Abstract
Background
Although several cardiovascular, demographic, genetic and lifestyle factors have been associated with cognitive function, little is known about what type of cognitive impairment they are associated with. The aim was to examine the associations between different risk factors and future memory and attention/executive functions, and their interaction with APOE genotype.
Methods
Participants from a large, prospective, population-based, Swedish study were included (n = 3,229). Linear regression models were used to examine baseline hypertension, body mass index (BMI), long-term glucose levels (HbA1c), different lipid levels, physical activity, alcohol consumption, smoking, education, APOE genotype, age and sex. All models were adjusted for follow-up time and basic demographics, and, in a second step, all significant predictors were included to examine independent effects. Follow-up outcomes were memory and attention/executive functions.
Results
The mean age at baseline was 56.1 (SD 5.7) years and 59.7% were women. The mean follow-up time was 17.4 (range 14.3–20.8) years. When examining independent effects, APOE ε4 genotype(p < 0.01), and higher HbA1c(p < 0.001), were associated with future low memory function. Higher BMI (p < 0.05), and HbA1c(p < 0.05), lower high-density lipoprotein cholesterol (HDL-C)(p < 0.05)and stroke(p < 0.001) were associated with future low attention/executive function. The strongest factors associated with both better memory and attention/executive functions were higher education and alcohol consumption. Further, significant interaction effects between predictors and APOE genotype were found. For memory function, the protective effects of education were greater among ɛ4-carriers(p < 0.05). For attention/executive function, the protective effects of alcohol were greater among ɛ2 or ɛ4-carriers(p < 0.05). Also, attention/executive function was lower among ɛ4-carriers with higher BMI(p < 0.05) and ɛ2-carriers with higher HbA1c-levels(p < 0.05).
Conclusions
Targeting cardiovascular risk factors in mid-life could have greater effect on future attention/executive functions rather than memory, whereas targeting diabetes could be beneficial for multiple cognitive domains. In addition, effects of different risk factors may vary depending on the APOE genotype. The varied cognitive profiles suggest that different mechanisms and brain regions are affected by the individual risk factors. Having detailed knowledge about the specific cognitive effects of different risk factors might be beneficial in preventive health counseling.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01497-6.
Introduction
Cognitive decline increases with age but is not an inevitable consequence of healthy aging. Understanding underlying mechanisms could be of great importance for targeting modifiable risk factors to delay or prevent the onset of cognitive impairment. Total cardiovascular risk has previously been associated with increased cognitive decline [1]. Specific co-morbidities, e.g. diabetes, have been shown to be a modifiable risk factor for cognitive impairment [2, 3], whereas targeting hypertension has presented conflicting results. Even though there are some well-established risk factors for dementia such as diabetes, hypertension, obesity, low education and harmful use of alcohol [4–6], large longitudinal studies on how various types of risk factors contribute to cognitive decline in different cognitive domains are lacking. A deeper understanding of which cognitive functions are affected by different risk factors could provide useful information about potential underlying mechanism of the cognitive impairment and more precise preventive health counceling about the dangers of certain conditions. The use of short follow-up periods and the absence of adjustment for a range of potential confounding factors in the previous studies also contribute to uncertain conclusions regarding the impact of various risk factors [7].
The aim of this study was to investigate how different risk factors contribute to future memory and attention/executive function, respectively. Furthermore, we examined how they interacted with APOE genotype, as APOE ɛ4 is a well-established risk factor/marker for Alzheimer’s disease (AD) [8] and APOE ɛ2 have been shown both being a risk factor for cardiovascular disease [9] but in a meta-analysis being a protective factor for myocardial infarction [10]. This was carried out in a large population-based study (n = 3,229) including extensively characterized participants with a follow-up of 14–21 years.
Methods
Study population
Data was obtained from the population-based prospective Malmö Diet and Cancer Study (MDCS). Between 1991 and 1996, men born 1923–1945 and women born 1923–1950 who were living in Malmö were invited to participate. Baseline examination included body composition measurements, blood pressure, baseline questionnaire and dietary assessments. In the self-administered baseline questionnaire (http://links.lww.com/WNL/A48) education, occupation, current medication and family history of diseases in close relatives were assessed. Dietary assessments included: (1) a prospective seven-day diet record, (2) a self-administered food frequency questionnaire and (3) a 45–60 min interview with trained personnel. Exclusion criteria for participating in Malmö Diet and Cancer Study (MDCS) included language difficulties and intellectual disability, which could preclude participants from filling in questionnaires properly. From MDCS, a randomly selected population were invited to further baseline examinations with fasting plasma samples and carotid artery examination, forming the cardiovascular cohort (MDSC-CV). In 2007–2012 participants of MDSC-CV were invited to a re-examination where 76% of the surviving population participated [11]. At follow-up, cognitive assessments were performed, including the Mini-Mental State Examination (MMSE) [12] and A Quick Test of cognitive speed (AQT) [13].
All participants received information about the study and gave written consent to participate. Ethical approval was given by the Ethical Committee of Lund University, Lund, Sweden (LU 51–90, LU 532–2006).
Demographic predictors of cognitive function
Sociodemographic factors included age, sex and level of education. Based on information from the baseline self-reported questionnaire, variables were divided as follows: Education level into three levels as per study design: primary/elementary school (≤ 8 years), secondary school/high school (9–12 years), or higher education/university (≥ 13 years); Smoking status into smokers, former smokers, and never smokers; and physical activity as metabolic equivalent hours/week (METh/week) where one METh is defined as the metabolic intensity when a person is at rest. METh/week was computed by multiplying time (hours) spent on each activity by the respective metabolic equivalent task (intensity) factor (MET) [14]. Information on alcohol consumption (g/day) was derived both from the food frequency questionnaire and the seven-day diet record. Alcohol consumption was divided into quartiles, and 14 g of pure ethanol was considered to represent one standard drink, from the National Institution on Alcohol Abuse and Alcoholism [15]. Zero-consumers had reported no consumption during the past year. In sensitivity analyses, other stratifications of alcohol consumption were used. Triglycerides (mmol/l), cholesterol (mmol/l), high-density lipoprotein cholesterol (HDL-C) (mmol/l), and low-density lipoprotein cholesterol (LDL-C) (mmol/l) and blood glucose were measured in serum after an overnight fast at the baseline visit, using standard procedures at the Department of Clinical Chemistry, Malmö, Sweden [16]. Systolic blood pressure was measured after 10 min of rest in a horizontal position. A diagnosis of hypertension was derived from the baseline questionnaire. We calculated body mass index (BMI) as kg/m2. APOE genotype analyzed from blood was divided into four groups: ɛ2-carrier (ɛ2/ɛ2, ɛ2/ɛ3), ɛ3/ɛ3, ɛ4-carrier (ɛ3/ɛ4, ɛ4/ɛ4) and ɛ2/ɛ4, with ɛ3/ɛ3 as reference group in the statistical models, since ɛ4 is associated with increased risk of AD [8] and ɛ2 with increased cardiovascular disease (but lower risk of AD) [17]. History of stroke was derived from the questionnaire (prevalent at baseline) or during follow-up from the Swedish National Patient Register (NPR). That is, the variable “stroke” denoted either prevalent or incident stroke. The NPR covers both the Swedish Inpatient Register and the hospital-based outpatient register.
