Public health officials rely on criteria developed by Wilson and Jungner for assessing whether or not to implement population screening programmes. These criteria were developed over 30 years ago, when screening primarily focused on detecting early stages or precursors of chronic disease. With the introduction of testing for genetic susceptibility, particularly for cancer, it is important to assess whether these criteria can continue to be applied in the decision making process. We report on a workshop that assessed criteria for population screening in the context of testing for genetic susceptibility to cancer.
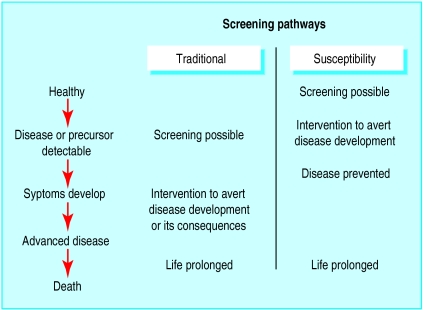
Many criteria for the evaluation of screening programmes have been proposed,1,2 and most are similar to those proposed by Wilson and Jungner in a 1968 World Health Organization report.3 The criteria are based on a simple linear model of disease progression (figure) in which screening tests primarily detect a preclinical asymptomatic phase.
Summary points
Screening has expanded from early detection of disease or its precursors to include testing for susceptibility, such as genetic testing for cancer
The Wilson and Jungner framework for evaluating screening tests, produced for the World Health Organization in 1968, is commonly used for population screening
The relevance of this framework for testing for genetic susceptibility to cancer has not previously been assessed
A modified Wilson and Jungner framework can continue to provide a robust approach to evaluating testing for genetic susceptibility
The continuum of screening has expanded to include a range of other states. The figure illustrates another model for screening—screening for risk factors or susceptibility, the detection of risk factors for disease4 (such as blood pressure or cholesterol concentration), or the identification, through the detection of genetic markers, of individuals who have increased susceptibility to disease.5 Separate consideration of these forms of screening is important as the type of interventions that can be offered to individuals who have positive test results can differ: treatment in the case of traditional screening, interventions to modify the risk factor, or counselling if susceptibility is identified. Thus, the criteria for evaluating programmes at the population level for such tests could differ.
Is it premature to discuss criteria for organised screening programmes for testing for genetic susceptibility to cancer? We consider that it is better to have a discussion about such criteria now, before such tests become widely adopted.6,7 We report on the Crossroads 99 conference, held in Toronto, Canada, in October 1999, in conjunction with an international symposium examining the ethical, legal, and sociobehavioural implications of notification of risk of breast, ovarian, and colorectal cancer. The workshop participants included consumers, healthcare providers (clinical geneticists, genetics counsellors, medical, surgical, and radiation oncologists, public health specialists, psychologists, psychiatrists, nurses, social workers, and primary care physicians), researchers (basic scientists, epidemiologists, social scientists), lawyers, and ethicists.
The objective was to develop a consensus on a framework to be used for introducing population based screening programmes based on notification of risk for cancer. An explicit purpose was to ensure that the framework examined ethical, legal, social, and economic implications and could be applied to current and future tests for susceptibility.
Method
The Wilson and Jungner 1968 framework (table 1) for evaluating screening tests was presented as an example of a type of framework, but workshop participants were not bound to follow its structure or content. The workshop proceeded in three steps: identification of the domains or the major category headings for the framework; identification of criteria or the specific items within each domain; and prioritisation and selection of the criteria.
Table 1.
Criteria for assessment of screening
| Wilson and Jungner | Crossroads 99* |
|---|---|
| Knowledge of disease | Knowledge of population and disease |
| Condition must be important problem | Burden of target disease should be important |
| Target population or population at risk identifiable | |
| Recognisable latent or early symptomatic stage | Considerable level of risk or latent or preclinical phase |
| Natural course of condition, including development from latent to declared disease, should be adequately understood | Natural course (from susceptibility to precursor, early disease, and advanced disease) should be adequately understood |
| Knowledge of test | Feasibility of screening procedures |
| Suitable test or examination | Suitable test or examination |
| Test acceptable to population | Entire screening procedure acceptable to population |
| Case finding should be continuing process and not “once and for all” project | Screening should be continuing process and encompass all elements of screening procedures |
| Treatment for disease | Interventions and follow up |
| Accepted treatment for patients with recognised disease | Interventions that have physical, psychological, and social net benefit available |
| Facilities for diagnosis and treatment available | Facilities for adequate surveillance, prevention, treatment, education, counselling, and social support available |
| Agreed on policy concerning whom to treat as patients | Consensus on accepted management for those with positive test results |
| Cost considerations | Societal and health system issues |
| Costs of case finding (including diagnosis and treatment of patients diagnosed) economically balanced in relation to possible expenditures on medical care as whole | Costs should be balanced in economic, psychological, social, and medical terms and with healthcare expenditures as whole |
| Appropriate screening services accessible to entire population without adverse consequences for non-participants | |
| Appropriate confidentiality procedures and antidiscrimination provisions for participants and non-participants |
Ethical, legal, and sociobehavioural issues are considered across all domains. Screening should be considered within framework that recognises fundamental human rights.
