Abstract
The aim of this study was to analyze the role of humoral immunity in early human immunodeficiency virus (HIV) infection. As neutralizing activities in HIV-positive sera are rarely detectable earlier than 9 to 12 months after infection using primary lymphocytes as target cells in neutralization assays, humoral immunity is generally thought not to contribute significantly to early virus control in the patients. Besides lymphocytes, cells of the monocyte/macrophage lineage are known to be important target cells for HIV in vivo during the establishment of the infection. Therefore, we studied the neutralization of early primary HIV isolates by autologous serum samples using primary macrophages as target cells in the neutralization assays. We analyzed neutralizing activities against the autologous HIV-1 isolates in 10 patients' sera taken shortly after seroconversion, both on primary macrophages and, for comparison, on lymphocytes. Viruses were isolated and expanded in primary mixed cultures containing macrophages and lymphocytes in order to avoid selection for one particular cell type. All viruses replicated to different degrees in macrophages and lymphocytes; nine had a nonsyncytium-inducing phenotype, and one was syncytium inducing. The detection of neutralizing antibodies in acute primary HIV infection depended on the target cells used. Confirming previous studies, we did not find neutralizing activities on lymphocytes at this early time point. In contrast, neutralizing activities were detectable in the same sera if primary macrophages were used as target cells. Differences in neutralizing activities on macrophages and lymphocytes were not due to different virus variants being present in the different cell systems, as gp120 sequences derived from both cell types were homogeneous. Neutralization activities on macrophages did not correlate with the amount of β-chemokines in the sera. As affinity-purified immunoglobulin G preparations from an early patient serum also exhibited neutralization of the autologous virus isolate on primary macrophages, but not on lymphocytes, neutralization is very likely due to antibodies against viral epitopes necessary for infection of macrophages but not for infection of lymphocytes. Our data suggest that, along with cell-mediated immunity, humoral immunity may contribute to the reduction of primary viremia in the patient. This was further supported by a certain association between neutralizing antibody titers on macrophages and viral load in the patients.
After infection with the human immunodeficiency virus (HIV), the virus replicates to high titers, with plasma viral load greater than 106 viral RNA copies/ml (8). At seroconversion viremia decreases by several log units and may even reach undetectable levels. The viral load established after seroconversion has prognostic value for the subsequent course of the disease (27). This setpoint is determined on the one side by the efficiency of the virus-specific host response and on the other side by the biological properties of the virus itself.
Due to immunological constraints, the virus population at the time point of seroconversion is homogenous with respect to sequences derived from the external viral glycoprotein gp120 (9, 40, 56). Generally, viruses isolated at this time point have non-syncytium-inducing (NSI) phenotype and are dualtropic for primary lymphocytes and macrophages (50, 58).
Different studies showed that HIV-specific antibodies, though present shortly after seroconversion, are not able to neutralize the autologous virus isolates in lymphocyte cultures (2, 31). Neutralizing antibodies against the early virus isolates are first detectable about 1 year after infection (25, 30). HIV-specific cytotoxic T lymphocytes (CTLs), however, are detectable as early as 3 weeks after infection, preceding the strong decline in viremia (4, 23). Consequently, CTL activity is thought to be the major factor in early control of viremia. The role of the humoral immune response in early virus control is still controversial (37).
All studies on the neutralization of primary HIV in early infection were performed using primary lymphocytes as target cells. Besides lymphocytes, cells of the monocyte/macrophage lineage are important target cells for HIV in vivo (15, 17, 24, 35, 43, 53, 54). These are among the first cells encountered by the virus after sexual transmission (29, 51). They also disseminate the virus to the lymphoid system and other organs such as the liver, the lung, the brain, the gut, etc. (19, 22, 43, 47). The same cells play a pivotal role in the activation and control of the immune response and are functionally disturbed after infection (12, 57).
Therefore, we compared the neutralizing activity of patients' sera shortly after seroconversion against the autologous virus isolates on both primary macrophages and lymphocytes. As viruses tend to adapt to given cells in vitro (28, 52), special emphasis was placed on maintaining the original phenotypes by isolating the virus in mixed-culture systems (primary monocytes/macrophages and lymphocytes) and by limiting virus cultivation times.
MATERIALS AND METHODS
Patients.
Ten patients with well-defined time points of infection were included in this study. Patients presented to the doctors with acute infection symptoms, had incomplete Western blots at the time point of sampling, or had sexual contacts with index partners with confirmed HIV infection at well-known points in time. Blood samples were taken shortly after seroconversion (Table 1) and about 12 months after infection. None of the patients received antiviral treatment at the time point of the first blood drawing.
TABLE 1.
Clinical and virological data of the patients included in the study
| Code | Risk | Date of infection | Date of sampling | Viral load (103 copies/ml) | Viral subtype |
|---|---|---|---|---|---|
| HR001 | Heterosexual | October 94 | March 95 | 42 | C |
| HR002 | Homosexual | October 94 | April 95 | 19 | B |
| HR003 | Homosexual | January 95 | June 95 | 650 | B |
| HR004 | Heterosexual | February 95 | June 95 | 9.3 | E |
| HR005 | Homosexual | February 95 | July 95 | 20 | B |
| HR008 | Homosexual | March 95 | September 95 | 18 | E |
| HR009 | Homosexual | January 95 | September 95 | 4.1 | E |
| HR010 | Homosexual | February 95 | September 95 | 32 | B |
| HR011 | Heterosexual | October 95 | December 95 | 2.2 | B |
| HR014 | Heterosexual | January 96 | May 96 | 70 | B |
Virus isolation.
Virus isolation was conducted in mixed-culture systems containing primary T4 lymphocytes (PBLs) and monocytes/macrophages in order to avoid selection for one of the cell types (53). Patients' peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradients as described previously (34) and mixed with PBMCs from healthy HIV-negative donors (1:10 to 1:30). Cells were cultured in Teflon bags, which allowed differentiation of monocytes to macrophages. No additives for stimulation of PBLs were included in the culture medium (RPMI 1640, 5% human AB serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 4 mM l-glutamine, 1 mM sodium pyruvate, 5 ml [100×] of minimal essential medium [MEM] nonessential amino acids per 500 ml, 2 ml [100×] MEM vitamins per 500 ml). Cultures were maintained for at least 10 days at 37°C and 5% CO2. At this time point p24 antigen production was monitored for the first time, and the assay was repeated every 5 days (Coulter [Fullerton, Calif.] assay). If virus production was positive, fresh uninfected donor cells were added to the Teflon bags to stimulate virus production. At reverse transcriptase (RT) activities of >100,000 cpm/90 min/ml of culture supernatant, viral aliquots were frozen at −80°C (36). All stocks were characterized by p24 antigen amount, RT activity, and 50% tissue culture infective dose (TCID50) on stimulated PBLs.