Cognitive outcomes
The primary outcomes were memory and attention/executive functions. Memory function was measured using the delayed word recall part (remembering 0–3 words previously presented) and orientation to time and location (0–10 points for remembering e.g. present year, month, day of the week, and what floor the examination is taking place on, name of the building etc.). These two parts of the MMSE (examining both episodic and semantic memory functions) have previously extensively been used for assessing the memory impairment seen in AD, e.g. for identifying early AD-related memory impairment in non-demented individuals [18–20] and for differential diagnosis of the memory impairment seen in AD versus the cognitive impairment seen in dementia with Lewy bodies [21].
Attention and executive functions were examined using A Quick Test of cognitive speed (AQT), which has a high sensitivity for impaired attention (processing speed) and executive function (set-shifting) [13]. The AQT score constitutes the number of seconds it takes to fulfil each test plate of colours and shapes of figures, thus higher score equals worse performance. Part three (naming first colour, then shape) of the AQT is the most extensively validated part as a sensitive measure of attention/executive function and was used as outcome [13, 22–24]. Both the memory score and the attention/executive score were converted to z-scores, based on the distribution in the present population, for easier comparison of estimates. The attention/executive score was also inverted so that higher scores equal better performance, for both cognitive domains.
Statistical analysis
Chi-square test (for binary/categorical) variables and the Mann-Whitney U test (for continuous variables) were used for group comparisons. Continuous data were converted to z-scores based on the distribution in the present population for easier comparison of estimates. Multivariate linear regression models were used to examine associations between non-modifiable/modifiable risk factors (i.e., predictors) and subsequent cognitive performance (either memory or attention/executive z-scores). Associations between the predictor and future cognitive performance were examined in three steps: (1) univariate models including just the predictor and time from baseline to cognitive testing at follow-up; (2) basic models, including the predictor, age, sex, education, prevalent (at baseline) or incident (during follow-up) stroke (from now on referred collectively as “stroke”), and time from baseline to cognitive testing at follow-up; and (3) multivariable models, including the predictors that were significant in the basic models, and the same adjusting co-variates as in the basic models. To avoid collinearity issues, HbA1c, diabetes, and plasma glucose levels were not entered in the same model (only HbA1c in the multivariable model). Logistic regression models were used to examine predictors of drop-out from the study (i.e., baseline data available but no follow-up examination), adjusted for age, sex, education, time to follow-up and stroke.
An APOE interaction analysis was performed to examine potential interactions between risk factor and APOE genotype. APOE genotype was divided into four groups: ɛ3/ɛ3 (reference), ɛ2/ɛ2 or ɛ2/ɛ3, ɛ3/ɛ4 or ɛ4/ɛ4, and ɛ2/ɛ4.
All statistical analyses were performed using R version 4.2.1 (R Foundation for Statistical Computing). A p-value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
Participation in the MDCS reached 40.8% of the eligible population [25]. In total 30,446 attended in at least a part of the baseline examination.
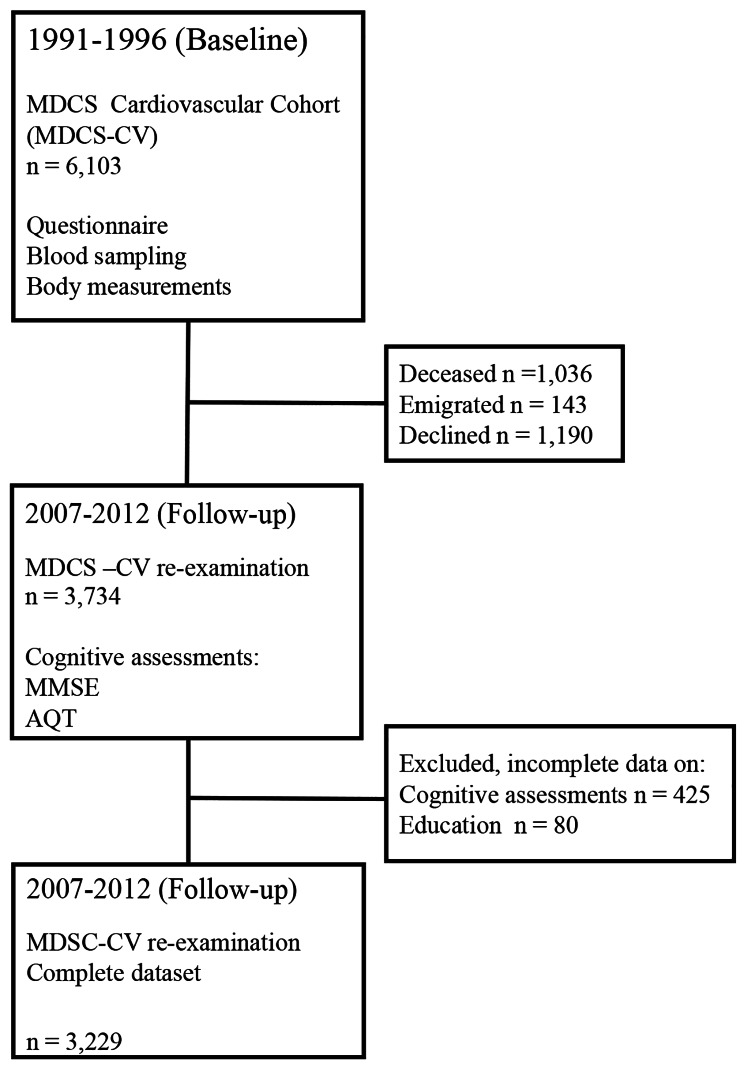
In 2007–2012 participants of MDSC-CV were invited to a re-examination where 3,734 people participated. Participants declining follow-up had generally poorer health status than the included participants. Reasons for non-participation included unwillingness, sickness, deceased or lack of contact information in registers. Individuals participating at follow-up in the MDSC-CV (2007–2012) were included in the study (n = 3,734). Reasons for non-participation at follow-up are shown in Fig. 1. Participants with incomplete data on cognitive assessments (n = 425) and education (n = 80) were excluded, resulting in a complete dataset of 3,229 individuals, which was used for the main analysis. A flow-chart of the inclusion is shown in Fig. 1. Table 1 presents characteristics of the study population. The mean age was 56.1 (SD 5.65, range 45.8–68.0) years at baseline and 59.7% were women. Mean time to the follow-up visit was 17.4 (SD 1.43, range 14.3–20.8) years [11].
