Abstract
Environmental mismatches are defined as changes in the environment that induce public health crises. Well known mismatches leading to chronic disease include the availability of technologies that facilitate unhealthy diets and sedentary lifestyles, both factors that adversely affect cardiovascular health. This commentary puts these mismatches in context with biota alteration, an environmental mismatch involving hygiene-related technologies necessary for avoidance of infectious disease. Implementation of hygiene-related technologies causes a loss of symbiotic helminths and protists, profoundly affecting immune function and facilitating a variety of chronic conditions, including allergic disorders, autoimmune diseases, and several inflammation-associated neuropsychiatric conditions. Unfortunately, despite an established understanding of the biology underpinning this and other environmental mismatches, public health agencies have failed to stem the resulting tide of increased chronic disease burden. Both biomedical research and clinical practice continue to focus on an ineffective and reactive pharmaceutical-based paradigm. It is argued that the healthcare of the future could take into account the biology of today, effectively and proactively dealing with environmental mismatch and the resulting chronic disease burden.
Keywords: Allergy, Autoimmune disease, Biota alteration, History, Hygiene
Introduction: Environmental Mismatch and the Biology of Non-infectious Disease
Environmental mismatches are defined as changes in the environment that lead to poor health in a population (see Box 1 for definitions). Such changes can include climate shifts, introduction of competing species, and loss of species upon which a population depends for food or other needs. Humans are in a unique position among species in that we ourselves have been responsible for introducing the environmental mismatches that profoundly affect us. In humans, widespread and highly impactful environmental mismatches can be identified that affect the health of a large swath of the population. Five such highly impactful environmental mismatches that consistently affect humans in high-income areas around the world at the present time are:
| Box 1. Definitions |
| Biota alteration: A widespread and highly impactful environmental mismatch imposed by systems hygiene. This dominant mismatch involves a dramatic decrease in the biodiversity of eukaryotic symbionts, primarily the almost complete loss of helminths and, possibly to a lesser extent, protists. |
| Biota reconstitution: The intentional, systematic, and controlled introduction of symbionts into a population which has lost those symbionts or related symbionts due to biota alteration. |
| Environmental mismatch: The condition in which a change in environment is incompatible with the health of an organism that was healthy prior to that change. A classic example is the development of agriculture and later food processing technology, which in turn lead to consumption of inflammatory diets characterized by high glycemic indexes, high fat, and low fiber. Consumption of this diet, in turn, contributes to pandemics of obesity, diabetes and cardiovascular disease. |
| Host: A symbiont that is typically larger and provides habitat to another, typically smaller, symbiont. |
| Hygiene hypothesis: An outdated term coined in the late 1980s which attempted to describe the role of hygiene in the increased prevalence of allergies [5]. Current theory describes the loss of specific symbionts and the effect of that loss on allergic, autoimmune, and neuropsychiatric disorders. (See the definition for biota alteration in this Box.) |
| Infection: A symbiotic relationship in which a symbiont causes a disease or pathological state in the host as a result of living on or within the host. |
| Mutualist: A symbiont that derives benefits from its host and, at the same time, provides benefits for its host. |
| Parasite: A symbiont that derives benefits from its host while harming its host. Whether a particular symbiotic relationship is mutualistic or parasitic may be conditional, depending on environmental conditions and other factors [6,7]. Further, some symbionts may provide both advantages and disadvantages for the host, blurring the lines between mutualist and parasite. |
| Pathogen: A symbiotic organism that causes disease in its host. This medical term is potentially ambiguous in some cases of symbiosis, as it may or may not be applied to symbionts even if they exist in most hosts without causing disease, a relatively common phenomenon [8-12]. |
| Symbiont: An organism physically associated with another organism. Such relationships may be temporary or long-term. |
| Systems hygiene: Use of technologies that prevent transmission of pathogens. Common examples of this type of hygiene are use of water purification technology, sewage and waste management facilities, food production and storage devices, and modern building construction methods. Systems hygiene is distinct from personal hygiene measures such as social distancing, wearing of face coverings, and reducing hand-to-face contact. |
• inflammatory diets, characterized by consumption of food high in energy density (calories per unit mass) and low in dietary fiber and nutrition [1];
• sedentary lifestyles, characterized by the use of energy saving technology that removes the need for physical labor [1];
• indoor work environments, characterized by protection against harsh climate conditions and dependence on artificial light sources [2];
• chronic psychological stress as a result of numerous social changes [3]; and
• biota alteration, characterized by the loss of symbiotic organisms such as protists and helminths as a result of the use of technologies aimed at preventing food and waterborne disease [4].
Other mismatches may vary in time or location. For example, the availability and widespread use of addictive substances (eg, alcohol, tobacco or betel (Areca) nut-derived products) is a mismatch that has affected the health of different cultures to varying degrees through time. These mismatches could be placed in a category along with inflammatory diets as factors that have arisen because modern agriculture and production processes have made highly pleasurable and often addictive substances widely available.
Major human-derived environmental mismatches often offer advantages for human culture, but they are classified as environmental mismatches because they carry with them biological consequences that lead to poor health (Figure 1). For example, the development of indoor work environments protects individuals from harsh climate conditions, but can lead to widespread deficiency of vitamin D, a factor necessary for adequate immune function. Similarly, the invention of labor-saving devices and food processing technology allows humans to focus on endeavors other than procuring food and shelter, but can lead to a sedentary lifestyle and, in turn, obesity and heart disease.
