Abstract
Background
Left ventricular assist devices (LVADs) have emerged as a successful treatment option for patients with end-stage heart failure. Compared with the best medical therapy, LVADs improve survival and enhance functional capacity and quality of life. However, two major complications compromise this patient population’s outcomes: thrombosis and bleeding. Despite technological innovations and better hemocompatibility, these devices alter the rheology, triggering the coagulation cascade and, therefore, require antithrombotic therapy. Anticoagulation and antiplatelet therapies represent the current standard of care. Still, inconsistency in the literature exists, especially whether antiplatelet therapy is required, whether direct oral anticoagulants can replace vitamin K antagonists and even whether phosphodiesterase type 5 inhibitors with their antithrombotic effects could be added to the regimen of anticoagulation.
Methods and analysis
We will perform a living systematic review with network meta-analysis and indirect comparison between current antithrombotic therapies, which have and have not been directly compared within clinical trials and observational studies. We will systematically search the following electronic sources: Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica Database (EMBASE). We will exclusively examine studies published in English from 2016 to the present. Studies conducted before 2016 will be omitted since our primary focus is evaluating continuous flow devices. Two independent reviewers will assess the articles by title, abstract and full text; any disagreement will be resolved through discussion, and a third reviewer will be involved if necessary. The Cochrane Risk of Bias tool will be used to assess the risk of bias. We will then conduct a pairwise meta-analysis; if the assumption of transitivity is satisfied, we will proceed with network meta-analysis using Bayesian methodology.
Ethics and dissemination
Formal ethical approval is not required as no primary data are collected. This systematic review and network meta-analysis will delineate the risks of stroke, thromboembolic events, pump thrombosis, gastrointestinal bleeding and mortality in patients equipped with LVADs who are subjected to various antithrombotic regimens. The findings will be disseminated via a peer-reviewed publication and presented at conference meetings. This will enhance clinical practice and guide future research on anticoagulation strategies within this distinct patient cohort.
PROSPERO registration number
CRD42023465288.
Keywords: Anticoagulation, Heart failure, Bleeding disorders & coagulopathies, Thromboembolism
STRENGTHS AND LIMITATIONS OF THIS STUDY.
In left ventricular assist device (LVAD) patients, anticoagulation practices, particularly concerning aspirin dosage, exhibit significant global variability, potentially introducing heterogeneity into the study and complicating analysis.
Variations in follow-up durations across studies, attributed to the absence of a standardised reporting protocol for major outcomes in LVAD patients, could affect outcome consistency.
The evidence base is restricted to a limited set of clinical trials; therefore, our analysis will encompass both clinical trials and observational studies. We recognise that observational studies’ inherent heterogeneity and biases could pose significant challenges when analysing the data.
Background and rationale
Heart failure is a global health crisis that appears to be on the rise, mainly due to the ageing population.1 Despite the availability of effective medical treatments for heart failure, a considerable number of patients progress to advanced congestive heart failure stages. For these individuals, cardiac transplantation is the optimal and conclusive treatment option. However, the chronic shortage of donor organs worldwide has led to a growing disparity between potential heart transplant recipients and available donor hearts. Consequently, left ventricular assist devices (LVADs) have emerged as a viable alternative not only to temporarily support heart function until a suitable heart becomes available2 but also as a definitive therapy.
In 2001, the landmark Randomised Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial demonstrated the effectiveness of the HeartMate XVE (Thoratec, Pleasanton, California, USA), a pulsatile-flow LVAD, in reducing all-cause mortality compared with optimal medical therapy (52% of survival compared with 25% in the medical group, p=0.002).3 Since then, LVADs have undergone considerable advancements, becoming smaller, more haemocompatible, silent and durable, making them increasingly suitable for long-term support.
