Abstract
Herpes simplex virus (HSV) has often been suggested as a suitable vector for gene delivery to the peripheral nervous system as it naturally infects sensory nerve terminals before retrograde transport to the cell body in the spinal ganglia where latency is established. HSV vectors might therefore be particularly appropriate for the study and treatment of chronic pain following vector administration by relatively noninvasive peripheral routes. However parameters allowing safe and efficient gene delivery to spinal ganglia following peripheral vector inoculation, or the long-term expression of delivered genes, have not been comprehensively studied. We have identified combinations of deletions from the HSV genome which allow highly efficient gene delivery to spinal dorsal root ganglia (DRGs) following either footpad or sciatic nerve injection. These vectors have ICP34.5 deleted and have inactivating mutations in vmw65. We also report that peripheral replication is probably necessary for the efficient establishment of latency in vivo, as fully replication-incompetent HSV vectors allow efficient gene expression in DRGs only after peripheral inoculation at a high virus dose. Very low transduction efficiencies are otherwise achieved. In parallel, promoters have been developed that allow the long-term expression of individual or pairs of genes in DRGs by using elements from the latently active region of the virus to confer a long-term activity onto a number of promoters which otherwise function only in the short term. This work further defines elements and mechanisms within the latently active region that are necessary for long-term gene expression and for the first time allows multiple inserted genes to be expressed from HSV vectors during latency.
Herpes simplex virus type 1 (HSV-1) and HSV-2 are large DNA viruses the life cycles of which are characterized by the ability to enter a lifelong latent state in sensory neurons from which reactivation of the virus can intermittently occur. During latency the HSV genome, which remains extrachromosomal, is largely quiescent, although the fact that a small part of the genome encoding the latency-associated transcripts (LATs) is transcribed during latency (38) shows that the development of gene delivery vectors allowing transgene expression during this time should be possible. During latency HSV is not naturally cleared by the immune system and infected cells remain undamaged, further showing the potential for a long-term therapeutic benefit with HSV as a vector. HSV is a large virus, potentially allowing the insertion of multiple therapeutic genes. It thus has a number of properties suggesting that it may ultimately provide an ideal vector for gene delivery to the nervous system (reviewed in references 6 and 13).
In this study we have attempted to optimize parameters allowing gene delivery to the spinal ganglia following inoculation of HSV vectors by peripheral routes, thus taking advantage of the natural life cycle of the virus, which usually infects axonal nerve terminals at peripheral sites. Neurotrophic herpesviruses such as HSV-1 and HSV-2 are unique among viruses currently under development as vectors in that they have the ability to infect axonal nerve terminals before retrograde transport to neuronal cell bodies where latency is established. While other viruses used as vectors, such as adenovirus, adeno-associated virus, and lentiviruses, can infect neurons in vitro and in vivo, none has evolved an ability to be efficiently transported to neuronal cell bodies in this way. In the peripheral nervous system there are a number of potential applications for vectors capable of axonal transport, including the stimulation of regrowth of damaged nerves, the study and treatment of various pain states, the protection of neurons from further degeneration in, e.g., motor neuron disease, the study and treatment of various neuropathies, the study of neuronal development, and the screening of the relevance of genes implicated as being important in any of these processes by a gene delivery approach.
Considerable previous work aimed at the development of HSV vectors has already been reported (reviewed in references 6 and 13). This has been largely targeted to overcoming two inherent problems with wild-type HSV as a vector system: how to disable the virus such that it is no longer pathogenic but such that it is still able to infect and remain latent in neurons and how to maintain transgene expression during HSV latency. For the use of HSV vectors which are to be introduced into the brain by direct injection or for gene delivery to cells in culture, it has become apparent that the expression of HSV genes from the vector must be minimized to prevent toxic effects (19, 20, 21, 37, 44). Here either the regulatory immediate-early (IE) genes encoding ICP4, ICP27, ICP22, and ICP0 must be deleted or their expression must be otherwise reduced or prevented (19, 20, 21, 37, 40, 44). This prevents toxicity from the IE proteins themselves, as each is highly cytotoxic, and also from the later genes the expression of which the IE genes stimulate. For gene delivery to spinal ganglia in vivo, however, usually a footpad or ear pinna inoculation route has been used, which requires transport from the inoculation site to the cell body in the spine. Here various viruses have been used, including those with the nonessential genes encoding ICP6 (16), ICP0 (24), thymidine kinase (TK) (18, 32), gC (9, 15, 25), or vmw65 (11) mutated or undisabled (22, 23, 28) or (by us) with ICP34.5 or ICP34.5 and vmw65 deleted (7). These viruses have each shown reasonable levels of at least short-term gene delivery and/or the ability to enter latency following inoculation of mice by the footpad or ear pinna route. All of these viruses, however, retain at least some degree of replication competence in vivo and in culture as no essential genes have been inactivated. So far it has not been reported whether viruses incapable of replication in any cell (i.e., with an essential gene or genes mutated) allow gene delivery to spinal ganglia following inoculation by peripheral routes such as the footpad or ear pinna. A virus with ICP4 deleted (i.e., replication incompetent) has, however, given low-efficiency delivery to dorsal root ganglia (DRGs) following direct inoculation into the sciatic nerve (9), and a virus with a partial deletion in ICP0, an inactivating mutation in vmw65, and a temperature-sensitive mutation in ICP4, providing a conditionally replication-competent virus, has allowed genes to be delivered following footpad inoculation (29) (see Discussion below). In that work (29) it was concluded that replication is probably not necessary for HSV to efficiently reach spinal ganglia following inoculation by peripheral routes. A primary aim of the present work, therefore, was to identify optimal combinations of mutations and further define parameters allowing efficient gene delivery to spinal ganglia by footpad inoculation or by inoculation directly into a peripheral nerve.
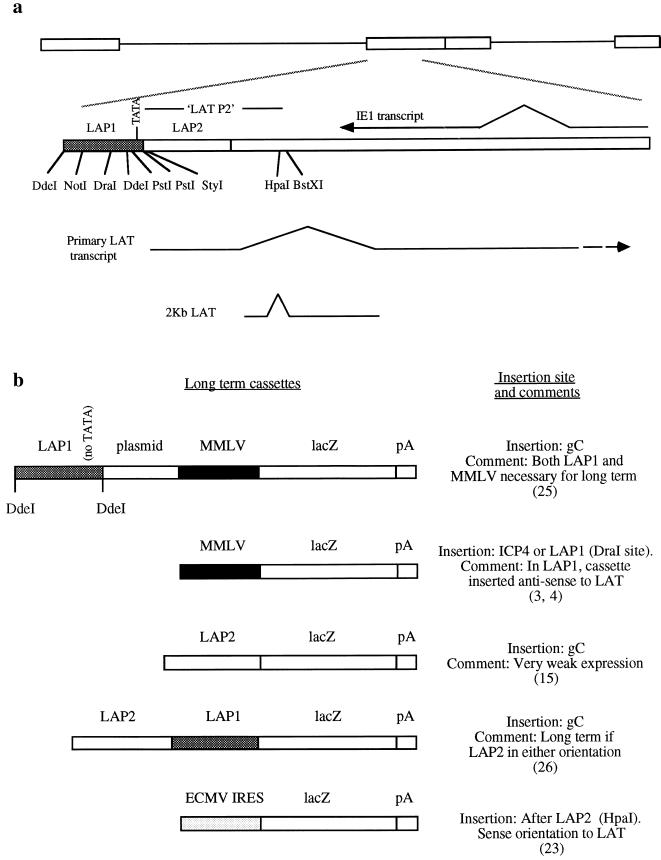
As discussed above, a second problem with HSV vectors has been an inability to retain expression of inserted genes during latency, as these genes under the control of most of the HSV and non-HSV promoters which have been tested, like the majority of the HSV genome during latency, become rapidly transcriptionally inactivated (see, e.g., references 22, 25, and 28). However a number of strategies allowing latent transgene expression have been reported. First, it was found that a Moloney murine leukemia virus (MMLV) promoter linked to a fragment of the LAT promoter (LAP1) (Fig. 1) and inserted in gC was able to drive expression during latency, although neither LAP1 nor the MMLV promoter alone, nor a number of other promoters linked to LAP1 or alone, allowed this to occur (25). MMLV alone inserted in ICP4 (3, 10) or in LAP1 (4), however, was active, which may be due to the proximity of these regions to the endogenous LATs in this case, in contrast to being distant from the LAT region (in gC) before (25). In other approaches it was found that LAP2 alone (Fig. 1) could give expression during latency when inserted in gC (15), but that this expression was very weak, and that LAP2 linked to LAP1, like MMLV linked to LAP1, could also maintain latent gene expression when inserted in gC (26). Finally, it was found that insertion of an internal ribosome entry site into the 2-kb LAT allowed expression of a downstream marker gene [with a poly(A) site] during latency (23). This strategy thus retained the natural LAT promoter structure, overcoming the problem that the RNA expressed during latency (the 2-kb LAT) would not usually allow protein expression as it is usually nuclear and nonlinear in nature (36, 45).