Fig. 1.
Flow chart describing the Malmö Diet and Cancer Study (MDCS) Cardiovascular Cohort at baseline 1991–1994 and follow-up 2007–2012
Table 1.
Characteristics of the Study population
| Total (n = 3,229) | |
|---|---|
| Age, y | 56.1 (5.65, 45.8–68.0) |
| Education level, n(%) | |
| < 8 years | 1304 (40.4) |
| 9–12 years | 1212 (37.5) |
| ≥ 13 years | 713 (22.1) |
| Time to follow-up visit, y | 17.4 (1.43, 14.3–20.8) |
| Follow-up AQT | |
| Part 3 (color and form, seconds) | 74.0 (20.2, 35–295) |
| Follow-up MMSE | |
| Total score | 28.1 (1.81, 11–30) |
| Orientation and memory scores | 12.1 (1.02, 4–13) |
| Hypertension | |
| Hypertension, from baseline questionnaire, n (%) | 444 (13.8) |
| Systolic blood pressure (mmHg) | 139 (18.0) |
| Lipid levels | |
| Total cholesterol (mmol/l) | 6.13 (1.07) |
| HDL-C (mmol/l) | 1.41 (0.372) |
| LDL-C (mmol/l) | 4.13 (0.976) |
| Triglycerides (mmol/l) | 1.29 (0.692) |
| Lipid lowering medication, n (%) | 63 (2.0) |
| Diabetes | |
| Diabetes at baseline, n (%) | 179 (5.5) |
| HbA1c (%) | 4.80 (0.598) |
| Fasting glucose (mmol/l) | 5.01 (0.997) |
| Stroke (prevalent or incident), n (%) | 241 (7.5) |
| Body Mass Index a | 25.4 (3.69) |
| Smoking, n(%) | |
| Current smoker | 723 (22.4) |
| Former smoker | 1137 (35.2) |
| Never smoker | 1368 (42.4) |
| Alcohol consumption, g/day | 10.8 (11.6) |
| Q1 (≤ 0.23 standard drinks/day) | 0.61 (0.77) |
| Q2 (0.24–0.56 standard drinks/day) | 5.15 (1.60) |
| Q3 (0.57–1.01 standard drinks/day) | 11.01 (2.09) |
| Q4 (≥ 1.02 standard drinks/day) | 26.33 (12.41) |
| APOE genotype, n (%) | |
| ε2/ε2 or ε2/ε3 | 397 (12.3) |
| ε3/ε3 | 1780 (55.1) |
| ε2/ε4 | 79 (2.4) |
| ε3/ε4 or ε4/ε4 | 812 (25.1) |
| Physical activity (METh/week) b | 3.41 (1.15) |
Data are shown as mean (SD, range) if not otherwise specified. All data represent baseline data except for MMSE and AQT scores at follow-up. Stroke is accounted for as prevalent at baseline or incident during follow-up
a Calculated as weight in kilograms divided by height in meters squared
b Physical activity as metabolic equivalent hours/week (METh/week). One METh is defined as the metabolic intensity when a person is at rest. METh/week was computed by multiplying time (hours) spent on each activity by the respective metabolic equivalent task (intensity) factor (MET).
Association between risk factors and future memory function
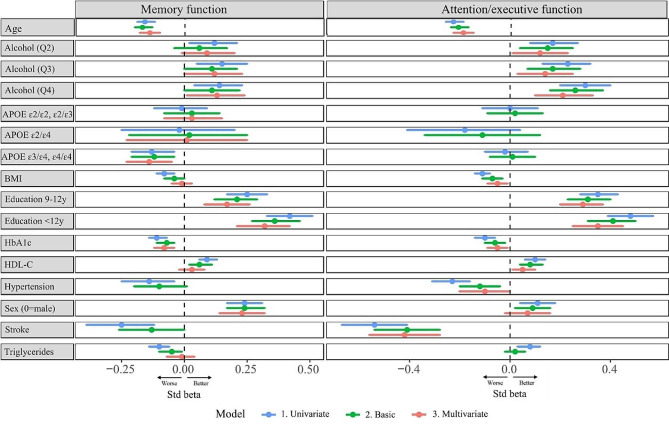
Associations between modifiable and non-modifiable risk factors in relation to future memory function are presented in Table 2; Fig. 2. The multivariable models examined the independent effects of the predictors on cognitive performance measured as z-scores (i.e., SD). They showed that the strongest protective predictor was education, where both 9–12 years (β = 0.17, 95%CI:0.08–0.26) and > 12 years of education (β = 0.32, 95%CI:0.21–0.42) were associated with better memory function (using ≤ 8 years as reference). The second strongest protective predictor was alcohol consumption, where consumption in both the second highest quartile, Q3 0.6-1.0 standard drinks/day, (β = 0.12, 95%CI:0.00-0.23) and highest quartile, Q4 ≥ 1.1 standard drinks/day, (β = 0.13, 95%CI:0.01–0.24) were associated with better memory function using the lowest consumption quartile, Q1 < 0,2 standard drinks/day, as reference. The robustness of this association was further examined in sensitivity analyses (end of Results section). Female sex was associated with better memory performance (β = 0.23, 95%CI:0.14–0.32). Presence of APOE ɛ4/ɛ4 or ɛ3/ɛ4 genotype (β=-0.14, 95%CI:-0.23– -0.05), age (β=-0.14, 95%CI:-0.18– -0.10), and higher HbA1c (β=-0.08, 95%CI:-0.12– -0.04), were all independently associated with lower memory function. Non-significant predictors are shown in eTable 1 and included for example a diagnosis of hypertension at baseline, physical activity and former/current smoking.
Table 2.
