Abstract
The mode and the site of action of the major antiscrapie drugs have been studied by investigating their effects on the abnormal protease-resistant isoform of PrP (PrPres) and on its accumulation in mouse spleen. Day-by-day PrPres accumulation in the spleen and in other peripheral organs was first monitored to describe the early steps of scrapie pathogenesis. Three phases were identified: the detection of scrapie inoculum on the day of scrapie infection, a clearance phase, and then the peripheral accumulation of PrPres. In a second step, the effects of the polyene antibiotic MS-8209, the polyanion dextran sulfate 500 (DS500), and Congo red were assessed on these phases, after the drugs were coincubated with scrapie inoculum. Highly different mechanisms and sites of action were apparent. MS-8209 had a weak effect on the accumulation of PrPres in spleen, suggesting another site of intervention for this drug. DS500 delayed the beginning of the clearance phase but then blocked PrPres synthesis for a long period of time, probably because of its immunological effects on the spleen. Surprisingly, Congo red suppressed the clearance phase of scrapie inoculum and then increased transiently accumulation of PrPres in spleen. We showed in vitro that this effect was related to a direct enhancement of the protease resistance of PrPres by the drug.
Transmissible spongiform encephalopathies (TSE) are a group of neurodegenerative diseases, including scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease in humans. Experimental models of scrapie have demonstrated that a host-encoded protein, PrP, plays a central role in the pathogenesis of these diseases (8). Indeed, an abnormal protease-resistant isoform of PrP (PrPres) accumulates proportionally to infectivity in the central nervous system during the development of TSE (7, 45). Unlike normal PrP (PrPc), PrPres sediments in detergents such as N-lauroyl-sarcosine and can aggregate into fibrils, designated scrapie-associated fibrils (SAF) or prion rods (41, 47). Such a purification has enabled the specific detection of PrPres by immunoblotting in the central nervous system (49), thereby becoming a tool to diagnose TSE. The lymphoreticular system (LRS) and the spleen in particular are infectious long before neuroinvasion occurs in most experimental scrapie mouse models (22, 32, 33). Infectivity can be detected in the spleen as soon as 1 week after intraperitoneal infection of mice, increasing to 4 weeks, when a plateau is reached, until the terminal stage of the disease (32, 33). In addition, scrapie infectivity has been detected in liver and spleen a few minutes after peripheral infection due to the inoculum itself (42). PrPres has been detected soonest at 1 to 2 weeks after intraperitoneal or intracerebral infection with two different scrapie strains in the spleen, large amounts of tissue having been necessary for detection (26, 48).
To date, no effective anti-TSE therapy is available. However, some molecules have prolonged survival or cured a few animals in experimental models of scrapie and BSE (for a review, see reference 3). Among them are polyene antibiotics such as amphotericin B (44) and its more efficient derivative MS-8209 (1–4, 14, 15), polyanions such as dextran sulfate 500 (DS500), heteropolyanions (such as HPA23) or sulfated polyanions (such as SP54) (19, 21, 23, 31, 34), and Congo red (CR) (30). Polyene antibiotics and polyanions prolong the survival time of scrapie-infected rodents and transiently reduce brain infectivity and PrPres accumulation (1, 4, 14, 21, 44). In addition, sulfated polyanions and CR inhibit PrPres accumulation and infectivity in a model of scrapie-infected mouse neuroblastoma cells (11–13). These data indicate that these drugs probably interfere with the formation of PrPres. It has been proposed (10) that polyanions and CR, which are sulfated glycosaminoglycan analogues, could specifically inhibit PrPres accumulation by impairing the association of PrPres with endogenous glycosaminoglycans, the latter being necessary to PrP amyloid plaque formation in natural TSE and in scrapie-infected mice (39, 50). A more direct interaction of CR with PrPres has also been suggested because this drug binds to PrPres fibrils (47), and in vitro incubation of CR with PrPres seems to overstabilize the conformation of the protein, therefore impairing its template function for the formation of new PrPres molecules (9). The antiscrapie effect of these molecules may also depend on their site of action and thus could be limited during the course of scrapie infection. Indeed, polyanions and CR are only efficient when administered around the time of scrapie infection (21, 23, 30). Polyene antibiotics are also efficient at this time and are the only drugs exerting benefits when given later in the infection (3, 4, 15). Polyanions, due to their effects on the immune system, probably act via the LRS (21). The sites of action of polyene antibiotics and CR remain more obscure.
In the present study, we analyzed the spleen as the site where polyene antibiotics, polyanions, and CR may act on scrapie infection. We investigated whether these molecules interact directly with PrPres. For this purpose, the kinetics of PrPres accumulation in the mouse spleen was established starting from the day of scrapie agent inoculation. Modifications of the kinetics induced by the coincubation of the drugs with scrapie inoculum or by control treatments performed before or after scrapie infection were studied in parallel. Understanding both early peripheral TSE pathogenesis and therapeutic action of antiscrapie drugs has become more crucial because peripheral organs seem also to be involved in the development of new-variant Creutzfeldt-Jakob disease, the human counterpart of BSE (28, 29). We showed that MS-8209 weakly modified the kinetics of PrPres accumulation in spleen, suggesting another site of intervention for this drug. In contrast, DS500 was a powerful inhibitor of PrPres synthesis in this tissue. The most surprising results were obtained with CR, which transiently increased PrPres accumulation in spleen in our scrapie mouse model. We investigated this situation and found that CR in vitro directly enhanced the resistance of PrPres to protease digestion.
MATERIALS AND METHODS
Chemicals.
MS-8209 is the N-methyl glucamine (NMG) salt of 1-deoxy-1-amino-4,6,O-benzylidine-d-fructosyl-amphotericin B (Mayoly-Spindler Laboratories, Chatou, France). MS-8209 and NMG (Sigma) were suspended in a 5% (wt/vol) sterile glucose solution. The sodium salt DS500 (Pharmacia) was resuspended in a sterile 0.9% NaCl solution. Ninety-nine-percent-pure Congo red (Sigma) was dissolved in sterile distilled water. The most efficient antiscrapie effect with MS-8209 has been observed at the dose of 25 mg/kg of body weight (15). All drugs were injected at this dose by the intraperitoneal route.
PrPres detection in the LRS.
