Abstract
The outcome and protective efficacy of maternal antibodies elicited by DNA immunization to the large (L) hepadnavirus envelope protein were studied using the duck hepatitis B virus (DHBV) model. Following genetic immunization of breeding ducks with a DHBV L protein gene-bearing plasmid, specific and highly neutralizing antibodies were transferred from the sera of immunized ducks, via the egg yolk, to the progeny of vaccinees. Interestingly, large amounts (60 to 100 mg/egg) of high-titer and L protein-specific yolk immunoglobulins (immunoglobulin Y) accumulated in the egg yolk. These results suggest that eggs from genetically immunized avians may represent a potent source of DNA-designed antibodies specific to viral antigen. Importantly, these antibodies are vertically transmitted and protect offspring against high-titer DHBV challenge.
Genetic vaccination is a promising novel approach which has been shown to elicit specific immunity against different pathogens, including hepatitis B virus (HBV) (reviewed in reference 8). However, it is not known whether maternal antibodies elicited by DNA immunization against hepadnavirus envelope proteins may protect progeny against viral infection. Moreover, the future of antibodies elicited in DNA-immunized avians and the protection of their offspring have not been investigated. Following protein vaccination of breeding ducks or chickens, only one class of maternal immunoglobulin, immunoglobulin Y (IgY), which is considered to be equivalent to mammalian IgG, is transferred from the blood circulation via the egg yolk to the offspring (13). In this regard, there is a growing interest in the avian egg as a potent supplier of antibodies, since following immunization with a given antigen, large amounts of antigen-specific IgY accumulate in the egg yolk, from which it can be easily isolated in purified form.
In this study we have chosen the duck HBV (DHBV) model to study the outcome of maternal antibodies elicited by DNA immunization against large (L) hepadnavirus envelope protein. The major DHBV neutralization epitopes, which are also involved in host cell interaction, map within the pre-S region of the 36-kDa L envelope protein (5, 17). We and others have previously reported that antibodies elicited by genetic immunization with a plasmid bearing genes expressing the DHBV envelope proteins neutralize DHBV infectivity when the antibodies are preincubated with virus before infection of primary duck hepatocytes (PDHs) or neonatal ducklings (16, 18). We investigated here the transfer of maternal anti-DHBV envelope antibodies from DNA-immunized ducks to hatchlings via the egg and their ability to confer protection to progeny of vaccinees.
Large amounts of specific and neutralizing antibodies can be purified from egg yolk following DNA immunization of ducks against DHBV L envelope protein.
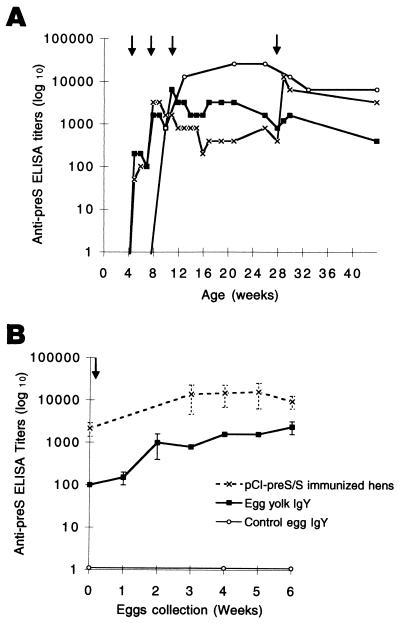
First, we investigated whether antibodies elicited by DNA immunization against DHBV L protein are transmitted to the egg yolk. Three laying Pekin ducks (Anas domesticus) were immunized intramuscularly with 100 μg of the recombinant pCI-preS/S plasmid, which expresses the DHBV L protein, or the empty pCI plasmid in saline buffer (NaCl, 0.9%) at weeks 4, 7, 10, as described previously (16), and boosted under the same conditions with 200 μg of plasmid at week 28. The kinetics of the anti-preS antibody response were tested by a previously described enzyme-linked immunosorbent assay (ELISA) (5). The results showed that DNA immunization of the ducks induced high titers of antibody that reached a plateau after the first boost (Fig. 1A), but anti-preS antibodies were not detected in the control ducks immunized with the empty pCI vector (data not shown). Eggs were collected weekly, during six consecutive weeks, from the laying ducks starting after the last DNA boost. Total immunoglobulins from egg yolk sac were extracted and purified by using the EGGstract IgY Purification System (Promega, Charbonnieres, France). As shown in Fig. 1B, no anti-preS antibodies were detected in eggs laid by pCI-immunized ducks. In contrast, the yolk antibodies purified from eggs laid by pCI-preS/S-immunized ducks had high ELISA titers of anti-preS antibody which paralleled those of the sera of the immunized ducks and no decrease in titer was seen during six consecutive weeks of follow-up without additional DNA boosts (Fig. 1B). Importantly, large amounts, i.e., 60 to 100 mg of purified IgY, were obtained from each egg yolk, and there was little variation in egg-to-egg anti-preS antibody titers at each time point.
