Abstract
Leptomeningeal carcinomatosis (LC) is a rare site of metastasis in solid tumors, and it is associated with poor prognosis due to disabling symptoms and a scarcity of treatment options. This condition is an uncommon entity in gastric cancer (GC). We present a case of primary LC manifestation in a patient with an incidental diagnosis of localized node-negative GC. We additionally perform a literature review and discuss the diagnostic and therapeutic challenges. In conclusion, LC from GC represents a rare condition with a dramatic prognosis. Its diagnosis might be very challenging. A multidisciplinary approach appears to be the best strategy for the management of LC from GC.
Keywords: leptomeningeal carcinomatosis, gastric cancer, brain metastases
1. Introduction
Gastric cancer (GC) is the sixth most common cancer, representing 5.6% of newly diagnosed cancers and the third largest cause of cancer-related deaths worldwide (7.7%) [1]. Its incidence and mortality are constantly decreasing over time due to screening policies and better hygienic condition worldwide [1]. Epidemiologically, GC displays a higher distribution in Asian countries [2], and some authors suggest using different therapeutical approaches between Eastern and Western patients presenting with GC [3,4,5]. Currently, the best treatment for gastric cancer amendable for resection is an interplay between perioperative chemotherapy followed by surgery [5,6,7]. For non-resectable/metastatic patients, current guidelines recommend various chemo- and immuno-therapy approaches [6,7].
The most common sites of GC metastases are represented by the locoregional and distant lymph nodes, liver, lung, and peritoneum [8]. Brain metastatic localizations of GC, associated with poor prognosis, usually involve cerebral parenchyma and are correlated with amplification of human epidermal growth factor receptor-2 (HER2), Caucasian ethnicity, proximal location, and histological signet cell ring subtype [9,10,11]. A more unusual and rarely reported site of metastatic spread is the meninges route, causing leptomeningeal carcinomatosis (LC). Very few cases in the literature describe instances where the first manifestation of GC is LC [12,13,14,15], and in only one patient was a locally advanced node negative GC observed [13]. The clinical presentation is characterized by common and non-specific neurological symptoms, including headache, nausea and vomiting, seizures, as well as isolated cranial nerve palsy. No specific therapies are available and systemic treatment has a scarce efficacy; therefore, therapeutic options mainly consist of symptom palliation with anti-depressants, anxiolytics, and opioid and non-opioid agents [16].
2. Materials and Methods
We present the case of 77-year-old man affected by localized gastric cancer with leptomeningeal spreading. Furthermore, a literature search through the PubMed database was conducted to identify articles regarding patients with a clinical primary manifestation of exclusive leptomeningeal carcinomatosis from gastric cancer, while patients without a positive anamnesis for gastric cancer and/or gastric resection were excluded. The search strategy was as follows: “stomach neoplasms [mesh] AND lepto* [tiab] AND carci* [tiab]”. Screening of titles, abstracts and articles in English language was performed. Articles in languages other than English were included, provided that they had an English abstract with sufficient information to extrapolate data for our review. Only studies published between 01/2000 and 02/2024 were included in our review.
3. Results
3.1. Case Presentation: Clinical History and Findings
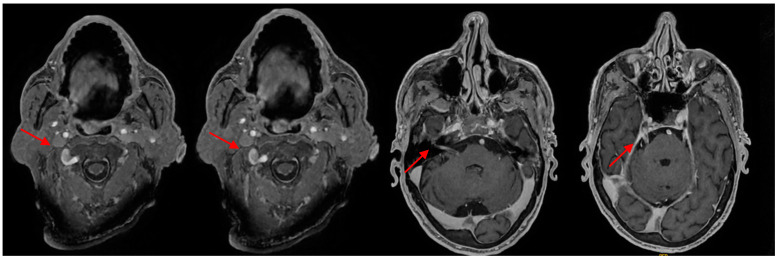
A 77-year-old Caucasian male referred to the emergency department (ED) of our hospital in December 2023 for worsening asthenia and cachexia over the previous two weeks, presenting paradoxical dysphagia and a weight loss of almost 20 kg in the last year (−30% of body weight). On his previous medical history, the patient had a systemic hypertension under medical treatment, and in 2020 he developed a left vocal cord palsy associated with dysphonia. The neurological exams revealed spontaneous fluent speech, appropriate in form and content, preserved extrinsic ocular motility without nystagmus, hyposthenia of the left superior and inferior facial muscles with left Bell phenomenon, ipsilateral hearing loss, and protruded tongue as a muscle deficit. Upon blood exam, the patient showed only mild anemia (hemoglobin 12.6 g/dL, red blood cell 4.2 × 106/μL). The patient was then admitted to the neurology department and a brain and encephalic trunk magnetic resonance (MRI) was requested. The patient’s MRI results showed a thickening of the intracisternal tract of the V pair of cranial nerves, with hypersignal in T2-FLAIR sequences and contrast enhancement. The same characteristic was also documented for the VII, VIII, IX, X, and XI pairs of cranial nerves. A linear enhancement of the intracisternal tract of the III and VI cranial nerves was noted. Also, an enhancement of the leptomeningeal surface of the spinal cord was documented in C1 and C2 (Figure 1).
