Abstract
Productive poliovirus infection of HeLa cells leads to the canonical cytopathic effect (CPE), whereas certain types of abortive infection result in apoptosis. To define the time course of commitment to the different types of poliovirus-induced death, inhibitors of viral replication (guanidine HCl) or translation (cycloheximide) were added at different times postinfection (p.i.). Early in the infection (during the first ∼2 h p.i.), predominantly proapoptotic viral function was expressed, rendering the cells committed to apoptosis, which developed several hours after viral expression was arrested. In the middle of infection, concomitantly with the onset of fast generation of viral progeny, the implementation of the viral apoptotic program was abruptly interrupted. In particular, activation of an Asp-Glu-Val-Asp (DEVD)-specific caspase(s) occurring in the apoptosis-committed cells was prevented by the ongoing productive infection. Simultaneously, the cells retaining normal or nearly normal morphology became committed to CPE, which eventually developed regardless of whether or not further viral expression was allowed to proceed. The implementation of the poliovirus-induced apoptotic program was suppressed in HeLa cells overexpressing the Bcl-2 protein, indicating that the fate of poliovirus-infected cells depends on the balance of host and viral pro- and antiapoptotic factors.
Depending on the conditions, poliovirus infection may trigger two different host cell responses, either the canonical cytopathic effect (CPE) or apoptosis (1, 34). The typical features of poliovirus CPE resulting from productive infection (22) include accumulation of membranous vesicles (3, 11), alterations in the plasma membrane permeability (8), rounding up of the infected cells, distortion and displacement of the nuclei, and condensation of chromatin (“pyknosis”). Some of these alterations appear to be manifestations of “true” cell-damaging effects, whereas others reflect at least in part changes in the cellular infrastructure induced by the virus for its own benefit. Thus, cytoplasmic vesicles contain all the components necessary for replication of the viral genome and in fact are the site of this replication (2, 5, 13). Little is known about the mechanisms underlying development of the CPE, although the involvement of nonstructural proteins encoded in the central region of the viral genome in the cytoplasmic vesiculation is well documented (4, 10, 31).
On the other hand, poliovirus infection of HeLa cells under restrictive conditions results in a typical apoptotic response: cell shrinkage, plasma membrane blebbing, a high level of chromatin condensation, degradation of the nuclear DNA into high-molecular-mass and oligonucleosome-sized species followed by fragmentation of the cells into membrane-surrounded “apoptotic bodies” (34). Apoptotic reaction, but not CPE, could be prevented by a specific caspase inhibitor, indicating that the two cellular responses to poliovirus infection are controlled separately (1). Apart from the involvement of activation of caspases, the mechanism of apoptosis triggered by poliovirus infection remains unknown. However, regardless of the mechanism, the fact that productive infection of the same cell leads to CPE rather than to apoptosis, along with other observations, allowed us to suggest that poliovirus encodes a distinct apoptosis-preventing function(s) in addition to the apoptosis-inducing function(s) (34). It is reasonable to assume that CPE can develop in the poliovirus-infected cells due to expression of the putative apoptosis-preventing function. Thus, it seems that proapoptotic and antiapoptotic functions may act together and compete with one another and with the CPE program during the viral reproduction cycle.
The main goal of this study was to establish the time course of development of these competing programs and to gain insight into their relationships. Accumulation of proapoptotic factors rendering cells committed to apoptosis was revealed early postinfection (p.i.). In the middle of the infectious cycle, however, the fate of the cell was shown to abruptly change due to the switching on the antiapoptotic and CPE programs.
MATERIALS AND METHODS
Reagents.
Caspase 3 was kindly donated by Y. A. Lazebnik (Cold Spring Harbor Laboratory), and caspases 7 and 8 were gifts from G. S. Salvesen (Burnham Institute, La Jolla, Calif.). Benzyloxycarbonyl-Val-Ala-Asp(OMe)fluoromethyl ketone (zVAD.fmk) was from Enzyme Systems Products (Dublin, Calif.), and other caspase inhibitors and chromogenic caspase substrates were from Calbiochem (La Jolla, Calif.).
Cells and virus.
A subline of HeLa-S3 cells designated HeLa-B (34) was used. Aliquots of cells treated with EDTA were plated onto 35- or 60-mm-diameter petri dishes (Corning-Costar) at a density of approximately 8.5 × 104 cells/cm2 and cultivated overnight under 5% CO2 at 37°C in basal Eagle's medium supplemented with 10% bovine serum. The growth medium was discarded, and a derivative of poliovirus type 1 Mahoney strain, Mgs (33), was added in a volume of 2 ml containing 1.5 × 109 PFU/ml. After a 30-min incubation at 18°C, the cells were washed with Earle's solution and 1 or 2 ml (for 35- or 60-mm-diameter dishes, respectively) of serum-free Eagle's medium was added. After incubation at 37°C for the indicated times, the viral harvest was determined by counting the plaques on RD cells.
Derivation of Bcl-2-expressing HeLa cells.