Figure 1.
Breaking the chains of environmental mismatch. Environmental mismatch describes a condition in which an environmental change leads to unhealthy biological consequences. The ability of humans to intervene in the biological consequences of environmental change offers us the opportunity to break the chains of environmental mismatch. Such approaches are rooted in sound biological science and are highly effective, generally reflecting a proactive approach to human health. Unfortunately, medical practice and current biomedical research often overlook opportunities to break the chains of mismatch, focusing instead on reactive, reductionistic, costly and ineffective approaches to human health.
Environmental mismatches profoundly affect almost all aspects of human health, including infectious disease, allergy, autoimmunity, diabetes, obesity, heart disease, digestive disorders, mental health disorders, and various cancers, including many lung, breast, and colon cancers. The World Health Organization (WHO) counts four lifestyle choices as the widespread and impactful drivers of disease: “tobacco use, physical inactivity, harmful use of alcohol and unhealthy diet” [1]. These factors, part of the cycle of environmental mismatch (Figure 1) are causing a “rising financial burden of [disease that] will reach levels that are beyond the capacity of even the wealthiest countries in the world to manage” according to Margaret Chan, who served as Director-General of the WHO for more than a decade [1].
The Environmental Mismatch of Biota Alteration
The lifestyle choices recognized by the WHO are the most generally recognized factors connected with environmental mismatch. However, “biota alteration” is another widespread and impactful environmental mismatch consistently seen in high-income areas of the world. Biota alteration is defined as alteration of the human biota by modern, urban lifestyles. The human biota is itself defined as the community of organisms associated with the human body and is typically discussed within the context of distinct categories based on the nature of the organisms involved. For example, the microbiota, or microscopic portion of the biota, contains viruses, bacteria, fungi, and protists. Other portions of the biota, comprised of larger, non-microscopic organisms, include a variety of cestodes (tapeworms) and nematodes (roundworms). Within the context of environmental mismatch and biota alteration, the human biota can be categorized based on how it is affected by urbanization and industrialization. Rook and colleagues first described such a categorization [13], dividing the biota into three groups, as shown in Table 1. Communicable, disease-causing microbes are more numerous as a result of increased population density, but the resulting infectious disease burden is partially mitigated by factors such as personal hygiene and vaccination. Beneficial microbes such as the gut microbiota, in contrast, are altered by a number of factors, including the processed diet and other lifestyle options that result from industrialization [14-16]. In addition, chronic inflammation resulting from several environmental mismatches likely leads to rapid and complex evolution-driven changes to the microbiota as it adapts to novel environments [17,18]. Other factors, including medical use of antibiotics, and perhaps other environmental factors such as the addition of synthetic compounds into the environment [19], can also contribute to alteration of the microbiota. In stark contrast, cestodes and nematodes of the gut, collectively termed helminths, have been greatly reduced in numbers by the development of “systems hygiene,” technologies ensuring pathogen-free food and water supplies as well as low levels of fecal-oral disease transmission [4]. Such technologies include water treatment facilities, food processing and storage technologies, and sewage handling processes.
Table 1. Alteration of Various Components of the Human Biota by Urban, Industrial Environments, and the Health Consequences of those Alterations.
| Component of the biota | Effect of industrialization | Results for human health |
| Pathogens: infectious disease-causing organisms | Increased exposure as a result of high population density | Pro-inflammatory: acts as triggers for chronic inflammatory disease. |
| Beneficial microbiota | Altered community composition as a result of processed foods, loss of contact with the soil, chronic inflammation, and other factors | Associated with inflammatory disease, although direct causal relationships between particular species and disease states are not evident in most cases. |
| Helminths and most protists | Almost complete loss of exposure as a result of systems hygiene | Pro-inflammatory: Profound loss of immune regulation in many individuals, leading to propensity for allergy, autoimmune disease, and neuropsychiatric conditions. |
These three divisions of the biota were first described by Rook and colleagues [13].
Just as distinct components of the biota are altered in different ways by urbanization and industrialization, so are the health-related consequences of those alterations different, depending on the component of the biota (Table 1). The increased presence of infectious disease as a result of increased population densities can act as triggers for autoimmune disease [5]. Further, alterations in the gut microbiota are often associated with chronic inflammatory disease, although specific community profiles associated with specific chronic inflammatory conditions are often difficult to identify, and causal relationships are not established [20-22]. In contrast, loss of helminths and protists results in a distinct phenotype, with increased propensities for allergic conditions, autoimmune diseases, and neuropsychiatric disorders [5,6]. Thus, it is the almost complete loss of helminths and protists [13] that will be considered here in the context of environmental mismatch and biota alteration. Importantly, this perspectives piece will not provide a detailed discussion of the field of helminth therapy. Several excellent reviews on the topic have been recently published [23-26]. Rather, this paper will contain a discussion of the biological observations that led to major innovations in the field of science and how biota alteration fits into a consistent historical trend of difficulty in translating those biological observations into clinical practice.