Determination of TCID50.
Phytohemagglutinin (PHA)-stimulated primary PBLs were adjusted to 4.5 × 106 cells/ml and infected with threefold dilutions of virus stocks in 2-ml Eppendorf cups rotated for 3 h at 37°C. Then cells were pelleted and washed twice with phosphate-buffered saline. Infected cells were adjusted to 106 cells/ml in PBL medium (RPMI 1640, 5% fetal calf serum, 4 mM l-glutamine, and 100 U of penicillin, 100 μg of streptomycin, and 0.4 U of interleukin 2/ml [Sigma, Deisenhofen, Germany]) and for each virus dilution a 200-μl cell suspension was added to each of 8 wells of a 96-well plate. Cultures were maintained at 37°C and 5% CO2 for 10 days. HIV-positive wells were identified by p24 antigen determinations. TCID50 values were calculated according to the Spearman-Kaerber formula (20, 44).
Determination of the replication of virus isolates on PBLs.
PHA-stimulated primary PBLs of one single donor were adjusted to 4.5 × 106 cells/ml and infected with 100 TCID50 of each virus isolate/ml in 2-ml Eppendorf cups rotated for 3 h at 37°C. Then cells were pelleted and washed carefully at least twice to remove the virus. For each virus, cells were adjusted to 106 cells/ml in PBL medium and a 200-μl suspension was seeded into each of 6 wells of a 96-well plate. Cultures were maintained at 37°C and 5% CO2 for 10 days. For each isolate, the replication activity was determined as the mean p24 antigen production over the six wells. These experiments were performed for all isolates using cell preparations from at least three donors to correct for donor dependency.
Determination of the replication of virus isolates on monocytes/macrophages.
PBMCs from a single HIV-negative donor were isolated by Ficoll gradient and adjusted to 4.5 × 106 cells/ml in macrophage medium (see medium of mixed-culture system). Then 200 μl of the cell suspension was added to each of 40 wells of a 96-well plate (4 wells per isolate) and incubated at 37°C for 30 min. Nonadherent cells were removed by intensive washing, leading to 95%-pure monocyte cultures (53). The adherent cells were cultured for 7 days at 37°C and 5% CO2 to allow differentiation to macrophages. Then four macrophage cultures (four wells) were each infected with 200 TCID50 of each virus isolate/ml for 2 days. Thereafter, virus was quantitatively removed by washing. After 4 days, cultures were monitored for p24 antigen production. For each isolate, the replication activity was determined as the mean p24 antigen production for the four wells. For all isolates the experiment was performed using cell preparations from three additional donors to assess donor dependency.
MT-2 assay for determination of syncytium-inducing (SI) and NSI phenotypes.
MT-2 cells were adjusted to 4.0 × 105 cells/ml in MT-2 medium (PBL medium without interleukin 2) and infected with 100,000 cpm of RT activity of each virus isolate/ml in Eppendorf cups rotated at 37°C for 6 h (21). For each virus isolate, cells were pelleted, washed twice, and adjusted to 2.0 × 105 cells/ml and 2-ml cell suspensions were seeded into duplicate wells of a 24-well plate. Cultures were monitored microscopically for syncytia.
Determination of β-chemokines MIP-1α and RANTES in patients' plasma.
Quantitative determinations of macrophage inflammatory protein 1β (MIP-1β) and RANTES (regulated upon activation, normal T-cell expressed and secreted) were performed by commercial enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Minneapolis, Minn.).
Affinity purification of serum IgG and preparation of Fab fragments.
Immunoglobulin G (IgG) was affinity purified from 500 μl of heat-inactivated patient serum by protein G columns (MAb Trap GII kit; Amersham-Pharmacia-Biotech, Freiburg, Germany). Five fractions were collected after elution, and aliquots were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) together with an aliquot of diluted patient serum to determine the quality and the relative amount of purified IgGs. One hundred microliters of IgG solution (0.71 mg of protein/ml) was digested with papain for 2 h at 37°C to obtain Fab fragments. After purification by Sepharose G columns to remove the Fc parts, an aliquot of the Fab fragments was subjected to SDS-PAGE to determine the quality and relative amount compared to those for IgG in the IgG fractions and in serum.
Neutralization studies.
Neutralizing activities of the 10 patients' sera against the autologous virus isolates were determined on primary monocytes/macrophages and lymphocytes derived from the same donor. Experiments were repeated for all 10 serum-virus pairs on macrophages and lymphocytes derived from two or three additional donors. All sera were heat inactivated at 57°C for 30 min to inactivate virus and complement factors. Neutralization with HIV-positive sera relative to that with HIV-negative sera was defined by the formula percent neutralization = (1 − p/n) × 100, where p is the mean amount of virus produced in cultures incubated with HIV-positive serum and n is the mean amount of virus produced in cultures incubated with HIV-negative serum.
Neutralization on macrophages.
PBMCs from the HIV-negative donors were isolated by Ficoll gradients and adjusted to 4.5 × 106 cells/ml in macrophage medium (see medium of mixed-culture system). For each serum-virus pair, 200 μl of cell suspension was added to each of 4 wells of a 96-well plate and incubated at 37°C for 30 min. Nonadherent cells were removed by intensive washing, and the adherent cells were cultured in 200 μl of macrophage medium for 7 days at 37°C and 5% CO2 to allow differentiation into macrophages. On day 8, 100 μl of supernatant was removed. Virus stocks were pelleted and resuspended in macrophage medium containing 20% HIV-positive serum of the corresponding patient at a concentration of 400 TCID50/ml. This virus-serum solution was preincubated for 30 min at 37°C before 100 μl was added to each of the four wells, leading to a final virus concentration of 200 TCID50/ml and a final patient serum concentration of 10%. After 2 days, virus was quantitatively removed by several washings, and 4 days later, cultures were monitored for p24 antigen production. Reductions in mean p24 antigen amounts (over the four wells) by more than 50% compared to the positive control (incubation of virus with HIV-negative sera) at 10% patient serum concentration were considered neutralization activities. In this case, increasing dilutions (two- to fourfold) of patients' sera were tested to determine 50% neutralization titers. Dilutions were made in HIV-negative serum in order to keep the serum concentration constant at 10%.
