6456.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visanji N., Marras C. The relevance of pre-motor symptoms in Parkinson’s disease. Expert Rev. Neurother. 2015;15:1205–1217. doi: 10.1586/14737175.2015.1083423. [DOI] [PubMed] [Google Scholar]
- 23.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Jellinger K.A. Is Braak staging valid for all types of Parkinson’s disease? J. Neural Transm. 2019;126:423–431. doi: 10.1007/s00702-018-1898-9. [DOI] [PubMed] [Google Scholar]
- 25.Bowler R.M., Koller W., Schulz P.E. Parkinsonism due to manganism in a welder: Neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Hui T., Guo S. Early onset Parkinson’s disease in the cycle of 3,4-methylenedioxymethamphetamine and substance use: A case report. J. Med. Case Rep. 2023;17:405. doi: 10.1186/s13256-023-04147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Acuña D., Shin S.J., Rhee K.H., Kim S.J., Lee S.J. α-Synuclein propagation leads to synaptic abnormalities in the cortex through microglial synapse phagocytosis. Mol. Brain. 2023;16:72. doi: 10.1186/s13041-023-01059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra S., Sahay S., Maji S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019;1867:890–908. doi: 10.1016/j.bbapap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Henderson M.X., Cornblath E.J., Darwich A., Zhang B., Brown H., Gathagan R.J., Sandler R.M., Bassett D.S., Trojanowski J.Q., Lee V.M.Y. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat. Neurosci. 2019;22:1248–1257. doi: 10.1038/s41593-019-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piroska L., Fenyi A., Thomas S., Plamont M.A., Redeker V., Melki R., Gueroui Z. α-Synuclein liquid condensates fuel fibrillar α-synuclein growth. Sci. Adv. 2023;9:eadg5663. doi: 10.1126/sciadv.adg5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuzumi A., Hatano T., Matsumoto G., Nojiri S., Ueno S.I., Imamichi-Tatano Y., Kimura H., Kakuta S., Kondo A., Fukuhara T., et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. 2023;29:1448–1455. doi: 10.1038/s41591-023-02358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vascellari S., Orrù C.D., Groveman B.R., Parveen S., Fenu G., Pisano G., Piga G., Serra G., Oppo V., Murgia D., et al. α-Synuclein seeding activity in duodenum biopsies from Parkinson’s disease patients. PLoS Pathog. 2023;19:e1011456. doi: 10.1371/journal.ppat.1011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prymaczok N.C., De Francesco P.N., Mazzetti S., Humbert-Claude M., Tenenbaum L., Cappelletti G., Masliah E., Perello M., Riek R., Gerez J.A. Cell-to-cell transmitted alpha-synuclein recapitulates experimental Parkinson’s disease. NPJ Park. Dis. 2024;10:10. doi: 10.1038/s41531-023-00618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giasson B.I., Covy J.P., Bonini N.M., Hurtig H.I., Farrer M.J., Trojanowski J.Q., Van Deerlin V.M. Biochemical and pathological characterization of Lrrk2. Ann. Neurol. 2006;59:315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 36.Gaig C., Martí M.J., Ezquerra M., Rey M.J., Cardozo A., Tolosa E. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. J. Neurol. Neurosurg. Psychiatry. 2007;78:626–628. doi: 10.1136/jnnp.2006.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling H., Kara E., Bandopadhyay R., Hardy J., Holton J., Xiromerisiou G., Lees A., Houlden H., Revesz T. TDP-43 pathology in a patient carrying G2019S LRRK2 mutation and a novel p.Q124E MAPT. Neurobiol. Aging. 2013;34:2889.e5–2889.e9. doi: 10.1016/j.neurobiolaging.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori H., Kondo T., Yokochi M., Matsumine H., Nakagawa-Hattori Y., Miyake T., Suda K., Mizuno Y. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51:890–892. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- 39.Segura-Aguilar J. Can we conclude a potential therapeutic action for Parkinson’s disease by using postmortem tissue and a preclinical model based on an exogenous neurotoxin? Cell Death Dis. 2018;9:748. doi: 10.1038/s41419-018-0798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinnell J.R., Cui M., Tieu K. Exosomes in Parkinson disease. J. Neurochem. 