FIG. 1.
The LAT region and promoter cassettes which have previously been identified as driving long-term gene expression. (a) Schematic representation of the LAT region showing relevant restriction sites, the promoter regions LAP1 and LAP2, and the LAT P2 region mentioned in the text. (b) Previous promoter cassettes giving gene expression during HSV latency. ECMV, encephalomyocarditis virus; IRES, internal ribosome entry site.
Thus, due to the factors described above, a second aim of the work reported here was to further define parameters enabling gene expression during latency and in particular to develop systems which would allow the long-term expression of multiple genes during latency and which could be inserted at any desired site within the HSV genome. This would take advantage of the large genome size of HSV, allowing insertion of ≈15 kb of exogenous sequence and of even larger insertions if the HSV sequence is first removed as is the case in most vectors.
MATERIALS AND METHODS
Cell culture and virus propagation.
Replication-competent and -incompetent viruses were propagated on BHK cells and 27/12/M:4 cells (40) (27/12/M:4 cells are BHK cells which complement mutations in ICP4, ICP27, and vmw65), respectively. Cells were cultured in Dulbecco modified Eagle medium plus 10% fetal calf serum with 800 μg of neomycin per ml and 750 μg of Zeocin per ml for 27/12/M:4 cells (40). HMBA (3 mM) was included for propagation of viruses containing the in1814 mutation (30).
Initial promoter constructs.
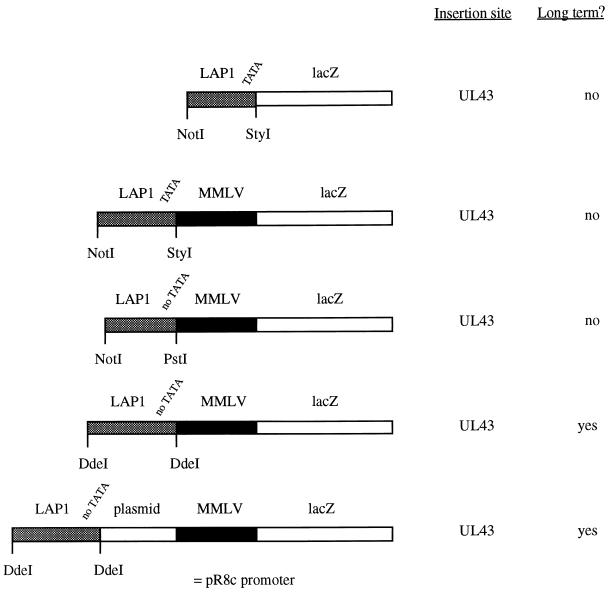
Six plasmids were constructed (see Fig. 2). These contained the LAP1 promoter (HSV-1 nucleotides [nt] 118443 to 118878 [NotI-StyI] [Fig. 1]) linked to lacZ (from pCH110 [Pharmacia]) and inserted into a plasmid allowing homologous recombination into UL43 at the unique NsiI site or the same plasmid except with the MMLV long terminal repeat (LTR) (from pJ4 [NheI-KpnI] [31]) inserted between the LAP1 sequence and the lacZ gene and with LAP1 sequences of either HSV-1 nt 118443 to 118878 (NotI-StyI), nt 118443 to 118671 (NotI-PstI), or nt 118181 to 118768 (DdeI-DdeI). Finally, a cassette consisting of a LAP1 sequence (nt 118181 to 118768 [DdeI-DdeI]), a ≈700-bp plasmid spacer sequence derived from pGem3Zf (NdeI-XbaI) (Promega), and an MMLV LTR promoter driving lacZ (similar to the promoter cassette reported by Lokensgard et al. [25]) was constructed and inserted into UL43 flanking regions (giving plasmid pR8c) or into a plasmid allowing recombination into the virion host shutoff protein (vhs)-encoding gene (UL41) at the unique NruI site (giving plasmid pR15). HSV nucleotide numbers refer to GenBank file HE1CG.
FIG. 2.
Initial promoter constructs tested and long-term expression characteristics. Positions of the restriction sites indicated in the LAT region are shown in Fig. 1.
Replication-competent viruses.
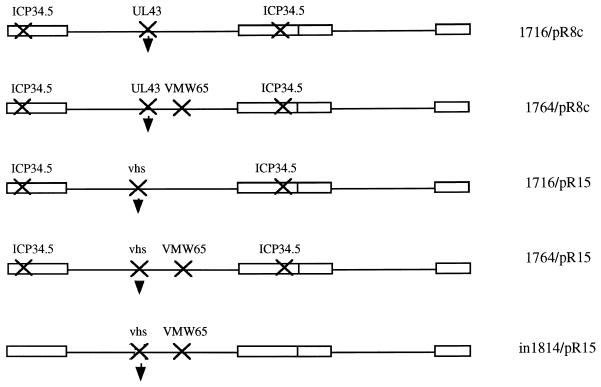
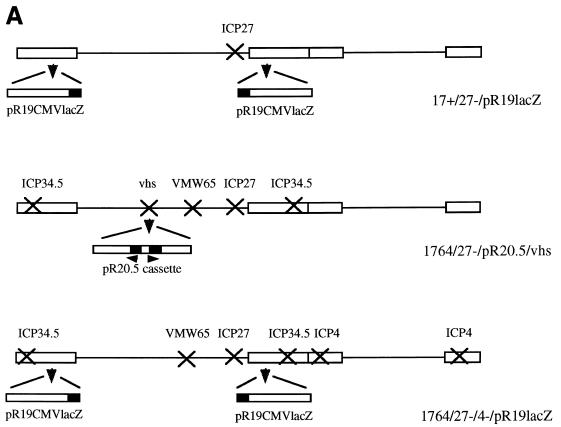
The plasmids described above allowing insertion into UL43 were inserted into virus strain 1764 (ICP34.5 and vmw65 deficient [7]) by standard techniques of homologous recombination into purified viral genomic DNA and selection of lacZ-expressing plaques followed by further purification and Southern blotting to confirm genome structure. The promoter-lacZ cassettes contained in plasmids pR8c and pR15 were also inserted into virus strains 1716, 1764, and in1814 (vmw65 deficient [1]) by homologous recombination. (The resulting virus strains generated are shown in Fig. 3.)
FIG. 3.
Replication-competent viruses tested for gene delivery to DRGs. In each case the indicated genes have been inactivated, with insertion of a chimeric LAP1-MMLV-lacZ promoter cassette either in the gene encoding vhs (UL41) or in UL43 as indicated by the arrowhead. This insertion forms the pR8c cassette when inserted in UL43 and the pR15 cassette when inserted in vhs.
Second-generation promoter constructs.
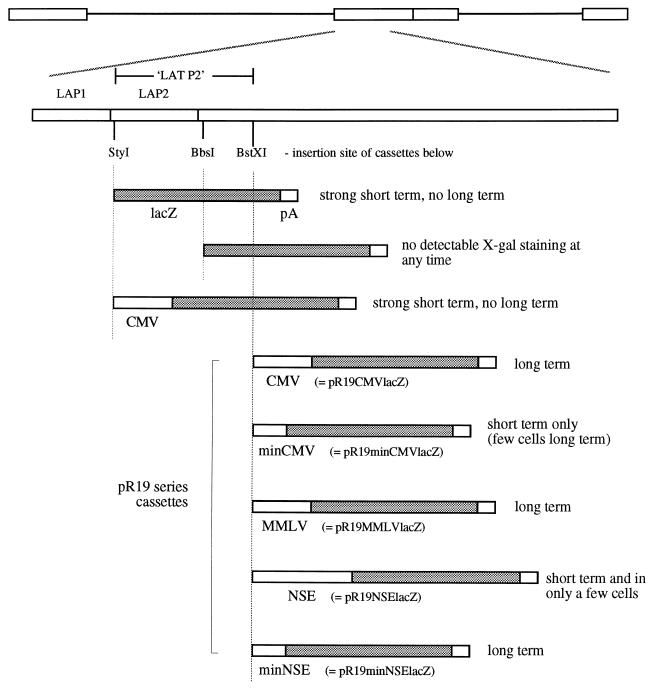
Plasmids were constructed allowing homologous recombination into the HSV-1 LAT region, using a 3.5-kb NotI fragment (HSV-1 nt 118443 to 122029) inserted into pGem5 (Promega). A lacZ gene (from pCH110, HindIII-BamHI [Pharmacia]) was inserted between the two StyI sites (between nt 118878 and 119248) or the two BbsI sites (between nt 119674 and 121328); a cytomegalovirus (CMV) promoter (from pcDNA3 [Invitrogen])-lacZ cassette was inserted between the StyI sites and between the BstXI sites (nt 120220 and 120408); and a minimal CMV promoter (156-bp SmaI-SmaI fragment from pUHC13-3 [17])-lacZ cassette, a neuron-specific enolase (NSE) promoter (1.8 kb [14]), a minimal NSE promoter (255-base 3′-terminal fragment from the 1.8-kb NSE promoter [41]), and an MMLV promoter (from pJ4)-lacZ cassette were inserted between the same BstXI sites. These were inserted into virus strain 1764 by homologous recombination (see above). (The resulting constructs are shown in Fig. 5.)
