The likely increases in availability of DNA based tests and demand by patients for genetic information and advice mean that primary care practitioners will need to become genetically literate.1,2 Genetic medicine is already beginning to enter the realms of primary care through the availability of testing for predisposition to certain cancers and carrier screening and diagnostic tests for common recessive disorders such as cystic fibrosis and hereditary haemochromatosis. For the near future these issues will probably remain the focus of genetic medicine in primary care, but this could shift if pharmacogenetic research fulfils even some of its early promises.
We discuss the implications of genetic advances for primary care, how genetic medicine could be integrated into primary care, and the skills that primary care practitioners will need to provide advice.
Summary points
Primary care practitioners need to become genetically literate
Currently the most important elements for primary care are prediction of risk of certain cancers and carrier screening for common autosomal recessive conditions such as cystic fibrosis
Pharmacogenetics will become increasingly relevant in decisions around prescribing
Integrating elements of genetic medicine into primary care will require the development of generic skills in genetic risk assessment and communication
A multifaceted approach, including community genetic counsellors, primary care genetic specialists, educational programmes, and computerised decision support, is required to support the acquisition of genetic skills in primary care
Methods
We searched Medline and Embase for relevant papers, combining terms relating to primary care and clinical genetics. We included papers identified from a previous systematic review of primary research on the role of family practice in genetics3 and relevant papers published subsequently. The papers were heterogeneous in their focus and methods, and, when appropriate, we state the evidence on which our statements are based.
Genetic medicine and the interface between primary and secondary care
As more is learned about the genetic aspects of common diseases, the question arises as to who is best placed to meet the incipient service need. Traditionally, clinical genetics has dealt mostly with rare conditions, counselling couples about risks for recurrence for subsequent pregnancies or families affected by adult onset disorders such as Huntington's disease. With the discovery of several genes that underlie certain familial cancers, referrals to clinical geneticists about family history of cancer have risen considerably. At Addenbrooke's Hospital, Cambridge, referrals to the cancer genetics clinic rose from 400 to almost 800 in the year to mid-1999, even though many of these patients were unlikely to carry a mutation in a known susceptibility gene for cancer.
The recent completion of the human genome project and current research into single nucleotide polymorphisms will lead to the identification of more genetic mutations that increase risk of common diseases such as cardiovascular disease, diabetes, and cancers. But clinical genetics departments will be unable to cope with a further rise in demand given their existing workforce4 and all branches of medicine, especially primary care, will be required to advise patients about genetic issues.
Role of primary care in genetic services
Rather than development of a “primary care genetics service,” specific elements of genetic medicine could be incorporated into the generalist role (box B1).5 In the United Kingdom primary care is well placed to support an integrated genetic service given the traditional focus on the family and relatively well computerised longitudinal health records. Qualitative studies of UK general practitioners show that identification of people at increased risk of a genetic disease, and reassuring those who are not, are accepted roles of primary care.6
Box 1.
Potential roles of primary care practitioners in genetic medicine7
- Identification of individuals who may benefit from genetics services, including those with a genetic disorder and those at increased risk for having or transmitting a genetic disorder
- Recognition of historical and physical features of common genetic conditions
- Monitoring the health of individuals with a genetic disorder, in conjunction with specialists in genetics
- Provision of basic genetics information to patients and families to help understanding and informed decision making
- Coordination of care for individuals with complex genetics service needs
- Recognition of the special psychosocial issues for a family in which one or more members are affected with a genetic disorder or susceptibility
- Knowledge of how to access the full range of genetics services from which patients might benefit
- Appropriate referral of patients with additional genetics services needs
- Facilitation of use of genetics services
This approach, however, centres on the traditional view that defines clinical genetics as a specialty and on how primary care could act as an effective gatekeeper for that service. It does not acknowledge how genetic medicine will become more pervasive and alter the practice of medicine in every specialty, including primary care.
Risk prediction for common disease
The family history can be used in primary care to indicate genetic risk factors for several common diseases (box B2).8 Its most important clinical use currently is in determining risk of specific cancers, such as breast, ovarian, and colorectal cancer, and in using this information to inform decisions about early screening or genetic testing for BRCA1, BRCA2, and HNPCC (hereditary non-polyposis colorectal cancer) mutations (box B3). Genetic counselling before testing for these genes is crucial because of uncertainty about existing interventions for carriers and the potential for harm, such as insurance or employment discrimination. Such testing should remain, at least for the near future, in the domain of specialist genetic clinics and among patients who present with concerns about their risk to their general practitioner.
