The clinical effectiveness of antiretroviral therapy has improved markedly over the last few years. Since 1996 in the developed world there have been dramatic falls in the incidence of new AIDS cases and AIDS associated deaths. Published data in the late 1990s estimated the mortality rate in patients with CD4 counts of less than 100 × 106/l had fallen by nearly two thirds to <8 per 100 patient years. Although the long term clinical efficacy of the current antiretroviral treatment regimens remains uncertain, the biological rationale for maintaining a clinical response has been established. Sustained inhibition of viral replication results in partial reconstitution of the immune system in most patients, substantially reducing the risk of clinical disease progression and death. Reservoirs of HIV in latently infected resting T lymphocytes and other long lived cell populations make it unlikely that HIV can be eradicated by antiretroviral therapy alone. Strategies to sustain suppression of viral replication in the long term will be necessary.
This article has been adapted from the forthcoming 5th edition of ABC of AIDS. The book will be available from the BMJ bookshop and at www.bmjbooks.com
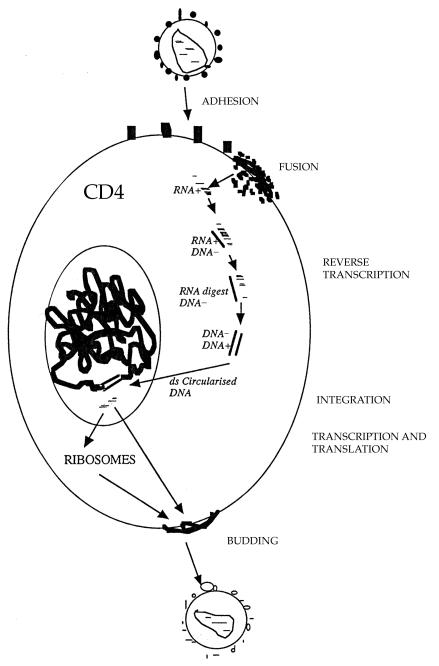
There are several potential targets for antiretroviral drugs in the viral replication cycle. Three classes of antiretroviral drugs are currently used in combination for the treatment of HIV infection, which target the activity of two viral enzymes. New therapeutic agents are constantly being evaluated.
Targets for antiretroviral therapy
| Target | Treatment |
|---|---|
| Virus receptor and entry | Fusion inhibitors, chemokine receptor blockers |
| Reverse transcriptase | Inhibitor/DNA chain terminators |
| RNAase | Inhibitors |
| Integration | Viral integrase inhibitors |
| Viral gene expression | Inhibitors of HIV regulatory genes and their products |
| Viral protein synthesis | Enzyme inhibitors, eg protease inhibitors |
| Viral budding | Interferons (also act at other sites of replication cycle), antibodies, and ligands |
Reverse transcriptase inhibitors
The first drugs made available for clinical use were inhibitors of the HIV reverse transcriptase. Before the virus can be integrated into the host cell genome DNA, a copy of the viral RNA has to be formed (proviral DNA). This is regulated by the specific HIV DNA polymerase: reverse transcriptase. If a DNA copy is not formed, the viral RNA genome becomes susceptible to destruction by cellular enzymes.
The nucleoside reverse transcriptase inhibitors are both competitive inhibitors of reverse transcriptase and DNA chain terminators. The normal 2′ deoxynucleosides which are substrates for DNA synthesis link to form a chain by phosphodiester linkages bridging the 5′ and 3′ positions on the five carbon sugar molecule. The 2′, 3′-dideoxynucleoside analogues are formed by the replacement of the 3′-hydroxy group by an azido (zidovudine), hydrogen, or other group. These nucleoside analogues as substrates will bind to the active site of the HIV reverse transcriptase and will be added to the growing HIV proviral DNA chain. However, once inserted, the normal 5′ to 3′ links will not occur, resulting in HIV proviral DNA chain termination.
Genotypic mutations at various codons in the reverse transcriptase gene result in decreased susceptibility of HIV to inhibition by the nucleoside reverse transcriptase inhibitors. Several nucleoside reverse transcriptase inhibitors are currently licensed for the treatment of HIV infection in combination regimens, and newer agents with better tolerability and resistance profiles are under evaluation.
