Abstract
(1) Background/Objectives: Dexmedetomidine is a sedative for patients receiving invasive mechanical ventilation (IMV) that previous single-site studies have found to be associated with improved survival in patients with COVID-19. The reported clinical benefits include dampened inflammatory response, reduced respiratory depression, reduced agitation and delirium, improved preservation of responsiveness and arousability, and improved hypoxic pulmonary vasoconstriction and ventilation-perfusion ratio. Whether improved mortality is evident in large, multi-site COVID-19 data is understudied. (2) Methods: The association between dexmedetomidine use and mortality in patients with COVID-19 receiving IMV was assessed. This retrospective multi-center cohort study utilized patient data in the United States from health systems participating in the National COVID Cohort Collaborative (N3C) from 1 January 2020 to 3 November 2022. The primary outcome was 28-day mortality rate from the initiation of IMV. Propensity score matching adjusted for differences between the group with and without dexmedetomidine use. Adjusted hazard ratios (aHRs) for 28-day mortality were calculated using multivariable Cox proportional hazards models with dexmedetomidine use as a time-varying covariate. (3) Results: Among the 16,357,749 patients screened, 3806 patients across 17 health systems met the study criteria. Mortality was lower with dexmedetomidine use (aHR, 0.81; 95% CI, 0.73–0.90; p < 0.001). On subgroup analysis, mortality was lower with earlier dexmedetomidine use—initiated within the median of 3.5 days from the start of IMV—(aHR, 0.67; 95% CI, 0.60–0.76; p < 0.001) as well as use prior to standard, widespread use of dexamethasone for patients on respiratory support (prior to 30 July 2020) (aHR, 0.54; 95% CI, 0.42–0.69; p < 0.001). In a secondary model that was restricted to 576 patients across six health system sites with available PaO2/FiO2 data, mortality was not lower with dexmedetomidine use (aHR 0.95, 95% CI, 0.72–1.25; p = 0.73); however, on subgroup analysis, mortality was lower with dexmedetomidine use initiated earlier than the median dexmedetomidine start time after IMV (aHR, 0.72; 95% CI, 0.53–0.98; p = 0.04) and use prior to 30 July 2020 (aHR, 0.22; 95% CI, 0.06–0.78; p = 0.02). (4) Conclusions: Dexmedetomidine use was associated with reduced mortality in patients with COVID-19 receiving IMV, particularly when initiated earlier, rather than later, during the course of IMV as well as use prior to the standard, widespread usage of dexamethasone during respiratory support. These particular findings might suggest that the associated mortality benefit with dexmedetomidine use is tied to immunomodulation. However, further research including a large randomized controlled trial is warranted to evaluate the potential mortality benefit of DEX use in COVID-19 and evaluate the physiologic changes influenced by DEX that may enhance survival.
Keywords: coronavirus disease 2019 (COVID-19), dexmedetomidine, invasive mechanical ventilation, mortality, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 7 million deaths worldwide as of March 2024 [1]. Mortality in critically ill patients with coronavirus disease 2019 (COVID-19) is high [2,3,4,5], so there is a need to improve survival in critically ill patients with COVID-19.
Dexmedetomidine (DEX) is an alpha-2 adrenergic receptor (α2-AR) agonist that was introduced in 1999 as a sedative in the intensive care unit (ICU) for patients receiving mechanical ventilation [6]. Since introduction, interest has mounted over whether DEX improves outcomes including survival compared to gamma-aminobutyric acid (GABA) receptor ligand sedatives such as propofol or benzodiazepines for ICU patients [6,7]. One of the foundational reasons for this interest is the immunomodulatory effects of DEX [6,8,9]. While there is a rationale for targeting the inflammatory response as a survival strategy for ICU patients with sepsis or acute respiratory distress syndrome (ARDS), randomized controlled trials (RCTs) have demonstrated mixed outcomes with anti-inflammatory pharmacologic strategies such as corticosteroids [10] or DEX [7] outside of the COVID-19 population. The COVID-19 pandemic created a global urgency for investigations, leading to improved mortality outcomes for patients with SARS-CoV-2 infection. A key finding was that COVID-19 deaths are in part attributed to an inflammatory response [11], and RCTs demonstrated a reduction in mortality with corticosteroid use among those receiving IMV or oxygen support [12,13].
No large RCTs have evaluated DEX use in COVID-19 outcomes. The potential for DEX to improve COVID-19 outcomes has been proposed through dampening the inflammatory response [14,15,16] such as through sympatholytic and vagomimetic pathways [6,14,15,16], maintaining endothelial cell junction and microcirculatory integrity [17,18,19] as well as other direct and indirect effects on immune cells and other cells types [14,15,16,20]. Other reported benefits of DEX as a sedative option include the lack of significant respiratory depression [21], analgesic properties with an opioid-sparing effect [22], reduced agitation and delirium [23], preserving a degree of responsiveness and arousability [24] as well as potential improvements in hypoxic pulmonary vasoconstriction and the ventilation–perfusion ratio [15,25].
DEX is reported to improve COVID-19 outcomes including improved oxygen saturation (SpO2) [25], improved partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) [26], shortened duration of mechanical ventilation [27], and reduced mortality [28,29]. However, these investigations have been limited in both scope and size. For this study, we utilized the National Institutes of Health’s National COVID Cohort Collaborative (N3C) Data Enclave that houses electronic health record (EHR) data for over 6 million COVID-19 positive patients across over 70 health systems in the United States [30,31]. In this retrospective multi-center cohort study, we hypothesized that DEX use would be associated with improved survival in critically ill patients with COVID-19 receiving IMV in the N3C database.
2. Materials and Methods
2.1. Study Design
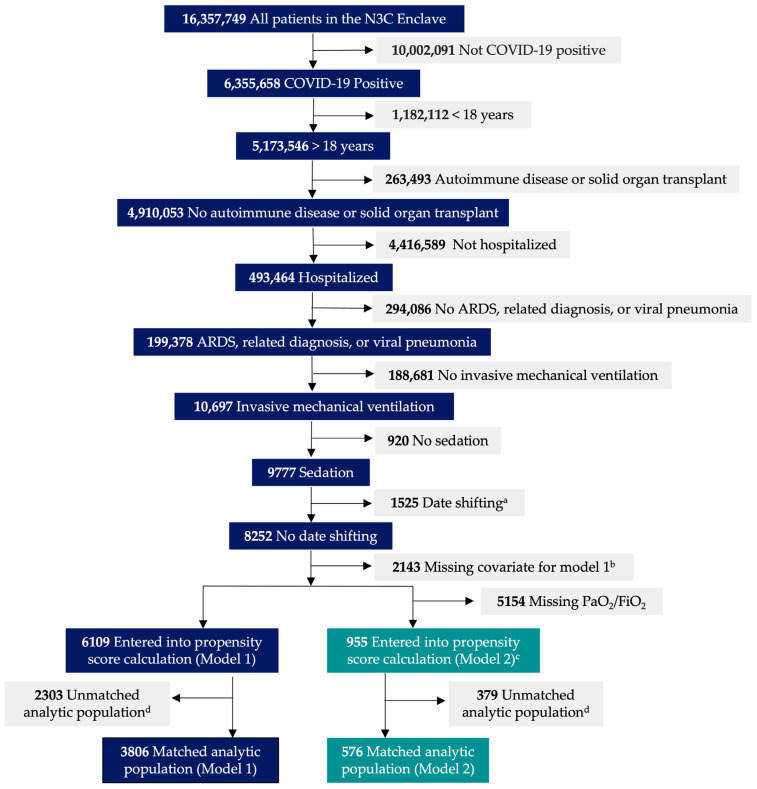
This retrospective multi-center cohort study was performed using EHR data in the N3C Data Enclave hosted by the National Center for Advancing Translational Sciences (NCATS). Patients were included if they had: (i) a diagnosis of COVID-19 or laboratory confirmed SARS-CoV-2 on polymerase chain reaction or antigen test results [32]; (ii) hospitalization within 21 days of first positive COVID-19 indication; (iii) ARDS, related diagnosis, or viral pneumonia; and (iv) received IMV and sedation. The median time between first COVID-19 indication and hospitalization are provided in Table S1. Exclusion criteria included: (i) younger than 18 years of age; (ii) diagnosis of autoimmune disease; and (iii) history of solid organ transplant. Phases of enrollment, exclusion, and data analysis are provided in Figure 1. This study was approved by the institutional review boards at Rush University Medical Center (22032001-IRB01), University of Chicago (IRB22-0681), and the NCATS N3C Data Access Committee (DUR-DODE010). The authors used the Strengthening the Reporting of Observational Research Studies in Epidemiology (STROBE) guidelines [33].
