Abstract
Background
Mismatch repair deficiency (dMMR) and microsatellite instability-high (MSI-H) occur in a subset of cancers and have been shown to confer sensitivity to immune checkpoint inhibition (ICI); however, there is a lack of prospective data in urothelial carcinoma (UC).
Methods and analysis
We performed a systematic review to estimate the prevalence of dMMR and MSI-H in UC, including survival and clinical outcomes. We searched for studies published up to 26 October 2022 in major scientific databases. We screened 1745 studies and included 110. Meta-analyses were performed if the extracted data were suitable.
Results
The pooled weighted prevalences of dMMR in bladder cancer (BC) and upper tract UC (UTUC) were 2.30% (95% CI 1.12% to 4.65%) and 8.95% (95% CI 6.81% to 11.67%), respectively. The pooled weighted prevalences of MSI-H in BC and UTUC were 2.11% (95% CI 0.82% to 5.31%) and 8.36% (95% CI 5.50% to 12.53%), respectively. Comparing localised versus metastatic disease, the pooled weighted prevalences for MSI-H in BC were 5.26% (95% CI 0.86% to 26.12%) and 0.86% (95% CI 0.59% to 1.25%), respectively; and in UTUC, they were 18.04% (95% CI 13.36% to 23.91%) and 4.96% (95% CI 2.72% to 8.86%), respectively. Cumulatively, the response rate in dMMR/MSI-H metastatic UC treated with an ICI was 22/34 (64.7%) compared with 1/9 (11.1%) with chemotherapy.
Conclusion
Both dMMR and MSI-H occur more frequently in UTUC than in BC. In UC, MSI-H occurs more frequently in localised disease than in metastatic disease. These biomarkers may predict sensitivity to ICI in metastatic UC and resistance to cisplatin-based chemotherapy.
INTRODUCTION
Approximately 83 000 cases of urothelial carcinoma (UC) are diagnosed annually in the USA, with upper tract (including renal pelvis and ureter) UC (UTUC) accounting for 5%–10% of diagnoses.1–3 About one-third of UC patients present with muscle-invasive disease which carries a significant risk of progression to metastatic disease. For most of these patients, radical surgery offers the best likelihood of cure, especially when combined with perioperative systemic therapies.4–7 In the metastatic setting, systemic options have expanded significantly in recent years, including immune checkpoint inhibitors (ICI), enfortumab vedotin, erdafitinib for patients with FGFR3-altered tumours, sacituzumab govitecan and new treatment combinations such as enfortumab vedotin with pembrolizumab8–12; however, UC remains an aggressive disease with generally poor outcomes.3
Mismatch repair deficiency (dMMR) occurs when there is a loss of one or more of the four MMR proteins: MLH1, MSH2, MSH6 and PMS2. This results in impaired correction of spontaneous mutations in repetitive DNA sequences, leading to a high frequency of nucleotide gain or loss from microsatellite tracts, known as microsatellite instability (MSI).13 14 Lynch syndrome is an autosomal-dominant inherited deficiency in MMR associated with malignancies including colorectal, endometrial and UTUC.15 16 A variant of Lynch syndrome, known as Muir-Torre syndrome, is characterised by sebaceous neoplasms or keratoacanthoma in addition to one or more Lynch syndrome-related malignancies.17 18 Tumours with dMMR or microsatellite instability-high (MSI-H) are associated with a higher expression of tumour neoantigens, which facilitates immune recognition. This immunogenic phenotype increases the susceptibility of tumours to reactivation of an anticancer immune response by ICI.19–21 Pembrolizumab has a tumour-agnostic FDA approval for use in dMMR/MSI-H metastatic cancers based on phase 2 data.22 23 Furthermore, recent clinical trials have demonstrated exquisite sensitivity of locally advanced dMMR/MSI-H rectal and colon cancer to ICI, making this a promising treatment modality that could avoid life-altering radical treatment for some patients.24–26 Based on mostly retrospective data, it is estimated that about 3%–10% of UC will have dMMR or MSI-H.27 28
This study aimed to conduct a systematic review and meta-analysis of UC with dMMR or MSI. Our objectives were (1) to estimate the prevalence of dMMR and MSI in localised and metastatic bladder cancer (BC) and UTUC, (2) to evaluate the activity and/or efficacy of ICI, cisplatin-based chemotherapy and/or other treatment modalities and (3) to estimate survival in this subgroup of patients.
MATERIALS AND METHODS
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see online supplemental material).29 The study protocol was approved by all authors and registered on the PROSPERO database (CRD42022365690). Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our study.
We included studies or publications which included patients with BC or UTUC and reported on dMMR and/or MSI-H. Types of studies or publications included clinical trials, prospective observational studies, retrospective studies, case series, case reports,and review articles.
A comprehensive search strategy was developed by a National Institutes of Health librarian (GB) and two authors (EBAC and GMI). The search was performed on 28 October 2022, by GB in PubMed, then translated into multiple databases and peer reviewed. We searched the following electronic databases: PubMed (National Library of Medicine), EMBASE (Elsevier), Cochrane Library (Wiley & Sons), CINAHL (EBSCOhost) and Web of Science (Clarivate Analytics). Grey literature was searched including conference proceedings, and preprints (bioRxiv and medRxiv). In addition to database searches, reference lists of the included publications were evaluated for potentially eligible publications by two authors (EBAC and GMI). The search strategy is available in online supplemental material.
Study selection and data collection
After completing database searches, results were imported by GB into EndNote V.2.0 (Clarivate Analytics), a citation management software. Records were screened using Covidence screening software (Covidence, Melbourne, Victoria, Australia). Selection of eligible publications was conducted independently by two authors (EBAC and GMI), beginning with title and abstract screening followed by full-text screening. Only studies of patients with UC histology were included. Studies which included patients with pure variant histology or other solid tumours were only included if individual patient data on UC patients were available. Studies reporting prevalence of dMMR or germline MMR gene alterations were only included if they tested and/or reported on all four MMR proteins or genes (MLH1, MSH2, MSH6 and PMS2). Given the smaller number of studies reporting prevalence of somatic MMR gene alterations, studies were included even if they did not report data on all four MMR genes. Studies reporting prevalence of MSI-H were included if they used a standardised definition of MSI-H, which was the presence of at least two unstable microsatellite loci either by PCR or next-generation sequencing (NGS). A pathologist (DA) assessed older studies which reported MSI-H based on PCR to ensure that they were included only if the methodology was comparable to more contemporary studies and suitable for meta-analysis. Studies reporting clinical outcomes or survival of dMMR/MSI-H UC patients were included. Case reports were included if the histology was UC and if they reported outcomes with either local or systemic treatment, or survival. Case reports of patients with multiple malignancies were included if they described the outcome of the UC with local or systemic treatment. Review articles were included if they were on MMR/MSI in UC. Studies reporting prevalence of dMMR/MSI-H/MMR gene alterations which only included patients with special characteristics for example, positive family history were also included in this study; however, their data were reported separately to avoid skewing the results. The exclusion of citations required the agreement of both authors, and in case of disagreements, a third author (ABA) was consulted.
Data extraction was performed independently by three authors (EBAC, GMI and SOA) using a Microsoft Excel template previously developed by all the authors. Meta-analyses were performed if the extracted data were suitable.
Definitions of outcomes
Prevalence was extracted as the number of patients with the characteristic of interest divided by the total number of patients tested. dMMR was defined as loss of expression in at least one MMR protein. The prevalence of dMMR or germline MMR gene alterations could be assessed if all four MMR proteins or genes were tested. Given the smaller number of studies reporting somatic MMR gene alterations in UC, the outcome of interest was modified to the frequency of patients with at least one somatic MMR gene alteration.
To assess the activity and/or efficacy of different modalities of therapy in dMMR/MSI-H UC, the following outcome measures were identified: objective response rate (ORR), disease-free survival (DFS), progression-free survival (PFS) and overall survival (OS). In studies which statistically compared the outcomes of dMMR/MSI-H patients with MMR-proficient (pMMR)/MSI-low (MSI-L)/microsatellite stable (MSS) patients, the summaries of their statistical analyses were extracted, such as HRs, ORs and/or p values.
Risk of bias (quality) assessment
For non-randomised studies, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Quasi-Experimental Studies30 was selected. For descriptive cross-sectional studies, the JBI Critical Appraisal Checklist for Prevalence Studies31 was used. For case reports and case series, the JBI Critical Appraisal Checklist for Case Reports32 was used. Assessment of the risk of bias was conducted by two authors (EBAC and SOA). Disagreements were resolved by a third author (ABA).