To prove that neutralization on macrophages is due to antibodies in the sera, we also performed neutralization assays with affinity-purified IgG and the corresponding Fab fragments for one patient. The amounts of IgG and Fab fragments used in the neutralization assays were adjusted to the amount of IgG in 10% patient serum as estimated by SDS-PAGE. Neutralization assays with the patient's serum, the corresponding IgGs, and Fab fragments were performed as described above on macrophages from the same donor in quadruplicate.
Neutralization on lymphocytes.
Virus stock (100 TCID50/ml) was incubated in PBL medium containing the corresponding patient serum (10%) for 30 min at 37°C. PHA-stimulated primary PBLs (4.5 × 106 cells/ml) from a single donor were infected with 100 TCID50 of the virus-serum solution/ml in a 2-ml Eppendorf cup rotated for 3 h at 37°C. Cells were pelleted and washed carefully to remove the virus. For each virus cells were adjusted to 106 cells/ml in PBL medium and 200 μl of suspension was seeded into each of 6 wells of a 96-well plate. After 10 days at 37°C and 5% CO2 (without medium change) the p24 amount was measured. Reduction in mean p24 antigen amounts (over the six wells) by more than 50% compared to that for the positive control (incubation of virus with HIV-negative sera) at 10% patient serum concentration were considered neutralization activities. In this case, increasing dilutions (two- to fourfold) of patients' sera were tested to determine 50% neutralization titers. Dilutions were made in HIV-negative serum in order to keep the serum concentration constant at 10%.
Sequence analysis.
Sequence analysis of about 300 bp of the V3 region was performed by direct sequencing of PCR products as described previously (16). PCR products were derived either from DNA of PBMCs and mixed cultures or, after RT, from viral supernatants of mixed cultures, PHA-stimulated lymphocytes, or macrophages. RT conditions were as follows: 50 U of Moloney murine leukemia virus reverse transcriptase, 1 μg of random hexamer primers, 0.5 mM deoxynucleoside triphosphates, and 5 mM MgCl2 in 100 mM Tris-HCl–500 mM KCl for 60 min at 37°C. Whole gp120 sequences were determined on multiple clones derived from culture supernatants of macrophages and lymphocytes. Sequences were evaluated on an A.L.F. automated sequencing device (Amersham-Pharmacia-Biotech).
RESULTS
Patients.
The clinical and virological characteristics of the patients are summarized in Table 1. Ten German patients, recently infected with HIV type 1 (HIV-1) heterosexually or homosexually, were included in this study (10). All samples were collected within 2 to 8 months after infection as determined by clinical data and incomplete Western blot patterns characteristic for seroconvertors. All patients were therapy naive at the first time point of sampling. Viral load was between 2.2 × 103 and 6.5 × 105 copies per ml of plasma by the Chiron assay. Viral subtypes were determined by V3-based differential serotyping (16) complemented by direct sequencing of about 300 bp including the V3 region. In all cases, the results of serotyping and genotyping were congruent. There were a total of six infections with HIV-1 subtype B, three with subtype E, and one with subtype C.
Characterization of primary virus isolates.
In contrast to current protocols for virus isolation (34), viruses were isolated in mixed cultures (53) in this study in order to avoid selection for one of the two cell types. These cultures contained nonactivated PBLs as well as monocytes/macrophages. To minimize in vitro adaptation, the cultivation time was limited to 4 to 5 weeks including the generation of virus stocks. Thus, in order to stay as close as possible to the in vivo situation, relatively low virus titers had to be dealt with due to the nature of early isolates (34) (Table 2). Infection and neutralization assays were optimized for these low-titer primary virus stocks (see Materials and Methods).
TABLE 2.
Characteristics of patients' virus isolates
| Subtype | Code | RT activity (104 cpm/ml/90 min) | p24 antigen concn (ng/ml) | TCID50 (log10/ml) | Phenotype |
|---|---|---|---|---|---|
| B | HR002 | 11.5 ± 1.2 | 31.3 ± 1.4 | 3.3 ± 0.4 | NSI |
| B | HR003 | 27.8 ± 1.7 | 64.0 ± 7.4 | 2.7 ± 0.3 | NSI |
| B | HR005 | 6.7 ± 0.2 | 18.9 ± 4.2 | 3.1 ± 0.3 | NSI |
| B | HR010 | 18.0 ± 1.1 | 33.3 ± 1.9 | 3.0 ± 0.3 | SI |
| B | HR011 | 12.9 ± 1.7 | 40.3 ± 3.1 | 2.4 ± 0.2 | NSI |
| B | HR014 | 8.7 ± 0.5 | 43.6 ± 2.1 | 3.1 ± 0.3 | NSI |
| C | HR001 | 12.6 ± 0.5 | 19.5 ± 1.2 | 2.7 ± 0.3 | NSI |
| E | HR004 | 10.7 ± 1.2 | 8.3 ± 1.1 | 3.2 ± 0.3 | NSI |
| E | HR008 | 10.1 ± 0.3 | 10.6 ± 1.8 | 1.7 ± 0.3 | NSI |
| E | HR009 | 8.0 ± 0.2 | 4.3 ± 0.5 | 2.1 ± 0.2 | NSI |
The stock characteristics are summarized in Table 2. Nine of the 10 virus isolates were of the NSI phenotype, and 1 was SI. The SI isolate, HR010, had a V3 loop sequence typical for SI viruses, i.e., an arginine at position 11 and high positive charge (+6).
The TCID50s ranged from 2.1 to 3.3 log10 units/ml. There was no correlation between the number of infectious particles in the virus stocks (TCID50s) and either the RT activity or the amount of p24 antigen, both of which measure infectious and noninfectious virus particles. In all non-B isolates the amounts of p24 antigen measured were smaller than those in subtype B isolates due to the reduced sensitivity of the p24 antigen assay for non-B isolates (data not shown).
Replicative capacities of the primary virus isolates on lymphocytes and monocytes/macrophages.