A History of Difficulty Transferring Simple Biological Observations into Medicine
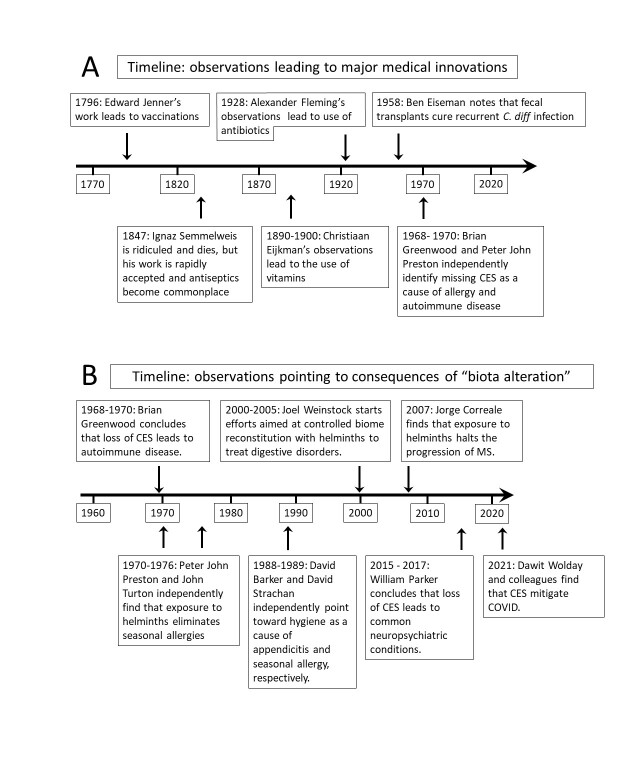
A timeline showing the discovery of some of the major, life-saving medical innovations in use today is shown in Figure 2A. An assessment of these historical milestones shows that (a) major medical innovations are generally triggered by simple biological observations, and (b) transferring biological observations into the field of medicine can be challenging. For example, Edward Jenner’s observations that individuals working closely with milk cows had less severe smallpox than did others led to his development of the first vaccines. However, when his work was rejected by the Royal Society, he self-published, coining the term vaccination [27]. The well-known experience of Ignaz Semmelweis is perhaps one of the most extreme individual examples of difficulty in translation from observations to the clinic. Although eventually hailed as the Father of Hand Hygiene, the Father of Infection Control, and the Savior of Mothers, his work on the prevention of infection using personal hygiene was heavily criticized and rejected, resulting in the death of thousands of patients and contributing to his own death in a mental asylum in 1865 [28]. In another example, assessment of bacterial growth inhibition by fungus on a petri dish led to the discovery of penicillin in 1928 by Alexander Fleming and marked the beginning of the antibiotic revolution, but technical difficulties in production of the chemical for clinical use were not overcome for more than a decade, and penicillin was largely forgotten. Fortunately, the successful production of another class of antibiotics, sulfanilamides, eventually attracted interest in antibiotics in general, encouraging Chain and Florey to develop a method of producing sufficient quantities of penicillin for clinical use [29]. In addition, the death of soldiers from infection during the early years of World War II was likely a major contributing factor motivating the commercial production of the antibiotic [29]. These pivotal historical events point toward systematic difficulties in moving simple biological observations into the clinic and suggest that medical infrastructure has generally lacked an effective process for accomplishing the task. Further, evidence points toward a history of pioneering individuals and perhaps even military authorities with specific goals working outside of the mainstream medical system to achieve the transition from observations to the clinic.
Figure 2.
Observations in biology and corresponding major medical innovations. A. A historical view of some observations that led to major medical innovations in the past. Simple observations rather than detailed scientific studies provided the impetus for most innovations: The milkmaids did not get severe smallpox, patients did not die when their physicians were clean, bacteria would not grow next to a fungus, the chickens were healthier eating brown rice, etc. B. A historical perspective on published studies providing new insights into the effects of biota alteration and, in some cases, biota restoration. Insights in this field were generally triggered by observations in individuals and/or in populations, then validated using laboratory animal models. Ongoing work looking at the effect of biota alteration on type 2 diabetes [49] and aging [50] is not included in the diagram. CES: Complex eukaryotic symbionts (helminths and protists).
A current example of extreme difficulty in transferring biological observations to the clinic involves the fecal transplant. Fecal transplantation, a process that occurs in all humans around the time of birth, involves the transfer of a fecal microbiome from one individual to another. The slow pace of incorporating fecal transplantation into the standard clinical treatment for recurrent Clostridium difficile colitis represents an ongoing and tragic failure in the history of medicine. Ben Eiseman’s team in Denver, Colorado discovered in 1958 that fecal transplantation effectively resolves recurrent C. difficile-associated colitis, a common and deadly condition that results when the intestinal microbiota fails to recover following treatment with antibiotics. Eiseman’s groundbreaking work was validated in the early 1960s by several independent studies, but despite his renown in the surgical field [30] and over 10 000 deaths per year from recurrent C. difficile colitis, fecal transplants took more than half a century to move forward into the mainstream [31], and are still not standard of care in all institutions. When the procedure eventually did become more popular, it was apparently independent of Eiseman’s ongoing influence [31]. Eiseman, for his part, published only a single paper on the topic, spending much of the latter part of his career on unrelated issues such as organization of medical resources for military combat. Poignantly, the number of US deaths from recurrent C. difficile colitis that could have been prevented by fecal transplants between 1958 and the present conservatively exceeds US deaths during the Vietnam war by a factor of 10.
