Abstract
Background
An increasing body of evidence suggests that home-based exercise (HBE) therapy has significant therapeutic effects on knee osteoarthritis (KOA) and hip osteoarthritis (HipOA), and it has advantages such as cost savings, strong operability, and good compliance compared with hospitalization and exercise courses.
Objective
To evaluate the efficacy of HBE in the treatment of KOA and HipOA.
Methods
A systematic search was conducted in PubMed, Cochrane, Web of Science, and Embase to collect randomized controlled trials. The retrieval time was from database establishment until March 6, 2024. Stata 15.1 software was used for data analysis.
Results
A total of 16 randomized controlled trials involving 3,015participants were included, with 1,519 participants in the intervention group and 1,496 in the control group. The meta-analysis showed that, compared to the control group, HBE can significantly improve pain [SMD=-0.38, 95% CI (-0.58, -0.18); P = 0.001], joint function [SMD=-0.60, 95% CI (-1.01, -0.19); P = 0.004], balance ability [SMD=-0.67, 95% CI (-1.00, -0.34); P = 0.001], mobility (ADL) [SMD = 0.51, 95% CI (0.19, 0.82); P = 0.002] in patients with KOA and HipOA. There is no statistical difference in the improvement of joint stiffness [WMD = -0.80, 95% CI (-1.61, 0.01); P = 0.052]. In addition, subgroup analysis showed that HBE significantly improved pain, joint function, and balance ability in KOA patients compared with the control group. HipOA patients showed significant improvement in pain and joint function; However, HBE only improved activity ability in patients with comorbidities of KOA and HipOA.
Conclusion
HBE can effectively alleviate pain, improve joint function, and enhance physical function in patients with KOA and HipOA. However, more high-quality randomized controlled trials (RCTs) with large sample sizes and long-term interventions are needed to validate the efficacy of HBE due to limitations in the methodology and consistency of indicator outcomes in the included RCTs.
Registration number
We’ve registered with PROSPERO, and the number is CRD42023443085.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-07585-w.
Keywords: Home-based exercise, Knee osteoarthritis, Hip osteoarthritis, Meta-analysis, Kinesitherapy
Introduction
Osteoarthritis (OA) is a common chronic joint disease characterized by joint pain and morning stiffness. In 2019, it affected 528 million people globally [1]. Currently, there is no effective treatment that has been proven to slow the progression of the disease [2]. The latest estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) found that the age-standardized prevalence and incidence of symptomatic, radiographically confirmed OA increased by 9.3% (95% UI 8–10.7%) and 8.2% (95% UI 7.1–9.4%), respectively, from 1990 to 2017 [3, 4]. In the 2019 Global Burden of Musculoskeletal Diseases survey, OA accounted for 20.1% of the demand for musculoskeletal rehabilitation, placing a huge economic and public healthcare burden on individuals, families, and society [5, 6]. Knee osteoarthritis (KOA) and hip osteoarthritis (Hip OA) are the two most common types of osteoarthritis in the lower limbs due to weight-bearing and mobility. The knee joint is the most complex joint in the human body, bearing the greatest load among all joints, making it the most prone to osteoarthritis [7]. Although all factors affecting joints in osteoarthritis have commonalities, unlike KOA, hip OA is often associated with hip dysplasia and acetabular impingement syndrome, two risk factors [8, 9].
Age, obesity, heavy manual labor, and high-intensity exercise are all risk factors for OA. Patients with certain comorbidities have a higher incidence of OA and an increased risk of activity limitations caused by OA, making it more difficult to manage their condition [6, 10]. In addition to surgery, medication, and physical therapy, exercise is considered the cornerstone of OA treatment. The Osteoarthritis Research Society International (OARSI) and American College of Rheumatology (ACR) guidelines recommend that regardless of whether there are comorbidities, diet weight management, regular and sustained exercise plans, and mind-body exercises (such as Tai Chi, yoga) should be regarded as the core therapeutic modalities for OA [11, 12].
Patients with OA may avoid exercise due to pain and fear of exercise-related injury. Shur et al. [13] found that a lack of physical activity can lead to age-related muscle loss and decreased muscle quality, which is unfavorable for the prognosis of patients with KOA [14]. Increasing evidence supports exercise as a maximally effective treatment for alleviating symptoms and related comorbidities of OA [15]. An international consensus study [16] has developed evidence-based recommendations for OA exercise, which include tailoring personalized exercise plans based on patients’ conditions, optimizing modes and dosages, and emphasizing compliance and exercise education. Despite the strong recommendation for exercise therapy in OA guidelines. However, it is difficult to form standardized exercise prescriptions, which are multidimensional and complex, due to insufficient research on clinical controlled trial data, resulting in difficulties in efficacy assessment and comparative research [15, 16]. Hospital-based treatment plans and exercise programs do not confer a long-term prognosis benefit over home-based exercise (HBE) therapy. HBE is an effective way to maintain rehabilitation and combination therapy after discharge. In contrast, HBE therapy offers several advantages, including cost savings, practicality, high compliance, improved comfort during rehabilitation, and reduced risk of injury associated with travel to clinics. Hurley M et al. [17] found that exercise can improve physical function, depression, and pain in patients with KOA and hip OA. Jönsson et al. [18] showed that early-stage and mild KOA/HipOA patients, particularly those who declined surgical intervention, experienced significant relief of clinically relevant pain when they participated in a self-management program that incorporated HBE. Currently, there is no substantial evidence indicating the clinical efficacy and superiority of HBE. Therefore, this meta-analysis aims to address this question and provide clinical physicians and patients with exercise therapy plans, data analysis, and references.
Methods
Search strategy
Two independent researchers searched four databases, PubMed, Embase, Cochrane, and Web of Science, using a combination of topic words and free words. The search was conducted from the establishment of the database until March 6, 2024. The search keywords mainly included Knee Osteoarthritis, Knee Arthritis, Hip OA, Coxarthrosis, and Home-based exercise. In addition, the two researchers also reviewed the references of similar studies to ensure the inclusion of relevant literature that was not searched. The detailed search strategy is shown in Supplementary Table 1.