Figure 1.
Brain MRI.
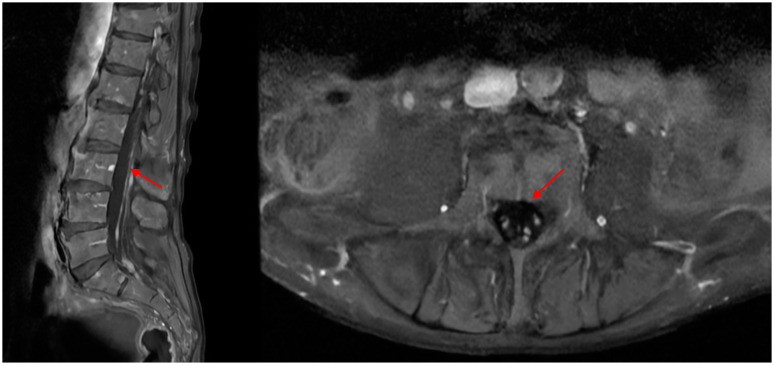
This finding was depicted as non-specific, and the differential diagnosis included inflammatory, neoplastic, neurolymphomatosis, or granulomatous disease. During hospitalization, the patient was tested for the extractable nuclear antigen antibodies (ENAs) profile (Anti-Ro52 (Sjögren Syndrome A, SSA), anti-Ro60 (SSA), anti-La (SS-B), anti-Smith (Sm), anti-Jo1, anti-systemic sceloris (Scl 70), anti-ribonucleoprotein (RNP), cytosplamatic-anti-neutrophil cytoplasmatic antibodies (cANCA), perinuclear ANCA (pANCA), anti-nucleus antibodies (ANAs)), the paraneoplastic antibody panel, and immunoglobulin G subclasses; all results were negative. Afterwards, the patient was scheduled for a lumbar MRI that showed pseudonodular enhancement after contrast injection of the medullaris conus and the origins of the cauda equina (Figure 2). A neurolymphomatosis was then suspected by the radiologist, rather than a neoplastic leptomeningitis, and for this reason a lumbar puncture was performed, showing a xanthocromic clear fluid with an elevated cell count (49 mm3, 70% lymphocytes, with some bigger lymphocytes containing nuclear abnormalities, 87% T lymphocyte with a T4/T8 ration of 5.2, 2% B lymphocyte, 12% natural killer), hypoglycorrhachia (34 mg/dL) and hyperproteinorrachia (350 mg/dL), negative for bacterial growth, negative for viral infections (herpes simplex virus-1, herpes simplex virus-2, cytomegalovirus, varicella zoster virus, Epstein–Barr virus, and human herpes virus 6). The cytologic exam of the liquor showed erythrocytes, rare neutrophilic granulocytes, and occasional histiocytes, with a slight increase in the lymphocyte quota noted.
Figure 2.
Lumbar MRI.