The mouse scrapie strain C506M3 (7.9 × 108 50% lethal dose/g of brain [36]) was obtained from brain homogenates of terminally ill animals. Eight-week-old C57BL/6 females (Centre d'Elevage R. Janvier, Le Genest-Saint-Isle, France) were intraperitoneally inoculated with 100 μl of a 2% (wt/vol) brain homogenate. Sacrifices were performed in triplicate at different hours and days postinoculation (dpi), by cervical column disruption. Various organs, including the spleen, pancreas, a part of the liver, and the thymus and salivary glands, were immediately removed, frozen in liquid nitrogen, and kept at −80°C until protein analysis. Some severely combined immunodeficient (SCID) mice and PrP0/0 mice were also sacrificed in duplicate as controls.
Coincubation of scrapie inoculum with antiscrapie drugs before administration to mouse.
Equal doses of MS-8209, CR, or DS500 (100 μl of a 7.5 mg/ml solution, i.e., 25 mg/kg of body weight for mice) were incubated with the C506M3 inoculum (100 μl of a 2% brain homogenate) for 2 h at room temperature under slight stirring. The solution (200 μl per mouse) was then inoculated intraperitoneally into 8-week-old C57BL/6 mice. As a control, 5% glucose was coincubated with the scrapie inoculum. Day 0 is the day of inoculation. Spleens were harvested in triplicate at various dpi, from day 0 to 100.
Control treatments around the time of inoculation.
Several complementary treatments using these drugs were also performed to compare their effects on PrPres in spleen to those observed with the coincubation regimen. In treatment 1, mice were treated with a 25 mg/kg dose of either MS-8209, CR, or DS500 2 h before scrapie inoculation (control mice were treated with 5% glucose). In treatment 2, single doses of DS500 (25 mg/kg) were administered 14, 21, and 35 days after scrapie inoculation, respectively. Finally, in treatment 3, 0.5 mg of CR was intraperitoneally administered to scrapie-infected mice twice weekly for 5 weeks from the day of inoculation. In treatments 2 and 3, control mice were untreated. The intraperitoneal route was used to infect mice by administration of 100 μl of a 2% brain homogenate. Spleens were harvested in triplicate at each time of analysis.
In vitro incubation of CR with scrapie inoculum.
Various CR concentrations (100 μl of a 7.5- to 0.37-mg/ml solution) were incubated with C506M3 scrapie inoculum (100 μl of a 2% brain homogenate) for 2 h at room temperature under slight stirring. PrPres from the inoculum was then purified by a SAF protocol (see below) or by proteinase K (PK) (10 μg/ml) digestion alone. CR was also incubated for 2 h with SAF-purified PrPres (from 100 μl of 2% brain homogenate). All samples were then resuspended in 4215 buffer (4% sodium dodecyl sulfate, 2% β-mercaptoethanol, 1% Tris-glycine [pH 8.8], 5% sucrose) before denaturation for 5 min at 100°C. A further step with cold acetone or methanol precipitation was added before the Western blotting procedure. The equivalent of 50 μg of brain homogenate was loaded onto a 12% polyacrylamide gel for PrPres detection. As a control, the C506M3 scrapie inoculum was similarly incubated with either 750 μg of MS-8209, its solvent (NMG), or DS500, before purification of PrPres by a SAF protocol.
Tissue PrPres detection and quantification.
Tissues were homogenized at 20% (wt/vol) in a 5% glucose (wt/vol) sterile solution with a Ribolyser (Hybaid). PrPres was extracted from 100 to 200 μl of tissue homogenate by using a previously reported SAF protocol (37). PK was used at a concentration of 10 μg/ml. Samples were denatured in 4215 buffer for 5 min at 100°C before further purification and concentration of PrPres with cold acetone. A 10- to 40-mg equivalent of tissue was run on 12% polyacrylamide gels, electrotransfered onto nitrocellulose membranes (Schleicher & Schuell) (51), and immunoblotted with a 1/5,000 dilution of the polyclonal anti-PrP antibody JB007 (15). Immunoreactivity was visualized with an enhanced chemiluminescence kit on autoradiographic films (Amersham). For each experiment, dilutions of a scrapie brain homogenate were submitted to the same SAF and Western blotting protocol and served for quantification (see Fig. 1 and 3B). PrPres from the tissue studied was compared to PrPres present in the dilution scale by quantifying both immunoreactivities with the NIH Image program for autoradiographic films (Wayne Rasband, National Institutes of Health, Bethesda, Md.). Therefore, the quantity of PrPres in the tissue studied was expressed as an equivalent of terminally ill scrapie brain mass (as the number of micrograms of brain equivalent).
FIG. 1.
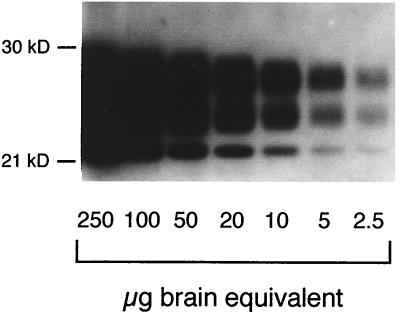
Sensitivity of PrPres detection. PrPres was purified from serial dilutions of mouse scrapie brain homogenate and submitted to Western blotting analysis, as described in Materials and Methods. PrPres quantification was expressed as the equivalent of scrapie-infected brain mass (as micrograms of brain equivalent). A similar dilution scale was prepared throughout the experiments to quantify PrPres levels in the spleen. kD, molecular mass markers in kilodaltons.
FIG. 3.