The workshop participants worked in multidisciplinary groups that identified key domains and criteria. Common themes from the groups were identified and the resulting model reviewed in a plenary session.
Results
The overall thrust of the Wilson and Jungner framework was strongly agreed with, although there was some broadening of the domains and criteria. The consensus was that ethical, legal, and social issues should be dealt with across all the domains, rather than treating them separately. These issues were viewed as essential for all considerations involving a screening programme. Creation of a separate category for them could lead to their being marginalised.
The participants also recommended that all screening programmes should observe the basic universal principles of human rights, such as those in the convention on human rights and biomedicine.8 This statement covers the primacy of the human being, equitable access to health care, privacy, right to information, non-discrimination, and use of predictive genetic tests. Considerations about new screening technologies and programmes that seek to apply them should pay attention to such fundamental principles.
Knowledge of population and disease
Table 1 lists the original and revised framework. The first domain has been broadened to include characteristics of the population. This acknowledges that in testing for susceptibility it is important not only to understand the disease but also the population that is to be tested. The first criterion is essentially unchanged except the more commonly used term “burden of disease” is used. The burden ultimately being considered should be that of the disease to be prevented, not the condition or marker status that is being screened for. For example, in genetic screening for ovarian cancer it is the incidence and prevalence of ovarian cancer that is important in determining burden, not the prevalence of the marker status for the condition. An analogy may be drawn with cholesterol screening, in which “hypercholesterolaemia” is often labelled as disease. The prevalence of this state is far greater than that of the target conditions such as heart disease.
The second criterion in this domain is new. There is need for acceptable and valid methods for identifying those individuals in the target population who are most likely to benefit from susceptibility testing. For example, the Amsterdam criteria identify those at risk for hereditary non-polyposis colorectal cancer.9 The target population may be individuals or families. The need to identify the target population is not necessarily a new concept when it comes to screening as all accepted screening tests to date have had some component of identification of a target population, usually on the basis of age. However, the Wilson and Jungner criteria did not explicitly specify that the mechanism for selecting those eligible for screening should be reliable and valid.
In addition to being able to identify a latent or preclinical phase, knowledge of a measurable level of risk should be a criterion. For example, tests for BRCA1 or 2 mutations may identify an increase in risk of disease. To make decisions about screening programmes, knowledge of the level of risk (that is, penetrance) and its relevance is important. Relevance will depend on context and will certainly have to be an issue of on going research.
Natural course is also included from the original framework but is extended to include knowledge of the natural course from the state of susceptibility to the state of precursor or early disease. The risk of developing a disease, the stages it goes through, and the time periods over which this occurs should be well understood.
Feasibility of screening procedures
This domain's title has been expanded, although the basic concepts are unchanged. Screening procedures involve more than the test alone. They include a range of activities from identification of the target population to recruitment, counselling, informed consent, administration of the actual test, and communication of the results.
In terms of the suitability of a test or examination, in addition to elements such as accuracy (sensitivity and specificity) and predictive value there needs to be an understanding of the value of the test results, standards for analysis, and a clear understanding of the limitations.
The test needs to be acceptable to the target or at risk population. This has conventionally been approached in terms of the discomfort of the test and its side effects. With testing for genetic susceptibility this approach needs to be broadened to include consideration of acceptability to the individual, family, and society in terms of psychological, ethical, legal, and social implications. An example of an issue that may need to be considered would be potential discrimination and denial of insurance. Potential side effects need to be considered in the context of the family. Even those individuals who do not participate in screening can suffer potential effects. For instance, individuals who decline to undergo a test may have relatives who have positive test results, and this information may not remain confidential. These individuals may then be identified as coming from the “positive family,” even though they did not want to be tested. For example, three sisters out of four may decide to undergo testing for susceptibility for breast cancer. If two out of the three who are tested are identified as having a mutation, then that family will be labelled as “positive.” The third sister, who has negative results, may disclose that she is not affected but that there is a mutation in the family. The fourth sister, who chose not to be tested, is then identifiable as being from an affected family and could suffer, even though she did not want to take part in testing at all.