Significant predictors of future memory function
| Baseline predictor a | Univariate models b | Basic models c | Multivariable model d |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | -0.16 (-0.19 – -0.12)*** | -0.17 (-0.20 – -0.13)*** |
-0.14 (-0.18 – -0.10)*** |
| Alcohol consumption e | |||
|
Q1 (≤ 0.23 standard drinks/day) Q2 (0.24–0.56 standard drinks/day) Q3 (0.57–1.01 standard drinks/day) Q4 (≥ 1.02 standard drinks/day) |
Reference 0.12 (0.02–0.21)* 0.15 (0.05–0.25)** 0.14 (0.04–0.23)** |
Reference 0.06 (-0.04–0.17) 0.11 (0.00–0.21)* 0.11 (-0.00–0.22) |
Reference 0.10 (-0.01–0.20) 0.12 (0.00– 0.23)* 0.13 (0.01–0.24)* |
| APOE genotype | |||
|
ɛ3/ɛ3 ɛ2/ɛ2 or ɛ2/ ɛ3 ɛ2/ɛ4 ɛ3/ɛ4 or ɛ4/ɛ4 |
Reference -0.01 (-0.12–0.09) -0.02 (-0.25–0.20) -0.13 (-0.21 – -0.04)** |
Reference 0.03 (-0.08–0.14) 0.02 (-0.22 – 0.25) -0.12 (-0.21 – -0.04)** |
Reference 0.03 (-0.08–0.15) 0.01 (-0.23–0.25) -0.14 (-0.23 – -0.05)** |
| BMI | -0.08 (-0.11 – -0.04)*** | -0.04 (-0.08 – -0.00)* | -0.01 (-0.05–0.03) |
| Diabetes f | -0.36 (-0.51 – -0.21)*** | -0.29 (-0.44 – -0.13)*** | Not included g |
| Education | |||
|
≤8 years 9–12 years >12 years |
Reference 0.25 (0.17–0.33)*** 0.42 (0.33–0.51)*** |
Reference 0.21 (0.12–0.29)*** 0.36 (0.27–0.46)*** |
Reference 0.17 (0.08–0.26)*** 0.32 (0.21–0.42)*** |
| Glucose (plasma) | -0.10 (-0.14 – -0.07)*** | -0.09 (-0.12 – -0.05)*** | Not included g |
| HbA 1c | -0.11 (-0.14 – -0.07)*** | -0.07 (-0.11 – -0.04)*** | -0.08 (-0.12 – -0.04)*** |
| HDL-C | 0.09 (0.06–0.13)*** | 0.06 (0.02–0.11)** | 0.03 (-0.02–0.08) |
| Sex (0 = men, 1 = women) | 0.24 (0.17–0.31)*** | 0.24 (0.17 − 0.32) *** | 0.23 (0.14–0.32)*** |
| Systolic blood pressure | -0.11 (-0.14 – -0.07)*** | -0.04 (-0.08 – -0.00)* | -0.03 (-0.07–0.01) |
| Triglycerides | -0.10 (-0.14 – -0.06)*** | -0.05 (-0.10 – -0.01)* | -0.01 (-0.07–0.04) |
Only predictors that were significant in the basic model are shown in Table 2. The predictors that were non-significant in the basic model are shown in eTable 1 and included: Carotid stenosis, hypertension, LDL-C, physical activity, prevalent or incident stroke and smoking. Predictors in bold represent the significant predictors from the multivariable model
a All continuous variables used as z-scores (i.e., not alcohol, APOE, diabetes, education, hypertension and sex)
b Including only the predictor and time between baseline and follow-up
c Including the predictor adjusted for age, sex, education, time between baseline and follow-up, and prevalent or incident stroke
d One model combining all significant predictors from the basic models, adjusted for age, sex, education, time between baseline and follow-up, prevalent or incident stroke and blood lipid lowering medication
e Alcohol consumption in quartiles with lowest quartile as reference: Q1 ≤ 0.23 standard drinks/day (0–3.37 g/day); Q2 0.24–0.56 standard drinks/day (3.38–7.83 g/day); Q3 0.57–1.01 standard drinks/day (7.84–15.2 g/day); Q4 ≥ 1.02 standard drinks/day, (≥ 15.3 g/day)
f Defined as a diabetes diagnosis entered in the baseline questionnaire or having fasting plasma glucose levels > 6mmol/L at baseline
g To avoid collinearity issues, HbA1c, diabetes, and plasma glucose levels were not entered in the same model (only HbA1c in the multivariable model)
* p < 0.05, ** p < 0.01, *** p < 0.001
Fig. 2.
Predictors of future memory and attention/executive function. All continuous variables used as z-scores. Alcohol consumption in quartiles with lowest quartile as reference: Q1 ≤ 0.23 standard drinks/day (≤ 3.37 g/day); Q2 0.24–0.56 standard drinks/day (3.38–7.83 g/day); Q3 0.57–1.01 standard drinks/day (7.84–15.2 g/day); Q4 ≥ 1.02 standard drinks/day, (≥ 15.3 g/day). Univariate model: Including only the predictor and time between baseline and follow-up. Basic model: Including the predictor, adjusted for age, sex, education, time between baseline and follow-up and prevalent or incident stroke. Multivariable model: One model combining all significant predictors from the basic models, adjusted for age, sex, education, time between baseline and follow-up, prevalent or incident stroke and blood lipid lowering medication
Association between risk factors and future attention/executive function
Associations between modifiable and non-modifiable risk factors and future attention/executive function are presented in Table 3; Fig. 2. The multivariable models examined repeatedly the independent effects of the predictors on cognitive performance measured as z-scores (i.e., SD). From the multivariable models, education was again shown to be the strongest protective predictor, where both 9–12 years (β = 0.29, 95%CI:0.20–0.37) and > 12 years of education (β = 0.35, 95%CI:0.24–0.45) were associated with better attention/executive function (using ≤ 8 years as reference). The second strongest protective factor was alcohol, where consumption in the upper three quartiles, Q2 0.2–0.5 standard drinks/day, (β = 0.12, 95%CI:0.02–0.23), Q3 0.6-1.0 standard drinks/day, (β = 0.14, 95%CI0.04-0.25) and highest Q4 ≥ 1.1 standard drinks/day, (β = 0.21, 95%CI:0.11–0.33) all were associated with a better attention/executive function using the lowest consumption quartile, Q1 < 0,2 standard drinks/day, as reference. The robustness of this association was further examined in sensitivity analyses (end of Results section). Furthermore, higher HDL-C was associated with better attention/executive performance (β = 0.06, 95%CI:0.01–0.10). Presence of stroke (β=-0.41, 95%CI:-0.56– -0.27), age (β= -0.19, 95%CI:-0.23– -0.15), higher BMI (β=-0.04, 95%CI:-0.09– -0.00), and higher HbA1c (β=-0.05, 95%CI:-0.09– -0.01) were all independently associated with lower attention/executive function. Non-significant predictors are shown in eTable 2 and included for example APOE-genotype, physical activity, and smoking. See also sensitivity analyses at the end of the Results section.
Table 3.