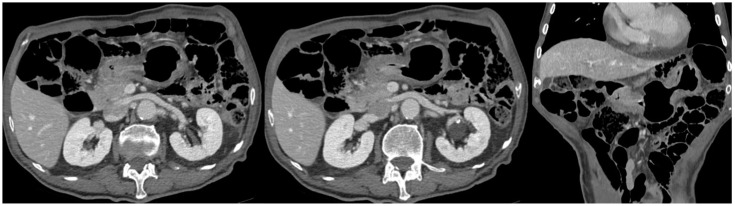
A full-body computed tomography (CT) scan with intravenous contrast showed a pre-pyloric contrast-enhanced thickening (9 mm) in the stomach without any locoregional lymphadenopathy (Figure 3).
Figure 3.
Abdominal CT.
To further investigate the gastric findings, the patients underwent a gastroscopy that confirmed a pre-pyloric lesion with a positive biopsy for signet ring cell gastric adenocarcinoma with microsatellite stability (MSS) and immunohistochemistry 1+ for HER2. An in-depth anamnestic investigation revealed no upper gastrointestinal tract symptoms, despite progressive and disabling neurological symptoms.
In the meantime, after a neurosurgery consultation, the patient was proposed for a biopsy of the causa equina, which showed metastatic spread of a signet ring cell gastric adenocarcinoma c-erb2/NEU 2+ (antibody c-ErbB2 DAKO); negative fluorescence in situ hybridization (FISH) was utilized. The mismatch repair (MMR) panel showed nuclear expression of the protein for human mutL homolog 1, Postmeiotic Segregation Increased 2, and MutS homologs 2 and 6 (hMLH1, PMS2, hMSH2 and hMSH6), demonstrating a phenotype with microsatellite stability (MSS).
During hospitalization, the patient’s neurological conditions worsened, with progressive dysarthria and cranial nerves deficit.
Due to the complexity of the clinical case and the need for several professional’s expertise, the gastrointestinal multidisciplinary team discussed the patient. Given their symptoms, comorbidities, and performance status PS (Karnofsky score 40%) a chemotherapy regimen was not deemed feasible, and whole-brain radiation therapy followed by best supportive care was suggested for the sole purpose of symptoms palliation, according to the current guidelines of the European Association of Neuro-Oncology (EANO) [17].
3.2. Literature Review
A total of 36 articles were found with the aforementioned search criteria; after title and abstract screening, 9 records were excluded for irrelevant topic, while were excluded 15 for a known positive anamnesis for operated or palliated gastric cancer. A total of 12 papers, all case reports, were included in our final analysis [10,11,12,13,15,16,17,18,19,20,21,22]. A total of 12 patients were retrieved from our analysis, 6 males and 5 females, and in one case the gender was not specified; the median age was 58.9 years (range 40–80). The histology reported a signet ring cell carcinoma and poorly differentiated gastric carcinoma in eight (67%) and four cases (33%), respectively; HER-2 status was investigated only in one case, and it was found to be negative [18]. The median overall survival from diagnosis, available for only eight cases, was 60 days. All the characteristics are listed in Table 1.
Table 1.
Literature review of cases with initial manifestation of meningeal carcinomatosis from gastric cancer. NR: not reported, RT: radiation therapy, S1: tegafur/gimeracil/oteracil, FLOT: fluorouracil, leucovorin, oxaliplatin, and docetaxel.
