Abstract
A frequent mutation at codon 97 of human hepatitis B virus core antigen has been shown to cause an “immature secretion” phenotype, featuring nonselective and excessive secretions of virions containing immature viral genome. Our current study demonstrates that this abnormality can be efficiently offset by another frequent core mutation, P130T.
Hotspot mutations of the human hepatitis B virus (HBV) core antigen gene (HBcAg) have been identified in HBV chronic carriers, such as mutations at codons 5, 13, 59, 60, 87, 97, 130, and 182 (1, 13). Among these hotspots, codon 97 has the highest mutation frequency (1, 3, 6–8, 11–15, 20, 22–24, 26, 28–31), changing from a phenylalanine (F) or isoleucine (I) into a leucine (L). From earlier studies, it has been observed that the wild-type hepadnavirus DNA replicative intermediates can be enveloped and secreted as virions only after completion of the minus-strand DNA synthesis or initiation of plus-strand synthesis (10, 25). However, we recently reported that the acquisition of a leucine residue at codon 97 (97L) of HBcAg enabled the virus to secrete an excessive amount of immature genome with nascent incomplete single-strand DNA (ssDNA) in an envelope-dependent manner (33, 34). This immature secretion phenomenon is subtype independent, since it can occur in the genetic context of either ayw or adr subtypes, as long as the core gene contains a leucine residue at amino acid 97 (34).
Hepadnavirus immature secretion does not appear to be limited to the tissue culture system. For example, it was observed in vivo in woodchuck hepatitis B virus in one woodchuck treated with acyclovir (27). The mechanism of such drug-induced immature secretion remains unclear. Most recently, virion-like particles containing an abundant level of immature ssDNA were also found in sera containing snow geese hepatitis B viruses (SGHBV) (4), suggesting that immature secretion could also be found in avian hepadnaviruses in vivo. Whether such an immature secretion phenotype of SGHBV is simply a species-specific feature or is caused by naturally occurring mutations remains unclear. As demonstrated in HBV, it could be encoded entirely by a single missense mutation within the core gene (33, 34) or equally likely by mutations within the envelope or polymerase genes. So far, immature secretion of virions has been observed in several different hepadnaviruses in vivo and in culture.
Besides the 97L mutation, another frequent missense mutation occurs at codon 130 of HBcAg in patients (1, 3, 6–8, 11–15, 20, 22–24, 26, 28–31). According to the published data compiled from 19 independent studies with 96 reported HBcAg sequences from 66 hepatitis B patients, the proline-to-threonine change (P130T) is the most frequent mutation at codon 130 (67 of 96 [70%]). Approximately 30% (29 of 96) of mutations at codon 130 of HBcAg in chronic carriers change from proline (P) to amino acids other than threonine (T). At a closer examination, we noted that the P130T mutation is frequently associated with the occurrence of mutation I97L (50 of 67 [75%]), although it also can occur by itself (17 of 67 [25%]). Note that in some reports, direct sequencing of total HBV DNA without cloning was used. In that case, only one HBV sequence was obtained from one patient. Because HBV variant populations often exist as a mixture in the same individual, we chose to best present the association between these two mutations quantitatively by the number of independent clones rather than by the number of patients. To date, the functional significance of mutation P130T, alone or in association with the mutation 97L, remains unclear. In this study, we examined the capability of DNA replication and virion secretion of the naturally occurring single (P130T) and double (I97L/P130T) mutants.
We introduced a P-to-T change to a wild-type HBV (subtype adr) at codon 130 of HBcAg (Altered Sites II In Vitro Mutagenesis Systems; Promega) (17). The P130T mutation was created by using the oligonucleotide 5′-TCG CAC TCC TAC CGC TTA CAG-3′. The mutant P130T monomer was subsequently dimerized (pP130T) as described elsewhere (34). To test the effects of the mutation P130T in association with the mutation I97L, the double mutant pI97L/P130T was created by introducing the P130T mutation into mutant I97L (34).