The packaging cell line Phoenix ampho and retroviral vector pLC-bcl-2 (containing the puromycin resistance gene under the control of the Moloney murine leukemia virus long terminal repeat and the human bcl-2 gene under the control of a cytomegalovirus promoter) were kindly donated by P. M. Chumakov (Institute of Molecular Biology, Moscow, Russia). To construct the control vector, the bcl-2 gene was excised from pLC-bcl-2 by treatment with BamHI and XhoI, and the resulting 5′ protruding ends were filled in with Klenow enzyme and blunt end ligated. Both vectors were used to produce stable transformed HeLa-bcl2 and HeLa-puro, respectively, as described elsewhere (http://www.sciencexchange.com/sxprotocols/molbiol/rapid.htm). Briefly, the Phoenix ampho cells were transfected with the plasmids by using the calcium phosphate technique, and the retrovirus-containing supernatant gathered 48 h later was used immediately for HeLa cell infection. Two days later, the cells were seeded onto the selective medium, Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1.5 μg of puromycin per ml. The resistant clones were combined and maintained in the same medium with 1 μg of puromycin per ml. The enhanced expression of Bcl-2 in these cells was confirmed by Western blotting.
Determination of caspase activities.
Following the appropriate treatment, the cells were harvested, washed with phosphate-buffered saline, and lysed with the cell lysis buffer from the Clontech CPP-32 colorimetric detection kit according to the manufacturer's protocol. The resulting lysates were stored in aliquots at −80°C. Caspase activities were determined in 100-μl reaction mixtures containing 45 μl of cell lysate, the appropriate caspase substrate (100 μM), 50 μl of 2× reaction buffer [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 7.0), 0.2 mM EDTA, 20% glycerol, 2 mM dithiothreitol] and where indicated, a 10 μM concentration of the broad-range caspase inhibitor, zVAD.fmk. The optical density at 405 nm was determined.
Search for potential caspase inhibitor(s).
The search for potential caspase inhibitor(s) was essentially as described above but exogenous caspases were added. In one set of experiments, recombinant caspases 3, 7, and 8 were added at concentrations of 150, 100, and 0.75 ng/ml, respectively, and 50-μl portions of lysates prepared at different times from the productively infected cells were used. In another set of experiments, caspases were represented by 22.5 μl of a lysate from apoptosis-committed cells (extracts prepared at 6 h p.i. from cells to which cycloheximide (CHI) had been added 2 h p.i.). Putative inhibitors were represented by 22.5 μl of lysates prepared from the productively infected cells at different times p.i. The amounts of individual caspases and caspase-containing lysates were checked to correspond to the linear region of the activity. In all these assays, Ac-Asp-Glu-Val-Asp-p-nitroaniline (Ac-DEVD-pNA) was used as a substrate.
DNA analysis.
DNA fragmentation was assayed as described previously (34). Cells treated with EDTA were suspended in a buffer containing 20 mM EDTA and 10 mM Tris-HCl (pH 7.4) were lysed with 0.5% Triton X-100 for 20 min in an ice bath. Nuclei were pelleted at 12,000 rpm for 15 min at 4°C in a 5415 C centrifuge (Eppendorf, Hamburg, Germany), and the resulting supernatants were treated with phenol or sodium dodecyl sulfate. After ethanol precipitation, nucleic acids were dissolved in 10 μl of H2O and treated with RNase A (10 μg/ml, 37°C, 30 min). Samples were subjected to electrophoresis on 1.5% agarose gels.
Light, fluorescence, and electron microscopy.
The proportions of dead cells and cells exhibiting cytoplasmic blebbing were determined by light microscopy using EDTA-treated cell preparations stained with 0.01% methylene blue. For fluorescence microscopy, the permeable fluorescent dye Hoechst-33342 (Sigma) was added at a final concentration of 5 μg/ml to experimental cultures 30 min before cell harvesting. The cells were fixed with Safe Fix (Curtin Matheson Scientific, Houston, Tex.) for 30 min and washed twice with H2O. A drop of a glycerol–1 M Tris-HCl (pH 7.5) mixture (9:1) was added to the fixed monolayer, and cells were covered with a coverslip and observed with an epifluorescence microscope (Leica DMLS equipped with filter cube A, or Nikon E-800 equipped with a DAPI [4′,6′-diamidino-2-phenylindole] filter). For the quantification of nuclear alterations, two fields of each sample were photographed at a magnification of ×200, and nuclei, at least 800 per each sample, were counted on slide projections. For electron microscopy, the EDTA-treated cells were fixed first with 2.5% glutaraldehyde and subsequently with 1% OsO4 and embedded into Epon-812. Ultrathin sections were examined with a Philips CM 100 or JEM-1200-II electron microscope.
RESULTS
Development of commitment to apoptosis.
