Abstract
Heterosexual transmission of human immunodeficiency virus (HIV) is the most frequent mode of infection worldwide. However, the immediate events between exposure to infectious virus and establishment of infection are still poorly understood. This study investigates parameters of HIV infection of human female genital tissue in vitro using an explant culture model. In particular, we investigated the role of the epithelium and virucidal agents in protection against HIV infection. We have demonstrated that the major target cells of infection reside below the genital epithelium, and thus HIV must cross this barrier to establish infection. Immune activation enhanced HIV infection of such subepithelial cells. Furthermore, our data suggest that genital epithelial cells were not susceptible to HIV infection, appear to play no part in the transfer of infectious virus across the epithelium, and thus may provide a barrier to infection. In addition, experiments using a panel of virucidal agents demonstrated differential efficiency to block HIV infection of subepithelial cells from partial to complete inhibition. This is the first demonstration that virucidal agents designed for topical vaginal use block HIV infection of genital tissue. Such agents have major implications for world health, as they will provide women with a mechanism of personal and covert protection from HIV infection.
Heterosexual transmission of human immunodeficiency virus (HIV) infection occurs through mucosal surfaces and is the major route of infection worldwide. This mode of transmission is increasing in prevalence more rapidly than any other in the West (31, 33, 42). Male-female transmission rates (per contact infectivity estimated to be 0.0009 in North American women) are reported to be approximately eight times more efficient than female-male rates, with history of concomitant sexually transmitted diseases (STDs) being the most strongly associated risk factor (22). Conflicting data have been reported on the selective pressure of mucosal transmission on the phenotypic and genotypic characteristics of transmitted viral isolates from a heterogeneous inoculum. Predominant isolation from peripheral blood of virus able to infect both macrophages and CD4+ T lymphocytes (non-syncytium inducing [NSI], M-tropic) over those that preferentially infect T lymphocytes (syncytium inducing [SI], T-tropic), during or near to acute HIV infection have lead to the suggestion that NSI viruses may be more readily transmitted via mucosal routes (40). The mechanisms for such selective transmission of HIV isolates may be one of either selective penetration or selective amplification within the infected host (33). Analyses of genital biopsies from HIV-infected women and preliminary studies from this laboratory, using cervical organ culture as a model of primary infection, have demonstrated that HIV-infected cells reside within subepithelial mucosa, with no evidence of HIV infection of epithelial cells (20, 21, 23, 27, 35). Furthermore, it has been demonstrated that the primary targets of simian immunodeficiency virus (SIV) infection, following intravaginal infection of macaques, are cervical and vaginal subepithelial cells (35). Such data indicate that establishment of HIV infection requires transepithelial penetration. Whether intact genital epithelium presents a barrier to, or is an active participant in, HIV transmission has not been tested in primary human mucosal tissue.
Epithelium along the female genital tract differs in structural cellular organization: the vagina and ectocervix, the site most exposed to a natural inoculum, are composed of stratified epithelium, whereas the endocervix is composed of a single epithelial monolayer. Multiple mechanisms for HIV transmission across genital epithelia have been proposed: direct HIV infection of epithelial cells, transcytosis of HIV through epithelial cells, epithelial transmigration of HIV-infected donor cells, uptake of HIV by intraepithelial Langerhans cells, or circumvention of epithelium via breaches in epithelial integrity (14, 33). Evidence for HIV infection of, or transcytosis through, epithelial cells is derived from in vitro studies using epithelial cell lines, which may bear little relation to primary intact genital epithelium (1, 38). Furthermore, infection or transcytosis in such models is dependent on cell-associated virus, an observation at odds with efficient mucosal cell-free SIV or feline immunodeficiency virus infection (3, 35). Strong epidemiological association of inflammatory ulcerative venereal disease with HIV transmission and observation that mucosal SIV transmission may be enhanced following thinning of vaginal epithelium by progesterone implants suggests a barrier role for genital epithelium (18, 31). Furthermore, recent studies have demonstrated that STDs increase both the number of CD4 cells in genital mucosa and the expression of chemokine receptors known to function as HIV coreceptors, thereby increasing the number of target cells (16, 24, 30). While HIV may achieve transepithelial penetration by more than one mechanism, the relatively low incidence of per-contact infectivity suggests that this is unlikely to reflect a constitutive mechanism. However, male-female transmission is also influenced by factors relating to the male partner, including seminal viral load and incidence of STDs (31), all of which have an impact on contact infectivity.
While condoms provide an effective barrier against transmission of HIV and other STDs, they require the consent of the male partner, which cannot always be negotiated by women at risk for infection. Thus, there is an urgent need to develop prevention strategies that are under the personal control of women. The potential of effective topical vaginal virucides to prevent sexual transmission of HIV and other STDs is widely recognized (8). However, proper evaluation of the efficacy of such agents in blocking HIV infection of female genital tissue has been hampered by the lack of appropriate experimental models.
Thus, understanding the first critical events in genital mucosal transmission of HIV infection is important in developing strategies to block or limit such transmission. In this study, human genital mucosal tissue from premenopausal seronegative women was been used to define primary target cells for HIV infection within genital mucosa, differential susceptibility of such tissue to M-tropic and T-tropic HIV isolates, and the interaction of HIV with genital epithelium. Furthermore, this in vitro model has been used to determine the efficacy of potential vaginal virucides designed to protect women from HIV infection.
MATERIALS AND METHODS
Virus culture and infection.
The following strains of HIV-1 were used in this study: BaL (grown in monocyte-derived macrophages), RF and IIIB (grown in H9 cells) (AIDS reagent project, National Institute for Biological Standards and Control, Potters Bar, United Kingdom), and SL-2, 2044, and 2076 (generously donated by Paul Clapham, Imperial Cancer Research Fund, United Kingdom) grown in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cell (PBMC) cultures. Cell-free viral preparations were passed through 0.2-μm-pore-size filters and treated with DNase I (Sigma Ltd., Poole, United Kingdom) prior to use (100 U/ml, 5 mM MgCl2, 30 min at 37°C). Chronically infected PM-1 T cells (AIDS reagent project) were produced by continual culture (RPMI 10% [RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin, streptomycin, and l-glutamine]) of these cells following exposure to the BaL or RF strain of HIV-1. Chronically infected PBMC cultures were established following infection of PHA-stimulated PBMC cultures with HIVBaL. Infection was monitored by p24 antigen release, measured by enzyme-linked immunosorbent assay (ELISA; DuPont NEN, Hounslow, United Kingdom) according to the manufacturers protocol. Chronically infected cells were treated with mitomycin C (200 μg/ml; Sigma) for 1 h at 37°C prior to use (38).
Culture of human genital tract tissue explants and HIV infection.