Inclusion and exclusion criteria
Specific inclusion criteria were as follows: (1) Participants meet one or more of the diagnostic criteria for KOA and HipOA in the Kellgren Lawrence classification (KL scale), ACR, and American Rheumatology Association (ARA), or had written diagnosis or clinical imaging evidence from a doctor to prove the diagnosis of KOA and HipOA [19–22]; (2) The intervention group received HBE, without restrictions on specific forms of exercise; The control group received blank controls, health education and publicity, and non-steroidal anti-inflammatory drugs, excluding exercise interventions; (3) The patient’s gender, age, race, and source of the case were not limited, without any restriction on whether to use a blinding method. Studies had to be published in English; (4) The outcome measures encompassed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analogue Scale (VAS), timed up and go test (TUG), timed chair stand (TCS), gait speed (GS), the six-minute walk test (6MWT), the five-times sit-to-stand test (FTSST) [23–25]; (5) The search design was a randomized controlled trial (RCT).
The specific exclusion criteria were as follows: (1) Literature review, meta-analysis, duplicate publications, conference abstracts, animal experiments, case reports, protocols, non-randomized controlled trials and interventions that do not meet the inclusion criteria, and unavailable full-text and data; (2) Patients with a history of knee joint trauma, surgery, or rheumatoid arthritis; (3) duplicate publications.
Literature screening and data extraction
The literature was screened by two independent reviewers, who read the title, abstract, and full text, extracted data, and cross-validated the findings. Any discrepancies were resolved through discussion with a third reviewer to reach a final decision. Duplicate publications were first automatically searched using Endnote software and then manually reviewed and removed. Titles and abstracts were screened before reading the full texts of selected articles. Subsequently, two evaluators independently extracted related information from the selected studies based on a standardized data extraction table (Table 1). The main extracted information included the first author’s name, publication year, country, sample size, gender, age, intervention measures, treatment period, and outcome measures.
Table 1.
Basic characteristics of included studies
| Study | Year | Country | Sample size | Gender (M/F) | Mean age(years) | Interventional protocol | outcome | Type | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBEG | CG | HBEG | CG | HBEG | CG | Time of treatment | ||||||
| Oh, S.L [32] | 2021 | South Korea | 40 | 20 | None | 72.44 ± 6.30 | 71.06 ± 5.41 |
HBE 2-3times/week HEP 50 min/month |
HEP 1 × 50 min/month |
20 W | F1,F4,F5,F6,F7,F9 | KOA |
| Krauss, I [33] | 2020 | Germany | 64 | 63 | 77/50 | 57.8 (9.6) | 60.3 (8.8) |
HBE 2 × 60 min/week |
NT | 12 W | F9 | Hip OA |
| Chen.H [38] | 2019 | China | 71 | 70 | 22/119 | 68.9(7.78) | 68.8(6.96) |
HBE 3 × 30 min/week HEP pre-6 weeks 60 min/2 weeks |
HEP pre-6 weeks 60 min/2 weeks |
12 W | F1,F5,F7,F8,F10 | KOA |
| Steinhilber, B [28] | 2017 | Germany | 70 | 68 | None | 58 ± 19 | 60 ± 9 |
HBE 2 × 60 min/week |
NT | 12 W | F3 | Hip OA |
| Takacs, J [27] | 2017 | Canada | 20 | 20 | 8/32 | 66.1 (8.7) | 67.1 (5.4) |
HBE 4 times/week |
NT | 10 W | F3,F4 | KOA |
| C.H. Teirlinck [39] | 2016 | Netherland | 101 | 102 | 84/119 | 64 (8.5) | 67 (9.6) |
HBE 1 × 30 min/week |
general practitioner care | 12 M | F3,F7 | Hip OA |
| Armagan.O [41] | 2015 | Turkey | 30 | 40 | 15/55 | 55.9 ± 4.9 | 56.8 ± 3.7 |
HBE 2 times/week |
Glucosamine 1.5 g/d |
24 W | F1,F2,F4,F5 | KOA |
| Zeng, R.M [26] | 2015 | China | 32 | 27 | 31/28 | 65.19 ± 2.61 | 64.81 ± 2.48 |
HBE 5 × (45 min Tai chi + 30 min cinesiatrics)/week HEP |
HEP | 12 W | F1,F4,F7,F8 | Hip OA |
| Eun-Lee Lee [31] | 2023 | Korea | 15 | 16 | None | 65.63 ± 3.7 | 68.27 ± 4.78 |
HBE 3 × 35 min/week |
NT | 8 W | F2,F7,F10 | KOA |
| Bennell, K.L [40] | 2010 | Australia | 45 | 44 | 46/43 | 64.5 (9.1) | 64.6 (7.6) |
HBE 5 times/week |
NT | 12 W | F1,F3,F4 | KOA |
| Doi T [37] | 2008 | Janpan | 63 | 58 | 31/90 | 67.4 ± 13.4 | 71.2 ± 22.2 |
HBE 2 × 15 min/day |
NSAIDs | 8 W | F1,F2 | KOA |
| Hughes, S. L [34] | 2006 | Chicago | 115 | 100 | 48/167 | 73.3 | 73.4 |
HBE 3 × 90 min/week |
HEP | 12 M | F1,F4,F5,F6,F8 | KOA, HipOA |
| Hughes, S.L [35] | 2004 | Chicago | 80 | 70 | 125/25 | 73.5 | 73.7 |
HBE 3 × 90 min/week |
HEP | 8 M | F1,F4,F5,F6,F8 | KOA, HipOA |
| Ravaud, P [30] | 2004 | France | 735 | 760 | 424/1071 | None | None |
HBE 4 × 30 min/week |
Usual care | 6 M | F1,F2,F4 | KOA, HipOA |
| Evcik, D [36] | 2002 | Turkey | 27 | 26 | 17/36 | 56.3 ± 6.1 | 55.8 ± 6.9 |
HBE 2 × 10 min/day |
NT | 12 W | F1,F2,F4 | KOA |
| Rogind, H [29] | 1998 | Denmark | 11 | 12 | 2/21 | 69.3 _+ 8.2 | 73.0 _+ 6.5 |
HBE 1 time/day HEP 2 times/week |
NT | 12 M | F3,F9 | KOA |
Abbreviations HBEG: home-based excises group; CG: control group; M/F: male/female; HBE: home-based excises; HEP: Health education program; NT: no treatment; F1: WOMAC pain; F2: VAS; F3: Knee pain/Hip pain (11-point NRS); F4: WOMAC function; F5: WOMAC stiffness; F6: TCS(timed chair stand); F7: TUG(timed up& go); F8:6MWT(The six-minute walk test); F9: GS( gait speed); F10: FTSST(The five-times sit-to-stand test)
Quality assessment
Two independent researchers adopted Cochrane Handbook for Systematic Reviews of Interventions was used to assess the methodological quality of the included studies. The assessment included random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias. Each domain was rated as “low risk of bias,” “high risk of bias,” or “unclear” (lack of relevant information or unclear bias). The evaluation results were confirmed by two researchers after cross checking. The evaluation results of the two researchers were tested for consistency using Kappa. A Kappa value less than 0.2 indicated poor consistency, 0.2–0.4 indicated average consistency, 0.4–0.6 indicated moderate consistency, 0.6–0.8 indicated strong consistency, and 0.8-1.0 indicated strong heterogeneity.