Statistical analyses
The extracted prevalences for dMMR, MSI-H and germline or somatic gene alterations were separately pooled. Pooled estimates were quantified if there were at least two studies reporting the results of the same outcome using a random intercept logistic regression model.33 Pooled weighted estimates were reported as prevalences with 95% CIs. The inconsistency index (I2) was calculated to measure heterogeneity. According to prespecified cutoffs, low heterogeneity was defined as an I2 of <25%, moderate heterogeneity when I2 fell between 25% and 75%, and high heterogeneity when I2 was >75%. Due to the potential heterogeneity among some of the pooled studies, subgroup analyses were explored but did not yield improved or clinicopathologically valid models. Summaries of these effect measures were calculated using a random effects model to remedy potential heterogeneity among the included studies. Publication bias was assessed using funnel plot asymmetry and Egger’s test. All statistical analyses were performed with R V.4.2.2 (The R Foundation for Statistical Computing, 2022), with the use of the meta package.34
RESULTS
Study selection and clinicopathological characteristics
The results of the literature search and the study selection process are shown in the PRISMA flow diagram29 (figure 1). A total of 1745 studies were screened, of which 110 were included: 2 clinical trials,35 36 86 retrospective studies,27 28 37–120 13 case reports121–133 and 9 review articles134–142 (online supplemental table S1). Among all included studies, sex was reported in 13 445 patients (73.4% male; 26.6% female). Age ranged from 17 to 97 years. Among 29 782 patients, primary sites were BC (69.8%), UTUC (19.7%) and not stated (10.5%). No studies included urethral tumours. Disease stage was reported in 7257 patients: localised (M0) in 66.3% and metastatic (M1) in 33.7%. Tumour stage was reported in 4009 patients with localised disease: Ta–T1 (36.7%); T2–T4 (63.3%).
Figure 1.
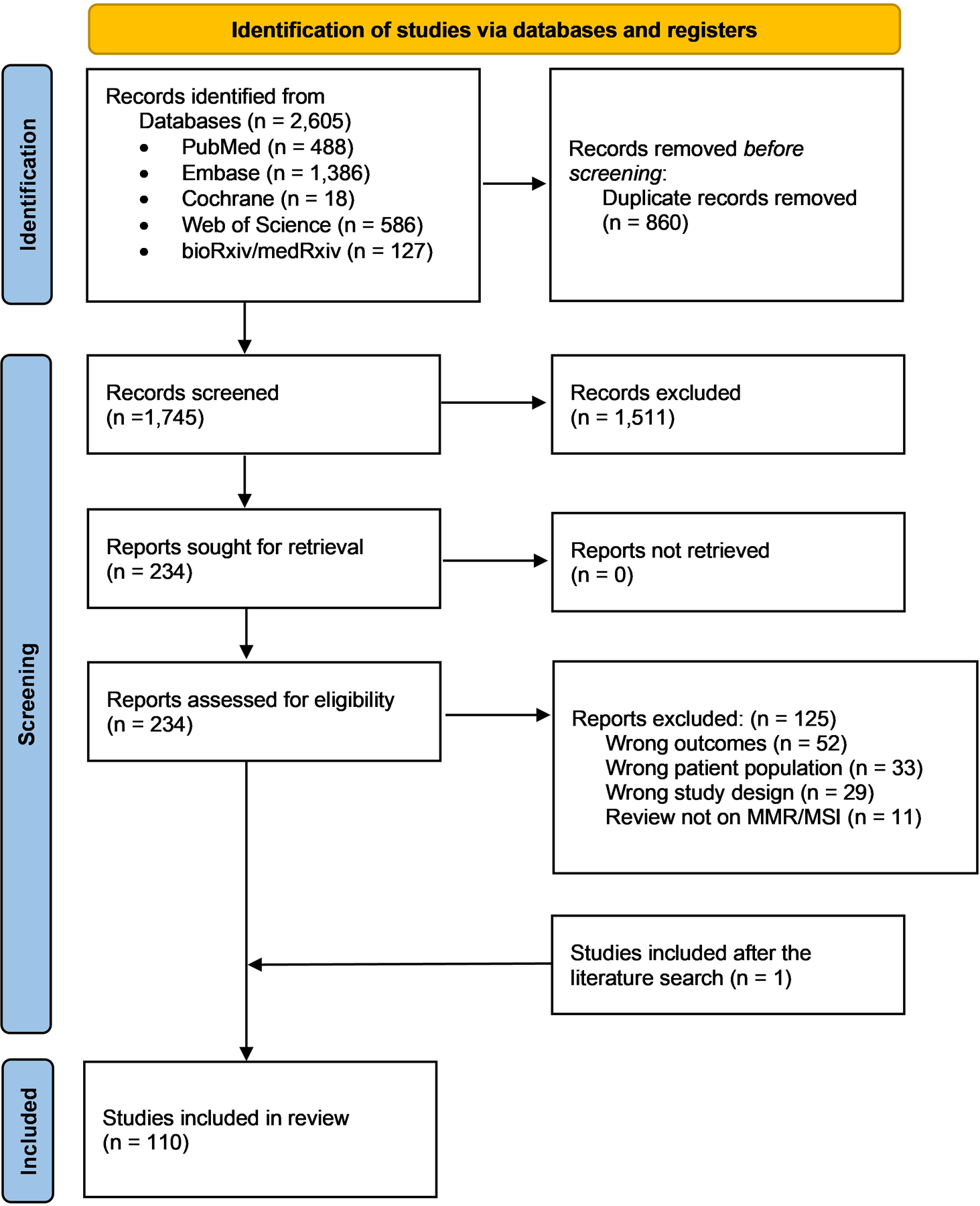
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results of quantitative analyses
Mismatch repair deficiency
29 studies (4759 patients) reported frequencies of dMMR. The pooled weighted prevalence of dMMR in UC patients was 6.03% (95% CI 4.17% to 8.64%) (figure 2). The three most frequent MMR protein loss patterns in UC, BC and UTUC are shown in table 1.
Figure 2.
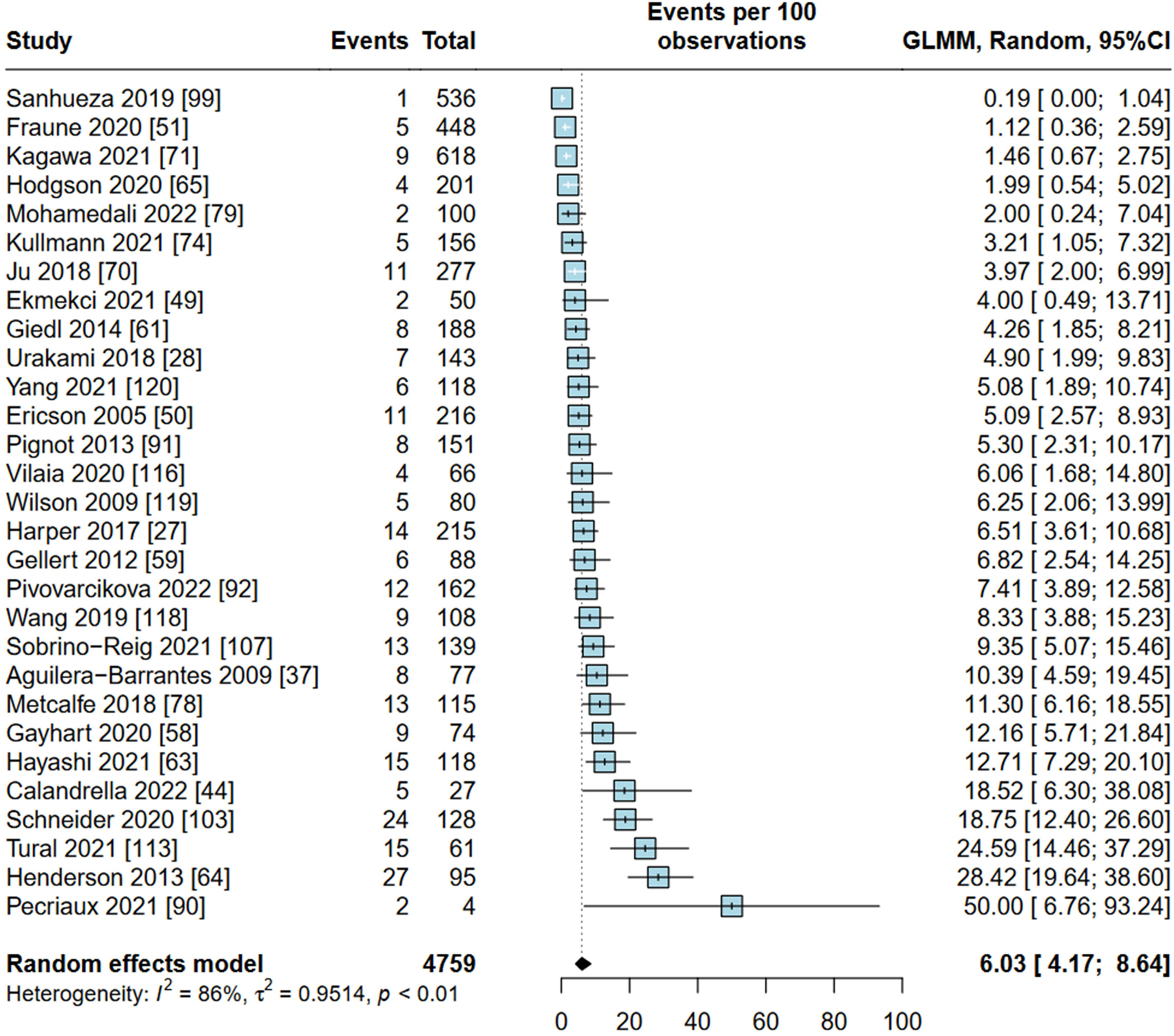
Forest plot of included studies reporting on prevalence of mismatch repair deficiency in urothelial carcinoma. GLMM, generalised linear mixed models.