Continuous-flow (CF) technology with minimal or no pulse physiology4 was a key factor in the miniaturisation of newer LVAD designs. Devices such as HeartMate 3 (HM3) (Abbott Laboratories, Abbott Park, Illinois, USA) and Heartware HVAD (Medtronic, Minneapolis, Minnesota, USA) exemplify CF-LVADs that no longer rely on large pneumatic extracorporeal pumps for generating pulses. Subsequently, survival after LVAD implantation has improved significantly over the last decade. However, this change in blood flow dynamics, characterised by laminar flow with reduced or absent pulsatility in CF-LVADs, is considered a major contributing factor to endothelial dysfunction, leading to potential occurrences of bleeding or thromboembolic events.5 Of note, in June 2021, Medtronic halted the worldwide distribution and sale of the Heartware HVAD device due to an elevated risk of neurological adverse events and mortality.6
To prevent thrombotic events and minimise bleeding incidence, careful antithrombotic management is necessary. In the past, pulsatile devices required only aspirin as antithrombotic therapy.3 Until recently, for newer CF-LVADs, the practice involved lifelong anticoagulation with a vitamin K antagonist (VKA) along with concomitant antiplatelet agents, mainly based on non-randomised evidence.7 8 The ARIES-HM3 trial,9 however, has recently challenged this approach by demonstrating that excluding aspirin from the antithrombotic regimen in patients with a fully magnetically levitated LVAD did not compromise safety and was associated with a reduction in bleeding events, with 74% of patients in the placebo group vs 68% in the aspirin group being alive and free of major nonsurgical haemocompatibility-related adverse events at 12 months. This aspirin avoidance led to a 34% reduction in nonsurgical bleeding events without an increase in stroke or other thromboembolic events. Similarly, the US-TRACE study observed 93.8% freedom from ischaemic stroke and 92.7% from device thrombosis at 1 year among HeartMate II patients on reduced antithrombotic therapy, despite a subsequent bleeding event in 52% of cases.10 The European TRACE study further supports managing HeartMate II patients with a VKA without antiplatelet therapy could reduce the incidence of major bleeding without increasing thromboembolic events, including ischaemic stroke and pump thrombosis, with an 81% freedom from bleeding and 96% freedom from ischaemic stroke at 2 years.11
These findings challenge the necessity of aspirin in antithrombotic regimens, especially with devices such as the HM3, which have significantly reduced the incidence of pump thrombosis. An observational study reported no bleeding events among patients discharged without aspirin, contrasting with a 39% bleeding occurrence in patients treated with aspirin, suggesting the potential safety and efficacy of primary warfarin monotherapy after discharge.12 Another study added insight to this ongoing discussion by examining the effects of discontinuing aspirin in patients equipped with the HeartMate 3 LVAD.13 Initially, 43 patients—after excluding 7 who died before leaving the hospital—received a combination of aspirin and warfarin. Based on personalised evaluations, three patients chose to continue this combined treatment while the remaining 40 switched to only warfarin. This change enabled the researchers to assess the safety and effectiveness of warfarin alone in managing the risks of blood clots and bleeding, with measures such as international normalised ratio (INR) and lactate dehydrogenase levels showing no significant changes after stopping aspirin. The study also tracked the performance of the LVAD, monitoring metrics such as blood pressure, pump speed and flow to ensure the device worked well without aspirin.13
More recently, as a result of enhanced blood compatibility of these devices, more conservative approaches to anticoagulation have been explored. The MAGENTUM-1 study validated lower INR levels without increasing the risk of adverse events.14 Newer direct-acting oral anticoagulants (DOACs) have emerged as a potential substitute for anticoagulation among LVAD patients.15 16 Additionally, observations suggest that a lower dosage of aspirin (81 mg daily) achieves comparable antithrombotic effects compared with the standard dose (325 mg).17 Consequently, a range of worldwide antithrombotic protocols have been investigated, including those excluding aspirin,9 12 13 18 using reduced aspirin doses,17 19 adopting DOACs15 16 and even using phosphodiesterase type 5 inhibitors for their antithrombotic properties.20–22 Due to the absence of direct comparisons between numerous antithrombotic regimens, clinical equipoise exists concerning the most suitable antithrombotic therapy for LVAD patients. Meaningful advancements in antithrombotic treatment will likely emerge only by implementing well-designed randomised trials that directly measure the effects of different treatments. In the interim, an indirect comparison may offer additional insights into this crucial and current aspect of the lives of many LVAD patients worldwide. Therefore, we plan to conduct a living systematic review and network meta-analysis (NMA) of comparative cohort studies and randomised controlled trials to assess the incidence of thrombotic events and bleeding between various antithrombotic regimes in patients implanted with LVADs.