All primary virus isolates of this study, irrespective of the subtype, productively infected primary macrophages as well as lymphocytes. This is in accordance with the amino acid sequences of the corresponding V3 loops, as described for dualtropic viruses by Westervelt et al. (55) and Shioda et al. (39), having either histidine, threonine, or asparagine at position 13, tyrosine at position 21, and glutamic acid, aspartic acid, or alanine at position 25 (data not shown).
The replicative capacity, measured by p24 antigen production, on primary macrophages and lymphocytes derived from four different donors was analyzed. Although for a given isolate p24 antigen production differed from donor to donor, in general, all isolates replicated better on lymphocytes than on macrophages. For lymphocytes, p24 production was in the range of 1 to 50 ng/ml depending on the donor and the isolate. In contrast, for macrophages the lowest value was 10 pg/ml and the highest was 1,000 pg/ml. However, isolates HR011 and HR014 reached equivalent p24 antigen amounts on both cell types. Three classes of replicative activity could be defined on both cell types, irrespective of the donor variations observed (Tables 3 and 4).
TABLE 3.
Replicative capacity of primary virus isolates on lymphocytesa
| Subtype | Isolates whose replicative capacity is:
|
||
|---|---|---|---|
| High (>10.0 ng/ml) | Medium (1.0–10.0 ng/ml) | Low (<1.0 ng/ml) | |
| B | HR010 | HR002, HR003 | HR005, HR011, HR014 |
| C | HR001 | ||
| E | HR004 | HR008, HR009 | |
Viral isolates were grouped into three classes according to the mean p24 antigen production on cells from four different donors.
TABLE 4.
Replicative capacity of primary virus isolates on macrophagesa
| Subtype | Isolates whose replicative capacity is:
|
||
|---|---|---|---|
| High (>0.2 ng/ml) | Medium (0.1–0.2 ng/ml) | Low (<0.1 ng/ml) | |
| B | HR010, HR011, HR014 | HR003 | HR002, HR005 |
| C | HR001 | ||
| E | HR008 | HR004, HR009 | |
Viral isolates were grouped into the three classes according to the mean p24 antigen production on cells from four different donors.
Neutralization of primary virus isolates by autologous serum samples on macrophages and lymphocytes.
Serum samples were taken shortly after seroconversion (Table 1) at the time point of virus isolation. For some patients additional serum samples could be taken about 12 months after infection. Neutralization activities of all sera against the autologous virus isolates were determined for both cell types.
First serum samples taken shortly after seroconversion.
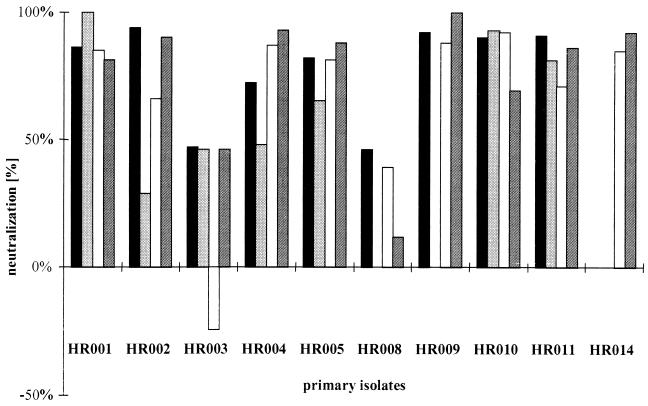
For macrophages, the neutralizing activity of the sera toward the autologous virus isolates was determined on quadruplicate macrophage cultures derived from four different donors. By using 10% serum, neutralization activities between 80 and 100% were found for 8 of the 10 isolates (Fig. 1). For the remaining two sera, neutralizing activities were close to 50% on macrophages from three and two out of four donors.
FIG. 1.
Neutralization activity of 10% serum against the autologous virus isolate from the first time point analyzed on primary macrophages derived from four different donors. Each bar represents neutralization activity on cells from one donor.
In order to quantitate the neutralizing activities, further dilutions of sera were tested and 50% neutralization titers were determined (Fig. 2). For the eight sera with >50% neutralizing activity, these ranged from 21 to 284.
FIG. 2.
Neutralization titers of early serum samples against the autologous virus isolates from the first time point on primary macrophages. Each bar represents the mean neutralization titer on cells from two different donors.
For control, neutralization studies on primary lymphocytes were also performed. In contrast to the results for macrophages, no neutralization activities (titer <10) could be demonstrated for any of the isolates using sera from the early time points (Table 5). This is in accordance with published studies showing no neutralization of primary virus isolates on lymphocytes before 1 year after infection.
TABLE 5.
Comparison of 50% neutralization titers of the first and second serum samples on lymphocytes and macrophages
| Virus isolate | 50% neutralization titer of:
|
|||
|---|---|---|---|---|
| First serum on:
|
Second serum on:
|
|||
| Lymphocytes | Macrophages | Lymphocytes | Macrophages | |
| Subtype B | ||||
| HR002 | <10 | 30 | >640 | 535 |
| HR003 | <10 | <10 | —a | — |
| HR005 | <10 | 26 | 85 | 796 |
| HR010 | <10 | 21 | <10 | 463 |
| HR011 | <10 | 284 | — | — |
| HR014 | <10 | 49 | >640 | 3,847 |
| Subtype C | ||||
| HR001 | <10 | 34 | 16 | 199 |
| Subtype E | ||||
| HR004 | <10 | 25 | 50 | 237 |
| HR008 | <10 | <10 | — | — |
| HR009 | <10 | 206 | <10 | 73 |
—, serum samples not available.
Second serum samples taken about 12 months after infection.
For seven patients a second serum sample was taken about 12 months after infection. Studies of the neutralization activity of these sera against the autologous virus isolates from the early time points were performed on primary macrophages and lymphocytes as described above. On macrophages, neutralization titers increased 6- to 30-fold compared to those for the first serum sample (Table 5). For HR014, a neutralization titer of nearly 4,000 was achieved. There was only one exception, HR009, where the neutralization titers decreased about threefold.
In contrast to the first serum samples, five of the seven follow-up sera showed neutralization activities against the early virus isolates also on lymphocytes. However, in general, neutralization titers of the second serum samples were lower on lymphocytes than on macrophages (Table 5).
Are there different virus subpopulations infecting macrophages and lymphocytes?