Significant predictors of future attention/executive function
| Baseline predictor a | Univariate model b | Basic model c | Multivariable model d |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | -0.23 (-0.26 – -0.19)*** | -0.20 (-0.24 – -0.17)*** | -0.19 (-0.23 – -0.15)*** |
| Alcohol consumption e | |||
|
Q1 (≤ 0.23 standard drinks/day) Q2 (0.24–0.56 standard drinks /day) Q3 (0.57–1.01 standard drinks/day) Q4 (≥ 1.02 standard drinks/day) |
Reference 0.17 (0.08–0.27)*** 0.23 (0.13–0.32)*** 0.30 (0.20–0.40)*** |
Reference 0.15 (0.04–0.25)** 0.17 (0.07–0.28)*** 0.26 (0.16–0.37)*** |
Reference 0.12 (0.02–0.23)* 0.14 (0.04–0.25)** 0.21 (0.11–0.33)*** |
| BMI | -0.11 (-0.14 – -0.08)*** | -0.07 (-0.11 – -0.03)*** | -0.04 (-0.09 – -0.00)* |
| Diabetes f | -0.44 (-0.59 – -0.29)*** | -0.37 (-0.52 – -0.21)*** | Not included g |
| Education | |||
|
≤8 years 9–12 years >12 years |
Reference 0.35 (0.28–0.43)*** 0.48 (0.39–0.57)*** |
Reference 0.31 (0.23–0.40)*** 0.41 (0.31–0.50)*** |
Reference 0.29 (0.20–0.37)*** 0.35 (0.24–0.45)*** |
| Glucose (plasma) | -0.10 (-0.13 – -0.06) *** | -0.07 (-0.10 – -0.03)*** | Not included g |
| HbA 1c | -0.10 (-0.14 – -0.06)*** | -0.06 (-0.10 – -0.02)*** | -0.05 (-0.09 – -0.01)* |
| HDL-C | 0.10 (0.06–0.14)*** | 0.08 (0.04–0.13)*** | 0.06 (0.01–0.10)* |
| Hypertension h | -0.26 (-0.36 – -0.16)*** | -0.14 (-0.24 – -0.03)* | -0.10 (-0.21–0.01) |
| Sex (0 = men, 1 = women) | 0.11 (0.04–0.18)** | 0.09 (0.02–0.16)* | 0.07 (-0.02–0.16) |
| Systolic blood pressure | -0.12 (-0.15 – -0.08)*** | -0.03 (-0.07–0.01) | Not included |
| Stroke (prevalent or incident) | -0.54 (-0.67 – -0.41)*** | -0.41 (-0.54 – -0.28)*** | -0.41 (-0.56 – -0.27)*** |
Only predictors that were significant in the basic model are shown in Table 3. The predictors that were non-significant in the basic model are shown in eTable 2 and included: APOE, carotid stenosis, LDL-C, physical activity, smoking, systolic blood pressure and triglycerides. Predictors in bold represent the significant predictors from the multivariable model
a All continuous variables are shown as z-scores, (i.e., not alcohol, diabetes, education and sex)
b Including only the predictor and time between baseline and follow-up
c Including the predictor adjusted for age, sex, education, time between baseline and follow-up and prevalent or incident stroke
d One model combining all significant predictors from the basic models, adjusted for age, sex, education, time between baseline, prevalent or incident stroke and follow-up and blood lipid lowering medication
e Alcohol consumption in quartiles with lowest quartile as reference: Q1 ≤ 0.23 standard drinks/day (0–3.37 g/day); Q2 0.24–0.56 standard drinks/day (3.38–7.83 g/day); Q3 0.57–1.01 standard drinks/day (7.84–15.2 g/day); Q4 ≥ 1.02 standard drinks/day, (≥ 15.3 g/day)
f Defined as a diabetes diagnosis entered in the baseline questionnaire or having fasting plasma glucose levels > 6mmol/L at baseline
g To avoid collinearity issues, HbA1c, diabetes, and plasma glucose levels were not entered in the same model (only HbA1c in the multivariable model)
h Defined as hypertension from baseline questionnaire
* p < 0.05, ** p < 0.01, *** p < 0.001
Interaction effects between APOE-genotype and future memory function
Interactions between significant predictors (from the multivariable models) and APOE genotype on future memory function are presented in eTable 3 and eFigure 1. The protective effect of education was significantly lower among APOE ɛ4/ɛ4 and ɛ3/ɛ4-carriers and those with > 12 years of education (interaction p-value < 0.05). Further, individuals with APOE ɛ2/ɛ4 and higher HDL-C had better memory function (p < 0.05). APOE ɛ3/ɛ4 and ɛ4/ɛ4-carriers with stroke had lower memory performance (p < 0.05).
Interaction effects between APOE-genotype and future attention/executive function
The protective effects of alcohol on attention/executive function were highest among ɛ2-carriers (ɛ2/ɛ3) and ɛ4-carriers (ɛ3/ɛ4 and ɛ4/ɛ4), (interaction p-values < 0.05). Individuals with APOE ɛ4/ɛ4 and ɛ3/ɛ4 with higher BMI had lower attention/executive function, (p < 0.05). ɛ2-carriers (ɛ2/ɛ2, ɛ2/ɛ3 or ɛ2/ɛ4) with increasing HbA1c-levels had lower attention/executive function (interaction p-value < 0.05). For memory function, APOE ɛ4/ɛ4 and ɛ3/ɛ4-carriers with stroke performed significantly lower on attention/executive function (p < 0.05). (eTable 3 and eFigure 2)
Sensitivity analyses of alcohol, lipids, stroke, hypertension and predictors of attending the follow-up visit
Using the original grouping of alcohol consumption in quartiles, participants reporting zero alcohol consumption were excluded (n = 400), since this group is known to introduce bias related to previous alcoholism or other co-morbidities causing the individual to stop drinking alcohol [26]. In this population of alcohol consumers (n = 2829), higher alcohol consumption, adjusted for age, sex, education and time to follow-up, was still associated with better attention/executive function for Q2 (p < 0.05), Q3 (p < 0.01) and Q4 (p < 0.001). All quartiles but the lowest (reference) were also significant in the multivariable step (p < 0.05 − 0.01). Alcohol consumption was however no longer a significant predictor of better memory function, when excluding zero-consumers (eTable 4). Finally, the population was stratified based on the cut-off of 168 g/week (1.7 standard drinks/day) from the Lancet Commission [5] (suggested to be associated with increased risk of dementia), to examine a potential U-shaped effect whereby the very highest consumption would have a negative effect on cognition. In this “risk consumption” group (n = 343) we did not, however, find a lower performance in any cognitive function, compared with those with consumption below the cut-off, even when excluding zero consumers (eTable 4).
To examine if the seemingly positive effect of alcohol consumption was caused by a “survival bias”, alcohol consumption was examined as a predictor of drop-out during study follow-up (adjusted for age, sex, education, and stroke). This showed the opposite relationship, i.e., that higher alcohol consumption was associated with higher likelihood of turning up at the follow-up visit (< 0.001 for quartiles 2–4; eTable 5). Participants with higher education were also more likely to participate at follow-up. We found, however, that higher HbA1c, a diagnosis of hypertension at baseline, higher age, and stroke were associated with lower likelihood of attending the follow-up visit (eTable 5). No associations were found for APOE genotype, BMI and cholesterol-levels, and attending the follow-up visit.