| Study | Country and Year |
Age and Sex | Clinical Presentation | Pathology | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Fuchizaki U et al. [23] | Japan, 2005 | 42, male | Unsteady gait, ataxia, dysmetria | Signet ring cell carcinoma | Chemotherapy | Deceased 49 days after diagnosis |
| Braeuninger S et al. [24] | Germany, 2005 | 68, male | Vertigo, diplopia, nausea, vomiting | Poorly differentiated carcinoma | Intrathecal methotrexate | Deceased 2 months after diagnosis |
| Lee G H et al. [25] | South Korea, 2007 | 49, female | Headache, dizziness, easy fatigability, and melena | Signet ring cell carcinoma | Supportive care | NR |
| Cresto N et al. [26] | Switzerland, 2007 | 57, NR | Nocturnal limb hypoesthesia and paresthesia, visual impairment | Signet ring cell carcinoma | NR | NR |
| Suto C et al. [27] | Japan, 2007 | 70, male | Optic neuropathy | Signet ring cell carcinoma | NR | Deceased 3 months after diagnosis |
| Yamada T et al. [28] | Japan, 2008 | 53, male | Anorexia, intermitting diplopia, general fatigue, headache, vertigo | Poorly differentiated carcinoma | Whole-brain RT | Deceased 127 days after diagnosis |
| Ohno T et al. [14] | Japan, 2010 | 62, male | Bilateral hearing loss | Poorly differentiated carcinoma | Whole-brain RT + S1 + Paclitaxel | Deceased 12 weeks after diagnosis |
| Kawasaki A et al. [12] | Japan, 2014 | 80, female | Headache, nausea, fever | Signet ring cell carcinoma | Supportive care | NR |
| Guo J-W et al. [13] | China, 2014 | 40, female | Headache and cervical pain | Signet ring cell carcinoma | Supportive care | Deceased 2 months after diagnosis |
| Vergoulidou M [15] | Germany, 2017 | 48, female | Headache and nausea | Signet ring cell carcinoma | Intrathecal methotrexate + systemic FLOT | Deceased 2 months after diagnosis |
| Ino R et al. [18] | Japan, 2021 | 77, female | General malaise, posterior neck pain, gait disturbance | Poorly differentiated carcinoma | NR | Deceased 25 days after diagnosis |
| Silverman A et al. [29] | USA, 2023 | 61, male | Positional headache, blurry vision, early satiety, weight loss | Signet ring cell carcinoma | NR | NR |
4. Discussion
Due to its clinical presentation and diagnostic difficulty, the incidence of meningeal carcinomatosis and its prevalence among cancer patients is uncertain, and the best estimate is that it occurs in 2% to up to 10% of patients affected by malignancies during the disease’s course [19].
Although frequent in leukemias and lymphomas, it represents a rare entity in solid tumors [30] and often occurs following the involvement of other parenchymal organs [20]. Lung (highest absolute number), breast (highest probability), and melanoma solid tumours develop meningeal carcinomatosis most frequently. In breast cancer patients, meningeal carcinomatosis is most commonly associated with young age, ductal carcinoma, HER-2-positive tumours, and triple-negative tumours. In lung cancer, meningeal carcinomatosis is frequently associated with adenocarcinoma histotype, oncogenic driver mutations like epidermal growth factor receptor (EGFR) mutation, and anaplastic lymphoma kinase (ALK) translocation. In the largest cohort of patients with melanoma, BRAF mutations were identified in 67% of patients with leptomeningeal carcinomatosis versus 47% in the general population of patients with melanoma [17,19,21,22]. Although a rare occurrence, meningeal carcinomatosis appears in 0.062% of patients with gastric cancer [31].
Typical signs and symptoms of LC are mainly headache, nausea and vomiting, altered mental status, and also cranial nerve palsy manifested through sensory loss or facial paralysis, as they depend on the central nervous system (CNS) area of meningeal carcinomatosis involvement. However, clinical presentation may be asymptomatic or very subtle, with minimal to no symptoms [17,30].
The largest database on LC currently available in the literature, constructed by Megid et al. [32], reports a rate of 0.61% LC over more than 3200 patients with esophago-gastric cancers. The authors report a higher rate of HER-2 positivity in brain metastases over LC from esophageal and gastric cancer. The authors concluded that the best treatment in LC appears to be whole-brain RT, but 35% of patients might be planned for best supportive care. A discrepancy between the two proposed treatments in terms of survival is clearly evident (2.8 months versus 0.7, p = 0.015).
According to the EANO-ESMO Clinical Practice Guidelines (2023) [17,30], leptomeningeal metastasis diagnostic work-up includes a detailed neurological examination; cerebrospinal MRI with and without contrast, as the gold standard imaging method in LC diagnosis and follow-up; and lumbar puncture, when possible. CT scan should be restricted to patients with contraindications to MRI or emergency settings. Leptomeningeal biopsies are often unnecessary but may be useful in cases of difficult differential diagnoses, such as patients without a history of cancer and negative cerebrospinal fluid (CSF) cytology [17].