Figure 1.
Flow diagram depicting the phases of enrollment, exclusion, and data analysis for Model 1 and Model 2. a Patients with implausible data points such as a COVID-19 diagnosis in 2017 or individuals in whom the date of death predated their date of hospital admission were excluded. b If a patient was missing a specific covariate for the Cox model, they were excluded for complete case analysis. These specific covariates included age, body mass index, modified Charlson comorbidity index, and modified Sequential Organ Failure Assessment score (central nervous system and respiratory component removed). c Compared to Model 1, additional Model 2 covariates included PaO2/FiO2 and modified Sequential Organ Failure Assessment score with respiratory component added for complete cases analyses. d Unmatched patients between the dexmedetomidine group and no dexmedetomidine group on propensity score calculation were removed.
2.2. Data Collection
The N3C aggregates and harmonizes EHR data for patients with laboratory confirmed or suspected COVID-19 during any encounter after 1 January 2020 from data partners (i.e., sites) across the U.S. [30,31]. Within the N3C, sites are asked to upload two years of health histories before the earliest COVID-19 test date for each patient. The design, sampling, and harmonization methods used in the N3C Enclave have been described previously [31]. This investigation used the N3C Limited Dataset (LDS) [30], which retained the dates of clinical services without shifting for all patients meeting the inclusion criteria. External (i.e., non-site) mortality data were incorporated by the N3C Privacy-Preserving Record Linkage [34]. Data collection within the N3C Data Enclave was performed from 1 January 2020 to 3 November 2022.
2.3. Outcomes
Cohorts included patients that received DEX (DEX group) and patients that did not receive DEX (no DEX group). The DEX group was defined as patients that received DEX between the start time of IMV and 28 days thereafter. Patients that started DEX before IMV were included but only if DEX was continued between the start of IMV and 28 days later. The primary outcome was 28-day mortality rate from the initiation of IMV. This outcome was calculated by performing propensity score matching between the DEX and no DEX group followed by multivariable Cox regression analysis. Based on our previous investigation [28], we hypothesized that DEX use would be associated with a lower 28-day mortality rate.
2.4. Covariates
Propensity score matching between the DEX and no DEX group was performed to adjust for significant covariate differences at hospital admission and ICU variables; pre-propensity score matched covariates at hospital admission and in the ICU are provided in Tables S2 and S3. A significant difference between groups was identified as a standardized mean difference (SMD) > 0.2 [35]. In addition, relevant clinical variables were identified a priori that could have the greatest confounding influence on mortality and were included in the multivariable Cox (proportional hazards) regression analysis for 28-day mortality, in addition to DEX use, as described previously [28]. These variables included factors at hospital admission: age, body mass index (BMI), and modified Charlson comorbidity index (mCCI). The mCCI was calculated as described by Quan et al. (2011) [36]. Additional variables included PaO2/FiO2 and modified Sequential Organ Failure Assessment (mSOFA) scores at the start time of IMV as well as dexamethasone and remdesivir use during hospitalization [28]. The PaO2/FiO2 ratio and mSOFA score were calculated as the worst value over 24 h from the start time of IMV [37]. In all mSOFA score calculations, the central nervous system component was removed, since patients were assessed while under sedation. Within the Cox models, DEX use was treated as a time-varying covariate to adjust for immortal time bias [28,38].
Interleukin-6 (IL-6) receptor antagonists (tocilizumab and sarilumab), Janus kinase (JAK) inhibitors (baricitinib and tofacitinib), and COVID-19 vaccination status prior to a COVID-19 diagnosis were initially identified as covariates due to their immunomodulatory effects and potential influences on mortality. However, between 0 and 6% of the patients received these treatments in either group in our models in the N3C database with no significant differences between groups (Tables S4–S6) and were removed as covariates and used in propensity score matching and Cox regression. Following propensity score matching, these variables remained between 0 and 6% between groups with no significant difference between groups (Tables S7–S9).
2.5. Subgroup Analysis
As a subgroup analysis, evaluation of the influence of the earlier or later initiation of DEX on survival was performed as previously described [28]. Earlier DEX start time was defined as DEX use initiated earlier than the median start time of DEX use relative to the start of IMV amongst the entire DEX group; later start time was defined as DEX use after the median start time. We hypothesized that earlier DEX start times relative to IMV initiation would be associated with a greater reduced risk of death, as previously found [28], which may be due to promoting early immunomodulatory benefits around the time of IMV, dampening COVID-19 progression.
This investigation was performed between 1 January 2020 and 3 November 2022. Starting in July 2020, there was a shift toward the standard usage of corticosteroids, in particular dexamethasone, at the time of IMV or oxygen support in patients with COVID-19, based on the RCT findings [12,13]. Since we hypothesized that DEX may have immunomodulatory benefits in critically ill patients with COVID-19, we questioned whether the shift in treatment protocols, starting around July 2020 incorporating dexamethasone, a powerful immunosuppressant, around the time of IMV, influenced the associated mortality benefit of DEX; dexamethasone may provide powerful immunosuppression to a degree where the addition of the immunomodulatory benefits of DEX are diminished. To further evaluate the impact of the current dexamethasone guidelines on the association between DEX use and mortality, we used the same propensity score matching and Cox regression models incorporating patients either prior to 30 July 2020 (pre-dexamethasone era) or post 30 July 2020 (current dexamethasone era).
2.6. Statistical Analysis
Data acquisition and analysis were performed using Palantir Foundry (Palantir Technologies Inc., Denver, CO, USA), Python (Python Software Foundation), version 3.6, and R, version 4.0.2 (R Foundation for Statistical Computing) using the survival package [39,40] hosted within the N3C Enclave. Continuous variables are presented as the mean and standard deviation (SD). Categorical variables were calculated as the number and percentage of patients; if the number was less than 20, the number and percentage was not reported for reasons of person privacy and N3C policy. For all variables, an SMD was calculated.
The 28-day mortality rate as an outcome was evaluated using propensity score matching followed by Cox regression. Propensity score matching was performed using 1:1 nearest neighbor matching, without replacement, with a caliper [41]. Model 1 (primary model) adjusted for variables at hospital admission and ICU variables with an SMD > 0.2 with propensity score matching and further adjusted a priori selected covariates in Cox regression including: (1) DEX use as a time-varying covariate, (2) age, (3) BMI, (4) mCCI, (5) mSOFA (central nervous system and respiratory component removed), (6) dexamethasone use, and (7) remdesivir use. The Model 2 (secondary model) covariates were identical to Model 1 with the addition of PaO2/FiO2 and the mSOFA respiratory component in the Cox regression model. The majority of sites in the N3C Enclave did not have PaO2/FiO2 data available, primarily because the N3C did not require that sites submit data on ventilator settings, where FiO2 is often documented. As of fall 2022, only six sites nationwide in the N3C Data Enclave had PaO2/FiO2 data, with 42.9% of that data coming from a single site. Due to this limitation, we removed the requirement of PaO2/FiO2 data for our primary model (Model 1). In both models, adjustment for site differences was performed by including a categorical variable with levels for each data partner that represented > 5% of our population and another level for all other sites grouped together [42].
Simple imputation using the mean of the immediate preceding and succeeding most severe value over 24 h was used for missing values for the component mSOFA scores (Model 1 and 2) and PaO2/FiO2 (Model 2) within the 24 h time period of interest—from initiation of IMV [37]. Within both models, a complete case analysis was performed, with exclusion of any patient with a missing covariate or implausible data points such as a COVID-19 diagnosis in 2017 or individuals in whom the date of death predated their date of hospital admission within the N3C database (Figure 1). Mortality outcome data over 28 days are presented as adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) with p < 0.05 considered significant.
