Abstract
Simple Summary
Vulvoperineal defect reconstruction after oncological resection often leads to complications, which affect approximately 30% of patients. In recent decades, flap design has evolved towards perforator-based approaches to reduce this complication rate and reduce functional deficits. The aim of our systematic review and meta-analysis was to assess and compare the complication rate between perforator- and non-perforator-based reconstructions. Among 2576 screened studies, 49 met our inclusion criteria, encompassing 1840 patients. We found that the overall short-term surgical complication rate was comparable in patients receiving a perforator or a non-perforator flap, with a tendency towards fewer complications when using a perforator flap. Perforator flap-based reconstruction allows the surgeon to spare relevant anatomical structures and is therefore associated with fewer functional deficits, with a comparable complication rate.
Abstract
Background: Patients with advanced vulvoperineal cancer require a multidisciplinary treatment approach to ensure oncological safety, timely recovery, and the highest possible quality of life (QoL). Reconstructions in this region often lead to complications, affecting approximately 30% of patients. Flap design has evolved towards perforator-based approaches to reduce functional deficits and (donor site) complications, since they allow for the preservation of relevant anatomical structures. Next to their greater surgical challenge in elevation, their superiority over non-perforator-based approaches is still debated. Methods: To compare outcomes between perforator and non-perforator flaps in female vulvoperineal reconstruction, we conducted a systematic review of English-language studies published after 1980, including randomized controlled trials, cohort studies, and case series. Data on demographics and surgical outcomes were extracted and classified using the Clavien–Dindo classification. We used a random-effects meta-analysis to derive a pooled estimate of complication frequency (%) in patients who received at least one perforator flap and in patients who received non-perforator flaps. Results: Among 2576 screened studies, 49 met our inclusion criteria, encompassing 1840 patients. The overall short-term surgical complication rate was comparable in patients receiving a perforator (n = 276) or a non-perforator flap (n = 1564) reconstruction (p* > 0.05). There was a tendency towards fewer complications when using perforator flaps. The assessment of patients’ QoL was scarce. Conclusions: Vulvoperineal reconstruction using perforator flaps shows promising results compared with non-perforator flaps. There is a need for the assessment of its long-term outcomes and for a systematic evaluation of patient QoL to further demonstrate its benefit for affected patients.
Keywords: vulvoperineal, perforator flap, non-perforator flap, cancer, reconstruction, female, surgical outcomes, quality of life, complications
1. Introduction
Vulvar cancer is rare among gynecological malignancies, accounting for less than 5% of gynecologic cancers. However, there has been a significant increase in incidence, especially in women under the age of 60 [1,2]. This imposes a serious physical and psychological burden on the affected women. The predominant subtype among vulvar carcinomas is squamous-cell carcinoma [3], which accounts for more than 90% of cases, followed by malignant melanoma, extramammary Paget’s disease, or, rarely, Bartolini gland carcinoma.
Over the past decades, the management of these neoplasms has significantly evolved. The advancement towards less radical surgical interventions has led to a noticeable reduction in complications, with a marked improvement in the quality of life (QoL) of patients [4,5]. A multidisciplinary approach integrating the expertise of gynecologic oncologists, radiologists, and plastic reconstructive surgeons is required to ensure optimal patient outcomes [5,6]. Surgeries such as wide local tumor excision, a standard procedure for squamous-cell carcinoma of the vulva, are performed up to the fascia. Partial or total vulvectomy and pelvic exenteration remain crucial for achieving local tumor control [7]. Depending on tumor size and the extent of the resected area, the resulting defect can be challenging to close. Traditionally, procedures without flap reconstruction have been associated with high complication rates, reaching nearly 60% [8]. The introduction of flap reconstructions, both perforator and non-perforator types, aimed to mitigate these adverse outcomes. However, despite these advancements, the complication rate remains concerningly high [9,10,11], with flap dehiscence, partial or complete flap loss, and infections at both the recipient and donor sites being the most prevalent issues.
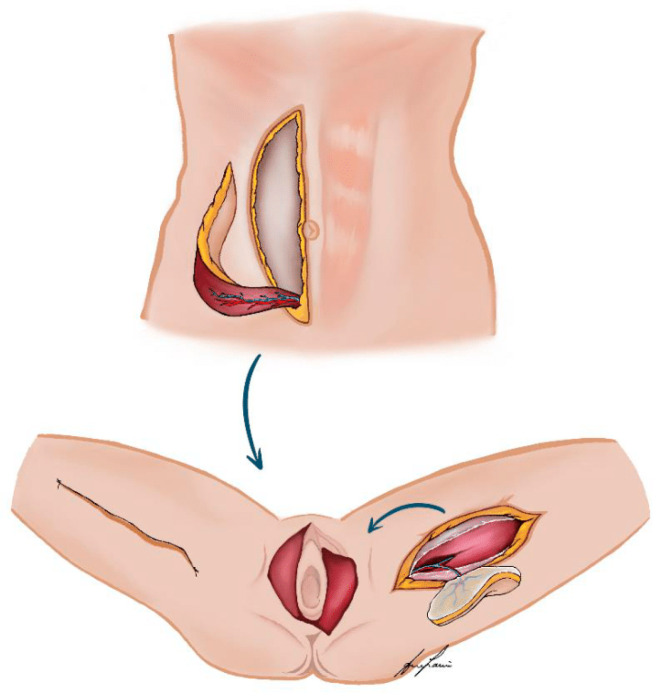
Aiming to further decrease complications, there has been a paradigm shift from using muscle- or musculocutaneous flaps to fasciocutaneous perforator-based flaps (Figure 1) [12], which preserve the underlying muscle, thus preventing the loss of functionality at the donor site, and are believed to result in fewer complications [12]. Despite their theoretical advantages and successful application in other anatomical regions, evidence supporting their superiority in vulvoperineal reconstruction remains sparse [13,14,15].
Figure 1.
Illustrated examples of muscle- or musculocutaneous flaps (VRAM—flap from the abdominal wall) and fasciocutaneous perforator-based flaps (PAP—flap from the left thigh, donor site closure as vertical thigh lift) as local vulvoperineal reconstruction options.
Moreover, the role of radiotherapy introduces additional complexity to the surgical outcomes of vulvoperineal reconstruction. These patients are usually heavily radiated, and their proportion ranges from 27.9% in primary cancer with residual tumor to 65.1% in recurrent cases [16]. The acute and chronic adverse effects of radiotherapy significantly impact the risk of complications, affecting the choice and success of reconstructive strategies [17].
The aim of this systematic review and meta-analysis is to address the existing knowledge gap by providing a comprehensive evaluation of the current literature on the use of perforator and non-perforator flaps for vulvoperineal reconstruction after oncologic resection. To our knowledge, this is the first analysis that specifically compares the postoperative surgical outcomes of these two types of flap reconstructions. We hypothesize that the use of perforator flaps is associated with a lower risk of complications, particularly at the donor site, thereby potentially providing a significant improvement in postoperative recovery and quality of life for patients undergoing vulvoperineal reconstruction.
2. Materials and Methods
This systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. It was registered in the PROSPERO International prospective register of systematic reviews. The registration took place on 1 March 2023 (ID CRD42023403496).
2.1. Search Strategy
We conducted a comprehensive systematic literature search to identify articles addressing pelvic cancers and reconstructive procedures for vulvoperineal defects, with a specific focus on the utilization of flap techniques. The search strategy was developed by a medical information specialist. The search syntax was composed and optimized in Embase (Elsevier, Amsterdam, The Netherlands), from where it was translated for other databases using publicly available macros [18]. The bibliographic databases Embase (Elsevier), Medline (Ovid, New York, NY, USA), and Web of Science Core Collection (Clarivate, Philadelphia, PA, USA) were searched using database-specific subject headings and text words (last search 28 September 2022). No language or publication date restrictions were applied but conference abstracts were excluded from the search. The full search strategies can be found in the electronic Supplementary Material Appendix S1.
2.2. Inclusion and Exclusion Criteria
The selection process focused on all articles considering either perforator or non-perforator flaps for vulvoperineal reconstruction after oncologic resection. To enhance clarity, we organized flaps into two distinct groups: perforator flaps, which encompass options like the pedicled profunda artery perforator (PAP), anterolateral thigh (ALT), deep inferior epigastric perforator (DIEP), superior gluteal (SGAP), and inferior gluteal (IGAP) flap, and non-perforator flaps, including muscle-based techniques such as the vertical rectus abdominis myocutaneous (VRAM) flap, gracilis flap, and tensor fasciae latae flap as well as local random-pattern fasciocutaneous flaps like the lotus petal flap, V-Y fasciocutaneous flaps, and the gluteal fold flap, among others.
The selected articles were expected to provide information on postoperative outcomes for each intervention group. These postoperative outcomes included various aspects, such as the overall complication rate and complications that occurred at both the donor and recipient sites. These complications were well defined and included perineal infections, flap necrosis, partial or complete flap loss, dehiscence rate, and infection rate. Studies were eligible if they reported one or more of these predefined outcomes. The inclusion and exclusion criteria are summarized in Table 1. Specifically, we excluded case reports and small case series with fewer than seven patients. In addition, reviews, commentaries, letters to the editor, cadaver studies, animal studies, and articles not written in English were excluded from our analysis.
Table 1.