Box 2.
Categories of genetic medicine relevant in primary care
- Reproductive risk—for example, haemoglobinopathies, cystic fibrosis, muscular dystrophies, and many rarer autosomal recessive conditions; chromosomal disorders (such as Down's syndrome and Edwards's syndrome)
- Adult onset genetic disorders with a mendelian inheritance pattern—for example, Huntington's disease, subsets of common diseases such as familial cancers (BRCA1, BRCA2), maturity onset diabetes of the young (MODY)
- Common diseases with a multifactorial aetiology—for example, ischaemic heart disease, asthma, diabetes
- Normal genetic variations in drug metabolism and immune response
Box 3.
Familial colorectal cancer
- A 31 year old man presented with rectal bleeding due to haemorrhoids and mentioned to his general practitioner that cancer ran in the family. The doctor took a family history and found that the patient's father had developed colorectal cancer at the age of 48 and his paternal grandmother at 60. His paternal aunt had developed endometrial cancer at 55. The doctor referred him to the regional genetics clinic on the basis of locally agreed guidelines. After confirmation of the family history, the patient's father was contacted and offered diagnostic genetic testing. His father was found to carry a mutation in the MLH1 gene which causes hereditary non-polyposis colorectal cancer. After genetic counselling the patient chose to undergo predictive genetic testing and was found to carry the same mutation. He now undergoes regular colonoscopic surveillance for the detection and removal of adenomatous polyps.
There are guidelines to help primary care practitioners to stratify risk of cancer on the basis of the family history, thus enabling them to identify those patients who may be reassured about their family history and refer people at increased risk for genetic counselling (further details on criteria for referral can be found on the BMJ 's website). Once there is better evidence for the efficacy of interventions for people at increased risk (for example, tamoxifen for prevention of breast cancer9), systematic identification of these patients in primary care will be appropriate, such as through postal questionnaires on family history.10 Predispositional genetic testing for common diseases is still in its infancy. Testing for apolipoprotein E genotype to predict risk of Alzheimer's disease is not recommended because of the lack of preventive treatments and uncertainties about the interpretation of results. Its performance in diagnostic testing in symptomatic patients is also under investigation, and it should not yet be used routinely.11 Predisposing genes are known for a number of rare subtypes of common diseases, such as diabetes and Parkinson's disease in specific families with early onset disease,12,13 but their use as predictive tests is also limited by absence of preventive treatment. Larger epidemiological studies are required to confirm associations reported14 between specific genetic polymorphisms and risk of conditions such as asthma and cardiovascular disease before their use can be evaluated in clinical settings. Once the genetic risks are confirmed and their interactions with environmental factors known, genotypic information can be incorporated into existing models for cardiovascular risk to inform advice on behaviour and prescribing.
Genetic screening programmes
Primary care could play an important part in the provision of various genetic screening programmes, particularly in supporting informed choices about antenatal diagnosis of common genetic disorders. In the United Kingdom there are community based programmes for neonatal screening for phenylketonuria and antenatal serum screening for Down's syndrome. In the United States neonatal screening for haemoglobinopathy occurs routinely in most states, and antenatal screening for cystic fibrosis carrier status has been recommended nationally. A series of pilot studies of antenatal and general population screening for cystic fibrosis carriers has shown that patients can be adequately counselled in primary care, but uptake of the test by patients depended heavily on how it was offered (box B4).15,16
Box 4.
Antenatal carrier screening
- A 24 year old primiparous Asian woman came to see her general practitioner saying that she was seven weeks pregnant. After discussion with the doctor, the woman and her partner decided to be tested for haemoglobinopathy status and both were found to be carriers for β thalassaemia. The couple were referred to a specialist centre and counselled about prenatal diagnosis. Chorionic villus sampling was performed at 11 weeks, and the fetus was heterozygous for β thalassaemia. A healthy child was delivered at term, and the couple will be offered prenatal diagnosis in subsequent pregnancies.
There have been calls in the United Kingdom for a national selective screening policy in primary care for thalassaemia and sickle cell disease in those at risk (for example, in African-Caribbeans, southern Mediterraneans, and Asians) and proposals for structured antenatal screening for haemoglobinopathies, which would enable counselling and antenatal diagnosis in the first trimester.17
Population screening for hereditary haemochromatosis has been promoted because of the relatively high prevalence of mutations in the HFE gene that cause the disease and because there is effective treatment through regular phlebotomy. Pilot studies in the United States have shown that primary care can provide an adequate screening service,18 but doubts exist about the epidemiology of mutation carriers, in particular the proportion who will develop significant disease. Diagnostic testing in primary care by genotype or serum transferrin for patients with possible symptoms of the disease (for example, arthralgia, diabetes, fatigue) has been proposed to enable earlier diagnosis,19 but the cost effectiveness of this strategy requires evaluation.