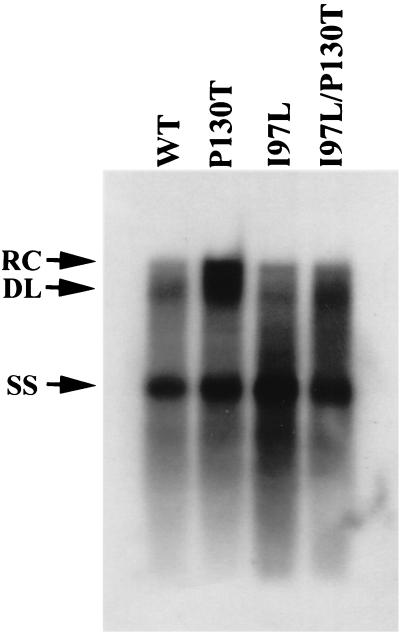
The intracellular core-associated HBV DNA was purified 7 days posttransfection and assayed by Southern blot analysis (Fig. 1). The total HBV specific replication signals were more or less similar among pWt, pP130T, pI97L, and pI97L/P130T. However, we noted that HBV DNA migrating near the 4.0-kb position, which is predominantly the near-full-length relaxed-circle (RC) form, is more enriched in mutant P130T than the other three genotypes. Although the increase in the degree of intracellular genome maturity is small in mutant P130T, it is reproducible from experiment to experiment. We compared the intensity on the X-ray film between the RC and double-strand linear (DL) forms versus the intensity of full-length ssDNA at the 1.5-kb position (33). Our measurement revealed an increased proportion of the near-full-length RC DNA of mutant P130T at the 4.0-kb position by two- to threefold (2.5 ± 0.6; an average of three independent experiments).
FIG. 1.
The intracellular hypermaturation phenotype of mutation P130T displays an increased relative amount of intracellular near-full-length RC-form DNA. Ten micrograms of plasmid DNA was adjusted to a total of 35 μg of DNA with a carrier and transfected to human hepatoma cell line HepG2. Core-associated HBV DNA was purified 7 days posttransfection as described previously (33). HBV DNA replication intermediates were separated by gel electrophoresis and detected by Southern blot analysis with a 3.2-kb HBV (adr) full-length probe. Characteristic HBV DNA replication intermediates are indicated by arrows. WT, wild type; SS, ssDNA.
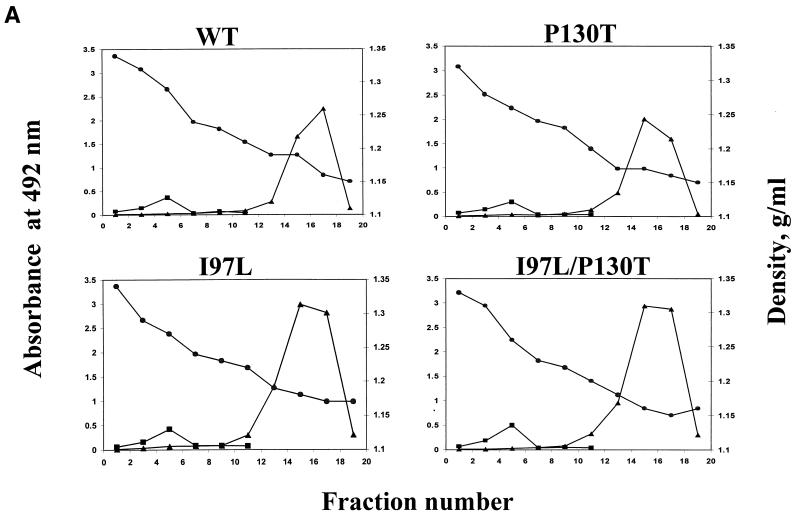
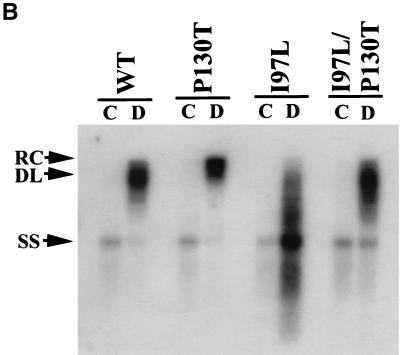
The media of each of the transfected cultures were collected and examined for their respective secretion profiles of virion particles, according to their buoyant density, by cesium chloride gradient centrifugation. Each fraction was assayed for its immunoreactivity by enzyme-linked immunosorbent assay specific for HBV surface antigen (HBsAg) and e or core antigen (HBeAg or HBcAg, respectively) (Fig. 2A) as well as for its degree of genome maturity by Southern blot analysis (Fig. 2B). The gradient distributions of HBsAg and HBcAg or HBeAg are similar among these four different genotypes (Fig. 2A). Likewise, the HBV DNA profiles of the naked core particle fractions are similar among these four different genotypes (Fig. 2B). Consistent with the intracellular results in Fig. 1, secreted mutant P130T viruses have a more enriched full-length RC-form DNA at the 4.0-kb position than the wild-type control. Perhaps, the most surprising finding to us is that the immature secretion pattern of mutant I97L appeared to be “cured” by the second mutation, P130T, in the genotype I97L/P130T (Fig. 2B).