Inclusion and exclusion criteria.
| PICOS | Inclusion | Exclusion |
|---|---|---|
| Populations | Female adults with vulvoperineal reconstruction with perforator or non-perforator flap | Cadaveric, animal studies |
| Intervention | Perforator or non-perforator flap for vulvoperineal reconstruction following oncologic resection | Other reconstruction techniques like primary closure or net implementation |
| Comparator | The study analysis compared postoperative surgical outcome parameters | |
| Outcomes | Main outcome: complications like infection rate, dehiscence, partial or total flap necrosis graded according to Clavien–Dindo classification | Studies that do not report main outcome |
| Study design | Randomized controlled trials, comparative studies, and case series ≥7 patients | Reviews, meta-analyses, case reports, unpublished studies, and non-English language studies |
2.3. Selection Process and Data Extraction
First, three authors performed a calibration phase with 100 abstracts to ensure that they uniformly applied the predefined inclusion and exclusion criteria. Subsequently, titles and abstracts were independently screened by two authors to identify all potentially relevant papers. The selected studies were then reviewed in full text by two independent authors. If the inclusion criteria were met, the data were independently extracted by both authors into a standardized Excel file. Any disagreements in the selection process and data extraction were resolved in discussion with the senior author. The following data were extracted: study design; country and timing of study; patient demographics, such as age, BMI, their oncologic diagnosis, details of the reconstruction procedures performed, and surgical outcomes, along with screening for complications at donor and recipient site; and assessments of patient quality of life.
2.4. Clavien–Dindo Classification
In order to ensure consistency in the assessment of complications across various studies, we standardized the categorization of complications using the Clavien–Dindo classification [19].
2.5. Statistics
To determine whether the use of perforator flaps is associated with a risk of complication, we used a random-effects meta-analysis to derive a pooled estimate of the frequency of complications as a percentage in patients who received at least one perforator flap and in patients who received non-perforator flaps. Pooled complication frequency was based on reported complication proportions and 95% Wilson confidence intervals. Studies reporting on either treatment were included as two one-arm studies in this analysis. We used the empirical Bayesian approach for estimation and assessed between-study variance (τ2), and heterogeneity as Cochran’s Q test, H2, and I2. We calculated a pooled effect size, e.g., risk difference, using meta-regression with treatment as the only independent variable and visualized the results as a forest plot. Two studies [20,21] reported complications only at the flap level, not at the patient level. For comparability, we approximated the proportion of patients with complications assuming each patient had at most one flap complication.
We performed three sensitivity analyses to assess whether the surgical procedure could explain the difference in complication rates. First, we included further variables as covariates in the meta-regression; “recent study” was defined as year of publication ≥ 2015 and “large study” was defined as sample size ≥50. Second, we grouped patients who received flaps of both types as “non-perforator flap” and repeated the main analysis based on this grouping. Finally, we restricted the analysis to studies which reported on either treatment and hence provided a risk difference and calculated a pooled effect size using a random-effects meta-analysis with empirical Bayesian estimation. We refrained from meta-analyzing quality-of-life data as only a few studies reported these outcomes.
All analyses were conducted using Stata 16.0 (StataCorp LLC, College Station, TX, USA).
3. Results
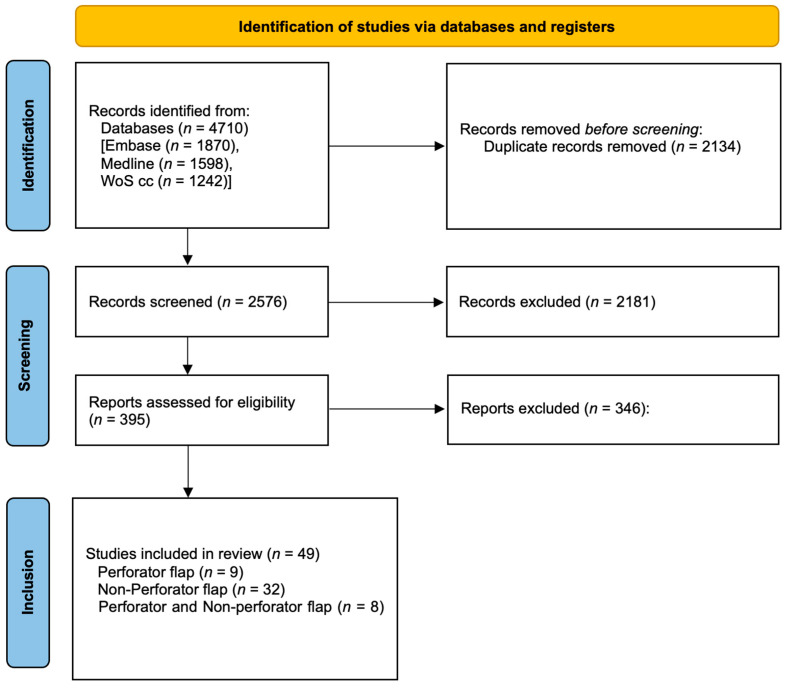
The initial search query resulted in 4710 records; after removing 2134 duplicates, 2576 records remained. After initial title and abstract screening, 395 articles were assessed in full text. Forty-nine studies fulfilled the selection criteria. Among them, thirty-two studies analyzed non-perforator flaps [9,10,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], only nine studies focused on perforator flaps [21,51,52,53,54,55,56,57,58], and eight studies focused on both [59,60,61,62,63,64,65,66] (Figure 2).
Figure 2.
PRISMA flow chart.
3.1. Study Characteristics
The 49 studies were conducted in Europe (n = 29) [9,10,20,22,24,25,26,27,28,30,31,34,35,36,38,39,40,41,42,43,45,47,50,52,54,57,58,62,65], the United States (n = 5) [29,44,46,48,49], and Asia (n = 15) [21,23,32,33,37,51,53,55,56,59,60,61,63,64,66] and were published between 1990 and 2022. Two studies were multicentric [28,50]; the others were monocentric. One study [46] was prospective and all others were retrospective. A total of 1840 female patients were included for analysis in our study, with 2077 (84%) non-perforator flaps and 384 (16%) perforator flaps (Table 2).
Table 2.
Study overview. # number.
| Study | # Flaps | Non-Perforator | Perforator |
|---|---|---|---|
| Abdulrahman 2022 [22] | 18 | Gracilis myocutaneous flap (7), IGAM (1), local fasciocutaneous flap (2), VRAM (8) | |
| Chen 2022 [23] | 30 | VRAM (30) | |
| Elia 2022 [59] | 55 | Gracilis myocutaneous flap (3), local fasciocutaneous flap (2), VRAM (1) | ALT (3), local perforator fasciocutaneous flap (23), PAP (23) |
