Abstract
Heterosubtypic immunity (HSI) is defined as cross-protection against influenza virus of a different serotype than the virus initially encountered and is thought to be mediated by influenza virus-specific cytotoxic T lymphocytes (CTL). Since gamma interferon (IFN-γ) stimulates cytotoxic cells, including antigen-specific CTL which may control virus replication by secretion of antiviral cytokines such as tumor necrosis factor alpha and IFN-γ, we have investigated the mechanism of HSI by analyzing the role of IFN-γ for HSI in IFN-γ gene-deleted (IFN-γ−/−) mice. It has been reported that IFN-γ is not required for recovery from primary infection with influenza virus but is important for HSI. Here, we conclusively show that IFN-γ is not required for induction of secondary influenza virus-specific CTL responses in mediastinal lymph nodes and HSI to lethal influenza A virus infection. Although T helper 2 (Th2)-type cytokines were upregulated in the lungs of IFN-γ−/− mice after virus challenge, either Th1- or Th2-biased responses could provide heterosubtypic protection. Furthermore, titers of serum-neutralizing and cross-reactive antibodies to conserved nucleoprotein in IFN-γ−/− mice did not differ significantly from those in immunocompetent mice. These results indicate that lack of IFN-γ does not impair cross-reactive virus-specific immune responses and HSI to lethal infection with influenza virus. Our findings provide new insight for the mechanisms of HSI and should be valuable in the development of protective mucosal vaccines against variant virus strains, such as influenza and human immunodeficiency virus.
Protection against challenge with influenza viruses of the same strain is normally attributed to neutralizing antibodies specific for two viral membrane glycoproteins, hemagglutinin and neuraminidase. Due to periodic antigenic shifts in these two glycoproteins, virus-neutralizing antibodies induced by primary infection fail to protect from secondary infection with a different subtype, and cross-protection between different subtypes of influenza A virus is mediated by heterosubtypic immunity (HSI) (30). Although the precise effector mechanisms for HSI remain undefined, HSI is thought to be mediated by subtype cross-reactive cytotoxic T lymphocytes (CTL) (34, 36, 41, 47). These CTL recognize conserved epitopes of internal proteins, such as nucleoprotein (NP) or matrix protein shared by influenza A virus subtypes. Passive transfer of large numbers of in vitro-activated T cells possessing subtype-specific cytotoxic activity to influenza virus-infected mice can reduce pulmonary influenza virus titers, promote their recovery, and provide protection against infection under certain circumstances (6, 18, 20, 33, 39, 44, 45). More recently, we have reported that antigen-specific CTL responses induced in mediastinal lymph nodes (MLN), a type of mucosa-associated lymphoid tissue (MALT), are associated with host recovery from lethal infection with heterosubtypic influenza A virus (25). The CTL responses may control virus infection through direct lysis of infected cells or by secretion of antiviral cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (for a review, see reference 13). While the direct lysis of virus-infected cells by CTL is thought to control infection with noncytopathic virus, secretion of antiviral cytokines by CTL is considered more effective in control of infection by cytopathic viruses such as influenza virus.
IFN-γ exerts pleiotropic effects, including direct antiviral activity and stimulation of antiviral immune responses. In virally infected cells, IFN-γ induces synthesis of proteins and enzymes that inhibit viral replication by impairing accumulation of virus-specific mRNA, double-stranded RNA, and proteins (26). In addition, IFN-γ stimulates antiviral immune responses by upregulating major histocompatibility complex (MHC) molecules on antigen-presenting cells (4), augmenting the proteolysis and peptide transport machinery in antigen-presenting cells (28, 42), and activating immune cells (5, 21). IFN-γ plays a protective role against infections by vaccinia (12, 15), herpes simplex (32), cytomegalovirus (11), murine hepatitis virus 3 (19), lymphocytic choriomeningitis virus (16), and adenovirus (43). Interestingly, IFN-γ is not necessary for recovery from primary infection with influenza virus (7) but has been reported to play a role in HSI (1). However, the latter study employed immunization as well as challenge protocols distinct from those routinely used for studies of HSI (3, 17, 25, 30, 46). Thus, the precise role of IFN-γ in HSI has not been unambiguously elucidated.
Antigen-specific MHC class I-restricted CTL secrete IFN-γ upon antigenic stimulation (14, 22), and differentiation to CTL effector cells is dependent on the action of IFN-γ (21, 31). The mutual interaction between antigen-specific CTL responses and IFN-γ and the association of CTL responses in HSI led us to assess this in mice deficient in IFN-γ. In this report, the effect of IFN-γ on induction of cellular and humoral immune responses and HSI to lethal influenza challenge was systematically studied using IFN-γ gene-deleted (IFN-γ−/−) mice.
MATERIALS AND METHODS
Viruses.
Influenza virus strains A/Udorn/307/72 (H3N2) (henceforth A/Udorn) (a gift from Brian Murphy, National Institutes of Health, Bethesda, Md.) and A/PR/8/34 (H1N1) (a gift from Thomas M. Moran, Mount Sinai School of Medicine, New York, N.Y.) were prepared as previously reported (25). Mouse-adapted virus A/PR/8/34 (H1N1), prepared as lung homogenates of intranasally infected mice, was used for a challenge.
Mice.