3. Results
For the primary analysis (Model 1), among the 16,357,749 patients screened, there were a total of 17 health systems that included 6109 patients, with 3002 patients in the DEX group and 3107 patients in the no DEX group meeting the inclusion and exclusion criteria. Following propensity score matching, there were 1903 patients in the DEX group and 1903 patients in the no DEX group (Figure 1). The mean age in Model 1 was 62.6 years; 61.6% were male, 17.4% were Black, 59.3% were White, and 17.7% were Hispanic. In the restricted analysis (Model 2), where only sites that provided PaO2/FiO2 data were included, there were six health system sites that included 955 patients (Figure 1), with 615 patients in the DEX group and 340 patients in the no DEX group meeting the inclusion and exclusion criteria. Following propensity score matching, there were 288 patients in both the DEX and no DEX group (Figure 1). The mean age in Model 2 was 63.6 years; 63.5% were male, 4.0% were Black, 77.8% were White, and 12.8% were Hispanic.
3.1. Covariate Balance before and after Propensity Score Matching
In Model 1, the SMDs before propensity score matching were considered significant (>0.2) in one out of twenty-eight variables at hospital admission (3.6%) and five out of twenty-five ICU variables (20.0%) (Tables S2 and S3). In Model 2, the SMDs before propensity score matching were considered significant (>0.2) in two out of twenty-eight variables at hospital admission (7.1%) and six out of twenty-six ICU variables (23.1%) (Tables S2 and S3). After propensity score matching was performed, all variables at hospital admission and the ICU variables were similar between groups, with an SMD less than 0.2 (Table 1 and Table 2).
Table 1.
Variables at hospital admission, propensity score matched cohort (1 January 2020 to 3 November 2022).
| Model 1 a | Model 2 b | |||||
|---|---|---|---|---|---|---|
| n = 3806 | n = 576 | |||||
| Variable | No DEX (n = 1903) | DEX (n = 1903) | SMD | No DEX (n = 288) | DEX (n = 288) | SMD |
| Age (SD) | 63.7 (13.6) | 61.6 (14.5) | 0.14 | 64.0 (12.7) | 63.1 (13.4) | 0.07 |
| Male sex (%) | 1169 (61.4) | 1176 (61.8) | 0.01 | 188 (65.3) | 178 (61.8) | 0.07 |
| Race (%) | ||||||
| Black | 325 (17.1) | 337 (17.7) | 0.08 | <20 * | <20 * | 0.11 |
| Other | 63 (3.3) | 82 (4.3) | <20 * | <20 * | ||
| Unknown | 354 (18.6) | 387 (20.3) | 37 (12.8) | 41 (14.2) | ||
| White | 1161 (61.0) | 1097 (57.6) | 227 (78.8) | 221 (76.7) | ||
| Ethnicity (%) | ||||||
| Hispanic | 329 (17.3) | 344 (18.1) | 0.03 | 43 (14.9) | 31 (10.8) | 0.13 |
| Not Hispanic | 1479 (77.7) | 1454 (76.4) | 240 (83.3) | 251 (87.2) | ||
| Unknown | 95 (5.0) | 105 (5.5) | <20 * | <20 * | ||
| Active cancer (%) | 179 (9.4) | 163 (8.6) | 0.03 | 24 (8.3) | 22 (7.6) | 0.03 |
| Cardiovascular disease (%) | ||||||
| Hypertension | 1424 (74.8) | 1355 (71.2) | 0.08 | 205 (71.2) | 206 (71.5) | 0.01 |
| Coronary artery disease | 435 (22.9) | 414 (21.8) | 0.03 | 71 (24.7) | 62 (21.5) | 0.07 |
| Congestive heart failure | 465 (24.4) | 457 (24.0) | 0.01 | 80 (27.8) | 71 (24.7) | 0.07 |
| Chronic respiratory disease (%) | ||||||
| Asthma | 225 (11.8) | 217 (11.4) | 0.01 | 32 (11.1) | 30 (10.4) | 0.02 |
| COPD | 590 (31.0) | 573 (30.1) | 0.02 | 97 (33.7) | 92 (31.9) | 0.04 |
| Interstitial lung disease | 49 (2.6) | 39 (2.0) | 0.04 | <20 * | <20 * | 0.07 |
| Obstructive sleep apnea | 370 (19.4) | 355 (18.7) | 0.02 | 53 (18.4) | 64 (22.2) | 0.10 |
| Immunosuppression (%) | ||||||
| HIV | <20 * | <20 * | 0.03 | <20 * | <20 * | 0.08 |
| Kidney disease (%) | ||||||
| Chronic | 495 (26.0) | 451 (23.7) | 0.05 | 64 (22.2) | 52 (18.1) | 0.10 |
| End-stage | 75 (3.9) | 80 (4.2) | 0.01 | <20 * | <20 * | 0.03 |
| Liver disease (%) | ||||||
| Cirrhosis | 65 (3.4) | 62 (3.3) | 0.01 | <20 * | <20 * | 0.04 |
| Hepatitis B | <20 * | <20 * | 0.05 | <20 * | <20 * | 0.08 |
| Hepatitis C | 37 (1.9) | 63 (3.3) | 0.09 | <20 * | <20 * | 0.07 |
| Metabolic disease | ||||||
| Obesity (%) | 763 (40.1) | 764 (40.1) | 0.00 | 118 (41.0) | 109 (37.8) | 0.06 |
| Morbid obesity (%) | 359 (18.9) | 355 (18.7) | 0.01 | 47 (16.3) | 54 (18.8) | 0.06 |
| BMI (SD) | 33.3 (9.6) | 33.4 (9.4) | 0.01 | 33.0 (9.6) | 33.5 (9.5) | 0.05 |
| Diabetes (%) | 876 (46.0) | 895 (47.0) | 0.02 | 127 (44.1) | 121 (42.0) | 0.04 |
| mCCI (SD) | 2.49 (2.39) | 2.41 (2.39) | 0.03 | 2.31 (2.30) | 2.14 (2.15) | 0.08 |
Abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease, DEX = dexmedetomidine; HIV = human immunodeficiency virus; mCCI = modified Charlson comorbidity index; SD = standard deviation; SMD = standardized mean difference. a Model 1: Health system sites included that lacked PaO2/FiO2 data. b Model 2: Only health system sites that had PaO2/FiO2 data were included. * Values less than 20 denoted as <20 as per N3C policy. Ranges used for complementary values to prevent <20 calculation.
Table 2.
ICU variables, propensity score matched cohort (1 January 2020 to 3 November 2022).