Typical MRI signs of LC include “contrast enhancement of cerebellar folia and sulci, basilar cisterns, cranial nerves, brain surface, the surface of the lateral ventricles and lumbar nerve roots, notably the cauda equina” [17], or they may be completely non-specific and should be interpreted in the context of clinical signs. According to a retrospective study conducted in 2020 by Le Rhun et al. [17,33], five groups of LC cases were described in MRI with different roles of prognostic and predictive value [34,35,36,37]. Abnormalities on MRI were observed in only 67% of patients with LC; therefore, a normal MRI might not rule out LC [35,36]. Cytological examination of the CSF is currently considered the gold standard for the diagnosis of LC, but its positivity does not exceed 60%, thus a repeated lumbar puncture might be needed [17,38].
The diagnosis of LC from GC is eased by radiological findings of a positive anamnesis for gastrointestinal manifestation, and it can be suspected in advanced disease with metastatic involvement of the liver, peritoneum, or brain. It should be taken into account that in our case, the patient did not report gastrointestinal symptoms, and the CT scan showed a locally confined GC without locoregional lymphadenopathy; this indolent and subclinical presentation may have slowed the diagnostic work-up.
A multimodal approach is required for treatment options [39,40], which include intrathecal chemotherapy in association with systemic chemotherapy, and radiation therapy according to the current EANO guidelines [17]. A patient’s performance status and comorbidities, histological and molecular tumor characteristics, and previous treatments should be considered. Regarding radiotherapy, a stereotactic approach may be preferable in nodular meningeal carcinomatosis, whereas whole-brain radiotherapy therapy is preferred in extensive nodular LC, although it is not associated with improved OS [17].
In our literature review, we found that two patients underwent intrathecal methotrexate, two patients underwent whole-brain radiation therapy, and only one underwent systemic treatment with the FLOT protocol [41], while most patients were assigned to best supportive care due to their associated poor performance status. In our case, the patient was proposed for whole-brain radiation therapy to palliate the neurological symptomatology, and no other treatment was proposed considering his performance status.
5. Conclusions
In conclusion, LC from GC represents a rare condition with a dramatic prognosis (usually less than 2 months). Diagnosis might be very challenging, and the available treatments are poorly effective due to the disease’s anatomical location and very rapid clinical worsening. A multidisciplinary approach with a focus on supportive care appears to be the best strategy for the management of LC.
Author Contributions
Conceptualization A.L., G.A. and L.D.C.; methodology A.L., F.S.L.C. and L.D.C.; execution A.L., G.A. and E.L.; acquisition of data A.L., E.L. and F.S.L.C.; writing—original draft preparation A.L. and E.L.; writing—review and editing G.A., F.M., G.B., M.F.O. and P.M.; supervision F.M., G.B., M.F.O. and P.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and ‘good clinical practice’ guidelines. Ethical approval was waived due to the nature of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. [(accessed on 5 May 2024)];Int. J. Cancer. 2021 149:778–789. doi: 10.1002/ijc.33588. Available online: https://pubmed.ncbi.nlm.nih.gov/33818764/ [DOI] [PubMed] [Google Scholar]
- 3.Russo A., Li P., Strong V.E. Differences in the multimodal treatment of gastric cancer: East versus west. J. Surg. Oncol. 2017;115:603–614. doi: 10.1002/jso.24517. [DOI] [PubMed] [Google Scholar]
- 4.Markar S.R., Karthikesalingam A., Jackson D., Hanna G.B. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: Comparison between West and East. Ann. Surg. Oncol. 2013;20:2328–2338. doi: 10.1245/s10434-012-2862-9. [DOI] [PubMed] [Google Scholar]