Methods
This protocol is reported following the Preferred Reporting Items for Systematic Review Protocols (PRISMA) Statement (online supplemental appendix A)23 24 and is registered in the International Prospective Register of Systematic Reviews (PROSPERO) database. The review will be conducted under the guidance of The Cochrane Handbook for Systematic Reviews of Interventions and will be reported following the PRISMA Extension Statement for NMA.23 Any protocol modifications will be described in the publication of the final report.
bmjopen-2023-080110supp001.pdf (167.6KB, pdf)
Types of studies
We will include randomised controlled trials and comparative cohort studies. The inclusion of non-randomised studies is justified by the predominance of observational studies over randomised trials in the literature, and the tendency of randomised trials to under-report rare or late-emerging adverse events.
Types of participants
Adult patients greater than 18 years old on continuous flow-LVAD support.
Types of interventions and comparators
Patients receiving VKA (INR goal between 2 and 3) with aspirin 325 mg will be the reference group (or comparator). As new interventions, we will include alternative antithrombotic regimens, such as:
VKAs (at varying INR levels), either in combination with different aspirin doses or without.
Direct thrombin inhibitors.
Phosphodiesterase type 5 inhibitors.
Factor Xa Inhibitors.
The absence of antithrombotic medications.
We will include any of these interventions irrespective of dose and duration of administration.
Outcomes of interest
Stroke, thromboembolic events and pump thrombosis are our primary outcomes; bleeding and mortality are our secondary outcomes. We will define outcomes according to the Interagency Registry for Mechanically Assisted Circulatory Support study25 :
Ischaemic stroke: ‘New acute neurological deficit of any duration associated with acute infarction on imaging corresponding anatomically to the clinical deficit’.
Haemorrhagic stroke: ‘New acute neurological deficit attributable to intracranial haemorrhage.’
Pump thrombosis: ‘Special case of major device malfunction and can be categorised as a suspected device thrombus or confirmed device thrombus.’
Bleeding: ‘Any overt, actionable sign of haemorrhage (eg, more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone).’
Studies with either primary and/or secondary outcomes will be included.
Search strategy and databases
The search strategy was developed with the assistance of an experienced librarian in systematic reviews and NMAs. The search strategy is described in online supplemental appendix B, and we systematically search the following electronic sources: Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE) and Excerpta Medica Database (EMBASE).
We will exclusively examine studies published in English from 2016 to the present. Studies conducted before 2016 will be omitted since our primary focus is evaluating continuous flow devices. It is noteworthy that studies before 2016 typically involved the assessment of pulsatile flow devices, which have since become obsolete.
We will conduct searches on ClinicalTrials.gov and clinicaltrialregister.eu to locate ongoing trials. Furthermore, we will find additional references by manually reviewing the citations of the included articles. Our database searches will be refreshed every 2 months until publication.
Study selection
All records identified by the search strategy will be uploaded to Covidence 2.0 software,26 and duplicates will be removed. Two independent reviewers (SHD vs OD or HS) will screen studies for eligibility based on titles, abstracts and full texts using the eligibility criteria. Any discrepancies in the inclusion criteria will be resolved through discussion and consensus between the reviewers. If necessary, a third reviewer (DT) will be involved. We will use the discrepancies between the reviewers to calculate a kappa statistic and assess inter-reviewer reliability; a kappa statistic >0.6 will be considered acceptable.
We will document the reasons for excluding full texts and present this information using Covidence to create a PRISMA flow chart.
Data extraction
We will design a standardised data extraction form that will be piloted on 10% of included studies. Two reviewers will independently extract the data, and any inconsistencies will be resolved through discussion or with a third reviewer, if necessary. If we need further information or the data appears insufficient, we will contact the authors. If it is impossible to reach the authors, we will discuss this limitation in the final manuscript.
Using the data extraction form, we will capture the following information: title, authors, journal, publication date, study period, number of participants, country, type of implanted device, study population characteristics, antithrombotic regimens and primary and secondary outcomes.