2021;157:413–428. doi: 10.1111/jnc.15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakabayashi K., Tanji K., Odagiri S., Miki Y., Mori F., Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 42.Ingelsson M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016;10:408. doi: 10.3389/fnins.2016.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du X.Y., Xie X.X., Liu R.T. The Role of α-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:8645. doi: 10.3390/ijms21228645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv Q.K., Tao K.X., Wang X.B., Yao X.Y., Pang M.Z., Liu J.Y., Wang F., Liu C.F. Role of α-synuclein in microglia: Autophagy and phagocytosis balance neuroinflammation in Parkinson’s disease. Inflamm. Res. 2023;72:443–462. doi: 10.1007/s00011-022-01676-x. [DOI] [PubMed] [Google Scholar]
- 45.Morales-Martínez A., Martínez-Gómez P.A., Martinez-Fong D., Villegas-Rojas M.M., Pérez-Severiano F., Del Toro-Colín M.A., Delgado-Minjares K.M., Blanco-Alvarez V.M., Leon-Chavez B.A., Aparicio-Trejo O.E., et al. Oxidative Stress and Mitochondrial Complex I Dysfunction Correlate with Neurodegeneration in an α-Synucleinopathy Animal Model. Int. J. Mol. Sci. 2022;23:11394. doi: 10.3390/ijms231911394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ordonez D.G., Lee M.K., Feany M.B. α-synuclein Induces Mitochondrial Dysfunction through Spectrin and the Actin Cytoskeleton. Neuron. 2018;97:108–124.e6. doi: 10.1016/j.neuron.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocha E.M., De Miranda B., Sanders L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Pt BNeurobiol. Dis. 2018;109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang R., Sun H., Ren H., Wang G. α-Synuclein aggregation and transmission in Parkinson’s disease: A link to mitochondria and lysosome. Sci. China Life Sci. 2020;63:1850–1859. doi: 10.1007/s11427-020-1756-9. [DOI] [PubMed] [Google Scholar]
- 49.Bigi A., Cascella R., Cecchi C. α-Synuclein oligomers and fibrils: Partners in crime in synucleinopathies. Neural Regen. Res. 2023;18:2332–2342. doi: 10.4103/1673-5374.371345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kordower J.H., Dodiya H.B., Kordower A.M., Terpstra B., Paumier K., Madhavan L., Sortwell C., Steece-Collier K., Collier T.J. Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol. Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valensin D., Dell’Acqua S., Kozlowski H., Casella L. Coordination and redox properties of copper interaction with α-synuclein. J. Inorg. Biochem. 2016;163:292–300. doi: 10.1016/j.jinorgbio.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Perissinotto F., Stani C., De Cecco E., Vaccari L., Rondelli V., Posocco P., Parisse P., Scaini D., Legname G., Casalis L. Iron-mediated interaction of alpha synuclein with lipid raft model membranes. Nanoscale. 2020;12:7631–7640. doi: 10.1039/d0nr00287a. [DOI] [PubMed] [Google Scholar]
- 55.Masato A., Plotegher N., Terrin F., Sandre M., Faustini G., Thor A., Adams S., Berti G., Cogo S., De Lazzari F., et al. DOPAL initiates αSynuclein-dependent impaired proteostasis and degeneration of neuronal projections in Parkinson’s disease. NPJ Park. Dis. 2023;9:42. doi: 10.1038/s41531-023-00485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma M., Sharma N., Khairnar A. Intranasal Rotenone Induces Alpha-Synuclein Accumulation, Neuroinflammation and Dopaminergic Neurodegeneration in Middle-Aged Mice. Neurochem. Res. 2023;48:1543–1560. doi: 10.1007/s11064-022-03847-y. [DOI] [PubMed] [Google Scholar]
- 57.Ma Z., Liu K., Zhang R.F., Xie Z.X., Liu W., Deng Y., Li X., Xu B. Manganese-induced α-synuclein overexpression promotes the accumulation of dysfunctional synaptic vesicles and hippocampal synaptotoxicity by suppressing Rab26-dependent autophagy in presynaptic neurons. Pt 1Sci. Total Environ. 2023;858:159753. doi: 10.1016/j.scitotenv.2022.159753. [DOI] [PubMed] [Google Scholar]