FIG. 1.
Follow-up of humoral anti pre-S antibody responses in sera of DNA-immunized laying ducks and in their eggs. (A) Individual kinetics of anti-preS antibody titers of three immunized female ducks following immunization with the pCI-preS/S plasmid. Arrows indicate DNA injections at weeks 4, 7, 10, and 28. (B) --X--, mean anti-preS antibody titers in the sera of these pCI-preS/S-immunized ducks after the last DNA boost (arrow); —■—, mean anti-preS yolk IgY titers from two to four eggs laid by these ducks at each time point; —○—, mean anti-preS IgY titers from eggs laid by control pCI-immunized ducks. Vertical bars represent standard deviations. Detection of endpoint anti-preS antibody titers, expressed in log 10 units, was performed by direct ELISA.
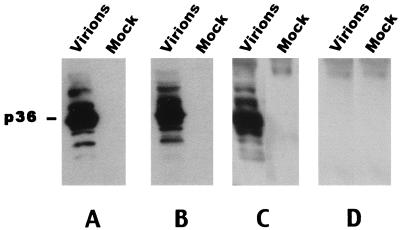
The specificities of egg yolk antibodies obtained from the DNA-immunized ducks were tested by immunoblot analysis using concentrated virions and mock sera from DHBV-infected and noninfected duck sera, respectively, followed by the detection of bound antibody with anti-rabbit or anti-duck immunoglobulin peroxydase-labeled antibody and a chemiluminescence detection kit (Amersham, Courtaboeuf, France), as described previously (3). The results showed that IgY from eggs laid by pCI-preS/S-immunized ducks recognized the 36-kDa DHBV L envelope protein in concentrated, serum-derived viral particles (Fig. 2C). The immunoblotting pattern was comparable to the one obtained with the sera from breeding ducks immunized with this plasmid (Fig. 2B) or with the control serum from a rabbit immunized with the recombinant DHB preS envelope protein (Fig. 2A). The IgY from eggs laid by pCI-immunized ducks were not reactive in this test (Fig. 2D).
FIG. 2.
Specificity of purified IgY. Proteins from DHBV-infected (lanes marked “Virions” and noninfected (lanes marked “Mock”) duck sera were revealed in an immunoblotting assay with polyclonal anti-DHB preS rabbit antiserum (A), serum from pCI-preS/S-immunized ducks (B), IgY from an egg laid by a pCI-preS/S-immunized duck (C), and IgY from an egg laid by a pCI-immunized duck (D) (control). The p36 DHBV L protein is indicated.
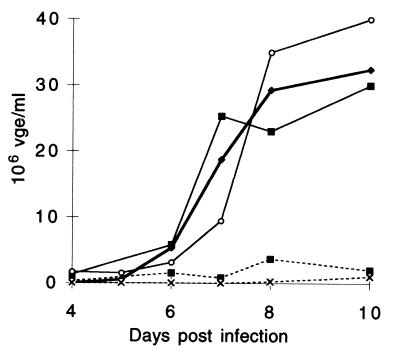
It was important to assess whether such egg yolk antibodies from DNA-vaccinated ducks are neutralizing. To this end, the capacity of these antibodies to neutralize DHBV infection was tested in vitro with PDHs obtained by in situ collagenase perfusion (3). The PDHs were infected with a DHBV inoculum (2 × 108 virus genome equivalents [vge]/well) which had been preincubated overnight at room temperature with either phosphate-buffered saline, duck serum, or purified egg yolk IgY. As illustrated in Fig. 3, a total virus release, reflected by the area under the curve, was not affected by preincubation of the inoculum with either control duck serum or control yolk IgY from eggs laid by empty pCI vector-immunized ducks (Fig. 3). By contrast, serum from ducks immunized with the pCI-preS/S plasmid and IgY from eggs laid by these ducks were highly neutralizing, since decreases by 96 and 99%, respectively, of released virions were observed in PDHs infected with DHBV preincubated with these antibodies (Fig. 3). Taken together, these results indicate that genetic immunization of ducks with the plasmid bearing the gene encoding hepadnavirus L envelope protein elicits large amounts of antigen-specific and highly neutralizing egg yolk antibodies.