FIG. 5.
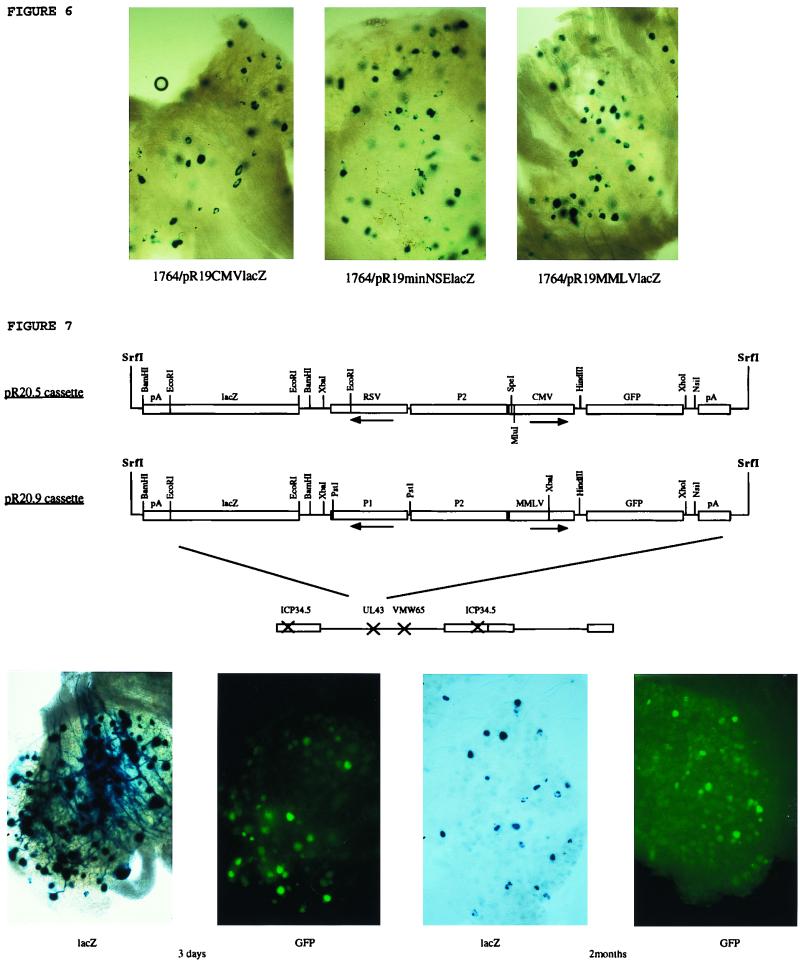
Second-generation promoter constructs tested for long-term gene expression. The LAT region and relevant restriction sites are shown, indicating the positions at which the cassettes below were inserted into the HSV genome. These cassettes were inserted into the LAT regions of viruses already deficient in ICP34.5 and vmw65 (virus strain 1764) and are thus present twice per virus genome due to the diploid nature of the LAT region. LAP1 and LAP2 refer to the sequences identified by Goins et al. (15), with LAT P2 being the region used later in this study by us. The pR19 series of cassettes referred to in the text is indicated, together with a summary of the results obtained following footpad inoculation of mice with these viruses (see Materials and Methods).
pR20.5 and pR20.9.
The pR20.5 cassette has previously been described (40). The pR20.9 cassette is identical except with the replacement of the CMV promoter by the MMLV LTR promoter from pJ4 (31) and of the Rous sarcoma virus promoter by the LAP1 promoter (HSV-1 nt 118181 to 118878, DdeI-StyI). These were inserted into plasmids containing the UL43 or vhs flanking regions as described above or into a plasmid containing sequences allowing recombination into US5 at the unique SacI site (nt 137945). (The structures of these plasmids are shown in Fig. 7.)
Replication-incompetent viruses.
Virus strain 17+/27−/pR19lacZ has previously been described (42) and contains a CMV-lacZ cassette inserted into the LAT region (as shown in Fig. 5) of HSV strain 17+ from which ICP27 has been deleted. Virus strains 1764/27−/pR20.5/vhs and 1764/27−/4−/pR20.5/vhs were constructed by homologous recombination of the pR20.5 cassette inserted into vhs gene-containing flanking regions (inserted into the NruI site as before) into virus strains with ICP34.5, ICP27, and the LAT P2 region deleted and containing the in1814 mutation, or also with ICP4 deleted, respectively (40). Deletion of endogenous LAT P2 regions prevents recombinational instability which is otherwise caused by insertion of LAT P2 elements (contained in pR20.5) in vhs. Virus strain 1764/27−/4−/pR19lacZ was constructed as was virus strain 17+/27−/pR19lacZ, except with recombination of the CMV-lacZ cassette into the LAT region of the multiply deleted virus backbone described above. Here LAT P2 elements are not deleted. Replication-incompetent viruses were propagated as previously described (40).
In vivo gene delivery.
Viruses were inoculated by the footpad route as previously described, and at various times postinoculation animals were killed and DRGs were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (for LacZ expression) as reported previously (7), or the X-Gal staining step was omitted for observation of green fluorescent protein (GFP) expression by fluorescence microscopy. When dual expression of GFP and lacZ was to be assessed, GFP fluorescence was examined prior to X-Gal staining, as this otherwise masks fluorescence from GFP. When DRGs were to be sectioned, animals were perfused rather than post-fixed in 4% paraformaldehyde prior to further processing. For sciatic nerve inoculation, animals were anesthetized, the sciatic nerve was exposed, and 2 to 5 μl of virus suspension was injected into the sciatic nerve using a glass micropipette. The quantity of 2 to 5 μl was used as by this procedure not all of the vector suspension tends to remain in the nerve. Injection was thus continued (up to 5 μl) until injection of ≈2 μl was concluded to have been achieved. Using smaller volumes did not give as reproducible results. The wounds were then closed, and animals were allowed to recover. For footpad inoculation (20-μl inoculation volume in all cases) viruses were usually used at a titer of 108 PFU/ml, and for sciatic nerve inoculation viruses were used at 107 PFU/ml, except when otherwise indicated.
In situ hybridization.
In situ hybridization was carried out exactly as described previously (33) except with a LAT-specific oligonucleotide probe complementary to HSV-1 nt 120691 to 120647 (5′-TAAAGGTTTACTTTTGTATCTTTTCCCTGTCTGTGTTGGATGTAT).
RESULTS
We have previously found that viruses with ICP34.5 deleted or with ICP34.5 deleted and also with an inactivating mutation in vmw65 (the in1814 mutation) can allow gene delivery to DRGs following footpad inoculation but that gene expression (lacZ activity) with the LAP1 promoter used in this case was transitory (7). These viruses were also mutated in UL43 (the lacZ insertion site), a gene previously found to not affect the latency process. We now aimed to improve the gene delivery efficiency of these viruses and also achieve long-term gene expression, optimally of multiple genes.
Initial promoter experiments.