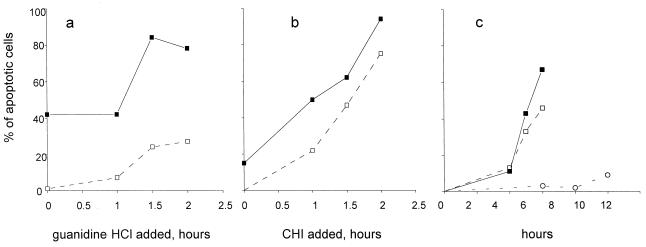
It has been shown previously that infection of HeLa cells under certain nonpermissive conditions triggers an apoptotic response (34). The development of commitment to apoptosis was studied by determining the proportion of apoptotic cells some time after the addition of inhibitors of viral reproduction. HeLa cells were infected with poliovirus at a high multiplicity, ensuring simultaneous infection of all the cells. The inhibitors were added at different times p.i. At 5.30 and 11.5 h p.i., the permeable nuclear fluorescent dye Hoechst-33342 was added, and the cells were fixed 30 min thereafter. The proportion of cells with nuclear manifestations of apoptosis was determined by fluorescence microscopy. The following observations were made upon adding a potent inhibitor of viral RNA replication, guanidine HCl, to the infected cells (final concentration of 100 μg/ml) (Fig. 1a). (i) In accordance with the previous results (34, 35), a significant proportion of the infected cells became apoptotic when the inhibitor was present from the onset of the infection, thereby preventing replication of the viral RNA. This result suggested that translation of the parental viral templates could generate quantities of the putative proapoptotic protein(s) sufficient to trigger the apoptotic response in some cells. (ii) A significantly greater proportion of apoptotic cells was observed when guanidine was added 1.5 to 2 h p.i. Thus, when early replication of the viral RNA was permitted, accumulation of active proapoptotic factor(s) took place. (iii) The proportion of apoptotic cells at 12 h p.i. was markedly higher than at 6 h p.i., regardless of when guanidine was added. Hence, the execution of the apoptotic program required many hours of metabolic activity, even though the commitment to apoptosis occurred very early p.i.
FIG. 1.
Development of apoptosis after interruption of productive poliovirus infection. Guanidine HCl (100 μg/ml) (a) or CHI (10 μg/ml) (b) was added to the infected HeLa cells at the indicated times, and the proportion of apoptotic cells was determined at either 12 h p.i. (solid squares) or 6 h p.i. (empty squares). In panel c, CHI (10 μg/ml) was added to the poliovirus-infected cells at 0 (circles), 1.5 (empty squares), and 2 (solid squares) h p.i., and the proportion of apoptotic cells was determined at the indicated times. Thirty minutes before fixation, Hoechst-33342 was added to the culture medium (to a final concentration of 5 μg/ml). Several low-magnification fields were photographed with a fluorescence microscope, and the proportion of the apoptotic cells was determined after counting at least 800 nuclei.
Guanidine HCl, at the concentration used, did not appear to affect translation of the viral RNA. Since the functional longevity of the input viral mRNA in the presence of guanidine was unknown, it was decided to determine the time course of the accumulation of proapoptotic factors by using a protein synthesis inhibitor rather than an inhibitor of viral RNA replication. This approach was hampered by the fact that translational inhibitors (e.g., CHI at a concentration of 100 μg/ml) proved to be potent inducers of apoptosis in uninfected HeLa cells (34, 35). However, at 10 μg/ml, CHI failed to induce apoptosis in an appreciable proportion of uninfected cells (see Fig. 2a), even though it produced ∼90% suppression of protein synthesis (compared to ∼95% suppression by a 100-μg/ml concentration) and nearly completely (more than 103-fold) inhibited the yield of infectious virus progeny (not shown). If CHI at this low concentration was added at the onset of infection, only a very small proportion of cells exhibited signs of apoptosis 12 h (but not 6 h) thereafter (Fig. 1b). Addition of the inhibitor at different time intervals early in infection resulted in a significant increase in the number of cells committed to apoptosis, so that that the overwhelming majority of the cells eventually become apoptotic when CHI was added 2 h p.i. (Fig. 1b). The following conclusions emerged from inspection of Fig. 1b and comparing it with Fig. 1a. (i) The proportion of the cells committed to apoptosis was low when CHI (versus guanidine) was added at the beginning of viral reproduction, suggesting that translation of the viral genome was required for the accumulation of the putative viral proapoptotic factor(s). (ii) During the first 2 h p.i., the proportion of cells committed to apoptosis increased roughly linearly, suggesting accumulation of putative proapoptotic factor(s) early in poliovirus reproduction.
FIG. 2.
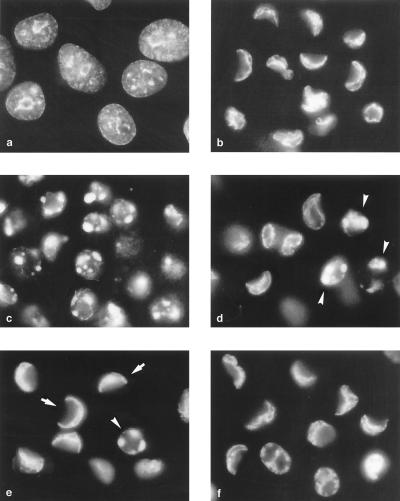
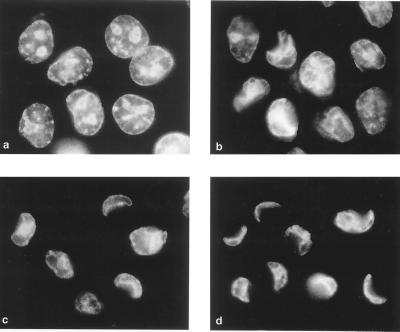
The switch in the cell commitment in the middle of poliovirus infection. Fluorescence microscopy of Hoechst-33342-stained HeLa cells was performed. (a) Uninfected cells treated with CHI (10 μg/ml) for 10 h; (b) infected cells at 8 h p.i. with no CHI added; and (c to f) cells to which CHI (10 μg/ml) was added 1.5 (c), 2 (d), 2.5 (e), and 3 (f) h p.i. Some of the apoptotic and CPE cells are indicated by arrowheads and arrows, respectively.