FIG. 3.
In vitro neutralization of DHBV infectivity with immunized-duck sera and egg yolk IgY. PDHs were infected with DHBV inoculum preincubated with phosphate-buffered saline (—⧫—), sera from pCI-immunized ducks ( ), egg yolk IgY from pCI-immunized ducks (
), egg yolk IgY from pCI-immunized ducks ( ), sera from pCI-preS/S-immunized ducks (---■---), and IgY from pCI-preS/S-immunized ducks (---×---). The release of viral particles was quantified in cell medium in virus genome equivalents by dot blot hybridization. Means of duplicate determinations are presented.
), sera from pCI-preS/S-immunized ducks (---■---), and IgY from pCI-preS/S-immunized ducks (---×---). The release of viral particles was quantified in cell medium in virus genome equivalents by dot blot hybridization. Means of duplicate determinations are presented.
Offspring of DNA-vaccinated ducks are protected against high-titer DHBV challenge.
To verify that maternal antibodies transmitted to the eggs reached the progeny circulation, fecund eggs from DNA vaccinees were placed in an SMA 120 incubator (SOCMA, Beaumont, France) and incubated at 37.5°C and 55% humidity during 28 days until hatching. Our results show that none of the ducklings from pCI-immunized ducks had detectable anti-preS antibodies at hatch as assessed by ELISA but that all ducklings from pCI-preS/S-immunized ducks presented anti-preS antibody titers ranging from 400 to 12,800 (Table 1).
TABLE 1.
In vivo protective effect of maternally transferred antibodies from DNA-vaccinated ducksa
| Plasmid used to immunize female ducks | No. of ducklings | Anti-preS antibody titerb at hatch | Viremiac (vge [107]/ml) |
|---|---|---|---|
| pCI | 927 | 0 | 118 |
| 928 | 0 | 5,468 | |
| 929 | 0 | 191 | |
| 930 | 0 | 7,726 | |
| 931 | 0 | 335 | |
| 932 | 0 | 2,361 | |
| pCI-preS/S | 924 | 3,200 | 867 |
| 925 | 400 | 9,135 | |
| 772 | 12,800 | 0 | |
| 773 | 6,400 | 0 | |
| 777 | 6,400 | 0 | |
| 778 | 6,400 | 0 | |
| 923 | 6,400 | 0 | |
| 926 | 6,400 | 0 |
Fecund eggs from DNA-vaccinated ducks were incubated until hatched, and progeny ducklings were challenged with DHBV.
End-point ELISA antibody titers were taken as the reciprocal of the highest serum dilution which gave an optical density signal greater than three standard deviations above the mean signal of four replicates of control sera (16).
Viremia, quantified in virus genome equivalents by dot blot hybridization, is expressed as the total viral release in serum during 17 days of follow-up.
To define whether maternal antibodies from DNA-immunized ducks can protect offspring against viral infection, progeny ducklings received a high-titer (5 × 107 vge) challenge with DHBV and the viremia of individual ducks was monitored for 17 days. As summarized in Table 1, all six progeny ducklings that hatched from control pCI-immunized ducks, which had no detectable anti-preS antibodies, became infected (Table 1). Out of eight ducklings hatched from pCI-preS/S-immunized ducks, two ducklings which had anti-preS antibody titers of ≤3,200 at hatch became infected, as evidenced by detection of DHBV DNA in their sera. By contrast, six out of six ducklings with anti-preS antibody titers of ≥6,400 at hatch were protected against DHBV challenge.