Pharmacogenetics
The commercial drive by the pharmaceutical industry to discover the biological explanations that underlie individual variation in drug response could potentially have a far greater impact on future practice in primary care.20 Early examples of how apolipoprotein E status affects response to lipid lowering drugs and treatment for Alzheimer's disease21,22 hint at the possibility of applying genetic testing to tailor drug treatment for a wide range of disorders. Pharmacogenetics could be used to identify patients who are at risk of severe adverse effects from drug treatment, such as bleeding complications of warfarin,23 or non-response due to inadequate dosing, such as with antidepressants.24 The cost effectiveness of testing for prothrombotic mutations such as factor V Leiden before prescription of oral contraceptives is uncertain but testing may be appropriate for women with a strong family history of thrombosis.25 Pharmacogenetic information may require less understanding of genetic principles than would be necessary, for example, to provide counselling for carrier screening for cystic fibrosis. Ultimately, therefore, pharmacogenetics may be a much greater driving force for the application of genetic medicine in primary care than specific genetic screening programmes.
Genetic skills in primary care and training needed
If primary care is to incorporate genetics, certain generic knowledge and skills must be acquired that focus initially on the ascertainment of genetic risk, in particular taking an adequate family history and annotating this in the form of a pedigree (box B5). Ideally this should span three generations and include details about the age of onset of disease and age of death for each relative. An awareness of associated cancers in specific familial cancers (for example, endometrial cancer in hereditary non-polyposis colorectal cancer) and the importance of ethnicity in determining risk for certain diseases (for example, haemoglobinopathies) would guide the collection of information on family history and its interpretation. Additional skills in assessment of risk and in communication are required for appropriate discussion of an individual's risk.
Box 5.
Genetic skills and knowledge required in primary care
- Taking, recording, and interpreting family history
- Recognition of common patterns of inheritance
- Awareness of the importance of ethnicity in determining risk (for example, Ashkenazi Jews and increased risk of BRCA mutations) and specific cancer clusters
- Communication of risk and counselling in a non-directive manner
- Understanding of the limitations of genetic testing, including the implications of testing for insurance
The recent and rapid developments in genetic medicine mean that many primary care practitioners have received minimal training in clinical genetics. Various methods will be required to support practitioners to develop these genetic skills and knowledge, including changes to the undergraduate curriculum—for example, discussing risk assessment of colorectal cancer as part of training in gastroenterology—to promote an integrated approach to genetic medicine. Postgraduate training and assessment in primary care should include approaches to developing and examining the outlined genetic skills. But these will depend first on the acquisition of genetic skills among all clinical teachers.
A randomised controlled trial of a multidisciplinary postgraduate educational programme showed limited increase in screening for haemoglobinopathy in primary care.26 Additional, more innovative methods are required, including management guidelines and computerised pedigree drawing and decision support.27 Online resources will become increasingly useful both for health professionals and their patients.28
While these slower acting educational processes take effect, additional approaches to integrate existing genetic services with primary care will be necessary. Studies in the United Kingdom are evaluating the use of community genetic counsellors acting as outreach workers from the genetics clinic to liaise with local general practices. Such practitioners are either genetic nurses or genetic counsellors, and they provide an initial filter for referrals to the geneticist and perform an educational role. However, there are probably not enough trained counsellors to support widespread adoption of such a model. An alternative approach is for primary care groups to support the development of primary care specialists, possibly working in conjunction with community genetic counsellors, to act as an intermediate point of referral between general practice and specialist genetic clinics. A third allied model is to train a specific practitioner from each practice in genetic skills to act as an in-house expert, supported by electronic resources.
Future directions
Integrating genetics into primary care requires a gradual adoption and incorporation of specific elements of genetic medicine. There are reasonable doubts as to how quickly the new genetics will deliver clinically useful tests and knowledge to primary care and whether it will initially raise more questions than answers.29 There are concerns about potential harm from inappropriate use of genetic testing in primary care30 and a reluctance by primary care practitioners to adopt these new responsibilities. Because of the uncertainties around the benefits and harm from genetic testing, the clinical implications of a specific genetic test will require careful evaluation, including information about cost effectiveness, before widespread adoption is recommended.