Table 1.
The three most common mismatch repair (MMR) protein losses and germline and somatic mismatch repair gene alterations in urothelial carcinoma (UC), bladder cancer (BC) and upper tract urothelial carcinoma (UTUC)
| Tumour | UC | BC | UTUC |
|---|---|---|---|
| MMR protein loss pattern (frequency) | MSH2 and MSH6 63/3903 (1.6%) | PMS2 9/1671 (0.5%) | MSH2 and MSH6 56/1465 (3.8%) |
| MSH6 57/3903 (1.5%) | MLH1 and PMS2 8/1671 (0.5%) | MSH6 49/1465 (3.3%) | |
| MLH1 and PMS2 13/3903 (0.3%) | MSH6 7/1671 (0.4%) | MLH1 and PMS2 4/1465 (0.3%) | |
| Germline MMR gene alteration (frequency) | MSH2 33/1084 (3.0%) | MSH2 2/463 (0.4%) | MSH2 31/621 (5.0%) |
| MSH6 10/1084 (0.9%) | Not available | MSH6 10/621 (1.6%) | |
| MLH1 8/1084 (0.7%) | Not available | MLH1 8/621 (1.3%) | |
| Somatic MMR gene alteration (frequency) | MSH6 70/3895 (1.8%) | MSH6 24/3181 (0.8%) | MSH6 36/765 (4.7%) |
| MLH1 41/3895 (1.1%) | MSH2 9/3181 (0.3%) | MSH2 25/765 (3.3%) | |
| MSH2 31/3895 (0.8%) | MLH1 8/3181 (0.3%) | MLH1 18/765 (2.4%) |
MLH1, MutL protein homolog 1; MSH2, MutS protein homolog 2; MSH6, MutS protein homolog 6; PMS2, postmeiotic segregation increased 2.
12 studies with 2542 patients reported prevalence of dMMR in BC. The pooled weighted prevalence of dMMR in BC was 2.30% (95% CI 1.12% to 4.65%) (online supplemental figure S1). Four studies reported prevalence of dMMR in localised BC, among which the pooled weighted prevalence of dMMR was 3.09% (95% CI 0.99% to 9.20) (online supplemental figure S2). One study of patients with localised BC had a significantly higher dMMR prevalence113; when the meta-analyses were rerun without it, the pooled weighted prevalences of dMMR were 1.78% (95% CI 1.04% to 3.02%) in BC and 1.52% (95% CI 0.98% to 2.34% in localised BC (online supplemental figures S1a and S2a, respectively). No studies specifically reported dMMR prevalence in metastatic BC.
18 studies (2074 patients) reported the prevalence of dMMR in UTUC. The pooled weighted prevalence of dMMR in UTUC was 8.95% (95% CI 6.81% to 11.67%) (online supplemental figure S3). Five studies reported prevalence of dMMR in localised UTUC, among which the pooled weighted prevalence of dMMR was 8.47% (95% CI 5.80% to 12.21%) (online supplemental figure S4). One study of four patients reported dMMR in metastatic UTUC with a prevalence of 25%.44
Microsatellite instability-high
38 studies (17 070 patients) reported the prevalence of MSI-H in UC; 21 used PCR and 17 used NGS. The pooled weighted prevalence of MSI-H in UC was 4.54% (95% CI 2.83% to 7.19%) (figure 3). In BC, the pooled weighted prevalence of MSI-H among 16 studies (12 292 patients) was 2.11% (95% CI 0.82% to 5.31%) (online supplemental figure S5). For localised BC, the pooled weighted prevalence was 5.26% (95% CI 0.86% to 26.12%) (online supplemental figure S6). For metastatic BC, it was 0.86% (95% CI 0.59% to 1.25%) (online supplemental figure S7).
Figure 3.
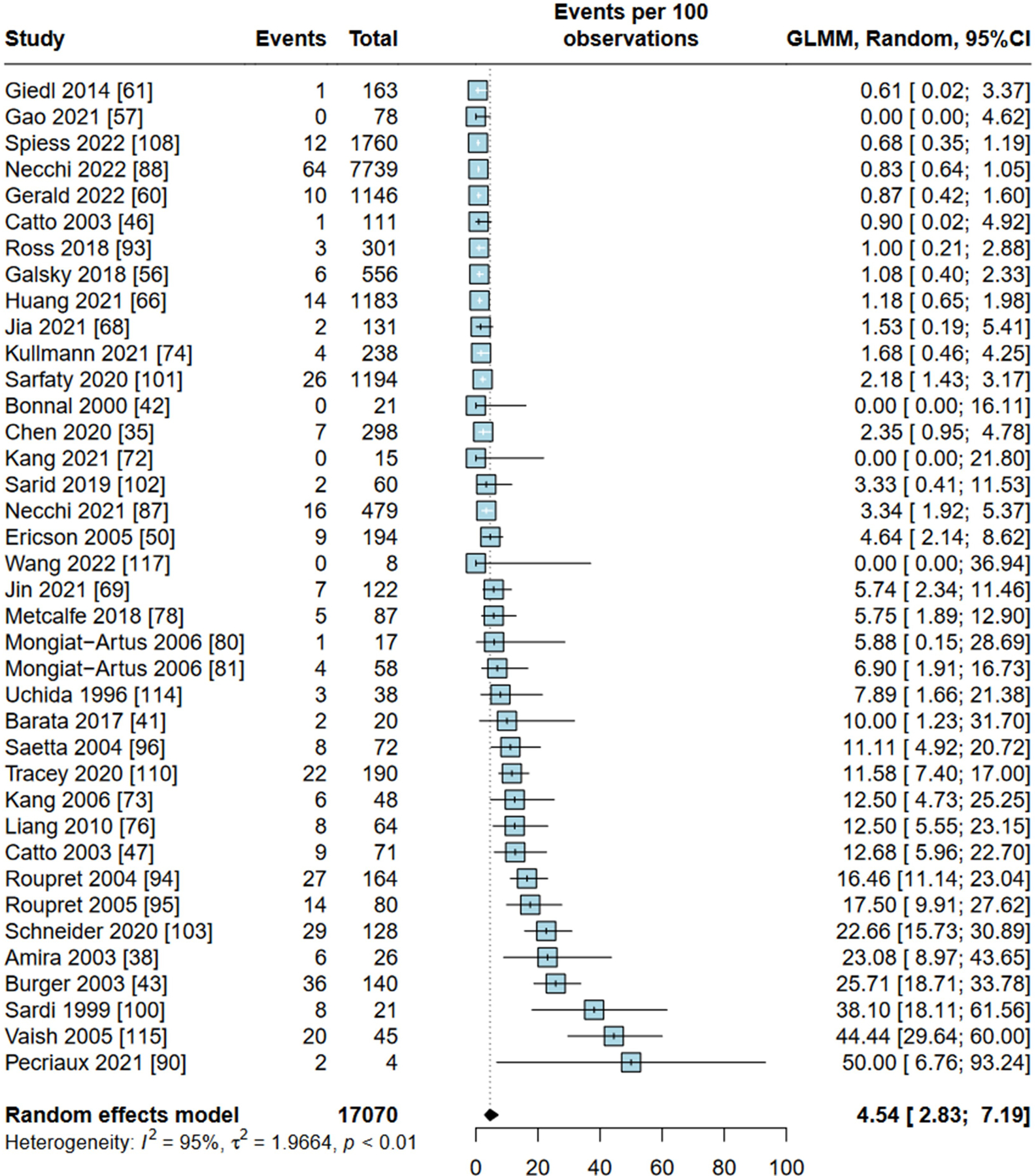
Forest plot of included studies reporting on prevalence of microsatellite instability-high in urothelial carcinoma. GLMM, generalised linear mixed models.
In UTUC, the pooled weighted prevalence of MSI-H among 17 studies (2427 patients) was 8.36% (95% CI 5.50% to 12.53%) (online supplemental figure S8), with pooled weighted prevalence for localised and metastatic UTUC of 18.04% (95% CI 13.36% to 23.91%) and 4.96% (95% CI 2.72% to 8.86%), respectively (online supplemental figures S9 and S10).
Studies of MSI among special populations, that is, patients with PD-L1 positivity, age ≤45 and with FGFR3 alterations are presented in online supplemental material.
Germline mismatch repair gene alterations
Nine studies (3077 patients) reported germline MMR gene alterations in UC, among which the pooled weighted prevalence was 4.81% (95% CI 2.52% to 8.98%) (online supplemental figure S11). In UTUC, it was 6.53% (95% CI 2.98% to 13.74%) among 6 studies (783 patients) (online supplemental figure S12). As there was a study with significantly outlying results,40 these meta-analyses were rerun without it, yielding pooled weighted prevalence of germline MMR gene alterations in UC of 3.82% (95% CI 2.36% to 6.12%) and in UTUC of 4.97% (95% CI 2.55% to 9.46%) (online supplemental figures S11a and S12a, respectively). Only one study reported the prevalence of germline MMR gene alterations in BC: 0.43%; 2/463 patients, both of which were MSH2 gene alterations.45 The most common germline MMR gene alterations are shown in table 1. Studies of germline MMR gene alterations among special populations, for example, patients with positive family history, are presented in online supplemental material.