| Muallem 2022 [24] | 126 | Flap combination (22), local fasciocutaneous flap (84), myocutaneous flap (20) | |
| Shin 2022 [51] | 27 | Local perforator fasciocutaneous flap (27) | |
| Zhang 2022 [61] | 34 | Local fasciocutaneous flap (30), VRAM (2) | ALT (2) |
| Aksan 2021 [25] | 31 | Local fasciocutaneous flap (29), skin graft (1), VRAM (1) | |
| Giannini 2021 [26] | 40 | Local fasciocutaneous flap (40) | |
| Tock 2019 [27] | 61 | Gluteal thigh flap (16), local fasciocutaneous flap (45) | |
| Fin 2018 [28] | 59 | Local fasciocutaneous flap (59) | |
| Hand 2018 [29] | 42 | Local fasciocutaneous flap (42) | |
| Hellinga 2018 [9] | 89 | Local fasciocutaneous flap (89) | |
| Confalonieri 2017 [10] | 365 | Local fasciocutaneous flap (365) | |
| Gentileschi 2017 [52] | 16 | ALT (16) | |
| Lange 2017 [20] | 114 | Local fasciocutaneous flap (114) | |
| Chang 2016 [21] | 19 | PAP (19) | |
| Conri 2016 [30] | 36 | Local fasciocutaneous flap (36) | |
| Herraiz Roda 2016 [31] | 17 | Local fasciocutaneous flap (17) | |
| Huang 2015 [53] | 27 | Local perforator fasciocutaneous flap (16), PAP (11) | |
| Kim 2015 [32] | 41 | Local fasciocutaneous flap (41) | |
| Negosanti 2015 [62] | 33 | Local fasciocutaneous flap (28) | DIEP (5) |
| Nomura 2015 [33] | 19 | Local fasciocutaneous flap (16), non-specified flap (3) | |
| Zhang 2015 [63] | 27 | Gracilis myocutaneous flap (1), TRAM (1) | ALT (24), DIEP (1) |
| Zhang 2015_2 [64] | 40 | Gracilis myocutaneous flap (2), TRAM (1) | ALT (24), DIEP (6), pudendal thigh fasciocutaneous flap (7) |
| Benedetti Panici 2014 [34] | 29 | Local fasciocutaneous flap (29) | |
| Tan 2014 [60] | 72 | Gracilis myocutaneous flap (36), local fasciocutaneous flap (21), skin graft (11), VRAM (3) | ALT (1) |
| Argenta 2013 [35] | 80 | Local fasciocutaneous flap (80) | |
| Kuokkanen 2013 [36] | 22 | Local fasciocutaneous flap (22) | |
| Lee 2013 [37] | 27 | Local fasciocutaneous flap (27) | |
| Al-Benna 2012 [54] | 13 | Local perforator fasciocutaneous flap (13) | |
| Buda 2012 [38] | 38 | Local fasciocutaneous flap (38) | |
| Misani 2011 [39] | 69 | Local fasciocutaneous flap (69) | |
| Zeng 2011 [66] | 14 | Local fasciocutaneous flap (3) | ALT (11) |
| Yun 2010 [55] | 24 | PAP (24) | |
| Zhou 2010 [56] | 10 | ALT (10) | |
| Franchelli 2009 [57] | 53 | Local perforator fasciocutaneous flap (53) | |
| Staiano 2009 [40] | 53 | Gracilis myocutaneous flap (4), latissimus dorsi muscle flap (1), local fasciocutaneous flap (26), tensor fasciae latae flap (1), VRAM (21) | |
| Weikel 2008 [41] | 207 | Gluteal thigh flap (54), gracilis myocutaneous flap (5), local fasciocutaneous flap (123), tensor fasciae latae flap (9), VRAM (16) | |
| Weikel 2006 [42] | 123 | Gluteal thigh flap (40), gracilis myocutaneous flap (4), local fasciocutaneous flap (58), tensor fasciae latae flap (8), VRAM (13) | |
| Salgarello 2005 [65] | 31 | Local fasciocutaneous flap (18), VRAM (4) | Pudendal thigh fasciocutaneous flap (9) |
| Ragoowansi 2004 [58] | 56 | Local perforator fasciocutaneous flap (56) | |
| Persichetti 2003 [43] | 26 | Local fasciocutaneous flap (26) | |
| Arkoulakis 2002 [44] | 36 | Local fasciocutaneous flap (36) | |
| Moschella 2000 [45] | 22 | Local fasciocutaneous flap (22) | |
| Loree 1997 [46] | 13 | Gracilis (2), local fasciocutaneous flap (10), VRAM (1) | |
| Niranjan 1996 [47] | 13 | Local fasciocutaneous flap (13) | |
| Burke 1995 [48] | 18 | Gracilis myocutaneous flap (18) | |
| Helm 1993 [49] | 30 | Local fasciocutaneous flap (30) | |
| Shepeherd 1990 [50] | 16 | VRAM (16) |
3.2. Overall Complications
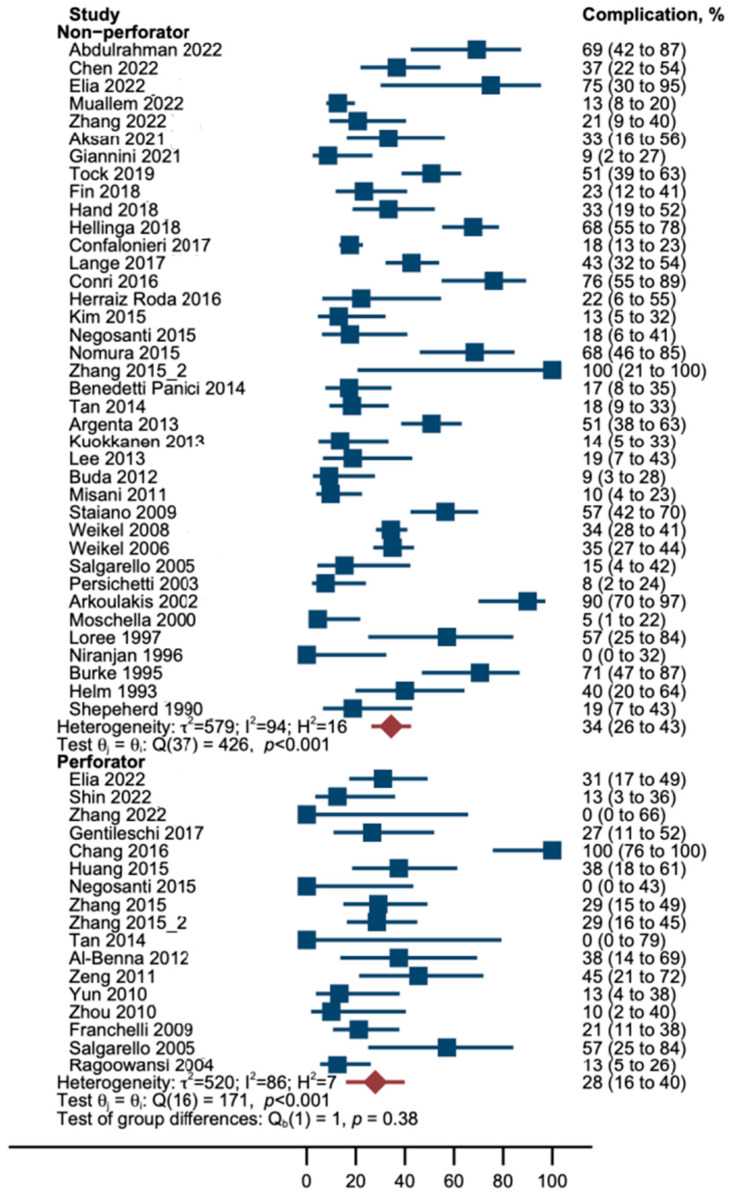
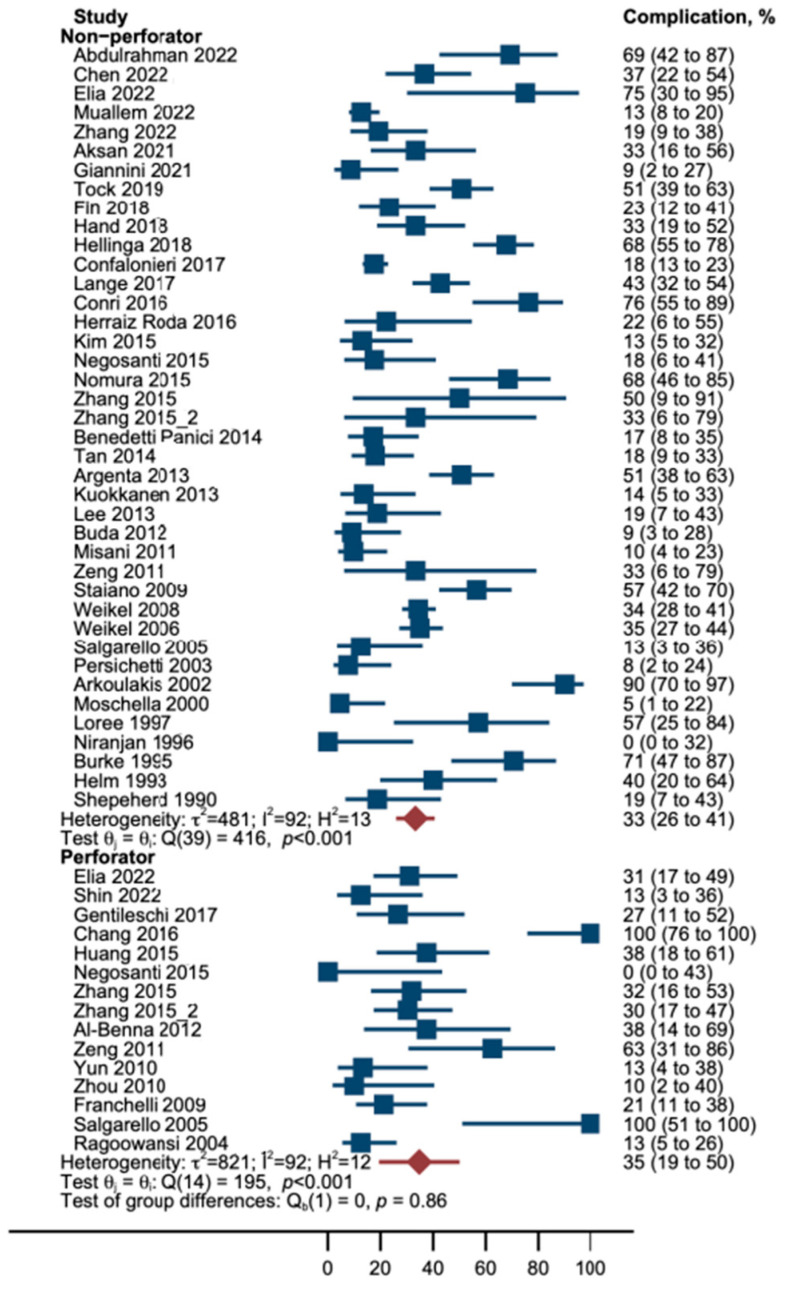
In this random-effects meta-analysis, the incidence of complications was lower in patients who underwent a perforator flap reconstruction, although this difference did not reach statistical significance. Specifically, the explained variance in the outcome (R2) was 0%, indicating that perforator flaps did not have an impact on the frequency of complications (*p > 0.05). Heterogeneity among the studies (I2) was as large as 91%; hence, 91% of the observed variation among studies is due to the heterogeneity of findings rather than chance. Between-study variance τ2 was 0.05; thus, it was larger than the estimated treatment effect as absolute. Cochran’s Q test showed that study results differed, as the studies reported significantly different frequencies of complications (**p < 0.001) (Table 3, Figure 3).
Table 3.
Meta-regression: Association of treatment and proportion of complications.
| Variable | Association with Outcome | Model Fit | Heterogeneity among Studies | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | R2 | p * | I2 (%) | H2 | τ2 | p ** | |
| Treatment | −0.06 (−0.20 to 0.09) | 0.459 | 0 | 0.450 | 91 | 11 | 0.05 | <0.001 |
| Treatment | −0.06 (−0.21 to 0.08) | 0.413 | 1 | 0.299 | 90 | 10 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.09 (−0.04 to 0.22) | 0.175 | ||||||
| Treatment | −0.04 (−0.20 to 0.11) | 0.599 | 0 | 0.617 | 90 | 10 | 0.05 | <0.001 |
| # Patients ≥ 50 | 0.06 (−0.13 to 0.25) | 0.540 | ||||||
| Treatment | −0.05 (−0.21 to 0.10) | 0.522 | 0 | 0.457 | 90 | 10 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.09 (−0.05 to 0.22) | 0.203 | ||||||
| # Patients ≥ 50 | 0.04 (−0.14 to 0.23) | 0.654 | ||||||
* p of regression model testing whether explanatory variables are associated with dependent variable. ** p of Cochran’s Q test of residual homogeneity testing the H0 that all study results are equal. # number
Figure 3.