The differences in neutralization activities found on macrophages and lymphocytes could be explained if different virus variants of a patient's quasispecies were infecting both cell types. To address this question, viral supernatants from two different macrophage cultures and two different lymphocyte cultures were analyzed genetically for patients HR003 (subtype B, NSI), HR004 (subtype E, NSI), HR010 (subtype B, SI), and HR011 (subtype B, NSI). In order to avoid contamination by input virus, the first supernatant was carefully removed and, after several washing steps, newly produced viruses in the second supernatant were obtained for genetic analysis. About 300 bp including the immunodominant V3 region were sequenced directly, and sequences were compared to the sequences of the respective viral stocks generated in mixed cultures. No differences between the V3 sequences derived from lymphocyte and macrophage cultures from the same patient at this early timepoint were found. Also, sequence data of the entire gp120 derived from the same cultures demonstrate extensive homogeneity (data not shown). Thus, neutralization differences found on macrophages and lymphocytes are unlikely to be due to different virus variants present in these cells.
Factors determining neutralization activities in sera.
HIV-positive sera contain antibodies against the virus, but β-chemokines also are known to have inhibitory effects on virus replication (7). Principally, both mechanisms could account for neutralizing effects.
(i) β-chemokines.
Due to limiting amounts of sera, we determined only the concentrations of the two β-chemokines MIP-1β and RANTES in all serum samples by commercial ELISAs.
The MIP-1β concentrations were between 19 and 47 pg/ml in the first serum samples (for the individual sera the concentrations [picograms per milliliter] were as follows: HR001, 19; HR002, 41; HR003, 40; HR004, 10; HR005, 47; HR008, 35; HR009, 33; HR010, 25; HR011, 46; HR014, 35), i.e., in the range of 21 to 100 pg/ml measured for HIV-negative sera from healthy donors, which were always used as positive controls in the neutralization assays. This excludes MIP-1β as a factor responsible for the neutralization activities observed. In the second serum samples, the MIP-1β concentrations were not elevated significantly, ranging from 27 to 157 pg/ml.
For RANTES, the concentrations were between 8.5 and 19.7 ng/ml in the first serum samples (the concentrations [nanograms per milliliter] were as follows: HR001, 11.2; HR002, 17.2; HR003, 12.9; HR004, 9.0; HR005, 11.4; HR008, 15.5; HR009, 19.7; HR010, 8.5; HR011, 17.8; HR014, 15.0). The concentrations of RANTES in HIV-negative sera from healthy donors were around 0.03 to 0.68 ng/ml. Thus, the RANTES concentration was significantly higher in HIV-positive sera than in HIV-negative sera, suggesting that RANTES could be a potential candidate for the neutralizing activities in the sera. However, there was no increase in RANTES concentration from the first to the second serum samples (range, 7.3 to 21.0 ng/ml), but there was increasing neutralizing activity. Therefore, neutralizing activities in the sera did not correlate with the concentrations of RANTES, which suggests that RANTES is not responsible for the neutralizing effects observed. Furthermore, chemokine concentrations in the early serum samples did not correlate with the 50% neutralizing titers of the corresponding sera as measured on macrophages. For example, the sera of HR008 and HR009 have similar levels of chemokines MIP-1β and RANTES, but serum HR008 does not show neutralization on macrophages (titer, <10), whereas HR009 has a 50% neutralizing titer of 206.
(ii) Antibodies.
In order to analyze the role of serum IgGs in virus neutralization, neutralization studies were performed with IgGs affinity purified from HIV-positive serum. As the amount of sera from the patients in this study was limited and had to be used for the assays of comparative neutralization on macrophages and lymphocytes, we selected an additional patient to address this question.
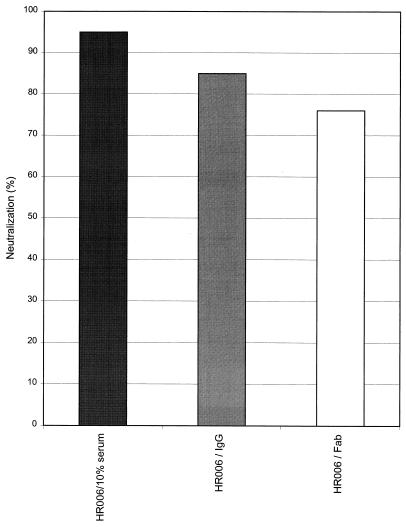
Patient HR006 was infected in November 1994 by homosexual exposure, and the first blood sample was obtained 7 months later. Like the other patients, HR006 was therapy naive at the time point of sampling. Virus isolation was performed in mixed cultures as described for the other patients. The virus isolate was dualtropic, NSI, and genetically HIV-1 subtype B. As described for the 10 patients analyzed above, the early serum sample of patient HR006 neutralized the autologous virus isolate on macrophages from different donors by more than 95% compared to the negative serum control, but virus was not neutralized on lymphocytes. We purified IgGs from the patient's serum by affinity chromatography and, in addition, Fab fragments were prepared from part of the IgG fractions. Parallel neutralization studies were performed with 10% serum as well as with the IgG and Fab fragment preparations on macrophages from the same donor. The amounts of IgG present in the serum and in the affinity-purified IgG preparation were estimated by SDS-PAGE and by titration in a V3 subtype B consensus peptide ELISA (16). Based on this, for the neutralization assays the amounts of IgG were adjusted so that serum and the IgG fraction contained similar quantities of IgG. As shown in Fig. 3, 10% serum, the affinity-purified IgGs, and the corresponding Fab fragments neutralized the autologous virus isolate (HR006) on macrophages. Furthermore, the extents of neutralization were in a similar range (95% for 10% serum; 85% for the affinity-purified IgG), suggesting that neutralizing antibodies in the serum are indeed responsible for neutralization.
FIG. 3.
Neutralizing activity of 10% serum of patient HR006 and the corresponding affinity-purified IgGs and Fab fragments against the autologous virus isolate on macrophages. Each bar represents the mean neutralization activity of six wells. Experiments were performed on cells from the same donor.
DISCUSSION
Difficulties imposed by primary virus isolates.
In this study, we used primary HIV-1 isolates to compare the neutralization activities of autologous serum samples obtained shortly after seroconversion on different primary target cells. In order to avoid in vitro selection and/or adaptation during virus isolation, we used mixed-culture systems including primary unactivated lymphocytes and monocytes/macrophages (53) and short cocultivation times. Titers of most viral stocks were low (Table 2) and needed to be compensated for by optimized in vitro infectivity assays.