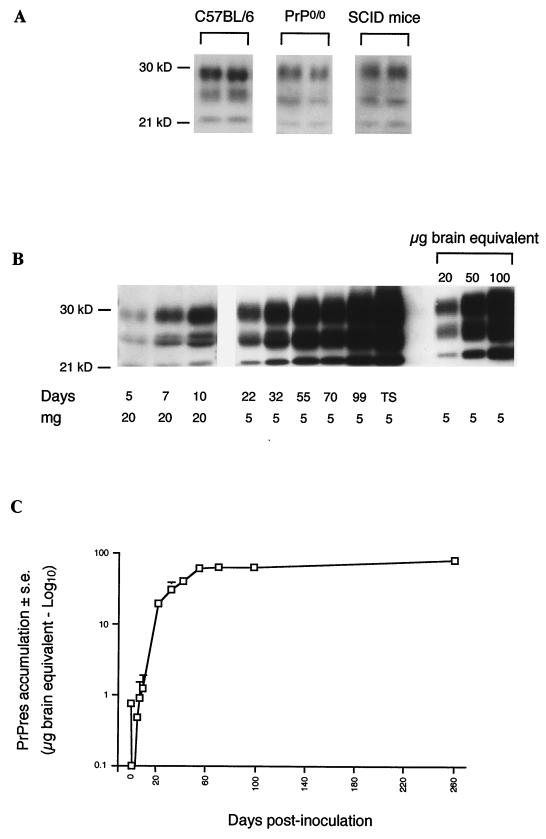
Accumulation of PrPres in the spleens of intraperitoneally scrapie-infected mice. (A) PrPres present in scrapie inoculum was detected in C57BL/6, PrP0/0, and SCID mice in spleen tissues harvested 3 (PrP0/0 mice) or 4 (C57BL/6 and SCID mice) h after scrapie infection. The equivalent of 20 mg of spleen was loaded in the sodium dodecyl sulfate-polyacrylamide electrophoresis gel. (B and C) C57BL/6 mice were infected with 200 μl of a 1% brain homogenate. (B) Neosynthesized PrPres appeared 5 days after inoculation; the amount increased over more than 2 months and plateaued until the terminal stage (TS) of the disease. The equivalent of 20 mg of spleen was loaded until 10 days (mg). Thereafter, 5 mg was sufficient. (C) Quantification of PrPres in spleen from day 0 until the terminal stage of the disease (logarithmic scale). Results are expressed as an equivalent of scrapie brain mass as in the legend to Fig. 1 (micrograms of brain equivalent). The standard deviations for results without error bars were too small to be represented. kD, molecular mass markers in kilodaltons.
RESULTS
Early detection of PrPres in the LRS.
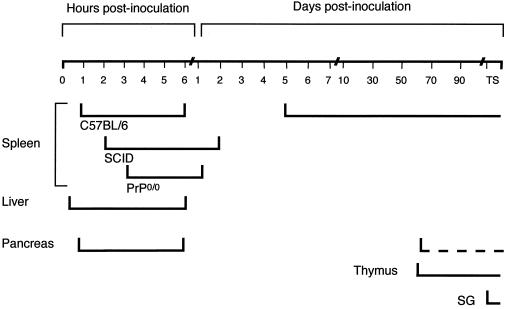
We first sought to determine if PrPres could be used as a sensitive predictor of early scrapie pathogenesis in the spleen, this organ being first infected in most experimental mouse models (32). To attain this goal, PrPres was purified with a SAF protocol. To establish our detection threshold, PrPres was purified from serial dilutions of scrapie brain homogenate. PrPres was detected routinely with 2.5 μg of brain equivalent (Fig. 1) and a maximal sensitivity of around 0.5 μg (see Fig. 4). PrPres was detected in the spleens of C57BL/6 mice as soon as 1 h after intraperitoneal scrapie infection until 6 h later (Fig. 2 and 3A). PrPres was also found in liver and pancreas 15 and 45 min after scrapie infection, respectively, to 6 h postinfection but not in the thymus or in salivary glands (Fig. 2). At day 1, PrPres was not detected in these organs (Fig. 2). PrPres was again detected at day 5, and increasing amounts accumulated in the spleen until 30 to 70 dpi, which corresponds to the beginning of a plateau level which was observed until the terminal stage of the disease. The 30- to 70-dpi interval observed in the spleen is the mean of seven different experiments. During the plateau, levels of PrPres in the spleen were 30 to 150 times lower than those found in the brain per gram of tissue. A representative immunoblot of PrPres detection and the quantification of PrPres accumulation in mouse spleen are shown in Fig. 3B and C. They correspond to PrPres detected after intraperitoneal scrapie infection of mouse with 200 μl of 1% brain homogenate (control of experiments described below). In other organs, PrPres was detected in the thymus and (inconsistently) in the pancreas from 55 dpi to the terminal stage of disease and weakly in a pool of salivary glands from mice at the terminal stage (Fig. 2). Detection in salivary glands has not been performed before. In addition, it is noteworthy that PrPres was not detected in mesenteric lymph nodes before day 21 or in Peyer's patches or auxillary lymph nodes before day 35. These tissues were then PrPres positive until the animals' death (38).
FIG. 4.
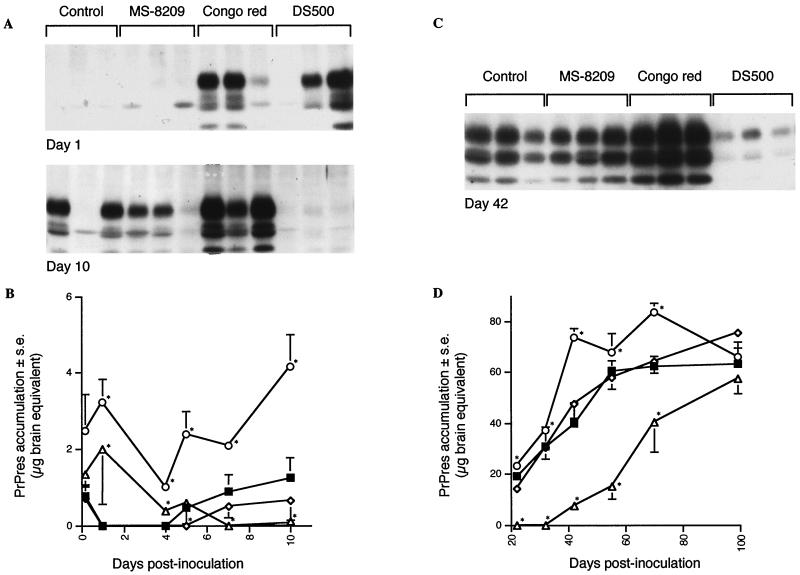
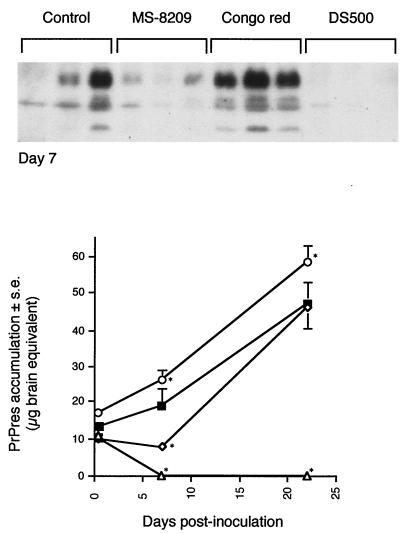
Effects of the coincubation of scrapie inoculum with either MS-8209, DS500, or Congo red on the kinetics of the accumulation of PrPres in spleen. MS-8209 (◊), DS500 (▵) or Congo red (○) solutions (7.5 mg/ml) were incubated with mouse scrapie inoculum (vol/vol) for 2 h at room temperature before intraperitoneal injection of the mixture to mice as described in Materials and Methods. Control mice (■) were infected with inoculum coincubated with 5% glucose. PrPres in spleen was purified and immunoblotted from 4 h to 100 days after scrapie infection. Representative immunoblots are shown in panels A and C at days 1, 10 and 42. (B and D) Quantification of PrPres in spleen. The standard deviations for results without error bars were too small to be represented. ∗, the difference in PrPres accumulation between treated and control mice was statistically significant (P < 0.05; Mann-Whitney u test).