The experiments with CHI also confirmed that the apoptosis commitment and apoptosis execution steps might be separated by several hours (Fig. 1). In the experiments with both guanidine and CHI, not only nuclear morphology but also hallmarks of apoptosis such as plasma membrane blebbing (by phase-contrast microscopy) and degradation of nuclear DNA were studied. The results of these studies were in full accord with those observed with Hoechst-33342 staining (data not shown).
The apoptosis-to-CPE commitment switch.
Although, as shown in the preceding section, accumulation of proapoptotic factors predominated and occurred nearly linearly, early in the reproduction cycle, the permissive poliovirus infection of HeLa cells ends up in CPE rather than apoptosis, suggesting that at some point the overall balance is switched to the antiapoptotic state. In an attempt to determine this switching point, the effects of adding CHI (10 μg/ml) in the middle of the reproductive cycle was investigated. In the experiment shown in Fig. 2, CHI was added at 1.5, 2, 2.5, and 3 h p.i., and the Hoechst-33342-stained cells were investigated at 8 h p.i. A significant proportion of cells in which normal viral reproduction was permitted for 1.5 to 2 h before protein synthesis was stopped eventually developed a typical nuclear apoptotic response, i.e., chromatin was heavily condensed and nuclei were fragmented into apoptotic bodies (Fig. 2c and d); nearly no cells exhibiting CPE could be observed in the sample that received CHI 1.5 h p.i. (Fig. 2c), but some such cells could be observed in the sample that received the inhibitor half an hour later (Fig. 2d). The proportion of apoptotic cells markedly diminished, whereas the proportion of CPE cells increased in the sample that was treated with CHI at 2.5 h p.i. (Fig. 2e). In the sample treated with CHI at 3 h p.i., CPE cells nearly exclusively accumulated (Fig. 2f). Thus, the switch in the cell commitment from apoptosis to CPE appeared to occur around 2 h p.i. (or around 2.5 h in other experiments).
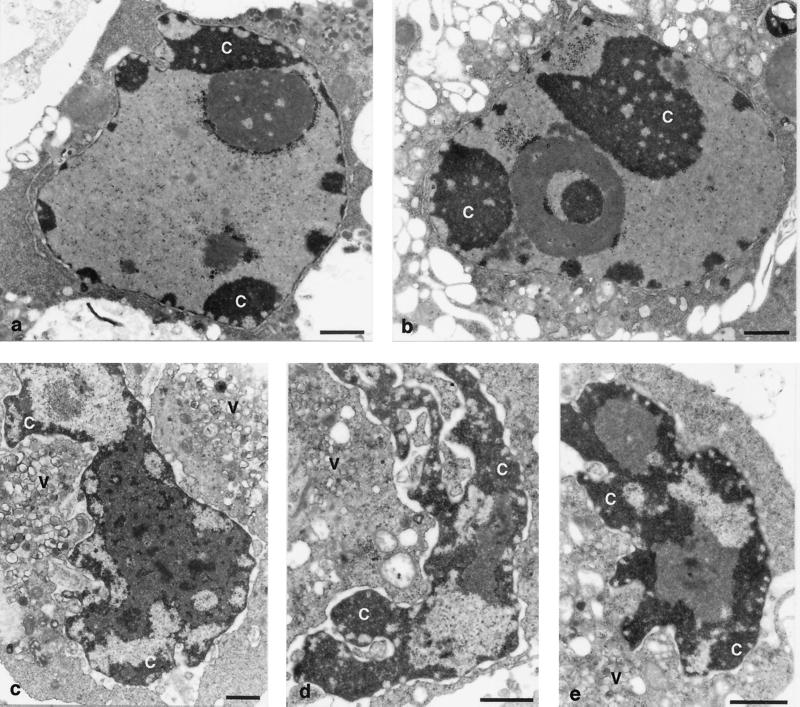
This switch could also be documented by electron microscopy. When CHI was added 1.5 h p.i. and the cells were investigated 8 h p.i., the chromatin in the majority of nuclei exhibited detachment from the nuclear membranes and condensation and fragmentation into round bodies, the features typical of apoptosis (Fig. 3a). If the productive infection was allowed to proceed a half an hour longer, both typical apoptotic cells (Fig. 3b) and cells exhibiting CPE (Fig. 3c) could be detected. Typical features of the latter included nuclear displacement to the cellular periphery, heavy deformation of the nuclei, partial chromatin condensation unaccompanied by its detachment from the nuclear membranes, and enlargement of the perinuclear space. The addition of CHI at 3 h p.i. resulted in the predominant accumulation of the cells exhibiting typical CPE (Fig. 3d) being indistinguishable from the cells productively infected for 6 h (Fig. 3e).
FIG. 3.
Electron microscopy of the cells in which poliovirus infection was interrupted at different times. Infected cells at 8 h p.i. to which CHI (10 μg/ml) was added 1.5 (a), 2 (b and c), and 3 (d) h p.i. An infected cell at 6 h p.i. of productive infection is shown in panel e. Some of the areas of condensed chromatin in both apoptotic cells (a and b) and cells exhibiting CPE (c and d) are labeled with c, and virus-induced vesicles are marked v. Bars, 1 μm.