Statistical analysis
Stata 15.0 was used for meta-analysis, and heterogeneity was tested using Cochran’s Q test and Higgins I² Quantitative statistics. Continuous variables were represented by mean difference (MD), and if the units were inconsistent, standardized mean difference (SMD) was used. The effect size and 95% confidence interval (CI) were used as the statistical measures for evaluating their effects. The I2 value was used to test the heterogeneity. If the homogeneity was good (I2 < 50%), a fixed-effects model was used. If the heterogeneity was large (I2 ≥ 50%), a random-effects model was used. When there was excessive heterogeneity, sensitivity analysis and subgroup analysis were used to explore the sources of heterogeneity. Funnel plots were used to visually reflect publication bias, and Egger’s test was used to statistically test publication bias; P > 0.05 indicates the existence of publication bias, and the trim and fill method was used. Further sensitivity analysis was conducted to examine the stability of the research results. The statistical significance of the merged statistics of the included studies was set at P < 0.05.
Results
Literature screening
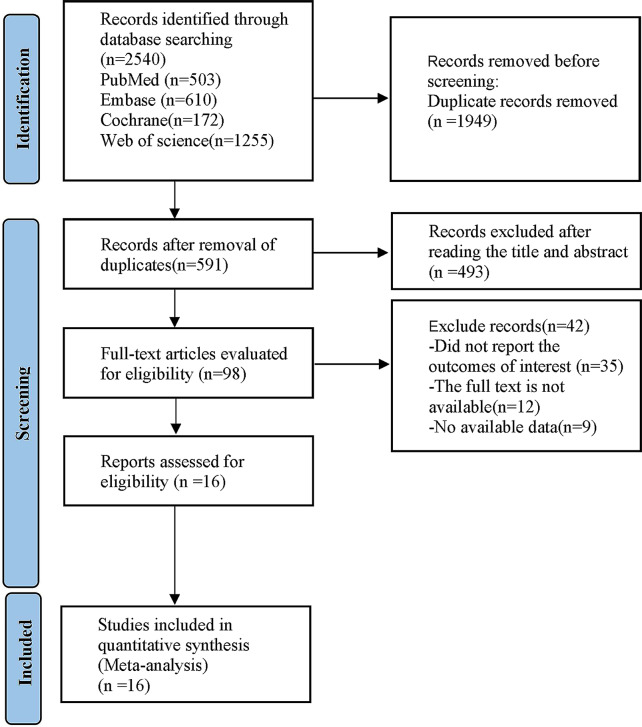
As shown in Fig. 1, a total of 2,540 articles were searched from the four databases, 1,949 of which were duplicate articles or marked as mismatched by automatic tool and were excluded. After reading the title and abstract, 493 articles that clearly did not meet the inclusion criteria were excluded. Subsequently, the full texts of the remaining 98 articles were searched and read. According to the inclusion criteria, 82 articles that did not meet the inclusion criteria were excluded, including reviews, meta-analyses, duplicate publications, conference abstracts, animal experiments, case reports, protocols, non-randomized controlled trials, and intervention measures. Finally, a total of 16 RCTs were included in this meta-analysis.
Fig. 1.
The whole literature selection process
Basic characteristics of included studies
A total of 3,015 KOA patients were involved in the 16 finally included studies, all of which were published in English between 1998 and 2023 [26–41]. The countries where the patients were located included China, the United States, South Korea, Germany, Canada, Türkiye, the Netherlands, Australia, Japan, France and Denmark. These patients all meet one or more diagnostic criteria for KOA or HipOA in the ARA, ACR, and KL scale.
The basic information of the participants involved in all 16 studies is as follows: the average age of these participants ranged from 55 to 74 years old; The participants in all 16 study consisted of males and females; Three studies reported the medication history of participants [27, 31, 35], and 5 studies reported the underlying diseases of participants [20, 26, 30, 35, 38]. Regarding intervention methods, the control group were treated with blank controls in 7 articles [26–28, 32, 35, 36, 40], health education lectures in 5 articles [26, 31, 33, 34, 37], routine care in 3 articles [29, 30, 38], and medication in 1 articles [37]. The follow-up time for these studies ranged from 8 to 12 months. Regarding the three main outcomes, one study did not report pain [32], 7 studies did not report joint function-related indicators [28, 29, 31, 33, 37–39], and 11 studies did not report joint stiffness [26–30, 32, 35–39]. The baseline characteristics of the included studies were comparable. The specific characteristics of the included studies are shown in Table 1.
Risk of bias assessment
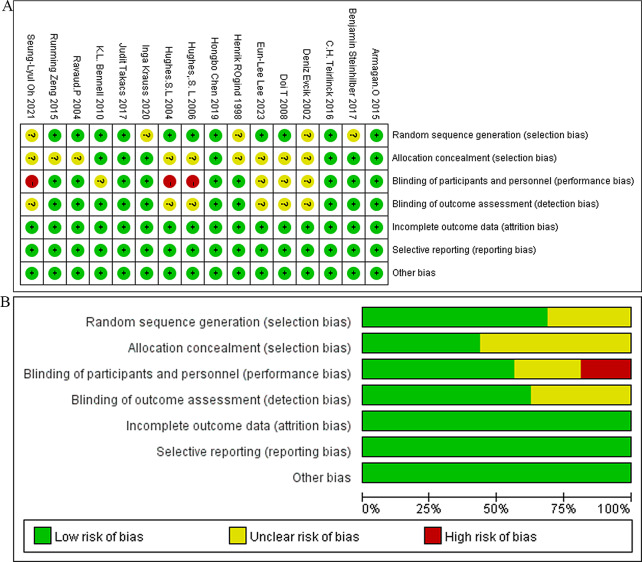
Based on the Cochrane bias assessment criteria, 16 studies were evaluated, with 11 articles providing a clear description of their specific methods for randomization and 7 articles explaining the blinding methods used. Three studies did not use blinding methods, while 10 studies blinded the outcome evaluators. The risk of bias assessment for the included studies is shown in Fig. 2A-B. Subsequently, Kappa test and paired chi square analysis were performed on the evaluation results of the two researchers. The results showed a Kappa value of 0.73 (P = 0.001) and paired chi square analysis P = 0.923, indicating good consistency in the evaluation results of the two researchers.