| Model 1 a | Model 2 b | |||||
|---|---|---|---|---|---|---|
| n = 3806 | n = 576 | |||||
| Variable | No DEX (n = 1903) | DEX (n = 1903) | SMD | No DEX (n = 288) | DEX (n = 288) | SMD |
| PaO2/FiO2 (SD) | -- | -- | 85.5 (57.1) | 84.8 (44.8) | 0.01 | |
| mSOFA score (SD) c | 4.75 (2.37) | 4.78 (2.23) | 0.01 | 8.74 (1.94) | 8.66 (1.75) | 0.04 |
| Sedative use (%) | ||||||
| GABA receptor ligand (%) | 1901 (99.9) | 1878 (98.7) | 0.14 | 288 (100.0) | 278 (96.5) | 0.27 |
| Propofol | 1637 (86.0) | 1696 (89.1) | 0.09 | 223 (77.4) | 232 (80.6) | 0.08 |
| Midazolam | 1483 (77.9) | 1581 (83.1) | 0.13 | 221 (76.7) | 234 (81.2) | 0.11 |
| Lorazepam | 922 (48.4) | 1004 (52.8) | 0.09 | 153 (53.1) | 167 (58.0) | 0.10 |
| Ketamine | 283 (14.9) | 365 (19.2) | 0.11 | 50 (17.4) | 68 (23.6) | 0.16 |
| Opioid use (%) | 1850 (97.2) | 1858 (97.6) | 0.03 | 280 (97.2) | 281 (97.6) | 0.02 |
| Corticosteroid (any) use (%) | 1559 (81.9) | 1567 (82.3) | 0.01 | 231 (80.2) | 217 (75.3) | 0.12 |
| Methylprednisolone | 314 (16.5) | 364 (19.1) | 0.07 | 55 (19.1) | 48 (16.7) | 0.06 |
| Dexamethasone | 1221 (64.2) | 1249 (65.6) | 0.03 | 173 (60.1) | 171 (59.4) | 0.01 |
| Hydrocortisone | 301 (15.8) | 303 (15.9) | 0.00 | 46 (16.0) | 53 (18.4) | 0.06 |
| Prednisone | 157 (8.3) | 199 (10.5) | 0.08 | 33 (11.5) | 38 (13.2) | 0.05 |
| Remdesivir use (%) | 598 (31.4) | 558 (29.3) | 0.05 | 71 (24.7) | 68 (23.6) | 0.02 |
| Antibiotic (any) use (%) | 1733 (91.1) | 1762 (92.6) | 0.06 | 228 (79.2) | 238 (82.6) | 0.09 |
| Anticoagulant (any) use (%) | 1833 (96.3) | 1868 (98.2) | 0.11 | 272 (94.4) | 269 (93.4) | 0.04 |
| Heparin | 1365 (71.7) | 1409 (74.0) | 0.05 | 191 (66.3) | 207 (71.9) | 0.12 |
| LMWH | 1285 (67.5) | 1354 (71.2) | 0.08 | 169 (58.7) | 174 (60.4) | 0.04 |
| Factor Xa inhibitor | 318 (16.7) | 346 (18.2) | 0.04 | 47 (16.3) | 44 (15.3) | 0.03 |
| Direct thrombin inhibitor | 50 (2.6) | 78 (4.1) | 0.08 | <20 * | <20 * | 0.08 |
| Warfarin | 52 (2.7) | 68 (3.6) | 0.05 | <20 * | <20 * | 0.05 |
| Inhaled NO Use (%) | <20 * | <20 * | 0.02 | <20 * | <20 * | <0.01 |
| Vasopressor Use (%) | 1569 (82.4) | 1685 (88.5) | 0.17 | 254 (88.2) | 263 (91.3) | 0.10 |
| Paralytic/NMB (%) | 1503 (79.0) | 1587 (83.4) | 0.11 | 204 (70.8) | 213 (74.0) | 0.07 |
| RRT (%) | 93 (4.9) | 159 (8.4) | 0.14 | 29 (10.1) | 30 (10.4) | 0.01 |
| ECMO (%) | 42 (2.2) | 78 (4.1) | 0.11 | <20 * | <20 * | 0.14 |
Abbreviations: DEX = dexmedetomidine; ECMO = extracorporeal membrane oxygenation; GABA = gamma-aminobutyric acid; LMHW = low molecular weight heparin; mSOFA = modified Sequential Organ Failure Assessment; NMB = neuromuscular blockade; NO = nitric oxide; PaO2/FiO2 = partial pressure of arterial oxygen to the fraction of inspired oxygen; RRT = renal replacement therapy; SD = standard deviation; SMD = standardized mean difference. a Model 1: Health system sites included that lacked PaO2/FiO2 data. b Model 2: Only health system sites that had PaO2/FiO2 data were included. c mSOFA score in model 1 had the central nervous system and respiratory component removed; mSOFA score in model 2 had the central nervous system component removed. * Values of less than 20 denoted as <20 as specified by the N3C Data Enclave policy.
3.2. Outcomes
Of the 3806 patients included in Model 1 following propensity score matching, 57.2% died between the start of IMV and 28 days thereafter. The percentage of patients that died was lower in the DEX group compared to the no DEX group (47.4% vs. 67.0%; relative risk reduction, 29.3%, p < 0.001). In Model 1, the 28-day mortality rate was lower with DEX use on multivariable regression (aHR, 0.81; 95% CI, 0.73–0.90; p < 0.001). Similarly, the 28-day mortality rate was lower with DEX use on univariable regression (aHR, 0.90; 95% CI, 0.81–0.99; p = 0.04) (Table 3). Of the 576 patients included in Model 2 following propensity score matching, 55.4% died between the start of IMV and 28 days thereafter. The percentage of patients that died was lower in the DEX group compared to the no DEX group (49.7% vs. 61.1%; relative risk reduction, 18.7%, p < 0.01), but DEX use was not associated with a significantly lower 28-day mortality rate with multivariable regression (aHR, 0.95; 95% CI, 0.72–1.25; p = 0.73) or univariable regression (aHR, 1.05; 95% CI, 0.80–1.36; p = 0.74 (Table 3). The survival curves between cohorts in Model 1 and Model 2 using multivariable regression are provided in Figures S1 and S2. The aHRs for all covariates in Model 1 and Model 2 are provided in Tables S10 and S11.
Table 3.
The 28-day mortality rate from initiation of invasive mechanical ventilation with dexmedetomidine use, propensity score matched cohort (1 January 2020 to 3 November 2022).
| Model 1 a | Model 2 b | |||
|---|---|---|---|---|
| n = 3806 | n = 576 | |||
| Cox Regression Model | aHR (95% CI) | p | aHR (95% CI) | p |
| Multivariable (DEX use) c | 0.81 (0.73, 0.90) | <0.001 | 0.95 (0.72, 1.25) | 0.73 |
| Univariable (DEX use) d | 0.90 (0.81, 0.99) | 0.04 | 1.05 (0.80, 1.36) | 0.74 |
Abbreviations: aHR = adjusted hazard ratio; DEX = dexmedetomidine; 95% CI = 95% confidence interval. a Model 1: Health system sites included that lacked PaO2/FiO2 data. b Model 2: Only health system sites that had PaO2/FiO2 data were included. c Model 1 adjusted for (i) dexmedetomidine as a time-varying covariate, (ii) age, (iii) BMI, (iv) mCCI, (v) mSOFA (central nervous system and respiratory component removed), (vi) dexamethasone use, and (vii) remdesivir use; Model 2 adjusted for (i) dexmedetomidine as a time varying covariate, (ii) age, (iii) BMI, (iv) mCCI, (v) PaO2/FiO2, (vi) mSOFA (central nervous system component removed), (vii) dexamethasone use, and (viii) remdesivir use. In both models, adjusting for site differences was performed by including a categorical variable with levels for each data partner representing >5% of our population and then all others grouped together. d Model 1 and Model 2 adjusted for dexmedetomidine use as a time-varying covariate only.
3.3. Subgroup Analysis
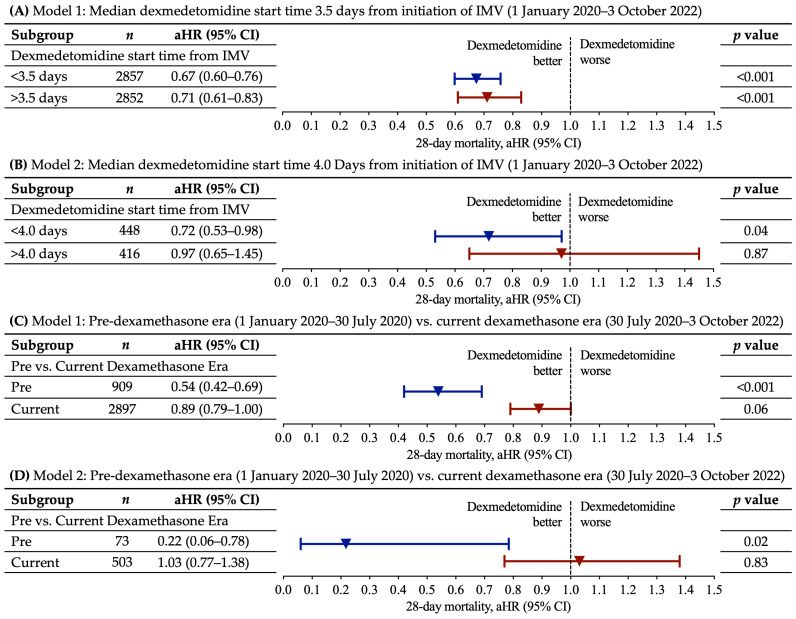
The median start time of DEX from the initiation of IMV was 3.5 days (interquartile range (IQR), 7.0 days) in Model 1 and 4.0 days (IQR, 5.0 days) in Model 2. Within the respective models, we evaluated whether the 28-day mortality rates were influenced by DEX start times earlier or later than the median start time of DEX from the initiation of IMV. Earlier DEX start time was associated with a reduction in the 28-day mortality rate in both Model 1 (aHR, 0.67; 95% CI, 0.60–0.76; p < 0.001) and Model 2 (aHR, 0.72; 95% CI, 0.53–0.98; p = 0.04) (Figure 2A,B). Later DEX start time was associated with a lower mortality rate in Model 1 (aHR, 0.71; 95% CI, 0.61–0.83; p < 0.001) but not Model 2 (aHR, 0.97; 95% CI, 0.65–1.45; p = 0.87) (Figure 2A,B). In both models, earlier DEX start times compared to later DEX start times were associated with a lower aHR (Figure 2A,B). The aHRs for all covariates in this subgroup analyses are provided in Tables S10 and S11. The unadjusted percentage of patients who died in the DEX group in Model 1 was 56.7% (early treatment) and 38.0% (late treatment) compared to 67.0% in the no DEX group (Table S12); in Model 2, the unadjusted percentage of patients who died in the DEX group was 54.4% (early treatment) and 43.8% (late treatment) compared to 61.1% deaths in the no DEX group (Table S12).