In summary, this brief historical perspective demonstrates that incorporating straightforward and intuitive biological observations into the clinic is not sufficiently incentivized by even a very large number of lives impacted. When considering this issue, the rationale behind ignoring apparent resolutions of far-reaching and impactful problems should be addressed. The quest for more information regarding biological mechanisms, financial considerations, and a lack of awareness seem to dominate a landscape of apathy. Regardless of the reasons, difficulty in converting groundbreaking discovery into standard medical practice has existed for over two centuries and does not appear to be changing, as is further illustrated in the next section.
Overcoming the Mismatch of Biota Alteration: Another Example of Difficulties in Transferring Observations into Clinical Practice
As described above (Table 1), the most prominent, medically important aspect of biota alteration is the environmental mismatch that involves the loss of complex eukaryotic symbionts, particularly helminths and protists, from the ecosystem of the human body as a result of “systems hygiene” [4]. Figure 2B shows a historical perspective of the milestones in understanding the impact of biota alteration on immune function and chronic disease. Observations published by Brian Greenwood working in Ibadan, Nigeria between 1968 and 1970 showed that an absence of helminths and protists could lead to autoimmune disease [32-34]. Simultaneous observations by Peter John Preston, working with the British Royal Navy, showed that the presence of helminths alleviates seasonal allergies [7], an observation soon confirmed by John Turton in Surrey, UK [35]. Thus, at least three independent observations made over half a century ago had demonstrated the importance of helminths and protists for prevention and even treatment of some common allergic and autoimmune conditions.
Almost 20 years after the observations made by Greenwood, Preston, and Turton, the field of immunology entered the discussion, and the term “hygiene hypothesis” was coined [36]. Unfortunately, the original observations of the importance of helminths and protists had been forgotten, and the field of immunology focused on cellular and molecular factors involved in disease rather than restoring an altered biota. Another decade passed before the late Joel Weinstock attempted to reconstitute the human biota with helminths in an effort to treat inflammatory bowel disease. Despite dramatic early successes prior to 2005 at the University of Iowa, the effort to bring biota reconstitution to the clinic eventually failed, likely due in part to a lack of understanding regarding the production of helminths by pharmaceutical manufacturers [37]. Subsequently, thousands of individuals began self-treating with helminths [37,38], but the practice has not yet reached mainstream clinical use.
Only within the past decade, while examining the reported outcomes of individuals self-treating with helminths, co-author Parker and colleagues observed that helminths were beneficial to neuropsychiatric function in humans [38]. Recent findings in rodent models of biota reconstitution support the importance of those organisms for mental health and function [39-41]. For example, Daniel Młocicki and Dogmara Mirowska-Guzel at the Medical University of Warsaw, Poland, showed in 2022 that laboratory rats colonized with the benign helminth Hymenolepis diminuta perform better in memory tests than helminth-free animals [40]. Independently, Marilyn Scott’s laboratory at McGill University in Quebec, Canada also showed in 2022 that offspring of laboratory mice colonized with the nematode Heligmosomoides bakeri have profoundly better spatial memory compared to offspring of uncolonized controls [39]. The fact that colonized animals perform better on memory tests and avoid dangerous (for their species) open fields more effectively brings into question the unknown mental health costs that humans without such symbionts may be facing.
Major depressive disorder, anxiety disorders, and several other neuropsychiatric disorders can be considered chronic inflammatory conditions [42,43], consistent with the view that the same environmental mismatches that derail immune function and lead to allergy and autoimmune disease can also adversely affect brain function (Figure 1). Multiple sclerosis (MS), the clearest example of dysfunctional crosstalk between the nervous and immune systems, has significant co-morbidity with depression and anxiety. MS relapse and progression is dramatically slowed and reduced in the presence of naturally occurring infections with helminths [44-46]. Other neuropsychiatric disorders, such as bipolar disorder, schizophrenia, and autism spectrum disorder, show hallmarks of neuroimmune dysfunction and are difficult to treat with current pharmaceutical treatments focused on the nervous system (Table 2). Systematic studies of individuals using intentional helminth exposure to reduce pathological immune reactivity indicated that the presence of helminths mitigates symptoms of both major depressive disorder and anxiety disorders [38,47]. These disorders and others can potentially be treated with biota alteration as a novel option. Mechanisms currently thought to underlie positive impact of biota reconstitution on brain health include short chain fatty acids (SCFA) acting on microglia, alterations in ratios, and activities of regulatory T cells versus inflammatory T-helper cell subsets (notably Th1 and Th17), a reduction in the neuroinflammatory cytokine response to infection [41], and alterations in bidirectional signaling of the nervous system via visceral, vagal, and spinal nerves [48].