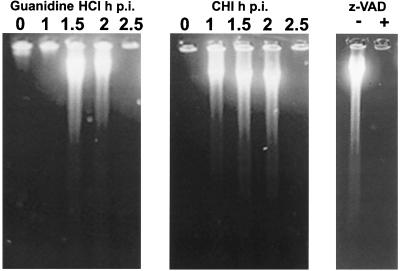
Degradation of DNA into the both (predominantly) high-molecular-mass and smaller fragments, typical of apoptosis, could be detected in the infected cells that received either guanidine or CHI 1.5 and 2 h p.i. and harvested 6 h p.i. but could no longer be detected (or was markedly suppressed) if the inhibitors were added at 2.5 h p.i. (Fig. 4). DNA degradation was prevented in the presence of zVAD.fmk (z-VAD) (Fig. 4). These observations lend additional support to the notion that a dramatic loss of commitment to apoptosis abruptly occurred in the middle of the infectious cycle (around 2 h p.i. or somewhat later).
FIG. 4.
DNA fragmentation in the cells with interrupted poliovirus infection. Guanidine HCl (100 μg/ml) or CHI (10 μg/ml) was added to the infected cells at the times (in hours) p.i. indicated over the lanes. CHI was added 2 h p.i. to the samples with (+) or without (−) zVAD.fmk (z-VAD) (100 μg/ml). DNA was extracted at 6 h p.i.
The time of commitment switch coincided with the onset of the fast phase of the infectious progeny accumulation (not shown).
Time course of the development of CPE.
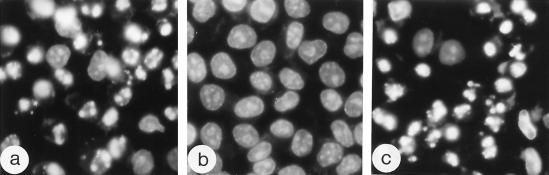
Interpretation of the data on the changing fate of the infected cells depended on the knowledge of the time course of development of CPE in our system under permissive conditions. Inspection of Hoechst-33342-stained productively infected HeLa cells revealed no appreciable nuclear morphological alterations before 3 h p.i. (not shown). At this time point, the shape of some nuclei began to change slightly, but no significant alterations in chromatin staining became apparent (Fig. 5a). By 4 h p.i., nuclear deformation affected a greater proportion of the cells (Fig. 5b), and typical crescent-like CPE nuclei could be seen in the 5-h p.i. sample (Fig. 5c). Even at this time point, a proportion of nuclei exhibited little, if any, signs of damage. The CPE was complete by 8 h p.i. (Fig. 5d). Phase-contrast microscopy showed that the cells had begun to lose their adherence to the substratum and to round up by 4 h p.i., and this process was close to completeness by 5 h p.i. (not shown).
FIG. 5.
Time course of development of CPE. Productively infected cells were stained with Hoechst-33342 and fixed at 3 (a), 4 (b), 5 (c), and 8 (d) h p.i.
Thus, the cells lost their commitment to apoptosis and became committed to CPE during the time interval (2 to 3 h p.i.) when their nuclei were still morphologically intact. Importantly, several hours were required after the commitment switch for the actual development of CPE.
Activation of caspases during apoptosis and CPE.
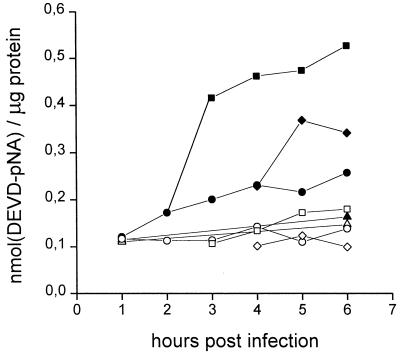
It was shown previously that a permeable irreversible inhibitor of caspases, zVAD.fmk, completely suppressed the development of poliovirus-triggered apoptosis (1). This fact suggested that one or more caspases should be involved in the realization of the viral proapoptotic function. The interruption of the implementation of the apoptotic program could be due to either prevention of caspase activation or inhibition of their activities. The activity of caspases during poliovirus-induced apoptosis was determined first. Extract from the infected cells destined for apoptosis (i.e., treated with CHI at 1.5, 2, and 3 h p.i.) were prepared at different times (i.e., 4 and 6 h) p.i. and were assayed for their abilities to cleave chromogenic peptide substrates of different caspases. The following compounds were investigated in this respect: Ac-DEVD-pNA (an optimal substrate of caspases 3 and 7), Ac-Tyr-Val-Ala-Asp-pNA (a substrate of caspases 1 and 4), Ac-Trp-Glu-His-Asp-pNA (a substrate of caspases 1, 4, and 5), Ac-Val-Glu-Ile-Asp-pNA (a substrate of caspase 6), and Ac-Ile-Glu-Thr-Asp-pNA (a substrate of caspase 8 and granzyme B) (32). Within the sensitivity of the assay, only Ac-DEVD-pNA produced a clear signal. A DEVD-specific caspase(s) activation could be seen as early as 3 h p.i. (Fig. 6), only one hour after the interruption of the productive infection. The appearance of the DEVD-dependent signal was completely prevented by the addition of zVAD.fmk (Fig. 6).
FIG. 6.