Somatic mismatch repair gene alterations
13 studies (5237 patients) reported somatic MMR gene alterations in UC. The pooled weighted frequency of patients with at least one somatic MMR gene alteration was 4.39% (95% CI 1.82% to 10.23%) (online supplemental figure S13). The most common somatic MMR gene alterations are shown in table 1.
In BC, the pooled weighted frequency of patients with at least one somatic MMR gene alteration was 3.02% (95% CI 0.46% to 17.46%) among 5 studies (3286 patients) (online supplemental figure S14). For localised BC, it was 23.81% (95% CI 17.61% to 31.35%); for metastatic BC, it was 1.60% (95% CI 1.13% to 2.25%); however, there were only two studies in each of these meta-analyses (online supplemental figures S15 and S16).
In UTUC, the pooled weighted frequency of patients with at least one somatic MMR gene alteration was 6.79% (95% CI 2.62% to 16.47%) among 6 studies (867 patients) (online supplemental figure S17). One study reported the frequency of at least one somatic gene alteration in localised UTUC (6%).53 For metastatic UTUC, the pooled weighted frequency between the two studies was 16.39% (95% CI 13.35% to 19.97%) (online supplemental figure S18).
Clinical outcomes of patients with dMMR or microsatellite instability
Eight retrospective studies reported outcomes of patients with localised disease undergoing local therapy (online supplemental table S2). 17 studies reported clinical outcomes of ICI in patients with dMMR/MSI-H UC: 2 clinical trials, 2 retrospective studies and 13 case reports (online supplemental tables S3 and S4). Between these studies, 36 patients had received ICI—35 as treatment for metastatic disease and one as adjuvant treatment post surgery. Disease response was reported in 34 of the 35 patients with metastatic disease. The pooled response rate was 22/34 (64.7%). Best overall response was reported in eight patients: complete response (CR) in 4/8, partial response (PR) in 3/8 and stable disease (SD) in 1/8. Nine patients received chemotherapy for metastatic disease, among whom the best overall responses were: PR in 1/9 (response rate 11.1%), SD in 1/9 and progressive disease in 7/9 (online supplemental tables S3 and S4).
Bias assessment
Assessments of risk of bias and publication bias are available in online supplemental material. Funnel plots were generated for dMMR and MSI-H in UC, BC and UTUC (online supplemental figures S19–S24). There is symmetry in the majority of assessments limiting publication bias, except for dMMR UC and UTUC (online supplemental figures S19 and S21, respectively) where publication bias may exist.
DISCUSSION
Detection of nuclear expression of MLH1, MSH2, PMS2 and MSH6 proteins by immunohistochemistry (IHC) is the gold standard for identifying MMR status, with nuclear loss of at least one of these markers within tumour cells considered dMMR.143–145 Notably, dMMR is increasingly being detected indirectly by somatic MMR gene alterations on NGS, and small studies have demonstrated concordance with IHC MMR protein loss.146 147 To determine MSI status, earlier studies employed PCR while more contemporary studies used NGS. The established Bethesda PCR panel consists of five mononucleotides (BAT25, BAT26, D2S123, D5S346 and D17S250), of which at least two must be altered to diagnose MSI-H.148 149 Although developed for use in colorectal carcinoma, the panel has been used in other solid tumours including UC to detect MSI status.47 87 95 When only one locus was altered, which defined microsatellite instability-low (MSI-L), the clinical and biological implications were equivocal. Additional mononucleotide repeats in the panel were suggested to increase sensitivity.149 NGS studies including whole exome sequencing and targeted sequencing assays can determine MSI status by sequencing around microsatellite regions with comparison between tumour and normal tissue.13 150–153 In large cohorts, validation with PCR and IHC showed 99.1%–99.4% concordance.154 155 NGS-based methods are more sensitive as they are capable of assessing hundreds to thousands of loci, compared with a limited panel with PCR, which reduces the likelihood of detection of MSI-L tumours.153 This overcomes the possible subjectivity that arises from using PCR by providing quantitative results. However, since the majority of MSI is caused by epigenetic changes, detecting these by NGS requires more complex analysis and interpretation.
Notably, the pooled weighted prevalences of MSI-H and somatic dMMR gene alterations were higher in localised BC (stage M0) compared with metastatic BC, and in UTUC, the pooled weighted prevalence of MSI-H was also higher in localised disease. The higher prevalence of these characteristics in localised disease has also been observed in colorectal cancer156 157 and may be due to higher neoantigen load of MSI-H/dMMR tumours stimulating an antitumour immune response; hence reducing the likelihood of metastases.157 Interestingly, there was also a difference in the pattern of MMR protein loss in UTUC compared with BC. In UTUC, MSH2 and MSH6 were the most frequent MMR proteins and/or genes lost or altered (both somatic and germline), whereas the trend was not as clear in BC. This contrasts with dMMR colorectal cancer, in which 90% of cases are due to loss of MLH1 and MSH2 genes.158 159
We found a higher prevalence of germline MMR gene alterations in patients with UTUC or a positive family history; hence, germline testing should be discussed in these situations. In colorectal cancer, in which the prevalence of Lynch syndrome is also relatively low, around 5%,160 universal germline testing is recommended.161 The increasingly routine performance of NGS, especially in metastatic UC, will identify more patients with either MSI-H or somatic MMR gene alterations, which will likely result in more germline testing being performed. Identification of a germline MMR gene alteration will have significant implications for patients and their families. A prior study showed that the standardised incidence ratio of developing BC was 8.2-fold and 16.2-fold higher in males and females, respectively, harbouring MMR germline variants compared with the general population.162 The risk was highest for MSH2 germline carriers. Moreover, the risk of developing subsequent urothelial cancers was >100 fold higher in MMR germline carriers. Another study showed that in patients harbouring pathogenic Lynch syndrome-associated variants, the risk of developing urinary tract tumours by age 75 was 24.9% for MSH2 carriers, 11% for MSH6 carriers and 8% for MLH1 carriers. In comparison, risks of developing colorectal cancer were 43%, 15% and 45.8%, for MSH2, MSH6 and MLH1 carriers, respectively.163 For individuals with germline MMR gene alterations, the National Comprehensive Cancer Network guidelines do not recommend routine surveillance for UC due to lack of clear supporting evidence, although it is recommended for individuals with a family history of UC. The considerably higher risk of developing BC, especially among MSH2 carriers, could inform surveillance guidelines. For colorectal cancer, the detection of germline MMR gene alterations has clear surveillance implications, including earlier and more frequent colonoscopies.161
We found that dMMR and MSI-H metastatic UC is responsive to ICI, with included studies reporting ORRs of 50%–90%,35 36 39 101 deep responses35 36 39 101 121 126 164 and long durations of response121 123 126 132 (online supplemental tables S3 and S4). These data show that dMMR and MSI-H may be predictive biomarkers for response to ICI in UC, which has already been prospectively demonstrated in solid tumours.20 Indeed, pembrolizumab received accelerated FDA approval for use in any metastatic solid tumours with dMMR or MSI-H based on clinical activity demonstrated in the phase 2 Keynote-158 trial.20 23 The response of dMMR/MSI-H metastatic UC to chemotherapy is less encouraging, with high rates of primary progression101 122 132 133 and short PFS.101 The high rates and long durations of response to ICI and the lower likelihood of disease control with chemotherapy shown in this systematic review raise the question of whether these patients should receive ICI monotherapy upfront for metastatic disease. This question remains even with the standard of care for untreated metastatic UC recently changing to enfortumab vedotin (EV) plus pembrolizumab as it is unknown whether this subset of patients will do just as well receiving the agents sequentially instead of concurrently, which will limit or delay treatment related toxicity.165 166 For localised disease, there is conflicting evidence. Some studies suggest superior DFS compared with pMMR/MSI-L/MSS patients and high rates of local control67 77; other studies suggest no difference in outcomes.96 98 Some studies suggest a propensity for dMMR/MSI-H patients to have multifocal, bilateral and/or both bladder and upper tract disease, either synchronously or metachronously.67 77 A novel approach for the treatment of localised dMMR/MSI-H tumours which has seen impressive results in rectal24 and colon cancer25 26 is definitive treatment with ICI, known as immunoablation. In the phase 2 dMMR rectal cancer trial, there was a 100% clinical CR rate among the first 14 participants with stage II and stage III disease who were treated with the anti-PD-1 antibody dostarlimab, and no recurrences at a median follow-up of 6.8 months. None of the patients had required chemotherapy, radiotherapy or surgery up to the latest data cut-off; however, longer follow-up is needed to confirm the success of this approach.24 167 In the phase 2 NICHE-2 trial which enrolled 112 patients with locally advanced dMMR colon cancer, patients were given two cycles of ICI (first ipilimumab/nivolumab, then nivolumab) before proceeding to surgery. Remarkably, 95% of patients attained a major pathological response, defined as <10% residual viable tumour, and 67% of patients attained a pathologic CR.26 A systematic review assessing neoadjuvant ICI in 423 patients with dMMR/MSI-H localised colorectal cancer reported CR (pathological CR plus clinical CR) of 72% and a complete resection (R0) rate of 99.3%.168 These results suggest a need to develop similar trials in dMMR/MSI-H muscle-invasive or locally advanced UC, as these could revolutionise the treatment landscape for these patients. This approach may allow patients to avoid the quality-of-life impacts of radical surgery or trimodality therapy and potentially be effective for multifocal localised disease without the need for multiple procedures.
