Abstract
H-2b mice are resistant to persistent infection of the central nervous system by Theiler's virus. They clear the infection 7 to 10 days after intracranial inoculation. Resistance maps to the H-2D gene and not to the H-2K gene and is associated with a potent antiviral cytotoxic T-lymphocyte (CTL) response. We used H-2b mice in which the H-2D or the H-2K gene had been inactivated to dissect the respective roles of these genes in resistance. We report that H-2D−/− but not H-2K−/− mice were susceptible to persistent infection. Furthermore, whereas H-2K−/− mice mounted a vigorous virus-specific CTL response, similar to that of control C57BL/6 mice, the CTL response of H-2D−/− mice was nil or minimal. Using target cells transfected with the H-2Db or the H-2Kb gene, we showed that the H-2K-restricted CTL response against the virus was minimal in H-2D−/− mice. These results demonstrate that the H-2Db and H-2Kb genes play nonredundant roles in the resistance to this persistent infection.
The DA strain of Theiler's virus, a picornavirus of mice, causes a persistent infection of the spinal cord with primary demyelination which is one of the best models of multiple sclerosis (19). The disease that follows intracranial inoculation is biphasic. An acute gray matter encephalomyelitis, which lasts 7 to 10 days, is followed by a low-grade persistent infection of the white matter with chronic inflammation and primary demyelination. The severity of clinical symptoms associated with persistent infection depends on the central nervous system (CNS) viral load (2). Susceptibility to viral persistence and the accompanying pathology varies greatly between inbred strains of mice (7). Strains which are resistant to persistent infection clear the infection after the acute encephalomyelitis. Although susceptibility is multigenic, the H-2D locus, but not the H-2K locus, has a major effect as shown by studying H-2 congenic mice and the dm1 mutation, a large deletion which fuses the H-2D and H-2L genes and which confers susceptibility to the resistant parental B10.D2 strain (9, 10, 28).
Because the H-2b resistant haplotype is dominant over the susceptible H-2q haplotype (9, 25), direct evidence that the major histocompatibility complex class I H-2D gene is responsible for susceptibility was obtained by showing that H-2q mice become resistant to viral persistence and to demyelination when they are transgenic for the H-2Db gene (3, 26). The role of the H-2Db gene is also shown by the fact that mutations in the gene modify susceptibility to demyelination (18). The generally held view is that the H-2Db gene brings about resistance by efficient viral epitope presentation resulting in viral clearance by cytotoxic T lymphocytes (CTL) and prevention of demyelination (13, 19).
Several observations support the role of the CTL response in viral clearance: CD8+ T cells invade the CNS at a later time in susceptible SJL/J mice than in resistant C57BL/6 mice (16), H-2b mice with an inactivated β2-microglobulin gene become susceptible to viral persistence and to demyelination (14, 24, 27), CD8+ T-cell depletion renders mice less efficient at clearing the infection (5), and resistance can be conferred on susceptible substrains of BALB/c mice by passive transfer of CD8+ T cells isolated from resistant substrains (20). H-2Db-restricted CTL have been isolated from the spleen and CNS of resistant C57BL/6 mice (11, 17), and an immunodominant H-2Db-restricted viral epitope has been characterized elsewhere (6, 12). Interestingly, CTL activity is detected very early after inoculation of resistant C57BL/6 mice and rises sharply thereafter, whereas it appears late in susceptible SJL/J mice and remains at a low level (11).
The genome of H-2b mice contains two classical H-2 class I genes, H-2D and H-2K, which are located in different regions. Both class I molecules are highly polymorphic and can present peptides efficiently. Yet, at least for the H-2b, H-2d, and H-2k haplotypes, resistance to Theiler's virus-induced disease maps to the H-2D and not the H-2K gene. This may suggest that, at least in the CNS, some functions of the H-2D and H-2K molecules other than their capacity to bind and present peptides are different in the course of viral infections. Interestingly, H-2D molecules are expressed at a higher level than H-2K molecules in the CNS of H-2s SJL/J mice during the acute phase of Theiler's virus infection, a difference which is not observed for H-2b mice (1). On the other hand, H-2D-, but not H-2K-, restricted CTL are found in the CNS of H-2b mice at that time, whereas neither H-2D- nor H-2K-restricted CTL are found in the CNS of H-2s mice (15). In the present work, we used H-2b mice in which the H-2D or the H-2K gene had been inactivated by homologous recombination to further dissect the respective roles of these genes in the resistance to persistent infection by Theiler's virus.