Six-week-old female BALB/c (H-2d) and C57BL/6 (H-2b) (wild-type [WT]) mice and mice homozygous for a targeted disruption of the IFN-γ gene (C57BL/6 IFN-γ−/− mice) were purchased from Jackson Laboratories (Bar Harbor, Maine). The BALB/c IFN-γ−/− mice were generously provided by Timothy A. Stewart of Genentech (San Francisco, Calif.). All mice were maintained under pathogen-free conditions in a flexible Trexler isolator and provided sterile food and water ad libitum. The mice used in our experiments were between 8 and 10 weeks of age.
Immunizations and challenge of mice with influenza virus.
All mice were immunized with 5 × 105 PFU of virus. Ketamine-anesthetized mice each received 50 μl of virus inoculum for total respiratory tract (TRT) infection. For intravenous (i.v.) immunization, 200 μl of virus suspension was injected into the tail vein of each mouse. Immunization by TRT and i.v. methods resulted in 100 and 98% of mice displaying antibody responses, respectively. The same pattern was observed in immunocompetent as well as in IFN-γ−/− mice. The absence of antibody (Ab) response in 2% of i.v.-immunized mice reflects occasional unsuccessful i.v. immunization. For the study of HSI, all mice must be successfully immunized (monitored by Ab response) with serotypes different from that used for challenge. Therefore, before virus challenge all mice were tested for the presence of virus-specific serum Ab by enzyme-linked immunosorbent assay (ELISA) to ensure that immune responses to the initial virus infection had been induced. Mice without virus-specific Abs were excluded from these experiments. For virus challenges, ketamine-anesthetized mice were infected by the TRT method with 250 PFU (5× the 50% lethal dose) resuspended in 50 μl of phosphate-buffered saline (PBS) per ml per mouse.
Cells.
EL-4 (H-2b) T-cell lymphoma and P815 (H-2d) mastocytoma cells (American Type Culture Collection, Rockville, Md.) were maintained in standard complete medium (RPMI 1640; Gibco, Grand Island, N.Y.) containing 10% fetal bovine serum (FBS) and antibiotics. Hybridoma 3.155 cells (ATCC) secrete a rat monoclonal immunoglobulin M (IgM) Ab specific for all mouse Lyt-2 (CD8) alleles. These Abs can inhibit T-cell-mediated cytolysis in the absence of complement (29).
Lung tissue extracts for cytokine analysis.
Lung tissue extracts were prepared as described by others (9). Intact lungs were collected for assessment of cytokines characteristic of Th1- or Th2-type responses. Prior to lung removal, the pulmonary vasculature was perfused with 1 ml of PBS containing 5 mM of EDTA via the right ventricle. After removal, whole lungs were homogenized in 3 ml of lysis buffer containing 0.5% Triton X-100, 150 mM Tris, 1 mM CaCl2, and 1 mM MgCl2 (pH 7.4), using a tissue homogenizer (Biospec Products, Inc., Racine, Wis.). Homogenates were incubated on ice for 30 min and then centrifuged at 1,000 × g for 10 min. Supernatants were collected after passage through a 0.45-μm-pore-size filter and stored at −20°C until assessed for cytokines.
Analysis of IFN-γ mRNA.
Whole lungs were homogenized, and total RNA was isolated by phenol-chloroform extraction with RNA STAT-60 reagent (Tel-test, Inc., Friendswood, Tex.). All reverse transcription and PCRs were performed with reagents from the GeneAmp RNA PCR Kit (Perkin-Elmer, Foster City, Calif.) according to the producer's protocols. Murine IFN-γ cDNA was amplified using 5′-TGAACGCTACACACTGCATCTTGG-3′ as a sense primer and 5′-CGACTCCTTTTCCGCTTCCTGAG-3′ as an antisense primer (8). PCR products were visualized by ethidium bromide-stained gels.
Virus neutralization assay.
Virus neutralization (VN) activity was assayed as previously reported (25). Briefly, heat-inactivated (30 min at 56°C) serum samples were serially diluted in 96-well tissue culture plates (Costar, Cambridge, Mass.) at 4 wells per dilution, starting at a 1:8 dilution in a total volume of 50 μl of RPMI 1640 medium without serum. Influenza virus (A/Udorn or A/PR/8/34) at a 50% tissue culture infective dose of 200 in 50 μl of RPMI 1640 medium was added to each well. Control wells contained either medium only instead of immune serum or medium only without virus. After incubation at room temperature for 1 h, 50 μl of RPMI 1640 medium containing 3.75 μg of trypsin per ml was added to each well and transferred into wells with an 80% monolayer of MDCK cells previously washed with PBS. The plates were incubated at 35°C in a 5% CO2 atmosphere. Three days later, 100 μl of 10% formalin was added, plates were incubated for 1 h, and cells were washed with PBS and stained with Coomassie brilliant blue for the cytopathic effect determination. VN titer was calculated as an average of 4 dilutions per serum sample at which 100% VN occurred.
ELISA.
The assay was performed as previously described (25). Briefly, 96-well MaxiSorp Nunc-Immuno plates (Nalge Nunc International, Naperville, Ill.) were coated with RNP of influenza A virus at a concentration of 0.5 μg/ml. The bound Ab was detected with enzyme-conjugated anti-mouse Ig (Southern Biotechnology Associates, Birmingham, Ala.). IgG1 and IgG2a subclass titers were determined using enzyme-conjugated monoclonal anti-mouse IgG1 (G1 7.3; 2 μg/ml), IgG2a (R19-15; 1 μg/ml) heavy-chain-specific Abs (PharMingen, San Diego, Calif.), respectively.