Figure 2.
Subgroup analysis examining 28-day mortality rate based on start time of dexmedetomidine as well as dexmedetomidine use pre-dexamethasone era vs. current dexamethasone era. (A,B) Shown are the prespecified subgroup analyses for Model 1 and Model 2 by dexmedetomidine start times pre or post median start time of dexmedetomidine from the start of invasive mechanical ventilation (IMV). The median start time of DEX is shown for both Model 1 and Model 2. (C,D) Shown are the prespecified subgroup analyses for Model 1 and Model 2 by dexmedetomidine use pre-dexamethasone era (1 January 2020 to 30 July 2020) vs. dexmedetomidine use during the current dexamethasone era (30 July 2020 to 3 November 2022). Within the models, propensity score matching and multivariable Cox regressions were performed, and the total number of patients are displayed for each subgroup. The adjusted hazard ratios for each subgroup are plotted as an inverted triangle, and 95% CIs are plotted as horizontal lines. p values < 0.05 are considered significant and correspond to an aHR and 95% CI below 1. Abbreviations: aHR = adjusted hazard ratio; 95% CI = 95% confidence interval.
To further evaluate the impact of the current dexamethasone guidelines on the association between DEX use and mortality, we used the same Model 1 and Model 2 incorporating patients either pre-dexamethasone era (1 January 2020 to 30 July 2020) or during the current dexamethasone era (30 July 2020 to 3 November 2022). There were substantially lower aHRs for DEX use in the pre-dexamethasone era as opposed to the current dexamethasone era in both Model 1 (pre-dexamethasone era aHR 0.54; 95% CI, 0.42–0.69; p < 0.001 vs. current dexamethasone era aHR 0.89; 95% CI, 0.79–1.00; p = 0.06) and Model 2 (pre-dexamethasone era aHR 0.22; 95% CI 0.06–0.78; p = 0.02 vs. current dexamethasone era 1.03; 95% CI, 0.77–1.38; p = 0.83) (Figure 2C,D). The aHRs for all covariates in this subgroup analyses are provided in Tables S13–S16. The unadjusted percentage of patients who died in the DEX group vs. no DEX group in Model 1 was as follows: 36.2% DEX group vs. 62.7% no DEX group (pre-dexamethasone era) and 51.2% DEX group vs. 68.2% no DEX group (current dexamethasone era) (Table S17). In Model 2, the percentage of patients who died in the current dexamethasone era was 51.0% DEX group and 61.4% no DEX group; in the pre-dexamethasone era, the no DEX group had 59.5% deaths, and the DEX group had less than 20 patients that died, and therefore the percentage was masked to protect patient privacy as per the N3C policy (Table S17).
4. Discussion
In this retrospective multi-center cohort study including 3806 patients (Model 1; primary model) in the N3C database with COVID-19 receiving IMV, DEX use was associated with a lower aHR for 28-day mortality from the start time of IMV. Within Model 1, on subgroup analysis, earlier initiation of DEX—DEX use within the median of 3.5 days from the start of IMV—was associated with a lower aHR; furthermore, DEX use prior to 30 July 2020 was associated with a lower aHR. In the secondary model (Model 2) that included 576 patients, with restriction to clinical sites providing PaO2/FiO2 data, mortality was not lower with DEX use; however, similar to Model 1, on subgroup analysis, associated mortality was lower with earlier initiation of DEX use and DEX use prior to 30 July 2020.
The differences between Model 1 and Model 2 with regard to overall associated mortality outcomes between the DEX and no DEX group could be multifactorial. Model 2 was restricted to substantially fewer patients and clinical sites due to the requirement of PaO2/FiO2 data, which was limited across many sites in the N3C database. Furthermore, these PaO2/FiO2 data as well as further incorporation of these data by the addition of the respiratory component in the mSOFA score were factored into the propensity score matching and multivariable Cox regression in Model 2. Therefore, while Model 2 had fewer patients and sites, it better accounted for COVID-19 severity. Intriguingly, while both Model 1 and Model 2 showed reduced mortality with early DEX use or use prior to 30 July 2020, the dichotomy in mortality outcomes compared to later DEX initiation time or DEX use post 30 July 2020 was greater within Model 2 than Model 1. As previously discussed, this dichotomy may be related to differences in the sample size, total clinical sites, or key covariates incorporated into the model.
Our findings are consistent with other observational studies that found lower mortality with DEX use in critically ill patients with COVID-19 [28,29]. The lower 28-day mortality rate with earlier DEX use relative to IMV is consistent with our previous investigation at Rush University System for Health (RUSH) hospitals [28]. Lower mortality with earlier DEX use might be attributed to the immunomodulatory effects of DEX [6,14,15,16,17,18,19]. Failure of the initial clearance of SARS-CoV-2 infection and/or a dysfunctional immune response can result in an inflammatory cascade, contributing to severe lung pathology and a major systemic inflammatory response [43]. Earlier initiation of DEX use relative to start time of IMV may further limit organ dysfunction, irreversible organ damage, and death.
If DEX improves COVID-19 mortality outcomes through immunomodulatory effects, we hypothesized that the incorporation of immunosuppressant corticosteroids, in particular dexamethasone, around the time of respiratory support would reduce the association between DEX and lower mortality—dexamethasone may suppress the immune response to a degree where immunomodulation by DEX provides limited added benefit. Starting in July 2020, there was a shift toward the standard usage of corticosteroids, in particular dexamethasone, at the time of IMV or oxygen support in COVID-19, based on RCT findings [12,13]. In dramatic fashion, the associated mortality benefit with DEX was the greatest during the pre-dexamethasone era (prior to 30 July 2020) in both Model 1 and Model 2. These results potentially suggest that DEX and dexamethasone might have overlapping effects that reduce mortality such as through dampening the inflammatory response.
The use of α2-AR agonists such as DEX have been widely reported to dampen the inflammatory response [14,15,16] such as through sympatholytic and vagomimetic pathways [6,14,15,16] and other direct and indirect effects on immune cells and other cells types [14,15,16,20]. Furthermore, α2-AR agonists have direct effects on the vascular endothelium [17,18,19] with the limitation of circulating leukocyte (neutrophil) extravasation to the site of inflammatory stimulus and tissue [17,44]. The use of corticosteroids, while beneficial around the time of respiratory support in COVID-19, has been of concern as a “double-edged sword” due to the potential to reduce anti-viral immunity and viral clearance through immunosuppression [45,46,47]. For specific infectious diseases, due to concerns of reduced pathogen clearance, DEX may provide an immunomodulatory effect that is overall less immunosuppressive than corticosteroids; however, this was not addressed in this investigation.