Survival in patients with dMMR/MSI-H may be favourable. In one study, all 10 patients with advanced or metastatic disease survived beyond 15.5 months after ICI39; in another study, 77% of 26 patients lived beyond 2 years.101 Univariate analyses have demonstrated superior OS in patients with dMMR/MSI-H compared with patients without these characteristics84 95 113; however, other studies show no difference in survival.96 109 Of note, most of these studies are small and retrospective, which increases the risk of bias. Comparatively, in localised and advanced colorectal cancer, MSI-H has been shown to be favourably prognostic for survival.169–171 In UC, it is likely that OS will depend on treatment for advanced disease. Newer studies with more patients receiving ICI may demonstrate a greater degree of improvement in survival compared with pMMR/MSI-L/MSS patients.
Study limitations
There was heterogeneity in MSI detection techniques among included studies due to their significant evolution over the last two decades. Consequently, the MSI-H prevalence data that were meta-analysed were likely not completely standardised. The focus of this systematic review was on dMMR and MSI-H in UC; however, our search results also yielded studies focusing on germline and somatic gene alterations, which we included due to their relevance to the topic. As this study was not primarily designed to search for genetic and genomic studies, our search may not have comprehensively included these studies. The response rates of dMMR/MSI-H patients to ICI and chemotherapy were pooled from separate studies; hence, they are only crude estimates. Lastly, the survival of patients with dMMR or MSI-H UC and the prognostic value of these biomarkers could not be estimated or meta-analysed due to a lack of uniformity in the data.
CONCLUSION
To the best of our knowledge, this is the first systematic review and meta-analysis to examine dMMR and MSI-H in UC. We found that these characteristics occur more commonly in UTUC than in BC and may occur more frequently in localised disease than in metastatic disease. In UC, dMMR or MSI-H may be predictive of response to ICI and negatively predictive of response to cisplatin-based chemotherapy. Our findings support the development of studies employing novel immunotherapeutic approaches for this small but important subset of patients. Finally, the role of universal germline testing in UC is still unclear; however, it should be offered in patients with a positive personal or family history of Lynch-related malignancies.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
In solid tumours, mismatch repair deficiency (dMMR) and microsatellite instability-high (MSI-H) confer a sensitivity to immune checkpoint inhibition (ICI); however, there is a paucity of prospective data on the prevalence of these characteristics in urothelial carcinoma, whether they are predictive for response to ICI or other treatment modalities, and whether they are prognostic. Their prevalence by primary tumour site (bladder vs upper tract) or stage (localised vs metastatic) is not well described.
WHAT THIS STUDY ADDS
Our study found that dMMR and MSI-Hare approximately three times more prevalent in upper tract urothelial carcinoma (dMMR 8.95%; MSI-H 8.36%) than in bladder cancer (dMMR 3.09%; MSI-H 2.11%), and that MSI-H occurs more frequently in localised disease compared with metastatic disease. We also found that dMMR or MSI-H may confer high sensitivity to ICI and resistance to chemotherapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings provide a rationale for clinical trials of ICI in dMMR/MSI-H UC which in the localised setting, may spare patients the morbidities of radical surgery, and in the metastatic setting, allow them to avoid or delay the toxicities of chemotherapy or antibody-drug conjugates.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. This research was supported by the Intramural Research Program of the National Institutes of Health (NIH).
Footnotes
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability statement
Data are available on reasonable request.
REFERENCES
- 1.Raman JD, Messer J, Sielatycki JA, et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int 2011;107:1059–64. [DOI] [PubMed] [Google Scholar]
- 2.Soria F, Shariat SF, Lerner SP, et al. Epidemiology, diagnosis, preoperative evaluation and Prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol 2017;35:379–87. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology, and End Results Program;. [Available from: Cancer STAT facts: bladder cancer. 2024. Available: https://seer.cancer.gov/statfacts/html/urinb.html
- 4.Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020;395:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. [DOI] [PubMed] [Google Scholar]
- 6.Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol 2020;203:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with Pt3-Pt4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an Intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell PH, Rosenberg JE, Hoimes CJ, et al. Enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (La/mUC): subgroup analyses of confirmed objective response rate (cORR) from EV-103 cohort K. JCO 2023;41:499. [Google Scholar]
- 9.Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021;384:1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 2021;39:2474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–30. [DOI] [PubMed] [Google Scholar]
- 12.Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–48. [DOI] [PubMed] [Google Scholar]
- 13.Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 2016;22:1342–50. [DOI] [PubMed] [Google Scholar]
- 14.Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017;2017:PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch HT, Snyder CL, Shaw TG, et al. Milestones of lynch syndrome: 1895–2015. Nat Rev Cancer 2015;15:181–94. [DOI] [PubMed] [Google Scholar]
- 16.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol 2019;37:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz RA, Torre DP. The muir-torre syndrome: a 25-year retrospect. J Am Acad Dermatol 1995;33:90–104. [DOI] [PubMed] [Google Scholar]
- 18.Nakrani RN, Ghosh A, Richard Lee CC, et al. New facial papules in a 66-year-old woman with bladder cancer. J Am Acad Dermatol 2014;71:1250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from checkmate 142. Ann Oncol 2022;33:1052–60. [DOI] [PubMed] [Google Scholar]
- 22.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food & Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017.
- 24.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N Engl J Med 2022;386:2363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–76. [DOI] [PubMed] [Google Scholar]
- 26.Chalabi M, Verschoor YL, van den Berg J, et al. Lba7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study. Ann Oncol 2022;33:S1389. [Google Scholar]
- 27.Harper HL, McKenney JK, Heald B, et al. Upper tract urothelial carcinomas: frequency of association with mismatch repair protein loss and lynch syndrome. Mod Pathol 2017;30:146–56. [DOI] [PubMed] [Google Scholar]
- 28.Urakami S, Inoshita N, Oka S, et al. Clinicopathological characteristics of patients with upper urinary tract urothelial cancer with loss of immunohistochemical expression of the DNA mismatch repair proteins in universal screening. Int J Urol 2018;25:151–6. [DOI] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Joanna Briggs Institute. Checklist for quasi-experimental studies. 2017. Available: https://jbi.global/sites/default/files/2019-05/JBI_Quasi-Experimental_Appraisal_Tool2017_0.pdf
- 31.The Joanna Briggs Institute. Checklist for prevalence studies. 2017. Available: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf
- 32.The Joanna Briggs Institute. Checklist for case reports. 2020. Available: https://jbi.global/sites/default/files/2020-08/Checklist_for_Case_Reports.pdf
- 33.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010;29:3046–67. [DOI] [PubMed] [Google Scholar]
- 34.Harrer M, Cuijpers P, Furukawa TA, et al. Doing Meta-Analysis with R: A Hands-on Guide. 1st edn. Boca Raton, FL and London: Chapman & Hall/CRC Press, 2021. Available: https://www.taylorfrancis.com/books/9781003107347 [Google Scholar]
- 35.Chen J, Quan M, Chen Z, et al. Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: an open-label prospective pivotal trial. J Cancer Res Clin Oncol 2020;146:2651–7. [DOI] [PubMed] [Google Scholar]
- 36.Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol 2022;33:929–38. [DOI] [PubMed] [Google Scholar]
- 37.Aguilera-Barrantes I, Hampel H, Westman R, et al. Immunohistochemical screening for lynch syndrome in patients with urothelial carcinoma of the renal pelvis and Ureter does not correlate with clinical screening parameters. Lab Invest 2009;89:155A. [Google Scholar]
- 38.Amira N, Rivet J, Soliman H, et al. Microsatellite instability in urothelial carcinoma of the upper urinary tract. J Urol 2003;170:1151–4. [DOI] [PubMed] [Google Scholar]