In summary, this study demonstrates for the first time that following DNA vaccination of laying ducks against large hepadnavirus envelope, specific, highly neutralizing, and protective antibodies are transferred from the sera of immunized ducks via the egg yolk to the progeny of vaccinees. The plasmid used in this study bears the gene that expresses the DHBV large envelope protein, which contains major virus neutralization epitopes (17). Interestingly, as shown here, DNA immunization of ducks with as little as 100 μg of plasmid allowed us to generate large amounts (i.e., 60 to 100 mg from each egg) of highly neutralizing and purified IgY. In addition, anti-preS egg yolk antibodies were long-lasting, with little egg-to-egg titer variation. Therefore, our study demonstrates that eggs from genetically immunized ducks may represent a potent source of DNA-designed antibodies specific to viral antigens. In this regard, DNA immunization is known to induce a robust immune response in murine models against viral antigens (7). However, the low antibody yield obtained in mice hampered the use of this approach as a source of antiviral antibodies. In contrast, daily egg collection from DNA-immunized ducks, or from chickens, which are reared more easily, allows the rapid and noninvasive production of considerable amounts of virus-specific IgY antibodies. Such egg yolk IgYs are known to be valuable immunodiagnostic reagents, since they do not react with mammalian globulins due to structural differences (4, 11, 12). Because mutations can easily be introduced into plasmid DNA by site-directed mutagenesis, genetic vaccination of breeding avians may permit the rapid generation of IgY antibodies specific to mutated viral proteins. Such custom-designed IgY antibodies may represent interesting tools for the detection of the growing number of human HBV variants which escape actual immunodiagnostic tests (9).
Importantly, we show here that hatchlings from DNA-immunized ducks were protected against DHBV infection. This is of particular interest, since, to date, the protective effect of maternal antibodies transmitted from DNA-vaccinated avians to offspring has not yet been investigated. In this regard, conventional protein vaccination of breeding avians is known to be a useful approach for conferring to their hatchlings passive immunity to viral infection, as was documented for avian reovirus (14). Our results are promising and suggest that genetic immunization may have a great potential for improving early life protection of domestic avian species against different viral infections.
The protective efficacy of a DNA vaccine against HBV envelope proteins has been studied with two chimpanzees and showed partial protection, since limited virus replication occurred after challenge (15). However, the effectiveness of maternal antibodies induced by a DNA vaccine for offspring protection against hepadnavirus infection has not yet been investigated. Our study demonstrates that DNA immunization of breeding ducks with the plasmid bearing the gene encoding DHBV L envelope protein confers a significant level of protection, since 75% of hatchlings resisted DHBV challenge. In addition, we have challenged ducklings with a particularly large amount of virus, i.e., 5 × 107 virus genomes, whereas neonatal ducklings, known to be highly sensitive to DHBV, can be infected with as little as 1 virus genome (10). The outcome of challenge was correlated with anti-preS antibody titers at hatch. Thus, only two ducklings with low anti-preS antibody titers at hatch (≤3,200) became infected whereas all six ducklings with higher anti-preS antibody titers were protected against DHBV challenge. A strong correlation between anti-HBV envelope antibody titers and the efficiency of protection has also been described for chimpanzees (6). These observations are consistent with the mechanism of antibody-mediated antiviral protection, which was analyzed in detail by Bachmann and coworkers (1).
Taken together, our results demonstrate that genetic immunization against L hepadnavirus envelope protein is capable of inducing maternal antibodies which are vertically transmitted and which confer protection to progeny against virus infection. Interestingly, the maternal transmission of anti-human immunodeficiency virus antibodies in response to DNA vaccination has been recently reported for pregnant chimpanzees by Bagarazzi et al. (2), and those authors have suggested the usefulness of genetic immunization for the reduction of mother-to-fetus virus transmission. Whether this approach can be applied for the control of HBV transmission requires further study.
Acknowledgments
This work was supported by grant 9720 from the ARC. Christine Rollier is a fellow of the Ministère de l'Education Nationale de la Recherche et de la Technologie.
We thank Christelle Deleage for excellent technical assistance. We are grateful to Alan Kay for critical reading of the manuscript.