While the lowest aHRs for mortality with earlier DEX use relative to IMV and use prior to the current dexamethasone era may suggest an immunomodulatory effect, we cannot rule out alternative potential benefits of DEX use influencing reduced mortality. Other reported benefits of DEX as a sedative option, which could influence mortality, include the following: lack of significant respiratory depression [21], reduced agitation and delirium [23], preserving a degree of responsiveness and arousability [24], and potential improvements in hypoxic pulmonary vasoconstriction and ventilation–perfusion ratio [15,25].
Other studies have predominately evaluated short-term outcomes of DEX use in patients with COVID-19 with severe or critical illness [25,26,27]. A case report found improvement in oxygen saturation (SpO2) on high flow nasal cannula with DEX use in the emergency room setting [25]. In the ICU, the initiation of DEX was associated with improvement in PaO2/FiO2 in a retrospective cohort analysis [26]. In a small double-blinded controlled clinical trial in the ICU, the DEX group compared to the propofol control group had a shorter duration of mechanical ventilation [27]. Clinical trials listed underway investigating DEX use in COVID-19 outcomes include NCT04358627, NCT04413864, and NCT05233605 (ClinicalTrials.Gov, accessed on 5 April 2024). Alternatively, the α2-AR agonist clonidine, which is used as an antihypertensive, appeared to limit the progression of moderate to severe COVID-19 in a case series, when initiated before or at the time of the requirement of oxygenation or hospitalization [48].
This study had a number of strengths including being a multi-center design, substantial sample size, with rigorous control for differences between the DEX and no DEX groups using propensity score matching and multivariable Cox regression. Following propensity score matching, there were no significant differences between the DEX and no DEX group with regard to variables at hospital admission or ICU variables. Furthermore, Cox regression further adjusted for a priori selected covariates that could have had the most confounding influence on mortality outcomes, which were the following: age, BMI, mCCI, mSOFA, dexamethasone use, remdesivir use as well as the addition PaO2/FiO2 and the incorporation of the respiratory component in the mSOFA score in Model 2. Furthermore, within the Cox regression models, DEX use was treated as a time-varying covariate to adjust for immortal time bias [28,38].
This study also had certain limitations. This work was retrospective in nature, and the N3C database has limitations with regard to the covariates that can be assessed. Due to ventilator setting data being available in the N3C database at only a limited number of sites, we removed the requirement of PaO2/FiO2 from the primary model (Model 1) but included PaO2/FiO2 in the restricted secondary model (Model 2); this restriction decreased the overall sample size and number sites in Model 2. Sample size was also further restricted on subgroup analysis in particular for Model 2 when assessing the pre-dexamethasone era vs. current dexamethasone era. The pre-dexamethasone era was the most restricted in sample size and took place from the initial start of the pandemic in the U.S. from 1 January 2020 to 30 July 2020, while the current dexamethasone era in this study (30 July 2020–3 October 2022) had more patients enrolled over a greater window of time. Prone positioning use was initially part of the variables selected for the Cox models as previously described [28] due to its reported benefits of improved oxygenation, which might improve survival in patients with COVID-19 [49]. However, these data were not available within the N3C Data Enclave. The ability to look at dose-dependent associated mortality outcomes with DEX was initially planned for assessment; however, this assessment was not possible in the N3C database. Alwakeel et al. (2024) was able to incorporate the association of individual sedative (propofol, ketamine, midazolam, and DEX) dose and in-hospital mortality in patients with COVID-19 requiring IMV in a multi-center retrospective investigation, and only DEX was associated with decreased odds of mortality, while the remaining sedatives were not associated with an increase or a decrease in odds of mortality [50].
Because this was an observational study, the timing of DEX use with regard to the start of the study (IMV) and assessment over 28 days was not standardized. Patients that started DEX later after IMV had to survive before starting DEX, which resulted in immortal time bias. Not adjusting for immortal time bias can cause substantial overestimations and underestimations of mortality outcome data [38,51,52,53]. Indeed, in this study, when looking at early vs. late DEX start times with regard to IMV, percent deaths were lower with later as opposed to earlier DEX treatment in Model 1 and Model 2; however, when DEX was treated as a time-varying covariate to adjust for immortal time bias in multivariable Cox regression, aHRs were lower with early rather than later DEX start times with regard to IMV. In this investigation, the basis of our conclusions were made treating DEX as a time-varying covariate to adjust for immortal time bias.
Addressing specific DEX associated immunomodulatory biomarkers was not planned or performed in this investigation. In clinical studies predominately evaluating DEX as a sedative in the perioperative environment or for use in critically ill patients with sepsis, DEX has been reported to decrease inflammatory markers and mediators including but not limited to the following: tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and C-reactive protein (CRP) [8,54]. Many of these inflammatory markers or mediators would not be possible to assess in the N3C database. However, given the overall finding of greatest lowered mortality in patients with earlier DEX use relative to IMV and the use of DEX prior to the standard utilization of dexamethasone, it would be of interest in the future to specifically evaluate markers and mediators of inflammation in this subgroup of patients. Clinical trials listed underway investigating DEX use in COVID-19 and immunomodulation with specific assessment of markers and mediators of inflammation include NCT04413864 and NCT05233605 (ClinicalTrials.Gov, accessed on 5 April 2024).
This investigation focused on mortality outcomes, and the consideration of adverse effects with DEX use was not evaluated. However, DEX can increase the risk of transient hypertension with rapid administration, bradycardia, and hypotension due to the peripheral vasoconstrictive and sympatholytic properties of the drug [55,56]. Therefore, DEX should be used with caution in specific patients [55,56].
5. Conclusions
In this retrospective multi-center cohort study, DEX use was associated with a reduced risk of death in patients with COVID-19 receiving IMV, particularly when initiated earlier relative to the start time of IMV and used prior to the current dexamethasone era (prior to 30 July 2020). These particular findings might suggest that DEX is improving the mortality outcomes through immunomodulatory effects. However, the immunomodulatory effects associated with DEX use were not assessed in this investigation and require further exploration. Results from a large RCT are warranted to clarify the potential mortality benefit of DEX use in COVID-19 and evaluate the physiologic changes influenced by DEX that could provide a mortality benefit.
6. Patents
J.L.H and M.A.W have filed a patent related to these studies (PCT/US2021/056580).