Fig. 2.
(A) Risk of bias summary; (B) Risk of bias summary
Meta-analysis results
Main outcome measures
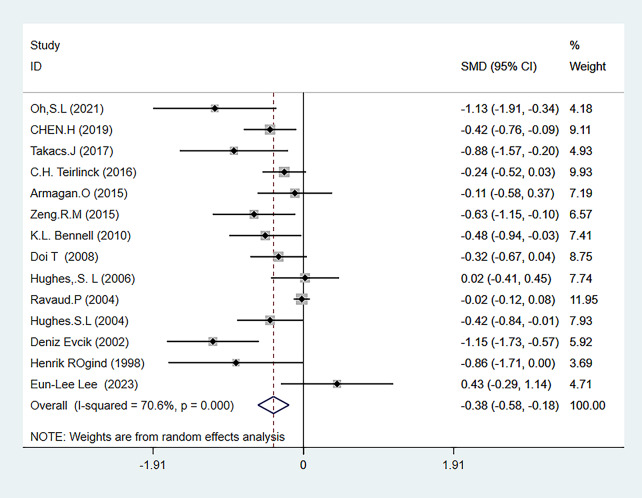
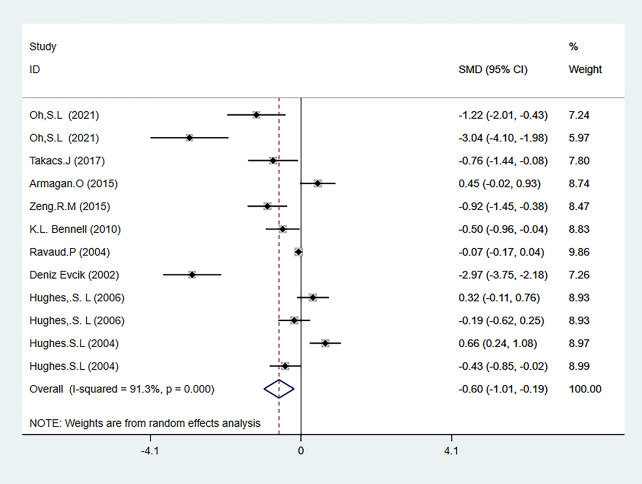
The three main outcome measures of this meta-analysis were pain (evaluated using the WOMAC pain subscale, VAS, and NARS 11-point combined assessment), WOMAC stiffness subscale, and joint function (evaluated using the WOAMC function subscale and TCS combined assessment). The results showed that HBE significantly improved pain [SMD=-0.38, 95% CI (-0.58, -0.18); P = 0.001] and joint function [SMD=-0.60, 95% CI (-1.01, -0.19); P = 0.004] in KOA and HipOA patients, but there was no statistically significant difference in the improvement of joint stiffness [WMD = 0.80, 95% CI (1.61, 0.01); P = 0.052]. The main outcome indicators are shown in Fig. 3-Fig. 5, and Table 2. The grade rating of the main outcome measures is shown in Table 3.
Fig. 3.
Forest map of pain meta-analysis
Fig. 5.
Forest map of meta-analysis of joint function
Table 2.
Summary of meta-analysis results
| Measurements | Number of included articles | Number of patients involved | I² value (%) | SMD/WMD (95%CI) | P-value |
|---|---|---|---|---|---|
| Primary outcome measures | |||||
| pain | 14 | 2526 | 70.6 | -0.38 (-0.58, -0.18) | 0.001 |
| stiffness | 5 | 429 | 89.8 | -0.80 (1.61,0.01) | 0.052 |
| function | 9 | 2007 | 91.3 | -0.60 (-1.01, -0.19) | 0.004 |
| Secondary outcome measures | |||||
| balance | 5 | 608 | 70.5 | -0.67 (-1.00, -0.34) | 0.001 |
| ADL | 7 | 66.5 | 66.5 | 0.51 (0.19, 0.82) | 0.002 |
Table 3.
Grade rating for outcome indicators
| Primary outcome measures | Grade |
|---|---|
| pain | Moderate |
| stiffness | Low |
| function | Moderate |
| Secondary outcome measures | |
| balance | Low |
| ADL | Moderate |
Pain
A total of 14 articles quantified the level of pain in patients [26, 27, 29–32, 34–41], involving 2,526 participants, with 1,280 in the HBE group and 1,246 in the control group. The meta-analysis showed heterogeneity (I2 = 70.6%), so a random-effects model was used to analyze the studies. The analysis results showed that HBE was more effective in reducing pain levels in KOA and HipOA patients than the control group [SMD=-0.38, 95%CI (-0.58, -0.18); P = 0.001] (Fig. 3).
WOMAC stiffness
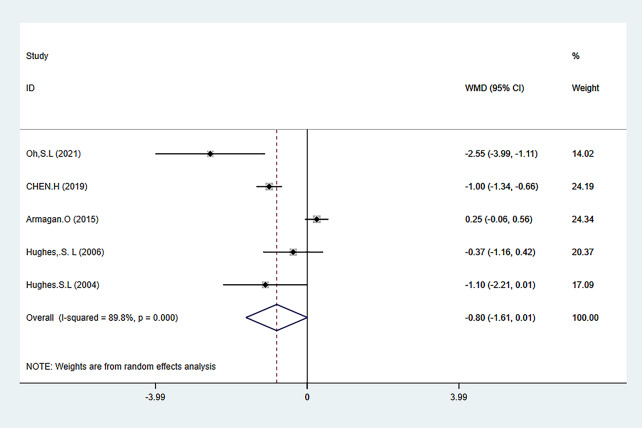
WOMAC stiffness scores were mentioned in 5 articles [32, 34, 35, 38, 41], involving 429 participants, with 240 in the HBE group and 189 in the control group. The meta-analysis showed heterogeneity (I2 = 89.8%), so a random-effects model was used to analyze the studies. The analysis results showed that no significant statistical differences were observed in stiffness levels between the HBE group and the control group [WMD=-0.80, 95% CI (-1.61,0.01); P = 0.052] (Fig. 4).