Cervical and vaginal tissue were obtained from premenopausal women undergoing planned therapeutic hysterectomy at St. George's and St. Helier Hospitals, London, United Kingdom. Cervical and vaginal explants, comprising of both epithelial and stromal tissue, were produced using either 3- or 8-mm-diameter biopsy punches as described below. Intact explants (3 mm) were cultured on squares of stainless-steel mesh, individually, in 24-well flat-bottomed plates so that a meniscus of culture medium (Eagle's minimal essential medium supplemented with penicillin, streptomycin, l-glutamine, and HEPES buffer [EMEM], Sigma) was in contact with the under surface of the grid, as previously described (23). In some experiments, where indicated, explants were directly cultured in 300 μl of RPMI 10% in a 96-well plate. Explants were incubated at 37°C in a humidified atmosphere containing 5% CO2, and two-thirds of the medium was changed every 2 to 3 days, taking care not to disturb any migratory cells within the culture wells. Two protocols were used for HIV infection of explant tissue. Initially, explants were cultured for 2 days prior to infection in either the absence or presence of PHA (5 μg/ml). Explants were then exposed to viral isolates by immersion in 1 ml of cell-free virus (103 to 106 50% tissue culture infective doses [TCID50]) for 2 h at 37°C. After incubation with infectious virus, explants were washed a minimum of five times in phosphate-buffered saline (PBS) and then cultured on grids as described above. Explants stimulated with PHA were cultured in the presence of interleukin-2 (IL-2; 10 U/ml; AIDS reagent project) throughout the experiment. After 8 days, explants were processed for immunohistochemistry or PCR. Remaining tissue was transferred to 96-well plates, cultured in medium (RPMI 10%) alone or restimulated by culture in the presence of PHA (5 μg/ml) for 2 days, and subsequently refed with medium containing IL-2. Culture medium was collected every 2 to 3 days and stored at −70°C before subsequent measurement of p24 antigen content by ELISA. For later experiments, explants were exposed to viral innoculum, by immersion in cell-free virus (103 to 106 TCID50) for 2 h at 37°C, within 4 h of isolation. Subsequently explant tissue was washed in PBS as described above, and tissue was directly transferred to 96-well plates, cultured in medium (RPMI 10%) alone or stimulated by culture in the presence of PHA (5 μg/ml) for 2 days, and subsequently refed with medium containing IL-2. In both culture models, any migratory cells were retained within the culture wells through out the experiment.
Preparation of epithelial sheets and primary epithelial cultures.
Cervical epithelial sheets were isolated from 8-mm biopsies following enzymatic digestion using dispase II (Boehringer Mannheim Ltd., Lewes, United Kingdom) and trypsin-EDTA (Sigma). Tissue was incubated in dispase overnight at 4°C. The following day, biopsies were washed three times in PBS to remove dispase and the epithelial cells were separated from the submucosa using fine forceps as previously described (15). Ectocervical epithelium (released as an intact sheet of cells) and endocervical epithelium (released as small clumps of cells) were placed in trypsin-EDTA for 20 min at 37°C with frequent agitation. The resulting cell suspensions were centrifuged and resuspended at 2 × 105 ectocervical cells and 105 endocervical cells per ml in keratinocyte-serum free medium (Gibco/Life Technology, Paisley, United Kingdom). Cells were grown in 12-well plates at 37°C in a humidified atmosphere containing 5% CO2 and refed every 2 days by replacing half of the existing medium. Epithelial monolayers were demonstrated to be viable and functional by growth characteristics, structural and ultrastructural morphology (light and electron microscopy), mitotic activity [incorporation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye], and presence of cytokeratin markers (confirming cell origin) [K13, stratified ectocervical cells; K18, columnar endocervical cells] [data not shown]). The intestinal epithelial cell line I407 (American Type Culture Collection) was cultured in RPMI 10%. Epithelial monolayers were exposed to cell-free virus (30 to 70 ng of HIV p24 per ml, pretreated with DNase) or chronically infected cells (pretreated with mitomycin C) for 2 or 24 h at 37°C in 5% CO2. Epithelial monolayers were washed five times immediately after exposure to cell-free HIV or chronically HIV-infected cells and washed again 48 h later. Culture medium was collected every 2 days for p24 ELISA. Eight days postinfection, monolayers were processed for PCR or cocultured with PM-1 T cells, seeded at a 1:1 ratio for 10 days for virus isolation. PM-1 T cells are susceptible to both M- and T-tropic HIV, expressing both CXCR4 and CCR5 coreceptors.
Virus transmission across polarized epithelial sheets.
Ectocervical epithelial sheets (8 mm), isolated as described above, were analyzed by light microscopy (×200) for any visible perforation of the epithelial cell surface. Viability and functional analysis of epithelial sheets was carried out as described above. In addition, presence of functional intercellular junctions (which exclude inulin) were demonstrated by the detection of inulin transfer following EGTA treatment. Epithelial sheets were clamped in diffusion chambers (12) consisting of two acrylic plates, each of which had a central aperture (3.5-mm diameter) connected to vertical chambers providing independent access to apical and basolateral surfaces. A leakproof seal was obtained by the means of concentrally positioned O-rings (6-mm diameter). Cell-free virus (30 to 70 ng of HIV p24 per ml, equivalent to 105 TCID50) or chronically infected cells in culture medium containing 0.5 mCi of [14C] inulin were added to the apical chamber (1 ml), while culture medium alone was added to the basolateral chamber, and polarized tissue was incubated for 2 h at 37°C in 5% CO2. Culture medium was subsequently withdrawn from apical and basolateral chambers, and the epithelial tissue was removed. Medium was tested for the presence of [14C]inulin, p24 antigen, and infectious virus by coculture with PM-1 T cells. Epithelial layers were washed, either processed for quantitative PCR or fixed in 3% glutaraldehyde in PBS, and sent for processing for transmission electron microscopy (TEM) (Department of Anatomy, St. George's Hospital Medical School).
Measurement of tissue viability.
Potential toxicity of virucidal agents was quantitated using the principle of MTT (Sigma) dye reduction by viable explant tissue into a methanol soluble formazan product. In brief, ectocervical explants (3-mm diameter) were incubated in virucidal agent (0.01 to 100 μg/ml) overnight and subsequently washed five times in PBS. Tissue was then either immediately tested for viability (T1) or cultured on grids in EMEM for a further 5 days before testing for viability (T6). To assess tissue viability, washed explants were incubated in medium containing MTT (250 μg/ml) for 3 h at 37°C. Tissue viability was determined by dividing the optical density of the formazan product at 570 nm by the dry weight of the explant. Toxicity was determined by comparison of viability between treated explants and untreated control tissue. Virucidal agents were considered to be nontoxic only at concentrations that demonstrated no reduction in tissue viability at either T1 or T2. A minimum of three independent experiments using tissue from separate donors were performed in duplicate for each condition.
Immunohistochemistry.
Tissues were fixed overnight in neutral buffered formalin, and 4-μm sections of paraffin-embedded tissue were prepared. Endogenous peroxidase was inactivated in deparaffinizsed sections with a 30-min treatment in methanol–0.3% (vol/vol) H2O2. Antigens in paraffin sections were unmasked with a 10-min treatment with pronase (Dako Ltd., High Wycombe, United Kingdom). Slides were washed between incubations with Tris-buffered saline. Slides were incubated with 20% (vol/vol) rabbit serum in Tris-buffered saline for 30 min followed by overnight incubation at 4°C with one of the following primary monoclonal antibodies diluted in 20% (vol/vol) rabbit serum: anti-p24 (Kal-1), CD2 (MT910), CD68 (PGM1), CD1a (NA1/34), CD45RO (OPD4), and mouse immunoglobulin G1 (Dako) and major histocompatibility class II (WR18 [Serotec, Oxford, United Kingdom] and L243 [American Type Culture Collection). Biotinylated rabbit anti-mouse immunoglobulin G antibody (Dako) was applied to the sections, followed by avidin-biotin peroxidase complex (Dako) and diaminobenzidine (DAB) substrate (Sigma). Sections were counterstained with Mayer's hematoxylin. For dual immunohistochemistry with primary antibodies raised in the same species, the above procedure was applied in two consecutive treatments. Sections were not counterstained. The first monoclonal antibody was detected with the peroxidase-DAB method. The procedure was then repeated, starting at the pronase step, for the second monoclonal antibody which was detected with avidin-biotin alkaline phosphatase (Dako) and nitroblue tetrazolium salt and bromochloroindolylphosphate substrates (Sigma).
PCR for proviral DNA.