- 5.Garbarino G.M., Laracca G.G., Lucarini A., Piccolino G., Mercantini P., Costa A., Tonini G., Canali G., Muttillo E.M., Costa G. Laparoscopic versus Open Surgery for Gastric Cancer in Western Countries: A Systematic Review and Meta-Analysis of Short- and Long-Term Outcomes. J. Clin. Med. 2022;11:3590. doi: 10.3390/jcm11133590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajani J.A., D’Amico T.A., Bentrem D.J., Chao J., Cooke D., Corvera C., Das P., Enzinger P.C., Enzler T., Fanta P., et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. [(accessed on 5 May 2024)];J. Natl. Compr. Cancer Netw. 2022 20:167–192. doi: 10.6004/jnccn.2022.0008. Available online: https://pubmed.ncbi.nlm.nih.gov/35130500/ [DOI] [PubMed] [Google Scholar]
- 7.Lordick F., Carneiro F., Cascinu S., Fleitas T., Haustermans K., Piessen G., Vogel A., Smyth E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. [(accessed on 5 May 2024)];Ann. Oncol. 2022 33:1005–1020. doi: 10.1016/j.annonc.2022.07.004. Available online: http://www.annalsofoncology.org/article/S0923753422018518/fulltext. [DOI] [PubMed] [Google Scholar]
- 8.Riihimaki M., Hemminki A., Sundquist K., Sundquist J., Hemminki K. Metastatic spread in patients with gastric cancer. [(accessed on 5 May 2024)];Oncotarget. 2016 7:52307–52316. doi: 10.18632/oncotarget.10740. Available online: https://pubmed.ncbi.nlm.nih.gov/27447571/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanna L., Seghini P., Di Nunzio C., Orlandi E., Michieletti E., Stroppa E.M., Mordenti P., Citterio C., Vecchia S., Zangrandi A. Gastric cancer with brain metastasis and the role of human epidermal growth factor 2 status. Oncol. Lett. 2018;15:5787–5791. doi: 10.3892/ol.2018.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Lin Y., Duan J., Xu K., Mao M., Wang X. A Population-Based Analysis of Distant Metastasis in Stage IV Gastric Cancer. [(accessed on 5 May 2024)];Med. Sci. Monit. 2020 26:e923867. doi: 10.12659/MSM.923867. Available online: https://pubmed.ncbi.nlm.nih.gov/32409630/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartelt S., Momm F., Weissenberger C., Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: Prognostic factors and survival. World J. Gastroenterol. 2004;10:3345–3348. doi: 10.3748/wjg.v10.i22.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki A., Suzuki K., Takekawa H., Nakamura T., Yamamoto M., Asakawa Y., Okamura M., Hirata K. Co-occurrence of multiple cerebral infarctions due to hypercoagulability associated with malignancy and meningeal carcinomatosis as the initial manifestation of gastric cancer. BMC Neurol. 2014;14:160. doi: 10.1186/s12883-014-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J.W., Zhang X.T., Chen X.S., Zhang X.C., Zheng G.J., Zhang B.P., Cai Y.F. Leptomeningeal carcinomatosis as the initial manifestation of gastric adenocarcinoma: A case report. World J. Gastroenterol. 2014;20:2120–2126. doi: 10.3748/wjg.v20.i8.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno T., Yokoyama Y., Aihara R., Mochiki E., Asao T., Kuwano H. Sudden bilateral sensorineural hearing loss as the presenting symptom of meningeal carcinomatosis of gastric cancer: Report of a case. Surg. Today. 2010;40:561–565. doi: 10.1007/s00595-009-4099-1. [DOI] [PubMed] [Google Scholar]
- 15.Vergoulidou M. Leptomeningeal Carcinomatosis in Gastric Cancer: A Therapeutical Challenge. Biomark. Insights. 2017;12:1177271917695237. doi: 10.1177/1177271917695237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S.R., Malapati S.J., Mattour A. Cancer Metastasis Through the Lymphovascular System. Springer International Publishing; Cham, Switzerland: 2023. Leptomeningeal Carcinomatosis; pp. 575–583. [Google Scholar]