FIG. 2.
Timing and tissue distribution of PrPres in the LRS of scrapie-infected mice. C57BL/6, PrP0/0, and SCID mice were intraperitoneally infected with the C506M3 mouse scrapie strain. PrPres was purified from various tissues harvested at the indicated hours and days postinoculation until the terminal stage of the disease (TS). PrPres detection in salivary glands (SG) was performed with a pool of three mice. Detection of PrPres in pancreas was inconstant from 55 days postinfection (dotted line).
As a control, the presence of PrPres was assessed in the spleens of PrP0/0 and SCID mice, because the spleen is not involved in scrapie pathogenesis in SCID mice (5, 35) and PrP0/0 mice are not susceptible to infection (8). PrPres was detected in the spleens of these mice a few hours after scrapie infection, as in C57BL/6 mice (Fig. 2 and 3A). Differently from them, spleen PrPres was also detected in decreasing amounts on days 1 and 2 but not later (Fig. 2). This result indicates that inoculum was detectable in the spleens of these mice for a few hours or days following inoculation.
The kinetics of PrPres accumulation in the spleen was used to study the molecular effect and to localize the site(s) of action of MS-8209, DS500, and CR.
Coincubation of inoculum with the polyene antibiotic MS-8209 modified slightly the kinetics of PrPres accumulation in the spleen.
Coincubation of MS-8209 with scrapie inoculum did not modify the rate of PrPres detection in spleen the day of inoculation or during the following 4 days when compared to control mice (Fig. 4A and B). MS-8209 weakly reduced spleen PrPres accumulation in spleen from day 5 to day 10, compared to its solvent (data not shown) or to the 5% glucose control (the reduction was statistically significant only at day 5 [P < 0.05; Mann-Whitney u test] (Fig. 4A and B). After this period, similar amounts of PrPres were present in both groups (Fig. 4C and D). A similar result, i.e., an effect restricted to 1 week after scrapie infection, was observed when the drug was administered 2 h before scrapie inoculation (Fig. 5).
FIG. 5.
Effects on the kinetics of accumulation of PrPres in spleen of a 2-h pretreatment with either MS-8209, DS500, or Congo red. Mice were treated with 25 mg/kg of either MS-8209 (◊), DS500 (▵), or Congo red (○) 2 h before intraperitoneal scrapie infection as described in Materials and Methods. Control mice (■) were treated with 5% glucose. PrPres purification and quantification were performed on spleen tissues harvested at 4 h and 7 and 22 days after infection. The standard deviations for results without error bars were too small to be represented. ∗, the difference between treated and control mice was statistically significant (P < 0.05; Mann-Whitney u test). A representative immunoblot at day 7 is shown.
Coincubation of inoculum with the polyanion DS500 strongly impaired PrPres accumulation in the spleen.
Coincubation of DS500 with scrapie inoculum before infection gave different results than those observed with MS-8209. First, PrPres was always detected in spleen from day 0 to 6, although amounts decreased slowly during this period (Fig. 4A and B). PrPres was not detected from day 7 to 42, except very slightly at day 10 (Fig. 4). At 42 dpi, PrPres in spleen was again detectable (Fig. 4C). Thereafter, PrPres accumulation increased gradually, reaching amounts close to controls at day 100 (Fig. 4D). From day 1 to 72, the amount of PrPres in spleen was significantly different from those found in control mice (P < 0.05; Mann-Whitney u test). A similar absence of PrPres was observed in the spleen from 7 to 22 dpi when DS500 was injected 2 h before scrapie inoculation (Fig. 5).
To assess the general efficiency of DS500 on spleen PrPres, treatments were also performed later than the day of inoculation. Single doses of DS500 (25 mg/kg) were administered 14, 21, or 35 days after scrapie infection. For all treatments, PrPres accumulation in spleen was transiently and significantly reduced compared to controls, although not cleared out as with the coincubation at day 0 (data not shown). The shortest effect was obtained when the drug was injected at 35 dpi: 1 month after the treatment, the amount of PrPres in spleen almost reached that in untreated mice.
Coincubation of inoculum with Congo red transiently increased PrPres accumulation in the spleen.
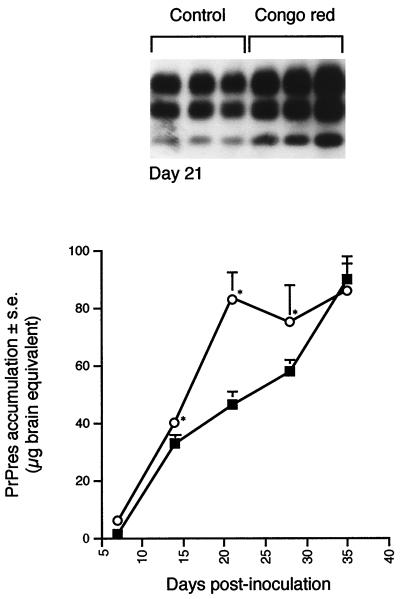
Coincubation of CR with scrapie inoculum before infection did not prevent visualization of PrPres in spleen from day 0 (Fig. 4). From day 0 to 70, the amount of PrPres in spleen was significantly higher than in control mice (P < 0.05; Mann-Whitney u test) (Fig. 4). At day 100, values were similar (Fig. 4D). A less pronounced but significant rise was observed after a single injection of CR 2 h before scrapie inoculation from day 7 to day 22 (Fig. 5). As the absence of PrPres inhibition in spleen could be linked to the CR treatment regimen, mice were also treated twice weekly from day 0 to 35 with 0.5 mg of CR per mouse. PrPres analysis was performed every week. Repeated CR injections transiently increased PrPres accumulation for 4 weeks (P < 0.05; Mann-Whitney u test) (Fig. 6). At the end of the treatment, PrPres amounts in untreated and CR-treated mice were similar (Fig. 6). Thus, in our model, CR did not slow PrPres accumulation in the spleen. On the contrary, CR increased it transiently. The most important effect occurred when CR was coincubated with inoculum. Therefore, we assessed the direct effects of CR on inoculum-associated PrPres.