Table 2. The Prevalence of Some of the Most Common Chronic, Inflammation-related Conditions in the US.
| Condition | Percentage of US Population* | Common Immunosuppressive Drugs |
| Lupus | 0.06% [51] | azathioprine, methotrexate and mycophenolate [52] |
| Multiple sclerosis (MS) | 0.12% [53] | azathioprine, cyclophosphamide, methotrexate, and mitoxantrone [54], as well as fingolimod and natalizumab among others [55] |
| Alopecia areata | 0.21% [56] | Oral cyclosporin, methotrexate, and sulphasalazine [57] |
| Inflammatory bowel disease (Crohn’s and ulcerative colitis) | 0.36% [58] | azathioprine, 6-mercaptopurine, methotrexate, and cyclosporin [59] |
| Rheumatoid arthritis | 0.40% [60] | azathioprine, cyclophosphamide, chlorambucil and methotrexate [61] |
| Psoriasis | 2.31% [62] | adalimumab, infliximab, etanercept, ustekinumab, and alefacept [63] |
| Eczema | 9.67% [64] | cyclosporin, azathioprine, methotrexate, mycophenolate mofetil, and pimecrolimus [65] |
| Depression | 13.2% [66] | Not applicable** |
| Migraine, severe headache | 15.3% [67] | Not applicable** |
| Anxiety disorders | 19.1% [68] | Not applicable** |
| Allergic rhinitis (allergies)*** | 15-30% [69] | dexamethasone, antihistamines and other mast-cell antagonists, decongestants |
Immunosuppressive drugs typically used to treat those conditions are listed, if applicable. This tally is not meant to be exhaustive, as for example it does not include food sensitivities or irritable bowel syndrome. Although these chronic conditions are associated with inflammation, immunosuppressive drugs are not commonly used to treat them. *Percentage calculations used the total number of patients divided by the total US population in the same year as the patient data. The total US population was obtained through data from The World Bank. **Although inflammation underpins depression and anxiety disorders [42,43] and migraine headaches [70], pharmaceutical management of these conditions typically targets neurological function rather than immune factors. ***The prevalence of allergic rhinitis is higher when patient self-reports are considered compared to physician reports. However, since allergic rhinitis is often treated with immunosuppression by the patient using over-the-counter medications, patient reports are pertinent for this assessment of the numbers of individuals using immunosuppression.
Most recently, the view that biota alteration affects the pathogenesis of viral disease [4] was shown to apply to COVID-19, revealing a profound and negative impact of biota alteration on the viral pandemic [5]. Recent work related to COVID-19 showed that symbiotic protists and helminths afforded roughly a 3-fold protection from severe clinical outcomes following COVID-19 infection [5]. Given the importance of biota alteration in such a diverse array of chronic, inflammation-associated diseases, the impact of biota alteration on human health is difficult to estimate. However, a summary of pharmaceutical use by the US population in an attempt to compensate for the health consequences of biota alteration can be tabulated (Table 2). In that tabulation, it is evident that many if not most individuals suffer from the adverse effects of biota alteration. Nevertheless, at the same time, we do not in any way suggest that systems hygiene be abandoned [4].
Conclusions and Outlook
The consequences of environmental mismatches need to be addressed immediately in the interest of public health. Unfortunately, even for mismatches for which the underlying biology is extremely well established, little progress has been made at the public health level. For example, despite the implementation of numerous programs by various agencies to improve diets and physical activity, the burden of disease associated with inflammatory diets and sedentary lifestyles continues to worsen [1]. Thus, the incentives for effectively addressing the problems of even the most well-established environmental mismatches are not adequate at the present time.
A straight-forward means of adjusting for the effects of biota alteration has been proposed. The systems hygiene that leads to biota alteration offers very substantial advantages to human society. This type of hygiene allows humans to live in high-density populations without suffering from repeated waves of deadly, water and food-borne infections and the stench of waste products from digestion [4]. Thus, no effort to eliminate the underlying cause of biota alteration is envisioned. Rather, it is biota reconstitution, the controlled reintroduction of benign, symbiotic helminths with desired immunomodulatory effects, that is required [5]. Although the impacts of biota alteration and other environmental mismatches for population health are evident, the pathway from environmental mismatch to disease is generally complex at the individual level, interacting with genetics, epigenetics, socioeconomic factors, and numerous lifestyle choices. Thus, not all individuals will be affected adversely by all environmental mismatches, including biota alteration. With this in mind, efforts to prevent the impact of biota alteration on the population as a whole must have a minimal negative impact on that population, involving the introduction of truly benign organisms.
Although scientific questions remain regarding the mechanisms by which biota alteration facilitates chronic disease, further basic science research regarding this environmental mismatch seems trite at best and misguided at worst. Additional information about the nature of chronic disease will add knowledge but will not change the underlying cause of disease or the options available for remedy of those underlying causes. Thus, despite unknowns, biota reconstitution should be advanced into clinical trials with urgency at the present time. As more and more individuals in high-income countries require pharmaceutical-based immunosuppression (for examples in the US population, see Table 2), the consequences of biota alteration have become dire. In addition, increased inflammation as a result of biota alteration can impact the effects of other environmental mismatches that are primarily associated with cardiovascular disease and infectious disease. Evaluation of potential solutions to the adverse consequences of biota alteration involves systematic evaluation of benign symbiotic organisms for their effect on disease, with a view of optimizing the benefit-to-cost ratio of the therapy. Unfortunately, as with other efforts to address environmental mismatch, incentives to proceed with available solutions are insufficient at the present time [37]. As highlighted above, data showing that complex eukaryotic symbionts profoundly benefit allergic and autoimmune disorders are now more than half a century old [7,32-34], suggesting that the consequences of biota alteration are as entrenched in the population as the consequences of other environmental mismatches.