Donor dependency is a critical issue to be considered when comparing biological properties of different virus isolates (5, 45). We determined replication rates as well as neutralization activities on cells from two to four different donors, in four to six parallel cultures each, to correct for these biological variations. Although biological variations were sometimes large for a given isolate, we were able to distinguish isolates with low, medium, and high replication capacities on lymphocytes as well as on macrophages (Tables 3 and 4).
Replication of primary virus isolates on primary lymphocytes and macrophages.
All primary virus isolates could productively infect primary lymphocytes as well as macrophages, independently of the SI or NSI phenotype. As different conditions for infection had to be used on lymphocytes and macrophages, replication rates in terms of absolute amounts of p24 produced by both culture systems cannot be compared directly, even when cells of the same donor were used. Generally, the replication rate was higher in lymphocytes than in macrophages, although two isolates, HR011 and HR014, produced similar p24 antigen amounts on both cell types.
In previous work primary HIV-1 isolates were classified according to their replicative capacities in lymphocytes (rapid/high and slow/low [14] or “a” to “d” [54]) and macrophages (α to δ [54]). According to this classification, our low-passage early primary virus isolates all belong into the slow/low or d/δ group. Nevertheless, we could still discern isolates with high, medium, and low replication capacities on lymphocytes and to a lesser degree on macrophages (Tables 3 and 4). As this characteristic was independent of the donor cells used, it might be a phenotypic property of the respective viral isolates themselves. Thus, early primary virus isolates from different individuals differ in their intrinsic replication capacities on lymphocytes. On macrophages, the same isolates lead to less-pronounced replication differences.
Neutralization of primary virus isolates by autologous serum samples on different target cells.
In accordance with published studies (2, 30, 31) in which neutralization of primary HIV-1 isolates by autologous serum samples shortly after seroconversion was studied, we did not find neutralizing activities before 10 to 14 months after infection, using primary lymphocytes as target cells. In contrast, when using the same isolates with primary macrophages as target cells, we found high levels of neutralizing activities in the same sera as early as 2 months after infection. Recently, Zhuge et al. reported similar results with plasma from macaques experimentally infected with macaque simian immunodeficiency virus (SIVmac) (59). Also, Stamatatos et al. reported that macrophages are more sensitive to neutralization than lymphocytes, when monoclonal antibodies are used as neutralizing agents (46). That neutralization activities depend on the target cells used in the assays could also be demonstrated for feline immunodeficiency virus (1).
The fact that neutralization depends on the target cells used might be explained by different virus variants from the quasispecies infecting macrophages or lymphocytes. However, sequence analyses of gp120 derived from culture supernatants of infected macrophages and lymphocytes showed extreme sequence conservation. We conclude that it is essentially the same virus variant which infects both target cells. Similar conclusions were drawn by Simmons et al., who showed that biological clones of HIV could infect primary macrophages as well as lymphocytes (42). Thus, differences in neutralization are not likely to be due to different virus variants infecting both cell types.
In our study, the neutralizing effects of the serum samples could principally be attributed to inhibitory β-chemokines or to antibodies specific for the virus. However, the concentration of the β-chemokine MIP-1β in the sera was too low to be responsible for any of the inhibitory effects observed. The concentration of RANTES was increased compared to that for HIV-negative individuals. However, different groups showed that RANTES, even at concentrations of 50 ng/ml, could not inhibit the infection of macrophages by HIV, although the corresponding chemokine receptor, CCR5, is expressed on these cells (38, 41, 48). Furthermore, the concentrations of RANTES among the different sera were comparable (8.5 to 19.7 ng/ml) and thus did not correlate with the observed differences in neutralizing activities on macrophages. Also, in the second serum samples an increase in neutralizing activities on macrophages was not paralleled by increasing RANTES concentrations (7.3 to 21.0 ng/ml).
Finally, since an affinity-purified IgG preparation and Fab fragments from serum of patient HR006 were able to neutralize the respective autologous virus isolate in a range similar to that for 10% serum, the neutralizing effects observed on macrophages can be attributed to antibodies. We therefore conclude that neutralizing antibodies are indeed present in patients' sera from very early on and are detectable if primary macrophages are used, instead of lymphocytes, as target cells for neutralization assays.
As mentioned above, Zhuge et al. also found neutralizing activities against the autologous virus isolates in early plasma samples from experimentally infected macaques when using primary simian macrophages as target cells (59). No neutralization activities on lymphocytes were found. However, in this study, neutralizing activities on macrophages depended on the continuous presence of plasma in the culture supernatant. Removing plasma resulted in virus production comparable to that of cultures infected with virus in the absence of plasma. The authors concluded that the neutralizing effect is due to a postentry step. In our study, serum was only present at the time point of infection and was subsequently completely removed together with the virus by vigorous washing. In contrast to findings of the study of Zhuge et al., virus production, measured as the amount of p24 antigen on days 4 and 8 after serum removal, did not increase in our study and always remained far below the level of the control (incubated with HIV-negative serum). Our results are therefore compatible with neutralizing activities at the level of virus entry, as would primarily be expected for antibodies, while the discrepancy with the conclusion by Zhuge et al. remains unresolved.
Based on previous considerations, neutralizing antibodies might be directed against viral epitopes which are necessary for the infection of macrophages (macrophage-tropic epitopes [MTE]) but which are not necessary for the infection of lymphocytes (lymphotropic epitopes [LTE]). Obviously, the mode of infection differs between macrophages and lymphocytes. Interestingly, β-chemokines RANTES, MIP-1α, and MIP-1β are able to inhibit the infection of lymphocytes, whereas inhibition of macrophages by β-chemokines is controversial (32, 38, 39). This also may indicate that virus entry is different in both cell types. Different coreceptors may be used on macrophages and lymphocytes. Early primary HIV-1 isolates usually utilize the CCR5 receptor for virus entry (6, 11). Although CCR5 is expressed on both primary lymphocytes and primary macrophages (3, 26), the active receptor forms used for virus entry may differ on different cells (18, 33). Alternatively, CCR5 may undergo different posttranslational modifications (13) in macrophages and lymphocytes. In both cases, different epitopes on the viral surface (MTE, LTE) can be postulated for CCR5 binding. This requires further investigation.