FIG. 2.
Mutation P130T can reverse the immature secretion phenotype of mutation I97L. (A) Secreted HBV particles were analyzed by CsCl gradient centrifugation. The media were collected on days 5 and 7 posttransfection. Virus particles were then purified through a 20% sucrose cushion and subjected to isopycnic centrifugation in a gradient of 20 to 50% (wt/vol) cesium chloride. Fractions were separated according to their buoyant density and then submitted to assays for HBsAg (▴) (Abbott Auszyme EIA [enzyme immunoassay] kit) and HBeAg (■) (Abbott HBe rDNA [recombinant DNA] EIA kit). The enveloped virions (HBsAg positive and HBcAg negative) band at a density near 1.24 g/cm3 around fractions 10 to 14. The nonenveloped core particles (HBsAg negative and HBcAg positive) band at a density near 1.35 g/cm3 around fractions 2 to 6. WT, wild type. (B) Extracellular HBV DNA was analyzed by Southern blotting. Extracellular HBV DNA was purified and collected into either the core (pooled from fractions 2, 4, and 6) or Dane (pooled from fractions 10, 12, and 14) particle fractions. HBV-specific signal was detected by Southern blot assay as described above. C, nonenveloped core particles; D, enveloped Dane particles; SS, ssDNA.
As described previously (33), the maturity of the HBV DNA genome can be operationally defined as the ratio of RC-form DNA (the signals from the 4.0-kb position to the position right above the 1.5-kb ssDNA form) to ssDNA (the signals at and below the 1.5-kb position). As shown in Fig. 2B, the degree of genome maturity (RC/ssDNA ratio) of the wild type is 14-fold higher than that of mutant I97L (9.7/0.7). When mutation I97L is accompanied by mutation P130T in double mutant I97L/P130T, the RC/ssDNA ratio increased by about 10-fold more than that of the single mutant I97L (8.0/0.7), which is nearly 83% (8.0/9.7) of the level of the wild type (77% ± 6% of the wild-type level in three independent experiments) (Fig. 2B).
Our current study focused on the adr subtype, since few mutations at HBcAg codon 130 have been reported in the ayw subtype. Interestingly, in one longitudinal study of two chronic active hepatitis patients, the viral genomes in the sera acquired mutation I97L before the P130T mutation (i.e., mutant I97L emerged before mutant I97L/P130T) (14). It is conceivable that mutation P130T probably occurred later in patients to offset the immature secretion effect of the I97L mutation, which could have been acquired earlier through an independent mechanism such as immune escape. At present, it remains unclear if additional amino acid changes of HBcAg, other than at positions 97 and 130, could affect the compensatory property of the 130T mutation: e.g., mutants with double mutations at both codons 97 and 130 reported in references 1, 3, 6–8, 11–15, 20, 22–24, 26, and 28–31 might contain additional mutations. Note that the mutations at codon 97 often change from an isoleucine to a leucine (L) in the adr/w subtype or from a phenylalanine to a leucine in the ayw subtype.
Artificially created compensatory mutations, which can restore the stem-loop structure of HBV encapsidation signal and thus the replication activity, have been reported (16, 18, 21, 32). Most recently, compensatory mutations for the replication of 3TC drug-resistant polymerase variants have been reported (2, 5, 9, 19). To the best of our knowledge, this double mutation, I97L/P130T, is the first example of a naturally occurring compensatory mutation of the human HBV without any drug treatment. Further studies of the intramolecular compensatory mutations, such as I97L/P130T, could lead to a better understanding of the factors involved in plus-strand DNA synthesis and genome maturation, in addition to viral evolution and the structure-function relationship of HBcAg in virion secretion.
Acknowledgments
We thank colleagues in C. Shih's laboratory for careful reading of the manuscript.
This study was mainly supported by NIH grants RO1 CA 70336 and CA 84217 to C.S.