If necessary, our team will contact study authors to obtain additional information for our review.
Living systematic review
We will perform updates to our search every 2 months. A range of antiplatelet and anticoagulation strategies are being used, but there is a lack of studies directly comparing them. We are of the opinion that ongoing clinical trials focused on antithrombotic therapies have the potential to offer new perspectives that will enrich our NMA.
Network meta-analysis
Before proceeding with the NMA, we will assess if sufficient statistical data exist to evaluate their consistency and the assumption of transitivity.
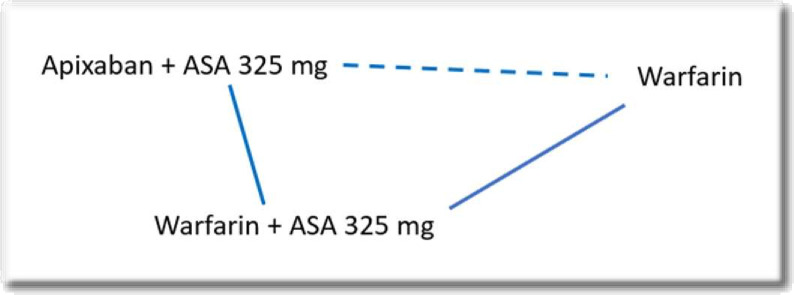
According to this assumption, we can only examine these trials when we have closed loops, and it assumes that the distribution of the effect modifiers is comparable across treatments. For instance, if studies of warfarin and aspirin versus apixaban and aspirin, and warfarin-aspirin versus warfarin differ with respect to their effect modifiers, then it would not be appropriate to make an indirect comparison between apixaban-aspirin and warfarin-only regimen (figure 1).
Figure 1.
The dashed line indicates an indirect comparison. ASA, aspirin.
If NMA is conducted, we will adopt a Bayesian approach and a random effects model for binomial and continuous outcomes, assessing the effect estimate of each anticoagulation therapy.
Mean differences and ORs with 95% CIs will be presented. Following unadjusted analysis, secondary analyses will be conducted to account for any imbalance in the distribution of effect modifiers, especially types of devices. Network meta-regression methods will be conducted to account for these differences.
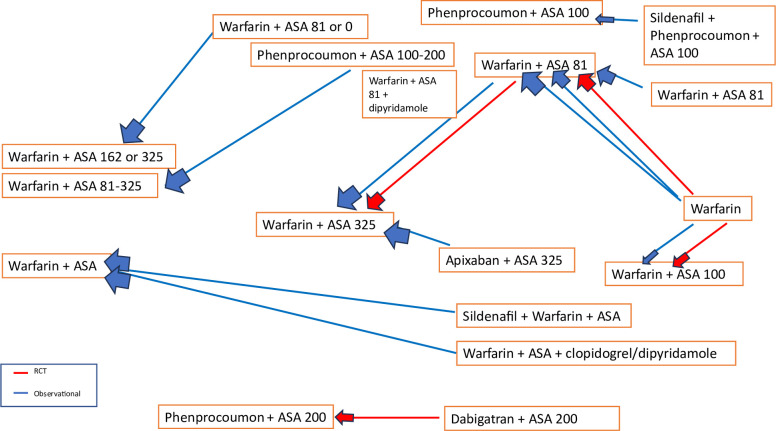
Geometry of the network
The network diagram will visually represent the available evidence of each comparison between different antithrombotic regimens. Figure 2 shows a draft of the possible network diagram for our future analysis.
Figure 2.
The network diagram depicts multiple comparisons among different antithrombotic regimens used in LVAD patients. Although warfarin combined with 325 mg of aspirin was the standard treatment until recently, there has been a trend towards more conservative strategies in current practice. ASA, aspirin; LVAD, left ventricular assist device; RCT, randomised controlled trial.
Risk of bias in individual studies
To determine methodological validity, we will assess the risk of bias of the included studies at a study level using the Revised Cochrane Collaborations Risk-of-Bias (RoB 2) tool and ROBINS-I (Risk Of Bias In Non-randomised Studies–of Interventions). Any discrepancies will be resolved through discussion until a consensus is reached.