FIG. 6.
Effects of repetitive administration of Congo red on accumulation of PrPres in the spleen of scrapie-infected mice. A total of 0.5 mg of Congo red per mouse was administered twice weekly during 5 weeks. PrPres purification and quantification were performed on spleen tissues harvested every week (○). The amount of PrPres was compared to that obtained in untreated scrapie-infected mice (■). The standard deviations for results without error bars were too small to be represented. ∗, the difference between treated and untreated mice was statistically significant (P < 0.05; Mann-Whitney u test). A representative immunoblot at day 21 is shown.
Increased detection of inoculum PrPres incubated with Congo red.
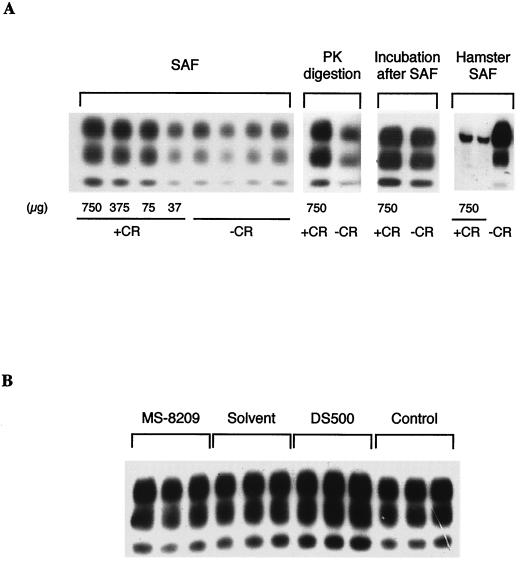
Equal volumes of CR and 2% mouse scrapie inoculum were incubated for 2 h at room temperature. CR amounts varied from 37 to 750 μg. After incubation, PrPres was purified by a SAF protocol. The detection of inoculum-associated PrPres was enhanced in a dose-dependent manner (Fig. 7A). A similar increase was also observed when PrPres was purified only by PK digestion, i.e., without the addition of detergents (Fig. 7A). Adding CR after the SAF protocol, i.e., after PK cleavage but before denaturation, did not modify the PrPres detection profile (Fig. 7A). This suggests that the drug directly increased the PK resistance of PrPres. A similar incubation of 750 μg of MS-8209 did not modify the detection of inoculum PrPres, compared to incubation with 5% glucose or its solvent (Fig. 7B). On the opposite, incubation with DS500 increased detection slightly (Fig. 7B).
FIG. 7.
Effects of Congo red (CR), MS-8209, and DS500 on the detection of PrPres present in scrapie inoculum. (A) Decreasing concentrations of CR (+CR) were incubated with mouse scrapie inoculum (strain C506M3) for 2 h at room temperature before PrPres purification by a SAF protocol (SAF). CR increased detection of inoculum PrPres in a dose-dependent manner. A similar increase was also observed when PrPres was purified with PK digestion but not when CR was added after the SAF protocol (Incubation after SAF). CR was similarly incubated with 2% brain homogenate from scrapie-infected Syrian hamsters (strain 263K). Inoculum-associated PrPres was purified by a SAF protocol and immunoblotted with JB007. In this case, a weak hamster PrPres signal was observed (Hamster SAF). Control samples (-CR) were incubated with 5% glucose. (B) Mouse scrapie inoculum was similarly incubated with either MS-8209, its solvent (NMG), or DS500 at the dose of 750 μg. Control mice were incubated with 5% glucose. PrPres was purified by a SAF protocol. MS-8209 did not increased detection of inoculum PrPres. On the opposite, DS500 did it slightly.
We also investigated the effects of CR incubation on PrPres present in hamster scrapie inoculum (strain 263K) to assess the scrapie strain specificity of our results. Unlike results with mouse PrPres, detection of hamster PrPres after SAF purification was strongly decreased (Fig. 7A), as previously described (9), suggesting that the effects of CR on PrPres are strain specific.
DISCUSSION
PrPres accumulation in mouse LRS after intraperitoneal scrapie infection.
Although scrapie is a neurodegenerative disease, in most experimental models and in some naturally infected species, the LRS and the spleen in particular play a major role in its pathogenesis (22, 32, 33). Previous studies demonstrated scrapie infectivity in peripheral organs from the day of infection up to the terminal stage of disease, (22, 32, 33, 42). The discovery of PrPres as a specific molecular hallmark of scrapie (7, 45) has permitted enhanced study of scrapie pathogenesis (24, 26, 48). We found PrPres in multiple tissues, including the LRS of scrapie-infected mice. PrPres was detected in liver, pancreas, and spleen shortly after intraperitoneal inoculation (Fig. 2). During the next 4 days, PrPres was not detected. PrPres reappeared in the spleen at day 5, and the amount of PrPres increased until a plateau was reached at 30 to 70 dpi. This plateau level persisted to the terminal stage of the disease (Fig. 3). Lymph nodes, Peyer's patches, thymus, salivary glands, and (inconsistently) pancreas became PrPres positive after the spleen (this study and reference 38). In PrP0/0 and SCID mice, where the scrapie agent could not replicate in the LRS (5, 8, 35). PrPres was detected at day 0 but not after day 2 (Fig. 2 and 3A). The detection of PrPres in organs classically involved in the capture and clearance of nonhost particles shortly after inoculation independently of mouse status strongly suggests that inoculum-associated PrPres was detected the day of inoculation. When the LRS is immunologically impaired, as in SCID mice, or does not express PrPc, inoculum-associated PrPres was quickly eliminated (within 3 days). When the LRS is functional and expresses PrPc, inoculum-associated PrPres clearance involving spleen macrophages (6) also occurred, but PrPres reappeared in spleen by day 5, representing neosynthetized PrPres. Lymph nodes, Peyer's patches, thymus, pancreas, and salivary glands may represent secondary replication centers, as PrPres is detected in them later.