MATERIALS AND METHODS
Animals and virus.
H-2Db−/− and H-2Kb−/− mice were bred at the Institut Pasteur animal facility in specific-pathogen-free sanitary conditions. C57BL/6 mice were purchased from Iffa Credo (Les Oncins, France) or Janvier (Le Genest-St. Isle, France). The construction of the H-2Db−/− and the H-2Kb−/− mice has been described in detail elsewhere (21, 23). C57BL/6 × 129/Sv mice heterozygous for the mutations were successively backcrossed toward the C57BL/6 mice and intercrossed to obtain homozygous null-mutant mice. The H-2Db−/− mice were backcrossed for four or six generations, depending on the experiment, and the H-2Kb−/− mice were backcrossed for six generations.
The DA strain of Theiler's virus was propagated in baby hamster kidney (BHK-21) cell monolayers grown in Dulbecco's modified Eagle's medium supplemented with 7.5% fetal calf serum and titrated using a standard plaque assay on BHK-21 cell monolayers. Four- to six-week-old mice were inoculated intracranially with 104 PFU of virus in 40 μl of phosphate-buffered saline (PBS).
Preparation of total RNA from brain or spinal cord and dot blot hybridization.
Total RNA was extracted from brain or spinal cord as described in detail elsewhere (9). Fivefold dilutions of total RNA, starting from 10 μg, were immobilized on duplicate Hybond C-Extra filters (Amersham Corp., Arlington Heights, Ill.) according to the manufacturer's recommendation. Filters were hybridized with 106 cpm of a random-primed 32P-labeled cDNA probe specific for the virus or for β-actin overnight at 65°C in 0.5 M sodium phosphate (pH 7.4)–7% sodium dodecyl sulfate. The filters were washed four times for 15 min at 65°C in 40 mM sodium phosphate (pH 7.4)–1% sodium dodecyl sulfate, dried, exposed overnight, and analyzed with a phosphorimager (Becton Dickinson). Hybridization to the β-actin probe was used to control the quality of the RNA samples. The highest dilution which gave a positive signal with the virus-specific probe was used as a measure of viral RNA content. To normalize the results of different experiments, dilutions of RNA samples previously analyzed were run as standards on the filters.
Immunohistochemistry.
The mice were anesthetized with ether, perfused through the left ventricle with 20 ml of PBS followed by 20 ml of 4% paraformaldehyde in PBS. The brain and spinal cord were dissected out, refixed by immersion, and embedded in paraffin using routine procedures. Serial coronal sections of the brain and longitudinal sections of the spinal cord were prepared. The sections were reacted with a rabbit hyperimmune serum against Theiler's virus capsid proteins, followed by a biotinylated anti-rabbit secondary antibody and an avidin-biotin–horseradish peroxidase complex (Vectastain; Vector Laboratories, Burlingame, Calif.). The slides were developed with 0.01% H2O2–diaminobenzidine-tetrahydrochloride (DAB; Sigma) in 0.05 Tris-HCl (pH 7.5) for 5 min and counterstained with Harris hematoxylin.
CTL assay.
The generation of CTL, the 51Cr labeling and preparation of target cells, and the CTL assay have been described in detail elsewhere (12). Briefly, mice were inoculated intraperitoneally with 106 PFU of Theiler's virus, and splenocytes were prepared 3 weeks later. Splenocytes were restimulated in vitro for 5 days by cocultivation with Theiler's virus-infected, irradiated, syngeneic splenocytes. Three kinds of target cells were used: C57SV (H-2b) fibroblasts and Ltk− cells stably transfected with either the H-2Db or the H-2Kb gene. Target cells were obtained by loading them with 51Cr followed by infection with Theiler's virus for 2 h. Cytolytic activity of restimulated splenocytes was measured by a standard 51Cr release assay.
Statistical analysis.
The means of the scores of viral persistence were compared among mouse strains using either the unpaired Student t test or the analysis of variance and its associated Scheffe test from the Statview F-4.5 package.
RESULTS
H-2Db−/−, but not H-2Kb−/−, mutant mice are susceptible to persistent infection and to late white matter disease.