To examine a potential U-shaped association of low-density lipoprotein cholesterol (LDL-C) and attention/executive function, sensitivity analysis with LDL-C-levels in quartiles was carried out. We found no significant association with attention/executive function with LDL levels and quartile one, three or four using quartile two as reference (data not shown).
Since stroke is a known risk factor for cognitive impairment, we also performed a sensitivity analysis excluding those with prevalent stroke at baseline. In the multivariable analysis for memory function, the significant predictors remained the same except for alcohol consumption which was no longer significant at any level of consumption. In the multivariable analysis for attention/executive function, the same variables were significant as in the main result (data not shown).
When adjusting the basic model for antihypertensive treatment, hypertension was still a significant predictor of attenttion/executive function, but not memory (data not shown), and it did not change any of the significant predictors in the multivariable models.
Discussion
In this longitudinal, population-based prospective cohort study of 3,229 individuals with 17 years of follow-up, we found that cardiovascular risk factors such as higher BMI, lower HDL-C and stroke were associated with lower attention/executive function, while having an APOE ɛ4-allele was associated with poorer memory function. Higher HbA1c was associated with lower performance in both cognitive domains. Higher education and higher alcohol consumption were associated with both better memory and attention/executive functions. Interaction effects were found between predictors and APOE genotype. For memory function, the protective effects of education were greater among ɛ4-carriers. For attention/executive function, the protective effects of alcohol were largest among ɛ2 or ɛ4-carriers. Also, attention/executive function were lower among ɛ4-carriers with higher BMI and ɛ2-carriers with higher HbA1c-levels.
The main strengths of this study were the prospective study design, large sample size, long follow-up time, and the analysis of a wide range of risk factors in multivariable models to examine independent effects. In a previous cross-sectional large population-based study, no association between APOE genotype and cognition were found, concluding the need for further longitudinal studies [27]. Here we show a significant relationship between APOE ɛ4 and future low memory function. The most likely mechanism for this association is the well-known association with β-amyloid accumulation, which in turn facilitates tau accumulation (the hallmark pathologies of Alzheimer’s disease) that starts around the entorhinal cortex and hippocampus (key regions for the memory function) [28, 29].
Several cardiovascular risk factors are known to be associated with cognitive impairment [30–32], but here we show that they are specifically associated with future attention/executive function (Table 3; Fig. 2). These risk factors included higher BMI, elevated HbA1c and low HDL-C. Hypertension has previously been associated with cognitive impairment in numerous studies [33–35], whereas others have not been able to replicate that [3, 36]. In this present study, we found an association, specifically with attention/executive function both in univariate analyses and when adjusting for age, gender, education and stroke. The association did, however, not remain significant in the multivariable model, suggesting that the effect to some extent could be mediated by any of the other risk factors, or that it co-varies with another stronger risk factor such as stroke. The association of cardiovascular risk factors with specifically attention/executive function, and not memory, suggest that there is another pathophysiological mechanism behind this type of cognitive decline. One possible explanation is that these risk factors are more related to arteriolosclerosis (arterial stiffness) and venous collagenosis [37] in the brain, which could contribute to attention/executive impairment. This suggests targeting cardiovascular risk factors in mid-life could have greater impact on future attention/executive function, but less so on memory function. High HbA1c levels were, however, associated with lower function in both cognitive domains, and consistent results were found with increasing plasma glucose and prevalence of diabetes. This indicates that high glucose levels are associated with lower cognitive functions in multiple cognitive domains, through potentially different mechanisms.
Higher education was, as expected, associated with better cognitive performance in both domains, as low education is a well-known risk factor for cognitive decline and dementia. In the present study, higher alcohol consumption was associated to both better memory and attention/executive function. After excluding zero-consumers this association was, however, only significant for attention/executive function, making this association more robust than that for memory. The sensitivity analysis suggests that the seemingly positive effect of alcohol on memory performance is not caused by increasing alcohol consumption, but instead by worse memory performance in the zero-consumer group (potentially related to co-morbidities and previous alcoholism). Similar positive effects of alcohol on cognitive function have previously been shown [38, 39]. The potential mechanism behind alcohol consumption and better cognitive performance may be related to potentially better cardiovascular health [40] even though this has not been confirmed in a recent study using Mendelian randomization [41]. The suggested beneficial cardiovascular effect has been indicated to be U-shaped, whereby both very low and very high consumption have a negative effect [42]. We could, however, not find that high consumption (as defined by the Lancet Commission [5]) was associated with worse cognitive function (eTable 4). Nonetheless, it is important to note that this study only examined the effect of alcohol on cognitive function and study adherence, but not other known negative effects of alcohol [43, 44]. Although we see a robust positive finding between alcohol consumption and attention/executive function, we do not make any claims that alcohol is overall beneficial or recommend increased alcohol consumption in low consumers. Further, alcohol consumption was self-reported which can introduce biases and it may also reflect other factors influencing cognition such as socioeconomic status, which was only indirectly adjusted for using for example education level [45]. Cultural or religious differences could possibly also affect the results.
An interaction effect between APOE genotype and diabetes on cognitive function and dementia has previously been shown, though primarily between APOE ɛ4 and diabetes [46, 47]. In this study, we instead found that APOE ɛ2-carriers with increased HbA1c had greater impairment of attention/executive function. APOE ɛ2 is a known mediator of hyperlipidaemia, because of its inaccurate binding to LDL receptors, and can in the presence of other environmental factors increase the risk of atherosclerosis [9], which could be an explanation of the observed interaction effect in that this provides a synergistic detrimental effect with HbA1c on the vascular system with a downstream effect on attention/executive function. Hyperglycaemia is known to be associated to arterial stiffness with negative effects on attention/executive function [48, 49]. APOE ɛ4-carriers with stroke had lower cognitive function in both cognitive domains, which is in agreement with the previously found synergistic effect of having both AD pathology (accelerated by ɛ4-carriership) and cerebrovascular disease [50–53].
In this study, we did not find any effects of physical activity on cognitive function. This is in line with a recent large review and meta-analysis [54], concluding that positive findings in previous studies may be due to low statistical power, publication bias, and unseemly adjustment for baseline differences.
This study has some limitations. As in the case of all non-randomized, observational studies, other confounding factors might be present, although we have adjusted for multiple potential confounding and mediating factors at baseline. Further, a potential survival bias could have been introduced at follow-up since hypertension and higher HbA1c levels were predictors of drop-out during follow-up (eTable 5) potentially minimizing their negative effect on cognition. They were, however, still significant predictors of cognition, so this potential bias would only have affected the effect size. Another limitation is the cognitive tests, which only capture some aspects of the cognitive domains memory and attentions/executive function. The lack of baseline cognitive assessments is also a limitation. The findings should be validated with more extensive neuropsychological tests. For alcohol consumption, a positive association of higher consumption and cognitive function can potentially be biased if only selected, more healthy, higher consumers attend follow-up due to a large drop-out related to the known negative health effects of high alcohol consumption [43, 44]. However, in our drop-out analysis we found the opposite, i.e., that higher alcohol consumption at baseline predicted higher probability of attending the follow-up visit (eTable 5). We can therefore exclude that such a survival bias caused the somewhat controversial finding of a positive effect of higher alcohol consumption on better cognitive function.