Interestingly, we could observe a certain association between neutralization titers on macrophages and viral load in the patients. As shown in Table 6, the highest neutralization titers (HR011 and HR009) correlated with the lowest viral load values. If neutralization titers were reduced by a factor of 7 to 10, viral load was increased about 10-fold in five of six patients (HR002, HR005, HR010, HR001, HR004). Neutralization titers below 10 correlated with very high viral load in patient HR003; however, in patient HR008 viral load was only 18,500 copies/ml of plasma. Thus, in 8 out of 10 patients there was good association between neutralization titers determined on macrophages and the viral load data in the respective serum samples. One explanation could be the observation by Tsai et al. that viruses released by macrophages are more infectious than viruses coming out of lymphocytes (49). These more-infectious viruses can then infect more target cells, and thus macrophages would indirectly influence the viral load in the patients. Consequently, neutralizing HIV on macrophages should result in a pronounced reduction in the viral load, as observed in 8 out of the 10 patients.
TABLE 6.
Relationship between neutralization titers of the early serum samples on macrophages and viral load in the patients
| Isolate | Viral load (103 copies/ml) | 50% neutralization titer of 1st serum sample on macrophages |
|---|---|---|
| Subtype B | ||
| HR002 | 19.4 | 30 |
| HR003 | 650.5 | 0 |
| HR005 | 19.6 | 26 |
| HR010 | 31.8 | 21 |
| HR011 | 2.2 | 284 |
| HR014 | 70.2 | 49 |
| Subtype C | ||
| HR001 | 42.2 | 34 |
| Subtype E | ||
| HR004 | 9.3 | 25 |
| HR008 | 18.5 | 0 |
| HR009 | 4.1 | 206 |
In summary, we could show for the first time that neutralizing antibodies are present in patients' sera shortly after seroconversion. So far, the role of humoral immunity in early virus control has been questioned, as neutralizing antibodies could not be detected before approximately 1 year after infection. However, these conclusions were drawn from studies using lymphocytes as target cells. We could now demonstrate by using primary macrophages as target cells that neutralizing antibodies are present much earlier. Thus, besides CTL activity, humoral immunity defined on macrophages may very well contribute to the early control of viremia in the patient. Therefore, future vaccines should include antigens which are able to induce neutralizing activity against HIV on primary macrophages.
ACKNOWLEDGMENTS
We especially acknowledge the help of the clinicians Heribert Knechten (Aachen) and Hans Jäger (Munich), who provided the clinical material analyzed in this study. Peter Müller from the Paul-Ehrlich-Institute (Langen) helped with measurements of β-chemokines. We thank Jolanta Juraszczyk, Margot Landersz, and Karin Becker-Peters for expert technical assistance. We thank Rolf Eckhardt (Coulter Immunotech Diagnostics, Germany) for special support regarding p24 antigen assays.
This work was supported by a grant from the Bundesministerium für Bildung und Forschung to U.D. (BMBF 01KI9408). The Georg-Speyer-Haus is supported by the Bundesministerium für Gesundheit and the Hessisches Ministerium für Wissenschaft und Kunst.
REFERENCES
- 1.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binley J M, Klasse P J, Cao Y Z, Jones I, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang J, Naif H M, Li S, Sullivan J S, Randle C M, Cunningham A L. Twin studies demonstrate a host cell genetic effect on productive human immunodeficiency virus infection of human monocytes and macrophages in vitro. J Virol. 1996;70:7792–7803. doi: 10.1128/jvi.70.11.7792-7803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Daar E S, Moudgil T, Meyer A D, Ho D D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 9.Delwart E L, Sheppard H W, Walker B D, Goudsmit J, Mullins J I. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J Virol. 1994;68:6672–6683. doi: 10.1128/jvi.68.10.6672-6683.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich U, Ruppach H, Gehring S, Knechten H, Knickmann M, Jäger H, Wolf E, Husak R, Orfanos C E, Brede H D, Rübsamen-Waigmann H, von Briesen H. Large proportion of non-B HIV-1 subtypes and presence of zidovudine resistance mutations among German seroconverters. AIDS. 1997;11:1532–1533. [PubMed] [Google Scholar]
- 11.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peoper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 12.Esser R, Glienke W, von Briesen H, Rübsamen-Waigmann H, Andreesen R. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with HIV. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- 13.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 14.Fenyö E M, Morfeldt-Manson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Asjo B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartner S, Markovitz P, Markovitz D M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 16.Gehring S, Maayan S, Ruppach H, Balfe P, Juraszczyk J, Yust I, Vardinon N, Rimlawi A, Polak S, Bentwich Z, Rübsamen-Waigmann H, Dietrich U. Molecular epidemiology of HIV in Israel. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:296–303. doi: 10.1097/00042560-199708010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gendelman H E, Orenstein J M, Baca L M, Weiser B, Burger H, Kalter D C, Meltzer M S. The macrophage in the persistence and pathogenesis of HIV-1 infection. AIDS. 1989;3:475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hill C M, Kwon D, Jones M, Davis C B, Marmon S, Daugherty B L, DeMartino J A, Springer M S, Unutmaz D, Littman D R. The amino terminus of human CCR5 is required for its function as receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology. 1998;248:357–371. doi: 10.1006/viro.1998.9283. [DOI] [PubMed] [Google Scholar]
- 19.Housset C, Boucher O, Girard P M, Leibowitch J, Saimot A G, Brechot C, Marche C. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol. 1990;21:404–408. doi: 10.1016/0046-8177(90)90202-g. [DOI] [PubMed] [Google Scholar]
- 20.Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol. 1931;152:380. [Google Scholar]