Cytokine ELISA assays.
Cytokine levels in lung extracts and culture supernatants were determined by an ELISA with capture and detection monoclonal Abs (PharMingen) specific for the murine cytokines interleukin-4 (IL-4) and IFN-γ. The cytokine ELISA assays were performed as previously described (38). To determine the amount of cytokine present in test samples, twofold dilutions of recombinant murine IL-4 (Endogen, Boston, Mass.) and IFN-γ (Genzyme Corp., Cambridge, Mass.) were used as standard curves, and values for the test samples were then interpolated. The actual values represent the mean of triplicate samples ± 1 standard deviation. The detection limits were 25 and 20 pg/ml for IFN-γ and IL-4, respectively.
Generation of antigen-specific CTL effector cells.
Stimulation of antigen-specific CTL effector cells was performed as previously described (25). Briefly, spleens and MLN were taken from five mice per experimental group, and single-cell suspensions were pooled for further analysis. A portion of the spleen cell suspension, stimulator cells, was infected with influenza virus A/Udorn at a multiplicity of infection (MOI) of 2 to 4 or with A/PR/8/34 at an MOI of 4 to 6 in 0.2 ml of PBS or RPMI 1640 medium without FBS. After incubation for 30 min at 37°C and with 5% CO2, RPMI 1640 containing 10% FBS, 10 mM HEPES, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.03% glutamine, and 3× 10−5 M 2-mercaptoethanol (complete medium) was added, and the cell suspension was incubated for 3 h, irradiated (3,000 R), washed, and mixed with the remaining splenocytes (responder cells (R), at an R-to-stimulator-cell ratio of 2:1 (3 × 106 to 4 × 106 R/ml) in CTL complete medium. Murine recombinant IL-2 (rIL-2) (R & D Systems, Inc., Minneapolis, Minn.) was added to cultures at day 3 (20 U/ml), followed by an additional 3-day incubation at 37°C with 5% CO2, after which effector cells were washed and tested with virus-infected MHC-matched target cells in a 51Cr release assay.
Preparation of target cells.
P815 (H-2d) and EL-4 (H-2b) cells were infected at an MOI of 5 with A/Udorn or A/PR/8/34 influenza virus in 100 μl of incomplete medium (without serum) for 20 min at 37°C. The cells were washed to remove unbound virus and were cultured for 2 h in 500 μl of complete medium containing 100 μCi of 51Cr per 106 cells. Prior to assessing cytotoxic activity, 51Cr-labeled cells were washed three times. The cells were counted and then used as target cells in the cytotoxic assay, as described below.
Cytotoxicity assay.
The 51Cr-labeled P815 or EL-4 target cells were washed three times and resuspended in complete medium at 105 cells/ml; 100-μl aliquots of the cell suspension were added to 96-well, round-bottom microtiter plates containing triplicate 100-μl samples of serially diluted effector cells. The microtiter plates were centrifuged at 400 × g for 5 min and then incubated for 4 h at 37°C and 5% CO2. The level of released radioactivity in 100 μl of supernatant from each well was measured in a gamma counter (Cobra II Auto-Gamma; Packard Instrument Co., Downers Grove, Ill.). Specific lysis was calculated from the 51Cr released in counts per minute with the formula: percentage of specific lysis = [(experimental cpm − spontaneous cpm)/(maximal release cpm − spontaneous cpm)] × 100. The value of 51Cr, as counts per minute for spontaneous and maximal release, was determined by incubating target cells with either 100 μl of medium or 100 μl of 5% Triton X-100, respectively. Spontaneous release of 51Cr in the absence of effector cells was usually less than 15%; standard errors of the mean (SEM) were less than 5% of the mean value and are not included.
Statistics.
The data are expressed as the mean ± 1 SEM and compared using a two-tailed student's t test and one-way analysis of variance. The results were analyzed using the InStat 2.00 statistical program (GraphPad Software, San Diego, Calif.) for Macintosh computers and were considered to be statistically significant if P values were less than 0.05.
RESULTS
Protection of IFN-γ−/− mice against challenge with mouse-adapted heterosubtypic influenza virus.