The impact of specific mismatches, including unhealthy diets, cigarette smoking, and sedentary lifestyles have been estimated [1]. In addition, the estimates presented herein (eg, Table 2) provide one perspective on the burden of disease resulting from biota alteration. However, the total burden of all environmental mismatch on human disease has yet to be estimated. The relative contribution of environmental mismatch to mortality, the loss of disability adjusted life years, the financial costs to healthcare, and impact on overall quality of life merits close examination. In the interest of public health, it is hoped that such knowledge will help policy makers provide incentives for effectively dealing with environmental mismatch at the population level. Prudent policy would emphasize population-wide, evidence-based strategies aimed at addressing all environmental mismatches and the health consequences of those mismatches. Although biota reconstitution can be readily envisioned to compensate for biota alteration, addressing other environmental mismatches may prove more difficult, as those mismatches often involve socioeconomic factors and lifestyle choices related to diet, exercise, and chronic stress. Initiatives are needed that include large-scale, interdisciplinary, longitudinal programs with stable funding sources aimed at more precisely determining the impact of mismatch on a population-wide basis, and further elucidating how genetic, epigenetic, and environmental factors affect the mismatch-induced pathogenesis of disease at the individual level.
The light of biological science provides a promise of disease treatment and prevention. Tragically, that light has been ignored as modern biomedical research focuses on small details without an awareness of the larger light. It is anticipated that continued attempts to achieve health using pharmaceutical-based approaches that do not address the underlying, biological causes of disease will continue to fail. This ongoing failure will be reflected in the continued growth of chronic disease burden, ever-increasing human suffering, and a financial cost that will eventually overwhelm even the wealthiest of countries. The question is, how long will humanity suffer under the burden of environmental mismatch before taking effective action in the light of biology?
Glossary
- WHO
World Health Organization
- CES
complex eukaryotic symbionts (helminths and protists)
- MS
Multiple sclerosis
Author ORCIDs
WP, ORCID: https://orcid.org/0000-0003-3644-9152; JK, ORCID: https://orcid.org/0000-0001-5411-6082; LW, ORCID: https://orcid.org/0000-0002-6006-8256; LA, ORCID: https://orcid.org/0000-0002-9091-7650; JDL, ORCID: https://orcid.org/0000-0001-5085-9807.
Competing interests
Co-author WP has a financial commercial interest in helminth therapy with incentives to conduct incisive clinical trials evaluating helminth therapy. Co-author EP is currently a student at Duke University, which also has a financial interest in helminth therapy. Co-authors KJ, LW, and JDL direct academic laboratories with incentives to maintain funding for those laboratories. Co-author LA has no competing interests.
References
- Alwin A. Global status report on noncommunicable diseases 2010. World Health Organization; 2011. [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007. Jul;357(3):266–81. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Brenner SL, Jones JP, Rutanen-Whaley RH, Parker W, Flinn MV, Muehlenbein MP. Evolutionary mismatch and chronic psychological stress. Journal of Evolutionary Medicine. 2015;3:art235885. 10.4303/jem/235885 [DOI] [Google Scholar]
- Parker W, Sarafian JT, Broverman SA, Laman JD. Between a hygiene rock and a hygienic hard place: avoiding SARS-CoV-2 while needing environmental exposures for immunity. Evol Med Public Health. 2021. Feb;9(1):120–30. 10.1093/emph/eoab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W, Patel E, Jirků-Pomajbíková K, Laman JD. COVID-19 morbidity in lower versus higher income populations underscores the need to restore lost biodiversity of eukaryotic symbionts. iScience. 2023. Mar;26(3):106167. 10.1016/j.isci.2023.106167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W, Ollerton J. Evolutionary biology and anthropology suggest biome reconstitution as a necessary approach toward dealing with immune disorders. Evol Med Public Health. 2013. Jan;2013(1):89–103. 10.1093/emph/eot008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston PJ. The biology of the atopic response. J R Nav Med Serv. 1970;56(3):229–35. 10.1136/jrnms-56-229 [DOI] [PubMed] [Google Scholar]
- Wolday D, Gebrecherkos T, Arefaine ZG, Kiros YK, Gebreegzabher A, Tasew G, et al. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. EClinicalMedicine. 2021. Sep;39:101054. 10.1016/j.eclinm.2021.101054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelkeba L, Mekonnen Z, Emana D, Jimma W, Melaku T. Prevalence of soil-transmitted helminths infections among preschool and school-age children in Ethiopia: a systematic review and meta-analysis. Glob Health Res Policy. 2022. Mar;7(1):9. 10.1186/s41256-022-00239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu G, Aschalew Z, Zerihun E. Burden of intestinal helminths and associated factors three years after initiation of mass drug administration in Arbaminch Zuria district, Southern Ethiopia. BMC Infect Dis. 2018. Aug;18(1):435. 10.1186/s12879-018-3330-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhotská Z, Jirků M, Hložková O, Brožová K, Jirsová D, Stensvold CR, et al. A study on the prevalence and subtype diversity of the intestinal protist blastocystis sp. in a gut-healthy human population in the Czech Republic. Front Cell Infect Microbiol. 