Summary measures
Primary outcome
Incidence of stroke and thromboembolic events will be reported as dichotomous outcomes occurring at any time after implantation of the LVAD until 3 years of follow-up. Relative risks with 95% CIs will be calculated to compare the incidence of stroke between different antithrombotic regimens.
Secondary outcomes
Bleeding will be reported as dichotomous data.
Pairwise meta-analysis
We will conduct a pairwise meta-analysis using random-effects model. Statistical heterogeneity within pairwise comparisons will be evaluated by visual inspection of forest plots and I2 measure. If there is a high amount of heterogeneity (I2>75%), then sources of heterogeneity will be examined through subgroup and sensitivity analyses.
Subgroup and sensitivity analyses
If the studies have high heterogeneity, subgroup analysis will be performed based on age, type of device and recalled devices from 2021.
Sensitivity analysis will be used to verify the reliability of the results. According to the Cochrane Handbook, sensitivity analysis will be conducted in the three aspects of methodological quality, sample size and statistical model. We will exclude studies with poor research quality, small sample size and high risk of bias.
Assessment of inconsistency
Inconsistency in the data will be assessed by fitting inconsistency model scatterplots and using Cochran’s Q test. A statistician with experience in systematic review and NMA will assist our team.
Ethics and dissemination
This study is SR protocol collecting data from published literature, and therefore, does not require institutional review board approval. Results from this SR and NMA will be published in a peer-reviewed journal.
Patient and public involvement
No patients or members of the public will be directly assessed. Only data already reported in the literature will be used in this study.
Discussion
This systematic review and NMA is conducted against a backdrop of evolving LVAD technology and antithrombotic therapy. With the HM3 emerging as the only available implantable pump in many regions and its notably low risk of thrombosis, the implications of antithrombotic management have never been more pivotal. The HM3’s technological advancements have reduced the incidence of pump thrombosis, shifting the focus towards managing bleeding risks. The ARIES study’s findings are particularly relevant here,9 as they underscore the safety and efficacy of excluding aspirin from the antithrombotic regimen, which could mark a paradigm shift in reducing bleeding events without increasing thromboembolic risks.
While we recognise the pivotal contributions of the ARIES study, particularly its insights into aspirin’s role in the antithrombotic regimens for LVAD recipients, it is critical to underline that its findings predominantly pertain to those with the HeartMate 3 device. This focus leaves a gap in our understanding of therapy for individuals with other devices, such as the still-used HeartMate 2. Furthermore, our analysis seeks to broaden the scope of investigation by assessing the impact of various treatments—including DOACs, phosphodiesterase type 5 inhibitors, and phenprocoumon—on both primary and secondary outcomes. This comprehensive approach is designed to offer a more nuanced understanding of antithrombotic therapy’s efficacy and safety across the diverse spectrum of LVAD technologies and patient needs.