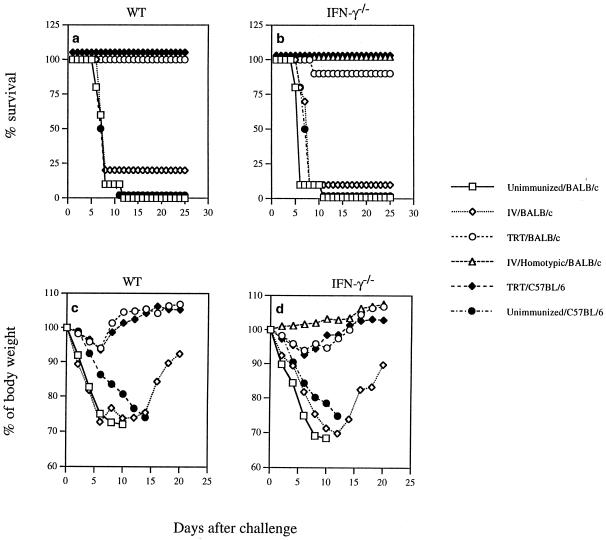
To determine the role of IFN-γ for heterosubtypic protection, IFN-γ−/− mice were immunized by TRT exposure to a sublethal dose of live A/Udorn (H3N2) virus. Four weeks later, the mice were challenged with a lethal dose of A/PR/8/34 (H1N1) virus. Ninety percent of IFN-γ−/− mice immunized previously via the TRT method with live A/Udorn virus survived the challenge (Fig. 1b). This survival rate is comparable to that seen in immunocompetent mice immunized previously via the TRT method (100%) (Fig. 1a). A much lower survival rate was observed in IFN-γ−/− and immunocompetent mice immunized i.v. (10 to 20%, respectively). As negative controls, unimmunized mice were challenged with virus and exhibited 100% mortality. IFN-γ−/− mice previously immunized i.v. with homologous but not heterosubtypic serotype virus were protected from TRT challenge with a lethal dose. No weight loss was seen in this group (Fig. 1d), and protection from secondary infection was presumably mediated by preexisting Ab specific for homologous outer membrane glycoproteins. A transient weight loss was observed in both immunocompetent and IFN-γ−/− mice immunized via the TRT method (Fig. 1c and d). During the first week after challenge, the i.v. immunized and unimmunized mice showed severe weight loss from day 2 to 6, when mortality was first observed. These results clearly demonstrate that IFN-γ−/− mice, like immunocompetent mice, developed complete HSI against lethal infection with influenza virus. A similar pattern of host recovery as a result of HSI was observed in both genetically distinct C57BL/6 and BALB/c strains of WT and IFN-γ−/− mice (Fig. 1). This indicates that both H-2b and H-2d MHC haplotypes are permissive with regard to infection, disease, and mortality.
FIG. 1.
HSI in IFN-γ−/− mice. IFN-γ−/− and WT mice were immunized with influenza virus A/Udorn (H3N2) by different routes, as indicated. Four weeks later, they were challenged with heterologous mouse-adapted influenza strain A/PR/8/34 (H1N1) via the TRT route. The mortality rate was monitored daily for at least 3 weeks (a and b), and body weight of surviving individual mice was measured every 2 days until all live animals regained their initial weight (c and d). Values are the mean of 10 mice per group.
Induction of subtype cross-reactive CTL responses in IFN-γ−/− mice.
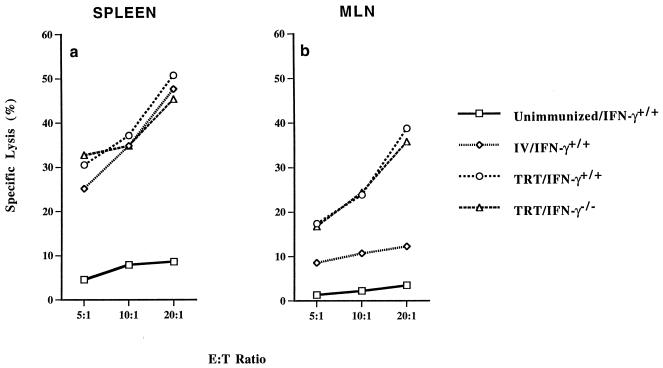
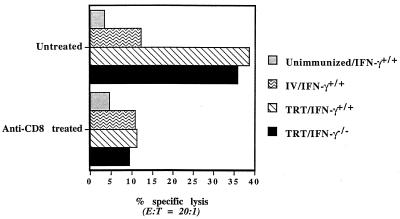
Since HSI is associated with virus-specific CD8+ CTL induced in MALT (25), the role of IFN-γ for induction of CTL responses was examined. Immunocompetent and IFN-γ−/− mice were immunized by TRT exposure to live A/Udorn virus. Four weeks later, the mice were challenged with a lethal dose of A/PR/8/34 (H1N1) virus. On day 3 after challenge, five mice from each subgroup were analyzed. Lymphocytes isolated from spleens and MLN were subjected to 6-day in vitro culture to generate antigen-specific CTL effector cells. All groups of immunized mice had strong heterosubtypic CTL activity against H1N1 (Fig. 2a) as well as H3N2 (data not shown) virus-infected target cells in the spleens. In contrast, when lymphocytes isolated from MLN, the draining lymph nodes of the lungs, were stimulated in vitro and assayed for CTL activity, positive heterosubtypic CTL activity was detected only in MLN of mice immunized via the TRT method. The CTL activities are similar in both immunocompetent and IFN-γ−/− mice (Fig. 2b). When the effector cells were treated with monoclonal Ab specific for the CD8 molecule before performing the cytotoxic assay, no antigen-specific lysis was detected (Fig. 3). These results indicated that heterosubtypic virus-specific CTL activity induced in MLN is mediated predominantly by CD8+ T cells. The data demonstrate that lack of IFN-γ does not impair induction and differentiation of virus-specific CD8+ CTL responses, observed in different lymphoid organs following nasal or parental routes of immunization. Furthermore, virus-specific CD8+ CTL responses induced in MLN of both WT and IFN-γ−/− mice were associated with HSI.
FIG. 2.