2020. Oct;10:544335. 10.3389/fcimb.2020.544335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RM, Leyden JJ, Margolis DJ. Colonisation rates of Streptococcus pyogenes and Staphylococcus aureus in the oropharynx of a young adult population. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2005;11(2):153-5. Epub 2005/02/01. https://doi.org/ 10.1111/j.1469-0691.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014. Jul;177(1):1–12. 10.1111/cei.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. Jan;505(7484):559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012. May;486(7402):222–7. Available from: http://www.nature.com/nature/journal/v486/n7402/abs/nature11053.html#supplementary-information 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018. Mar;555(7695):210–5. 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A, Holzknecht ZE, Holzknecht R, Bowles DE, Kotzé SH, Modliszewski JL, et al. Evolution of bacteria in the human gut in response to changing environments: an invisible player in the game of health. Comput Struct Biotechnol J. 2021. Jan;19:752–8. 10.1016/j.csbj.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Jirků M, Corcoran DL, Parker W, Jirků-Pomajbíková K. Altered gut ecosystems plus the microbiota’s potential for rapid evolution: A recipe for inevitable change with unknown consequences. Comput Struct Biotechnol J. 2021. Oct;19:5969–77. 10.1016/j.csbj.2021.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sharma P, Pal N, Kumawat M, Shubham S, Sarma DK, et al. Impact of environmental pollutants on gut microbiome and mental health via the gut-brain axis. Microorganisms. 2022. Jul;10(7):1457. 10.3390/microorganisms10071457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelenburg HJ, Lucassen PJ, Sarafian JT, Parker W, Laman JD. Multiple sclerosis and the microbiota: progress in understanding the contribution of the gut microbiome to disease. Evol Med Public Health. 2022. Jun;10(1):277–94. 10.1093/emph/eoac009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloquin JM, Nguyen DD. The microbiota and inflammatory bowel disease: insights from animal models. Anaerobe. 2013. Dec;24:102–6. 10.1016/j.anaerobe.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019. Aug;8(3):126. 10.3390/pathogens8030126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H. Human helminth therapy to treat inflammatory disorders - where do we stand? BMC Immunol. 2015. Mar;16(1):12. 10.1186/s12865-015-0074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Xu N, Wang X, Vallée I, Liu M, Liu X. Helminth Therapy for Immune-Mediated Inflammatory Diseases: Current and Future Perspectives. J Inflamm Res. 2022. Jan;15:475–91. 10.2147/JIR.S348079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020. May;16(5):e1008508. 10.1371/journal.ppat.1008508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotková K, Parker W, Levá J, Růžková J, Lukeš J, Jirků Pomajbíková K. Helminth Therapy - From the Parasite Perspective. Trends Parasitol. 2019. Jul;35(7):501–15. 10.1016/j.pt.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc Bayl Univ Med Cent. 2005. Jan;18(1):21–5. 10.1080/08998280.2005.11928028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi U, Barwal KC. Ignac Semmelweis-Father of Hand Hygiene. Indian J Surg. 2020. Jun;82(3):276–7. 10.1007/s12262-020-02386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg Infect Dis. 2017;23(5):849–53. 10.3201/eid2305.161556 [DOI] [Google Scholar]
- Beger HG. Ben Eiseman, M.D., Emeritus Professor of Surgery and Medicine, University of Colorado Medical School, Denver, USA. hans.beger@medizin.uni-ulm.de. Langenbecks Arch Surg. 2004. Aug;389(4):311–2. 10.1007/s00423-004-0457-z [DOI] [PubMed] [Google Scholar]
- Rao K, Young VB. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect Dis Clin North Am. 2015. Mar;29(1):109–22. 10.1016/j.idc.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM. Autoimmune disease and parasitic infections in Nigerians. Lancet. 1968. Aug;2(7564):380–2. 10.1016/S0140-6736(68)90595-3 [DOI] [PubMed] [Google Scholar]
- Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB x NZW) F1 hybrid mice by infection with malaria. Nature. 1970. Apr;226(5242):266–7. 10.1038/226266a0 [DOI] [PubMed] [Google Scholar]
- Greenwood BM, Voller A, Herrick EM. Suppression of adjuvant arthritis by infection with a strain of the rodent malaria parasite Plasmodium berghei. Ann Rheum Dis. 1970. May;29(3):321–3. 10.1136/ard.29.3.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton JA. Letter: IgE, parasites, and allergy. Lancet. 1976. Sep;2(7987):686. 10.1016/S0140-6736(76)92492-2 [DOI] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989. Nov;299(6710):1259–60. 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan A, Sarafian JT, Jirků-Pomajbíková K, Parker W. Socio-medical studies of individuals self-treating with helminths provide insight into clinical trial design for assessing helminth therapy. Parasitol Int. 2022. Apr;87:102488. 10.1016/j.parint.2021.102488 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Jaint D, Thomas S, Wilson J, Parker W. Overcoming evolutionary mismatch by self-treatment with helminths: current practices and experience. Journal of Evolutionary Medicine. 2015;3:235910. 10.4303/jem/235910 [DOI] [Google Scholar]