Not only is there a need for informed consent and counselling that is inclusive of families and, when appropriate, communities (for example, ethnic groups), screening programmes need to ensure that there are staff and facilities available for such services. Delivery of the screening programme should include provision of education for consumers and health professionals.
Interventions and follow up
This domain underwent considerable expansion, although the criteria are conceptually the same. Rather than considering only treatments for disease we consider a range of interventions and follow up strategies. The evaluations of these interventions should examine social and psychological aspects as well as physical aspects. The interventions need to have demonstrable net benefit on these dimensions. For example, prophylactic mastectomy may be an effective intervention for reducing risk of cancer in carriers of the BRCA mutation, but evidence would also be required on its psychological impact. Physical benefits would need to be traded off against potential adverse psychological or social effects.
Facilities for all aspects of follow up need to be available. There are many services required in addition to diagnosis and treatment. Prevention includes primary means through dietary modification, chemoprevention, and prophylactic surgery. Surveillance, such as colonoscopy in those at high risk for colorectal cancer or the CA 125 blood test or transvaginal ultrasound for women at high risk for ovarian cancer, can itself be a series of screening tests. A screening programme needs to ensure that such services are available, accessible, and of high quality. The population tested will require education, counselling, and social support. Resources to support the practitioners who deliver the services, particularly those at the primary care level, need to be available.
Societal and health system issues
The last category of the original framework examined costs. We propose expansion to societal and health system issues. A full range of costs, including psychological and social, need to be considered.
The principle of equity needs to be observed to ensure that appropriate screening services are accessible. It is vital to ensure that non-participation in such programmes does not adversely affect individuals. Conversely, it will be important to ensure that there is not the potential for conflict between those who are affected with the disease or susceptibility to the disease and the population at large.
Such screening programmes must ensure that there are no injustices based on test results and that there is adequate provision to ensure privacy and confidentiality of the information collected.
Discussion
The Crossroads 99 conference confirmed the overall usefulness of the original Wilson and Jungner framework. Given that it was developed well before modern testing for genetic susceptibility, its robustness is a testament to the foresight of the authors. Table 2 shows the hypothetical application of the Crossroads 99 framework to testing for susceptibility to colorectal cancer.10–12 This illustrates which of the new criteria are not yet met and the usefulness of the new framework in identifying issues for further evaluation and research.
Table 2.
Example of application of new criteria to screening for genetic susceptibility to colorectal cancer
| Crossroads 99 criteria | Testing for susceptibility to colorectal cancer | Met by new criteria |
|---|---|---|
| Knowledge of population and disease | ||
| Burden of target disease should be important | Colorectal cancer is third commonest malignancy in United Kingdom, incidence is increasing | Yes |
| Ability to identify target population or population at risk | Amsterdam criteria are generally accepted; further research on accuracy of criteria is required; no large series of patients fulfilling Amsterdam criteria has mutation detection rate >70% | Partially |
| Considerable level of risk or latent or preclinical phase | Lifetime risk of colorectal cancer in those with two first degree relatives is 1 in 6; in those with autosomal dominant pedigree it is 1 in 2 (population risk is 1 in 50) | Yes |
| Natural course (from susceptibility to precursor, early disease, and advanced disease) should be adequately understood | Natural course from premalignant adenomatous polyps to malignant polyps is understood; whether progression is different in those with different mutations needs to be more clearly established | Partially |
| Feasibility of screening procedures | ||
| Suitable test or examination | Known mutations can be screened for, familial testing protocols are required; acceptability in population is being assessed | Partially |
| Entire screening procedure acceptable to population | Acceptability of familial assessment and mutation testing in general population is not yet established | No |
| Screening should be continuing process and encompass all elements of screening procedures | Complete range of services required for screening (education, counselling, support) not in place | No |
| Interventions and follow up | ||
| Interventions that have physical, psychological, and social net benefit available | Surveillance and treatment available but balance of physical, psychological, and social benefits not established; interventions such as chemoprevention are being evaluated | No |
| Facilities for adequate surveillance, prevention, treatment, education, counselling, and social support available | Level of services required and infrastructure not in place | No |
| Consensus on accepted management for those with positive test results | Emerging consensus on management of mutation carriers | Partially |
| System issues | ||
| Costs should be balanced in economic, psychological, social, and medical terms and with healthcare expenditure as whole | Cost effectiveness in economic, psychological, social, and medical terms not established | No |
| Appropriate screening services accessible to entire population without adverse consequences for non-participants | Familial assessment and testing available only in specialised centres | No |
| Appropriate confidentiality procedures and antidiscrimination provisions for participants and non-participants | Appropriate procedures and provisions not yet completely studied or established | No |
While the workshop primarily considered testing for genetic susceptibility to cancer, the new criteria should be valuable in the consideration of other genetic or susceptibility testing. For example, screening for hereditary haemochromatosis has emerged as a controversial issue.13 Among the issues to be examined with this test are the criteria for selecting the target population; the natural course and burden of disease; the interventions that could be offered to those who are screened, particularly in young people; the psychological and social effects of being tested; and the balance of economic, psychological, and social effects. System issues are paramount: screening programmes should be efficient, accessible, of high quality, ensure consumer choice, and respect the fundamental principles of human rights.