For PCR, DNA was extracted from cells and tissues by an overnight digestion at 55°C and 5 min at 95°C with 500 μg of proteinase K per ml, 0.1% (vol/vol) Nonidet P-40 (Sigma), and PCR buffer II (Perkin-Elmer, Warrington, United Kingdom). Viral DNA was purified using QIAamp spin columns (Qiagen, Crawley, United Kingdom). Qualitative PCR for HIV-1 DNA was carried out by amplification of a 540-bp long terminal repeat LTR sequence (5) which had a sensitivity of 10 copies per 105 cells. To control for sample quality and loading, duplicate PCRs were carried out using primers for β-actin (forward primer, 5′-GAA GAT CAA GAT CAT TGC TCC TCC-3′; reverse primer, 5′-CTG GTC TCA AGT CAG TGT ACA GG-3′) (GenBank accession no. M10277). Quantitative proviral PCR for HIV-1 LTR DNA was carried out by real-time PCR on a ABI Prism 7700 sequence detection system as previously described (9). The following reagents were used for this assay: LTR forward primer, 5′-CAC ACA AGG CTA CTT CCC TGA-3′ (59 to 79); LTR reverse primer, 5′-TCT CTG GCT CAA CTG GTA CTA GCT T-3′ (141 to 165); probe 5′-(6-carboxy-fluorescein [FAM])AGA ACT ACA CAC CAG GGC CAG GGA TCA G(6-carboxy-tetramethyl-rhodamine [TAMRA])-3′ (85 to 112) (based on the reference sequence for HIV-1, isolate HXB2; GenBank accession no. K03455); β-actin forward primer, 5′-TCA CCC ACA CTG TGC CCA TCT ACG A-3′ (486 to 510); reverse primer 5′-AGT CAG TCA GGT CCC GGC C-3′ (567 to 585); probe 5′-(VIC proprietary dye [PE Applied Biosystems])ATG CCC TCC CCC ATG CCA TCC TGC GT(Q) (TAMRA)-3′ (GenBank accession no. AB004047). Primers and FAM-labeled probe were obtained from MWG Biotech, Ebersberg, United Kingdom, and VIC-labeled β-actin probe was obtained from PE Applied Biosystems, Warrington, United Kingdom. β-actin was used as an internal control allowing normalization of HIV copy number relative to the number of genome equivalents in the specimen. The threshold sensitivity was 10 DNA copies per reaction.
RESULTS
HIV infection of genital mucosa is potentiated by immune activation.
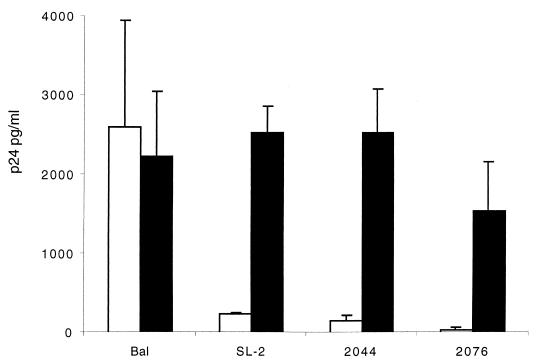
Susceptibility of female genital tract mucosa to HIV infection was investigated in vitro using mucosal tissue explants, obtained from seronegative premenopausal women undergoing hysterectomy as previously described (23). Ectocervical, endocervical, and vaginal explants (3 mm3) were cultured under resting (medium alone) or activating (in the presence of PHA and IL-2) conditions. Explant tissue was exposed, in a nonpolarized manner, to primary NSI and laboratory-adapted SI HIV-1 isolates with known coreceptor restriction: BaL (NSI, CCR5/CCR3 restricted), IIIB, and RF (SI, predominantly CXCR4 restricted). Ectocervical and endocervical explant cultures were demonstrated to be susceptible to infection with HIV-1BaL as indicated by increased accumulation of HIV p24 release independent of culture conditions (Fig. 1). In contrast, T-tropic strains of HIV (IIIB and RF) induced significant levels of productive HIV infection only in immune activated tissue. However, productive infection with either IIIB or RF could still be rescued if tissue stimulation (with PHA) was delayed by 8 days after exposure to HIV (Table 1). Persistence of HIV within explants for 8 days prior to stimulation was dependent on HIV infection, as these effects were inhibited in the presence of zidovudine. A similar pattern of HIV replication was also observed in vaginal explants (data not shown). To determine whether other primary isolates of HIV replicated similarly to HIV-1BaL, ectocervical explants were exposed to primary isolates SL-2 (NSI, predominantly CCR5 restricted), 2044 (SI, CXCR4 restricted), and 2076 (SI, dualtropic, able to use CCR5, CCR3, and CXCR4). Explants were inoculated with virus within 4 h of obtaining tissue from surgery to minimize any potential changes in coreceptor expression and cultured in the presence or absence of activating conditions. Unlike HIV-1BaL (at an equivalent dose [data not shown]), all three primary strains required immune activation to induce significant levels of viral replication (Fig. 2).
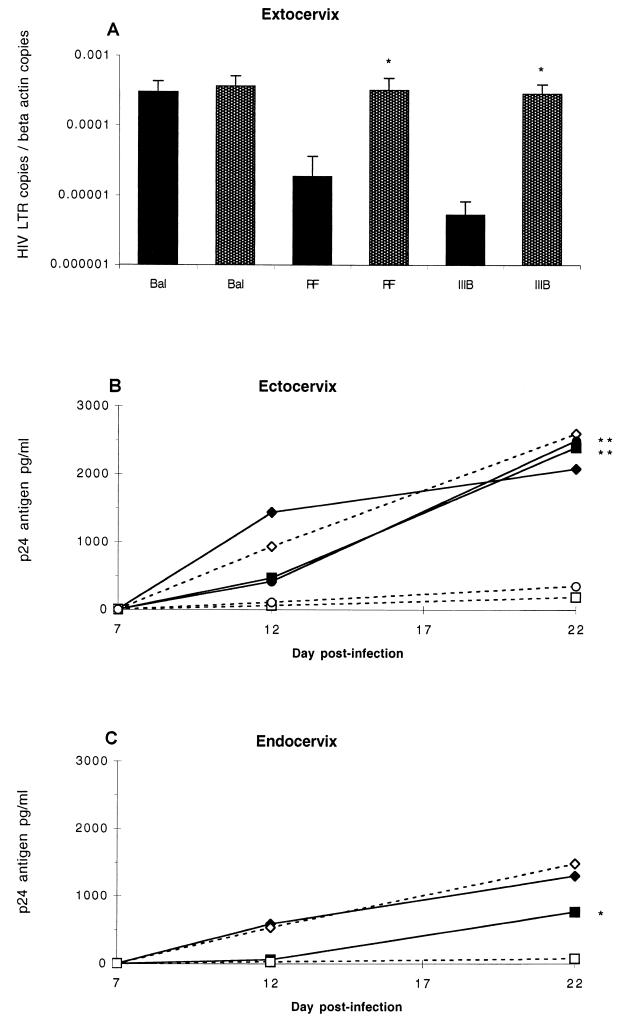
FIG. 1.
Kinetics of HIV replication in cervical explant tissue. (A) Accumulation of HIV-1 DNA in ectocervical explants was determined 7 days after infection with HIV-1BaL, HIV-1IIIB, or HIV-1RF as assayed for LTR DNA by quantitative real-time PCR as described in Materials and Methods. Explants either were prestimulated with PHA 2 days prior to HIV infection and subsequently cultured with IL-2 (filled bars) or were cultured in medium alone before and after infection (open bars). Data represents the mean and standard error of three independent experiments using paired explants from separate donors. p24 antigen release from ectocervical (B) and endocervical (C) explants was measured by ELISA. Explants were cultured either alone (open symbols, solid lines) or prestimulated with PHA (5 μg/ml) 2 days prior to viral exposure and restimulated 8 days post exposure. Stimulated explants were cultured in the presence of IL-2 (10 U/ml) (closed symbols, broken lines). Explants were exposed to HIV-1BaL (105 TCID50) without (◊) or with (⧫) (PHA, HIV-1IIIB (106 TCID50) without (□) or with (■) PHA, or HIV-1RF (106TCID50) without (○) or with (●) PHA. Data represent the mean from a minimum of three independent experiments using paired explants from separate donors. (t test; ∗, P = <0.05; ∗∗, P = <0.01).