Antiscrapie effects of the polyene antibiotic MS-8209.
The precise mechanisms of action of polyene antibiotics, polyanions, and Congo red, as well as their site of intervention remain unclear. Moreover, the effects of these drugs have never been compared together in one single scrapie strain-mouse combination. We first studied MS-8209, as this drug represents one of the most efficient antiscrapie drugs (3, 4, 15). For both the coincubation of MS-8209 with scrapie inoculum and the single injection 2 h before scrapie inoculation, PrPres levels at day 0 were similar to those of control mice, suggesting the absence of a direct effect of the drug on PrPres itself (Fig. 4 and 5). This was confirmed by the fact that in vitro incubation of MS-8209 with scrapie inoculum did not modify PrPres detection, although DS500 and, more particularly, CR increased it (Fig. 7B). Both treatments also only weakly decreased spleen PrPres accumulation a few days after scrapie inoculation (Fig. 4 and 5). Longer treatment periods increased the effects of MS-8209 (unpublished data), but the reduction observed in spleen PrPres accumulation did not account for the benefits to survival time observed (1, 3, 14). Thus, our study suggests that the antiscrapie effects of MS-8209 are not mainly dependent on spleen interactions, at least during the early stages of infection. Moreover, the treatment of scrapie-infected SCID mice with MS-8209 has shown that the drug was efficient in the absence of a functional LRS, further suggesting that spleen was not involved in a critical way (5). These findings, together with results showing an efficiency of MS-8209 even after neuroinvasion has occurred and in mice expressing only PrPc in neurons (15, 17), indicate that the drug effects may no mainly involve scrapie replication in the spleen.
Spleen-specific effects of the polyanion DS500.
The poor effects of MS-8209 on the spleen were highlighted, when compared to DS500, although both drugs exhibit similar efficiency on the survival time of scrapie-infected rodents (3). Indeed, DS500 did seem to involve the spleen, the strongest inhibiting effects being seen around the time of inoculation. Both coincubation with scrapie inoculum and the 2 h pretreatment reduced PrPres synthesis for long periods of time (Fig. 4 and 5). After inoculation with the DS500-inoculum mixture, PrPres levels in spleen decreased slowly in the first days after inoculation and were undetectable from day 7 to 42 (Fig. 4). This result suggests that coincubation of scrapie agent with DS500 induced the clearance of the majority of inoculum-associated PrPres. The time to clear scrapie inoculum was much longer than that observed in untreated mice (1 day), in SCID mice (3 days), or in PrP0/0 mice (2 days). PrPres stability was slightly increased in vitro when the drug was mixed with scrapie brain homogenate (Fig. 7B). It is possible that this stabilized DS500 resisted clearance for a few days. As spleen macrophages are involved in the clearance of inoculum PrPres at the time of infection (6), another explanation could be that DS500 directly impaired their functions, as the toxic effects of polyanions on phagocytic cells of the LRS have been reported (25). Disappearance of PrPres from the spleen from day 7 is then likely to be due to the pleiotropic effects of DS500 on immune cells of the spleen (18, 20, 23, 40), probably altering PrPres-cell interactions. Moreover, it is noteworthy that despite a 1-month period of nondetection, PrPres reappeared, and its amount reached levels similar to those of controls around 3 months after scrapie infection (Fig. 4). This suggests that some cells are resistant to DS500 treatment and can be a reservoir of infectivity. In this situation, PrPres in spleen was probably derived from a tissue other than spleen, or PrPres persisted in undetectable amounts in the spleen.
Absence of inhibiting effects of Congo red on accumulation of PrPres in spleen.
Finally, we tested the action of Congo red in our scrapie mouse model, expecting a reduction in accumulation of PrPres in spleen, since this drug has been shown to inhibit PrPres accumulation in scrapie-infected cells and prolong the survival time of scrapie-infected hamsters and has frequently been compared to polyanions (10–13, 30). CR coincubation with scrapie inoculum before mouse infection was of particular interest because CR effects have often been linked to a binding of PrPres to the drug (9, 16, 47). Surprisingly, the coincubation of CR with mouse scrapie inoculum transiently increased spleen PrPres synthesis. As with DS500, decreased amounts of PrPres were seen in the spleen from day 1 to 4, but thereafter significantly more PrPres was accumulated in spleens of treated than of control mice (Fig. 4). This result could indicate that CR renders inoculum-associated PrPres resistant to the 0- to 4-day clearance. Consequently, more PrPres would be present to initiate infection. This suggests also that CR has no effects on immune cells as DS500 could have, altering PrPres synthesis.
Twice-weekly injections of mice with CR and pretreatment with the drug 2 h before scrapie infection did not decrease PrPres accumulation (Fig. 5 and 6), suggesting that, in our model, CR may not exert any benefit. In addition, no increase in survival time of scrapie-infected mice treated with CR was observed in earlier studies (R. Race, unpublished data). Thus, the in vivo protective effects of CR seem, to date, to be limited to hamster scrapie (30).
Increase of mouse PrPres detection after incubation of Congo red with scrapie inoculum.
To support our hypothesis that CR coincubation renders PrPres resistant to clearance after scrapie infection, we studied the effects of CR directly on inoculum-associated PrPres. CR enhanced the detection of mouse PrPres present in the inoculum in a dose-dependent manner (Fig. 7A). This increase was always observed regardless of the purification protocol with (SAF) or without (PK digestion) detergents. If CR was added after the SAF protocol (i.e., after PK cleavage) but before denaturation, the resulting PrPres detection was not increased (Fig. 7A). This indicates that CR enhanced the protease resistance of PrPres rather than its insolubility or its denaturation susceptibility. CR is known to intercalate into the β sheet of proteins. As PrPres is characterized by high β-sheet content (43), CR could directly bind to PrPres and thereby overstabilize the complex, making it more resistant to proteolysis.
Could the binding of Congo red to PrPres always explain its efficiency in vivo?