Fig. 4.
Forest map of meta-analysis of joint stiffness
Joint function
Joint function was evaluated using two tests, WOAMC function and TCS. A total of 9 articles reported relevant tests [26, 27, 30, 32, 34–36, 40, 41], involving 2007 participants, with 1,019 in the HBE group and 988 in the control group. The meta-analysis showed heterogeneity (I2 = 91.3%), so a random-effects model was used to analyze the studies. The analysis results showed that HBE was more effective in improving joint function in KOA and HipOA patients than the control group [SMD=-0.60, 95% CI (-1.01, -0.19); P = 0.004] (Fig. 5).
Meta-analysis of the secondary outcome measures
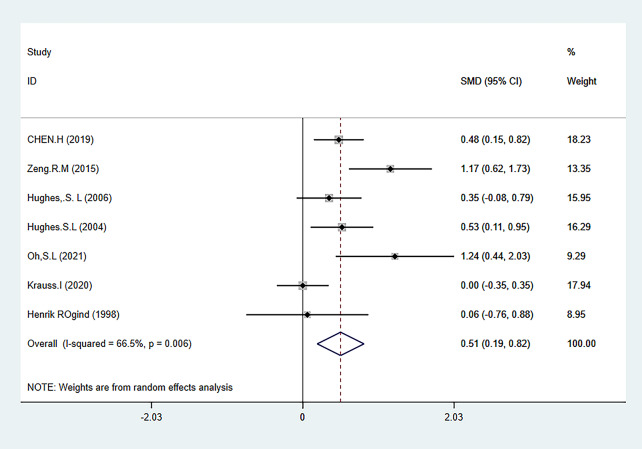
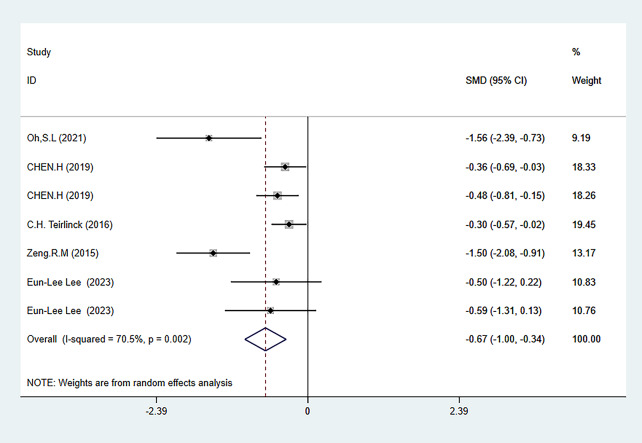
The two secondary outcome indicators of this meta-analysis were activity level (ADL) (evaluated using GS and 6MWT combined) and balance level (evaluated using TUG and FTSST combined). The results showed that HBE can significantly improve the balance ability ADL of KOA and HipOA patients [SMD = 0.51, 95% CI (0.19, 0.82); P = 0.002], [SMD=-0.67, 95% CI (-1.00, -0.34); P = 0.001]. The results of secondary outcome measures are shown in Figs. 6 and 7, and Table 2. The grade rating of the secondary outcome measure is shown in Table 3.
Fig. 6.
Forest map of activity capability meta-analysis
Fig. 7.
Forest map of meta-analysis of balancing ability
Mobility: ADL
ADL was evaluated using a combination of GS and 6MWT tests. A total of 7 articles reported relevant tests [26, 29, 32–35, 38] involving 568 individuals, including 317 in the HBE group and 251 in the control group. The heterogeneity test showed I2 = 66.5%. Therefore, a random effects model was used to analyze the included studies, and the analysis results showed that HBE had a better effect on improving the activity ability of KOA and HipOA patients than the control group [SMD = 0.51, 95% CI (0.19, 0.82); P = 0.002] (Fig. 6).
Balance ability
The balance ability was evaluated through a combination of TUG and FTSST tests. A total of 5 articles reported relevant tests [26, 31, 32, 38, 39], and 608 patients were involved, including 311 in the HBE group and 297 in the control group. The heterogeneity test showed I2 = 70.5%. Hence, a random-effects model was used to analyze the included studies. The analysis results showed that HBE was more effective in improving the balance ability of KOA and HipOA patients than the control group [SMD=-0.67, 95% CI (-1.00, -0.34); P = 0.001] (Fig. 7).
Subgroup analysis
Subgroup analysis was conducted based on the location of arthritis to further explore the therapeutic effect of HBE on different types of arthritis. Meanwhile, the sources of heterogeneity were explored through subgroup analysis due to the significant heterogeneity in the results of meta-analysis. According to the comprehensive subgroup analysis results, compared with the control group, HBE can significantly improve the joint function [SMD=-0.91, 95% CI (-1.66, -0.17); P = 0.016], balance ability [SMD=-0.58, 95% CI (-0.88, -0.27); P = 0.001], and ADL [SMD = 0.57, 95% CI (0.04,1.11); P = 0.036] in KOA patients, joint function in HipOA patients [SMD=-0.92, 95% CI (-1.45, -0.38); P = 0.001], and ADL in patients with comorbidities of KOA and HipOA [SMD = 0.44, 95% CI (0.14, 0.74); P = 0.004]. There was no significant difference in other outcomes compared with the control group. In addition, according to the I2 values of each subgroup analysis, the type of arthritis in different parts may be the reason for the high heterogeneity in the meta-analysis results of pain, balance ability, and ADL, but it is not the reason for the high heterogeneity in the meta-analysis results of joint stiffness and joint function. The results of subgroup analysis are shown in Supplementary Figs. 1–5 and Table 4.
Table 4.