TABLE 1.
HIV-1 replication in ectocervical explantsa
| HIV-1 strain | No. of patients | Mean p24 (pg/ml) (SEM)
|
||
|---|---|---|---|---|
| Control | PHA
|
|||
| 2 days preexposure | 8 days postexposure | |||
| BaL | 6 | 2,597 (1,246) | 2,227 (770) | 2,079 (607) |
| RF | 5 | 347 (146) | 2,454 (1,247) | 2,098 (810) |
| IIIB | 4 | 185 (78) | 2,428 (1,060) | 2,255 (1,270) |
p24 release from ectocervical explants 22 days postinfection. Explants were either cultured in medium alone (control), prestimulated with PHA 2 days prior to viral exposure and restimulated 8 days postexposure, or only stimulated with PHA 8 days postexposure. Stimulated explants were cultured in the presence of IL-2. Infection with M-tropic HIV-1BaL was independent of culture conditions. In contrast, significant infection with T-tropic strains of HIV occurred only in explants stimulated with PHA. However, such productive infection could still be induced if PHA stimulation was delayed by 8 days after exposure to HIV. Data represent the mean from a minimum of four independent experiments using two or more explants from separate donors.
FIG. 2.
Replication of primary HIV isolates in cervical explant tissue. Ectocervical explants were exposed to HIV-1 isolates SL-2 (M-tropic, NSI, CCR5 restricted), 2044 (T-tropic, SI, CXCR4 restricted), or 2076 (dualtropic, SI, able to use CCR5, CCR3, and CXCR4) by immersion in cell-free virus (103 TCID50) for 2 h at 37°C. Subsequently explant tissue was washed in PBS, and tissue was directly transferred to 96-well plates, where they were cultured in medium alone (RPMI 10%) (open bars) or stimulated by culture in the presence of PHA (5 μg/ml) for 2 days and subsequently refed with medium containing IL-2 (filled bars). Data are shown as p24 antigen release (mean ± standard error) 14 days postinfection and represent the mean from a minimum of three independent experiments using paired explants from separate donors.
Primary target cells of HIV infection within genital mucosa.
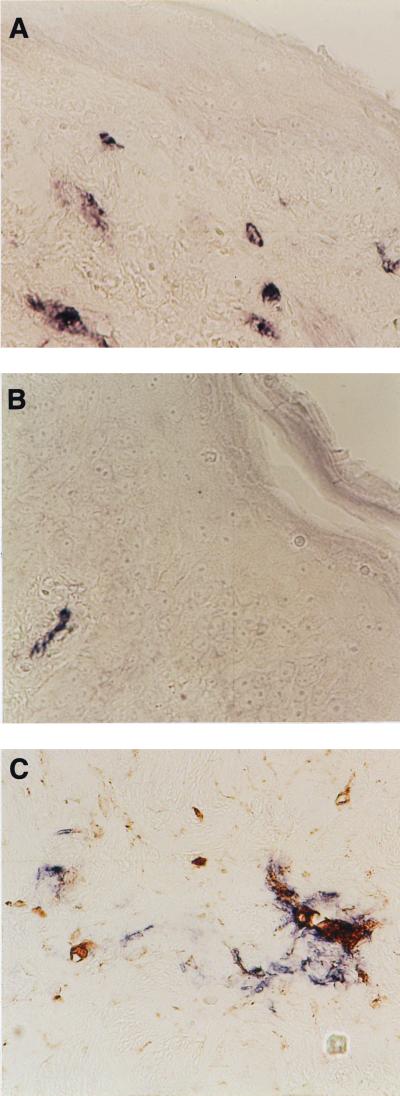
Mucosal explants were processed for immunohistochemistry 7 days after in vitro exposure to HIV isolates to determine the primary target cells of HIV infection within genital mucosa. In a previous study, we have described the normal cellular distribution of immune cells in such cultures (23). Such studies demonstrated that CD1a Langerhans cells are exclusively found in cervical eithelium, CD3 cells are predominantly detected in the mucosa, closely associated with the epithelium, and CD14/68-positive macrophages are restricted to subepithelial mucosa. Analysis of tissue infected with M-tropic HIV-1BaL, in the absence or presence of stimulation, demonstrated numerous cells, within cervical subepithelial mucosa, positive for HIV p24 expression (Fig. 3A). The majority (>90%) of these cells were dual positive for p24 expression and the macrophage marker CD68 (Fig. 3C). Few p24 cells were positive for CD3 expression, and there was no difference in their frequency between activated or resting explants. Some p24-positive cells were detected in immune activated cervical tissue exposed to T-tropic HIV isolates (IIIB and RF); however, their frequency was far lower than that seen with M-tropic strain of BaL, and it was not possible to determine their phenotype (Fig. 3B). HIV-infected epithelial cells or CD1a-positive Langerhans cells were not detected by immunohistochemistry in any HIV-exposed explants; furthermore, in all experiments no p24-positive cells were ever detected in the epithelium regardless of their phenotype.
FIG. 3.
(A and B) Immunohistochemical staining of p24 expression (blue/purple) in an ectocervical explant 7 days after infection with HIV-1BaL (A) and HIV-1RF (B). (C) Dual staining for p24 expression (blue/purple) and macrophage marker CD68 (brown) in an ectocervical explant 7 days after infection with HIV-1BaL. Original magnification for all panels, ×400.
Lack of evidence for epithelial infection or transcytosis of HIV.
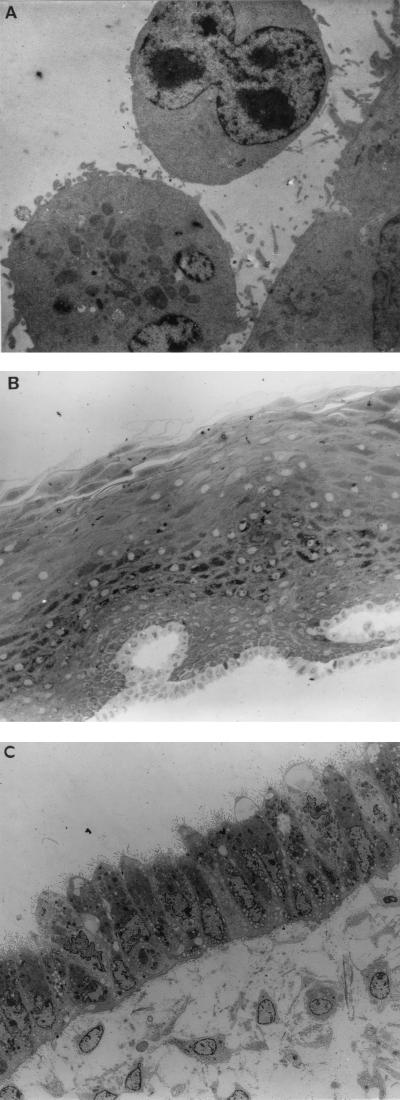
Further investigations were carried out to exclude a low level of HIV infection of genital epithelial cells. In vitro cultures of primary ectocervical and endocervical monolayers were established based on previously described methods (37). Epithelial monolayers were exposed to either cell-free M-tropic HIV-1BaL or T-tropic HIV-1RF, or to cell-associated HIV in the form of PM-1 cells (a T-cell line expressing both CCR5 and CXCR4 coreceptors) or human PBMC, chronically infected with either BaL or RF. The human intestinal epithelial cell line I407, previously demonstrated to be susceptible to HIV infection and to transcytose HIV (1, 25) was used as a positive control. Primary cultures of ectocervical and endocervical epithelial monolayers, unlike I407 cells, were resistant to HIV infection with either cell-free or cell-associated M- or T-tropic virus (Table 2). Furthermore, no evidence of endocytosis was detected by TEM of primary ectocervical, endocervical, or vaginal epithelium following 6 and 24 h of exposure to either cell-associated or cell-free HIV (data not shown). In contrast, I407 cells were susceptible to infection with cell-free or cell-associated HIV-1RF but resistant to HIV infection with either cell-free or cell-associated HIV-1BaL. Furthermore, while transient adherence of lymphocytes to I407 cells was detectable (Fig. 4A), adherence of such cells to primary genital epithelium was either very infrequent or not detectable by either light or electron microscopic analysis of primary epithelial cultures (Fig. 4B and C).