- 39.Andreev-Drakhlin A, Shah AY, Adriazola AC, et al. Efficacy of immune checkpoint blockade in patients with advanced upper tract urothelial cancer and mismatch repair deficiency or microsatellite instability (MSI). JCO 2021;39:487. [Google Scholar]
- 40.Audenet F, Isharwal S, Cha EK, et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res 2019;25:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barata PC, Koshkin VS, Funchain P, et al. Next-generation sequencing (NGS) of cell-free circulating tumor DNA and tumor tissue in patients with advanced urothelial cancer: a pilot assessment of concordance. Ann Oncol 2017;28:2458–63. [DOI] [PubMed] [Google Scholar]
- 42.Bonnal C, Ravery V, Toublanc M, et al. Absence of microsatellite instability in transitional cell carcinoma of the bladder. Urology 2000;55:287–91. [DOI] [PubMed] [Google Scholar]
- 43.Burger M, Filbeck T, Wieland W, et al. Histopathological, clinical and molecular characterization of carcinomas of the upper urinary tract with mutator phenotype. Eur Urol Suppl 2003;2:9. [Google Scholar]
- 44.Calandrella ML, Francesconi S, Caprera C, et al. Nectin-4 and DNA mismatch repair proteins expression in upper urinary tract urothelial carcinoma (UTUC) as a model for tumor targeting approaches: an Imgo pilot study. BMC Cancer 2022;22:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlo MI, Ravichandran V, Srinavasan P, et al. Cancer susceptibility mutations in patients with urothelial malignancies. J Clin Oncol 2020;38:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catto JWF, Xinarianos G, Burton JL, et al. Differential expression of Hmlh1 and Hmsh2 is related to bladder cancer grade, stage and prognosis but not microsatellite instability. Int J Cancer 2003;105:484–90. [DOI] [PubMed] [Google Scholar]
- 47.Catto JWF, Azzouzi A-R, Amira N, et al. Distinct patterns of Microsatellite instability are seen in tumours of the urinary tract. Oncogene 2003;22:8699–706. [DOI] [PubMed] [Google Scholar]
- 48.Chen H, Zhang R, Chen S, et al. Comprehensive genomic landscape in Chinese patients with urothelial carcinoma. JCO 2020;38:e17009. [Google Scholar]
- 49.Ekmekci S, Küçük Ü, Kaya Ö, et al. The association between the histopathological features and microsatellite instability in young patients with urothelial carcinoma of the bladder. Rev Assoc Med Bras 1992;67:64–70. [DOI] [PubMed] [Google Scholar]
- 50.Ericson KM, Isinger AP, Isfoss BL, et al. Low frequency of defective mismatch repair in a population-based series of upper urothelial carcinoma. BMC Cancer 2005;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraune C, Simon R, Hube-Magg C, et al. MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass. Urol Oncol 2020;38:488–95. [DOI] [PubMed] [Google Scholar]
- 52.Friedrich MG, Chandrasoma S, Siegmund KD, et al. Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Eur J Cancer 2005;41:2769–78. [DOI] [PubMed] [Google Scholar]
- 53.Fujii Y, Sato Y, Suzuki H, et al. Comprehensive analysis of upper urinary tract urothelial carcinoma. J Urol 2018;199:e776. [Google Scholar]
- 54.Furihata M, Shuin T, Takeuchi T, et al. Missense Mutation of the Hmsh6 and P53 genes in sporadic urothelial transitional cell carcinoma. Int J Oncol 2000;16:491–6. [PubMed] [Google Scholar]
- 55.Furihata M, Takeuchi T, Ohtsuki Y, et al. Genetic analysis of Hmlh1 in transitional cell carcinoma of the urinary tract: promoter methylation or Mutation. J Urol 2001;165:1760–4. [PubMed] [Google Scholar]
- 56.Galsky MD, Banchereau R, Kadel EE, et al. Biological features and clinical outcomes in atezolizumab (Atezo)-treated patients (Pts) with metastatic urothelial cancer (mUC) of the upper vs lower urinary tract (UTUC vs LTUC). Ann Oncol 2018;29:viii321. [Google Scholar]
- 57.Gao G, Zhang X-D, Qu H, et al. A comprehensive pan-cancer analysis of Cd274 gene amplification, tumor mutation burden, microsatellite instability, and PD-L1 expression in Chinese cancer patients. Ann Transl Med 2021;9:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gayhart MG, Johnson N, Paul A, et al. Universal mismatch repair protein screening in upper tract urothelial carcinoma. Am J Clin Pathol 2020;154:792–801. [DOI] [PubMed] [Google Scholar]
- 59.Gellert LL, Mehra R, Shia J, et al. DNA mismatch repair deficiency in urothelial carcinoma: an immunohistochemical study in upper versus lower genitourinary tract tumors. Lab Invest 2012;92:207A. [Google Scholar]
- 60.Gerald T, Margulis V, Meng X, et al. Actionable genomic landscapes from a real-world cohort of localized urothelial carcinoma patients. JCO 2022;40:525. [DOI] [PubMed] [Google Scholar]
- 61.Giedl J, Schneckenpointner R, Filbeck T, et al. Low frequency of HNPCC-associated microsatellite instability and aberrant MMR protein expression in early-onset bladder cancer. Am J Clin Pathol 2014;142:634–9. [DOI] [PubMed] [Google Scholar]
- 62.Guan B, Wang J, Li X, et al. Identification of germline mutations in upper tract urothelial carcinoma with suspected lynch syndrome. Front Oncol 2022;12:774202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayashi T, Ikeda K, Taniyama D, et al. Clinicopathological characteristics of upper tract urothelial cancer with loss of immunohistochemical expression of mismatch repair proteins. Eur Urol 2021;79:S1078. [Google Scholar]
- 64.Henderson SA, Heitzman JP, Czerniak B, et al. Microsatellite instability in urothelial carcinoma of the upper urinary tract. Lab Invest 2013;93:217A. [Google Scholar]
- 65.Hodgson A, Vesprini D, Liu SK, et al. Correlation of mismatch repair protein deficiency, PD-L1 and CD8 expression in high-grade urothelial carcinoma of the bladder. J Clin Pathol 2020;73:519–22. [DOI] [PubMed] [Google Scholar]
- 66.Huang RSP, Haberberger J, Severson E, et al. A Pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and Microsatellite instability in 48,782 cases. Mod Pathol 2021;34:252–63. [DOI] [PubMed] [Google Scholar]
- 67.Hubosky SG, Boman BM, Charles S, et al. Ureteroscopic management of upper tract urothelial carcinoma (UTUC) in patients with lynch syndrome (hereditary Nonpolyposis colorectal cancer syndrome). BJU Int 2013;112:813–9. [DOI] [PubMed] [Google Scholar]
- 68.Jia Y, He N, Yang Y, et al. Tumor mutation burden and immune microenvironment analysis of urothelial carcinoma. JCO 2021;39:494. [Google Scholar]
- 69.Jin S, Wei Y, Wu J, et al. The genomic landscape of Chinese patients with upper tract urothelial carcinoma. JCO 2021;39:e16583. [Google Scholar]
- 70.Ju JY, Mills AM, Mahadevan MS, et al. Universal lynch syndrome screening should be performed in all upper tract urothelial carcinomas. Am J Surg Pathol 2018;42:1549–55. [DOI] [PubMed] [Google Scholar]
- 71.Kagawa M, Kawakami S, Yamamoto A, et al. Identification of lynch syndrome-associated DNA mismatch repair-deficient bladder cancer in a Japanese hospital-based population. Int J Clin Oncol 2021;26:1524–32. [DOI] [PubMed] [Google Scholar]
- 72.Kang SY, Kim KM. Highly sensitive duplex MSI test and Bat40 germline polymorphism. APMIS 2021;129:607–15. [DOI] [PubMed] [Google Scholar]
- 73.Kang TW, Lee J-G, Jung SI, et al. A study of microsatellite instability of upper urinary tract transitional cell carcinoma. Korean J Urol 2006;47:1269. [Google Scholar]
- 74.Kullmann F, Strissel PL, Strick R, et al. Frequency of microsatellite instability (MSI) in upper tract urothelial carcinoma: comparison of the Bethesda panel and the Idylla MSI assay in a consecutively collected, multi-institutional cohort. J Clin Pathol 2023;76:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Labbate C, Saporito D, Mork M, et al. Universal germline genetic testing of patients with newly diagnosied upper tract urothelial carcinoma: an interim analysis. J Urol 2022;207:e818–9. [Google Scholar]
- 76.Liang J-F, Zheng H-X, Li N, et al. Fluorescent microsatellite analysis of urine sediment in patients with urothelial carcinoma. Urol Int 2010;85:296–303. [DOI] [PubMed] [Google Scholar]
- 77.Liang L, Rao P. Urothelial carcinoma with loss of Msh2 and/or Msh6 expression: a clinicopathologic study of 11 cases. Lab Invest 2017;97:237A–8A. [Google Scholar]
- 78.Metcalfe MJ, Petros FG, Rao P, et al. Universal point of care testing for lynch syndrome in patients with upper tract urothelial carcinoma. J Urol 2018;199:60–5. [DOI] [PubMed] [Google Scholar]
- 79.Mohamedali R, Adhya AK, Mandal S, et al. Expression of mismatch repair proteins in urothelial carcinoma of the urinary bladder. Indian J Cancer 2022;59:279–81. [DOI] [PubMed] [Google Scholar]
- 80.Mongiat-Artus P, Miquel C, van der Aa M, et al. Infrequent microsatellite instability in urothelial cell carcinoma of the bladder in young patients. Eur Urol 2006;49:685–90. [DOI] [PubMed] [Google Scholar]
- 81.Mongiat-Artus P, Miquel C, Van der Aa M, et al. Microsatellite instability and mutation analysis of candidate genes in urothelial cell carcinomas of upper urinary tract. Oncogene 2006;25:2113–8. [DOI] [PubMed] [Google Scholar]
- 82.Mossanen M, Nassar AH, Stokes SM, et al. Incidence of germline variants in familial bladder cancer and among patients with cancer predisposition syndromes. Clin Genitourin Cancer 2022;20:568–74. [DOI] [PubMed] [Google Scholar]