H-2b mice are resistant to persistent infection by Theiler's virus. Because H-2bβ2m−/− mutant mice are susceptible (14, 24, 27), it has been assumed that H-2b-associated resistance was due to a class I-restricted CTL response. We tested the role of class I genes in resistance directly by examining the phenotype of mice in which the H-2Db or the H-2Kb gene had been inactivated by homologous recombination (21, 23). H-2Db−/− mice were obtained from C57BL/6 × 129/Sv H-2Db+/− mice after backcrossing toward the C57BL/6 background for four or six generations, depending on the experiment, followed by intercrossing to obtain homozygous mutants. The H-2Kb−/− mutant mice were obtained in a similar way after backcrossing toward the C57BL/6 inbred strain for six generations. The mutant mice, as well as control C57BL/6 mice, were inoculated with 104 PFU of Theiler's virus, strain DA, and sacrificed 45 days later. Neither mutants nor controls showed clinical symptoms. Their spinal cords were examined for viral RNA levels and histopathology, as described in Materials and Methods. The results of the experiment are shown in Table 1.
TABLE 1.
Level of viral RNA in brain and spinal cord
| Mouse strain | Day p.i. | Organa | n | Viral RNA (mean ± SEM) |
|---|---|---|---|---|
| C57BL/6 | 7 | b | 3 | 0.17 ± 0.17 |
| sc | 3 | 0.0 ± 0.0 | ||
| 21 | b | 4 | 0.00 ± 0.00 | |
| sc | 4 | 0.13 ± 0.13 | ||
| 45 | sc | 11 | 0.00 ± 0.00 | |
| H-2Db−/− | 7 | b | 6 | 0.25 ± 0.25 |
| sc | 6 | 0.67 ± 0.31 | ||
| 21 | b | 7 | 0.00 ± 0.00 | |
| sc | 7 | 0.86 ± 0.32 | ||
| 45 | sc | 14 | 1.3 ± 0.3 | |
| 90 | sc | 7 | 2.5 ± 0.0 | |
| H-2Kb−/− | 45 | sc | 6 | 0.0 ± 0.0 |
b, brain; sc, spinal cord.
Measuring the level of viral RNA in the spinal cord with a dot blot assay showed that there was significantly more viral RNA in the case of H-2Db−/− mice than in the case of C57BL/6 mice (mDb−/− = 1.3 ± 0.3 [n = 14], mC57BL/6 = 0.0 ± 0.0 [n = 11], P = 0.0034) and of H-2Kb−/− mice (mKb−/− = 0.0 ± 0.0 [n = 6], P = 0.0164). The variation in RNA level between individual H-2Db−/− mice might have been due to the fact that the animals were obtained after only four to six backcrosses toward the C57BL/6 strain. The level of viral RNA was also examined 90 days postinfection (p.i.) in the spinal cord of H-2Db−/− mice (Table 1). The level was higher than at 45 days p.i. (mDb−/− = 2.5 ± 0.0 [n = 7]), and there was less variation from mouse to mouse. This result confirmed that the DA strain of Theiler's virus persists in the CNS of H-2Db−/− mice.
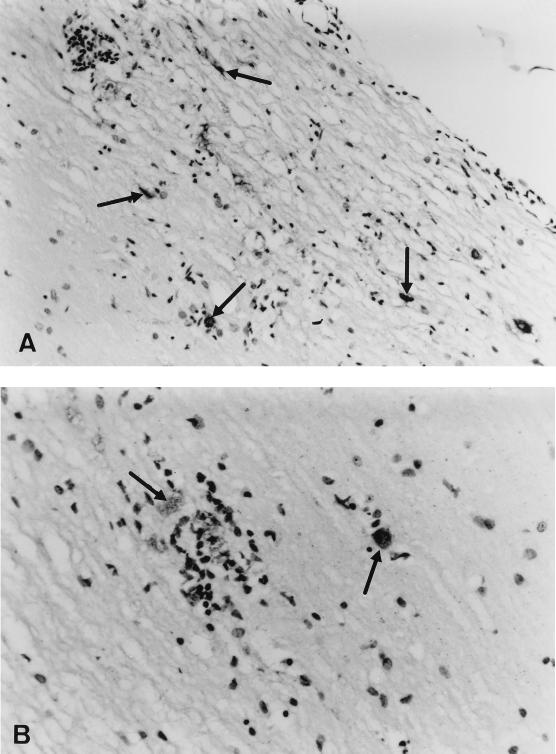
Using immunohistochemistry, infected cells were observed in the white matter of the spinal cord of 10 out of 15 H-2Db−/− mice examined at 45 days p.i. Infected cells were accompanied by meningitis, perivascular cuffs, and diffuse parenchymal inflammation (Fig. 1). At the same time p.i., a small number of antigen-containing cells—fewer than 10 cells per longitudinal section of the entire spinal cord—was observed in the spinal cord of only two out of six H-2Kb−/− mice examined, exclusively in the gray matter (data not shown).