Forest plot; Proportion of patients who suffered from complications (blue boxes) by treatment. Note that confidence intervals of proportions (horizontal lines) are asymmetrical because we used Wilson’s method for calculation of interval boundaries to account for small samples and few events. Red diamonds and lines represent pooled proportions with Wilson CI [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
3.3. Complications According to the Clavien–Dindo Classification
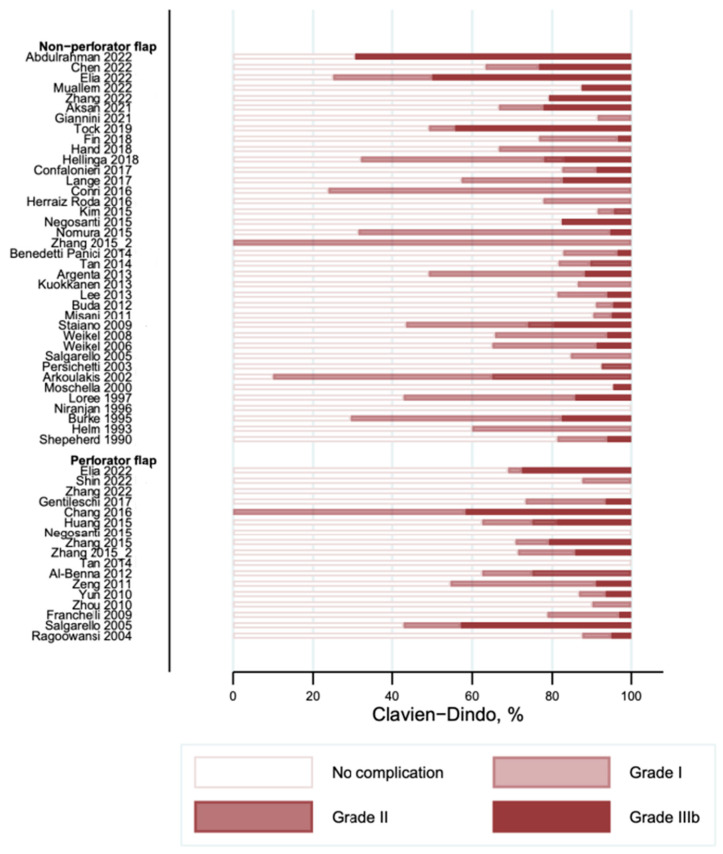
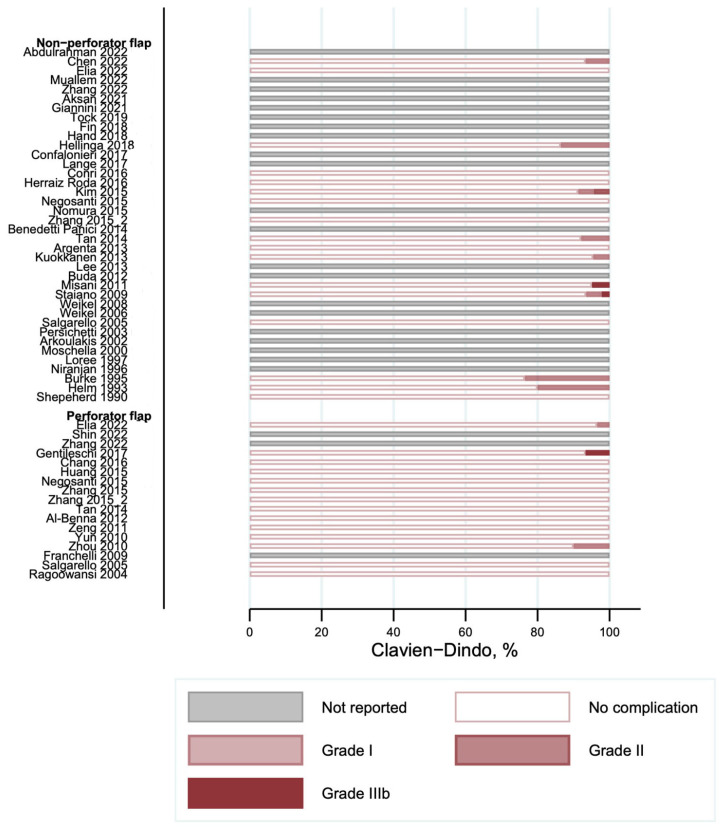
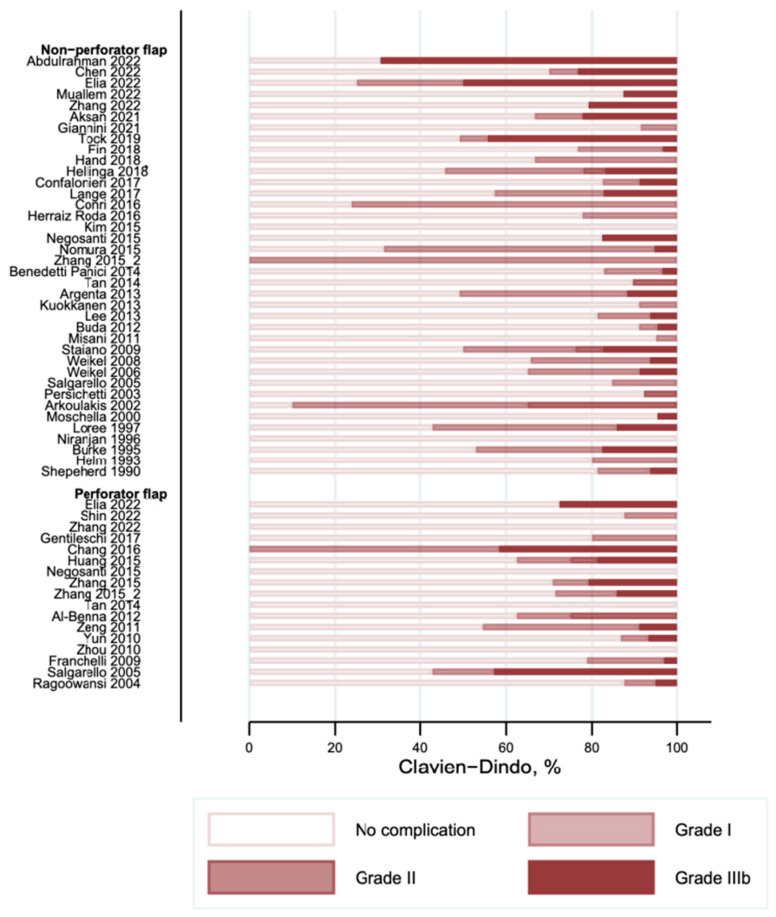
The overall complications graded according to the Clavien–Dindo classification are shown in Figure 4. Most of the reported complications were minor (Clavien–Dindo I) and were observed with a frequency of occurrence between 0 and 90% in the studies. The frequency of the second most commonly reported complication (Clavien–Dindo III) was observed in between 0 and 69% of cases. We did not find any complications with Clavien–Dindo grade IV or higher. Donor site complications are shown in Figure 5. Several studies did not report on their donor site complications (“Not reported” in Figure 5). Nine studies in the non-perforator flap [9,23,32,36,39,40,48,49,60] and three studies in the perforator flap group [52,56,59] reported on complications at the donor site. The frequency of donor site complications ranged from 0 to 10% in the included studies.
Figure 4.
Overall complications according to Clavien–Dindo classification [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
Figure 5.
Donor site complications according to Clavien–Dindo classification [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
Recipient site complications are shown in Figure 6. The distribution of complications on the recipient site according to the Clavien–Dindo classification is comparable to the overall complications.
Figure 6.
Recipient site complications according to Clavien–Dindo classification [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
3.4. Sensitivity Analysis
First, adjustment for year of publication and study size did not show an association with the frequency of complications and did not alter the effect estimate of treatment, model fit, and heterogeneity parameters. Secondly, patients who received both a perforator and a non-perforator flap were grouped as non-perforator flap, which yielded similar results as the main analysis (Table 4, Figure 7).
Table 4.
Sensitivity analysis 2: association of treatment and proportion of complications.
| Association with Outcome | Model Fit | Heterogeneity among Studies | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p | R2 | *p | I2 (%) | H2 | τ2 | **p |
| Treatment | 0.01 (−0.14 to 0.17) | 0.880 | 0 | 0.871 | 91 | 11 | 0.05 | <0.001 |
| Treatment | 0.01 (−0.14 to 0.16) | 0.917 | 0 | 0.561 | 91 | 11 | 0.05 | <0.001 |
| Year ≥ 2015 | 0.06 (−0.07 to 0.20) | 0.368 | ||||||
| Treatment | 0.03 (−0.13 to 0.19) | 0.737 | 0 | 0.753 | 91 | 11 | 0.05 | <0.001 |
| # Patients ≥ 50 | 0.07 (−0.12 to 0.26) | 0.472 | ||||||
| Treatment | 0.02 (−0.14 to 0.18) | 0.792 | 0 | 0.745 | 91 | 10 | 0.06 | <0.001 |
| Year ≥ 2015 | 0.06 (−0.08 to 0.19) | 0.421 | ||||||
| # Patients ≥ 50 | 0.06 (−0.13 to 0.25) | 0.545 | ||||||
* p of regression model testing whether explanatory variables are associated with dependent variable. ** p of Cochran’s Q test of residual homogeneity testing the H0 that all study results are equal. # number.
Figure 7.