- 83.Mullane SA, de Velasco G, Choueiri TK, et al. Genomic alterations in upper tract urothelial carcinoma (UTUC) versus urothelial carcinoma of the bladder (UBC). JCO 2016;34:431. [Google Scholar]
- 84.Mylona E, Zarogiannos A, Nomikos A, et al. Prognostic value of microsatellite instability determined by immunohistochemical staining of hMSH2 and hMSH6 in urothelial carcinoma of the bladder. APMIS 2008;116:59–65. [DOI] [PubMed] [Google Scholar]
- 85.Nassar A, Mouw KW, Esplin ED, et al. Germline alterations in urothelial carcinoma (UC) patients with family history of UC. JCO 2019;37:474. [Google Scholar]
- 86.Nassar AH, Abou Alaiwi S, AlDubayan SH, et al. Prevalence of pathogenic germline cancer risk variants in high-risk urothelial carcinoma. Genet Med 2020;22:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Necchi A, Madison R, Pal SK, et al. Comprehensive genomic profiling of upper-tract and bladder urothelial carcinoma. Eur Urol Focus 2021;7:1339–46. [DOI] [PubMed] [Google Scholar]
- 88.Necchi A, Murugesan K, Burn T, et al. Co-mutational landscape of key fibroblast growth factor receptor (FGFR) alterations in intra-hepatic cholangiocarcinoma (iCCA), bladder cancer (BC) and glioma. Ann Oncol 2022;33:S584–5. [Google Scholar]
- 89.Pal SK, Ali SM, Ennis R, et al. Characterization of mutational load in patients with advanced urothelial cancer. JCO 2016;34:460. [Google Scholar]
- 90.Pécriaux A, Favre L, Calderaro J, et al. Detection of Microsatellite instability in a panel of solid tumours with the Idylla MSI test using extracted DNA. J Clin Pathol 2021;74:36–42. [DOI] [PubMed] [Google Scholar]
- 91.Pignot G, Rouquette A, Vieillefond A, et al. Should we systematically screen for HNPCC in patients with upper urinary tract transitional cell carcinoma. Eur Urol Suppl 2013;12:e235. [Google Scholar]
- 92.Pivovarcikova K, Pitra T, Buchova K, et al. Systematic screening of upper tract lynch syndrome-related urothelial carcinoma: analysis of 162 patients from a single European institution. Mod Pathol 2022;35:656–7. [Google Scholar]
- 93.Ross JS, Gay LM, Ferry EK, et al. Fgfr3 driven metastatic urothelial carcinoma of the urinary bladder (mUCB): a comprehensive genomic profiling study. JCO 2018;36:4531. [Google Scholar]
- 94.Roupret M, Catto J, Coulet F, et al. Microsatellite instability as indicator of MSH2 gene mutation in patients with upper urinary tract transitional cell carcinoma. J Med Genet 2004;41:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rouprêt M, Fromont G, Azzouzi AR, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005;65:1233–7. [DOI] [PubMed] [Google Scholar]
- 96.Saetta AA, Goudopoulou A, Korkolopoulou P, et al. Mononucleotide markers of microsatellite instability in carcinomas of the urinary bladder. Eur J Surg Oncol 2004;30:796–803. [DOI] [PubMed] [Google Scholar]
- 97.Sanders JA, Frasier C, Matulay JT, et al. Genomic analysis of response to bacillus calmette-guérin (BCG) treatment in high-grade stage 1 bladder cancer patients. Transl Androl Urol 2021;10:2998–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanguedolce F, Cormio A, Massenio P, et al. Altered expression of HER-2 and the mismatch repair genes Mlh1 and Msh2 predicts the outcome of T1 high-grade bladder cancer. J Cancer Res Clin Oncol 2018;144:637–44. [DOI] [PubMed] [Google Scholar]
- 99.Sanhueza T, Gómez MC, Musulèn E, et al. Low frequency of mismatch repair protein expression in a series of bladder carcinoma. Virchows Arch 2019;475:S184. [Google Scholar]
- 100.Sardi I, Bartoletti R, Occhini I, et al. Microsatellite alterations in superficial and locally advanced transitional cell carcinoma of the bladder. Oncol Rep 1999;6:901–5. [DOI] [PubMed] [Google Scholar]
- 101.Sarfaty M, Teo MY, Al-Ahmadie H, et al. Microsatellite instability (MSI-H) in metastatic urothelial carcinoma (mUC): a biomarker of divergent responses to systemic therapy. JCO 2020;38:566. [Google Scholar]
- 102.Sarid DL, Berger R, Levertovsky M, et al. Genomic analysis of urothelial cancer and associations with treatment choice and outcome. Ann Oncol 2019;30:v377. [Google Scholar]
- 103.Schneider B, Glass Ä, Jagdmann S, et al. Loss of mismatch-repair protein expression and microsatellite instability in upper tract urothelial carcinoma and clinicopathologic implications. Clin Genitourin Cancer 2020;18:e563–72. [DOI] [PubMed] [Google Scholar]
- 104.Sekine Y, Iwasaki Y, Hakozaki N, et al. Prevalence and risk estimation of cancer-predisposing genes for upper urinary tract urothelial carcinoma in Japanese. Jpn J Clin Oncol 2022;52:1441–5. [DOI] [PubMed] [Google Scholar]
- 105.Silagy AW, DiNatale RG, Chowell D, et al. Genomic biomarkers for immune-checkpoint blockade response in metastatic upper tract urothelial carcinoma. J Urol 2020;203:e379. [Google Scholar]
- 106.Smith N, Sloan M, Wei J, et al. The association between germline mutations in DNA damage response and repair (DDR) genes and bladder cancer: results from the UK biobank. J Urol 2021;206. [Google Scholar]
- 107.Sobrino-Reig E, Meizoso T, García J, et al. Morphological predictors for microsatellite instability in urothelial carcinoma. Diagn Pathol 2021;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spiess PE, Bratslavsky G, Grivas P, et al. 1778P comparative genomic alterations (GA) landscape in urothelial carcinoma of the bladder (UCB) in patients of South Asian ancestry (SAS). Ann Oncol 2022;33:S1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Therkildsen C, Eriksson P, Höglund M, et al. Molecular subtype classification of urothelial carcinoma in lynch syndrome. Mol Oncol 2018;12:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tracey A, Wong N, Clinton T, et al. Identifying the frequency of actionable genomic alterations in localized and metastatic upper tract urothelial carcinoma. J Urol 2020;203:e379–80. [Google Scholar]
- 111.Truong H, Clements M, Sheikh R, et al. Clinical characteristics associated with high-risk cancer-predisposition germline mutations in patients with urothelial carcinoma: implications for genetic testing. J Urol 2021;206. [Google Scholar]
- 112.Truong H, Sheikh R, Kemel Y, et al. Defining hereditary upper tract urothelial carcinoma: implications for genetic testing and clinical management. JCO 2022;40:523. [Google Scholar]
- 113.Tural D, Akar E, Baytekin HF, et al. Relationship between survival outcomes and microsatellite instability, tumor infiltrating lymphocytes and programmed cell death Ligand-1 expression in patients with bladder cancer and radical cystectomy. J BUON 2021;26:2117–25. [PubMed] [Google Scholar]
- 114.Uchida T, Wang C, Wada C, et al. Microsatellite instability in transitional cell carcinoma of the urinary tract and its relationship to clinicopathological variables and smoking. Int J Cancer 1996;69:142–5. [DOI] [PubMed] [Google Scholar]
- 115.Vaish M, Mandhani A, Mittal RD, et al. Microsatellite instability as prognostic marker in bladder tumors: a clinical significance. BMC Urol 2005;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vilaia A, Peter L, Mocanu D, et al. Mismatch repair protein status and Gata3 expression in bladder urothelial carcinoma. Virchows Arch 2020;477:S170–1. [Google Scholar]
- 117.Wang M, Chen X, Dai Y, et al. Concordance study of a 520-gene next-generation sequencing-based genomic profiling assay of tissue and plasma samples. Mol Diagn Ther 2022;26:309–22. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Zhang J, Wang Y, et al. Expression status of Gata3 and mismatch repair proteins in upper tract urothelial carcinoma. Front Med 2019;13:730–40. [DOI] [PubMed] [Google Scholar]
- 119.Wilson AL, Watkin WG, Thornburg R, et al. MMR IHC in upper urinary tract urothelial carcinoma. Lab Invest 2009;89:201A. [Google Scholar]
- 120.Yang K, Yu W, Liu H, et al. Comparison of genomic characterization in upper tract urothelial carcinoma and urothelial carcinoma of the bladder. Oncologist 2021;26:e1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castro MP, Goldstein N. Mismatch repair deficiency associated with complete remission to combination programmed cell death ligand immune therapy in a patient with sporadic urothelial carcinoma: Immunotheranostic considerations. J Immunotherapy Cancer 2015;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng Y, Cao Y, Yuan M, et al. Different responses to anti-programmed cell death protein 1 (PD-1) Immunotherapy in a patient with lynch syndrome and Metachronous urothelial and colon cancer: a case report. Oncol Lett 2019;18:5085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hsieh-Wong J, Liu J, Huynh J, et al. Immunotherapy in synchronous MSI-H Rectal adenocarcinoma and upper tract urothelial carcinoma: a case report. J Gastrointest Oncol 2022;13:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Janavicius R, Elsakov P. Novel Germline Msh2 mutation in lynch syndrome patient surviving multiple cancers. Hered Cancer Clin Pract 2012;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kafka M, Zanier J, Horninger W. Endoscopy: minimal-invasive treatment approach of bilateral upper tract urothelial carcinoma associated with lynch syndrome-a case report. J Endourol Case Rep 2019;5:110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mancuso JG, Foulkes WD, Pollak MN. Cancer immunoprevention: a case report raising the possibility of “Immuno-Interception Cancer Prev Res (Phila) 2020;13:351–6. [DOI] [PubMed] [Google Scholar]