FIG. 1.
Longitudinal section of the spinal cord of an H-2Db−/− mouse, 45 days p.i. with Theiler's virus. Viral antigens were detected by immunoperoxidase staining (arrows). Counterstaining was done with hematoxylin. (A) Magnification, ×270. (B) Magnification, ×431.
In conclusion, these experiments showed that H-2Db−/− mice were susceptible to persistent infection of the white matter and to chronic inflammation, whereas H-2Kb−/− mice and, as expected, C57BL/6 control mice were resistant.
Viral replication and histopathology in the CNS of H-2Db−/− mice at early times after inoculation.
Resistant C57BL/6 mice clear the infection at the end of the early, gray matter, encephalomyelitis, whereas H-2Db−/− mice remain persistently infected. To compare the patterns of CNS infection in both mouse strains at the time when virus is cleared in the C57BL/6 strain, H-2Db−/− mutant mice and wild-type C57BL/6 mice were inoculated with 104 PFU of Theiler's virus, strain DA, and sacrificed 6 and 21 days later. Levels of viral RNA and histopathology were examined in brain and spinal cord. The results are shown in Table 1. At the peak of the early phase of the disease (6 days p.i.), the levels of viral RNA were not significantly different in the brain of control and H-2Db−/− mice (mC57BL/6 = 0.17 ± 0.17 [n = 3], mDb−/− = 0.25 ± 0.25 [n = 6]). On the other hand, a significant amount of viral RNA was already detected at this time in the spinal cord of H-2Db−/− mice, whereas no viral RNA could be found in the spinal cord of C57BL/6 mice (mC57BL/6 = 0.00 ± 0.00 [n = 3], mDb−/− = 0.67 ± 0.31 [n = 6]). Histopathological examination showed many cells containing viral antigens in the gray matter of the brain of five out of six wild-type mice and six out of eight mutant mice examined. Infected cells were located mainly along the needle track but also in scattered cortical and subcortical foci as well as in the hippocampus in some of the mice, regardless of the genotype (data not shown).
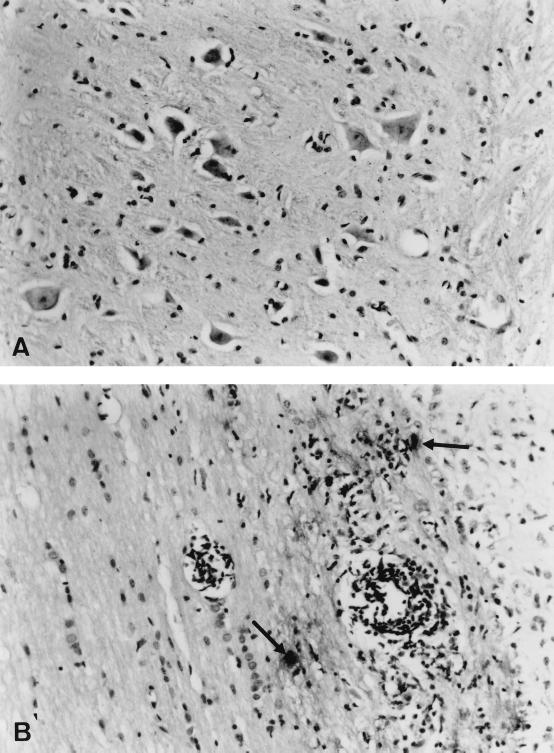
At 21 days p.i., no viral RNA was detected with the dot blot assay in the brain and spinal cord of three out of four C57BL/6 mice and only a low RNA level was detected in one out of four (mC57BL/6 = 0.13 ± 0.13 [n = 4]). Mild inflammation of the gray matter, and some meningitis, was observed in the spinal cord of all six C57BL/6 mice examined. No viral antigen was present in the gray matter in any of these mice (Fig. 2A). In two of them, there was mild inflammation and an occasional infected cell in the white matter (only two infected cells were detected in the white matter of one mouse). At the same time p.i., the level of viral RNA was moderate to high, depending on the animal, in the spinal cord of the seven H-2Db−/− mice examined (mDb−/− = 0.86 ± 0.32 [n = 7]). Large numbers of viral antigen-containing cells and severe inflammation were observed in the white matter of spinal cord of five out of six H-2Db−/− mice examined (Fig. 2B). Intense meningitis was also present. Although inflammatory foci were present in the gray matter, only a few infected cells were observed in this compartment. One of the six H-2Db−/− mice examined had lesions that were less extensive, but otherwise similar.