Forest plot of sensitivity analysis 2: Association of treatment and proportion (blue boxes) of complications. Note that confidence intervals of proportions (horizontal lines) are asymmetrical because we used Wilson’s method for calculation of interval boundaries to account for small samples and few events. Red diamonds and lines represent pooled proportions with Wilson CI [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
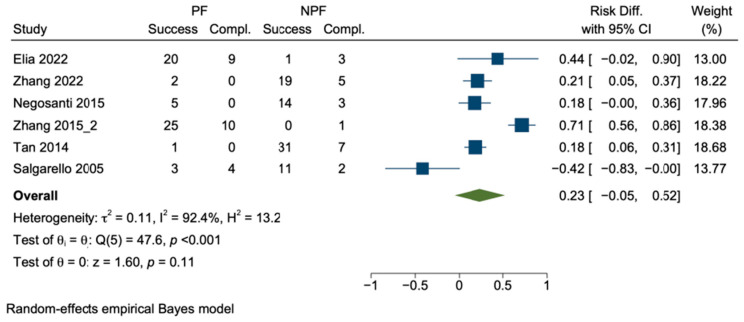
Thirdly, when we limited the analysis to studies that specifically addressed both perforator and non-perforator flap reconstructions, we were left with six studies only. This exclusion resulted in the omission of a substantial number of studies, precisely 43 out of the total 49 studies initially considered. The pooled risk difference suggested a potential advantage of perforator flap reconstruction; however, the reported risk difference was different (p of Cochran’s Q test was <0.001) and the heterogeneity was very high (I2 was 92%) (Figure 8).
Figure 8.
Sensitivity analysis 3: Pooled risk difference. Boxes represent differences in the risk of complication after perforator vs. non-perforator flap reconstruction with confidence interval, e.g., effect sizes. Green diamond shows pooled risk difference. PF perforator flap; NPF non–perforator flap [59,60,61,62,64,65].
As a result, a meta-regression of these six studies was neither feasible nor sensible, as the pooled estimate and heterogeneity measures from the meta-analysis above correspond exactly to the results derived from a constant-only meta-regression model. The criterion “size of study ≥ 50 patients” would yield just one group, and “publication after 2014” would comprise one single study only. Hence, adjustments as performed in the main analysis would not be appropriate.
3.5. Satisfaction and Quality of Life
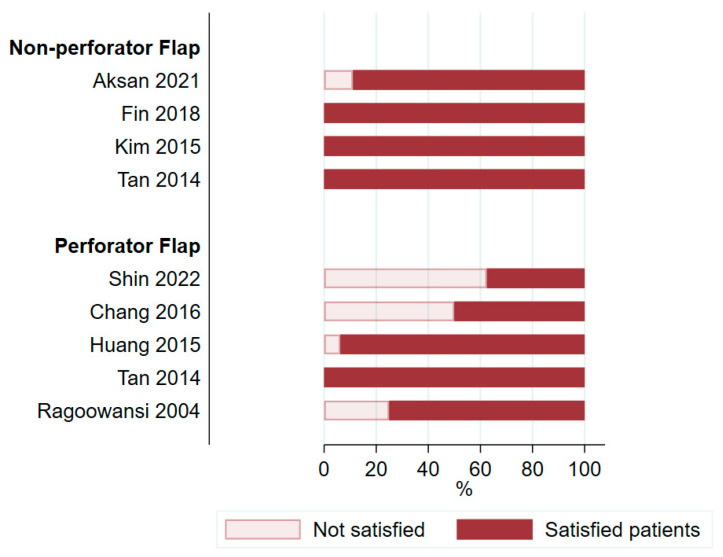
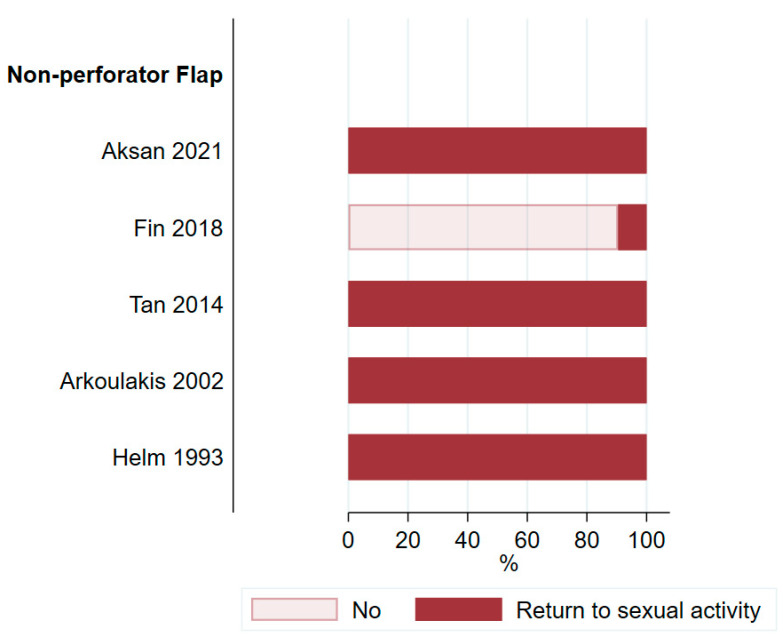
Patient satisfaction was analyzed in eight studies [21,25,28,32,51,53,58,60] including only 177 patients (9.6%). These eight studies showed a wide range in the proportion of satisfied patients, ranging from 38% to 100% (Figure 9). The low percentage of satisfied patients in the study by Shin et al. [51] and Chang et al. [21] is explained by the fact that only a small proportion of patients reported their level of satisfaction to the study team. Five studies [25,28,44,49,60] from the non-perforator group addressed returning to sexual activity after surgery (Figure 10). Tan et al. [60] also used one perforator flap but did not report on the sexual activity of this patient specifically. Although not reported in the analyzed studies, the effect of adjunctive radiotherapy may lead to radiation dermatitis, with a long-term negative impact on QoL [17].
Figure 9.
Patient satisfaction after surgery [21,25,28,32,51,53,58,60].
Figure 10.
Proportion of patients returning to sexual activity after surgery [25,28,44,49,60].
4. Discussion
With the continuous development of well-established systemic treatments, such as immunotherapy, and with the improvement in radiation therapy as well as development of surgical treatment options over the past decades, there is a significant increase in cancer survivors wishing for good QoL. Novel and innovative treatment approaches in the field of plastic surgery often encounter obstacles in their acceptance (process) and take a long time to become established across partner disciplines. It is therefore crucial for reconstructive surgeons to show collaborating specialties that exceptional results can be achieved with minimal complications. We must also constantly focus on innovation and improvement in our work. Thirty years ago, in 1994, arguably the first autologous breast reconstruction was performed with a DIEP flap, which at that time had already demonstrated its advantages over conventional methods, with lower donor site morbidity [67]. Nowadays, perforator flaps like the DIEP flap have been established as the gold standard in autologous breast reconstruction and are integral components in the armamentarium of high-volume medical centers. This illustrates the inertia in implementing innovations in existing systems.
The innovation of perforator flaps in general and the convincing results from other anatomical regions, coupled with the improvement in patients’ QoL, drove us to investigate the role of perforator flaps in the reconstruction of vulvoperineal defects. We therefore believe that this systematic review is, to the best of our knowledge, not only the first, but also the most comprehensive analysis of surgical outcomes comparing perforator and non-perforator flaps in female patients with vulvoperineal defects. Of the 2576 studies identified by the literature search, only 49 studies could ultimately be included in this review. Most of these studies assessed the outcomes of non-perforator flaps, with only eight studies specifically examining the results of perforator flaps. This shows that the literature on reconstructive procedures with perforator flaps in the vulvoperineal region is sparse.
4.1. Surgical Complications
There is a large range of surgical options available for closing vulvoperineal defects. These include primary closure, locoregional random-patterned, axially patterned, and perforator-based flaps, as well as musculocutaneous or perforator flaps.
The introduction of flap reconstruction as such has already been shown to reduce the complication rate significantly [68]. However, the high complication rate remains a challenge because the available reconstructive procedures are still associated with high morbidity at the recipient and donor sites. A major advantage of perforator flaps is their design, consisting only of the skin and subcutaneous fat tissue, leaving the underlying muscles and nerves intact [69]. Harvesting perforator flaps may keep other surgical options open, compared to random-pattern flaps, and they show a stable vascular territory compared to axial pattern flaps.
Consequently, there is usually less morbidity at the donor site, as muscle function is maintained. In many of the included studies (49%), there is unfortunately no clear distinction made between complications at the donor vs. the recipient site. On the other hand, research has demonstrated that muscle-based reconstructions have a high rate of donor site morbidity [70,71]. We therefore presume that the proportion of donor site complications in the included studies is under-reported.
For an effective comparison of the surgical outcomes of different studies, it is crucial that the complications are reported in a standardized manner. One approach commonly employed for this purpose is the Clavien–Dindo classification, which assesses the severity of complications according to the treatment they require [19]. In the studies we included, only a minority of them reported surgical outcomes using this classification method, which makes the comparison of surgical complications challenging.
4.2. Potential Importance of Perforator Flaps in Female Vulvoperineal Reconstruction
Our results potentially suggest that the use of perforator flaps in vulvoperineal reconstruction is associated with fewer complications compared to conventional flap reconstruction. The fact that this is a trend only may be attributable to the lack of studies directly comparing perforator versus non-perforator flaps and the high heterogeneity of the studies, which may lead to a loss of predictive power. It is interesting to note, however, that perforator flaps have become established as one of the leading procedures in breast reconstruction and show high success rates with a relatively low complication rate [72,73,74]. The same observation has been made in head and neck reconstructive surgery, with convincing results pertaining to the use of perforator flaps [75,76].
4.3. Assessment of Patient Satisfaction and Quality of Life
Only eight of forty-nine studies examined patient satisfaction and QoL. The finding that QoL continues to be under-reported and therefore is not the focus of outcomes is consistent with the current literature in the field of pelvic reconstruction. Witte et al. [77] compared the various options for flap reconstruction after pelvic exenteration, one of their secondary outcomes being the assessment of QoL. QoL was recorded by <10% of the included studies, again consistent with our findings. The vulvoperineal region plays an essential role in women’s QoL because it is responsible for vital functions such as micturition, defecation, reproduction, and psychosexual integrity. The vulvoperineal area is essential for the functioning of basic needs, such as intestinal voiding, urination, and sexual intercourse. Sexual function is an especially important factor of psychological well-being. Therefore, the assessment of QoL as an outcome parameter in this anatomical region should not be underestimated.