Activation of a DEVD-specific caspase(s) during apoptosis and CPE. The DEVD-specific caspase activity was determined as described in Materials and Methods in extracts from mock-infected cells (triangles), productively infected cells (circles), and infected cells to which CHI (10 μg/ml) was added 2 h (squares) and 3 h (diamonds) p.i. The enzyme activity was measured in samples without (solid symbols) and with (open symbols) zVAD.fmk (10 μg/ml) and is expressed as nanomoles of DEVD-pNA hydrolyzed per microgram of protein.
When extracts from the infected cells treated with CHI at 3 h p.i. (i.e., destined for CPE) were investigated similarly, a less prominent increase in the DEVD-specific caspase activity could be detected at 5 h p.i. (Fig. 6). The level of this activity varied somewhat between the experiments, but it was always lower than in the apoptosis-committed cells. An even lower level of DEVD-specific caspase activation was observed during the productive infection (Fig. 6). It was not clear whether this (low-level) activation was related to a small proportion of apoptotic cells present in the infected cultures or was an intrinsic characteristic of the infected cells.
No appreciable cleavage of the other investigated caspase substrates could be seen under any conditions investigated here (data not shown).
Search for a caspase inhibitor in the productively infected cells.
Lower levels of a DEVD-specific caspase activity in cells destined for CPE, compared those in cells committed to apoptosis (Fig. 6) could be due to either prevention of the enzyme activation or inhibition of the enzyme activity (or both). To determine whether a caspase inhibitor could be detected in the productively infected cells, extracts from the infected cells were added either to purified preparations of caspases 3, 7, and 8 incubated with their preferred substrates (Ac-DEVD-pNA for caspases 3 and 7 and Ac-IETD-pNA for caspase 8) or to extracts from the apoptosis-committed cells (infected cells to which CHI was added 2 h p.i. and incubated for an additional 4 h; in these samples, Ac-DEVD-pNA served as the substrate). Within the sensitivity of the assay, no evidence for the presence of a specific caspase inhibitor in the extracts of productively infected cells was obtained (not shown).
Effects of Bcl-2 expression on the development of apoptosis and CPE.
The enforced expression of the host antiapoptotic protein Bcl-2 has been demonstrated to suppress and delay apoptosis exerted by many RNA-containing viruses (16, 18, 20, 23, 25, 27, 29, 30). Similarly, development of the poliovirus-induced apoptosis in Bcl-2-expressing cells treated with CHI 1.5 h p.i. was severely inhibited, as judged by the essentially normal nuclear (Fig. 7b) or cytoplasmic (not shown) morphology at 6 h p.i. when a significant proportion of similarly treated control cells (not expressing Bcl-2) exhibited chromatin margination and fragmentation (Fig. 7a) as well as cytoplasmic blebbing (not shown). No accumulation of cells exhibiting CPE could be seen at this time. Bcl-2 expression did not prevent apoptotic death of the majority of the infected cells by 24 h p.i. (Fig. 7c). When the poliovirus-infected Bcl-2-expressing cells were treated with CHI at 2 h p.i. and observed at 6 h p.i., no appreciable changes in nuclear morphology could be seen (data not shown).
FIG. 7.
Suppressing effect of Bcl-2 expression on the development of poliovirus-induced apoptosis. Normal (a) and Bcl-2-expressing (b and c) HeLa cells were infected with poliovirus, and CHI (10 μg/ml) was added at 1.5 h p.i. The Hoechst-33342-stained cells were fixed at 6 (a and b) or 24 (c) h p.i., respectively.
Thus, the implementation of the viral apoptotic program depended on the balance of nonviral pro- and antiapoptotic factors in the infected cell. Importantly, a delay of the execution of viral apoptosis by Bcl-2 did not result in the development of CPE, as could have been expected if the outcome of the competition between the two death programs depended merely on the relative lengths of their cryptic (preexecution) phases.
DISCUSSION
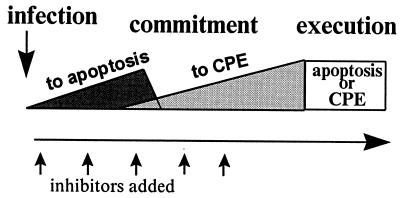
This work was aimed primarily at studying the relationship between the two death programs, apoptosis and CPE, triggered by poliovirus infection of HeLa cells. Even minimal amounts of the viral proteins produced by translation of the input viral RNA were sufficient to turn on the apoptotic program, as evidenced by the development of apoptosis in the cells infected in the presence of guanidine. The proportion of infected cells committed to apoptosis increased roughly linearly during the first 1.5 to 2 h p.i., provided further expression of the viral genome was severely hampered. However, events occurring during the next stage of viral reproduction (after 2 h p.i.) changed the destination of the infected cells toward CPE. Thus, a major observation of this study was the occurrence of a switch in the commitment of the poliovirus-infected cells in the middle of the infectious cycle (Fig. 8). Obviously, the outcome of competition between the two death programs triggered by infection depended nonlinearly on the expression of the viral genome.
FIG. 8.
Schematic representation of the time course of commitment of poliovirus-infected HeLa cells to apoptosis and CPE. The type of death and the proportion of dying cells depend on the time of addition of inhibitors of viral gene expression.
Another regulatory switch occurring in the middle of infection is the development of an antiapoptotic state. Indeed, cells undergoing productive poliovirus infection could be demonstrated, before the appearance of CPE, to be resistant to nonviral apoptotic stimuli (34, 35). Also, as shown here, activation of caspase(s) is suppressed in the course of productive infection.