FIG. 2.
Longitudinal section of the spinal cord, 21 days p.i. with Theiler's virus. Viral antigens were detected by immunoperoxidase staining. Counterstaining was done with hematoxylin. (A) Mild inflammation in the gray matter of a control C57BL/6 mouse, in the absence of viral antigens. Magnification, ×431. (B) Intense inflammation and viral antigens (arrows) in the white matter of an H-2Db−/− mouse. Magnification, ×270.
In conclusion, the H-2Db−/− null mutation does not affect the pattern of the early disease in brain. As for susceptible mice, the establishment of viral persistence coincides with the localization of the infection to the white matter of spinal cord (2, 4).
Anti-Theiler's virus CTL activity in null-mutant and control mice.
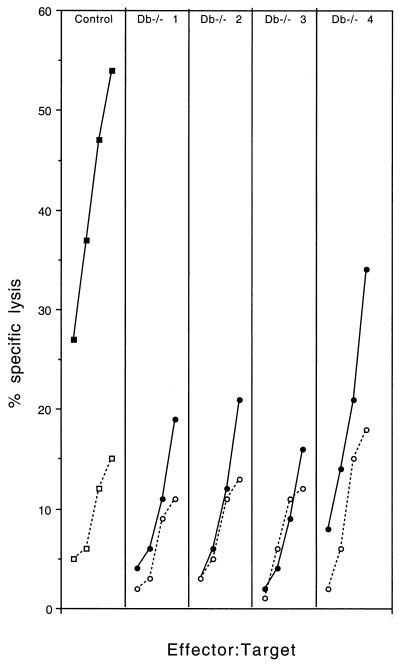
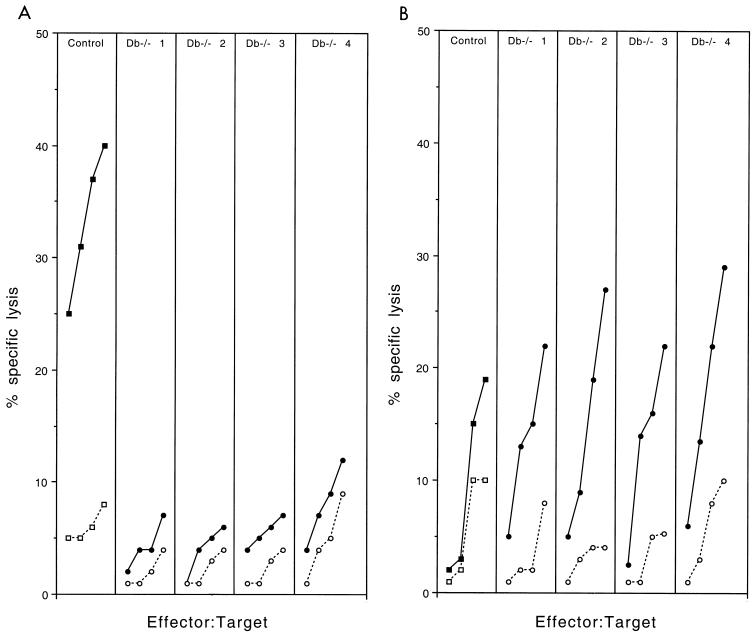
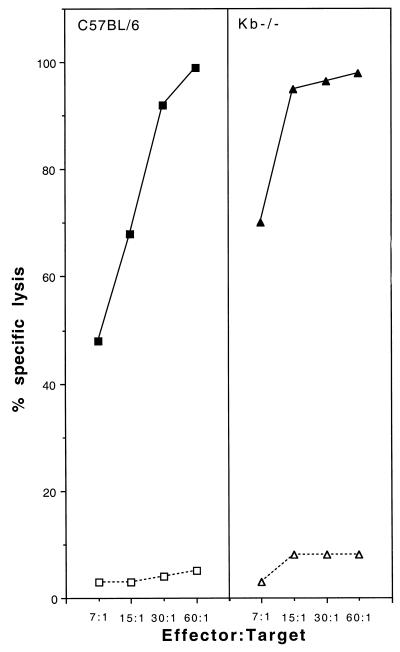
Resistant C57BL/6 mice develop a fast and intense H-2Db-restricted CTL response directed at an immunodominant epitope and only a weak H-2Kb-restricted response (11). The fact that H-2Db−/− mice become susceptible and that H-2Kb−/− mice remain resistant is consistent with the role of H-2Db-restricted CTL in resistance and suggests that H-2Kb-restricted CTL are unable to clear the infection. To examine these points further, we studied the virus-specific H-2Db- and H-2Kb-restricted CTL responses of H-2Db−/− mice. Briefly, 6- to 8-week-old H-2Db−/− and control C57BL/6 mice were inoculated intraperitoneally with 106 PFU of Theiler's virus. Spleen cells were prepared from individual mice 9 days later and restimulated in vitro with syngeneic infected splenocytes. Restimulated splenocytes were tested, in a 51Cr release assay, against infected C57SV fibroblasts or infected L cells expressing the H-2Db or the H-2Kb molecule. Figure 3 shows the results for one control C57BL/6 mouse and four individual H-2Db−/− mice. As shown by the figure, the splenocytes of H-2Db−/− mice had a much lower cytolytic activity against infected C57SV fibroblasts than did those of control C57BL/6 mice. However, a low but significant cytolytic activity was observed, for all four H-2Db−/− mice, at effector-to-target ratios of 60:1. In one case, mouse number 4, the activity was detected even at low effector-to-target ratios. Figure 4A shows that no H-2Db-restricted cytolytic activity was detected in H-2Db−/− mice, whereas splenocytes from C57BL/6 mice could lyse infected L cells expressing the H-2Db molecule. On the other hand, low but measurable cytolytic activity was observed in H-2Db−/− mice against L cells expressing the H-2Kb molecule (Fig. 4B). This activity probably explains the cytolytic activity of splenocytes from H-2Db−/− mice against infected C57SV fibroblasts (Fig. 3). Lastly, the splenocytes of infected H-2Kb−/− mice lysed infected C57SV fibroblasts as efficiently as did splenocytes of control C57BL/6 mice (Fig. 5). In summary, these results confirm that the majority of the CTL activity of C57BL/6 mice against Theiler's virus is H-2Db restricted. Inactivating the H-2Db gene causes only a slight increase in the H-2Kb-restricted response, which is insufficient to prevent persistence of the infection.