The non-nucleoside reverse transcriptase inhibitors are a group of structually diverse agents which bind to reverse transcriptase at a site distant to the active site resulting in confirmational changes at the active site and inhibition of enzyme activity. These agents show high antiviral activity in vitro and have relatively low toxicity. They are also highly specific, inhibiting the reverse transcriptase of HIV-1 but not HIV-2. In monotherapy, rapid emergence of resistant strains associated with single point mutations of the reverse transcriptase gene, high level phenotype resistance and loss of antiviral effect occurs. The drugs therefore need to be combined with other antiretroviral agents, usually two nucleoside reverse transcriptase inhibitors, to achieve and maintain an effective long term treatment response.
Antiretroviral regimens
2 nucleoside reverse transcriptase inhibitors, eg, zidovudine or stavudine + lamivudine or didanosine
Plus either
1 non-nucleoside reverse transcriptase inhibitor: nevirapine or efavirenz
or
1 protease inhibitor: indinavir, nelfinavir or saquinavir soft gel
or
2 protease inhibitors, eg, ritonavir + saquinavir
3 nucleoside reverse transcriptase inhibitors: zidovudine + lamivudine + abacavir
Recommended antiretroviral regimens for the initial treatment of chronic infection in adults (2001). Choice will depend on efficacy, tolerability, adherence, and resistance profile of regimen. Treatment guidelines are constantly reviewed and updated.
Protease inhibitors
The protease inhibitors bind competitively to the substrate site of the viral protease. This enzyme is responsible for the post-translational processing and cleavage of a large structural core protein during budding from the infected cell. Inhibition results in the production of immature virus particles. Their potent anti-HIV activity and introduction to clinical use from 1996 was one of the main reasons for the observed substantial falls in morbidity and mortality associated with HIV infection in the developed world. However, tolerability, relatively high pill burden, and poor adherence were frequent problems with the initial protease inhibitor containing regimens. Specific genotypic mutations in the protease gene can result in high levels of phenotype resistance to individual protease inhibitors and cross resistance. New protease inhibitors are under evaluation.
Treatment of chronic adult infection
In the mid-1990s, several large clinical endpoint studies demonstrated a strong association between falls in plasma HIV RNA levels (plasma viral load) in the first few weeks on therapy and clinical outcome at one year. It is now accepted that falls in plasma viral load combined with increases in CD4 count are predictive of the clinical treatment response on different combination regimens at 1-2 years, although changes in the markers probably do not fully predict the observed clinical effect.
Factors determining when to start and choice of therapy
Risk of clinical disease progression (CD4 count, viral load)
Willingness of patient to start therapy
Clinical effectiveness of combination regimen
Ability and motivation of patient to adhere to therapy
Drug toxicity profile
Pill burden and dosing schedule
Transmitted drug resistance
Future therapy options
Likelihood of drug resistance
Drug-drug interactions
Where possible an objective of antiretroviral therapy is to reduce and sustain plasma viral load levels to below the level of detectability of the current ultrasensistive viral load assays (<50 copies/ml). If patients are adherent to therapy, the likelihood of a viral load rebound and drug resistance is minimal. Despite inhibition of viral replication in plasma, lymph nodes, and at other sites, reservoirs of HIV infection in latently infected resting T lymphocytes remain. Continued activation of these cells will theoretically result in the reduction of this reservoir, however new cells probably continue to be infected as a result of either localised small bursts of viral replication or loss of the antiretroviral effect of the treatment regimen. Even in patients who have sustained, undetectable levels of plasma viral load (<50 copies/ml) for three years or more, discontinuation of antiretroviral therapy results in rapid rebound of plasma viral load to pretreatment levels.
Recommendations for starting antiretroviral therapy in adults: 2001
| Disease stage | BHIVA | USDHHS |
|---|---|---|
| Symptomatic | Treat | Treat |
| Asymptomatic | ||
| CD4 <200 × 106/l | Treat | Treat |
| CD4 200-350 × 106/l | Consider therapy depending on rate of CD4 count decline, symptoms, and patient's wishes | Therapy should generally be offered |
| CD4 >350 × 106/l | Defer | Defer or consider therapy if high viral load |
BHIVA, British HIV Association; USDHHS, United States Department of Health and Human Services.