- 58.Kissani N., Naji Y., Mebrouk Y., Chraa M., Ghanima A. Parkinsonism and chronic manganese exposure: Pilot study with clinical, environmental and experimental evidence. Clin. Park. Relat. Disord. 2020;3:100057. doi: 10.1016/j.prdoa.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mor D.E., Daniels M.J., Ischiropoulos H. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov. Disord. 2019;34:167–179. doi: 10.1002/mds.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bisaglia M., Mammi S., Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: Analysis of the interactions with alpha-synuclein. J. Biol. Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- 61.Sivakumar P., Nagashanmugam K.B., Priyatharshni S., Lavanya R., Prabhu N., Ponnusamy S. Review on the interactions between dopamine metabolites and α-Synuclein in causing Parkinson’s disease. Neurochem. Int. 2023;162:105461. doi: 10.1016/j.neuint.2022.105461. [DOI] [PubMed] [Google Scholar]
- 62.Muñoz P., Cardenas S., Huenchuguala S., Briceño A., Couve E., Paris I., Segura-Aguilar J. DT-Diaphorase Prevents Aminochrome-Induced Alpha-Synuclein Oligomer Formation and Neurotoxicity. Toxicol. Sci. 2015;145:37–47. doi: 10.1093/toxsci/kfv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S., Wang R., Wang G. Impact of Dopamine Oxidation on Dopaminergic Neurodegeneration. ACS Chem. Neurosci. 2019;10:945–953. doi: 10.1021/acschemneuro.8b00454. [DOI] [PubMed] [Google Scholar]
- 64.Latif S., Jahangeer M., Maknoon Razia D., Ashiq M., Ghaffar A., Akram M., El Allam A., Bouyahya A., Garipova L., Ali Shariati M., et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta. 2021;522:114–126. doi: 10.1016/j.cca.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Segura-Aguilar J., Paris I., Muñoz P., Ferrari E., Zecca L., Zucca F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- 66.Bisaglia M., Soriano M.E., Arduini I., Mammi S., Bubacco L. Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: Implications for mitochondrial dysfunction in Parkinson disease. Biochim. Biophys. Acta. 2010;1802:699–706. doi: 10.1016/j.bbadis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Segura-Aguilar J., editor. Clinical Studies and Therapies in Parkinson’s Disease: Translations from Preclinical Models. Elsevier; Cambridge, MA, USA: 2021. Dopamine oxidation to neuromelanin and neurotoxic metabolites; pp. 213–223. [Google Scholar]
- 68.Herrera A., Muñoz P., Steinbusch H.W.M., Segura-Aguilar J. Are Dopamine Oxidation Metabolites Involved in the Loss of Dopaminergic Neurons in the Nigrostriatal System in Parkinson’s Disease? ACS Chem. Neurosci. 2017;8:702–711. doi: 10.1021/acschemneuro.7b00034. [DOI] [PubMed] [Google Scholar]
- 69.Chagraoui A., Anouar Y., De Deurwaerdere P., Arias H.R. To what extent may aminochrome increase the vulnerability of dopaminergic neurons in the context of Parkinson’s disease. Int. J. Biochem. Cell Biol. 2024;168:106528. doi: 10.1016/j.biocel.2024.106528. [DOI] [PubMed] [Google Scholar]
- 70.Zafar K.S., Siegel D., Ross D. A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol. Pharmacol. 2006;70:1079–1086. doi: 10.1124/mol.106.024703. [DOI] [PubMed] [Google Scholar]
- 71.Xiong R., Siegel D., Ross D. Quinone-induced protein handling changes: Implications for major protein handling systems in quinone-mediated toxicity. Toxicol. Appl. Pharmacol. 2014;280:285–295. doi: 10.1016/j.taap.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Araújo F.M., Frota A.F., de Jesus L.B., Macedo T.C., Cuenca-Bermejo L., Sanchez-Rodrigo C., Ferreira K.M.S., de Oliveira J.V.R., de Fatima Dias Costa M., Segura-Aguilar J., et al. Aminochrome Induces Neuroinflammation and Dopaminergic Neuronal Loss: A New Preclinical Model to Find Anti-inflammatory and Neuroprotective Drugs for Parkinson’s Disease. Cell Mol. Neurobiol. 2023;43:265–281. doi: 10.1007/s10571-021-01173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein D.S. The “Sick-but-not-Dead” phenomenon applied to catecholamine deficiency in neurodegenerative diseases. Semin. Neurol. 