These experiments were aimed at identifying latently active promoters suitable for testing the various HSV mutants described below for long-term gene delivery in vivo, based on our and others' previous work. Further promoter optimization would then be possible. In these experiments (Fig. 2) a LAP1 promoter (437 bases, NotI-StyI), a LAP1 (437 bases, NotI-StyI)-MMLV LTR fusion, a LAP1 (223 bases, no TATA, NotI-PstI)-MMLV LTR fusion, a LAP1 (585 bases, no TATA, DdeI-DdeI)-MMLV LTR fusion, and a LAP1 (585 bases, no TATA)–≈700 bases of plasmid DNA-MMLV LTR fusion were tested for the long-term expression of lacZ when inserted into UL43. The last construct is very similar to the promoter identified as having long-term promoter activity by Lokensgard et al. (25) (Fig. 1), where ≈700 bases derived from the BAG vector (34) separated the LAP1 and MMLV LTR sequences. These experiments showed (Fig. 2) that as expected, LAP1 sequences alone could not drive long-term gene expression. They also showed that, similar to the work of Lokensgard et al. (25), a LAP1-MMLV fusion could give long-term expression. However this was entirely dependent on the length of LAP1 sequence used, as 585 bases without the LAP1 TATA box (DdeI-DdeI) could give long-term expression when fused to the MMLV LTR, but a shorter LAP1 fragment either with or without the TATA box (437 bases [NotI-StyI] or 223 bases [NotI-PstI]) could not. Thus, at least some of the sequences necessary for long-term expression under such circumstances are present within LAP1 between bases −343 and −603 (NotI-DdeI) with respect to the LAT transcriptional start site (HSV-1 nt 118180 to 118440). Moreover, while effective long-term gene expression could be obtained by a direct LAP1-MMLV LTR fusion, this was enhanced if the MMLV and LAP1 sequences were separated by ≈700 bases of plasmid DNA. Thus, these results, as well as further defining the regions necessary for LAP1 long-term activity when combined with the MMLV LTR, also provided an appropriate promoter for the further optimization of gene delivery experiments described below.
Deletion of vhs or inactivation of vmw65 in replication-competent HSV vectors.
It was thought likely that some degree of replication competence might be necessary for the efficient establishment of latency when HSV is inoculated at peripheral sites, but for a safe and effective vector the virus should include multiple disabling mutations. Both the ICP34.5 and the vmw65 mutation significantly reduce pathogenicity in vivo by both intracerebral and footpad inoculation but still allow some replication (1, 27). ICP34.5 is a neurovirulence factor, and vmw65 is the virion protein responsible for transactivation of IE genes soon after infection of a permissive cell. A characteristic of ICP34.5 mutants, however, is that while they can be reactivated from latency by DRG explant, they do so at considerably reduced efficiency compared to wild-type virus (35). We therefore speculated that such reduced efficiency may be reflected in a less-than-optimal efficiency of gene delivery, i.e, that latency establishment, which would be thought to be necessary for efficient gene expression, was also compromised. A further gene giving reduced virulence in vivo if mutated but not affecting growth in culture is that encoding vhs, (39). vhs mutants, like vmw65 mutants (12), reactivate at near-wild-type levels in DRG explant assays (39). This suggested to us that combining vhs mutations with mutations to ICP34.5 might increase the number of latent sites, and thus increase the resulting gene delivery efficiency, above those provided by mutation of ICP34.5 alone or ICP34.5 together with vmw65.
Optimization of HSV gene deletions allowing marker gene delivery to DRGs.
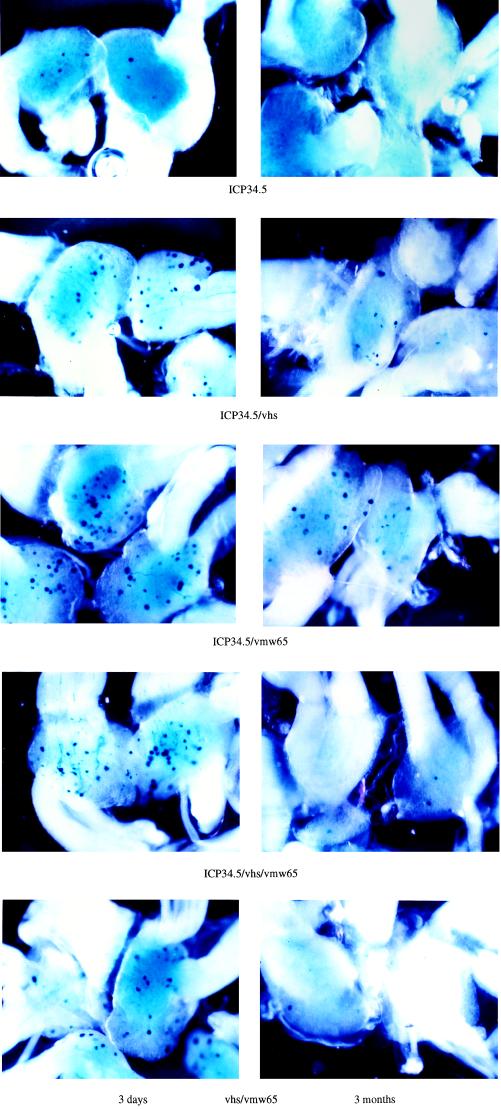
Based on the rationale described above, a number of viruses with mutations of ICP34.5, vmw65, vhs, and UL43 singly and in most combinations were generated (Fig. 3). Each of these viruses contained a lacZ reporter gene driven by the pR8c promoter construct, which is known to give latent gene expression (Fig. 2) (called pR15 when inserted in vhs), inserted in either UL43 or the gene encoding vhs (UL41). These were then tested for lacZ activity in a time course of 3 days to 3 months following footpad inoculation of mice. Example DRGs (L4 and L5) from these experiments are shown in Fig. 4. Here it was shown that an enhanced efficiency of gene delivery was not accompanied by inactivation of vhs. However, mutation of ICP34.5 together with vmw65 consistently gave a greater number of lacZ-positive cells than with the other viruses tested at all time points. Thus, while essentially wild-type virus (UL43 deleted) can give gene delivery, enhanced delivery (possibly due to reduced toxicity or immunogenicity) is observed with viruses singly mutated in ICP34.5, vhs, or vmw65. This is further enhanced by mutation of ICP34.5 together with vmw65 but is not further improved by mutation of vmw65 and vhs, ICP34.5 and vhs, or ICP34.5, vmw65, and vhs, where gene delivery efficiency is reduced compared to that for the single mutant viruses. It thus appears that the best gene delivery efficiency using any combination of ICP34.5, vmw65, or vhs mutations is obtained by mutation of vmw65 and ICP34.5. These viruses are also mutated in UL43 due to the lacZ insertion, although this does not itself appear to affect gene delivery efficiency, as insertion into US5 (another nonessential gene not effecting the latency phenotype [2]) is equally effective (see below).
FIG. 4.
Comparative gene delivery to DRGs using various replication-competent HSV vectors. The vectors shown in Fig. 3 were footpad inoculated into 3-week-old BALB/c mice (see Materials and Methods), and DRGs were removed and stained with X-Gal at either 3 days or 3 months postinoculation. L4 and L5 ganglia on the side of injection were removed from pairs of animals. Gene expression levels in L4 and L5 ganglia were in each case found to be similar. Not all DRGs are shown in some panels. In each case the genes inactivated in the respective viruses are indicated.
Promoter optimization.
Work by ourselves (described above) and others has shown that a number of different arrangements of LAT-derived elements, either alone or in combination with the MMLV LTR, can give transgene expression during latency (15, 25, 26). However, the mechanism of action of these elements is not yet known. It seems likely that attributes of the HSV DNA sequence, which is highly G+C rich (≈80% G+C) and has an unusual dinucleotide composition (5), are involved in the shutdown of HSV and non-HSV promoters during latency and also in the stable chromatin-like structure maintained during this time (8). Thus, for the HSV LAT region to remain transcriptionally active during latency, the LAT region may take up a DNA structure different from that of the remainder of the genome, as otherwise the LAT region might also be expected to become transcriptionally inactive during this time. Indeed, the LAT regions of HSV-1 and other alphaherpesviruses have a considerably different dinucleotide content than the rest of their respective genomes while remaining similarly G+C rich (5). Such an altered dinucleotide content could reflect an altered DNA structure during latency. If such a hypothesis is correct, it follows that the properties of the LAT region which allow gene expression during latency would not be promoter activity related but would be structure related, and thus non-LAT promoters placed in the LAT region at an appropriate position may be able to maintain gene expression during latency due to a locally altered DNA structure caused by LAT sequences. The possibility that such a hypothesis might be correct was tested by the construction of a number of viruses (Fig. 5) (the promoters each drive lacZ) in which either a CMV promoter (654 bases) was placed directly after LAP1 or a CMV promoter (654 bases), a minimal CMV promoter (156 bases), an MMLV LTR promoter, a full-length NSE promoter (1.8 kb), or a minimal NSE promoter (255 bases) was placed 1.4 kb downstream of the LAP1 TATA (after a region referred to by us as LAT P2, inserted between the two BstXI sites). A lacZ marker gene was also placed directly after LAP1 (between the two StyI sites) and directly after LAP2 (15) (at the BbsI site). The constructs with insertions after the BstXI site form the pR19 series of cassettes referred to below.