The optimal time to initiate therapy with the current antiretroviral drugs has not been established in clinical studies. CD4 count and plasma viral load are predictors of the estimated risk of progression to AIDS, which is a factor in determining when to start treatment. The motivation of a patient to start and adhere to therapy and the known effectiveness of current regimens are also important. Clinical practice across Europe and North America varies, but most clinicans would consider initiating therapy at some point when the CD4 count is 200-350 × 106/l and in all patients who are symptomatic. Even in patients who initiate therapy with CD4 counts of <100 × 106/l, substantial increases in CD4 count and clinical benefit can be achieved. Patients on therapy should have CD4 count and plasma viral load levels monitored at regular intervals. On effective therapy, plasma viral load falls rapidly as viral replication is inhibited. By four weeks a fall of greater than 1 log and by 3-6 months a fall to <50 copies/ml should be expected.
Drug toxicities
The tolerability and side effects of a combination regimen is very important in determining the antiviral response. In clinical practice 40-50% of patients will not have sustained falls in plasma viral load by one year of therapy and a major factor contributing to this is poor tolerability.
Drug toxicities
| Drug | Toxicity |
|---|---|
| Nucleoside reverse transcriptase inhibitors | |
| Class associated | Lactic acidosis |
| Hepatitic steatosis | |
| Lipodystrophy (peripheral fat wasting) | |
| Drug specific: | |
| Zidovudine | Bone marrow suppression, nausea, vomiting, myopathy |
| Stavudine | Peripheral neuropathy, hepatitis |
| Zalcitabine | Peripheral neuropathy, mouth ulcers |
| Didanosine | Pancreatitis, dry mouth, peripheral neuropathy |
| Lamivudine | Few side effects |
| Abacavir | Hypersensitivity reaction, nausea |
| Non-nucleoside reverse transcriptase inhibitors | |
| Nevirapine | Rash, hepatitis, Steven-Johnson syndrome |
| Efavirenz | Rash, dysphoria, mood changes, vivid dreams, hypercholesterolaemia |
| Protease inhibitors | |
| Class specific | Lipodystrophy (fat wasting or accumulation) |
| Hyperlipidaemia, diabetes mellitus | |
| Drug specific: | |
| Nelfinavir | Diarrhoea, rash |
| Saquinavir | Few side effects |
| Indinavir | Hyperbilirubinaemia, nephrolithiasis, nail changes, dry skin |
| Ritonavir | Perioral dysathesia, flushing, hepatitis, diarrhoea, nausea, vomiting |
| Amprenavir | Rash, nausea, diarrhoea |
| Lopinavir | Diarrhoea |
Future agents
For the reasons of poor tolerability, suboptimal antiviral potency, and long term drug toxicity, it is important that new antiretroviral agents and therapeutic strategies are developed and evaluated. New formulations of current drugs which improve tolerability and reduce pill burden will help to improve adherence in patients. New protease and reverse transcriptase inhibitors which in vitro appear to be effective against viral isolates which are resistant to different drugs are currently undergoing clinical trials. Whether these agents will prove to be clinically effective will be important in treating those patients who have previously failed combination therapies.
New classes of drugs are also being developed. Fusion inhibitors which block the activity of the GP41 viral transmembrane protein are in Phase III clinical trials and are likely to be the first new class of drug to reach the bedside.
As well as specific drugs that inhibit targets in the viral replication cycle, immunotherapeutic approaches are also being assessed. Treatment with cycles of the cytokine interleukin 2 results in substantial increase in CD4 counts but has little effect on plasma viral load levels. Interleukin 2 may also improve immune responses to HIV and a large randomised international trial is under way to assess its efficacy in combination with effective antiretroviral combination regimens. Therapeutic vaccines are also under evaluation which might improve specific immune responses and assist immunological control of HIV replication. Their clinical effectiveness remains uncertain.
Few areas of medicine have seen such dramatic changes in treatment, with a resulting reduction in morbidity and mortality, as there has been in the management of HIV infection. It is very likely that therapeutic options will continue to improve, although the long term efficacy of treatment over many years still remains uncertain.
Figure.
HIV replication