CTL responses induced in different lymphoid organs of BALB/c IFN-γ−/− and WT mice following immunization by different routes. Mice were infected with a sublethal dose of strain A/Udorn (H3N2). Four weeks later, the mice were challenged with a lethal dose of A/PR/8/34 (H1N1) virus. On day 3 after the challenge, five mice from each group were sacrificed. Lymphocytes were isolated from different lymphoid organs and stimulated in vitro. After 6-day in vitro stimulation, the cells were assayed for CTL activity against H1N1 virus-infected P815 target cells. Specific CTL activities were determined by subtracting the nonspecific cytotoxic activity against mock-infected P815 target cells from cytotoxic activity against virus-infected P815 target cells. The data represent results from two independent experiments of five mice per group.
FIG. 3.
Heterosubtypic CTL responses in MLN are mediated by CD8+ antigen-specific CTL effector cells. Pretreatment of CTL effector cells with monoclonal Ab specific for CD8 inhibits antigen-specific CTL activity (see the legend to Fig. 2 for details).
Cytokine responses in the absence of IFN-γ.
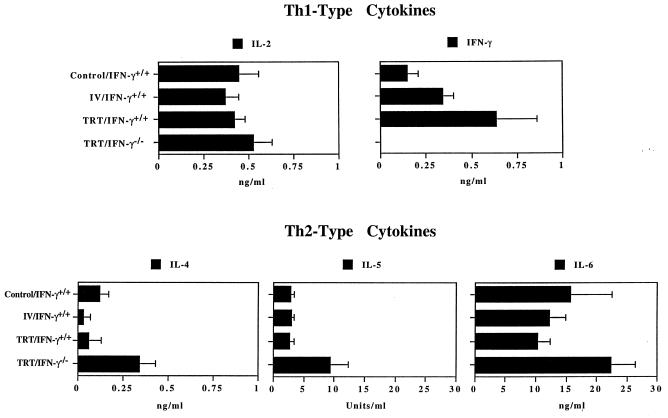
Since pneumonia is the cause of death after influenza virus infection, the regulation of immune responses at the site of infection may be essential for recovery from viral infection and the disease. Lung extracts from IFN-γ−/− and immunocompetent mice were analyzed after the challenge for Th1- and Th2-type cytokine production. To ensure that IFN-γ−/− did not produce IFN-γ, lung homogenates were analyzed for expression of mRNA specific for IFN-γ, and no IFN-γ-mRNA was detected in the lung homogenates of IFN-γ−/− mice (data not shown). The amount of IFN-γ varied among different groups of immunocompetent mice, but significantly elevated production of IFN-γ was observed in lung homogenates of heterosubtypically immune mice previously immunized by the TRT route (Fig. 4). In the absence of IFN-γ, the production of Th2-type cytokines (IL-4, IL-5, and IL-6) was significantly increased. However, there was no statistical difference in the amount of IL-2 produced in the lung extracts of IFN-γ−/− mice versus those of immunocompetent mice. The data indicate that the lack of IFN-γ switches cytokine responses to a Th2 cytokine profile at the site of infection. Furthermore, either Th1- or Th2-type cytokine responses in the lungs can provide heterosubtypic protection, since immunocompetent mice with a dominant Th1-type cytokine response and IFN-γ−/− mice with a dominant Th2-type cytokine response developed complete recovery from challenge with a lethal dose of influenza virus.
FIG. 4.
Th1- and Th2-type cytokine production in lung extracts. BALB/c IFN-γ−/− and WT mice were immunized with influenza virus A/Udorn (H3N2) by different routes, as indicated. Four weeks later, the mice were challenged with the heterologous mouse-adapted A/PR/8/34 (H1N1) influenza virus via the TRT route. On day 3 after challenge, mice were sacrificed, and lung extracts were collected and assayed for the level of cytokines as determined by ELISA. The values represent the mean and SEM of the amount of cytokines for five mice in each group.
Induction of humoral immune responses to influenza virus in IFN-γ−/− mice.
VN activity is required for Ab-mediated protection, while nonneutralizing Abs, which bind to virus-infected cells, can reduce the production of progeny virus (24). Thus, Abs could have a potential role as mediators of HSI. IFN-γ has been reported to be crucial in Ab class switching during maturation of humoral immune responses (5, 35). The impact of IFN-γ on induction of VN and serotype cross-reactive Abs was studied in our system. Immunocompetent and IFN-γ−/− mice were immunized by the i.v. or TRT routes with live A/Udorn virus. One month later, sera from immunized mice were assayed for the presence of VN and anti-NP Abs. Both immunocompetent and IFN-γ−/− mice displayed significant and comparable titers of VN Abs specific for the immunizing but not the challenge virus (Table 1) (one-way analysis of variance with a P value of <0.0001). This was true for sera collected 3 days after the challenge with heterologous influenza virus (data not shown). This result supports the observations described above, where IFN-γ−/− mice immunized with a homologous virus serotype were protected from challenge with a lethal dose of influenza virus. The protection of these mice from secondary infection is presumably mediated by VN Abs induced by primary infection. The results clearly demonstrate that lack of IFN-γ does not impair the induction of neutralizing Abs. However, the titers of VN Abs specific for immunizing virus did not correlate with protection against challenge with a lethal dose of heterologous influenza virus.
TABLE 1.