We hope that this revised framework will facilitate debate and comment regarding the development of screening programmes based on testing for genetic susceptibility. Ultimately, criteria such as these will be the basis for evidence based policy decisions for genetic screening programmes. Such a framework can provide an organised approach to the institution of screening programmes for susceptibility to ensure that fundamental issues, such as adequacy of resources for informed consent, capacity for follow up interventions, and systems issues are examined. While the potential benefits of such programmes are huge, the risks are considerable, and indiscriminate use could overwhelm our health systems.
Supplementary Material
Figure.
Screening pathways compared over simplified natural course of disease. Traditional screening identifies early stage disease or precursors to disease. Susceptibility screening moves the process earlier and identifies risk of disease in individuals who are healthy. Thus resulting interventions aim to reduce risk of disease developing rather than trying to reduce severity or consequences of disease
Acknowledgments
The Crossroads 99 meeting was held in November, 1999, in Toronto, in memory of Kathryn Taylor, PhD, who conceived the idea, developed the proposals, and garnered the support for it.
Footnotes
Funding: Crossroads 99 Symposium and Conference was supported by the Canadian Institute for Health Research, the Social Sciences and Humanities Research Council, the National Cancer Institute of Canada with funds raised by the Canadian Cancer Society, the US National Cancer Institute, and the University of Toronto Interdepartmental division of oncology.
Competing interests: None declared.
Details of the workshop participants can be found on the BMJ's website
References
- 1.Cadman D, Chambers L, Feldman W, Sackett D. Assessing the effectiveness of community screening programs. JAMA. 1984;251:1580–1585. [PubMed] [Google Scholar]
- 2.Kizer KW. Guidelines for community-based screening for chronic health conditions. Am J Prev Med. 1991;7:117–120. [PubMed] [Google Scholar]
- 3.Wilson JM. Principles and practice of screening for diseases. Geneva: World Health Organization; 1968. [Google Scholar]
- 4.Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562–1565. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell J. The new genetics in clinical practice. BMJ. 1998;316:618–620. doi: 10.1136/bmj.316.7131.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke AJ. Population screening for genetic susceptibility to disease. BMJ. 1995;311:35–38. doi: 10.1136/bmj.311.6996.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtzman NA, Shapiro D. The new genetics: genetic testing and public policy. BMJ. 1998;316:852–856. doi: 10.1136/bmj.316.7134.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Council of Europe. Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: convention on human rights and biomedicine. Strasbourg, France: Council of Europe Publishing: 1997 (ETS No 164). http://conventions.coe.int (accessed 28 February 2001).
- 9.Burke W, Petersen G, Lynch P, Botkin J, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. JAMA. 1997;277:915–919. [PubMed] [Google Scholar]
- 10.Hardy RG, Meltzer SJ, Jankowski JA. ABC of colorectal cancer: molecular basis for risk factors. BMJ. 2000;321:886–889. doi: 10.1136/bmj.321.7265.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole TR, Sleightholme HV. ABC of colorectal cancer: the role of clinical genetics in management. BMJ. 2000;321:943–946. doi: 10.1136/bmj.321.7266.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholefield JH. ABC of colorectal cancer: screening. BMJ. 2000;321:1004–1006. doi: 10.1136/bmj.321.7267.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddow JE, Bradley LA. Hereditary haemochromatosis: to screen or not. BMJ. 1999;319:531–532. doi: 10.1136/bmj.319.7209.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.