REFERENCES
- 1.Akarca U S, Lok A S F. Naturally occurring hepatitis B virus core gene mutations. Hepatology. 1995;22:50–60. [PubMed] [Google Scholar]
- 2.Allan M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine clinical investigation group. Hepatology. 1998;27:1670–1677. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 3.Carman W F, Boner W, Fattovich G, Colman K, Dornan E S, Thursz M, Hadziyannis S. Hepatitis B virus core protein mutations are concentrated in B cell epitopes in progressive disease and in T helper cell epitopes during clinical remission. J Infect Dis. 1997;175:1093–1100. doi: 10.1086/516447. [DOI] [PubMed] [Google Scholar]
- 4.Chang S-F, Netter H J, Bruns M, Schneider R, Frolich K, Will H. A new hepadnavirus infecting snow geese (anser caerulescens) produces a significant fraction of virions containing single-stranded DNA. Virology. 1999;262:39–54. doi: 10.1006/viro.1999.9844. [DOI] [PubMed] [Google Scholar]
- 5.Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Koida I, Arase Y, Saitoh S, Murashima N, Ikeda K, Kumada H. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–1716. doi: 10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- 6.Chuang W L, Omata M, Ehata T, Yokosuka O, Ito Y, Imazeki F, Lu S N, Chang W Y, Ohto M. Precore mutations and core clustering mutations in chronic hepatitis B virus infection. Gastroenterology. 1993;104:263–271. doi: 10.1016/0016-5085(93)90861-6. [DOI] [PubMed] [Google Scholar]
- 7.Ehata T, Omata M, Yokosuka O, Hosoda K, Ohto M. Variations in codons 84-101 in the core nucleotide sequence correlate with hepatocellular injury in chronic hepatitis B virus infection. J Clin Investig. 1992;89:332–338. doi: 10.1172/JCI115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehata T, Omata M, Chuang W L, Yokosuka O, Ito Y, Hosoda K, Ohto M. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J Clin Investig. 1993;91:1206–1213. doi: 10.1172/JCI116281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu L, Cheng Y C. Role of additional mutations outside the YMDD motif of hepatitis B virus polymerase in L(−)SddC (3TC) resistance. Biochem Pharmacol. 1998;55:1567–1572. doi: 10.1016/s0006-2952(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 10.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2703–2737. [Google Scholar]
- 11.Gotoh K, Mima S, Uchida T, Shikata T, Yoshizawa K, Irie M, Mizui M. Nucleotide sequence of hepatitis B virus isolated from subjects without serum anti-hepatitis B core antibody. J Med Virol. 1995;46:201–206. doi: 10.1002/jmv.1890460306. [DOI] [PubMed] [Google Scholar]
- 12.Gunther S, Paulij W, Meisel H, Will H. Analysis of hepatitis B virus population in interferon-a-treated patients reveals predominant mutations in the C-gene and changing e-antigenicity. Virology. 1998;244:146–160. doi: 10.1006/viro.1998.9079. [DOI] [PubMed] [Google Scholar]
- 13.Hosono S, Tai P C, Wang W, Ambrose M, Hwang D, Yuan T T, Peng B H, Yang C S, Lee C S, Shih C. Core antigen mutations of human hepatitis B virus in hepatomas accumulate in MHC class II-restricted T cell epitopes. Virology. 1995;212:151–162. doi: 10.1006/viro.1995.1463. [DOI] [PubMed] [Google Scholar]
- 14.Hur G M, Lee Y I, Sun D J, Lee J H, Lee Y I. Gradual accumulation of mutations in precore/core region of HBV in patients with chronic active hepatitis: implication of clustering changes in a small region of the HBV core region. J Med Virol. 1996;48:38–46. doi: 10.1002/(SICI)1096-9071(199601)48:1<38::AID-JMV6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Karasawa T, Shirasawa T, Okawa Y, Kuramoto A, Shimada N, Aizawa Y, Zeniya M, Toda G. Association between frequency of amino acid changes in core region of hepatitis B virus (HBV) and the presence of precore mutation in Japanese HBV carriers. J Gastroenterol. 1997;32:611–622. doi: 10.1007/BF02934110. [DOI] [PubMed] [Google Scholar]
- 16.Knaus T, Nassal M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 1993;17:3967–3975. doi: 10.1093/nar/21.17.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi M, Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984;30:227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- 18.Li J-S, Tong S-P, Wen Y-M, Vitvitski L, Zhang Q, Trépo C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: possible contribution of a single nucleotide in the precore region. J Virol. 1993;67:5402–5410. doi: 10.1128/jvi.67.9.5402-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melegari M, Scaglioni P P, Wands J R. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology. 1998;27:628–633. doi: 10.1002/hep.510270243. [DOI] [PubMed] [Google Scholar]