TABLE 2.
Primary cervical epithelial monolayers are resistant to HIV infectiona
| Origin of epithelial cells | Virus
|
No. positive/no. tested
|
||||
|---|---|---|---|---|---|---|
| Type | Strain | p24 antigen | PCR
|
Virus isolation by coculture | ||
| HIV LTR | β-Actin | |||||
| Ectocervix | ||||||
| Cell free | IIIB | 0/3 | 0/5 | 5/5 | 0/3 | |
| RF | 0/3 | 0/4 | 4/4 | 0/3 | ||
| BaL | 0/4 | 0/4 | 4/4 | 0/3 | ||
| Cell associated | RF | 0/5 | 0/5 | 5/5 | ND | |
| BaL | 0/8 | 0/8 | 8/8 | 0/6 | ||
| Endocervix | ||||||
| Cell free | IIIB | 0/3 | 0/6 | 6/6 | 0/3 | |
| RF | 0/5 | 0/4 | 4/4 | 0/5 | ||
| BaL | 0/7 | 0/8 | 8/8 | 0/6 | ||
| Cell associated | RF | 0/6 | 0/5 | 5/5 | 0/4 | |
| BaL | 0/7 | 0/7 | 7/7 | 0/7 | ||
| Intestine | ||||||
| Cell free | RF | 3/3 | 3/3 | 3/3 | 3/3 | |
| BaL | 0/3 | 0/3 | 3/3 | 0/3 | ||
| Cell associated | RF | 3/3 | 3/3 | 3/3 | 3/3 | |
| BaL | 0/6 | 0/6 | 6/6 | 0/6 | ||
Monolayers of primary ectocervical, endocervical, and intestinal (I407) epithelial cells were established as described in the text, and duplicate wells were exposed to either cell-free or cell-associated HIV-1BaL, HIV-1RF, or HIVIIIB for 2 h. Monolayers were washed at least five times with PBS and refed every 2 days. p24 antigen levels were monitored over 10 days by ELISA. After 10 days, either ectocervical and endocervical primary epithelial cells were trypsinized and the cell pellets tested for viral DNA, or they were cocultured with PM-1 cells for a further 10 days for viral isolation. p24 levels remained below detection in the period following exposure of primary cervical epithelial monolayers to virus, and after 10 days virus could not be detected by coculture or by PCR. Similar results were obtained when primary epithelial layers were exposed to cell-free or cell-associated virus for 24 h. Cell-associated virus in the form of chronically infected PM-1 cells or PBMC produced identical results. The intestinal cell line I407 became productively infected with both cell-free and cell-associated HIV-1RF but not HIV-1BaL. ND, not determined.
FIG. 4.
(A) TEM showing lymphocyte adherence to I407 epithelial monolayer (original magnification, ×7,800). Experiments representative of a minimum of five explants for each condition. (B) TEM showing ultrastructure of stratified cervical ectocervical epithelium following exposure to PM-1 T cells infected with HIV-1BaL (original magnification, ×260). The epithelial sheet has been removed from underlying stroma following overnight treatment with dispase. Epithelial tissue was exposed to cell-associated virus for 2 h in the described diffusion chamber and gently washed before processing for TEM. There is no evidence of adherence or penetration of PM-1 T cells into the epithelium (experiment representative of three). (C) TEM showing single endocervical epithelial monolayer following exposure to PBMC infected with HIV-1BaL (original magnification, ×1,200). Epithelial cells contain multiple mucus-containing vesicles and express multiple microvilli on their apical surface. Endogenous mononuclear cells can be seen in the underlying stromal tissue. The epithelial tissue was exposed to PBMC infected with HIV-1BaL for 2 h and processed as described above. There is no evidence of adherence of paracellular migration of donor PBMC (experiment representative of five).
Polarized genital epithelium is impervious to cell-free or cell-associated HIV.
To determine whether HIV can cross intact genital epithelium, epithelial sheets were polarized using a diffusion chamber allowing independent bathing of apical and basolateral epithelial surfaces as previously described (12). Such a system facilitated apical exposure of epithelial surfaces to HIV, as presented in vivo. Ectocervical stratified epithelial sheets were isolated following overnight digestion with dispase as previously described. Integrity of epithelial surfaces was assessed prior to experiments by direct light microscopic examination of the mucosal surface and permeability assessed during experiments by [14C]inulin diffusion. Polarized cultures of ectocervical epithelial sheets were exposed to cell-free or cell-associated HIV as described above. Measurement of basolateral p24 release and infectious coculture assays demonstrated that stratified epithelium, which excluded [14C]inulin, was impervious to cell-free or cell-associated HIV (Table 3). Furthermore, TEM analysis of polarized ectocervical and endocervical tissue exposed to either cell-free or cell-associated HIV demonstrated no evidence of HIV transcytosis or paracellular penetration of either cell-free HIV or HIV-infected cells. In addition, no interaction between exogenous HIV-infected mononuclear cells and epithelium was observed. Lack of paracellular penetration of cervical epithelial sheets by HIV-infected mononuclear cells was confirmed by demonstration that epithelial sheets were negative, by PCR, for proviral DNA following exposure to such infected cells (data not shown).
TABLE 3.
Polarized ectocervical epithelium is impervious to HIVa
| Epithelium | Virus | No. of patients | Mean % apical (SEM)
|
|
|---|---|---|---|---|
| [14C]inulin | p24 antigen | |||
| Intact | Cell free | 6 | 0.01 (0.00) | 0.03 (0.01) |
| Cell associated | 6 | 0.00 (0.00) | 0.03 (0.02) | |
| Perforated | Cell free | 4 | 3.20 (1.11) | 3.67 (1.86) |
| Cell associated | 3 | 4.98 (2.95) | 3.96 (1.35) | |
Ectocervical epithelial layers were placed in diffusion chambers, and the apical side was exposed to either cell-free or cell-associated HIV-1BaL in culture medium containing [14C]inulin for 2 h. Medium was withdrawn from apical and basolateral chambers, and the epithelium was removed. Medium was tested for the presence of [14C]inulin and p24 antigen (data shown as basolateral percentage of apical concentration) and cocultured with PM-1 cells for 10 days for viral isolation. Epithelial layers were processed for TEM. When epithelial layers were intact, no [14C]inulin or p24 antigen was detected in the basolateral chambers and no virus was detected by coculture. However, if epithelial integrity was disrupted, [14C]inulin and p24 antigen could be detected in basolateral chambers and virus could be detected by coculture in all samples. Similar results were obtained using cell-free (n = 5) or cell-associated (n = 3) HIV-1RF, both chronically infected PM-1 cells and PBMC produced identical results.