Induction of serum Absa
| Mice | Immunization route | Mean anti-NP Ab titerb (log2) | Mean titersb (log2) of neutralizing Ab to influenza virus strain
|
|
|---|---|---|---|---|
| A/Udorn (H3N2) | A/PR/8/34 (H1N1) | |||
| IFN-γ+/+ | No virus | 10.96 | <3 | <3 |
| IFN-γ+/+ | i.v. | 18.52 ± 0.72 | 9.35 ± 0.22 | <5 |
| IFN-γ+/+ | TRT | 19.56 ± 0.54 | 9.30 ± 0.59c | <5 |
| IFN-γ−/− | TRT | 18.16 ± 0.83 | 8.65 ± 0.45d | <5 |
IFN-γ−/− and immunocompetent (IFN-γ+/+) BALB/c mice were immunized by i.v. or TRT exposure with 5 × 105 PFU of influenza virus A/Udorn (H3N2) per mouse. Four weeks later, sera were collected and assayed for levels of Abs.
The values represent the means of Ab titers for five mice in each group, and (where shown) SEM.
c,d The two-tailed P value is 0.0943, considered not significant.
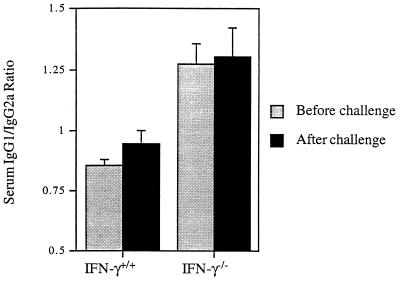
Lack of IFN-γ did not impair induction of anti-NP Abs. No statistically significant difference in serum anti-NP Ab titers was found between IFN-γ−/− and immunocompetent mice (Table 1). The level of Abs measured before the challenge did not differ from that detected 3 days after the challenge (data not shown). No statistically significant difference in serum anti-NP Ab titers was found between groups of immunized mice. Also, no statistically significant difference in titers of subtype cross-reactive serum Ab, as determined by ELISA with whole virus subtypes as antigens, was found between groups of immunized mice (data not shown). The results confirm previous results reported by our group (25). Thus, no correlation between anti-NP- or subtype-specific serum Ab titers and HSI was observed. In IFN-γ−/− mice, an Ab class switch to IgG1 was observed. Anti-NP IgG1/IgG2a Ab ratios in IFN-γ−/− mice were significantly higher than the ratios determined in WT mice (Fig. 5), although the levels of anti-NP IgG2a were not significantly lower than those of normal mice (data not shown). No significant differences were found between ratios determined before and after challenge of each group of mice (Fig. 5). Thus, our results most likely reflect a dominant Th2-type cytokine response in IFN-γ−/− mice.
FIG. 5.
Ratios of serum anti-NP IgG1 and IgG2a Abs in BALB/c IFN-γ−/− and WT mice. Mice were immunized with influenza virus A/Udorn (H3N2) by TRT. Four weeks later, the mice were challenged with the heterologous mouse-adapted A/PR/8/34 (H1N1) influenza virus. Sera were collected before and 3 days after challenge and assayed for levels of anti-NP IgG1 and IgG2a Abs as determined by ELISA. The values represent the ratios of the mean and SEM of antibody titers for five mice in each group.
DISCUSSION
In this study, the importance of IFN-γ in HSI to lethal influenza infection was explored using a model of IFN-γ−/− mice. Several important conclusions can be drawn from this study: (i) IFN-γ is not required for HSI to lethal influenza virus infection, since HSI was observed in both immunocompetent and IFN-γ−/− mice; (ii) IFN-γ-independent induction of virus-specific CTL responses induced in MALT was associated with HSI; (iii) Ab responses measured by titers of VN Ab and cross-reactive Abs to influenza A NP were not impaired in IFN-γ−/− mice; and (iv) in the absence of IFN-γ, Th2-type cytokines were upregulated in the lungs upon challenge. However, either Th1- or Th2-biased responses can be associated with heterosubtypic protection.
Recovery from primary virus infection involves both innate and acquired immunity. The latter is the major component of HSI, since it requires previous immunization. Memory T cells are a major source of IFN-γ in heterosubtypically immune mice. HSI to lethal influenza virus infection in the absence of IFN-γ indicates that T cells function in HSI by direct lysis of virus-infected cells or by secretion of alternative compensatory antiviral cytokines other than IFN-γ. Although IFN-γ plays a protective role against infections for a number of intracellular bacteria (2, 12) and viruses (11, 12, 15, 16, 19, 32, 43), it is not necessary for recovery from primary infection with influenza virus (7). Therefore, it was suggested that the importance of IFN-γ in clearance of a viral pathogen and recovery from infection depends upon the particular microorganisms. This notion is supported by our findings. On the other hand, the correlation between the level of IFN-γ and HSI observed in immunocompetent mice suggests a contribution of IFN-γ to a complex process involving possibly redundant host immune mechanisms. The contribution of IFN-γ may be substituted by other cytokines in the absence of IFN-γ. For example, TNF-α is known to participate in recovery from hepatitis B virus infection (10). The role of TNF-α for HSI in influenza virus infection is currently under investigation by our group.