- 17.Le Rhun E., Weller M., Bent M.v.D., Brandsma D., Furtner J., Rudà R., Schadendorf D., Seoane J., Tonn J.-C., Wesseling P., et al. Leptomeningeal metastasis from solid tumours: EANO–ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open. 2023;8:101624. doi: 10.1016/j.esmoop.2023.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ino R., Sada K.-E., Miyauchi A., Hashimoto D., Nojima S., Yamanaka S., Kawamura M. Added Diagnostic Value of Cerebrospinal Fluid Carcinoembryonic Antigen in a Patient with Leptomeningeal Carcinomatosis as the Initial Manifestation of Gastric Cancer. Acta Medica Okayama. 2021;75:659–661. doi: 10.18926/AMO/62781. [DOI] [PubMed] [Google Scholar]
- 19.Mittica G., Senetta R., Richiardi L., Rudà R., Coda R., Castellano I., Sapino A., Cassoni P. Meningeal carcinomatosis underdiagnosis and overestimation: Incidence in a large consecutive and unselected population of breast cancer patients. [(accessed on 5 May 2024)];BMC Cancer. 2015 15:1021. doi: 10.1186/s12885-015-2042-y. Available online: https://pubmed.ncbi.nlm.nih.gov/26715407/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pentheroudakis G., Pavlidis N. Management of leptomeningeal malignancy. Expert Opin. Pharmacother. 2005;6:1115–1125. doi: 10.1517/14656566.6.7.1115. [DOI] [PubMed] [Google Scholar]
- 21.Rubió-Casadevall J., Carbó-Bagué A., Puigdemont M., Osca-Gelis G., Oliveras G., Vilar-Coromina N., Ferrer-Fabrega B., Urban A., Llobet-Roma M., Martín-Romero F., et al. Population-based analysis of the prevalence of BRAF mutation in patients diagnosed with cutaneous melanoma and its significance as a prognostic factor. [(accessed on 5 May 2024)];Eur. J. Dermatol. 2021 31:616–622. doi: 10.1684/ejd.2021.4136. Available online: https://pubmed.ncbi.nlm.nih.gov/34789445/ [DOI] [PubMed] [Google Scholar]
- 22.Ferguson S.D., Bindal S., Bassett R.L., Haydu L.E., McCutcheon I.E., Heimberger A.B., Li J., O’brien B.J., Guha-Thakurta N., Tetzlaff M.T., et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD) [(accessed on 5 May 2024)];J. Neuro-Oncol. 2019 142:499–509. doi: 10.1007/s11060-019-03121-2. Available online: https://pubmed.ncbi.nlm.nih.gov/30847840/ [DOI] [PubMed] [Google Scholar]
- 23.Fuchizaki U., Ohta H., Kaneko S. Image of the Month: David M. Warshauer, Section Editor. Gastroenterology. 2005;128:1773. doi: 10.1053/j.gastro.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Braeuninger S., Mawrin C., Malfertheiner P., Schildhaus H.-U., Seiler C., Dietzmann K., Lins H. Gastric adenocarcinoma with leptomeningeal carcinomatosis as the presenting manifestation: An autopsy case report. Eur. J. Gastroenterol. Hepatol. 2005;17:577–579. doi: 10.1097/00042737-200505000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.G., Lee B., Kim S.M., Suh B.J., Yu H.J. A Case of Gastric Adenocarcinoma Presenting as Meningeal Carcinomatosis. Korean J. Intern. Med. 2007;22:304–307. doi: 10.3904/kjim.2007.22.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cresto N., Barth A., Arnold M., Weimann R., Gschossmann J., Ochsenbein A.F., Kolotas C., Peter H.J., Schiemann U. Extraordinary manifestation of a gastric carcinoma by leptomeningeal carcinomatosis and spinal metastasis. Med. Klin. 2007;102:255–258. doi: 10.1007/s00063-007-1030-7. [DOI] [PubMed] [Google Scholar]
- 27.Suto C., Oohira A., Funaki C., Kanno S., Mori Y. Pathological findings of optic neuropathy from metastatic leptomeningeal carcinomatosis. Jpn. J. Ophthalmol. 2007;51:396–398. doi: 10.1007/s10384-007-0466-x. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T., Furukawa K., Yokoi K., Ohaki Y., Okada S., Tajiri T. A case of meningeal carcinomatosis with gastric cancer which manifested meningeal signs as the initial symptom; the palliative benefit of radiotherapy. J. Nippon. Med. Sch. 2008;75:216–220. doi: 10.1272/jnms.75.216. [DOI] [PubMed] [Google Scholar]