These viruses were tested for gene expression during latency (results are also summarized in Fig. 5). This showed that neither a CMV promoter placed directly after LAP1 nor a full-length NSE promoter placed after LAT P2 could drive latent gene expression. However, a full-length CMV promoter, an MMLV promoter, and a minimal NSE promoter placed after LAT P2 (pR19CMVlacZ, pR19MMLVlacZ, and pR19minNSElacZ cassettes, respectively; the promoter insertion was made 1.4 kb downstream of the LAP1 TATA sequence) could give gene expression during latency (Fig. 6). The minimal CMV promoter (pR19minCMVlacZ cassette) gave latent gene expression when placed after LAT P2 but in considerably fewer cells than with the full-length CMV promoter or minimal NSE promoter. Thus, at this position after LAT P2 sequences either acting as latent gene expression enhancer elements or which might alter local DNA structure are appropriately positioned to allow gene expression from at least some non-LAT promoters during latency in DRGs.
It had previously been found that a CMV promoter placed at an essentially identical position in the HSV genome could not give lacZ expression during latency (22). However, this CMV promoter (370 bases) was closer in length to the minimal promoter used by us (156 bases; inactive during latency except in a very few cells) than to the full-length promoter (654 bases, including enhancer) which was active in the long term, possibly explaining this difference. Other work with the CMV promoter-enhancer-lacZ inserted into the TK locus of an otherwise wild-type virus (43) or into the TK locus of a virus mutated in vmw65 (11) showed very weak long-term expression in a small number of cells with an unusual speckled X-Gal staining appearance. The reason for this is unknown, but such a speckled pattern of staining was not seen in the work reported here.
Multiple long-term gene delivery.
The above results have for the first time demonstrated that the LAT region contains sequences which can allow long-term gene expression in a relatively non-promoter-specific fashion. This and other work (15, 23, 25, 26) has thus shown that long-term elements are contained within or around the LAP2 region, and elements in this region are essential for conferring long-term activity on promoters other than the MMLV LTR. Uniquely, the MMLV LTR can give long-term expression in combination with elements from LAP1 (25). We were interested, based on this information, in developing promoter systems which would be active during latency when inserted into the HSV genome outside the LAT region and which would preferably also allow the expression of multiple genes. To achieve this, a number of cassettes were constructed (Fig. 7). These cassettes have the general structure of a central LAT P2 element flanked by pairs of promoters facing away from this central element in a back-to-back orientation. The pair of promoters drive lacZ and GFP genes, respectively, which can be easily replaced by other genes when required. The pR20.5 cassette has Rous sarcoma virus and CMV promoters on either side of the LAT P2 element, and the pR20.9 cassette has an MMLV LTR and a LAP1 promoter similarly positioned. The complete cassettes are flanked by sites for the rare blunt-cutting restriction enzyme SrfI, allowing excision of the entire cassette and insertion into appropriate HSV DNA flanking regions and thus allowing recombination into the HSV genome at any chosen site. These cassettes were tested for gene expression during latency when inserted into either UL43 or US5 of a virus with ICP34.5 deleted and with an inactivating mutation in vmw65 (virus strain 1764, the optimal virus identified above).
These experiments showed that latent gene expression could be obtained with pR20.5 and pR20.9 of both GFP and lacZ (Fig. 7 shows expression from pR20.9) following footpad inoculation of mice. Insertions into US5 (results not shown) gave results identical to those for the insertions in UL43. However, pR20.9 gave consistently more GFP- and lacZ-positive cells than did pR20.5, indicating the likely possibility that further elements involved in gene expression during latency are located outside LAT P2, probably in LAP1, which is included in pR20.9 but not pR20.5 (Fig. 7). Further identification of the active elements in pR20.5 and pR20.9 is under way.
pR20.9 also has the interesting property that in BHK cells when the virus is replicating, even though GFP fluorescence can easily be detected, only very low-level lacZ activity (very pale staining with X-Gal) is evident (data not shown). As can be seen, X-Gal staining is strong in vivo. However, this effect is not a neuronal specificity of pR20.9 but appears to be an active repression by a viral protein, as in BHK cells together with acyclovir, when the virus does not replicate, X-Gal staining is again strong in individual transduced cells. The most likely candidate proteins for this are ICP4 and ICP0, both of which have previously been shown to repress the LAP2 promoter (15). Such a repression, however, is not been seen when using only the LAP1 or the MMLV LTR as a promoter or LAP1-MMLV chimeric promoters, and thus sequences in the LAT P2 region must participate in what appears to be a replication-dependent transcriptional repression.
Gene transfer following peripheral nerve injection.
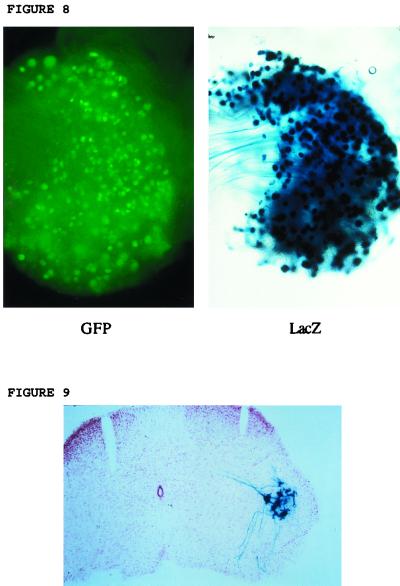
Another means of vector inoculation to allow gene delivery to spinal ganglia which might improve gene delivery efficiency is direct inoculation into a peripheral nerve. To test this, viruses were injected into the sciatic nerves of mice at various doses. This showed (Fig. 8) that very high-level efficiency of gene delivery to DRGs, better than by footpad inoculation, could be achieved, which further increased with dose. At later times (2 weeks to 3 months), the numbers of X-Gal-staining and/or GFP-fluorescent cells were reduced to levels similar to that achieved by footpad inoculation with same viruses. With 1764/pR20.9/UL43, however, only low-level lacZ activity could be detected 3 days to 1 week after sciatic nerve inoculation, but at later times this virus also showed gene expression at levels comparable to that achieved by footpad inoculation; i.e., the numbers of cells expressing lacZ and GFP with 1764/pR20.9/UL43 increased from essentially none to a maximum at approximately 2 weeks, with this number of cells then remaining relatively constant at all times tested thereafter. This result with 1764/pR20.9/UL43 may be for a reason similar to that for the only low-level gene expression seen in BHK cells with this virus. After sciatic nerve inoculation, there may be higher levels of ICP4 and/or ICP0 expressed in transduced cells compared to after footpad inoculation. This could, by virtue of an ICP4- and/or ICP0-mediated repressor activity, reduce levels of expression from the pR20.9 cassette at early times after inoculation.
Gene delivery to motor neurons following peripheral inoculation.
Footpad or sciatic nerve inoculation might be expected to result in infection of motor as well as sensory axons, and thus spinal cord sections were prepared following inoculation. This showed (Fig. 9) that motor neurons in the spinal cord were also effectively transduced following peripheral inoculation with the ICP34.5- and vmw65-deficient viruses (Fig. 9 shows transduction after footpad inoculation with 1764/pR19CMVlacZ; see Materials and Methods), but surprisingly, the number of transduced motor neurons was not further increased by sciatic nerve inoculation. Gene delivery was limited to motor neuron cell bodies in the L4-L5 region of the spinal cord, with no evidence of trans-synaptic spread to other cell types, to other regions of the spinal cord or brain stem, or to motor neurons or other cells contralateral to the injection site. This suggests that these viruses do not replicate after reaching motor neurons, although some replication would be theoretically possible with these ICP34.5- and vmw65-deficient viruses. Further work to optimize gene delivery to motor neurons is underway.
Replication-incompetent HSV vectors in the peripheral nervous system.