2020;40:502–514. doi: 10.1055/s-0040-1713874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jinsmaa Y., Sullivan P., Sharabi Y., Goldstein D.S. DOPAL is transmissible to and oligomerizes alpha-synuclein in human glial cells. Auton. Neurosci. 2016;194:46–51. doi: 10.1016/j.autneu.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jinsmaa Y., Sullivan P., Gross D., Cooney A., Sharabi Y., Goldstein D.S. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci. Lett. 2014;569:27–32. doi: 10.1016/j.neulet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grünblatt E., Mandel S., Jacob-Hirsch J., Zeligson S., Amariglo N., Rechavi G., Li J., Ravid R., Roggendorf W., Riederer P., et al. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome heat shock protein iron and oxidative stress regulated proteins cell adhesion/cellular matrix and vesicle trafficking genes. J. Neural Transm. 2004;111:1543–1573. doi: 10.1007/s00702-004-0212-1. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein D.S., Kopin I.J., Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huenchuguala S., Segura-Aguilar J. Single-neuron neurodegeneration as a degenerative model for Parkinson’s disease. Neural Regen. Res. 2024;19:529–535. doi: 10.4103/1673-5374.380878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagatsu T., Nakashima A., Ichinose H., Kobayashi K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Transm. 2019;126:397–409. doi: 10.1007/s00702-018-1903-3. [DOI] [PubMed] [Google Scholar]
- 80.Ren L.Q., Wienecke J., Hultborn H., Zhang M. Production of Dopamine by Aromatic l-Amino Acid Decarboxylase Cells after Spinal Cord Injury. J. Neurotrauma. 2016;33:1150–1160. doi: 10.1089/neu.2015.4037. [DOI] [PubMed] [Google Scholar]
- 81.Segura-Aguilar J., Paris I. Mechanisms of Dopamine Oxidation and Parkinson’s Disease. In: Kostrzewa R., editor. Handbook of Neurotoxicity. Springer; New York, NY, USA: 2014. pp. 865–883. [DOI] [Google Scholar]
- 82.Zecca L., Casella L., Albertini A., Bellei C., Zucca F.A., Engelen M., Zadlo A., Szewczyk G., Zareba M., Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J. Neurochem. 2008;106:1866–1875. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- 83.Zucca F.A., Capucciati A., Bellei C., Sarna M., Sarna T., Monzani E., Casella L., Zecca L. Neuromelanins in brain aging and Parkinson’s disease: Synthesis, structure, neuroinflammatory, and neurodegenerative role. IUBMB Life. 2023;75:55–65. doi: 10.1002/iub.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zucca F.A., Segura-Aguilar J., Ferrari E., Muñoz P., Paris I., Sulzer D., Sarna T., Casella L., Zecca L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017;155:96–119. doi: 10.1016/j.pneurobio.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segura-Aguilar J., Metodiewa D., Welch C.J. Metabolic activation of dopamine o-quinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim. Biophys. Acta. 1998;1381:1–6. doi: 10.1016/S0304-4165(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 86.Paris I., Perez-Pastene C., Cardenas S., Iturriaga-Vasquez P., Muñoz P., Couve E., Caviedes P., Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox. Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- 87.Briceño A., Muñoz P., Brito P., Huenchuguala S., Segura-Aguilar J., Paris I.B. Aminochrome Toxicity is Mediated by Inhibition of Microtubules Polymerization Through the Formation of Adducts with Tubulin. Neurotox. Res. 2016;29:381–393. doi: 10.1007/s12640-015-9560-x. [DOI] [PubMed] [Google Scholar]
- 88.Segura-Aguilar J., Lind C. On the mechanism of the Mn3+-induced neurotoxicity of dopamine: Prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem. Biol. Interact. 1989;72:309–324. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 89.Lozano J., Muñoz P., Nore B.F., Ledoux S., Segura-Aguilar J. Stable expression of short interfering RNA for DT-diaphorase induces neurotoxicity. Chem. Res. Toxicol. 2010;23:1492–1496. doi: 10.1021/tx100182a. [DOI] [PubMed] [Google Scholar]