The early commitment of infected cells to apoptosis may be regarded as a defensive measure aiming at elimination of such cells prior to completion of the viral reproductive cycle. In other words, the cell may posses a very sensitive sensor(s) able to recognize limited amounts of a virus-specific product(s) and to turn on the suicide program. On the other hand, poliovirus has evolved a counterdefensive mechanism activating an antiapoptotic program.
Obviously, the complex pattern of alterations in the infected cells depends on the interplay between virus-specific and host components. With regards to the viral products, the switch from the proapoptotic state to the antiapoptotic state may involve several not mutually exclusive mechanisms. It is not unlikely that the completely processed, “mature” polypeptides may be underrepresented at early steps, and one may speculate that the effects of “immature” and “mature” viral proteins on the apoptotic system as well as on CPE development may be different. On the other hand, it may be assumed that a lesser amount of viral proteins is required to turn on the apoptotic program than to trigger development of the antiapoptotic state and to commit cells to CPE. Also, the expression of proapoptotic and antiapoptotic poliovirus functions may be differently related to the rearrangement of intracellular infrastructure known to develop in the middle of the infectious cycle (22).
The commitment to apoptosis is accompanied by a relatively early (e.g., by 3 h p.i.) activation of a DEVD-specific caspase(s). This group of caspases includes caspase 3, which is believed to be an enzyme involved primarily in the final execution step of apoptosis (28, 38). It is likely that caspase 3 also plays this role in poliovirus-induced apoptosis, as it does in the apoptosis induced by some other RNA-containing viruses (6, 9, 17). However, the involvement of another caspase with a similar substrate specificity (e.g., caspase 7) cannot be excluded either. The data available do not permit the discrimination between two possible causes of a low-level activation of a DEVD-specific caspase(s) in productively infected cells. It may reflect either (i) implementation of the apoptosis execution step in a small proportion of truly apoptotic cells present in any (even uninfected and nontreated) population of HeLa cells, or (ii) “footprints” of the interrupted commitment of the majority of the cells to apoptosis that developed early in infection.
Since no appreciable amount of a free caspase inhibitor could be detected in the productively infected cells, it seems reasonable to assume that viral products accumulating after 2 h of infection (or appropriate virus-induced cellular rearrangements) prevented activation rather than directly inactivated the enzyme. As a result, the implementation of the apoptotic program is interrupted. The research aimed at identification of the viral antiapoptotic protein(s) as well as at elucidation of the mechanism(s) involved is under way.
An important point concerns relationships between the induction of the antiapoptotic state and commitment to CPE. Although at the moment we are aware of no means to uncouple these two processes in the virus-infected cells, the antiapoptotic activity of certain virus-specific proteins in uninfected cells (N. Neznanov et al., unpublished data) suggests that they reflect distinct viral functions.
In the system described, the type of death of the poliovirus-infected cells, apoptosis or CPE, was related to the abortive or productive character of infection, respectively. However, this should not necessarily be the case in other poliovirus-cell systems. Since the apoptotic and CPE programs depend nonlinearly on expression of the viral genome, quantitative differences in the rates of viral translation and/or replication may result in qualitative changes of the infection outcome. The real situation is even more complicated, and in fact hardly predictable, due to intervention of a multitude of host proapoptotic and antiapoptotic factors, as evidenced for example by experiments with the Bcl-2-expressing cells. Our preliminary observations suggest that in certain host cells productive poliovirus infection might be accompanied by apoptosis, whereas in other host cells abortive infection failed to trigger apoptosis (unpublished data). Thus, the model shown in Fig. 8 reflects the situation in certain cells but will not necessarily be as “clean” in others. Also, there is a claim that, in the central nervous systems of mice undergoing poliovirus-induced paralytic disease, infected neurons may die of apoptosis (15). A system in which some apoptotic manifestations resulted from productive infection with another enterovirus, coxsackievirus B3, has recently been reported (9), but again, this is not necessarily the case in other coxsackievirus systems. Nonuniform susceptibility of different host cells to the apoptosis-inducing activity of a given virus is perhaps a general phenomenon (see references 12, 21, and 27). Furthermore, more or less related viruses may differ in their proapoptotic and/or antiapoptotic activities (7, 37), and some viruses (e.g., certain strains of Theiler's murine encephalomyelitis virus) may express unique antiapoptotic proteins (14).
Such a complex control of the fate of infected cells should obviously have important implications for pathogenesis of viral diseases (reviewed in reference 16). For example, the ability of picornaviruses (15, 36) and some other neuropathogenic RNA-containing viruses (e.g., 19, 20, 24, 26, 27) to induce apoptosis in the central nervous system should likely be of clinical importance. Indeed the pattern of the disease should be affected, in particular, by the strength of the inflammatory reaction, which in turn should vary depending on the type of death of the infected neural cells (apoptotic cells do not generally elicit an inflammatory response).
ACKNOWLEDGMENTS
We thank Yuri Lazebnik for the generous gift of caspase 3 and caspase assay kits and Guy Salvesen for providing caspases 7 and 8.
This research was supported in part by grants from INTAS (to V.I.A. and K.B.), the Russian Foundation for Basic Research (to L.I.R.), and the Swiss National Science Foundation (to K.B.). V.I.A. is a Soros Professor.