In our mouse model, CR effects on PrPres accumulation in spleen seem therefore to be linked to the direct interaction of the drug with PrPres. As CR was efficient in prolonging survival time in hamster scrapie infection (30), we wondered if, under our purification conditions, CR would also act on hamster PrPres. In this case, a very weak PrPres signal was observed when 263K hamster scrapie inoculum was preincubated with CR (Fig. 7A). Thus, depending on the scrapie strain and on the host, CR could either stabilize or disrupt inoculum-associated PrPres.
Taken together, these data suggest that CR binds with PrPres and that the consequences of this in vitro binding are strongly correlated to the anti-scrapie effect observed in vivo. Whether CR specificity is associated to the sequence of PrP, to the structural conformation of PrPres, or both remains to be determined. Studies incubating CR with other experimental TSE agent strains would be of particular interest and are required to verify if the link observed between the in vivo and in vitro effects of this drug is a general phenomenon.
ACKNOWLEDGMENTS
We thank M. Seman and K. Cherifi (Mayoly Spindler Laboratories) for the gift of MS-8209, C. Weissmann for providing PrP0/0 mice, J. Y. Cesbron for SCID mice, R. Demaimay for helpful scientific discussion, and J. C. Mascaro, D. Farrant, and R. Rioux for excellent animal care.
This work was supported by a grant from the Institut de Formation Supérieure Biomédicale (IFSBM).
REFERENCES
- 1.Adjou K T, Demaimay R, Lasmézas C I, Deslys J P, Seman M, Dormont D. MS-8209, a new amphotericin B derivative, provides enhanced efficacy in delaying hamster scrapie. Antimicrob Agents Chemother. 1995;39:2810–2812. doi: 10.1128/aac.39.12.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjou K T, Demaimay R, Lasmézas C I, Seman M, Deslys J P, Dormont D. Differential effects of a new amphotericin B derivative, MS-8209, on mouse BSE and scrapie: implications for the mechanism of action of polyene antibiotics. Res Virology. 1996;147:213–218. doi: 10.1016/0923-2516(96)89651-8. [DOI] [PubMed] [Google Scholar]
- 3.Adjou K T, Deslys J P, Demaimay R, Seman M, Dormont D. Prospects for the pharmacological treatment of human prion diseases. CNS Drugs. 1998;10:83–89. [Google Scholar]
- 4.Beringue V, Demaimay R, Adjou K T, Demart S, Lamoury F, Seman M, Lasmézas C I, Deslys J P, Dormont D. Polyene antibiotics in experimental transmissible subacute spongiform encephalopathies. In: Morrison D R O, editor. Prions and brains diseases in animals and humans. Vol. 295. New York, N.Y: Plenum Press; 1998. pp. 177–185. [Google Scholar]
- 5.Beringue V, Lasmézas C I, Adjou K T, Demaimay R, Lamoury F, Deslys J P, Seman M, Dormont D. Inhibiting scrapie neuroinvasion by polyene antibiotic treatment of SCID mice. J Gen Virol. 1999;80:1873–1877. doi: 10.1099/0022-1317-80-7-1873. [DOI] [PubMed] [Google Scholar]
- 6.Beringue V, Demoy M, Lasmézas C I, Gouritin B, Weingarten C, Deslys J P, Andreux J P, Couvreur P, Dormont D. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J Pathol. 2000;190:495–502. doi: 10.1002/(SICI)1096-9896(200003)190:4<495::AID-PATH535>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Bolton D C, McKinley M P, Prusiner S B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 8.Büeler H, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 9.Caspi S, Halimi M, Yanai A, BenSasson S, Taraboulos A, Gabizon R. The anti-prion activity of Congo red. Putative mechanism. J Biol Chem. 1998;273:3484–3489. doi: 10.1074/jbc.273.6.3484. [DOI] [PubMed] [Google Scholar]
- 10.Caughey B, Brown K, Raymond G J, Katenstein G E, Thresher W. Binding of the protease-sensitive form of prion protein PrP to sulfated glycosaminoglycan and Congo red. J Virol. 1994;68:2135–2141. doi: 10.1128/jvi.68.4.2135-2141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey B, Ernst D, Race R E. Congo red inhibition of scrapie agent replication. J Virol. 1993;67:6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey B, Race R E. Potent inhibition of scrapie-associated PrP accumulation by Congo red. J Neurochem. 1992;59:768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 13.Caughey B, Raymond G J. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaimay R, Adjou K T, Lasmézas C I, Lazarini F, Cherifi K, Seman M, Deslys J P, Dormont D. Pharmalogical studies of a new derivative of amphotericin B, MS-8209, in mouse and hamster scrapie. J Gen Virol. 1994;75:2499–2503. doi: 10.1099/0022-1317-75-9-2499. [DOI] [PubMed] [Google Scholar]
- 15.Demaimay R, Adjou K T, Beringue V, Demart S, Lasmézas C I, Deslys J P, Seman M, Dormont D. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J Virol. 1997;71:9685–9689. doi: 10.1128/jvi.71.12.9685-9689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaimay R, Harper J, Gordon H, Weaver D, Chesebro B, Caughey B. Structural aspects of Congo red as an inhibitor of protease-resistant prion protein formation. J Neurochem. 1998;71:2534–2541. doi: 10.1046/j.1471-4159.1998.71062534.x. [DOI] [PubMed] [Google Scholar]
- 17.Demaimay R, Race R, Chesebro B. Effectiveness of polyene antibiotics in treatment of transmissible spongiform encephalopathy in transgenic mice expressing syrian hamster PrP only in neurons. J Virol. 1999;73:3511–3513. doi: 10.1128/jvi.73.4.3511-3513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamanstein T, Wagner B, Beyso I, Odenwald M V, Schulzt G. Stimulation of humoral antibody formation by polyanions. II. The influence of sulfate esters of polymers on the immune response in mice. Eur J Immunol. 1971;1:340–343. doi: 10.1002/eji.1830010507. [DOI] [PubMed] [Google Scholar]
- 19.Diringer H, Ehlers B. Chemoprophylaxis of scrapie in mice. J Gen Virol. 1991;72:457–460. doi: 10.1099/0022-1317-72-2-457. [DOI] [PubMed] [Google Scholar]
- 20.Dorries R, Schimpl A, Wecker E. Action of dextran sulfate as a direct and general B-mitogen. Eur J Immunol. 1974;4:230–233. doi: 10.1002/eji.1830040315. [DOI] [PubMed] [Google Scholar]