Supplementary Material
Footnotes
Contributors: SHD, DT and GAW conceived the idea and design for this systematic review. SHD, OD, DT and GAW developed the methodology for the systematic review protocol. The contents of this manuscript were drafted by SHD, OD and GAW with input from all members of the authorship team. The manuscript was reviewed by SHD, OD, DT, HS and GAW for important intellectual content. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 4. Lescroart M, Hébert J-L, Vincent F, et al. Pulsatility in ventricular assistance devices: a translational review focused on applied haemodynamics. Arch Cardiovasc Dis 2020;113:461–72. 10.1016/j.acvd.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 5. Khalil F, Asleh R, Perue RK, et al. Vascular function in continuous flow lvads: implications for clinical practice. Biomedicines 2023;11:757. 10.3390/biomedicines11030757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urgent Medical Device Communication Notification Letter Medtronic HVADTM System, Available: https://www.medtronic.com/content/dam/medtronic-com/global/HCP/Documents/hvad-urgent-medical-device-notice-june-2021.pdf
- 7. Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International society for heart and lung transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157–87. 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 8. Potapov EV, Antonides C, Crespo-Leiro MG, et al. EACTS expert consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 2019;56:230–70. 10.1093/ejcts/ezz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehra MR, Netuka I, Uriel N, et al. Aspirin and hemocompatibility events with a left ventricular assist device in advanced heart failure: the ARIES-Hm3 randomized clinical trial. JAMA 2023;330:2171–81. 10.1001/jama.2023.23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in heartmate II patients: a one-year analysis of the US-TRACE study. J Heart Lung Transplant 2015;34:1542–8. 10.1016/j.healun.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 11. Netuka I, Litzler P-Y, Berchtold-Herz M, et al. Outcomes in heartmate II patients with no antiplatelet therapy: 2-year results from the European TRACE study. Ann Thorac Surg 2017;103:1262–8. 10.1016/j.athoracsur.2016.07.072 [DOI] [PubMed] [Google Scholar]
- 12. Consolo F, Raimondi Lucchetti M, Tramontin C, et al. Do we need aspirin in heartmate 3 patients? European J of Heart Fail 2019;21:815–7. 10.1002/ejhf.1468 [DOI] [PubMed] [Google Scholar]
- 13. Lim HS, Ranasinghe A, Mascaro J, et al. Discontinuation of aspirin in heartmate 3 left ventricular assist device. ASAIO J 2019;65:631–3. 10.1097/MAT.0000000000000859 [DOI] [PubMed] [Google Scholar]
- 14. Connors JM, Gregor S, Crandall D, et al. Low-intensity anti-coagulation using vitamin K antagonists and factor X activity: a validation analysis of the MAGENTUM-1 study. J Heart Lung Transplant 2019;38:668–9. 10.1016/j.healun.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 15. Andreas M, Moayedifar R, Wieselthaler G, et al. Increased thromboembolic events with dabigatran compared with vitamin K antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ Heart Fail 2017;10:e003709. 10.1161/CIRCHEARTFAILURE.116.003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitehouse KR, Avula D, Kahlon T, et al. Apixaban: alternative anticoagulation for heartmate 3 ventricular assist device. ASAIO J 2022;68:318–22. 10.1097/MAT.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 17. Saeed O, Colombo PC, Mehra MR, et al. Effect of aspirin dose on hemocompatibility-related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant 2020;39:518–25. 10.1016/j.healun.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarzia V, Tessari C, Bagozzi L, et al. Anticoagulation alone as an effective and safe antithrombotic therapy in LVAD: when less is more. Curr Probl Cardiol 2023;48:101506. 10.1016/j.cpcardiol.2022.101506 [DOI] [PubMed] [Google Scholar]
- 19. Simone PB, Bullard H, Siddiqi U, et al. Impact of aspirin dosing on thrombotic outcomes in patients with the HVAD. ASAIO J 2021;67:e153–6. 10.1097/MAT.0000000000001421 [DOI] [PubMed] [Google Scholar]
- 20. Xanthopoulos A, Tryposkiadis K, Triposkiadis F, et al. Postimplant phosphodiesterase type 5 inhibitors use is associated with lower rates of thrombotic events after left ventricular assist device implantation. J Am Heart Assoc 2020;9:e015897. 10.1161/JAHA.119.015897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xanthopoulos A, Wolski K, Wang Q, et al. Postimplant phosphodiesterase-5 inhibitor use in centrifugal flow left ventricular assist devices. JACC Heart Fail 2022;10:89–100. 10.1016/j.jchf.2021.09.008 [DOI] [PubMed] [Google Scholar]
- 22. Krim SR, Bennett A, Pfeffer M, et al. Triple antithrombotic therapy in patients with left ventricular assist devices. Curr Probl Cardiol 2022;47:100940. 10.1016/j.cpcardiol.2021.100940 [DOI] [PubMed] [Google Scholar]
- 23. Hutton B, Catalá-López F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) 2016;147:262–6. 10.1016/j.medcli.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 24. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 25. INTERMACS User’s guide, Available: https://intermacs.kirso.net/intermacs-documents/
- 26. Covidence systematic review software, veritas health innovation. 2023. Melbourne, Australia; 2023. Available: at www.covidence.org [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-080110supp001.pdf (167.6KB, pdf)