Conclusion
In this prospective study of 3,229 middle-aged individuals with 17-years of follow-up, APOE ε4 genotype was associated with future lower memory function. High BMI, low HDL-C and stroke were associated with future lower attention/executive functions, but not future memory function. Diabetes was associated with lower cognitive function in both domains. Protective factors included higher education and alcohol consumption. These results suggest that targeting cardiovascular risk factors in interventions in mid-life may have a greater effect on future attention/executive function than memory function, whereas targeting hyperglycemia or diabetes could be beneficial for preserving multiple cognitive domains. In addition, effects of different risk factors may vary depending on the APOE genotype.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
I.G. wrote the main manuscript, with revision from S.P. I.G. prepared all figures. All authors reviewed the manuscript.
Funding
The study was supported by the Alzheimer’s Association (SG-23-1061717), Swedish Research Council (2022 − 00775, 2021 − 00905, 2018–02052, and 2017 − 01541), ERA PerMed (ERAPERMED2021-184), the Knut and Alice Wallenberg foundation (2017 − 0383), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-981132, AF-980907 and AF-968598), the Swedish Brain Foundation (FO2021-0293 and FO2022-0204), The Parkinson foundation of Sweden (1280/20 and 1412/22), the Cure Alzheimer’s fund, the Konung Gustaf V: s and Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2022 − 1259 and 2022 − 1346) and the Swedish federal government under the ALF agreement (2022-Projekt0080, 2022-Projekt0085 and 2018-Projekt0279). The MDCS-CV re-examination study was supported by grants from the Swedish Research Council (K2008-65X-20752-01-3, K2011-65X-20752-04-6), the Lundstroms Foundation, the Swedish Heart-Lung Foundation (2010 − 0244; 2013 − 0249) and ALF government grants (Dnr: 2012/1789) to PMN.
Open access funding provided by Lund University.
Data availability
Data was acquired from the MPP/MDCS Steering Committee and they have not given their permnission for researchers to share their data. Detailed instructions for data requests can be found on: https://www.malmo-kohorter.lu.se/malmo-cohorts.
Declarations
Ethical approval and consent to participate
All participants received information about the study and gave written consent to participate. Ethical approval was given by the Ethical Committee of Lund University, Lund, Sweden (LU 51–90, LU 532–2006).
Consent for publication
Not applicable.
Competing interests
SP has acquired research support (for the institution) from ki elements / ADDF. In the past two years, he has received consultancy/speaker fees from Bioartic, Biogen, Lilly, and Roche. OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past two years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. KN has received consultancy fee from Biogen in the past two years. The remaining authors have no disclosures relevant to the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Isabelle Glans, Email: isabelle.glans@med.lu.se.
Sebastian Palmqvist, Email: sebastian.palmqvist@med.lu.se.
References
- 1.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80:1300–6. doi: 10.1212/WNL.0b013e31828ab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–78. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 3.Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Impact of cardiovascular risk factors on cognitive function: the Tromsø study. Eur J Neurol. 2011;18:737–43. doi: 10.1111/j.1468-1331.2010.03263.x. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global action plan on the public health repsonse to dementia 2017–2025 [online]. https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025. Accessed 2023-02-14.
- 5.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with Incident Dementia or Cognitive Impairment: a systematic review and Meta-analysis. JAMA. 2020;323:1934–44. doi: 10.1001/jama.2020.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assuncao N, Sudo FK, Drummond C, de Felice FG, Mattos P. Metabolic syndrome and cognitive decline in the elderly: a systematic review. PLoS ONE. 2018;13:e0194990. doi: 10.1371/journal.pone.0194990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slooter AJ, Breteler MB, Ott A, Van Broeckhoven C, van Duijn CM. APOE genotyping in differential diagnosis of Alzheimer’s disease. Lancet. 1996;348:334. doi: 10.1016/S0140-6736(05)64501-1. [DOI] [PubMed] [Google Scholar]
- 9.Mahley RW, Apolipoprotein E. From cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl) 2016;94:739–46. doi: 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao A, Shi J, Liang Z, et al. Meta-analysis of the association between apolipoprotein E polymorphism and risks of myocardial infarction. BMC Cardiovasc Disord. 2022;22:126. doi: 10.1186/s12872-022-02566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosvall M, Persson M, Östling G, et al. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: the Malmö Diet and Cancer Study. Atherosclerosis. 2015;239:615–21. doi: 10.1016/j.atherosclerosis.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Palmqvist S, Minthon L, Wattmo C, Londos E, Hansson O. A quick test of cognitive speed is sensitive in detecting early treatment response in Alzheimer’s disease. Alzheimers Res Ther. 2010;2:29. doi: 10.1186/alzrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutie PM, Drake I, Ericson U, et al. Different domains of self-reported physical activity and risk of type 2 diabetes in a population-based Swedish cohort: the Malmö diet and Cancer study. BMC Public Health. 2020;20:261. doi: 10.1186/s12889-020-8344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute on Alcohol Abuse and Alcoholism. What Is A Standard Drink? [online]. https://www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink. Accessed 2023-02-14.