FIG. 3.
Virus-specific CTL responses in one C57BL/6 mouse (squares) and four individual H-2Db−/− mice (circles). The target cells were infected (closed symbols) or uninfected (open symbols) C57SV fibroblasts. Effector-to-target ratios were 7:1, 15:1, 30:1, and 60:1.
FIG. 4.
Virus-specific CTL responses in one C57BL/6 mouse (squares) and four individual H-2Db−/− mice (circles). The target cells were infected (closed symbols) or uninfected (open symbols) Ltk− cells transfected with the H-2Db gene (A) or the H-2Kb gene (B). Effector-to-target ratios were 7:1, 15:1, 30:1, and 60:1.
FIG. 5.
Virus-specific CTL responses in C57BL/6 and H-2Kb−/− mice (squares and triangles, respectively). The target cells were infected (closed symbols) or uninfected (open symbols) C57SV fibroblasts.
DISCUSSION
The resistance of H-2b mice to persistent infection by Theiler's virus maps to the H-2Db locus and not to the H-2Kb locus. Interestingly, only H-2D-restricted virus-specific CTL are found in the CNS of resistant C57BL/10 mice at the time of viral clearance, although both H-2D- and H-2K-restricted CTL are found in spleen (17). The results presented in this article show that inactivating the H-2Db, but not the H-2Kb, gene makes the animal susceptible to persistent infection. Therefore, they confirm the unique properties of the H-2Db gene in this system. This uniqueness is puzzling since both the H-2D and the H-2K genes code for class I molecules with similar potentials for peptide presentation. It could be explained in at least two ways. Since class I genes are not expressed in healthy CNS tissue, it is possible that the expression of the H-2D and H-2K molecules in CNS is regulated differently upon infection by Theiler's virus. This hypothesis is not upheld by the results of Altintas et al., who observed the same transient increase of expression for both class I molecules after intracranial inoculation of C57BL/6 mice with Theiler's virus (1). Another possibility is that the viral immunodominant H-2Db-restricted epitope (6, 12) is much more efficient at eliciting an immune response than are viral H-2Kb-restricted epitopes. This hypothesis is supported by recent data showing that the exons coding for the α1 and α2 domains of the H-2Db molecule, domains which bind the peptide epitope, are responsible for resistance (A. Azoulay-Cayla et al., unpublished data). The unique role played by the H-2D gene in Theiler's virus infection has been demonstrated for the b haplotype. At present, we do not know if it exists also for other haplotypes.