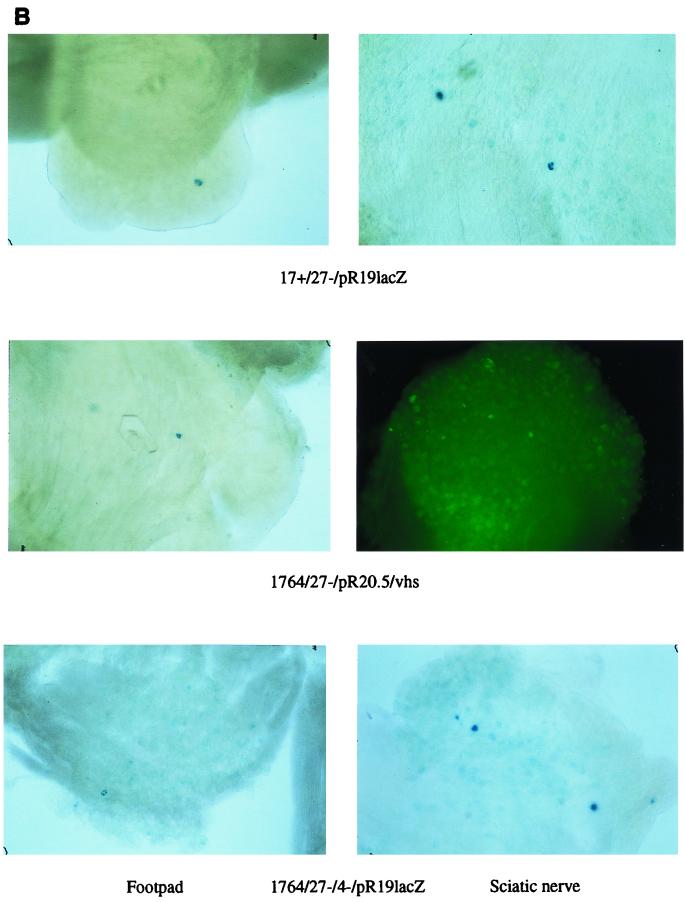
In addition to the viruses described above, a number of viruses which cannot replicate at all in vivo were tested for gene delivery to DRGs following footpad and sciatic nerve inoculation. These viruses either had ICP27 alone deleted, had ICP27 and ICP34.5 deleted and had the in1814 vmw65 mutation; or had ICP27, ICP4, and ICP34.5 deleted and had the in1814 vmw65 mutation (40, 42) (Fig. 10A). The virus with the pR20.5 cassette (Fig. 10A) also has the endogenous LAT P2 regions deleted to prevent recombinational instability which is otherwise caused by introduction of the LAT P2 region in the pR20.5 cassette. These results showed that following footpad or sciatic nerve inoculation at the titers usually used (108 and 107 PFU/ml, respectively [see Materials and Methods]), only very few X-Gal- or GFP-positive cells could be identified at any time point (Fig. 10B).
FIG. 10.
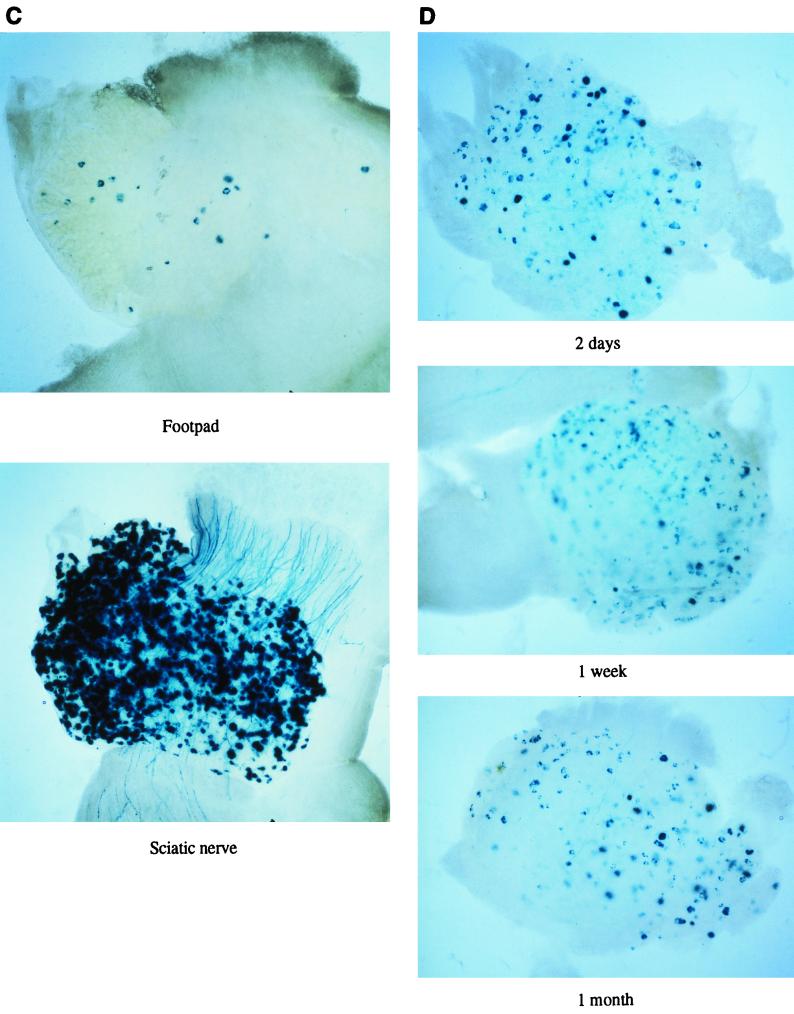
Gene delivery to DRGs using-replication incompetent HSV vectors. (A) Viruses used in the experiments (see Materials and Methods; the pR19 cassette where used in these viruses contains the CMV promoter). (B) Results (lacZ or GFP) obtained 1 week after footpad or sciatic nerve injection with the indicated viruses. (C) Results 1 week following footpad or sciatic nerve inoculation using 1764/27−/4−/pR19CMVlacZ at a higher titer (5 × 108 PFU/ml rather than 1 × 107 PFU/ml as used elsewhere for sciatic nerve inoculation). (D) Results 2 days, 1 week, and 1 month following sciatic nerve inoculation with 1764/27−/4−/pR19CMVlacZ at 108 PFU/ml.
These results suggest that some level of replication competence is necessary for efficient gene delivery to DRGs by peripheral routes, either by footpad inoculation or direct inoculation into a nerve. Indeed, limited replication might be expected to be necessary for the virus to penetrate through the various cell types in the footpad and also the cells surrounding the axonal bundles in the sciatic nerve. However, the finding that replication appeared to aid the virus in reaching DRGs following direct nerve injection was unexpected.
Following these somewhat disappointing results with replication-incompetent viruses as far as gene delivery was concerned, we further explored whether gene delivery could occur at greater efficiency with replication-incompetent viruses if higher titers of virus were used. Here, stocks at a titer of 5 × 108 PFU/ml were used. This gave a considerable improvement in gene delivery following sciatic nerve inoculation and some improvement following footpad inoculation (Fig. 10C). Thus, at an appropriate titer, replication-incompetent viruses can give effective gene delivery to DRGs by sciatic nerve inoculation. Moreover, gene expression continues during latency, using in this case the pR19CMV promoter cassette (Fig. 10D).
Reduction in gene expression over time is not due to promoter inactivation.
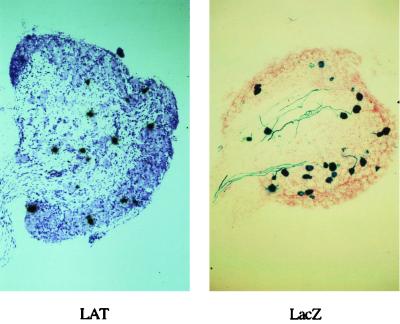
It is evident from the data presented that while significant gene expression with the promoters used does continue in the long term, this is reduced compared to that at early times after inoculation, at least with the replication-competent viruses used. This could be for a number of reasons, including promoter inactivation during latency. To see if promoter inactivation might be occurring, rather than either clearance of the virus by immune effects or cell death caused by toxic effects of the virus, in situ hybridization was performed to detect the LAT RNAs in infected cells at 1 month after inoculation with 1764/pR20.9/UL43. The LAT region in this virus has not been altered, and thus latently infected cells should express LAT RNAs detectable by such in situ techniques. This work showed that there were similar numbers of LAT-positive cells and lacZ-positive cells (Fig. 11), thus indicating that the pR20.9 cassette maintained gene expression during latency as efficiently as the endogenous LAT promoters maintained expression of the LATs. Thus, the pR20.9 cassette is not transcriptionally inactivated over time to any greater extent than the endogenous LATs, and hence reduced gene expression at later times is probably not due to promoter inactivation. This is further suggested for the pR19 cassette, as with a fully replication-incompetent virus, gene expression from this cassette remains relatively constant from 2 days to 1 month following inoculation (Fig. 10D).