Summary of subgroup analysis
| Measurements | Number of included articles | Subgroup | I² value (%) | SMD/WMD (95% CI) | P-value |
|---|---|---|---|---|---|
| primary outcome measures | |||||
| pain | 10 | KOA | 61.3 | -0.44(-0.71, -0.18) | 0.001 |
| 2 | HipOA | 37.8 | -0.37(-0.72, -0.02) | 0.041 | |
| 2 | KOA&HipOA | 70.4 | -0.17(-0.55, 0.21) | 0.384 | |
| stiffness | 4 | KOA | 92 | -0.75(-1.66,0.17) | 0.109 |
| 1 | KOA&HipOA | None | -1.10(-2.21, 0.01) | 0.053 | |
| function | 6 | KOA | 92.5 | -0.91 (-1.66, -0.17) | 0.016 |
| 1 | HipOA | None | -0.92(-1.45, -0.38) | 0.001 | |
| 2 | KOA&HipOA | 85.8 | 0.04 (-0.44, 0.52) | 0.864 | |
| Secondary outcome measures | |||||
| balance | 3 | KOA | 43.4 | -0.58 (-0.88, -0.27) | 0.001 |
| 2 | HipOA | 92.5 | -0.87 (-2.04, 0.30) | 0.147 | |
| ADL | 3 | KOA | 54.2 | 0.57 (0.04, 1.11) | 0.036 |
| 2 | HipOA | 91.9 | 0.57 (-0.58, 1.72) | 0.334 | |
| 2 | KOA&HipOA | 0 | 0.44 (0.14, 0.74) | 0.004 |
Sensitivity analysis
Sensitivity analysis was conducted on the data results of pain, joint stiffness, joint function, ADL, and balance ability in 16 articles to determine the stability of the comprehensive results. The results showed that the circles representing each study were within the range of the original confidence interval effect values, indicating that the analysis results were relatively stable, as shown in Supplementary Fig. 6.
Publication bias evaluation
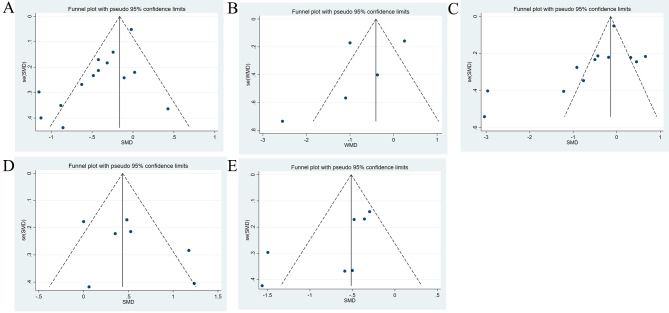
A funnel plot was used to evaluate publication bias for the outcomes. Subsequently, Egger’s test was further used to statistically test the publication bias. The results showed that the Egger’s test P-values for joint stiffness (P Egger = 0.127), joint function (P Egger = 0.096), ADL (P Egger = 0.383), and balance ability (P Egger = 0.144) were all > 0.05, indicating that there may be no publication bias. The P value of Egger’s test for pain was less than 0.05 (P Egger = 0.003), and no study was added after two iterations of the trim and fill method. The results showed no difference from the original results, indicating that there may be publication bias. However, the publication bias has little effect on the results of this study. The funnel plot is shown in Fig. 8A-E, and the Egger’s test results are shown in Supplementary Fig. 7.
Fig. 8.
Funnel diagram. (A) Pain; (B) Joint stiffness; (C) Joint function; (D) ADL; (E) Balance ability
Discussion
For patients with KOA and HipOA, HBE is a cost-effective and easily-promoted mode of physical activity, which is convenient and simple to perform with no use or minimal use of medical equipment. It can reduce psychological fear in patients and alleviate the economic burden on those with transportation difficulties or financial distress to visit physical therapists. Several studies have demonstrated the effectiveness of HBE in relieving joint pain, improving physical function, and enhancing quality of life [42, 43]. Chronic pain patients often tend to have a vicious cycle of physical inactivity, prolonged sitting, and disability exacerbation [44]. For patients suffering from long-term OA, pain may become a source of exercise phobia, and muscle strength may directly or indirectly influence their physical activities [45]. Studies have found no significant correlation between HBE and central sensitization or pain intensity, and exercise intensity does not induce more adverse reactions [46–48]. Low educational level has been identified as an important factor contributing to catastrophic pain and exercise phobia in OA patients [49], highlighting the need for healthcare providers to pay attention to psychological behavior induction and pain neuroscience education to eliminate patient fear of exercise. The efficacy of HBE is also related to patient compliance. Future research on behavioral interventions is needed to increase long-term exercise adherence. Patient compliance is influenced by factors such as supervision, family support, emotional involvement, and trust in physical therapists [50, 51], which can be improved through self-management plans, personalized programs, monitoring and feedback, cognitive-behavioral techniques, and other interventions [52]. However, some studies have shown that there is no specificity in treatment outcomes between supervised and unsupervised HBE, and compared to short-term supervised physical therapy, long-term HBE programs have better long-term outcomes for limb function [30, 53–55]. Therefore, how to improve patient motivation and provide HBE programs that are easy to adhere to in the long-term is a problem that needs further attention. This study has several imitations. First, the intervention methods were relatively single, and we did not take into account comprehensive factors such as self-management, supervised exercise, and health education that may affect patient compliance and final treatment outcomes. Further analysis can be conducted to analyze the efficacy of HBE for patients with severe pain after using combined drug or physical interventions. Second, our outcome indicators mainly focused on pain, and somatic function, with a lack of evaluation of psychological health, life quality. Third, the HBE programs should be personalized according to the different locations of joint wear and muscle and ligament injuries in patients. This study only partially summarizes the therapeutic effects of HBE, and more high-quality, large-scale studies are needed to explore the true efficacy of HBE.