- 21.Karlsson A, Parsmyr K, Sandström E, Fenyö E M, Albert J. MT-2 cell tropism as a prognostic marker for disease progression in HIV-1 infection. J Clin Microbiol. 1994;32:364–370. doi: 10.1128/jcm.32.2.364-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig S, Gendelman H E, Orenstein J M, dal Canto M C, Pezeshkpour G H, Yungbluth M, Janota R, Aksamed A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 23.Koup R A, Safrit J T, Cao Y Z, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kühnel H, von Briesen H, Dietrich U, Adamski M, Mix D, Biesert L, Kreutz R, Immelmann A, Henco K, Meichsner C, Andreesen R, Gelderblom H, Rübsamen-Waigmann H. Molecular cloning of two West African human immunodeficiency virus type 2 isolates that replicate well on macrophages: a Gambian isolate, from a patient with neurologic acquired immunodeficiency syndrome and a highly divergent Ghanian isolate. Proc Natl Acad Sci USA. 1989;86:2383–2387. doi: 10.1073/pnas.86.7.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackewitz C E, Yang L C, Lifson J D, Levy J A. Non cytolytic CD8 T-cell anti-HIV responses in primary HIV-1 infection. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 26.Marzio P D, Tse J, Landau N R. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retrovir. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 27.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 28.Meyerhans A, Cheynier R, Albert J, Seth M, Kwok S, Sninsky J, Morfeldt-Manson L, Asjo B, Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolates. Cell. 1989;58:901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- 29.Milman G, Sharma O. Mechanisms of HIV/SIV mucosal transmission. AIDS Res Hum Retovir. 1994;10:1305–1312. doi: 10.1089/aid.1994.10.1305. [DOI] [PubMed] [Google Scholar]
- 30.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore J P, Cao Y Z, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriuchi H, Moriuchi M, Combadiere C, Murphy P, Fauci A S. CD8+ T-cell derived soluble factors, but not β-chemokines RANTES, MIP-1α and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson W C, Rabut G E E, Nagashima K A, Tran D N H, Anselma D J, Monard S P, Segal J P, Thompson D A D, Kajumo F, Guo Y, Moore J P, Maddon P J, Dragic T. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73:4145–4155. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rübsamen-Waigmann H, von Briesen H, Holmes H, Bjorndal A, Korber B, Esser R, Ranjbar S, Tomlinson P, Galvao-Castro B, Karita E, et al. Standard conditions of virus isolation reveal biological variability of HIV type 1 in different regions of the world. AIDS Res Hum Retrovir. 1994;10:1401–1408. doi: 10.1089/aid.1994.10.1401. [DOI] [PubMed] [Google Scholar]
- 35.Rübsamen-Waigmann H, Willems W R, Bertram U, von Briesen H. Reversal of HIV-phenotype to fulminant replication on macrophages in perinatal transmission. Lancet. 1989;ii:1155–1156. doi: 10.1016/s0140-6736(89)91518-3. [DOI] [PubMed] [Google Scholar]
- 36.Rübsamen-Waigmann H, Becker W B, Helm E B, Brodt R, Fischer H, Henco K, Brede H D. Isolation of variants of lymphocytopathic retroviruses from the peripheral blood and cerebrospinal fluid of patients with ARC or AIDS. J Med Virol. 1986;19:335–344. doi: 10.1002/jmv.1890190406. [DOI] [PubMed] [Google Scholar]
- 37.Sattentau Q J. Neutralization of HIV-1 by antibody. Curr Opin Immunol. 1996;8:540–545. doi: 10.1016/s0952-7915(96)80044-6. [DOI] [PubMed] [Google Scholar]
- 38.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Dend H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyö E M, Lusso P. In vivo evolution of HIV-1 coreceptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 39.Shioda T, Levy J A, Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;15:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shpaer E G, Delwart E L, Kuiken C L, Goudsmit J, Bachmann M H, Mullins J. Conserved V3 loop sequences and transmission of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1994;10:1679–1684. doi: 10.1089/aid.1994.10.1679. [DOI] [PubMed] [Google Scholar]
- 41.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 42.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith P D, Meng G, Shaw G M, Li L. Infection of gastrointestinal tract macrophages by HIV-1. J Leukoc Biol. 1997;62:72–77. doi: 10.1002/jlb.62.1.72. [DOI] [PubMed] [Google Scholar]
- 44.Spearman C. The method of “right or wrong cases” (constant stimuli) without Gauss's formulae. Br J Psychol. 1908;2:227. [Google Scholar]
- 45.Spira A I, Ho D D. Effect of different donor cells on human immunodeficiency virus type 1 replication and selection in vitro. J Virol. 1995;69:422–429. doi: 10.1128/jvi.69.1.422-429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatatos L, Zolla-Pazner S, Gorny M K, Cheng-Mayer C. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology. 1997;229:360–369. doi: 10.1006/viro.1997.8443. [DOI] [PubMed] [Google Scholar]
- 47.Toossi Z, Nicolacakis K, Xia L, Ferrari N A, Rich E A. Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-1 infected individuals in vitro. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:325–331. doi: 10.1097/00042560-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 48.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai W P, Conley S R, Kung H F, Garrity R R, Nara P L. Preliminary in vitro growth cycle and transmission studies of HIV-1 in an autologous primary cell assay of blood-derived macrophages and peripheral blood mononuclear cells. Virology. 1996;226:205–216. doi: 10.1006/viro.1996.0648. [DOI] [PubMed] [Google Scholar]
- 50.Valentin A, Albert J, Fenyoe E M, Asjo B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Briesen H, Grez M, Ruppach H, Raudonat I, Unger R E, Becker K, Panhans B, Dietrich U, Rübsamen-Waigmann H. Selection of HIV-1 genotypes by cultivation in different primary cells. AIDS. 1999;13:307–315. doi: 10.1097/00002030-199902250-00002. [DOI] [PubMed] [Google Scholar]
- 53.von Briesen H, Andreesen R, Esser R, Bruger W, Meichsner C, Becker K, Rübsamen-Waigmann H. Infection of monocytes/macrophages by HIV in vitro. Res Virol. 1990;141:225–231. doi: 10.1016/0923-2516(90)90025-e. [DOI] [PubMed] [Google Scholar]
- 54.von Briesen H, Andressen R, Rübsamen-Waigmann H. Systematic classification of HIV biological subtypes on lymphocytes and monocytes/macrophages. Virology. 1990;178:597–602. doi: 10.1016/0042-6822(90)90361-t. [DOI] [PubMed] [Google Scholar]
- 55.Westervelt P, Trowbridge D B, Epstein L G, Blumberg B M, Li Y, Hahn B H, Shaw G M, Price R W, Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992;66:2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolinsky S M, Korber B T, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 57.Yoo J, Chen H, Krais T, Hirsch D, Plyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 58.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 59.Zhuge W, Jia F, Adany I, Narayan O, Stephens E B. Plasmas from lymphocyte- and macrophage-tropic SIVmac-infected macaques have antibodies with a broader spectrum of virus neutralization activity in macrophage versus lymphocyte cultures. Virology. 1997;227:24–33. doi: 10.1006/viro.1996.8300. [DOI] [PubMed] [Google Scholar]