4.4. Limitations
Many of the included studies lack precise descriptions of complications and their management, which can result in both the over- and underestimation of complication rates, thereby complicating the Clavien–Dindo classification. To limit these biases, we extracted complications as they were listed. In cases where the management of complications was not provided, we made assumptions based on best practices and subsequently categorized them accordingly. Some studies did not explicitly report donor site complications. In these cases, we assumed that the complications occurred at the recipient site, as complications are more frequent there. This assumption may have led to an overestimation of the complication rates at the recipient site of the studies concerned. Furthermore, the included studies showed a notably high degree of heterogeneity, which can be explained by the small sample sizes, the population’s complexity, and the intervention being studied.
A major limitation of this study is that the impact of radiation was not analyzed, mainly due to lack in reporting in the chosen studies. In the included literature, the effect of adjuvant or neoadjuvant radiation is not extensively addressed, despite playing an important part in outcomes and QoL.
Finally, the small proportion of studies that compared perforator and non-perforator flaps in a direct manner limits the conclusiveness of our sensitivity analysis.
4.5. Perforator Flaps as a Valid Choice in the Reconstructive Armamentarium
Perforator flaps have become established as a safe method with a good functional and aesthetic outcomes in multiple areas of reconstructive surgery. Perforator flaps can be chosen based on the reconstruction demands. The ALT flap provides stability due to its thick fascia. The PAP flap, with its thin dermis, is more pliable, if needed. Furthermore, sensitized flaps (ALT and PAP) can be performed.
It is noteworthy that the complication rates between perforator and non-perforator flaps are comparable, with a promising trend indicating fewer complications with perforator flaps. However, these results should be interpreted with the reservation of a possible bias due to the large heterogeneity between the analyzed studies. With careful consideration of the patient’s individual circumstances, wishes, and expectations and the surgeon’s level of experience, perforator flaps should be considered a viable option for the treatment of vulvoperineal defects.
5. Conclusions and Future Direction
Vulvoperineal reconstruction using perforator flaps shows promising results with a trend towards lower complication rates compared with conventional procedures. While technically challenging, this approach offers the potential to preserve important anatomical structures and, consequently, maintain functionality. To demonstrate the efficacy of perforator flaps in vulvoperineal reconstruction as successfully as in the field of breast and head and neck reconstruction and to validate or challenge the trend we have presented, it is essential to further investigate this in more extensive, direct comparative studies between perforator and non-perforator flaps in vulvoperineal reconstruction. Moreover, it is critical to comprehensively assess patient QoL through the systematic implementation and assessment of patient-reported outcome measures in clinical practice and research.
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QoL | quality of life |
| PAP | profunda artery perforator |
| ALT | anterolateral thigh |
| SGAP | superior gluteal artery perforator |
| IGAP | inferior gluteal artery perforator |
| DIEP | deep inferior epigastric artery perforator |
| VRAM | vertical rectus abdominis myocutaneous |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16122213/s1, Table S1. Statistics 2023-Complications after Perforator vs. non-Perforator Flap Transplanation. Appendix S1: Search strategies. References [9,10,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] are cited in the Supplementary Material.
Author Contributions
Conceptualization, E.A.K.; methodology, E.A.K. and C.A.-H.; pilot screening, S.W., L.K., and J.S.; abstract and full-text screening, S.W. and L.K.; data extraction, S.W. and L.K.; investigation, S.W. and L.K.; data curation, S.W. and L.K.; statistical analysis, B.G.; writing—original draft preparation, S.W. and L.K.; writing—review and editing, E.A.K., T.I., N.S., C.A.-H., C.M., V.H.-S., D.J.S., J.S., S.W. and L.K.; visualization, A.L.; supervision, E.A.K. and T.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the study design of a systematic review and meta-analysis of already existing literature.
Informed Consent Statement
Patient consent was waived due to the study design of a systematic review and meta-analysis of already existing literature.
Data Availability Statement
Data are contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This systematic review and meta-analysis was supported by the Department of Surgery of the University Hospital of Basel.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kang Y.-J., Smith M., Barlow E., Coffey K., Hacker N., Canfell K. Vulvar Cancer in High-Income Countries: Increasing Burden of Disease. Int. J. Cancer. 2017;141:2174–2186. doi: 10.1002/ijc.30900. [DOI] [PubMed] [Google Scholar]
- 2.Judson P.L., Habermann E.B., Baxter N.N., Durham S.B., Virnig B.A. Trends in the Incidence of Invasive and In Situ Vulvar Carcinoma. Obstet. Gynecol. 2006;107:1018. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 3.Olawaiye A.B., Cotler J., Cuello M.A., Bhatla N., Okamoto A., Wilailak S., Purandare C.N., Lindeque G., Berek J.S., Kehoe S. FIGO Staging for Carcinoma of the Vulva: 2021 Revision. Int. J. Gynecol. Obstet. 2021;155:43–47. doi: 10.1002/ijgo.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan A., Bieber A.K., Stein J.A., Pomeranz M.K. Diagnosis and Management of Vulvar Cancer: A Review. J. Am. Acad. Dermatol. 2019;81:1387–1396. doi: 10.1016/j.jaad.2019.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Barton D.P.J. The Prevention and Management of Treatment Related Morbidity in Vulval Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2003;17:683–701. doi: 10.1016/S1521-6934(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 6.Rogers L.J. Management of Advanced Squamous Cell Carcinoma of the Vulva. Cancers. 2021;14:167. doi: 10.3390/cancers14010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison J., Baldwin P., Buckley L., Cogswell L., Edey K., Faruqi A., Ganesan R., Hall M., Hillaby K., Reed N., et al. British Gynaecological Cancer Society (BGCS) Vulval Cancer Guidelines: Recommendations for Practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:502–525. doi: 10.1016/j.ejogrb.2020.05.054. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell R.L., Verleye L., Ratnavelu N., Galaal K., Fisher A., Naik R. Locally Advanced Vulva Cancer: A Single Centre Review of Anovulvectomy and a Systematic Review of Surgical, Chemotherapy and Radiotherapy Alternatives. Is an International Collaborative RCT Destined for the “Too Difficult to Do” Box? Gynecol. Oncol. 2017;144:438–447. doi: 10.1016/j.ygyno.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Hellinga J., Khoe P.C.K.H., Stenekes M.W., Eltahir Y. Complications after Vulvar and Perineal Reconstruction with a Lotus Petal Flap. Ann. Plast. Surg. 2018;80:268–271. doi: 10.1097/SAP.0000000000001271. [DOI] [PubMed] [Google Scholar]
- 10.Confalonieri P.L., Gilardi R., Rovati L.C., Ceccherelli A., Lee J.H., Magni S., Del Bene M., Buda A. Comparison of V-Y Advancement Flap Versus Lotus Petal Flap for Plastic Reconstruction After Surgery in Case of Vulvar Malignancies: A Retrospective Single Center Experience. Ann. Plast. Surg. 2017;79:186–191. doi: 10.1097/SAP.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 11.Hellinga J., Rots M., Werker P.M.N., Stenekes M.W. Lotus Petal Flap and Vertical Rectus Abdominis Myocutaneous Flap in Vulvoperineal Reconstruction: A Systematic Review of Differences in Complications. J. Plast. Surg. Hand Surg. 2021;55:67–82. doi: 10.1080/2000656X.2020.1828902. [DOI] [PubMed] [Google Scholar]
- 12.Geddes C.R., Morris S.F., Neligan P.C. Perforator Flaps: Evolution, Classification, and Applications. Ann. Plast. Surg. 2003;50:90. doi: 10.1097/00000637-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Largo R.D., Bhadkamkar M.A., Asaad M., Chu C.K., Garvey P.B., Butler C.E., Yu P., Hanasono M.M., Chang E.I. The Profunda Artery Perforator Flap: A Versatile Option for Head and Neck Reconstruction. Plast. Reconstr. Surg. 2021;147:1401–1412. doi: 10.1097/PRS.0000000000007977. [DOI] [PubMed] [Google Scholar]