- Noel SC, Fortin-Hamel L, Haque M, Scott ME. Maternal gastrointestinal nematode infection enhances spatial memory of uninfected juvenile mouse pups. Sci Rep. 2022. Jun;12(1):9796. 10.1038/s41598-022-13971-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecharz-Klin K, Świerczyńska M, Piechal A, Wawer A, Joniec-Maciejak I, Pyrzanowska J, et al. Infection with intestinal helminth (Hymenolepis diminuta) impacts exploratory behavior and cognitive processes in rats by changing the central level of neurotransmitters. PLoS Pathog. 2022. Mar;18(3):e1010330. 10.1371/journal.ppat.1010330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, et al. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav Immun. 2016. Jan;51:14–28. 10.1016/j.bbi.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016. Jan;16(1):22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013. Sep;11(1):200. 10.1186/1741-7015-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008. Aug;64(2):187–99. 10.1002/ana.21438 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007. Feb;61(2):97–108. 10.1002/ana.21067 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011. Apr;233(1-2):6–11. 10.1016/j.jneuroim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Liu J, Morey RA, Wilson JK, Parker W. Practices and outcomes of self-treatment with helminths based on physicians’ observations. Journal of Helminthology. 2016;FirstView:1-11. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Margolis KG. The gut, its microbiome, and the brain: connections and communications. J Clin Invest. 2021. Sep;131(18):e143768. 10.1172/JCI143768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter K, Tahapary DL, Sartono E, Soewondo P, Supali T, Smit JW, et al. Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol. 2017. May;39(5):e12404. 10.1111/pim.12404 [DOI] [PubMed] [Google Scholar]
- Zhang B, Gems D. Gross ways to live long: parasitic worms as an anti-inflammaging therapy? eLife. 2021. Feb;10:e65180. 10.7554/eLife.65180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmirly PM, Parton H, Wang L, McCune WJ, Lim SS, Drenkard C, et al. Prevalence of Systemic Lupus Erythematosus in the United States: Estimates From a Meta-Analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021. Jun;73(6):991–6. 10.1002/art.41632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon KP, Jiang SH. Treatment of systemic lupus erythematosus. Aust Prescr. 2020;43(3):85-90. Epub 06/02. https://doi.org/ 10.18773/austprescr.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86(11):1014-21. Epub 02/17. https://doi.org/ 10.1212/WNL.0000000000002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. Immunosuppressive treatment in multiple sclerosis. J Neurol Sci. 2004. Aug;223(1):1–11. 10.1016/j.jns.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Bierhansl L, Hartung HP, Aktas O, Ruck T, Roden M, Meuth SG. Thinking outside the box: non-canonical targets in multiple sclerosis. Nat Rev Drug Discov. 2022. Aug;21(8):578–600. 10.1038/s41573-022-00477-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigno M, Anastassopoulos KP, Mostaghimi A, Udall M, Daniel SR, Cappelleri JC, et al. A large cross-sectional survey study of the prevalence of Alopecia Areata in the United States. Clin Cosmet Investig Dermatol. 2020. Apr;13:259–66. 10.2147/CCID.S245649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries MJ, Sun J, Paus R, King LE Jr. Management of alopecia areata. BMJ. 2010. Jul;341;jul23 1:c3671–c. 10.1136/bmj.c3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58(2):519-25. Epub 08/29. https://doi.org/ 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol. 2014. Mar;20(12):3146–52. 10.3748/wjg.v20.i12.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. National Arthritis Data Workgroup . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008. Jan;58(1):15–25. 10.1002/art.23177 [DOI] [PubMed] [Google Scholar]
- Rainer F. Immunosuppressive therapy in rheumatoid arthritis. Acta Med Austriaca. 1988;15(5):137–40. [PubMed] [Google Scholar]
- Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CE. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021. Aug;157(8):940–6. 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JW, Koo JYM. The safety of systemic treatments that can be used for geriatric psoriasis patients: a review. Dermatol Res Pract. 2012;2012:367475-. Epub 05/28. https://doi.org/ 10.1155/2012/367475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin JM, Reed ML. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18(2):82-91. Epub 2007/05/15. https://doi.org/ 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- Lee JH, Son SW, Cho SH. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. 2016. May;8(3):181–90. 10.4168/aair.2016.8.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Gu Q. Antidepressant use among adults: United States, 2015–2018. In: Statistics NCHS, editor. Hyattsville, MD: Center for Disease Control and Prevention; 2020.
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018. Apr;58(4):496–505. 10.1111/head.13281 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health . Mental Health Information: Statistics: Any Anxiety Disorder. NIMH Information Resource Center. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder. accessed 2023.
- Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015. Jan;372(5):456–63. 10.1056/NEJMcp1412282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekker E, Tanaka M, Szabó Á, Vécsei L. Neurogenic inflammation: the participant in migraine and recent Advancements in translational research. Biomedicines. 2021. Dec;10(1):76. 10.3390/biomedicines10010076 [DOI] [PMC free article] [PubMed] [Google Scholar]