Acknowledgments
We gratefully acknowledge N3C Consortium investigator Christopher G. Chute at Johns Hopkins University for the following contributions at a consortial level: clinical data model expertise; data curation; data integration; data quality assurance; funding acquisition; governance; critical revision of the manuscript for important intellectual content; N3C Phenotype definition; project evaluation; project management; regulatory oversight/administration. Individual Acknowledgements For Core Contributors. We gratefully acknowledge the following core contributors to N3C: Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O’Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O’Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors. Data Partners with Released Data. The following institutions whose data are released or pending: Available: Advocate Health Care Network—UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus—UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University—U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic—UL1TR003015: iTHRIV Integrated Translational Health Research Institute of Virginia • Charleston Area Medical Center—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado—UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center—UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University—UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University—UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine—UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University—UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine—Loyola University Medical Center • Loyola University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham—UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester—UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina—UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center—UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours—U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem—UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago—UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN—INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University—UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center—UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center—UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey—UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University—U24TR002306 • The Ohio State University—UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo—UL1TR001412: Clinical and Translational Science Institute • The University of Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa—UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine—UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor—UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston—UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston—UL1TR001439: The Institute for Translational Sciences • The University of Utah—UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center—UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University—UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham—UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences—UL1TR003107: UAMS Translational Research Institute • University of Cincinnati—UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus—UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago—UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center—UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky—UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester—UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota—UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center—U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center—U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill—UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center—U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester—UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California—UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont—U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia—UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington—UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison—UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center—UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University—UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences—UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis—UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University—UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University—U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI). Submitted: Icahn School of Medicine at Mount Sinai—UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler—UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis—UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine—UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles—UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego—UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco—UL1TR001872: UCSF Clinical and Translational Science Institute. Pending: Arkansas Children’s Hospital—UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine—None (Voluntary) • Children’s Hospital of Philadelphia—UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center—UL1TR001425: Center for Clinical and Translational Science and Training • Emory University—UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth—None (Voluntary) • Loyola University Chicago—UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin—UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute—UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth—None (Voluntary) • Montana State University—U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center—UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center—U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute—UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research—None (Voluntary) • Stanford University—UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University—UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute—UL1TR002550: Scripps Research Translational Institute • University of Florida—UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center—UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio—UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital—UL1TR001863: Yale Center for Clinical Investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13123429/s1, Supplementary Information. Table S1. Median Time Between First COVID-19 Indication and Hospitalization Following Propensity Score Matching. Table S2. Pre Propensity Score Matching Variables at Hospital Admission. Table S3. Pre Propensity Score Matching ICU Variables. Table S4. Vaccinated Prior to a COVID-19 Diagnosis (Pre Propensity Score Matching). Table S5. Interleukin-6 (IL-6) Inhibitor Use (Tocilizumab and Sarilumab) (Pre Propensity Score Matching). Table S6. Janus Kinase (JAK) Inhibitor Use (Baricitinib and Tofacitinib) (Pre Propensity Score Matching). Table S7. Vaccinated Prior to a COVID-19 Diagnosis (Post Propensity Score Matching). Table S8. Interleukin-6 (IL-6) Inhibitor Use (Tocilizumab and Sarilumab) (Post Propensity Score Matching). Table S9. Janus Kinase (JAK) Inhibitor Use (Baricitinib and Tofacitinib) (Post Propensity Score Matching. Table S10. Model 1: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Full Study: 1 January 2020 to 3 November 2022), Propensity Score Matched Cohort. Table S11. Model 2: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Full Study: 1 January 2020 to 3 November 2022), Propensity Score Matched Cohort. Table S12. Number and Percent Deaths in Model 1 and Model 2 in Subgroup Analysis of Early vs. Late Dexmedetomidine Start Time. Table S13. Model 1: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Pre-Dexamethasone Era, 1 January 2020 to 30 July 2020), Propensity Score Matched Cohort. Table S14. Model 1: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Current Dexamethasone Era, 30 July 2020 to 3 November 2022), Propensity Score Matched Cohort. Table S15. Model 2: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Pre-Dexamethasone Era, 1 January 2020 to 30 July 2020), Propensity Score Matched Cohort. Table S16. Model 2: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Current Dexamethasone Era, 30 July 2020 to 3 November 2022), Propensity Score Matched Cohort. Table S17. Number and Percent Death in Model 1 and Model 2 in Subgroup Analysis of Pre-Dexamethasone Era and Current Dexamethasone Era. Figure S1. Survival curve—Model 1: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Full Study: 1 January 2020 to 3 November 2022), Propensity Score Matched Cohort. Figure S2. Survival curve—Model 2: Multivariable Cox Regression with Dexmedetomidine as a Time-Varying Covariate (Full Study: 1 January 2020 to 3 November 2022), Propensity Score Matched Cohort.
Author Contributions
Conceptualization, J.L.H., M.A.W. and R.A.B.; Methodology, All authors; Software, R.B. and T.J.B.; Validation, J.L.H., R.B., T.J.B. and R.A.B.; Formal analysis, J.L.H., R.B., and T.J.B.; Investigation, J.L.H., R.B., T.J.B., P.D., A.L., J.C.R., M.A.W. and R.A.B.; Resources, J.L.H., R.B.,T.J.B. and M.A.W.; Data curation, R.B. and T.J.B.; Writing—original draft preparation, J.L.H. and R.B.; Writing—review and editing, All authors; Visualization, J.L.H. and R.B.; Supervision, J.L.H., R.B., T.J.B., M.A.W. and R.A.B.; Project administration, J.L.H., R.B. and T.J.B.; Funding acquisition, J.L.H., R.B., T.J.B. and M.A.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the institutional review boards at Rush University Medical Center (22032001-IRB01) Initial institutional review board approval date: 21 March 2022; University of Chicago (IRB22-0681) Initial institutional review board approval date: 20 May 2022 and NCATS N3C Data Access Committee (DUR-DODE010) Initial data access committee approval date: 7 May 2022.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data collected with this study could be shared through the National Covid Cohort Collaborative (N3C) Data Enclave following appropriate approvals and permissions to access the data.
Conflicts of Interest
J.L.H and M.A.W have filed a patent related to these studies (PCT/US2021/056580). No other conflicts of interest are reported.
Funding Statement
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by CD2H-The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306. The N3C data transfer to NCATS was performed under a Johns Hopkins University Reliance Protocol #IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the ongoing development of this community resource (https://doi.org/10.1093/jamia/ocaa196). This project was supported by the NCATS of the NIH through grant number UL1TR002389 (to Julian Solway, Karen Kim, Joshua Jacobs) that funds the Institute for Translational Medicine (ITM), which provided an N3C Analyst Award to J.L.H. Further support was provided through the Grainger Directorship of the Rush Arthritis and Orthopedic Institute to M.A.W.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . COVID-19 Dashboard. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 20 March 2024)]. Available online: https://data.who.int/dashboards/covid19/deaths?n=c. [Google Scholar]