- 14.Mayo-Yáñez M., Rodríguez-Pérez E., Chiesa-Estomba C.M., Calvo-Henríquez C., Rodríguez-Lorenzo A. Deep Inferior Epigastric Artery Perforator Free Flap in Head and Neck Reconstruction: A Systematic Review. J. Plast. Reconstr. Aesthetic Surg. 2021;74:718–729. doi: 10.1016/j.bjps.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Cohen Z., Azoury S.C., Matros E., Nelson J.A., Allen R.J. Modern Approaches to Alternative Flap-Based Breast Reconstruction: Profunda Artery Perforator Flap. Clin. Plast. Surg. 2023;50:289–299. doi: 10.1016/j.cps.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zobec Logar H.B. Long Term Results of Radiotherapy in Vulvar Cancer Patients in Slovenia between 1997–2004. Radiol. Oncol. 2017;51:447–454. doi: 10.1515/raon-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrelli M.R., Shen A.H., Lee G.K., Momeni A., Longaker M.T., Wan D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019;83:S59–S64. doi: 10.1097/SAP.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramer W.M., Rethlefsen M.L., Mast F., Kleijnen J. Evaluation of a New Method for Librarian-mediated Literature Searches for Systematic Reviews. Res. Synth. Methods. 2018;9:510–520. doi: 10.1002/jrsm.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dindo D., Demartines N., Clavien P.-A. Classification of Surgical Complications. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange M., Hage J.J., van Beurden M. A Prospective Assessment of Surgical Risk Factors in 114 Gluteal Fold Flap Reconstructions After Oncological Vulvoperineal Resection. Ann. Plast. Surg. 2017;79:53–59. doi: 10.1097/SAP.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 21.Chang T.N.-J., Lee C.-H., Lai C.-H., Wu C.-W., Chang C.-S., Cheng M.-H., Huang J.-J. Profunda Artery Perforator Flap for Isolated Vulvar Defect Reconstruction after Oncological Resection. J. Surg. Oncol. 2016;113:828–834. doi: 10.1002/jso.24233. [DOI] [PubMed] [Google Scholar]
- 22.Abdulrahman G.O., Das N., Chandrasekaran T.V., Khot U., Drew P.J., Bose P., Vet J.N., Tofazzal N., Roberts S., Lutchman Singh K. Pelvic Exenteration for the Treatment of Locally Advanced Vulvar Cancer in South West Wales. Cancers. 2022;14:1767. doi: 10.3390/cancers14071767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q., Dong R., Zeng A., Teng Y., Liu Z., Zhu L., Long F., Si L., Yu N., Wang X. The Reconstructive Strategy for Pelvic Oncological Surgery with Various Types of MS-VRAM Flaps. J. Plast. Reconstr. Aesthetic Surg. 2022;75:2090–2097. doi: 10.1016/j.bjps.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Muallem M.Z., Sehouli J., Miranda A., Plett H., Sayasneh A., Diab Y., Muallem J., Hatoum I. Reconstructive Surgery versus Primary Closure Following Vulvar Cancer Excision: A Wide Single-Center Experience. Cancers. 2022;14:1695. doi: 10.3390/cancers14071695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aksan T., Karateke A., Uzuneyüpoğlu O., Öztürk M.B., Küçükbaş M. Reconstruction of Vulva and Perineal Defects After Gynecological Oncological Surgery and Effectiveness of Local Flaps. Düzce Tıp Fakültesi Derg. 2021;23:164–169. doi: 10.18678/dtfd.911107. [DOI] [Google Scholar]
- 26.Giannini A., Di Donato V., D’Oria O., Schiavi M.C., May J., Benedetti Panici P., Congiu M.A. The V-Y Gluteal Fold Advancement Flap: Outcomes Following Radical Surgery for Vulvar Malignancies. Int. J. Gynecol. Obstet. 2021;152:421–424. doi: 10.1002/ijgo.13430. [DOI] [PubMed] [Google Scholar]
- 27.Tock S., Wallet J., Belhadia M., Hudry D., Ghesquière L., Narducci F., Leblanc E. Outcomes of the Use of Different Vulvar Flaps for Reconstruction during Surgery for Vulvar Cancer. Eur. J. Surg. Oncol. 2019;45:1625–1631. doi: 10.1016/j.ejso.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Fin A., Rampino Cordaro E., Guarneri G.F., Revesz S., Vanin M., Parodi P.C. Experience with Gluteal V-Y Fasciocutaneous Advancement Flaps in Vulvar Reconstruction after Oncological Resection and a Modification to the Marking: Playing with Tension Lines. Int. Wound J. 2018;16:96–102. doi: 10.1111/iwj.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hand L.C., Maas T.M., Baka N., Mercier R.J., Greaney P.J., Rosenblum N.G., Kim C.H. Utilizing V-Y Fasciocutaneous Advancement Flaps for Vulvar Reconstruction. Gynecol. Oncol. Rep. 2018;26:24–28. doi: 10.1016/j.gore.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conri V., Casoli V., Coret M., Houssin C., Trouette R., Brun J.-L. Modified Gluteal Fold V-Y Advancement Flap for Reconstruction After Radical Vulvectomy. Int. J. Gynecol. Cancer. 2016;26:1300–1306. doi: 10.1097/IGC.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 31.Herraiz Roda J.L., Llueca Abella J.A., Maazouzi Y., Bouché Babiloni A., Cañete Mota A., Guijarro Colomer M., Serra Rubert A. Vulvar Reconstruction in Vulvar Cancer: “Lotus Petal” Suprafascial Flap. Gynecol. Surg. 2016;13:51–55. doi: 10.1007/s10397-015-0911-7. [DOI] [Google Scholar]
- 32.Kim S.W., Lee W.M., Kim J.T., Kim Y.H. Vulvar and Vaginal Reconstruction Using the “Angel Wing” Perforator-Based Island Flap. Gynecol. Oncol. 2015;137:380–385. doi: 10.1016/j.ygyno.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Nomura H., Maeda T., Usami T., Abe A., Yamamoto A., Matoda M., Okamoto S., Kondo E., Omatsu K., Kato K., et al. Vulvar Reconstruction Following Surgery for Vulvar Cancer Using a Stepladder V-Y Advancement Medial Thigh Flap. Int. J. Gynecol. Cancer. 2015;25:1484–1487. doi: 10.1097/IGC.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti Panici P., Di Donato V., Bracchi C., Marchetti C., Tomao F., Palaia I., Perniola G., Muzii L. Modified Gluteal Fold Advancement V-Y Flap for Vulvar Reconstruction after Surgery for Vulvar Malignancies. Gynecol. Oncol. 2014;132:125–129. doi: 10.1016/j.ygyno.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Argenta P.A., Lindsay R., Aldridge R.B., Siddiqui N., Burton K., Telfer J.R.C. Vulvar Reconstruction Using the “Lotus Petal” Fascio-Cutaneous Flap. Gynecol. Oncol. 2013;131:726–729. doi: 10.1016/j.ygyno.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Kuokkanen H., Mikkola A., Nyberg R.H., Vuento M.H., Kaartinen I., Kuoppala T. Reconstruction of the Vulva with Sensate Gluteal Fold Flaps. Scand. J. Surg. 2013;102:32–35. doi: 10.1177/145749691310200107. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.H., Shin J.W., Kim S.W., Oh D.Y., Park J.S., Hur S.Y., Rhie J.W., Ahn S.T. Modified Gluteal Fold V-Y Advancement Flap for Vulvovaginal Reconstruction. Ann. Plast. Surg. 2013;71:571–574. doi: 10.1097/SAP.0b013e31824f23e4. [DOI] [PubMed] [Google Scholar]
- 38.Buda A., Confalonieri P.L., Rovati L.C.V., Fruscio R., Giuliani D., Signorelli M., Dell’Anna T., Pirovano C., Milani R. Better Anatomical and Cosmetic Results Using Tunneled Lotus Petal Flap for Plastic Reconstruction after Demolitive Surgery for Vulvar Malignancy. Int. J. Gynecol. Cancer. 2012;22:860–864. doi: 10.1097/IGC.0b013e318249bf02. [DOI] [PubMed] [Google Scholar]
- 39.Misani M., Rovati L.C.V., Confalonieri P., Buda A., Giuliani D., Del Bene M. Modified Lotus Petal Flap for Vulvo-Vaginal Reconstruction after Resection for Vulvar Cancer: A Single Institution Experience. Handchir. Mikrochir. Plast. Chir. 2011;43:250–254. doi: 10.1055/s-0031-1280802. [DOI] [PubMed] [Google Scholar]
- 40.Staiano J.J., Wong L., Butler J., Searle A.E., Barton D.P.J., Harris P.A. Flap Reconstruction Following Gynaecological Tumour Resection for Advanced and Recurrent Disease—A 12 Year Experience. J. Plast. Reconstr. Aesthetic Surg. 2009;62:346–351. doi: 10.1016/j.bjps.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 41.Weikel W., Schmidt M., Steiner E., Knapstein P.-G., Koelbl H. Reconstructive Plastic Surgery in the Treatment of Vulvar Carcinomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;136:102–109. doi: 10.1016/j.ejogrb.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Weikel W., Schmidt M., Steiner E., Knapstein P.-G., Koelbl H. Surgical Therapy of Recurrent Vulvar Cancer. Am. J. Obstet. Gynecol. 2006;195:1293–1302. doi: 10.1016/j.ajog.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Persichetti P., Simone P., Berloco M., Casadei R.M., Marangi G.F., Cagli B., Di Lella F. Vulvo-Perineal Reconstruction: Medial Thigh Septo-Fascio-Cutaneous Island Flap. Ann. Plast. Surg. 2003;50:85–89. doi: 10.1097/00000637-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Arkoulakis N.S., Angel C.L., DuBeshter B., Serletti J.M. Reconstruction of an Extensive Vulvectomy Defect Using the Gluteus Maximus Fasciocutaneous V-Y Advancement Flap. Ann. Plast. Surg. 2002;49:50–54. doi: 10.1097/00000637-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Moschella F., Cordova A. Innervated Island Flaps in Morphofunctional Vulvar Reconstruction. Plast. Reconstr. Surg. 2000;105:1649–1657. doi: 10.1097/00006534-200004050-00008. [DOI] [PubMed] [Google Scholar]