REFERENCES
- 1.Agol V I, Belov G A, Bienz K, Egger D, Kolesnikova M S, Raikhlin N T, Romanova L I, Smirnova E A, Tolskaya E A. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology. 1998;252:343–353. doi: 10.1006/viro.1998.9438. [DOI] [PubMed] [Google Scholar]
- 2.Bienz K, Egger D, Rasser Y, Bossart W. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology. 1980;100:390–399. doi: 10.1016/0042-6822(80)90530-9. [DOI] [PubMed] [Google Scholar]
- 3.Bienz K, Egger D, Rasser Y, Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983;131:39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- 4.Bienz K, Egger D, Troxler M, Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990;64:1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitzer M, Prinz F, Bauer M, Spiegel M, Neubert W J, Gregor M, Schulze-Osthoff K, Lauer U. Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32) J Virol. 1999;73:702–708. doi: 10.1128/jvi.73.1.702-708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack K, Frings W, Dotzauer A, Vallbracht A. A cytopathogenic, apoptosis-inducing variant of hepatitis A virus. J Virol. 1998;72:3370–3376. doi: 10.1128/jvi.72.4.3370-3376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrasco L. Modification of membrane permeability by animal viruses. Adv Virus Res. 1995;45:61–112. doi: 10.1016/S0065-3527(08)60058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carthy C M, Granville D J, Watson K A, Anderson D R, Wilson J E, Yang D, Hunt D W, McManus B M. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol. 1998;72:7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho M W, Teterina N L, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 11.Dales S, Eggers H J, Tamm I, Palade G E. Electron microscopic study of the formation of poliovirus. Virology. 1965;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 12.Duncan R, Muller J, Lee N, Esmaili A, Nakhasi H L. Rubella virus-induced apoptosis varies among cell lines and is modulated by Bcl-x1 and caspase inhibitors. Virology. 1999;255:117–128. doi: 10.1006/viro.1998.9562. [DOI] [PubMed] [Google Scholar]
- 13.Egger D, Pasamontes L, Bolten R, Boyko V, Bienz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghadge G D, Ma L, Sato S, Kim J, Roos R P. A protein critical for a Theiler's virus-induced immune system-mediated demyelinating disease has a cell type-specific antiapoptotic effect and a key role in virus persistence. J Virol. 1998;72:8605–8612. doi: 10.1128/jvi.72.11.8605-8612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard S, Couderc T, Destombes J, Thiesson D, Delpeyroux F, Blondel B. Poliovirus induces apoptosis in the mouse central nervous system. J Virol. 1999;73:6066–6072. doi: 10.1128/jvi.73.7.6066-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin D E, Hardwick J M. Regulators of apoptosis on the road to persistent alphavirus infection. Annu Rev Microbiol. 1997;51:565–592. doi: 10.1146/annurev.micro.51.1.565. [DOI] [PubMed] [Google Scholar]
- 17.Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel M R. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998;17:1268–1278. doi: 10.1093/emboj/17.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson A C, Rossiter J P. Apoptotic cell death is an important cause of neuronal injury in experimental Venezuelan equine encephalitis virus infection of mice. Acta Neuropathol. 1997;93:349–353. doi: 10.1007/s004010050626. [DOI] [PubMed] [Google Scholar]
- 20.Jackson A C, Rossiter J P. Apoptosis plays an important role in experimental rabies virus infection. J Virol. 1997;71:5603–5607. doi: 10.1128/jvi.71.7.5603-5607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelachich M L, Lipton H L. Theiler's murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch F, Koch G. The molecular biology of poliovirus. Vienna, Austria: Springer Verlag; 1985. [Google Scholar]
- 23.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 24.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao C L, Lin Y L, Wang J J, Huang Y L, Yeh C T, Ma S H, Chen L K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberhaus S M, Smith R L, Clayton G H, Dermody T S, Tyler K L. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J Virol. 1997;71:2100–2106. doi: 10.1128/jvi.71.3.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pekosz A, Phillips J, Pleasure D, Merry D, Gonzalez-Scarano F. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J Virol. 1996;70:5329–5335. doi: 10.1128/jvi.70.8.5329-5335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter A G, Janicke R U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 29.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scallan M F, Allsopp T E, Fazakerley J K. Bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teterina N L, Gorbalenya A E, Egger D, Bienz K, Ehrenfeld E. Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J Virol. 1997;71:8962–8972. doi: 10.1128/jvi.71.12.8962-8972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 33.Tolskaya E A, Romanova L I, Kolesnikova M S, Agol V I. Intertypic recombination in poliovirus: genetic and biochemical studies. Virology. 1983;124:121–132. doi: 10.1016/0042-6822(83)90295-7. [DOI] [PubMed] [Google Scholar]
- 34.Tolskaya E A, Romanova L I, Kolesnikova M S, Ivannikova T A, Smirnova E A, Raikhlin N T, Agol V I. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J Virol. 1995;69:1181–1189. doi: 10.1128/jvi.69.2.1181-1189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolskaya E A, Romanova L I, Kolesnikova M S, Ivannikova T A, Agol V I. A final checkpoint in the drug-promoted and poliovirus-promoted apoptosis is under post-translational control by growth factors. J Cell Biochem. 1996;63:422–431. doi: 10.1002/(SICI)1097-4644(19961215)63:4%3C422::AID-JCB4%3E3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda I, Kurtz C I, Fujinami R S. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 37.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaux D L, Korsmeyer S J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]