- 90.Segura-Aguilar J., Muñoz P., Inzunza J., Varshney M., Nalvarte I., Mannervik B. Neuroprotection against Aminochrome Neurotoxicity: Glutathione Transferase M2-2 and DT-Diaphorase. Antioxidants. 2022;11:296. doi: 10.3390/antiox11020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segura-Aguilar J., Baez S., Widersten M., Welch C.J., Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J. Biol. Chem. 1997;272:5727–5731. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- 92.Dagnino-Subiabre A., Cassels B.K., Baez S., Johansson A.S., Mannervik B., Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem. Biophys. Res. Commun. 2000;274:32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 93.Cheng F.C., Kuo J.S., Chia L.G., Dryhurst G. Elevated 5-S-cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: Possible markers for and potential insights into the pathoetiology of Parkinson’s disease. J. Neural Transm. 1996;103:433–446. doi: 10.1007/BF01276419. [DOI] [PubMed] [Google Scholar]
- 94.Rosengren E., Linder-Eliasson E., Carlsson A. Detection of 5-S-cysteinyldopamine in human brain. J. Neural Transm. 1985;63:247–253. doi: 10.1007/BF01252029. [DOI] [PubMed] [Google Scholar]
- 95.Cuevas C., Huenchuguala S., Muñoz P., Villa M., Paris I., Mannervik B., Segura-Aguilar J. Glutathione transferase-M2-2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox. Res. 2015;27:217–228. doi: 10.1007/s12640-014-9500-1. [DOI] [PubMed] [Google Scholar]
- 96.Valdes R., Armijo A., Muñoz P., Hultenby K., Hagg A., Inzunza J., Nalvarte I., Varshney M., Mannervik B., Segura-Aguilar J. Cellular Trafficking of Glutathione Transferase M2-2 Between U373MG and SHSY-S7 Cells is Mediated by Exosomes. Neurotox. Res. 2021;39:182–190. doi: 10.1007/s12640-020-00327-5. [DOI] [PubMed] [Google Scholar]
- 97.Segura-Aguilar J., Mannervik B., Inzunza J., Varshney M., Nalvarte I., Muñoz P. Astrocytes protect dopaminergic neurons against aminochrome neurotoxicity. Neural Regen. Res. 2022;17:1861–1866. doi: 10.4103/1673-5374.335690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller D.B., O’Callaghan J.P. Biomarkers of Parkinson’s disease: Present and future. Metabolism. 2015;64((Suppl. S1)):S40–S46. doi: 10.1016/j.metabol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burke R.E., O’Malley K. Axon degeneration in Parkinson’s disease. Exp. Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segura-Aguilar Sulzer D., Zucca F.A., Zecca L. Overexpression of Vesicular Monoamine Transporter-2 may Block Neurotoxic Metabolites from Cytosolic Dopamine: A Potential Neuroprotective Therapy for Parkinson’s Disease. Clin. Pharmacol. Transl. Med. 2019;3:143–148. [PMC free article] [PubMed] [Google Scholar]
- 101.Biosa A., Arduini I., Soriano M.E., Giorgio V., Bernardi P., Bisaglia M., Bubacco L. Dopamine Oxidation Products as Mitochondrial Endotoxins, a Potential Molecular Mechanism for Preferential Neurodegeneration in Parkinson’s Disease. ACS Chem. Neurosci. 2018;9:2849–2858. doi: 10.1021/acschemneuro.8b00276. [DOI] [PubMed] [Google Scholar]
- 102.Cartier E.A., Parra L.A., Baust T.B., Quiroz M., Salazar G., Faundez V., Egaña L., Torres G.E. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J. Biol. Chem. 2010;285:1957–1966. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egaña L.A., Cuevas R.A., Baust T.B., Parra L.A., Leak R.K., Hochendoner S., Peña K., Quiroz M., Hong W.C., Dorostkar M.M., et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang C.L., Nelson O., Yazdani U., Pasbakhsh P., German D.C. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: Human midbrain dopamine neurons. J. Comp. Neurol. 2004;473:97–106. doi: 10.1002/cne.20098. [DOI] [PubMed] [Google Scholar]
- 105.Sulzer D., Bogulavsky J., Larsen K.E., Behr G., Karatekin E., Kleinman M.H., Turro N., Krantz D., Edwards R.H., Greene L.A., et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Athauda D., Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 107.Segura-Aguilar J. Neurotoxins as Preclinical Models for Parkinson’s Disease. Neurotox. Res. 2018;34:870–877. doi: 10.1007/s12640-017-9856-0. [DOI] [PubMed] [Google Scholar]