Vaginal virucides block HIV infection of genital mucosa.
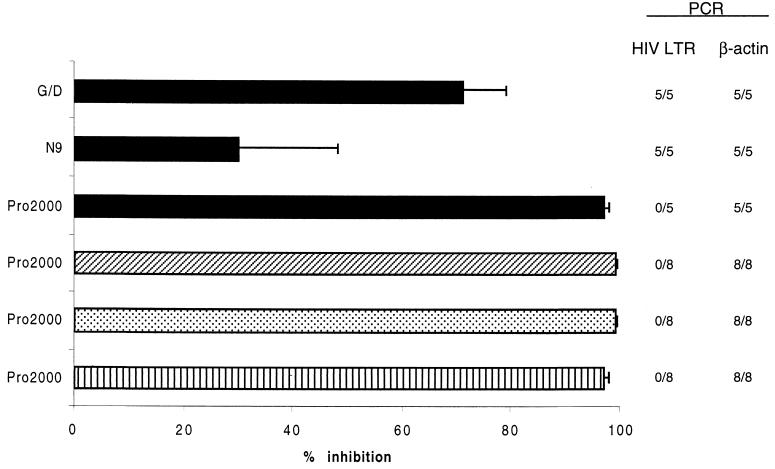
Further investigations were carried out to determine the efficacy of potential virucides to block HIV infection of cervical explants. Compounds included nonoxynol-9 (N-9; a nonionic surfactant known to be active against HIV and other sexually transmitted agents [36]), gramicidin (GD; a peptide antibiotic with virucidal activity [2]), and PRO 2000 (a naphthalene sulfonate polymer that appears to disrupt the initial binding and fusion events of HIV infection [32]). Agents were tested (range, 100 to 0.01 μg/ml) for potential tissue toxicity following overnight culture with ectocervical explants. Viability of cervical explants, assessed by MTT assay, was reduced following exposure to N-9 or GD at concentrations of >1 μg/ml, while PRO 2000 demonstrated no toxicity at 100 μg/ml (data not shown). As potential virucidal agents should demonstrate no tissue toxicity, which could reduce barrier effects of genital epithelium, for all subsequent investigations we used N-9 and GD at 1 μg/ml and PRO 2000 at 100 μg/ml. To determine the efficacy of these agents to block HIV infection of cervical tissue, explants were preincubated with virucides for 1 h prior to overnight exposure to virus in the continued presence of virucidal agent. Explants were exposed to virus in a nonpolarized manner, allowing direct access to both subepithelial and epithelial cells, analogous to conditions of compromised genital epithelium in vivo. Following overnight exposure to virus, explants were washed to remove any exogenous virus and virucidal agent and subsequently cultured for 10 days in the presence or absence of activating conditions prior to measurement of p24 production and proviral accumulation. Presence of N9, GD, and PRO 2000 during exposure of ectocervical explants to HIV-1BaL (105 TCID50/ml) resulted in a 30%, 71%, and 97% inhibition of p24 production, respectively. PRO 2000 was the only agent to completely block proviral formation, as determined by PCR, and this agent was also able to efficiently block HIV infection with three other primary HIV strains, SL-2, 2044, and 2076, under activating conditions (PHA and IL-2) (Fig. 5).
FIG. 5.
Effect of vaginal virucides on HIV-infected genital mucosa. Cervical explants were preincubated with the virucidal agent N-9 (1 μg/ml), GD (1 μg/ml), or PRO 2000 (100 μg/ml) for 1 h prior to overnight exposure to HIV-1 strain BaL (■; 105 TCID50/ml), SL2 (▨), 2044 (░⃞), or 2076 (▥) (all 103 TCID50/ml) in the continued presence of the virucidal agent. Explants were exposed to virus in a nonpolarized manner, allowing direct access to both subepithelial and epithelial cells. Following overnight exposure to viral inoculum, explants were washed extensively in PBS, stimulated by culture in the presence of PHA (5 μg/ml) for 2 days, subsequently refed with medium containing IL-2, and cultured for 14 days. Data represent percent inhibition of p24 production at day 14 of culture, compared to control cultures exposed to virus in the absence of virucidal agent, and are presented as the mean of five independent experiments using explants from separate donors. Detection of proviral DNA by PCR at the right is shown as number of experiments with detectable proviral DNA out of the total tested (limit of detection, 10 copies/explant).
DISCUSSION
HIV infection of genital mucosa is enhanced by immune activation.
The observation that immune activation (PHA and IL-2) enhanced HIV infection, using a range of viral isolates (RF, IIIB, 2044, 2076, and SL-2), suggests that transmission and productive infection of genital mucosa are likely to be enhanced by concomitant immune activation in vivo. Thus, infection with STDs resulting in local inflammation, and activation of local inflammatory cells potentially renders an individual more susceptible to HIV infection. Such observations fit with reported epidemiological evidence of a correlation between STDs and HIV infection (10, 19), although this was not observed by others (41). Furthermore, our in vitro studies are supported by recent studies demonstrating an association between STDs and increased CD4 levels and coreceptor expression in genital mucosa (16, 24, 30). Demonstration that these isolates can establish productive HIV infection of cervical explants even if immune activation is delayed up to 8 days after in vitro exposure suggests that immune stimulation caused by venereal infection in vivo potentially facilitates and amplifies localized HIV infection subsequent to the transmission event. It is beyond the scope of this experimental model to determine how long such a reservoir of localized HIV infection may persist; however, the potential ability of venereal infection to activate localized HIV infection in this manner has serious implications for transmission of HIV.
In contrast to the isolates mentioned above, HIV-1BaL (able to use both CCR5 and CCR3 coreceptors) infected and replicated in both immunologically silent and activated cervical tissue with equal efficiency. The ability of HIV-1BaL to replicate efficiently in cervical tissue, without immune activation, is unlikely to be coreceptor dependent, determined by V3 hypervariable regions of the viral envelope, since both SL-2 and 2076 can efficiently utilize CCR5, and 2076 can utilize CCR3 (34). Furthermore, such preferential replication is unlikely to be dependent on macrophage tropism, as SL-2, 2044, and 2075 can all infect macrophages in vitro (34). It is possible that the observed preferential replication of HIV-1BaL may reflect expression of sequences within V1 and V2 hypervariable regions that appear to modulate efficiency of viral spread in macrophages (39). Indeed, recent observations that a concentration-dependent direct or indirect interaction between CCR5 and CD4 governs HIV infection of macrophages (26) suggests that in the absence of increased CD4 and CCR5 levels stimulated by concomitant immune activation (16, 24, 30), such regions outside V3 may provide a selective advantage for infection of genital tissue. The contribution of such regions to HIV infection of cervical tissue is the subject of ongoing investigations. However, primary isolates of HIV demonstrating a similar replicative fitness for infection of genital tissue macrophages in vivo would have a selective advantage for both infection and amplification within genital mucosa and would be likely to predominate in peripheral blood during acute infection. This would be in keeping with a selective amplification model of HIV transmission (33).
In contrast, isolates lacking such a potential selective advantage for replication in mucosal tissue might remain localized in genital mucosa at undetectable levels until amplified by immune activation following coincidental or subsequent infection with other STDs. Such findings correlate with the observation that recently infected women display a genotypic diversity in HIV populations isolated from genital secretions not reflected in peripheral blood (28).
Prime target cells for HIV infection of the female genital tract.