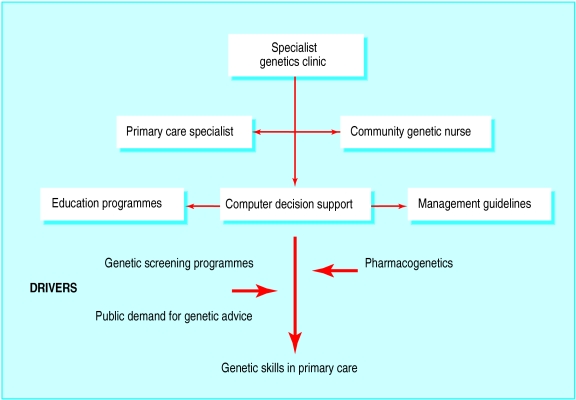
None the less, genetic medicine will shortly be arriving in general practice. For the next five years this will centre around screening for carriers of haemoglobinopathies and cystic fibrosis and assessment of risk for certain cancers. Beyond that there will be increasing use of genetic information to tailor drug selection and dosage and to predict risk of common conditions such as diabetes and cardiovascular disease. A multifaceted approach is essential to support the procurement of these skills in primary care (figure). The impact of genetics on primary care is currently small and primary care practitioners may not yet perceive genetic education as relevant. It will require increased public demand for genetic advice or the development of genetic tests of direct clinical value to drive the acquisition of genetic skills. If even a fraction of the claims made about the impending impact of genetics on clinical practice came true, clinical genetics services would be overwhelmed. We must not miss this opportunity to prepare primary care for the new genetics.
Additional educational resources
Rose P, Lucassen A. Practical genetics for primary care. Oxford: Oxford University Press, 1999.
GeneClinics. www.geneclinics.org (provides clinical summaries of many genetic disorders and role of genetic testing including information resources for patients. Educational section for healthcare professionals on indications, benefits, and limitations of genetic testing)
NSW Genetics Education Programme. www.genetics.com.au (factsheets about range of genetic disorders and guide to drawing family trees)
Supplementary Material
Figure.
Potential routes to development of genetic medicine in primary care
Acknowledgments
We thank Rhyddian Hapgood, Ron Zimmern, and Hilary Harris for helpful comments on earlier drafts of this paper.
Footnotes
Competing interests: JE and SH are members of the Medical Advisory Board of FamilyGenetix, an interactive web-based genetic risk assessment service.
An additional box of referral criteria can be found on the BMJ's website
References
- 1.North West England Faculty of the RCGP. Genetics in primary care. A report from the faculty genetics group. London: Royal College of General Practitioners; 1998. (Occasional Paper 77). [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews LB, Fullarton JE, Holtzman NA, Motulsky AG. Assessing genetic risks: implications for health and social policy. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 3.Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract. 1999;16:426–445. doi: 10.1093/fampra/16.4.426. [DOI] [PubMed] [Google Scholar]
- 4.Harper PS, Hughes HB, Raeburn JA. Clinical genetics services into the 21st century. Summary of a report of the clinical genetics committee of the Royal College of Physicians. J R Coll Physicians Lond. 1996;30:296–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S. Resisting revolution: generalism and the new genetics. Lancet. 1999;354:1992–1993. doi: 10.1016/S0140-6736(99)04073-8. [DOI] [PubMed] [Google Scholar]
- 6.Watson E, Shickle D, Qureshi N, Emery J, Austoker J. The ‘new genetics’ and primary care: general practitioners' views on their role and educational needs. Fam Pract. 1999;16:420–425. doi: 10.1093/fampra/16.4.420. [DOI] [PubMed] [Google Scholar]
- 7.Hayflick S, Eiff M. Role of primary care providers in the delivery of genetics services. Community Genetics. 1998;1:18–22. doi: 10.1159/000016131. [DOI] [PubMed] [Google Scholar]
- 8.Scheuner MT, Wang SJ, Raffel LJ, Larabell SK, Rotter JI. Family history: a comprehensive genetic risk assessment method for the chronic conditions of adulthood. Am J Med Genet. 1997;71:315–324. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 10.Leggatt V, Mackay J, Yates JR. Evaluation of questionnaire on cancer family history in identifying patients at increased genetic risk in general practice. BMJ. 1999;319:757–758. doi: 10.1136/bmj.319.7212.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post SG, Whitehouse PJ, Binstock RH, Bird TD, Eckert SK, Farrer LA, et al. The clinical introduction of genetic testing for Alzheimer disease. An ethical perspective. JAMA. 1997;277:832–836. doi: 10.1001/jama.277.10.832. [DOI] [PubMed] [Google Scholar]