FIG. 11.
LAT and lacZ are expressed in similar numbers of cells with 1764/pR20.9/43. Mice were footpad inoculated, and 1 month later DRG sections were either probed for LAT expression (see Materials and Methods; section was counterstained with thionin) or stained with X-Gal (section was counterstained with neutral red).
DISCUSSION
HSV mutants have been demonstrated to be capable of delivering genes to the peripheral nervous system on a number of occasions (see the introduction) and indeed have been shown to be capable of altering responsiveness to painful stimuli in a gene-specific fashion following inoculation of mice with a TK-negative vector encoding preproenkephalin (43). However, a number of questions as to the necessary properties the virus must retain to allow this to occur have remained unanswered, and in most cases mutants which retain some degree of pathogenicity in vivo have been used. Thus, viruses mutated in either gC or TK have been extensively used, which, while being somewhat attenuated compared to wild-type virus, are also replication competent and capable of pathogenicity if inoculated at a sufficient titer. Otherwise, viruses with mutations in vmw65 or ICP0 which are nonpathogenic but again capable of peripheral replication have been used. Dobson et al. (9) have previously shown that gene delivery could be achieved by sciatic nerve inoculation with an ICP4 deletion virus, but this delivery was of low efficiency (≈60 cells transduced per animal). Recently a virus with a partial deletion of ICP0, an inactivating mutation in vmw65, and a temperature-sensitive mutation to ICP4 has been shown to give relatively efficient long-term gene expression in DRGs following footpad inoculation (29). The study concluded that this strongly suggested that replication was not necessary for gene delivery following peripheral inoculation. However, this virus still retains replication competence in some circumstances, notably at temperatures below the fully nonpermissive temperature for the ICP4 mutation of 38.5°C. Thus, during inoculation of a mouse footpad, which is likely to be at a temperature below 37°C, some replication might occur, although none was detected in the reported study (29). Results with fully replication-incompetent viruses other than by sciatic nerve inoculation (9) have not been reported, and thus the question as to whether fully replication-incompetent HSV can reach spinal ganglia following peripheral inoculation has not previously been answered.
The second main area of study relevant to the development of HSV vectors for gene delivery to the peripheral nervous system has been the study and identification of promoters capable of driving gene expression during HSV latency. Thus, while expression of the LATs continues during latency, the expression of genes under the control of most other promoters does not. Recent work has shown that DNA elements from the LAT region, an internal ribosome entry site within the LAT region, or particular elements from the LAT region together with the MMLV promoter (but not other promoters) can allow a heterologous gene to be expressed in the long term (see the introduction), but the precise requirements for long-term gene expression or the mechanism for promoter shutdown which otherwise occurs has remained unknown.
The work presented here aimed to identify safe, nonpathogenic vectors which could allow gene delivery to spinal ganglia following peripheral inoculation and such that gene expression continued during HSV latency. Thus, the work on promoters aimed to identify the general principles required for long-term gene expression, particularly such that expression cassettes could be used outside the LAT region and such that multiple genes could be expressed in the long term.
For these reasons we therefore tested either a number of HSV mutants which were attenuated by the introduction of mutations which prevent pathogenicity in vivo, but which do not completely prevent replication (combinations of mutations in ICP34.5, vmw65, and vhs), or various viruses with mutations in ICP27 and/or ICP4 which cannot replicate in any cell (unless cells are artificially engineered to express ICP27 and/or ICP4). The first set of mutants were used because it was known that each could establish latency effectively, but each also reduced pathogenicity in vivo considerably. The highest gene delivery efficiency was achieved by the use of viruses with the combined deletion of ICP34.5 and an inactivating mutation in vmw65, by either footpad or sciatic nerve inoculation. A number of fully replication-incompetent viruses were essentially incapable of gene delivery to DRGs by either of these routes, surprisingly even when directly injected into the sciatic nerve, unless high virus titers were used. With high virus titers efficient long-term gene delivery could be achieved with replication-incompetent viruses, but only when mice were inoculated in the sciatic nerve and not by footpad inoculation. Thus, it appears that some degree of replication competence is necessary for HSV to usually reach spinal ganglia and enter latency, as in natural infections a high virus titer such as used for these experiments (1 × 108 to 5 × 108 PFU/ml) would not be expected. These results conflict with the conclusions of Marshall et al. (29), who concluded that replication is probably not necessary for HSV to efficiently reach spinal ganglia following peripheral (footpad) inoculation. However, as the virus used by Marshall et al. can replicate at below 37°C, some limited replication may have occurred following footpad inoculation (although none was detected), possibly explaining this difference.
The present work has therefore identified optimized, safe, and nonpathogenic backbone viruses for gene delivery to the peripheral nervous system by peripheral injection routes. These viruses are safe, because they cannot under any circumstances revert to a wild-type phenotype, as mutations in genes such as ICP34.5 and vmw65 cannot be repaired by recombination during growth in culture (the mutated genes are not included in the cells for growth), and they are nonpathogenic because multiple genes reducing or preventing pathogenicity have been mutated. Fully replication-incompetent viruses can also be used for gene delivery to spinal ganglia by direct intranerve injection at a sufficient titer, and these may be considered to have an improved safety profile if gene therapy (for example, for chronic pain) is to be considered for human use.
Our studies aimed at identifying parameters allowing promoters to remain active during latency have shown that the LAT region has the capability to allow long-term gene expression in a relatively non-promoter-specific fashion. Thus, the LAT region as a whole can confer a long-term activity to the LAP1 promoter itself, a minimal NSE promoter, a CMV promoter, and an MMLV promoter. This suggests that the LAT region might act to locally remodel the chromatin, allowing an altered DNA structure compared to the rest of the HSV genome during latency, and thus allows at least some promoters placed within it to remain active in the long term. It has previously been shown that LAP1 can confer long-term activity to the MMLV promoter and that LAP2 can confer long-term activity to LAP1, both outside the LAT region (inserted into gC [25, 26]). We have shown that sequences between positions −343 and −603 with respect to the LAT transcript start site are necessary for this LAP1-mediated activity, further defining active elements necessary for long-term gene expression. We are currently identifying active regions in and around both LAP1 and LAP2/LAT P2. Previous work had also shown that LAP2 could confer a long-term activity on LAP1 in a direction-independent fashion (26). Combining these studies suggested to us that promoter cassettes might be designed to allow the expression of pairs of genes from non-LAT sites within the HSV genome by placing pairs of promoters in a back-to-back orientation away from a centrally positioned element including LAP2. This was found to be the case, for the first time providing HSV vectors allowing the long-term expression of multiple heterologous genes during latency. Thus, while HSV has often been suggested to have advantages over other vectors due to its ability to package large inserts and thus potentially deliver multiple genes, this capability has not been able to be exploited in the long term, as only individual genes have previously been able to be expressed during latency.
Thus, overall the present work has further defined parameters allowing gene delivery to spinal ganglia by peripheral routes and has provided nonpathogenic, safe viruses suitable for this purpose. It has also further defined parameters allowing transgene expression during latency and provided promoter cassettes allowing the long-term expression of multiple heterologous genes. These cassettes can be inserted at non-LAT sites within the HSV genome. Such vectors have a number of potential uses in the study of processes occurring in or mediated via spinal ganglia. These include the study of pain states, which might ultimately allow the development of a gene therapy approach to long term analgesia, or the identification and characterization of genes important in these processes. The ability to deliver multiple genes can allow either the delivery of a marker gene together with the gene of interest or the delivery of pairs of genes such that interactions can be studied. Such approaches have not previously been possible using HSV or other vectors unless multiple viruses (which may not necessarily infect the same cell) are used.
FIG. 6-7.
Latent gene expression using second-generation promoters. X-Gal staining of DRGs was after footpad inoculation with ICP34.5- and vmw65-deficient viruses 1764/pR19CMVlacZ, 1764/pR19minNSElacZ, and 1764/pR19MMLVlacZ. Expression from each of the viruses 1 month after inoculation is shown. (Top) pR20.5 and pR20.9 expression cassettes inserted into vectors deficient in ICP34.5 and vmw65. (Bottom) Gene expression characteristics at 3 days and 2 months after footpad inoculation with the pR20.9-containing virus (1764/pR20.9/43). DRGs were either stained with X-Gal for lacZ expression or viewed by fluorescence microscopy for GFP expression. RSV, Rous sarcoma virus.
FIG. 8-9.