- 16.Nilsson PM, Engström G, Hedblad B. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects–a population-based study comparing three different definitions. Diabet Med. 2007;24:464–72. doi: 10.1111/j.1464-5491.2007.02142.x. [DOI] [PubMed] [Google Scholar]
- 17.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–4. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 18.Palmqvist S, Hertze J, Minthon L, et al. Comparison of brief cognitive tests and CSF biomarkers in predicting Alzheimer’s disease in mild cognitive impairment: six-year follow-up study. PLoS ONE. 2012;7:e38639. doi: 10.1371/journal.pone.0038639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe YM, Lee BC, Choi IG, Suh GH, Lee DY, Kim JW. MMSE Subscale scores as useful predictors of AD Conversion in mild cognitive impairment. Neuropsychiatr Dis Treat. 2020;16:1767–75. doi: 10.2147/NDT.S263702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmqvist S, Insel PS, Zetterberg H, et al. Accurate risk estimation of β-amyloid positivity to identify prodromal Alzheimer’s disease: cross-validation study of practical algorithms. Alzheimers Dement. 2019;15:194–204. doi: 10.1016/j.jalz.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmqvist S, Hansson O, Minthon L, Londos E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer’s disease with common cognitive tests. Int J Geriatr Psychiatry. 2009;24:1405–12. doi: 10.1002/gps.2277. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Pyo S, Shin SA, et al. A quick test of cognitive speed in older adults with Alzheimer’s disease and mild cognitive impairment: a preliminary behavioral and brain imaging study. Psychiatry Res Neuroimaging. 2018;280:30–8. doi: 10.1016/j.pscychresns.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Jalakas M, Palmqvist S, Hall S, et al. A quick test of cognitive speed can predict development of dementia in Parkinson’s disease. Sci Rep. 2019;9:15417. doi: 10.1038/s41598-019-51505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afshar PF, Wiig EH, Malakouti SK, Shariati B, Nejati S. Reliability and validity of a quick test of cognitive speed (AQT) in screening for mild cognitive impairment and dementia. BMC Geriatr. 2021;21:693. doi: 10.1186/s12877-021-02621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manjer J, Carlsson S, Elmståhl S, et al. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10:489–99. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Stockwell T, Greer A, Fillmore K, Chikritzhs T, Zeisser C. How good is Science? Bmj 2012;344:e2276; author reply e2294. [DOI] [PubMed]
- 27.Lyall DM, Ward J, Ritchie SJ, et al. Alzheimer disease genetic risk factor APOE e4 and cognitive abilities in 111,739 UK Biobank participants. Age Ageing. 2016;45:511–7. doi: 10.1093/ageing/afw068. [DOI] [PubMed] [Google Scholar]
- 28.Berron D, Vogel JW, Insel PS, et al. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain. 2021;144:2771–83. doi: 10.1093/brain/awab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iso-Markku P, Kaprio J, Lindgren N, Rinne JO, Vuoksimaa E. Education as a moderator of middle-age cardiovascular risk factor-old-age cognition relationships: testing cognitive reserve hypothesis in epidemiological study. Age Ageing 2022;51. [DOI] [PMC free article] [PubMed]
- 31.Zheng L, Matthews FE, Anstey KJ. Cognitive health expectancies of cardiovascular risk factors for cognitive decline and dementia. Age Ageing. 2021;50:169–75. doi: 10.1093/ageing/afaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassing LB, Hofer SM, Nilsson SE, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33:355–61. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- 33.Okusaga O, Stewart MC, Butcher I, Deary I, Fowkes FG, Price JF. Smoking, hypercholesterolaemia and hypertension as risk factors for cognitive impairment in older adults. Age Ageing. 2013;42:306–11. doi: 10.1093/ageing/afs193. [DOI] [PubMed] [Google Scholar]
- 34.Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing. 2013;42:338–45. doi: 10.1093/ageing/afs166. [DOI] [PubMed] [Google Scholar]
- 35.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA. 1999;281:438–45. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y, Huang MY, Mao CH, et al. Arteriolosclerosis differs from venular collagenosis in relation to cerebrovascular parenchymal damages: an autopsy-based study. Stroke Vasc Neurol; 2022. [DOI] [PMC free article] [PubMed]
- 38.Lang I, Wallace RB, Huppert FA, Melzer D. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing. 2007;36:256–61. doi: 10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- 39.Solfrizzi V, D’Introno A, Colacicco AM, et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–9. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 40.Snow WM, Murray R, Ekuma O, Tyas SL, Barnes GE. Alcohol use and cardiovascular health outcomes: a comparison across age and gender in the Winnipeg Health and drinking Survey Cohort. Age Ageing. 2009;38:206–12. doi: 10.1093/ageing/afn284. [DOI] [PubMed] [Google Scholar]
- 41.Larsson SC, Burgess S, Mason AM, Michaëlsson K. Alcohol Consumption and Cardiovascular Disease: a mendelian randomization study. Circ Genom Precis Med. 2020;13:e002814. doi: 10.1161/CIRCGEN.119.002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu A, Cooke AB, Scheffler P, Doonan RJ, Daskalopoulou SS. Alcohol exerts a shifted U-Shaped Effect on Central Blood pressure in young adults. J Gen Intern Med. 2021;36:2975–81. doi: 10.1007/s11606-021-06665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive Alcohol Use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5:e2239485. doi: 10.1001/jamanetworkopen.2022.39485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackenbach JP, Kulhanova I, Bopp M, et al. Inequalities in Alcohol-related mortality in 17 European countries: a retrospective analysis of Mortality registers. PLoS Med. 2015;12:e1001909. doi: 10.1371/journal.pmed.1001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towers A, Philipp M, Dulin P, Allen J. The Health benefits of Moderate drinking in older adults may be better explained by Socioeconomic Status. J Gerontol B Psychol Sci Soc Sci. 2018;73:649–54. doi: 10.1093/geronb/gbw152. [DOI] [PubMed] [Google Scholar]
- 46.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 47.Dore GA, Elias MF, Robbins MA, Elias PK, Nagy Z. Presence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse Study. Diabetologia. 2009;52:2551–60. doi: 10.1007/s00125-009-1497-2. [DOI] [PubMed] [Google Scholar]
- 48.Gottsäter M, Hindy G, Orho-Melander M, Nilsson PM, Melander O. A genetic risk score for fasting plasma glucose is independently associated with arterial stiffness: a mendelian randomization study. J Hypertens. 2018;36:809–14. doi: 10.1097/HJH.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilsson ED, Elmståhl S, Minthon L, et al. Nonlinear association between pulse wave velocity and cognitive function: a population-based study. J Hypertens. 2014;32:2152–7. doi: 10.1097/HJH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 50.Ballard CG, Morris CM, Rao H, et al. APOE epsilon4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63:1399–402. doi: 10.1212/01.WNL.0000141851.93193.17. [DOI] [PubMed] [Google Scholar]
- 51.Pendlebury ST, Poole D, Burgess A, Duerden J, Rothwell PM. APOE-ε4 genotype and dementia before and after transient ischemic attack and stroke: Population-based Cohort Study. Stroke. 2020;51:751–8. doi: 10.1161/STROKEAHA.119.026927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Cerebrovascular disease, the apolipoprotein e4 allele, and cognitive decline in a community-based study of elderly men. Stroke. 1996;27:2230–5. doi: 10.1161/01.STR.27.12.2230. [DOI] [PubMed] [Google Scholar]
- 53.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. Nun Study Jama. 1997;277:813–7. doi: 10.1001/jama.1997.03540340047031. [DOI] [PubMed] [Google Scholar]
- 54.Ciria LF, Román-Caballero R, Vadillo MA et al. An umbrella review of randomized control trials on the effects of physical exercise on cognition. Nat Hum Behav 2023. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data was acquired from the MPP/MDCS Steering Committee and they have not given their permnission for researchers to share their data. Detailed instructions for data requests can be found on: https://www.malmo-kohorter.lu.se/malmo-cohorts.