- 127.Mongiat-Artus P, Miquel C, Fléjou J-F, et al. Spectrum of molecular alterations in colorectal, upper urinary tract, endocervical, and renal carcinomas arising in a patient with hereditary non-polyposis colorectal cancer. Virchows Arch 2006;449:238–43. [DOI] [PubMed] [Google Scholar]
- 128.Sanwal C, Nooruddin K, Nguyen T, et al. A unique case of transitional cell carcinoma of renal pelvis in a patient with lynch syndrome. Int Med 2019;1:286. [Google Scholar]
- 129.Stewart MJ, Guerra GR, Sutherland TR, et al. Abdominal wall metastasis following open nephroureterectomy for upper tract urothelial carcinoma in a patient with lynch syndrome. BMJ Case Rep 2016;2016:bcr2016214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Winer A, Ghatalia P, Bubes N, et al. Dual Checkpoint inhibition with Ipilimumab plus nivolumab after progression on sequential PD-1/PDL-1 inhibitors pembrolizumab and atezolizumab in a patient with lynch syndrome, metastatic colon, and localized urothelial cancer. Oncologist 2019;24:1416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang H, Cheng JS. Segmental ureterectomy with buccal mucosa graft reconstruction in a patient with lynch syndrome and upper tract urothelial carcinoma. J Urol 2018;199:e409. [Google Scholar]
- 132.Yang Y, Jain RK, Glenn ST, et al. Complete response to anti-PD-L1 antibody in a metastatic bladder cancer associated with novel Msh4 mutation and microsatellite instability. J Immunother Cancer 2020;8:e000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yhim HY, Jeon SY, Lee CH, et al. Bilateral pleural effusion associated with atezolizumab in a patient with lynch syndrome-related urothelial carcinoma: a case report. Ann Palliat Med 2022;11:2162–9. [DOI] [PubMed] [Google Scholar]
- 134.Arzimanoglou II, Gilbert F, Barber HR. Microsatellite instability in human solid tumors. Cancer 1998;82:1808–20. [DOI] [PubMed] [Google Scholar]
- 135.Berndl F, Hassler MR. Molecular intricacies of upper tract urothelial carcinoma and their relevance for therapy considerations. Curr Opin Urol 2022;32:48–53. [DOI] [PubMed] [Google Scholar]
- 136.Catto JWF, Meuth M, Hamdy FC. Genetic instability and transitional cell carcinoma of the bladder. BJU Int 2004;93:19–24. [DOI] [PubMed] [Google Scholar]
- 137.Hamilou Z, Lavaud P, Loriot Y. Atezolizumab in urothelial bladder carcinoma. Future Oncol 2018;14:331–41. [DOI] [PubMed] [Google Scholar]
- 138.Moon C, Gordon M, Moon D, et al. Microsatellite instability analysis (MSA) for bladder cancer: past history and future directions. IJMS 2021;22:12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phelan A, Lopez-Beltran A, Montironi R, et al. Inherited forms of bladder cancer: a review of lynch syndrome and other inherited conditions. Future Oncol 2018;14:277–90. [DOI] [PubMed] [Google Scholar]
- 140.Rasmussen M, Madsen MG, Therkildsen C. Immunohistochemical screening of upper tract urothelial carcinomas for lynch syndrome diagnostics: a systematic review. Urology 2022;165:44–53. [DOI] [PubMed] [Google Scholar]
- 141.Wadhwa N, Mathew BB, Jatawa SK, et al. Genetic instability in urinary bladder cancer: an evolving hallmark. J Postgrad Med 2013;59:284–8. [DOI] [PubMed] [Google Scholar]
- 142.Rouprêt M, Azzouzi AR, Cussenot O. Microsatellite instability and transitional cell carcinoma of the upper urinary tract. BJU International 2005;96:489–92. [DOI] [PubMed] [Google Scholar]
- 143.Dietmaier W, Wallinger S, Bocker T, et al. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 1997;57:4749–56. [PubMed] [Google Scholar]
- 144.Shia J Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. J Mol Diagn 2008;10:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vikas P, Messersmith H, Compton C, et al. Mismatch repair and microsatellite instability testing for immune checkpoint inhibitor therapy: ASCO endorsement of college of American pathologists guideline. J Clin Oncol 2023;41:1943–8. [DOI] [PubMed] [Google Scholar]
- 146.Shimozaki K, Hayashi H, Tanishima S, et al. Concordance analysis of Microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep 2021;11:20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Albayrak A, Garrido-Castro AC, Giannakis M, et al. Clinical pan-cancer assessment of mismatch repair deficiency using tumor-only, targeted next-generation sequencing. JCO Precis Oncol 2020;4:1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 149.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim TM, Park PJ. A genome-wide view of microsatellite instability: old stories of cancer mutations revisited with new sequencing technologies. Cancer Res 2014;74:6377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Niu B, Ye K, Zhang Q, et al. Msisensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 2014;30:1015–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nowak JA, Yurgelun MB, Bruce JL, et al. Detection of mismatch repair deficiency and microsatellite instability in colorectal adenocarcinoma by targeted next-generation sequencing. J Mol Diagn 2017;19:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salipante SJ, Scroggins SM, Hampel HL, et al. Microsatellite instability detection by next generation sequencing. Clin Chem 2014;60:1192–9. [DOI] [PubMed] [Google Scholar]
- 154.Middha S, Zhang L, Nafa K, et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol 2017;2017:PO.17.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pearlman R, Frankel WL, Swanson BJ, et al. Prospective statewide study of universal screening for hereditary colorectal cancer: the Ohio colorectal cancer prevention initiative. JCO Precis Oncol 2021;5:PO.20.00525:779–91:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang C, Ding H, Sun S, et al. Incidence and detection of high microsatellite instability in colorectal cancer in a Chinese population: a meta-analysis. J Gastrointest Oncol 2020;11:1155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gutierrez C, Ogino S, Meyerhardt JA, et al. The prevalence and prognosis of microsatellite instability-high/mismatch repair-deficient colorectal adenocarcinomas in the United States. JCO Precis Oncol 2023;7:e2200179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue Hmlh1 is associated with hereditary non-polyposis colon cancer. Nature 1994;368:258–61. [DOI] [PubMed] [Google Scholar]
- 159.Papadopoulos N, Nicolaides NC, Wei YF, et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994;263:1625–9. [DOI] [PubMed] [Google Scholar]
- 160.Abu-Ghazaleh N, Kaushik V, Gorelik A, et al. Worldwide prevalence of lynch syndrome in patients with colorectal cancer: systematic review and meta-analysis. Genet Med 2022;24:971–85. [DOI] [PubMed] [Google Scholar]
- 161.National Comprehensive Cancer Network. Genetic/familial high-risk assessment: colorectal. 2023. Available: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
- 162.Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol 2012;30:4409–15. [DOI] [PubMed] [Google Scholar]
- 163.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in Path_Mmr carriers by gene and gender up to 75 years of age: a report from the prospective lynch syndrome database. Gut 2018;67:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yuan Z, Yang C, Chen J, et al. Comprehensive profiling of genomic in 80 Chinese patients with bladder cancer. JCO 2020;38:e17024. [Google Scholar]
- 165.US Food and Drug Administration. FDA APPROVES enfortumab vedotin-Ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. 2023. Available: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer
- 166.Powles TB, Perez Valderrama B, Gupta S, et al. Lba6 EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (La/mUC). Ann Oncol 2023;34:S1340. [Google Scholar]
- 167.Cercek A, Lumish MA, Sinopoli JC, et al. Single agent PD-1 blockade as curative-intent treatment in mismatch repair deficient locally advanced rectal cancer. JCO 2022;40:LBA5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chakrabarti S, Grewal US, Vora KB, et al. Outcome of patients with early-stage mismatch repair deficient colorectal cancer receiving neoadjuvant immunotherapy: a systematic review. JCO Precis Oncol 2023;7:e2300182. [DOI] [PubMed] [Google Scholar]
- 169.Petrelli F, Ghidini M, Cabiddu M, et al. Microsatellite instability and survival in stage II colorectal cancer: a systematic review and meta-analysis. Anticancer Res 2019;39:6431–41. [DOI] [PubMed] [Google Scholar]
- 170.Hou J, Zhao L, Zhang D, et al. Prognostic value of mismatch repair genes for patients with colorectal cancer: meta-analysis. Technol Cancer Res Treat 2018;17:153303381880850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jin Z, Sinicrope FA. Prognostic and predictive values of mismatch repair deficiency in non-metastatic colorectal cancer. Cancers (Basel) 2021;13:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.