- 21.Ehlers B, Diringer H. Dextran sulphate 500 delays and prevents mouse scrapie by impairment of agent replication in spleen. J Gen Virol. 1984;65:1325–1330. doi: 10.1099/0022-1317-65-8-1325. [DOI] [PubMed] [Google Scholar]
- 22.Eklund C M, Kennedy R C, Hadlow W J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967;117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Farquhar C F, Dickinson A G. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J Gen Virol. 1986;67:463–473. doi: 10.1099/0022-1317-67-3-463. [DOI] [PubMed] [Google Scholar]
- 24.Farquhar C F, Dornan J, Somerville R A, Tunstall A M, Hope J. Effect of Sinc genotype, agent isolate and route of infection on the accumulation of protease-resistant PrP in non-central nervous system tissues during the development of murine scrapie. J Gen Virol. 1994;75:495–504. doi: 10.1099/0022-1317-75-3-495. [DOI] [PubMed] [Google Scholar]
- 25.Fowler E F, Thomson A W. Effect of carrageenan on activity of the mononuclear phagocyte system in the mouse. Br J Exp Pathol. 1978;59:213–219. [PMC free article] [PubMed] [Google Scholar]
- 26.Grathwohl K U D, Horiuchi M, Ishiguro N, Shinagawa M. Improvement of PrPsc-detection in mouse spleen early at the preclinical stage of scrapie with collagenase-completed tissue homogenization and Sarkosyl-NaCl extraction of PrPsc. Arch Virol. 1996;141:1863–1874. doi: 10.1007/BF01718200. [DOI] [PubMed] [Google Scholar]
- 27.Hadlow W J, Kennedy R C, Race R E. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 28.Hill A F, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet. 1997;349:99–100. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- 29.Hilton D A, Fathers E, Edwards P, Ironside J W, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352:703–704. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]
- 30.Ingrosso L, Ladogana A, Pocchiari M. Congo red prolongs the incubation period in scrapie-infected hamsters. J Virol. 1995;69:506–508. doi: 10.1128/jvi.69.1.506-508.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimberlin R H, Walker C A. The antiviral compound HPA-23 can prevent scrapie when administered at the time of infection. Arch Virol. 1983;78:9–18. doi: 10.1007/BF01310854. [DOI] [PubMed] [Google Scholar]
- 32.Kimberlin R H, Walker C A. Pathogenesis of experimental scrapie. Ciba Found Symp. 1988;135:37–62. doi: 10.1002/9780470513613.ch4. [DOI] [PubMed] [Google Scholar]
- 33.Kimberlin R H, Walker C A. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J Comp Pathol. 1979;89:551–562. doi: 10.1016/0021-9975(79)90046-x. [DOI] [PubMed] [Google Scholar]
- 34.Kimberlin R H, Walker C A. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob Agents Chemother. 1986;30:409–413. doi: 10.1128/aac.30.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasmézas C I, Cesbron J Y, Deslys J P, Demaimay R, Adjou K T, Rioux R, Lemaire C, Locht C, Dormont D. Immune system-dependent and -independent replication of the scrapie agent. J Virol. 1996;70:1292–1295. doi: 10.1128/jvi.70.2.1292-1295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasmézas C I, Deslys J P, Demaimay R, Adjou K T, Hauw J J, Dormont D. Strain specific and common pathogenic events in murine models of scrapie and bovine spongiform encephalopathy. J Gen Virol. 1996;77:1601–1609. doi: 10.1099/0022-1317-77-7-1601. [DOI] [PubMed] [Google Scholar]
- 37.Lasmézas C I, Deslys J P, Robain O, Jaegly A, Beringue V, Peyrin J M, Fournier J G, Hauw J J, Rossier J, Dormont D. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 38.Maignien T, Lasmézas C I, Beringue V, Dormont D, Deslys J P. Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J Gen Virol. 1999;80:3035–3042. doi: 10.1099/0022-1317-80-11-3035. [DOI] [PubMed] [Google Scholar]
- 39.McBride P A, Wilson M I, Eikelenboom P, Tunstall A, Bruce M E. Heparan sulfate proteoglycan is associated with amyloid plaques and neuroanatomically targeted PrP pathology throughout the incubation period of scrapie-infected mice. Exp Neurol. 1998;149:447–454. doi: 10.1006/exnr.1997.6740. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy R E, Arnold L W, Babcock G F. Dextran sulfate: an adjuvent for cell-mediated immune responses. Immunology. 1977;32:963–974. [PMC free article] [PubMed] [Google Scholar]
- 41.Merz P A, Somerville R A, Wisniewski H M, Manuelidis L, Manuelidis E E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983;306:474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- 42.Millson G C, Kimberlin R H, Manning E J, Collis S C. Early distribution of radioactive liposomes and scrapie infectivity in mouse tissues following administration by different routes. Vet Microbiol. 1979;4:89–99. [Google Scholar]
- 43.Pan K M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocchiari M, Casaccia P, Ladogana A. Amphotericin B: a novel class of antiscrapie drugs. J Infect Dis. 1989;160:795–802. doi: 10.1093/infdis/160.5.795. [DOI] [PubMed] [Google Scholar]
- 45.Prusiner S B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 46.Prusiner S B, Cochran S P, Groth D F, Downey D E, Bowman K A, Martinez H M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 47.Prusiner S B, McKinley M P, Bowman K A, Bolton D C, Bendheim P E, Groth D F, Glenner G G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35:349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- 48.Race R E, Ernst D. Detection of proteinase K-resistant prion protein and infectivity in mouse spleen by 2 weeks after scrapie agent inoculation. J Gen Virol. 1992;73:3319–3323. doi: 10.1099/0022-1317-73-12-3319. [DOI] [PubMed] [Google Scholar]
- 49.Rubenstein R, Kascsak R J, Merz P A, Papini M C, Carp R I, Robakis N K, Wisniewski H M. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J Gen Virol. 1986;67:671–681. doi: 10.1099/0022-1317-67-4-671. [DOI] [PubMed] [Google Scholar]
- 50.Snow A D, Wight T N, Nochlin D, Koike Y, Kimata K, DeArmond S J, Prusiner S B. Immunolocalization of heparan sulfate proteoglycans to the prion protein amyloid plaques of Gerstmann-Straussler syndrome, Creutzfeldt-Jakob disease and scrapie. Lab Investig. 1990;63:601–611. [PubMed] [Google Scholar]
- 51.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]