- 46.Loree T.R., Hempling R.E., Eltabbakh G.H., Recio F.O., Piver M.S. The Inferior Gluteal Flap in the Difficult Vulvar and Perineal Reconstruction. Gynecol. Oncol. 1997;66:429–434. doi: 10.1006/gyno.1997.4790. [DOI] [PubMed] [Google Scholar]
- 47.Yii N.W., Niranjan N.S. Lotus Petal Flaps in Vulvo-Vaginal Reconstruction. Br. J. Plast. Surg. 1996;49:547–554. doi: 10.1016/s0007-1226(96)90132-0. [DOI] [PubMed] [Google Scholar]
- 48.Burke T.W., Morris M., Roh M.S., Levenback C., Gershenson D.M. Perineal Reconstruction Using Single Gracilis Myocutaneous Flaps. Gynecol. Oncol. 1995;57:221–225. doi: 10.1006/gyno.1995.1129. [DOI] [PubMed] [Google Scholar]
- 49.Helm C.W., Hatch K.D., Partridge E.E., Shingleton H.M. The Rhomboid Transposition Flap for Repair of the Perineal Defect after Radical Vulvar Surgery. Gynecol. Oncol. 1993;50:164–167. doi: 10.1006/gyno.1993.1186. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd J.H., Van Dam P.A., Jobling T.W., Breach N. The Use of Rectus Abdominis Myocutaneous Flaps Following Excision of Vulvar Cancer. Br. J. Obstet. Gynaecol. 1990;97:1020–1025. doi: 10.1111/j.1471-0528.1990.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 51.Shin J., Kim S.A., Rhie J.-W. Perineal Perforator Switch Flap for Three-Dimensional Vulvovaginal Reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2022;75:3208–3216. doi: 10.1016/j.bjps.2022.04.052. [DOI] [PubMed] [Google Scholar]
- 52.Gentileschi S., Servillo M., Garganese G., Simona F., Scambia G., Salgarello M. Versatility of Pedicled Anterolateral Thigh Flap in Gynecologic Reconstruction after Vulvar Cancer Extirpative Surgery. Microsurgery. 2017;37:516–524. doi: 10.1002/micr.30077. [DOI] [PubMed] [Google Scholar]
- 53.Huang J.-J., Chang N.-J., Chou H.-H., Wu C.-W., Abdelrahman M., Chen H.-Y., Cheng M.-H. Pedicle Perforator Flaps for Vulvar Reconstruction—New Generation of Less Invasive Vulvar Reconstruction with Favorable Results. Gynecol. Oncol. 2015;137:66–72. doi: 10.1016/j.ygyno.2015.01.526. [DOI] [PubMed] [Google Scholar]
- 54.Al-Benna S., Tzakas E. Postablative Reconstruction of Vulvar Defects with Local Fasciocutaneous Flaps and Superficial Fascial System Repair. Arch. Gynecol. Obstet. 2012;286:443–448. doi: 10.1007/s00404-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 55.Yun I.S., Lee J.H., Rah D.K., Lee W.J. Perineal Reconstruction Using a Bilobed Pudendal Artery Perforator Flap. Gynecol. Oncol. 2010;118:313–316. doi: 10.1016/j.ygyno.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q., Chen X., Yang Y. The Application of Anterolateral Thigh Flap in Post-Operative Repairing of Vulva Tumor. Chin. -Ger. J. Clin. Oncol. 2010;9:539–542. doi: 10.1007/s10330-010-0661-y. [DOI] [Google Scholar]
- 57.Franchelli S., Leone M.S., Bruzzone M., Muggianu M., Puppo A., Gustavino C., Di Capua E., Centurioni M.G. The Gluteal Fold Fascio-Cutaneous Flap for Reconstruction after Radical Excision of Primary Vulvar Cancers. Gynecol. Oncol. 2009;113:245–248. doi: 10.1016/j.ygyno.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Ragoowansi R., Yii N., Niranjan N. Immediate Vulvar and Vaginal Reconstruction Using the Gluteal-Fold Flap: Long-Term Results. Br. J. Plast. Surg. 2004;57:406–410. doi: 10.1016/j.bjps.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 59.Elia J., Do N.T.K., Chang T.N.-J., Lai C.-H., Chou H.-H., Chang F.C.-S., Huang J.-J. Redefining the Reconstructive Ladder in Vulvoperineal Reconstruction: The Role of Pedicled Perforator Flaps. J. Reconstr. Microsurg. 2022;38:10–26. doi: 10.1055/s-0041-1727199. [DOI] [PubMed] [Google Scholar]
- 60.Tan B.-K., Kang G.C.-W., Tay E.H., Por Y.C. Subunit Principle of Vulvar Reconstruction: Algorithm and Outcomes. Arch. Plast. Surg. 2014;41:379–386. doi: 10.5999/aps.2014.41.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J., Zhang J., Ge J., Su Z., Xiao X., Chen C., Shi F., Wang Y., Zhang J., Liang W. Experience with Flap Repair after Vulvar Carcinoma Resection: A Retrospective Observational Study of 26 Cases. Transl. Cancer Res. 2022;11:1740–1749. doi: 10.21037/tcr-22-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Negosanti L., Sgarzani R., Fabbri E., Palo S., Oranges C.M., De Iaco P., Zannetti G., Contedini F., Cipriani R. Vulvar Reconstruction by Perforator Flaps: Algorithm for Flap Choice Based on the Topography of the Defect. Int. J. Gynecol. Cancer. 2015;25:1322–1327. doi: 10.1097/IGC.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W., Zeng A., Yang J., Cao D., Huang H., Wang X., You Y., Chen J., Lang J., Shen K. Outcome of Vulvar Reconstruction by Anterolateral Thigh Flap in Patients with Advanced and Recurrent Vulvar Malignancy. J. Surg. Oncol. 2015;111:985–991. doi: 10.1002/jso.23908. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Zeng A., Yang J., Cao D., He X., Wang X., You Y., Chen J., Lang J., Shen K. Outcome of Vulvar Reconstruction in Patients with Advanced and Recurrent Vulvar Malignancies. BMC Cancer. 2015;15:851. doi: 10.1186/s12885-015-1792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salgarello M., Farallo E., Barone-Adesi L., Cervelli D., Scambia G., Salerno G., Margariti P.A. Flap Algorithm in Vulvar Reconstruction after Radical, Extensive Vulvectomy. Ann. Plast. Surg. 2005;54:184–190. doi: 10.1097/01.sap.0000141381.77762.07. [DOI] [PubMed] [Google Scholar]
- 66.Zeng A., Qiao Q., Zhao R., Song K., Long X. Anterolateral Thigh Flap-Based Reconstruction for Oncologic Vulvar Defects. Plast. Reconstr. Surg. 2011;127:1939–1945. doi: 10.1097/PRS.0b013e31820e9223. [DOI] [PubMed] [Google Scholar]
- 67.Allen R.J., Treece P. Deep Inferior Epigastric Perforator Flap for Breast Reconstruction. Ann. Plast. Surg. 1994;32:32. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Devulapalli C., Jia Wei A.T., DiBiagio J.R., Baez M.L., Baltodano P.A., Seal S.M., Sacks J.M., Cooney C.M., Rosson G.D. Primary versus Flap Closure of Perineal Defects following Oncologic Resection: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016;137:1602. doi: 10.1097/PRS.0000000000002107. [DOI] [PubMed] [Google Scholar]
- 69.Blondeel P.N., Van Landuyt K.H.I., Monstrey S.J.M., Hamdi M., Matton G.E., Allen R.J., Dupin C., Feller A.-M., Koshima I., Kostakoglu N., et al. The “Gent” Consensus on Perforator Flap Terminology: Preliminary Definitions. Plast. Reconstr. Surg. 2003;112:1378–1383. doi: 10.1097/01.PRS.0000081071.83805.B6. discussion 1384–1387; quiz 1383, 1516. [DOI] [PubMed] [Google Scholar]
- 70.Eseme E.A., Scampa M., Viscardi J.A., Ebai M., Kalbermatten D.F., Oranges C.M. Surgical Outcomes of VRAM vs. Gracilis Flaps in Vulvo-Perineal Reconstruction Following Oncologic Resection: A Proportional Meta-Analysis. Cancers. 2022;14:4300. doi: 10.3390/cancers14174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E., Fernando C., McCombie A., Bailey W., Frizelle F., Glyn T., Porter C., Wakeman C., Creagh T. Abdominal and Perineal Hernia Rates Following Vertical Rectus Abdominis Myocutaneous (VRAM) Flap Reconstruction - a Supraregional Experience. J. Plast. Reconstr. Aesthetic Surg. 2022;75:1158–1163. doi: 10.1016/j.bjps.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Qian B., Xiong L., Li J., Sun Y., Sun J., Guo N., Wang Z. A Systematic Review and Meta-Analysis on Microsurgical Safety and Efficacy of Profunda Artery Perforator Flap in Breast Reconstruction. J. Oncol. 2019;2019:9506720. doi: 10.1155/2019/9506720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knox A.D.C., Ho A.L., Leung L., Tashakkor A.Y., Lennox P.A., Van Laeken N., Macadam S.A. Comparison of Outcomes following Autologous Breast Reconstruction Using the DIEP and Pedicled TRAM Flaps: A 12-Year Clinical Retrospective Study and Literature Review. Plast. Reconstr. Surg. 2016;138:16–28. doi: 10.1097/PRS.0000000000001747. [DOI] [PubMed] [Google Scholar]
- 74.Tielemans H.J.P., van Kuppenveld P.I.P., Winters H., Hupkens P., Ulrich D.J.O., Hummelink S. Breast Reconstruction with the Extended Profunda Artery Perforator Flap. J. Plast. Reconstr. Aesthetic Surg. 2021;74:300–306. doi: 10.1016/j.bjps.2020.08.109. [DOI] [PubMed] [Google Scholar]
- 75.Wei F., Jain V., Celik N., Chen H., Chuang D.C.-C., Lin C. Have We Found an Ideal Soft-Tissue Flap? An Experience with 672 Anterolateral Thigh Flaps. Plast. Reconstr. Surg. 2002;109:2219–2226. doi: 10.1097/00006534-200206000-00007. discussion 2227–2230. [DOI] [PubMed] [Google Scholar]
- 76.Chana J.S., Odili J. Perforator Flaps in Head and Neck Reconstruction. Semin. Plast. Surg. 2010;24:237–254. doi: 10.1055/s-0030-1263066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witte D.Y.S., van Ramshorst G.H., Lapid O., Bouman M.-B., Tuynman J.B. Flap Reconstruction of Perineal Defects after Pelvic Exenteration: A Systematic Description of Four Choices of Surgical Reconstruction Methods. Plast. Reconstr. Surg. 2021;147:1420–1435. doi: 10.1097/PRS.0000000000007976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and the Supplementary Materials.