Differentiation of precursor CTL to activated CTL effectors is dependent on IFN-γ (21, 31); however, our results show that IFN-γ is not required for activation of memory CD8+ CTL. The differentiation of memory precursors to activated CTL effectors occurs in the absence of IFN-γ, since subtype-specific CD8+ CTL responses were induced in immunocompetent as well as in IFN-γ−/− mice. In addition, when CTL precursors were isolated from these mice, they differentiated into CTL effector cells during in vitro stimulation in the absence of IFN-γ. Previous studies have shown that IFN-γ is not required for induction of effective CTL responses after primary virus infection (7). Thus, IFN-γ does not appear to be necessary for either the in vivo or in vitro differentiation and effector function of CD8+ CTL. Several host cytokines, including IL-2, IL-12, IFN-γ, and TNF-α, contribute to antiviral activity (for a review, see reference 27). While IFN-γ and TNF-α contribute to direct antiviral effects, IL-2 and IL-12 activate the host defense through induction of CTL and NK cells. Although the level of IL-12 was not measured in our study, unimpaired production of IL-2 in IFN-γ−/− mice indicates that IL-2 may be an important factor for induction of cellular immune responses in these mice. In addition, increased levels of IL-4 observed in IFN-γ−/− mice suggests that IL-4 should be taken in account for the IFN-γ-independent pathway of induction and differentiation of subtype-specific CD8+ CTL, since IL-4 is able to prime CTL (37, 40).
The association between antigen-specific CD8+ CTL responses in MLN and HSI in IFN-γ−/− mice suggests that these CTL may play an important role in HSI to infection with influenza virus. Since CTL have been shown to secrete IFN-γ after antigenic stimulation (22) and IFN-γ is not involved in the host recovery from lethal infection with a heterosubtypic strain of cytopathic influenza virus, as shown in this study, it is likely that direct lysis of infected cells by CD8+ T cells is the dominant effector mechanism for virus clearance in HSI to influenza virus infection. This is supported by previous findings in which adoptive transfer of CTL clones generated from immunized IFN-γ−/− mice to naive immunocompetent mice can promote recovery from lethal viral challenge (7). Although the systemic spread of the influenza virus strains was not analyzed in detail, it is likely that the virus strains used would spread systemically, since influenza-virus-specific Abs were detected in serum after systemic or mucosal immunization. However, immunization by TRT exposure was much more effective than i.v. immunization for induction of antigen-specific CD8+ CTL responses in MLN and HSI in immunocompetent as well as IFN-γ−/− mice. These findings suggest that mucosal immunization preferentially induces immune responses in MALT that are important for host defense against mucosal pathogens, including influenza virus. Using C57BL/6 IFN-γ−/− mice, a recent study has shown that IFN-γ played a protective role in HSI (1). In that system, the immunization and challenge strategies were distinct from our protocol. Immunocompetent as well as IFN-γ−/− mice were immunized intraperitoneally and challenged via the aerosol route. Intraperitoneal immunization is less effective in induction of HSI in immunocompetent mice (25). TRT exposure to live virus has been the route of choice for induction of HSI to influenza virus since the earliest studies (3, 17, 25, 30, 46). Although immune responses were in the BALB/c strain predominantly analyzed, HSI was already observed in C57BL/6 IFN-γ−/− mice with a genetically distinct background. These results indicate that the genetic background does not affect IFN-γ-independent induction of HSI.
Although IFN-γ has potent effects on B-cell stimulation and antibody secretion (5), there were no differences noted in the induction of serum Ab responses to influenza virus between IFN-γ−/− and WT mice after infection, as measured by serum VN as well as anti-NP Ab titers. Our results are in accord with those obtained from the study of primary influenza virus infection (7). In addition, the lack of IFN-γ resulted in a switch of cytokine production from Th1 to Th2 type, with significantly enhanced IL-4 and IL-5 levels. This Th2-type cytokine switch is normally associated with humoral immune responses (23). Indeed, we observed an increased level of anti-NP IgG1 in IFN-γ−/− mice (Fig. 5). This response was accompanied by increased production of IL-4. Enhancement of Th2-type cytokine production in IFN-γ−/− mice was also observed in one study (7) but not in another (1). This inconsistency may be the result of different protocols used for bulk cultures on which cytokine analysis was performed. In our study, the levels of cytokines in lung extracts were determined. This allows better understanding of the regulation of immune responses at the site of infection itself. Since serum titers of cross-reactive and anti-NP Abs were not impaired in IFN-γ−/− mice and did not correlate with host recovery from challenge, the role of nonneutralizing Abs in the mucosal compartment (the site of influenza virus infection) for HSI may be important and needs to be further analyzed. In addition, although IFN-γ−/− mice are able to develop functional HSI comparable with immunocompetent mice and it is anticipated that long-term HSI may not be affected in IFN-γ−/− mice, the long-term memory CD8+ CTL responses in MLN and HSI in IFN-γ−/− mice need to be further explored. These points are currently under investigation in our laboratory.
In summary, this study demonstrated that IFN-γ is not required for HSI to lethal influenza virus infection. Along with other available knowledge, our findings suggest that HSI is a complex process which may involve multiple biological factors. It is noteworthy that reduction of virus replication in the lung varies from one study (17) to another (3), and HSI mice exhibit only modest reductions in lung virus titer (3). However, significant differences in host morbidity and survival among these groups of mice suggest that other factors controlling host resistance against virus infection, rather than virus clearance, may play an important role in HSI. In addition, the question of how effective virus-specific CD8+ CTL responses are for protection needs to be further explored, since adoptive transfer requires enormous numbers of in vitro-cloned virus-specific CD8+ CTL. On the other hand, it has been reported recently that Abs specific for internal viral proteins expressed on infected cells reduce the production of progeny virus and inhibit the spread of infection in infected SCID mice (24). The role of nonneutralizing Abs in MALT should be considered for HSI.