The SJL/J strain is the prototypic strain susceptible to the persistence of Theiler's virus. Although we did not compare the H-2Db−/− and SJL/J strains in the same experiment, the level of viral RNA in the former, 45 days p.i., was lower, and there was more variation between individuals, than what we routinely observe in the latter. This difference might be due to the existence of several susceptibility loci which are found in only the SJL/J strain (8). It is interesting that, by 90 days p.i., the level of viral RNA in H-2Db−/− mice becomes similar to that observed in SJL/J mice 45 days p.i.
The results presented in this article demonstrate that the H-2Db gene is required for clearance of Theiler's virus, whereas the H-2Kb gene is not. There is strong evidence in the literature that H-2b mice clear Theiler's virus infection through a vigorous H-2Db-restricted CTL response (6, 11, 12, 15). The fact that, in the experiments reported here, resistance is associated with such a virus-specific CTL response supports this conclusion. However, it has not been determined if these CTL target infected gray matter neurons or white matter glial cells as they become infected at the end of the early encephalomyelitis. The fact that the early neuronal infection was of the same magnitude in C57BL/6 mice and in H-2Db−/− mice suggests that the CTL do not clear the infection from infected neurons. The fact that H-2Db−/− mice do not mount a significant antiviral CTL response and are susceptible to persistent infection suggests very strongly that CTL are critical for viral clearance in wild-type mice. We cannot formally exclude a role of the H-2Db gene in the virus-specific CD4 and antibody responses, especially since it has been shown that circulating antibodies contribute to the resistance of the C57BL/6 strain (22, 29).
As shown in this article, H-2Db−/− mice were unable to mount an efficient, compensatory H-2Kb-restricted CTL response. This is shown by the susceptibility to persistent infection of the H-2Db−/− mice and, more directly, by the low specific cytotoxicity of splenocytes of infected H-2Db−/− mice against H-2Kb-expressing target cells (Fig. 4B). This result is in striking contrast with that obtained when the same mutant mice were infected with lymphocytic choriomeningitis virus. In this case, H-2Db−/− mice developed a normally subdominant H-2Kb-restricted CTL response which resulted in fatal choriomeningitis (23). This difference in behavior of the same mouse strain when infected by two different viruses emphasizes the danger of generalizing results for the immune responses to viral infections.
ACKNOWLEDGMENTS
We thank Sylvie Syan for excellent technical assistance and Mireille Gau for secretarial help.
Arièle Azoulay-Cayla was supported by fellowships from the Association pour la Recherche sur la Sclérose en Plaques and the Fondation pour la Recherche Médicale. Research on Theiler's virus in the Unité des Virus Lents is supported by grants from Institut Pasteur, CNRS, the Association pour la Recherche sur la Sclérose en Plaques, and the National Multiple Sclerosis Society, United States.
REFERENCES
- 1.Altintas A, Cai Z, Pease L R, Rodriguez M. Differential expression of H-2K and H-2D in the central nervous system of mice infected with Theiler's virus. J Immunol. 1993;151:2803–2812. [PubMed] [Google Scholar]
- 2.Aubagnac S, Brahic M, Bureau J-F. Viral load and a locus on chromosome 11 affect the late clinical disease caused by Theiler's virus. J Virol. 1999;73:7965–7971. doi: 10.1128/jvi.73.10.7965-7971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay A, Brahic M, Bureau J-F. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler's virus. J Virol. 1994;68:4049–4052. doi: 10.1128/jvi.68.6.4049-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bihl F, Pena-Rossi C, Guénet J-L, Brahic M, Bureau J-F. The shiverer mutation affects the persistence of Theiler's virus in the central nervous system. J Virol. 1997;71:5025–5030. doi: 10.1128/jvi.71.7.5025-5030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Tonks P, Welsh C J R, Nash A A. The role of CD8+ T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73:1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- 6.Borson N D, Paul C, Lin X, Nevala W K, Strausbauch M A, Rodriguez M, Wettstein P J. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J Virol. 1997;71:5244–5250. doi: 10.1128/jvi.71.7.5244-5250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahic M, Bureau J-F. Genetics of susceptibility to Theiler's virus infection. Bioessays. 1998;20:627–633. doi: 10.1002/(SICI)1521-1878(199808)20:8<627::AID-BIES5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Bureau J-F, Montagutelli X, Bihl F, Lefebvre S, Guénet J-L, Brahic M. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat Genet. 1993;5:87–91. doi: 10.1038/ng0993-87. [DOI] [PubMed] [Google Scholar]