The described study has confirmed that subepithelial cells are the prime target cells for HIV infection in female genital tract mucosal tissue in organ culture. Furthermore dual immunohistochemistry identified the majority of HIV-infected cells as subepithelial macrophages. Thus, these cells may represent the main target cells for HIV infection in the female genital tract. There was no evidence of HIV infection of cells within the cervical epithelium. This is in keeping with numerous previous studies demonstrating that following vaginal transmission of either HIV or SIV, infection is exclusively restricted to the subepithelial cells (20, 21, 23, 27, 35). The lack of HIV infection within the epithelium is highly likely to reflect recent observations that expression of CCR5, CCR3, and CXCR4 in cervical tissue is predominantly restricted to subepithelial cells (24, 44). However, it cannot be excluded that cell populations within the epithelium can harbor viral infection below limits of detection by immunohistochemistry.
Resistance of human endocervical, ectocervical, and vaginal epithelial cells to HIV infection, on exposure to cell-free or cell-associated HIV, was confirmed by PCR (able to detect 10 copies per 105 cells), infectious coculture, and p24 release using primary epithelial cell lines. While an extremely low level of infection cannot be completely excluded, such observations contrast to previous reports of productive infection utilizing transformed epithelial cell lines (38). Chenine et al. recently reported that intestinal epithelial cells could be productively infected with laboratory (HIV-1NDK) but not primary HIV isolates (4). Such data suggest that strain differences may determine differing tropism for epithelial cells. However, our observation that HIV-1RF and HIV-1NDK (data not shown), able to infect intestinal cells, did not infect cervical epithelial cells suggests that the adaptation required for infection of intestinal cells is not transferable to those of the cervix. Such differences are more likely to reflect the lack of CXCR4 expression within the cervical epithelium (24, 44), previously demonstrated to be required for infection of intestinal epithelial (6). These results are in agreement with the consistent inability to detect HIV infection of genital epithelial cells in vivo (20, 21, 23, 27, 35) but contrast to reported detection of infected intestinal cells in vivo (17). One study has previously suggested that genital epithelial cells may be susceptible to HIV infection ex vivo (13), as demonstrated by immunohistochemical localization. Differences between this previous report and ours demonstrating a lack of infection by immunohistochemistry, proviral PCR, p24 production, and infectious coculture assay may reflect differences in the purity of epithelial cultures and/or effectiveness in the elimination of nonspecific sticking of virus to the membrane of epithelial cells in the absence of productive infection. While transcytosis of HIV has been demonstrated in intestinal epithelial cell lines (1), extrapolation of these mechanisms to heterosexual transmission via intact genital mucosa would be premature. Indeed, data presented in this study demonstrate no evidence for transcytosis of HIV across primary human endocervical, ectocervical, and vaginal epithelial cell layers, strongly suggesting that transcytosis is not a major mechanism of transepithelial penetration across the female genital mucosa. Such findings are perhaps unsurprising since a principal strategic function of genital epithelium is protection from infection. Indeed, intestinal epithelial cells bear little resemblance to genital epithelial cells, being derived from endoderm, expressing no keratin markers, lacking stratification, and unlike genital epithelium, having a highly active endocytic phenotype. Thus, it is unlikely that genital epithelium would take on the transport function of specialized intestinal cells such as enterochromaffin cells reported to be infected in the rectal mucosa of HIV-positive subjects (17).
Other studies, using epithelial cell lines or animal models, have suggested that donor HIV-infected cells may themselves invade genital mucosa (14, 43). In this study, we observed that while PBMC transiently adhered to the I407 intestinal epithelial cell line, adherence to primary endocervical or ectocervical epithelial cultures was not detected. Furthermore, there was no evidence of HIV-infected donor cell migration into either ectocervical, endocervical, or vaginal tissue, as assessed by light and electron microscopy and by PCR for proviral DNA. Thus, an active role for either epithelia or migration of donor cells in transmucosal penetration of HIV appears unlikely in our in vitro model. In contrast, the weight of evidence presented in this study suggests that intact normal cervical and vaginal epithelial cells, in the absence of inflammatory stimuli, provide a barrier to both cell-free and cell-associated HIV. Thus, transmission of HIV infection at such tissue sites is likely enhanced by any physical breach in epithelial integrity, such as might be caused by physical abrasion, ulceration, or inflammation. This conclusion is supported by observations that factors which have the potential to decrease epithelial integrity, in particular, (i) epithelial ulceration following venereal infection and (ii) cervical ectopy which may leave tissue more friable, are associated with increased rates of HIV transmission (31, 33). Furthermore, while this study provides no evidence of HIV infection of epithelial Langerhans cells, their potential role in passive transfer of HIV across genital epithelium cannot be excluded (29).
The demonstration in this study that the target cells for HIV infection in genital mucosal tissue are exclusively found directly below genital epithelium, and that genital epithelial cells are resistant to HIV infection, strongly suggest that intact genital epithelium provides a barrier to HIV transmission. Strategies designed to protect genital epithelium are highly likely to have a major impact on heterosexual transmission rates. In this respect, aggressive syndromic management of STDs has been demonstrated in a Tanzanian trial to reduce HIV transmission rates by 42% (10). Entry of HIV through epithelial breaches would be unlikely to provide selective genotypic or phenotypic pressure on the heterogeneity of transmitted virus. Indeed, data reported here suggest that such pressure is likely to come from localized levels of immune activation, influencing coreceptor expression, and/or selective amplification of strains able to efficiently replicate in genital tissue in the absence of immune stimulation. Thus, potential virucides designed to block HIV infection should demonstrate no tissue toxicity, which could reduce barrier effects of genital epithelium, should not induce inflammation, and should be active even when epithelial integrity is compromised and/or under inflammatory conditions. Recent studies to evaluate safety and tolerability of intravaginal N-9 have suggested that its use may cause adverse effects including inflammation and reduction in numbers of lactobacilli (36). Thus, in this study, all virucidal agents were used at concentrations demonstrated not to be toxic to cervical tissue. N-9 and GD, when used at nontoxic concentrations, did not provide complete protection from HIV infection in vitro. In contrast, the virucidal agent PRO 2000 at a concentration of 100 μg/ml efficiently blocked HIV infection of cervical tissue under conditions which mimic both compromised epithelial integrity and inflammatory conditions. Previous studies have detected up to 107 HIV-1 RNA copies/ml of semen (11); however, this is unlikely to reflect the level of infectious virions. Indeed, quantitative microculture methods have demonstrated levels of only up to 104 infectious units per ejaculate (7). Thus, the demonstration that PRO 2000, at a concentration of 100 μg/ml, provided complete protection against a viral inoculum with a TCID50 of 105/ml suggests that this agent is highly likely to provide protection against any natural inoculum. Furthermore, such studies were carried out using a concentration of PRO 2000 that is 400 times less than that currently proposed for intravaginal use.
These studies demonstrate that the described cervical explant model of HIV infection represents a suitable model for evaluation of potential virucidal agents. Furthermore, this is the first demonstration that virucidal agents can effectively block HIV infection of genital tissue. In the absence of the imminent approval of an effective mucosal vaccine, use of such virucidal agents, designed to provide women with unobtrusive protection, is likely to have a major impact on global heterosexual transmission rates and may also prove useful in prevention of vertical transmission.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We are grateful to A. Wilson and R. Moss for electron microscopy, to Isaac Manyonda and Deborah Moncrieff and the Obstetrics and Gynaecology Departments of St. George's and St. Helier's Hospitals for assistance in obtaining cervical tissue, and to C. Corbishley and staff of the Histopathology Department at St. George's Medical School. We thank Paul Clapham (Institute for Cancer Research, London) for viral isolates SL2, 2044, and 2076, Procept for donation of PRO 2000, and London International Group for N9. We also thank the Medical Research Council (MRC) AIDS Reagent Project for supply of many reagents used in this study.