ACKNOWLEDGMENTS
We thank Michael W. Russell for critical reading and evaluation of the manuscript.
This work was supported in part by U.S. PHS grants AI 28147, AI 43197, T32 AI 07150, and T32 HL 07553 and contract AI 65298.
REFERENCES
- 1.Bot A, Bot S, Bona C A. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalton D, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 3.Epstein S L, Lo C Y, Misplon J A, Lawson C M, Hendrickson B A, Max E E, Subbarao K. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. [PubMed] [Google Scholar]
- 4.Fellous M, Nir U, Wallach D, Merlin G, Rubinstein M, Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci USA. 1982;79:3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelman F D, Katona I M, Mosmann T R, Coffman R L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 6.Fu T M, Guan L, Friedman A, Schofield T L, Ulmer J B, Liu M A, Donnelly J J. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999;162:4163–4170. [PubMed] [Google Scholar]
- 7.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray P W, Goeddel D V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci USA. 1983;80:5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Goodman R E, Standiford T J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 10.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 11.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 14.Klein J R, Raulet D H, Pasternack M S, Bevan M J. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982;155:1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohonen-Corish M R, King N J, Woodhams C E, Ramshaw I A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990;20:157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- 16.Leist T P, Eppler M, Zinkernagel R M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 18.Lin Y L, Askonas B A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucchiari M A, Modolell M, Eichmann K, Pereira C A. In vivo depletion of interferon-gamma leads to susceptibility of A/J mice to mouse hepatitis virus 3 infection. Immunobiology. 1992;185:475–482. doi: 10.1016/S0171-2985(11)80089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maraskovsky E, Chen W F, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989;143:1210–1214. [PubMed] [Google Scholar]
- 22.Morris A G, Lin Y L, Askonas B A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999;254:138–146. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen H H, Moldoveanu Z, Novak M J, van Ginkel F W, Ban E, Kiyono H, McGhee J R, Mestecky J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999;254:50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- 26.Pestka S, Langer J A, Zoon K C, Samuel C E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 27.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 28.Restifo N P, Esquivel F, Kawakami Y, Yewdell J W, Mule J J, Rosenberg S A, Bennink J R. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarmiento M, Dialynas D P, Lancki D W, Wall K A, Lorber M I, Loken M R, Fitch F W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Schulman J L, Kilbourne E D. Induction of partial specific heterotypic immunity in mice by a single infection with influenza virus. J Bacteriol. 1965;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon M M, Hochgeschwender U, Brugger U, Landolfo S. Monoclonal antibodies to interferon-gamma inhibit interleukin 2-dependent induction of growth and maturation in lectin/antigen-reactive cytolytic T lymphocyte precursors. J Immunol. 1986;136:2755–2762. [PubMed] [Google Scholar]
- 32.Stanton G J, Jordan C, Hart A, Heard H, Langford M P, Baron S. Nondetectable levels of interferon gamma is a critical host defense during the first day of herpes simplex virus infection. Microb Pathog. 1987;3:179–183. doi: 10.1016/0882-4010(87)90094-5. [DOI] [PubMed] [Google Scholar]
- 33.Taylor P M, Askonas B A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor P M, Davey J, Howland K, Rothbard J B, Askonas B A. Class I MHC molecules rather than other mouse genes dictate influenza epitope recognition by cytotoxic T cells. Immunogenetics. 1987;26:267–272. doi: 10.1007/BF00346521. [DOI] [PubMed] [Google Scholar]
- 35.Teale J M, Estes D M. Immunoglobulin isotype regulation. In: Callard R E, editor. Cytokines and B lymphocytes. San Diego, Calif: Academic Press, Inc.; 1990. p. 173. [Google Scholar]
- 36.Townsend A R M, McMichael A J, Carter N P, Huddleston J A, Brownlee G G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;9:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 37.Trenn G, Takayama H, Hu-Li J, Paul W E, Sitkovsky M V. B cell stimulatory factor 1 (IL-4) enhances the development of cytotoxic T cells from Lyt-2+ resting murine T lymphocytes. J Immunol. 1988;140:1101–1106. [PubMed] [Google Scholar]
- 38.van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 39.Wells M A, Daniel S, Djeu J Y, Kiley S C, Ennis F A. Recovery from a viral respiratory tract infection. IV. Specificity of protection by cytotoxic T lymphocytes. J Immunol. 1983;130:2908–2914. [PubMed] [Google Scholar]
- 40.Widmer M B, Grabstein K H. Regulation of cytolytic T-lymphocyte generation by B-cell stimulatory factor. Nature. 1987;326:795–798. doi: 10.1038/326795a0. [DOI] [PubMed] [Google Scholar]
- 41.Wraith D C, Vessey A E, Askonas B A. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol. 1987;68:433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Waters J B, Fruh K, Peterson P A. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci USA. 1992;89:4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yap K L, Ada G L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immunol. 1978;7:389–397. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 45.Yap K L, Ada G L, McKenzie I F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 46.Yetter R A, Lehrer S, Ramphal R, Small P A J. Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980;29:654–662. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yewdell J W, Bennink J R, Smith G L, Moss B. Influenza virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]