- 9.Bureau J-F, Montagutelli X, Lefebvre S, Guénet J-L, Pla M, Brahic M. The interaction of two groups of murine genes determines the persistence of Theiler's virus in the central nervous system. J Virol. 1992;66:4698–4704. doi: 10.1128/jvi.66.8.4698-4704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clatch R J, Melvold R W, Miller S D, Lipton H L. Theiler's murine encephalomyelitis virus (TMEV) induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1413. [PubMed] [Google Scholar]
- 11.Dethlefs S, Brahic M, Larsson-Sciard E L. An early, abundant cytotoxic T-lymphocyte response against Theiler's virus is critical for preventing viral persistence. J Virol. 1997;71:8875–8878. doi: 10.1128/jvi.71.11.8875-8878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dethlefs S, Escriou N, Brahic M, van der Werf S, Larsson-Sciard E-L. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J Virol. 1997;71:5361–5365. doi: 10.1128/jvi.71.7.5361-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drescher K M, Pease L R, Rodriguez M. Antiviral immune responses modulate the nature of central nervous system (CNS) disease in a murine model of multiple sclerosis. Immunol Rev. 1997;159:177–193. doi: 10.1111/j.1600-065x.1997.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 14.Fiette L, Aubert C, Brahic M, Pena Rossi C. Theiler's virus infection of β2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Pease L R, Rodriguez M. Differential generation of class I H-2D- versus H-2K-restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur J Immunol. 1997;27:963–970. doi: 10.1002/eji.1830270424. [DOI] [PubMed] [Google Scholar]
- 16.Lindsley M D, Rodriguez M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler's virus. J Immunol. 1989;142:2677–2682. [PubMed] [Google Scholar]
- 17.Lindsley M D, Thiemann R, Rodriguez M. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler's virus. J Virol. 1991;65:6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton H L, Melvold R, Miller S D, Dal Canto M C. Mutation of a major histocompatibility class I locus, H-2D, leads to an increased virus burden and disease susceptibility in Theiler's virus-induced demyelinating disease. J Neurovirol. 1995;1:138–144. doi: 10.3109/13550289509113960. [DOI] [PubMed] [Google Scholar]
- 19.Monteyne P, Bureau J-F, Brahic M. The infection of mouse by Theiler's virus: from genetics to immunology. Immunol Rev. 1997;159:163–176. doi: 10.1111/j.1600-065x.1997.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson S M, Dal Canto M C, Miller S D, Melvold R W. Adoptively transferred CD8+ T lymphocytes provide protection against TMEV-induced demyelinating disease in BALB/c mice. J Immunol. 1996;156:1276–1283. [PubMed] [Google Scholar]
- 21.Pascolo S, Bervas N, Ure J M, Smith A G, Lemonnier F A, Pérarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8 (+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pena Rossi C, Cash E, Aubert C, Coutinho A. Role of the humoral immune response in resistance to Theiler's virus infection. J Virol. 1991;65:3895–3899. doi: 10.1128/jvi.65.7.3895-3899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérarnau B, Saron M F, San Martin B R, Bervas N, Ong H, Soloski M J, Smith A G, Ure J M, Gairin J E, Lemonnier F A. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Pullen L C, Miller S D, Dal Canto M C, Kim B S. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur J Immunol. 1993;23:2287–2293. doi: 10.1002/eji.1830230935. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez M, David C S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- 26.Rodriguez M, David C S. H-2Dd transgene suppresses Theiler's virus-induced demyelination in susceptible strains of mice. J Neurovirol. 1995;1:111–117. doi: 10.3109/13550289509111015. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez M, Dunkel A J, Thiemann R L, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J Immunol. 1993;151:255–276. [PubMed] [Google Scholar]
- 28.Rodriguez M, Leibowitz J L, David C S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez M, Patick A K, Pease L R. Abrogation of resistance to Theiler's virus-induced demyelination in C57BL mice by total body irradiation. J Neuroimmunol. 1990;26:189–199. doi: 10.1016/0165-5728(90)90001-4. [DOI] [PubMed] [Google Scholar]