- 12.Yki-Jarvinen H. MODY genes and mutations in hepatocyte nuclear factors. Lancet. 1997;349:516–517. doi: 10.1016/S0140-6736(97)80078-5. [DOI] [PubMed] [Google Scholar]
- 13.Jarman P, Wood N. Parkinson's disease genetics comes of age. BMJ. 1999;318:1641–1642. doi: 10.1136/bmj.318.7199.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridker PM, Stampfer M. Assessment of genetic markers for coronary thrombosis: promise and precaution. Lancet. 1999;353:687–688. doi: 10.1016/S0140-6736(99)00067-7. [DOI] [PubMed] [Google Scholar]
- 15.Harris H, Scotcher D, Hartley N, Wallace A, Craufurd D, Harris R. Cystic fibrosis carrier testing in early pregnancy by general practitioners. BMJ. 1993;306:1580–1583. doi: 10.1136/bmj.306.6892.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekker H, Modell M, Denniss G, Silver A, Mathew C, Bobrow M, et al. Uptake of cystic fibrosis testing in primary care: supply push or demand pull? BMJ. 1993;306:1584–1586. doi: 10.1136/bmj.306.6892.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modell B, Petrou M, Layton M, Varnavides L, Slater C, Ward RH, et al. Audit of prenatal diagnosis for haemoglobin disorders in the United Kingdom: the first 20 years. BMJ. 1997;315:779–784. doi: 10.1136/bmj.315.7111.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonnell SM, Phatak PD, Felitti V, Hover A, McLaren GD. Screening for hemochromatosis in primary care settings. Ann Intern Med. 1998;129:962–970. doi: 10.7326/0003-4819-129-11_part_2-199812011-00007. [DOI] [PubMed] [Google Scholar]
- 19.Adams P, Brissot P, Powell LW. EASL international consensus conference on haemochromatosis. J Hepatol. 2000;33:485–504. doi: 10.1016/s0168-8278(01)80874-6. [DOI] [PubMed] [Google Scholar]
- 20.Wolf CR, Smith G, Smith RL. Science, medicine, and the future: pharmacogenetics. BMJ. 2000;320:987–990. doi: 10.1136/bmj.320.7240.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallman D, Ellsworth D, Boerwinkle E. Molecular and genetic approaches to the study of cardiovascular disease. J Cardiovasc Risk. 1997;4:325–331. [PubMed] [Google Scholar]
- 22.Richard F, Helbecque N, Neuman E, Guez D, Levy R, Amouyel P. APOE genotyping and response to drug treatment in Alzheimer's disease. Lancet. 1997;349:539. doi: 10.1016/S0140-6736(97)80089-X. [DOI] [PubMed] [Google Scholar]
- 23.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 24.Chou WH, Yan FX, de Leon J, Barnhill J, Rogers T, Cronin M, et al. Extension of a pilot study: impact of the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol. 2000;20:246–251. doi: 10.1097/00004714-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Vandenbroucke JP, van der Meer FJ, Helmerhorst FM, Rosendaal FR. Factor V Leiden: should we screen oral contraceptive users and pregnant women? BMJ. 1996;313:1127–1130. doi: 10.1136/bmj.313.7065.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modell M, Wonke B, Anionwu E, Khan M, Tai SS, Lloyd M, et al. A multidisciplinary approach for improving services in primary care: randomised controlled trial of screening for haemoglobin disorders. BMJ. 1998;317:788–791. doi: 10.1136/bmj.317.7161.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery J, Walton R, Murphy M, Austoker J, Yudkin P, Chapman C, et al. Computer support for interpreting family histories of breast and ovarian cancer in primary care: comparative study with simulated cases. BMJ. 2000;321:28–32. doi: 10.1136/bmj.321.7252.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FamilyGenetix. www.familygenetix.com (accessed 1 February 2001).
- 29.Holtzman NA, Marteau TM. Will genetics revolutionize medicine? N Engl J Med. 2000;343:141–144. doi: 10.1056/NEJM200007133430213. [DOI] [PubMed] [Google Scholar]
- 30.Giardiello FM, Brensinger JD, Petersen GM, Luce MC, Hylind LM, Bacon JA, et al. The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. N Engl J Med. 1997;336:823–827. doi: 10.1056/NEJM199703203361202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.