REFERENCES
- 1.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 2.Bagarazzi M L, Boyer J D, Javadian M A, Chattergoon M A, Shah A R, Cohen A D, Bennett M K, Ciccarelli R B, Ugen K E, Weiner D B. Systemic and mucosal immunity is elicited after both intramuscular and intravaginal delivery of human immunodeficiency virus type 1 DNA plasmid vaccines to pregnant chimpanzees. J Infect Dis. 1999;180:1351–1355. doi: 10.1086/314978. [DOI] [PubMed] [Google Scholar]
- 3.Borel C, Sunyach C, Hantz O, Trepo C, Kay A. Phosphorylation of DHBV pre-S: identification of the major site of phosphorylation and effects of mutations on the virus life cycle. Virology. 1998;242:90–98. doi: 10.1006/viro.1997.9004. [DOI] [PubMed] [Google Scholar]
- 4.Camenisch G, Tini M, Chilov D, Kvietikova I, Srinivas V, Caro J, Spielmann P, Wenger R H, Gassmann M. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. FASEB J. 1999;13:81–88. doi: 10.1096/fasebj.13.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Chassot S, Lambert V, Kay A, Godinot C, Trepo C, Cova L. Identification of major antigenic domains of duck hepatitis B virus pre-S protein by peptide scanning. Virology. 1994;200:72–78. doi: 10.1006/viro.1994.1164. [DOI] [PubMed] [Google Scholar]
- 6.Davis H L, Mancini M, Michel M L, Whalen R G. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–915. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Encke J, zu Putlitz J, Wands J R. DNA vaccines. Intervirology. 1999;42:117–124. doi: 10.1159/000024971. [DOI] [PubMed] [Google Scholar]
- 9.Howard C R. The structure of hepatitis B envelope and molecular variants of hepatitis B virus. J Viral Hepatitis. 1995;2:165–170. doi: 10.1111/j.1365-2893.1995.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Jilbert A R, Miller D S, Scougall C A, Turnbull H, Burrell C J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology. 1996;226:338–345. doi: 10.1006/viro.1996.0661. [DOI] [PubMed] [Google Scholar]
- 11.Larsson A, Wejaker P E, Forsberg P O, Lindahl T. Chicken antibodies: a tool to avoid interference by complement activation in ELISA. J Immunol Methods. 1992;156:79–83. doi: 10.1016/0022-1759(92)90013-j. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Ametani A, Shimizu M, Hatta H, Yamamoto T, Kaminogawa S. Production and characterization of anti-human insulin antibodies in the hen's egg. Agric Biol Chem. 1991;55:2141–2143. [PubMed] [Google Scholar]
- 13.Liu S S, Higgins D A. Yolk-sac transmission and post-hatching ontogeny of serum immunoglobulins in the duck (Anas platyrhynchos) Comp Biochem Physiol B. 1990;97:637–644. doi: 10.1016/0305-0491(90)90100-8. [DOI] [PubMed] [Google Scholar]
- 14.Meanger J, Wickramasinghe R, Enriquez C E, Wilcox G E. Immune response to avian reovirus in chickens and protection against experimental infection. Aust Vet J. 1997;75:428–432. doi: 10.1111/j.1751-0813.1997.tb14348.x. [DOI] [PubMed] [Google Scholar]
- 15.Prince A M, Whalen R, Brotman B. Successful nucleic acid based immunization of newborn chimpanzees against hepatitis B virus. Vaccine. 1997;15:916–919. doi: 10.1016/s0264-410x(96)00248-4. [DOI] [PubMed] [Google Scholar]
- 16.Rollier C, Sunyach C, Barraud L, Madani N, Jamard C, Trepo C, Cova L. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology. 1999;116:658–665. doi: 10.1016/s0016-5085(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 17.Sunyach C, Rollier C, Robaczewska M, Borel C, Barraud L, Kay A, Trepo C, Will H, Cova L. Residues critical for duck hepatitis B virus neutralization are involved in host cell interaction. J Virol. 1999;73:2569–2575. doi: 10.1093/gao/9781884446054.article.t048360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triyatni M, Jilbert A R, Qiao M, Miller D S, Burrell C J. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J Virol. 1998;72:84–94. doi: 10.1128/jvi.72.1.84-94.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]