- 108.Jing L., He M.T., Chang Y., Mehta S.L., He Q.P., Zhang J.Z., Li P.A. Coenzyme Q10 protects astrocytes from ROS-induced damage through inhibition of mitochondria-mediated cell death pathway. Int. J. Biol. Sci. 2015;11:59–66. doi: 10.7150/ijbs.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beal M.F. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors. 1999;9:261–266. doi: 10.1002/biof.5520090222. [DOI] [PubMed] [Google Scholar]
- 110.Park H.W., Park C.G., Park M., Lee S.H., Park H.R., Lim J., Paek S.H., Choy Y.B. Intrastriatal administration of coenzyme Q10 enhances neuroprotection in a Parkinson’s disease rat model. Sci. Rep. 2020;10:9572. doi: 10.1038/s41598-020-66493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parkinson Study Group QE3 Investigators. Beal M.F., Oakes D., Shoulson I., Henchcliffe C., Galpern W.R., Haas R., Juncos J.L., Nutt J.G., Voss T.S., et al. Randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014;71:543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 112.Ghosh A., Chandran K., Kalivendi S.V., Joseph J., Antholine W.E., Hillard C.J., Kanthasamy A., Kanthasamy A., Kalyanaraman B. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic. Biol. Med. 2010;49:1674–1684. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xi Y., Feng D., Tao K., Wang R., Shi Y., Qin H., Murphy M.P., Yang Q., Zhao G. MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Pt BBiochim. Biophys. Acta Mol. Basis Dis. 2018;1864:2859–2870. doi: 10.1016/j.bbadis.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 114.Solesio M.E., Prime T.A., Logan A., Murphy M.P., Del Mar Arroyo-Jimenez M., Jordán J., Galindo M.F. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson’s disease. Biochim. Biophys. Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Snow B.J., Rolfe F.L., Lockhart M.M., Frampton C.M., O’Sullivan J.D., Fung V., Smith R.A., Murphy M.P., Taylor K.M., Protect Study Group A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 116.Gong L., Zhang Q.L., Zhang N., Hua W.Y., Huang Y.X., Di P.W., Huang T., Xu X.S., Liu C.F., Hu L.F., et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: Linking to Akt/GSK3β signaling pathway. J. Neurochem. 2012;123:876–885. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- 117.Huang T.T., Hao D.L., Wu B.N., Mao L.L., Zhang J. Uric acid demonstrates neuroprotective effect on Parkinson’s disease mice through Nrf2-ARE signaling pathway. Biochem. Biophys. Res. Commun. 2017;493:1443–1449. doi: 10.1016/j.bbrc.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Crotty G.F., Ascherio A., Schwarzschild M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Pt BExp. Neurol. 2017;298:210–224. doi: 10.1016/j.expneurol.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parkinson Study Group SURE-PD3 Investigators. Schwarzschild M.A., Ascherio A., Casaceli C., Curhan G.C., Fitzgerald R., Kamp C., Lungu C., Macklin E.A., Marek K., et al. Effect of Urate-Elevating Inosine on Early Parkinson Disease Progression: The SURE-PD3 Randomized Clinical Trial. JAMA. 2021;326:926–939. doi: 10.1001/jama.2021.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni A., Ernst C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell Neurosci. 2022;16:826193. doi: 10.3389/fncel.2022.826193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bucher M.L., Barrett C.W., Moon C.J., Mortimer A.D., Burton E.A., Greenamyre J.T., Hastings T.G. Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson’s disease. NPJ Park. Dis. 2020;6:34. doi: 10.1038/s41531-020-00134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clarke C.E., Guttman M. Dopamine agonist monotherapy in Parkinson’s disease. Lancet. 2002;360:1767–1769. doi: 10.1016/s0140-6736(02)11668-0. [DOI] [PubMed] [Google Scholar]