- 20.Miska S, Gunther S, Vassilev M, Meisel H, Pape G, Will H. Heterogeneity of hepatitis B virus C-gene sequences: implications for amplification and sequencing. J Hepatol. 1993;18:53–61. doi: 10.1016/s0168-8278(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 21.Pollack J R, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Frias F, Buti M, Jardi R, Cotrina M, Viladomiu L, Esteban R, Guardia J. Hepatitis B virus infection: precore mutants and its relation to viral genotypes and core mutations. Hepatology. 1995;22:1641–1647. doi: 10.1002/hep.1840220605. [DOI] [PubMed] [Google Scholar]
- 23.Sterneck M, Gunther S, Santantonio T, Fischer L, Broelsch C E, Greten H, Will H. Hepatitis B virus genomes of patients with fulminant hepatitis do not share a specific core mutation. Hepatology. 1996;24:300–306. doi: 10.1002/hep.510240203. [DOI] [PubMed] [Google Scholar]
- 24.Sterneck M, Gunther S, Gerlach J, Naoumov N V, Santantonio T, Fischer L, Rogiers X, Greten H, Williams R, Will H. Hepatitis B virus sequence changes evolving in liver transplant recipients with fulminant hepatitis. J Hepatol. 1997;26:754–764. doi: 10.1016/s0168-8278(97)80239-5. [DOI] [PubMed] [Google Scholar]
- 25.Summers J, Mason W S. Replication of the genome of a hepatitis-B like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Akahane Y, Hino K, Ohata Y, Mishiro S. Hepatitis B virus genomic sequence in the circulation of hepatocellular carcinoma patients: comparative analysis of 40 full-length isolates. Arch Virol. 1998;143:2313–2326. doi: 10.1007/s007050050463. [DOI] [PubMed] [Google Scholar]
- 27.Tencza M G, Newbold J E. Heterogeneous response for a mammalian hepadnavirus infection to acyclovir: drug-arrested intermediates of minus-strand viral DNA synthesis are enveloped and secreted from infected cells as virion-like particles. J Med Virol. 1997;51:6–16. [PubMed] [Google Scholar]
- 28.Tsubota A, Kumada H, Takaki K, Chayama K, Kobayashi M, Kobayashi M, Suzuki Y, Saitoh S, Arase Y, Murashima N, Ikeda K. Deletions on the hepatitis B virus core gene may influence the clinical outcome in hepatitis B e antigen-positive asymptomatic healthy carriers. J Med Virol. 1998;56:287–293. doi: 10.1002/(sici)1096-9071(199812)56:4<287::aid-jmv1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Uchida T, Aye T T, Becher S O, Hirashima M, Shikata T, Komine F, Moriyama M, Arakawa Y, Takase S, Mima S. Detection of precore/core-mutant hepatitis B virus genome in patients with acute or fulminant hepatitis without serological markers for recent HBV infection. J Hepatol. 1993;18:369–372. doi: 10.1016/s0168-8278(05)80283-1. [DOI] [PubMed] [Google Scholar]
- 30.Uchida T, Aye T T, Shihata T, Yano M, Yatsuhashi H, Koga M, Mima S. Evolution of the hepatitis B virus gene during chronic infection in seven patients. J Med Virol. 1994;43:148–154. doi: 10.1002/jmv.1890430209. [DOI] [PubMed] [Google Scholar]
- 31.Wakita T, Kakumu S, Shibata M, Yoshioka K, Ito Y, Shinagawa T, Ishikawa T, Takayanagi M, Morishima T. Detection of pre-c and core region mutants of hepatitis B virus in chronic hepatitis B virus carriers. J Clin Investig. 1991;88:1793–1801. doi: 10.1172/JCI115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan T T, Faruqi A, Shih J W K, Shih C. The mechanism of natural occurrence of two closely-linked HBV precore predominant mutations. Virology. 1995;211:144–156. doi: 10.1006/viro.1995.1387. [DOI] [PubMed] [Google Scholar]
- 33.Yuan T T-T, Sahu G K, Whitehead W E, Greenberg R, Shih C. The mechanism of an immature secretion phenotype of a highly frequent naturally occurring missense mutation at codon 97 of human hepatitis B virus core antigen. J Virol. 1999;73:5731–5740. doi: 10.1128/jvi.73.7.5731-5740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan T T-T, Tai P-C, Shih C. Subtype-independent immature secretion and subtype-dependent replication deficiency of a highly frequent, naturally occurring mutation of human hepatitis B virus core antigen. J Virol. 1999;73:10122–10128. doi: 10.1128/jvi.73.12.10122-10128.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]