- 2.Lim Z.J., Subramaniam A., Ponnapa Reddy M., Blecher G., Kadam U., Afroz A., Billah B., Ashwin S., Kubicki M., Bilotta F., et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-Analysis. Am. J. Respir. Crit. Care Med. 2021;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karagiannidis C., Hentschker C., Westhoff M., Weber-Carstens S., Janssens U., Kluge S., Pfeifer M., Spies C., Welte T., Rossaint R., et al. Observational study of changes in utilization and outcomes in mechanical ventilation in COVID-19. PLoS ONE. 2022;17:e0262315. doi: 10.1371/journal.pone.0262315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandel A., Leazer S., Alcover K.C., Farley J., Berk J., Jayne C., McNutt R., Olsen M., Allard R., Yang J., et al. Intensive Care and Organ Support Related Mortality in Patients with COVID-19: A Systematic Review and Meta-Analysis. Crit. Care Explor. 2023;5:e0876. doi: 10.1097/CCE.0000000000000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maze M. From Bench to Bedside and Back Again: A Personal Journey with Dexmedetomidine. Anesthesiology. 2016;125:590–594. doi: 10.1097/ALN.0000000000001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Chen Q., Wang P., Jin W., Zhong C., Ge Z., Xu K. The Effect of Dexmedetomidine as a Sedative Agent for Mechanically Ventilated Patients with Sepsis: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:776882. doi: 10.3389/fmed.2021.776882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanders C.A., Rocke A.S., Edwardson S.A., Baillie J.K., Walsh T.S. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: A systematic review of animal and human studies. Crit. Care. 2019;23:402. doi: 10.1186/s13054-019-2690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lankadeva Y.R., Shehabi Y., Deane A.M., Plummer M.P., Bellomo R., May C.N. Emerging benefits and drawbacks of alpha(2)-adrenoceptor agonists in the management of sepsis and critical illness. Br. J. Pharmacol. 2021;178:1407–1425. doi: 10.1111/bph.15363. [DOI] [PubMed] [Google Scholar]
- 10.Rochwerg B., Oczkowski S.J., Siemieniuk R.A.C., Agoritsas T., Belley-Cote E., D’Aragon F., Duan E., English S., Gossack-Keenan K., Alghuroba M., et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit. Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 11.Wu M., Chen Y., Xia H., Wang C., Tan C.Y., Cai X., Liu Y., Ji F., Xiong P., Liu R., et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl. Acad. Sci. USA. 2020;117:28336–28343. doi: 10.1073/pnas.2018030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-Analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H., Davies R., Ma D. Potential therapeutic value of dexmedetomidine in COVID-19 patients admitted to ICU. Br. J. Anaesth. 2021;126:e33–e35. doi: 10.1016/j.bja.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain A., Lamperti M., Doyle D.J. Dexmedetomidine: Another arrow in the quiver to fight COVID-19 in intensive care units. Br. J. Anaesth. 2021;126:e35–e38. doi: 10.1016/j.bja.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safari S., Jahangirifard A., Zali A., Salimi A., Bastanhagh E., Aminnejad R., Jalili Khoshnood R., Ommi D. Potential Sedative and Therapeutic Value of Dexmedetomidine in Critical COVID-19 Patients. Pharm. Sci. 2021;27:S86–S93. doi: 10.34172/ps.2021.26. [DOI] [Google Scholar]
- 17.Herrera-Garcia A.M., Dominguez-Luis M.J., Arce-Franco M., Armas-Gonzalez E., Alvarez de La Rosa D., Machado J.D., Pec M.K., Feria M., Barreiro O., Sanchez-Madrid F., et al. Prevention of neutrophil extravasation by alpha2-adrenoceptor-mediated endothelial stabilization. J. Immunol. 2014;193:3023–3035. doi: 10.4049/jimmunol.1400255. [DOI] [PubMed] [Google Scholar]
- 18.Miranda M.L., Balarini M.M., Bouskela E. Dexmedetomidine attenuates the microcirculatory derangements evoked by experimental sepsis. Anesthesiology. 2015;122:619–630. doi: 10.1097/ALN.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 19.Yeh Y.C., Wu C.Y., Cheng Y.J., Liu C.M., Hsiao J.K., Chan W.S., Wu Z.G., Yu L.C., Sun W.Z. Effects of Dexmedetomidine on Intestinal Microcirculation and Intestinal Epithelial Barrier in Endotoxemic Rats. Anesthesiology. 2016;125:355–367. doi: 10.1097/ALN.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 20.Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int. Immunopharmacol. 2021;97:107709. doi: 10.1016/j.intimp.2021.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belleville J.P., Ward D.S., Bloor B.C., Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Venn R.M., Hell J., Grounds R.M. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit. Care. 2000;4:302–308. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng K.T., Shubash C.J., Chong J.S. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: Systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74:380–392. doi: 10.1111/anae.14472. [DOI] [PubMed] [Google Scholar]
- 24.Jakob S.M., Ruokonen E., Grounds R.M., Sarapohja T., Garratt C., Pocock S.J., Bratty J.R., Takala J., Dexmedetomidine for Long-Term Sedation Investigators Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 25.Stockton J., Kyle-Sidell C. Dexmedetomidine and worsening hypoxemia in the setting of COVID-19: A case report. Am. J. Emerg. Med. 2020;38:2247.e1–2247.e2. doi: 10.1016/j.ajem.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uusalo P., Valtonen M., Jarvisalo M.J. Hemodynamic and respiratory effects of dexmedetomidine sedation in critically ill COVID-19 patients: A retrospective cohort study. Acta Anaesthesiol. Scand. 2021;65:1447–1456. doi: 10.1111/aas.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghajarzadeh K., Fard M.M., Otaghvar H.A., Faiz S.H.R., Dabbagh A., Mohseni M., Kashani S.S., Fard A.M.M., Alebouyeh M.R. Effects of Dexmedetomidine and Propofol on Hemodynamic Stability and Ventilation Time in Patients Suffering COVID-19 Admitting to Intensive Care Units. Ann. Rom. Soc. Cell Biol. 2021;25:2457–2465. [Google Scholar]
- 28.Hamilton J.L., Vashi M., Kishen E.B., Fogg L.F., Wimmer M.A., Balk R.A. The Association of an Alpha-2 Adrenergic Receptor Agonist and Mortality in Patients with COVID-19. Front. Med. 2021;8:797647. doi: 10.3389/fmed.2021.797647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreychenko S.A., Bychinin M.V., Mandel I.A., Klypa T.V. The effectiveness of dexmedetomidine in patients with severe COVID-19. J. Clin. Pract. 2021;12:5–11. doi: 10.17816/clinpract88180. [DOI] [Google Scholar]
- 30.About the National COVID Cohort Collaborative. [(accessed on 9 December 2022)]; Available online: https://ncats.nih.gov/n3c/about.
- 31.Haendel M.A., Chute C.G., Bennett T.D., Eichmann D.A., Guinney J., Kibbe W.A., Payne P.R.O., Pfaff E.R., Robinson P.N., Saltz J.H., et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J. Am. Med. Inform. Assoc. 2021;28:427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latest Phenotype · National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition Wiki (github.com) [(accessed on 1 April 2024)]. Available online: https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition/wiki/Latest-Phenotype.
- 33.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 34.N3C Privacy-Preserving Record Linkage. [(accessed on 9 December 2022)]. Available online: https://covid.cd2h.org/PPRL.
- 35.Chow J.H., Rahnavard A., Gomberg-Maitland M., Chatterjee R., Patodi P., Yamane D.P., Levine A.R., Davison D., Hawkins K., Jackson A.M., et al. Association of Early Aspirin Use with In-Hospital Mortality in Patients with Moderate COVID-19. JAMA Netw. Open. 2022;5:e223890. doi: 10.1001/jamanetworkopen.2022.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., Januel J.M., Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 37.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 39.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [(accessed on 20 December 2022)]. Available online: https://www.R-project.org/
- 40.Therneau T.M. A Package for Survival Analysis in R. [(accessed on 20 December 2022)]. Available online: https://CRAN.R-project.org/package=survival.
- 41.Stuart E.A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen L.C., Yang D., Nicolaescu V., Best T.J., Gula H., Saxena D., Gabbard J.D., Chen S.N., Ohtsuki T., Friesen J.B., et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022;8:eabi6110. doi: 10.1126/sciadv.abi6110. [DOI] [PubMed] [Google Scholar]
- 43.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham E., Kaneko D.J., Shenkar R. Effects of endogenous and exogenous catecholamines on LPS-induced neutrophil trafficking and activation. Am. J. Physiol. 1999;276:L1–L8. doi: 10.1152/ajplung.1999.276.1.L1. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: A double-edged sword? Lancet. 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang X., Feng Y.M., Ni J.X., Zhang J.Y., Liu L.M., Hu K., Wu X.Z., Zhang J.X., Chen J.W., Zhang J.C., et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021;100:116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahsoun A., Fakih Y., Zareef R., Bitar F., Arabi M. Corticosteroids in COVID-19: Pros and cons. Front. Med. 2023;10:1202504. doi: 10.3389/fmed.2023.1202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyoju S.K., Baral B., Jha P.K. Central catecholaminergic blockade with clonidine prevent SARS-CoV-2 complication: A case series. IDCases. 2021;25:e01219. doi: 10.1016/j.idcr.2021.e01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathews K.S., Soh H., Shaefi S., Wang W., Bose S., Coca S., Gupta S., Hayek S.S., Srivastava A., Brenner S.K., et al. Prone Positioning and Survival in Mechanically Ventilated Patients with Coronavirus Disease 2019-Related Respiratory Failure. Crit. Care Med. 2021;49:1026–1037. doi: 10.1097/CCM.0000000000004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alwakeel M., Wang Y., Torbic H., Sacha G.L., Wang X., Abi Fadel F., Duggal A. Impact of Sedation Practices on Mortality in COVID-19-Associated Adult Respiratory Distress Syndrome Patients: A Multicenter Retrospective Descriptive Study. J. Intensive Care Med. 2024;39:646–654. doi: 10.1177/08850666231224395. [DOI] [PubMed] [Google Scholar]
- 51.Martinuka O., von Cube M., Wolkewitz M. Methodological evaluation of bias in observational coronavirus disease 2019 studies on drug effectiveness. Clin. Microbiol. Infect. 2021;27:949–957. doi: 10.1016/j.cmi.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones M., Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J. Crit. Care. 2016;36:195–199. doi: 10.1016/j.jcrc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Suissa S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 54.Wang K., Wu M., Xu J., Wu C., Zhang B., Wang G., Ma D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br. J. Anaesth. 2019;123:777–794. doi: 10.1016/j.bja.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 55.Weerink M.A.S., Struys M., Hannivoort L.N., Barends C.R.M., Absalom A.R., Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keating G.M. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75:1119–1130. doi: 10.1007/s40265-015-0419-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected with this study could be shared through the National Covid Cohort Collaborative (N3C) Data Enclave following appropriate approvals and permissions to access the data.