- 123.Schapira A.H., McDermott M.P., Barone P., Comella C.L., Albrecht S., Hsu H.H., Massey D.H., Mizuno Y., Poewe W., Rascol O., et al. Pramipexole in patients with early Parkinson’s disease (PROUD): A randomised delayed-start trial. Lancet Neurol. 2013;12:747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan T., Xie W., Jankovic J., Le W. Biological effects of pramipexole on dopaminergic neuron-associated genes: Relevance to neuroprotection. Neurosci. Lett. 2005;377:106–109. doi: 10.1016/j.neulet.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 125.Ulasov A.V., Rosenkranz A.A., Georgiev G.P., Sobolev A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tossetta G., Marzioni D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022;183:106365. doi: 10.1016/j.phrs.2022.106365. [DOI] [PubMed] [Google Scholar]
- 127.Tossetta G., Marzioni D. Targeting the NRF2/KEAP1 pathway in cervical and endometrial cancers. Eur. J. Pharmacol. 2023;941:175503. doi: 10.1016/j.ejphar.2023.175503. [DOI] [PubMed] [Google Scholar]
- 128.Tossetta G., Fantone S., Piani F., Crescimanno C., Ciavattini A., Giannubilo S.R., Marzioni D. Modulation of NRF2/KEAP1 Signaling in Preeclampsia. Cells. 2023;12:1545. doi: 10.3390/cells12111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang C., Cui C., Li N., Sun X., Wen L., Gao E., Wang F. Antioxidant activity and protective effect of wheat germ peptides in an in vitro celiac disease model via Keap1/Nrf2 signaling pathway. Food Res. Int. 2022;161:111864. doi: 10.1016/j.foodres.2022.111864. [DOI] [PubMed] [Google Scholar]
- 130.Ucar B.I., Ucar G., Saha S., Buttari B., Profumo E., Saso L. Pharmacological Protection against Ischemia-Reperfusion Injury by Regulating the Nrf2-Keap1-ARE Signaling Pathway. Antioxidants. 2021;10:823. doi: 10.3390/antiox10060823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu L.Y., Cui J.B., Xu X.M., Huang Z.H., Jiao H.T. Expression of Nrf2-Keap1-ARE signal pathway in traumatic lung injury and functional study. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1402–1408. doi: 10.26355/eurrev_201803_14486. [DOI] [PubMed] [Google Scholar]
- 132.Yang B., Wang G., Li Y., Yang T., Guo H., Li P., Li J. Hydroxycitric acid prevents hyperoxaluric-induced nephrolithiasis and oxidative stress via activation of the Nrf2/Keap1 signaling pathway. Cell Cycle. 2023;22:1884–1899. doi: 10.1080/15384101.2023.2247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mann G.E., Bonacasa B., Ishii T., Siow R.C. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009;9:139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Zheng Y., Lu H., Huang H. Desflurane Preconditioning Protects against Renal Ischemia-Reperfusion Injury and Inhibits Inflammation and Oxidative Stress in Rats through Regulating the Nrf2-Keap1-ARE Signaling Pathway. Drug Des. Devel Ther. 2020;14:1351–1362. doi: 10.2147/DDDT.S223742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Segura-Aguilar J., Mannervik B. A Preclinical Model for Parkinson’s Disease Based on Transcriptional Gene Activation via KEAP1/NRF2 to Develop New Antioxidant Therapies. Antioxidants. 2023;12:673. doi: 10.3390/antiox12030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Torrente L., Prieto-Farigua N., Falzone A., Elkins C.M., Boothman D.A., Haura E.B., DeNicola G.M. Inhibition of TXNRD or SOD1 overcomes NRF2-mediated resistance to β-lapachone. Redox Biol. 2020;30:101440. doi: 10.1016/j.redox.2020.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamaki Y., Tabuchi T., Takahashi T., Kosaka K., Satoh T. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–688. doi: 10.1055/s-0029-1240622. [DOI] [PubMed] [Google Scholar]
- 138.Muñoz P., Huenchuguala S., Paris I., Cuevas C., Villa M., Caviedes P., Segura-Aguilar J., Tizabi Y. Protective effects of nicotine against aminochrome-induced toxicity in substantia nigra derived cells: Implications for Parkinson’s disease. Neurotox. Res. 2012;22:177–180. doi: 10.1007/s12640-012-9326-7. Erratum in Neurotox. Res. 2012, 22, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liou S.F., Nguyen T.T.N., Hsu J.H., Sulistyowati E., Huang S.E., Wu B.N., Lin M.C., Yeh J.L. The Preventive Effects of Xanthohumol on Vascular Calcification Induced by Vitamin D3 Plus Nicotine. Antioxidants. 2020;9:956. doi: 10.3390/antiox9100956. [DOI] [PMC free article] [PubMed] [Google Scholar]