This work was supported by MRC grants G9428495 and G9828308 and is part of the MRC cooperative grant on intracellular pathogens (COGG-G9814061).
REFERENCES
- 1.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 2.Bourinbaiar A, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9, and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 3.Burkhard M, Obert L, O'Neil L, Diehl L, Hoover E. Mucosal transmission of cell-associated and cell-free feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13:347–355. doi: 10.1089/aid.1997.13.347. [DOI] [PubMed] [Google Scholar]
- 4.Chenine A, Matouskova E, Sanchez G, Reischig J, Pavlikova L, LeContel C, Chermann J, Hirsch I. Primary intestinal epithelial cells can be infected with laboratory-adapted strain HIV type 1 NDK but not with clinical primary isolates. AIDS Res Hum Retroviruses. 1998;14:1235–1238. doi: 10.1089/aid.1998.14.1235. [DOI] [PubMed] [Google Scholar]
- 5.Collin M, James W, Gordon S. Development of techniques to analyse the formation of HIV provirus in primary human macrophages. Res Virol. 1991;142:105–112. doi: 10.1016/0923-2516(91)90045-5. [DOI] [PubMed] [Google Scholar]
- 6.Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 1997;11:1311–1318. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Dyer J, Gilliam B, Eron J, Grossi L, Cohen M, Fiscus S. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with qantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 8.Elias C, Coggins C. Female-controlled methods to prevent sexual transmission of HIV. AIDS. 1996;10:S43–S51. [PubMed] [Google Scholar]
- 9.Esser M, Toshiyuki M, Mondor I, Sttentau Q, Dey B, Berger E, Boyd M, Lifson J. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type-1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73:4360–4371. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, Mayaud P, Changalucha J, Nicoll A, ka-Gina G, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Mellors J, Kingsley L, Riddler S, Singh M, Schreiber S, Cronin M, Rinaldo C. Hifh viral load in semen of human immunodeficiency virus type-1 infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Infect Dis. 1998;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert C, Edwards D, Boot J, Robinson C. In vitro modulation of the eosinophil-dependent enhancement of the permeability of the bronchial mucosa. Br J Pharmacol. 1991;104:391–398. doi: 10.1111/j.1476-5381.1991.tb12441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell A, Edkins R, Rier S, Yeaman G, Stern J, Fanger M, Wira C. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–3506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibata B, Parr E, King N, Parr M. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol Reprod. 1997;56:537–543. doi: 10.1095/biolreprod56.2.537. [DOI] [PubMed] [Google Scholar]
- 15.Kitano Y, Okada N. Separation of the epidermal sheet by dispase. Br J Dermatol. 1983;108:555–560. doi: 10.1111/j.1365-2133.1983.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 16.Levine W, Pope V, Bhoomkar A, Tambe P, Lewis J, Zaidi A, Farshy C, Mitchell S, Talkington D. Increase in endocervical CD4 lymphocytes amoung women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–174. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 17.Levy J, Margaretten W, Nelson J. Detection of HIV in enterochromaffin cells in the rectal mucosa of an AIDS patient. Am J Gastroenterol. 1989;84:787–789. [PubMed] [Google Scholar]
- 18.Marx P, Spira A, Gettie A, Dailey P, Veazey R, Lackner A, Mahoney C, Miller C, Claypool L, Ho D, Alexander N. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 19.Miller C. Host and viral factors influencing heterosexual HIV transmission. Rev Reprod. 1998;3:42–51. doi: 10.1530/ror.0.0030042. [DOI] [PubMed] [Google Scholar]
- 20.Miller C, Vogel P, Alexander N, Sutjipto S, Hendickx A, Marx P. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 21.Nuovo G, Forde A, MacConnell P, Fahrenwald R. In situ detection pf PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Padian N, Shiboski S, Glass S, Vittinghoff E. Heterosexual transmission of human immunodeficiency viruss (HIV) in northern California: results of a ten-year study. Am J Epidemiol. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 23.Palacio J, Souberbielle B, Shattock R, Robinson G, Manyonda I, Griffin G. In vitro HIV-1 infection of human cervical tissue. Res Virol. 1994;145:155–161. doi: 10.1016/s0923-2516(07)80017-3. [DOI] [PubMed] [Google Scholar]
- 24.Patterson B, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski M, Garcia P. Repertoire of chemokine receptor expression in the female genital tract. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips D, Bourinbaiar A. Mechanism of HIV spread from lymphocytes to epithelia. Virology. 1992;186:261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 26.Platt E, Wehrly K, Kuhmann S, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immundeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomerantz R, de la Monte S, Donegan S, Rota T, Vogt M, Craven D, Hirch M. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- 28.Poss M, Martin H, Kreiss J, Granville L, Chohan B, Nyange P, Mandaliya K, Overbaugh J. Diversity of populations from genital secretions and peripheral blood from women recently infected with human immunodeficiency virus type 1. J Virol. 1995;69:8118–8122. doi: 10.1128/jvi.69.12.8118-8122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reece J, Handley A, Anstee E, Morrison W, Crowe S, Cameron P. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rottman J, Ganley K, Williams K, Wu L, Mackay C, Ringler D. Cellular localization of the chemokine receptor CCR5. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- 31.Royce R, Sena M, Cates W, Cohen M. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 32.Rusconi S, Moonis M, Merrill D, Pallai P, Neidhardt E, Singh S, Willis K, Osburne M, Profy A, Jenson J, Hirsch M. Naphthalene sulphonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob Agents Chemother. 1996;40:234–236. doi: 10.1128/aac.40.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shattock R, Griffin G. Mucosal transmission of human immunodeficiency virus. In: Lever A, editor. The molecular biology of HIV/AIDS. Chichester, England: John Wiley & Sons, Ltd.; 1996. pp. 25–38. [Google Scholar]
- 34.Simmons G, Reeves J, McKnight A, Dejucq N, Hibbitts S, Power C, Aarons E, Schols D, Clercq E, Proudfoot A, Clapham P. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–84577. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–25. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stafford M, Ward H, Flanagan A, Rosenstein I, Taylor-Robinson D, Smith J, Weber J, Kitchen V. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Stanley M, Greenfield I. Culture of human cervical epithelial cells. In: Freshney R, editor. Culture of epithelial cells. New York, N.Y: Wiley-Liss, Inc.; 1992. pp. 135–158. [Google Scholar]
- 38.Tan X, Philips D. Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch Virol. 1996;141:1177–1189. doi: 10.1007/BF01718823. [DOI] [PubMed] [Google Scholar]
- 39.Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. Human immunodeficiency envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology. 1995;213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- 40.Van't Wout A, Kootstra N, Mulder-Kampinga G, Lent N, Scherpbier H, Veenstra J, Boer K, Coutinho R, Miedema F, Schuitemaker H. Macrophage-tropic varients initiate human immunodeficiency virus type-1 infection after sexual, parenteral and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wawer M, Sewankambo N, Serwadda D, Quinn T, Paxton L, Kiwanuka N, Wabwire-Mangen F, Li C, Lutalo T, Nalugoda F, Gaydos C, Moulton L, Meehan M, Ahmed S, Gray R. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomized community trial. Lancet. 1999;353:525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 42.Wortley P, Flemming P. AIDS in women in the United States. JAMA. 1997;278:911–916. [PubMed] [Google Scholar]
- 43.Zacharopoulis V, Perotti M-E, Phillips D. A role for cell migration in the sexual transmission of HIV-1. Curr Biol. 1997;7:534–537. doi: 10.1016/s0960-9822(06)00225-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, He T, Talal A, Wang G, Frankel S, Ho D. In vivo distribution of human immunodeficiency virus/simian immunodeficiency virus coreceptors:CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]