Conclusion
The present study shows that HBE can significantly improve pain, joint function, balance ability, and mobility in patients with KOA and HipOA. Due to the limitations of this study, further clinical data and high-quality research are needed in the future to confirm our conclusions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- 6MWT
Six-minute walk test
- ACR
American College of Rheumatology
- ADL
Activity level
- ARA
American Rheumatology Association
- CI
Confidence interval
- FTSST
Five-times sit-to-stand test
- GS
Gait speed
- HBE
Home-based exercise
- HipOA
Hip osteoarthritis
- KL scale
Kellgren Lawrence classification
- KOA
Knee osteoarthritis
- MD
Mean difference
- OARSI
Osteoarthritis Research Society International
- SMD
Standardized mean difference
- TCS
Timed chair stand
- TUG
Timed up and go test
- VAS
Visual Analogue Scale
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- RCT
Randomized controlled trial
Author contributions
Conceptualization: Yichen Mao, Boyuan Qiu, Weiwei Wang; Methodology: Yichen Mao, Boyuan Qiu, Weiwei Wang; Formal analysis and investigation: Yichen Mao, Boyuan Qiu; Writing - original draft preparation: Yichen Mao, Boyuan Qiu; Writing - review and editing: Zhixue Ou; Funding acquisition: Zhixue Ou; Resources: Boyuan Qiu, Pengwei Zhou; Supervision: Zhixue Ou, Weiwei Wang, Pengwei Zhou. Yichen Mao and Boyuan Qiu contributed equally to this work and should be considered as co-first authors. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Funding
This work was supported by the Guangxi Appropriate Technology Development and Promotion of Chinese Medicine Project (GZSY21-78).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Declarations
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yichen Mao MD and Boyuan Qiu MD contributed equally to this work and should be considered as co-first authors.
References
- 1.Organization WH. Musculoskeletal health. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.Accessed 14 July.
- 2.Jang S, Lee K, Ju JH. Recent updates of diagnosis, pathophysiology, and treatment on Osteoarthritis of the knee. Int J Mol Sci. 2021;22(5). [DOI] [PMC free article] [PubMed]
- 3.Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–28. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Fong DYT, Wong JYH. Secular trends in Musculoskeletal Rehabilitation needs in 191 countries and territories from 1990 to 2019. JAMA Netw Open. 2022;5(1):e2144198. doi: 10.1001/jamanetworkopen.2021.44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015;67(2):203–15. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter DJ, Bierma-Zeinstra S, Osteoarthritis Lancet. 2019;393(10182):1745–59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 7.Geng R, Li J, Yu C, Zhang C, Chen F, Chen J, et al. Knee osteoarthritis: current status and research progress in treatment (review) Exp Ther Med. 2023;26(4):481. doi: 10.3892/etm.2023.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther. 2016;33(11):1921–46. doi: 10.1007/s12325-016-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall M, van der Esch M, Hinman RS, Peat G, de Zwart A, Quicke JG, et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthritis Cartilage. 2022;30(1):32–41. doi: 10.1016/j.joca.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: Prevalence of Doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–53. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, hip, and Knee. Arthritis Rheumatol. 2020;72(2):220–33. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Shur NF, Creedon L, Skirrow S, Atherton PJ, MacDonald IA, Lund J, et al. Age-related changes in muscle architecture and metabolism in humans: the likely contribution of physical inactivity to age-related functional decline. Ageing Res Rev. 2021;68:101344. doi: 10.1016/j.arr.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):145–76. doi: 10.1016/j.rdc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Bamman MM, Wick TM, Carmona-Moran CA, Bridges SL. Jr. Exercise Medicine for Osteoarthritis: Research Strategies to maximize effectiveness. Arthritis Care Res (Hoboken) 2016;68(3):288–91. doi: 10.1002/acr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden MA, Metcalf B, Lawford BJ, Hinman RS, Boyd M, Button K, et al. Recommendations for the delivery of therapeutic exercise for people with knee and/or hip osteoarthritis. An international consensus study from the OARSI Rehabilitation Discussion Group. Osteoarthritis Cartilage. 2023;31(3):386–96. doi: 10.1016/j.joca.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N, et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4(4):Cd010842. doi: 10.1002/14651858.CD010842.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jönsson T, Eek F, Hansson EE, Dahlberg LE, Dell’Isola A. Factors associated with clinically relevant pain reduction after a self-management program including education and exercise for people with knee and/or hip osteoarthritis: data from the BOA register. PLoS ONE. 2023;18(2):e0282169. doi: 10.1371/journal.pone.0282169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minetti GA, Parodi M, Banderali S, Silvestri E, Garlaschi G, Cimmino MA. Magnetic resonance imaging as a structural refinement to the American College of Rheumathology clinical classification criteria for knee osteoarthritis. Reumatismo. 2022;74(3). [DOI] [PubMed]
- 20.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–78. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed up & go: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: self-paced Walk Test (SPWT), Stair climb test (SCT), six-Minute Walk Test (6MWT), Chair stand Test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and Car Task. Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S350–70. doi: 10.1002/acr.20538. [DOI] [PubMed] [Google Scholar]
- 26.Zeng R, Lin J, Wu S, Chen L, Chen S, Gao H, et al. A randomized controlled trial: preoperative home-based combined Tai Chi and Strength Training (TCST) to improve balance and aerobic capacity in patients with total hip arthroplasty (THA) Arch Gerontol Geriatr. 2015;60(2):265–71. doi: 10.1016/j.archger.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Takacs J, Krowchuk NM, Garland SJ, Carpenter MG, Hunt MA. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98(8):1586–93. doi: 10.1016/j.apmr.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Steinhilber B, Haupt G, Miller R, Janssen P, Krauss I. Exercise therapy in patients with hip osteoarthritis: effect on hip muscle strength and safety aspects of exercise-results of a randomized controlled trial. Mod Rheumatol. 2017;27(3):493–502. doi: 10.1080/14397595.2016.1213940. [DOI] [PubMed] [Google Scholar]
- 29.Røgind H, Bibow-Nielsen B, Jensen B, Møller HC, Frimodt-Møller H, Bliddal H. The effects of a physical training program on patients with osteoarthritis of the knees. Arch Phys Med Rehabil. 1998;79(11):1421–7. doi: 10.1016/S0003-9993(98)90238-6. [DOI] [PubMed] [Google Scholar]