- 29.Silverman A., Loube D., Lovall M., Cheronis C., Madill E., Karch C. Teaching NeuroImage: Cutaneous Lesions and Leptomeningeal Carcinomatosis in Gastric Signet-Ring Cell Carcinoma. Neurology. 2023;101:E1104–E1105. doi: 10.1212/WNL.0000000000207448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Rhun E., Devos P., Winklhofer S., Lmalem H., Brandsma D., Kumthekar P., Castellano A., Compter A., Dhermain F., Franceschi E., et al. Prospective validation of a new imaging scorecard to assess leptomeningeal metastasis: A joint EORTC BTG and RANO effort. [(accessed on 5 May 2024)];Neuro Oncol. 2022 24:1726–1735. doi: 10.1093/neuonc/noac043. Available online: https://pubmed.ncbi.nlm.nih.gov/35157772/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M. Intracranial involvement by metastatic advanced gastric carcinoma. [(accessed on 5 May 2024)];J. Neuro-Oncol. 1999 43:59–62. doi: 10.1023/A:1006156204385. Available online: https://pubmed.ncbi.nlm.nih.gov/10448872/ [DOI] [PubMed] [Google Scholar]
- 32.Baccili Cury Megid T., Baskurt Z., Ma L.X., Barron C.C., Farooq A., Saltiel M.P., Wang X., Bach Y., Ayoama H., Jang R.W., et al. Leptomeningeal carcinomatosis and brain metastases in gastroesophageal carcinoma: A real-world analysis of clinical and pathologic characteristics and outcomes. [(accessed on 5 May 2024)];J. Neurooncol. 2024 167:111–122. doi: 10.1007/s11060-024-04576-8. Available online: https://pubmed.ncbi.nlm.nih.gov/38372902/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Rhun E., Devos P., Weller J., Seystahl K., Mo F., Compter A., Berghoff A.S., Jongen J.L.M., Wolpert F., Rudà R., et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. [(accessed on 5 May 2024)];Neuro-Oncol. 2021 23:1100–1112. doi: 10.1093/neuonc/noaa298. Available online: https://pubmed.ncbi.nlm.nih.gov/33367859/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S.K., Agris J.M., Leeds N.E., Ginsberg L.E. Intracranial leptomeningeal metastases: Comparison of depiction at FLAIR and contrast-enhanced MR imaging. Radiology. 2000;217:50–53. doi: 10.1148/radiology.217.1.r00oc3550. [DOI] [PubMed] [Google Scholar]
- 35.Clarke J., Perez H., Jacks L., Panageas K., DeAngelis L. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Oostenbrugge R., Twijnstra A. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology. 1999;53:382. doi: 10.1212/WNL.53.2.382. [DOI] [PubMed] [Google Scholar]
- 37.Balm M., Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch. Neurol. 1996;53:626–632. doi: 10.1001/archneur.1996.00550070064013. [DOI] [PubMed] [Google Scholar]
- 38.Weston C.L., Glantz M.J., Connor J.R. Detection of cancer cells in the cerebrospinal fluid: Current methods and future directions. Fluids Barriers CNS. 2011;8:14. doi: 10.1186/2045-8118-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucarini A., Garbarino G.M., Orlandi P., Garofalo E., Bragaglia L., Laracca G.G., Canali G., Pecoraro A., Mercantini P. From “Cure” to “Care”: The Role of the MultiDisciplinary Team on Colorectal Cancer Patients’ Satisfaction and Oncological Outcomes. [(accessed on 5 May 2024)];J. Multidiscip. Health. 2022 15:1415–1426. doi: 10.2147/JMDH.S362550. Available online: https://www.dovepress.com/from-cure-to-care-the-role-of-the-multidisciplinary-team-on-colorectal-peer-reviewed-fulltext-article-JMDH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercantini P., Lucarini A., Mazzuca F., Osti M.F., Laghi A. How technology can help in oncologic patient management during COVID-19 outbreak. [(accessed on 5 May 2024)];Eur. J. Surg. Oncol. (EJSO) 2020 46:1189–1191. doi: 10.1016/j.ejso.2020.04.050. Available online: https://pubmed.ncbi.nlm.nih.gov/32389524/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Batran S.E., Homann N., Pauligk C., Illerhaus G., Martens U.M., Stoehlmacher J., Schmalenberg H., Luley K.B., Prasnikar N., Egger M., et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients with Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237–1244. doi: 10.1001/jamaoncol.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.