Gene delivery following sciatic nerve injection. Virus 1764/pR20.5/43 was inoculated into the mouse sciatic nerve. GFP and lacZ expression in the same DRG (L4) is shown 3 days after inoculation.Gene delivery to motor neurons. A spinal cord section (L4-L5 region) is shown 1 week after footpad inoculation with 1764/pR19CMVlacZ (see Materials and Methods). lacZ is shown to be expressed in motor neurons following X-Gal staining. Sections were counterstained with neutral red.
ACKNOWLEDGMENTS
J. Palmer and R. Branston contributed equally to this work.
We are grateful to the Medical Research Council for the support of parts of this work.
REFERENCES
- 1.Ace C I, McKee T A, Ryan M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan P, Davispoynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes-simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 3.Bloom D C, Maidment N T, Tan A, Dissette V B, Feldman L T, Stevens J G. Long-term expression of a reporter gene from latent herpes simplex virus in the rat hippocampus. Mol Brain Res. 1995;31:48–60. doi: 10.1016/0169-328x(95)00031-m. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D E, Stevens J G. Long-term expression of a foreign gene from a unique position in the latent herpes simplex virus genome. Hum Gene Ther. 1996;7:1447–1454. doi: 10.1089/hum.1996.7.12-1447. [DOI] [PubMed] [Google Scholar]
- 5.Coffin R S, Howard M K, Latchman D S. Altered dinucleotide content within the latently transcribed regions of the DNA of alpha herpes viruses—implications for latent RNA expression and DNA structure. Virology. 1995;209:358–365. doi: 10.1006/viro.1995.1267. [DOI] [PubMed] [Google Scholar]
- 6.Coffin R S, Latchman D S. Herpes simplex virus based vectors. In: Latchman D S, editor. Genetic manipulation of the nervous system. London, United Kingdom: Academic Press; 1995. pp. 99–112. [Google Scholar]
- 7.Coffin R S, Maclean A R, Latchman D S, Brown S M. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 1996;3:886–891. [PubMed] [Google Scholar]
- 8.Deshmane S L, Fraser N W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J Virol. 1989;63:943–947. doi: 10.1128/jvi.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson A T, Margolis T P, Gomes W A, Feldman L T. In vivo deletion analysis of the herpes simplex virus type 1 latency-associated transcript promoter. J Virol. 1995;69:2264–2270. doi: 10.1128/jvi.69.4.2264-2270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson A T, Margolis T P, Sederati F, Stevens J G, Feldman L T. A latent, non-pathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 11.Ecob-Prince M S, Hassan K, Denheen M T, Preston C M. Expression of β-galactosidase in neurons of dorsal root ganglia which are latently infected with herpes simplex virus type 1. J Gen Virol. 1995;76:1527–1532. doi: 10.1099/0022-1317-76-6-1527. [DOI] [PubMed] [Google Scholar]
- 12.Ecob-Prince M S, Rixon F J, Preston C M, Hassan K, Kennedy P G E. Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency-associated transcripts. J Gen Virol. 1993;74:995–1002. doi: 10.1099/0022-1317-74-6-995. [DOI] [PubMed] [Google Scholar]
- 13.Fink D J, Deluca N A, Goins W F, Glorioso J C. Gene transfer to neurons using herpes simplex virus vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 14.Forss-Petters S, Danielson P E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J G. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 15.Goins W F, Sternberg L R, Croen K D, Krause P R, Hendricks R L, Fink D J, Straus S E, Levine M, Glorioso J C. A novel latency active promoter is contained within the herpes simplex type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein D J, Weller S K. Herpes simplex virus type-1-induced ribonucleotide reductase activity is dispensible for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho D Y, Mocarski E S. β-Galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988;167:279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P A, Miyanohara A, Levine F, Cahill T, Friedman T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson P A, Wang M J, Friedman T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krisky D M, Wolfe D, Goins W F, Marconi P C, Ramakrishnan R, Mata M, Rouse R J D, Fink D J, Glorioso J C. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann R H, Brown C, Efstathiou S. A murine RNA polymerase 1 promoter inserted into the herpes simplex virus type 1 genome is functional during lytic, but not latent, infection. J Gen Virol. 1996;77:2575–2582. doi: 10.1099/0022-1317-77-10-2575. [DOI] [PubMed] [Google Scholar]
- 23.Lachmann R H, Efstathiou S. Utilization of the herpes simplex virus type 1 latency-associated regulatory region to drive stable reporter gene expression in the nervous system. J Virol. 1997;71:3197–3207. doi: 10.1128/jvi.71.4.3197-3207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokensgard J R, Bloom D C, Dobson A T, Feldman L T. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lokensgard J R, Berthomme H, Feldman L T. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J Virol. 1997;71:6714–6719. doi: 10.1128/jvi.71.9.6714-6719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maclean A R, Ul-Fareed M, Robertson L, Harland J, Brown S M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediately early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 28.Margolis T P, Bloom D C, Dobson A T, Feldman L T, Stevens J G. Decreased reporter gene expression during latent infection with HSV LAT promoter constructs. Virology. 1993;197:585–592. doi: 10.1006/viro.1993.1632. [DOI] [PubMed] [Google Scholar]
- 29.Marshall K R, Lachmann R H, Ethstathiou S, Rinaldi A, Preston C M. Long-term transgene expression in mice infected with a herpes simplex virus type 1 mutant severely impaired for immediate-early gene expression. J Virol. 2000;74:956–964. doi: 10.1128/jvi.74.2.956-964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes-simplex virus immediate-early gene expression in the absence of trans-induction by VMW65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 31.Morgenstern J P, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palella T D, Silverman L J, Schroll C T, Homa F L, Levine M, Kelley W M. Herpes simplex virus-mediated human hypoxanthine-guanine phosphoribosyl transferase gene transfer into neuronal cells. Mol Cell Biol. 1988;8:457–460. doi: 10.1128/mcb.8.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer J A, De Felipe C, O'Brien J A, Hunt S P. Disruption of the substance P receptor (neurokinin-1) gene does not prevent upregulation of preprotachykinin-A mRNA in the spinal cord of mice following peripheral inflammation. Eur J Neurosci. 1999;11:3531–3538. doi: 10.1046/j.1460-9568.1999.00771.x. [DOI] [PubMed] [Google Scholar]
- 34.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson L M, Maclean L M, Maclean A R, Brown S M. Peripheral replication and latency reactivation kinetics of the non-neurovirulent herpes-simplex virus type-1 variant-1716. J Gen Virol. 1992;73:967–970. doi: 10.1099/0022-1317-73-4-967. [DOI] [PubMed] [Google Scholar]
- 36.Rodahl E, Haarr L. Analysis of the 2-kilobase latency associated transcript expressed in PC12 cells productively infected with herpes simplex virus type 1: evidence for a stable nonlinear structure. J Virol. 1997;71:1703–1707. doi: 10.1128/jvi.71.2.1703-1707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samaniego L A, Neiderhiser L, Deluca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus α gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 39.Strelow L I, Leib D A. Role of the virion host shutoff (VHS) protein of herpes simplex virus type-1 in latency and pathogenesis. J Gen Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas S K, Lilley C E, Latchman D S, Coffin R S. Equine herpesvirus 1 gene 12 can substitute for vmw65 in the growth of herpes simplex virus (HSV) type 1, allowing the generation of optimized cell lines for the propagation of HSV vectors with multiple immediate-early gene defects. J Virol. 1999;73:7399–7409. doi: 10.1128/jvi.73.9.7399-7409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Twyman R M, Jones E A. Sequences in the proximal 5′ flanking region of the rat neuron-specific enolase (NSE) gene are sufficient for cell type-specific reporter gene expression. J Mol Neurosci. 1997;8:63–73. doi: 10.1007/BF02736864. [DOI] [PubMed] [Google Scholar]
- 42.Wagstaff M J D, Lilley C E, Smith J, Robinson M J, Coffin R S, Latchman D S. Gene transfer using a disabled herpes virus vector containing the EMCV IRES allows multiple gene expression in vitro and in vivo. Gene Ther. 1998;5:1566–1570. doi: 10.1038/sj.gt.3300754. [DOI] [PubMed] [Google Scholar]
- 43.Wilson S P, Yeomans D C, Bender M A, Lu Y, Goins W F, Glorioso J C. Antihyperalgesic effects of infection with preproenkephalin-encoding herpes viruses. Proc Natl Acad Sci USA. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu N, Watkins S C, Schaffer P A, Deluca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T-T, Su Y-H, Block T M, Taylor J M. Evidence that two latency-associated transcripts of herpes simplex virus are nonlinear. J Virol. 1996;70:5962–5967. doi: 10.1128/jvi.70.9.5962-5967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]