- 30.Ravaud P, Giraudeau B, Logeart I, Larguier JS, Rolland D, Treves R, et al. Management of osteoarthritis (OA) with an unsupervised home based exercise programme and/or patient administered assessment tools. A cluster randomised controlled trial with a 2 × 2 factorial design. Ann Rheum Dis. 2004;63(6):703–8. doi: 10.1136/ard.2003.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EL, Jang MH, Lee BJ, Han SH, Lee HM, Choi SU, et al. Home-based Remote Rehabilitation leads to Superior outcomes for older women with knee osteoarthritis: a Randomized Controlled Trial. J Am Med Dir Assoc. 2023;24(10):1555–61. doi: 10.1016/j.jamda.2023.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Oh SL, Kim DY, Bae JH, Lim JY. Effects of rural community-based integrated exercise and health education programs on the mobility function of older adults with knee osteoarthritis. Aging Clin Exp Res. 2021;33(11):3005–14. doi: 10.1007/s40520-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 33.Krauss I, Hein T, Steinhilber B, Janßen P. A 12-week exercise program for patients with hip osteoarthritis has no influence on gait parameters: a secondary analysis of a randomized controlled trial. Gait Posture. 2020;78:6–12. doi: 10.1016/j.gaitpost.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Hughes SL, Seymour RB, Campbell RT, Huber G, Pollak N, Sharma L, et al. Long-term impact of fit and strong! On older adults with osteoarthritis. Gerontologist. 2006;46(6):801–14. doi: 10.1093/geront/46.6.801. [DOI] [PubMed] [Google Scholar]
- 35.Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44(2):217–28. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- 36.Evcik D, Sonel B. Effectiveness of a home-based exercise therapy and walking program on osteoarthritis of the knee. Rheumatol Int. 2002;22(3):103–6. doi: 10.1007/s00296-002-0198-7. [DOI] [PubMed] [Google Scholar]
- 37.Doi T, Akai M, Fujino K, Iwaya T, Kurosawa H, Hayashi K, et al. Effect of home exercise of quadriceps on knee osteoarthritis compared with nonsteroidal antiinflammatory drugs: a randomized controlled trial. Am J Phys Med Rehabil. 2008;87(4):258–69. doi: 10.1097/PHM.0b013e318168c02d. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Zheng X, Huang H, Liu C, Wan Q, Shang S. The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: a quasi-experimental study. BMC Musculoskelet Disord. 2019;20(1):160. doi: 10.1186/s12891-019-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teirlinck CH, Luijsterburg PA, Dekker J, Bohnen AM, Verhaar JA, Koopmanschap MA, et al. Effectiveness of exercise therapy added to general practitioner care in patients with hip osteoarthritis: a pragmatic randomized controlled trial. Osteoarthritis Cartilage. 2016;24(1):82–90. doi: 10.1016/j.joca.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Bennell K, Hunt M, Wrigley T, Hunter D, McManus F, Hodges P, et al. Hip strengthening reduces symptoms but not knee load in people with medial knee osteoarthritis and varus malalignment: a randomised controlled trial. Osteoarthritis Cartilage. 2010;18(5):621–8. doi: 10.1016/j.joca.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Armagan O, Yilmazer S, Calısir C, Ozgen M, Tascioglu F, Oner S, et al. Comparison of the symptomatic and chondroprotective effects of glucosamine sulphate and exercise treatments in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2015;28(2):287–93. doi: 10.3233/BMR-140516. [DOI] [PubMed] [Google Scholar]
- 42.Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28(7):1655–65. [PubMed] [Google Scholar]
- 43.Ksibi I, Lebib S, Ben Salah FZ, Miri I, Koubaa S, Dziri C. [The contribution of home based exercise programme in case of osteoarthritis of the knee] Tunis Med. 2008;86(10):881–9. [PubMed] [Google Scholar]
- 44.Borisovskaya A, Chmelik E, Karnik A. Exercise and Chronic Pain. Adv Exp Med Biol. 2020;1228:233–53. doi: 10.1007/978-981-15-1792-1_16. [DOI] [PubMed] [Google Scholar]
- 45.Aydemir B, Huang CH, Foucher KC. Strength and physical activity in osteoarthritis: the mediating role of kinesiophobia. J Orthop Res. 2022;40(5):1135–42. doi: 10.1002/jor.25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Santana GN, Dibai-Filho AV, da Silva Júnior JEF, da Silva ACB, de Jesus SFC, Dos Santos PG, et al. Association between adherence to a home exercise program and central sensitization, pain intensity, and functionality in individuals with knee osteoarthritis. BMC Musculoskelet Disord. 2022;23(1):989. doi: 10.1186/s12891-022-05959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;2015(10):Cd010203. doi: 10.1002/14651858.CD010203.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keogh JWL, Grigg J, Vertullo CJ. Is Home-Based, high-intensity interval Training Cycling feasible and safe for patients with knee osteoarthritis? Study protocol for a Randomized Pilot Study. Orthop J Sports Med. 2017;5(3):2325967117694334. doi: 10.1177/2325967117694334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aily JB, de Almeida AC, Ramírez PC, da Silva Alexandre T, Mattiello SM. Lower education is an associated factor with the combination of pain catastrophizing and kinesiophobia in patients with knee osteoarthritis? Clin Rheumatol. 2021;40(6):2361–7. doi: 10.1007/s10067-020-05518-1. [DOI] [PubMed] [Google Scholar]
- 50.Loew L, Brosseau L, Kenny GP, Durand-Bush N, Poitras S, De Angelis G, et al. Factors influencing adherence among older people with osteoarthritis. Clin Rheumatol. 2016;35(9):2283–91. doi: 10.1007/s10067-015-3141-5. [DOI] [PubMed] [Google Scholar]
- 51.Campbell R, Evans M, Tucker M, Quilty B, Dieppe P, Donovan JL. Why don’t patients do their exercises? Understanding non-compliance with physiotherapy in patients with osteoarthritis of the knee. J Epidemiol Community Health. 2001;55(2):132–8. doi: 10.1136/jech.55.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan JL, Holden MA, Mason EE, Foster NE. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;2010(1):Cd005956. doi: 10.1002/14651858.CD005956.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deyle GD, Allison SC, Matekel RL, Ryder MG, Stang JM, Gohdes DD, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85(12):1301–17. doi: 10.1093/ptj/85.12.1301. [DOI] [PubMed] [Google Scholar]
- 54.Dell’Isola A, Jönsson T, Ranstam J, Dahlberg LE, Ekvall Hansson E, Education Home Exercise, and supervised Exercise for people with hip and knee osteoarthritis as part of a nationwide implementation program: data from the Better Management of patients with Osteoarthritis Registry. Arthritis Care Res (Hoboken) 2020;72(2):201–7. doi: 10.1002/acr.24033. [DOI] [PubMed] [Google Scholar]
- 55.Bieler T, Siersma V, Magnusson SP, Kjaer M, Beyer N. Exercise induced effects on muscle function and range of motion in patients with hip osteoarthritis. Physiother Res Int. 2018;23(1). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).