Abstract
Background:
Driving while intoxicated (DWI) is a serious public health problem. However, treatment for DWI arrestees is not readily available. This study examines the effectiveness of a contingency management (CM) procedure using transdermal alcohol concentration (TAC) monitoring to reduce drinking among DWI arrestees.
Method:
The study participants were 216 DWI arrestees under pretrial and included both Mandated participants undergoing court-ordered TAC monitoring and Non-Mandated participants wearing a study-provided TAC monitor. Participants were randomly assigned to either a CM (Mandated = 35; Non-Mandated = 74) or a Control condition (Mandated = 37; Non-Mandated = 70) and completed the 8-week intervention. CM participants received $50/week for not exceeding a TAC of 0.02 g/dL during the previous week. Payments to Controls were yoked to the CM group.
Results:
Among Non-Mandated participants, the probability of meeting the contingency was higher and remained stable (about 65%) over time in the CM group, whereas the probability was lower and declined in the Control group, widening the gaps in the probability between the study conditions (16.7%–24.1% greater in the CM group from visit 4 to 8, all p < 0.05). Among Mandated participants, the probability was not significantly different between conditions (p = 0.06–0.95). Furthermore, among Non-Mandated participants, the percentage of heavy drinking days remained low (9.16%–11.37%) in the CM group, whereas it was greater and increased over time (17.43%–26.59%) in the Control group. In Mandated participants, no significant differences in percent heavy drinking days were observed between conditions (p = 0.07–0.10).
Conclusion:
We found that contingency effects on alcohol use are more pronounced among frequent and heavy alcohol users, i.e., Non-Mandated DWI arrestees. However, for individuals whose drinking was already suppressed by existing contingencies (i.e., court-mandated TAC monitoring), our CM procedure did not produce additional reductions in drinking.
Keywords: contingency management, driving while intoxicated, remote alcohol monitoring, transdermal alcohol concentration
INTRODUCTION
Driving while intoxicated (DWI) is a prevalent and serious threat to public health, exacerbated by a high incidence of recidivism. DWI is among the most frequently committed offenses in the U.S. with an estimated more than 1 million arrests per year (Federal Bureau of Investigation, 2020), yet this may reflect only 0.1% of actual alcohol-impaired driving (Zaloshnja et al., 2013). Moreover, DWI is associated with severe consequences. For the past decade, over 10,000 people have been killed every year in alcohol-involved crashes (NHTSA, 2020). Social costs for alcohol-impaired driving are estimated at $50.9 billion (West et al., 2019).
An estimated 90% of DWI arrestees meet the criteria for alcohol use disorder (AUD; McCutcheon et al., 2009; Nelson et al., 2015). However, opportunities for treatment are limited for DWI arrestees (Mullen et al., 2015) and recidivism rates are high among them (Cavaiola et al., 2007). About one in three alcohol-impaired drivers appear to recidivate within 2 years of their first DWI conviction (Brinkmann et al., 2002). Repeat offenses among alcohol-impaired drivers account for one-third of all DWI offenses (Warren-Kigenyi & Coleman, 2014), with a 62% higher likelihood of being involved in fatal crashes than first-time alcohol-impaired drivers (Fell, 2014). Given the higher risk of recidivism, there have been calls for developing effective interventions for alcohol-impaired drivers (Brown et al., 2010; Cavaiola & Wuth, 2016).
Contingency management (CM) is a behavioral therapy that has been used to reduce harmful drug use (Oluwoye, Kriegel, et al., 2020; Petry et al., 2017). Using operant conditioning principles, CM prompts abstinence or reduction in use by providing positive reinforcement (e.g., cash, prizes) for providing objective evidence of meeting predefined treatment goals (Higgins, 1997; Prendergast et al., 2006). The majority of research examining the efficacy of CM has focused on substances for which use is easily measured, such as cocaine (Festinger et al., 2014; Yoon et al., 2020) and opioids (Bickel et al., 2008; Carroll et al., 2002). Subsequently, changes in breath expired-air carbon monoxide (CO) criterion (Javors et al., 2005; Lamb et al., 2005) and the use of cellular monitoring of CO results (Dallery et al., 2019) facilitated the use of CM for the treatment of smoking (for review, see Sigmon et al., 2008). Until recently using CM to reduce problem drinking has been similarly limited by the need for multiple daily measurements to reliably detect all or most dangerous drinking (Alessi & Petry, 2013).
Advances in the technology to objectively detect drinking include portable breath alcohol (BrAC) monitoring and electronic monitoring of transdermal alcohol concentration (TAC), which can overcome many obstacles to implementing CM to reduce heavy drinking (Dallery et al., 2019; Fairbairn & Kang, 2021). Although portable breathalyzers are more acceptable for consumers because of the relatively low cost of monitoring and less physical discomfort or stigma (Alessi & Petry, 2013), daily BrAC monitoring is invasive, requires active participation, and has limited capacity to detect alcohol use during nighttime (Oluwoye, Reneau, et al., 2020). TAC monitoring uses devices worn on the ankle or wrist to detect ethanol excreted through the skin (Swift, 2003), which enables an objective assessment of alcohol consumption that is measured passively and near-continuously (Fairbairn & Kang, 2021). While TAC monitoring can be less acceptable to patients due to physical (e.g., rash, itchiness) and emotional (e.g., embarrassment, stigma) discomfort (Barnett et al., 2017), emerging research has found that CM based on TAC monitoring can be effective to reduce excessive alcohol use among community-recruited heavy drinkers (Barnett et al., 2011, 2017; Dougherty et al., 2014; Dougherty, Karns, et al., 2015; Dougherty, Lake, et al., 2015). Furthermore, recent innovation enables the distinction between low, moderate, and heavy drinking levels (Karns-Wright et al., 2018; Roache et al., 2019). Thus, TAC monitoring may be useful for implementing CM interventions designed to reduce problematic drinking.
Compared to community-recruited samples, the DWI population has more complex needs and limited resources for treatment (Mullen et al., 2015). In addition, despite inherent contingencies (e.g., incarceration, fines), as reported in a study of the vehicle ignition interlock device for the DWI population (Vanlaar et al., 2010), rates of alcohol use are still alarmingly high among the DWI population even during pretrial supervision, that is, the period from arrest to trial. Taken together, the limited treatment options and intractable patterns of ongoing alcohol use may require an application specific for the DWI population. The current study aims to translate previous findings demonstrating effectiveness of CM to reduce alcohol use among community-recruited heavy drinkers (Dougherty et al., 2014; Dougherty, Lake, et al., 2015) into an examination of the efficacy of TAC-based CM to reduce alcohol use among adults arrested for DWI.
METHODS
Participants and criteria
Sample size estimation
Our pilot data showed that DWI offenders who met our inclusion criteria had an average of 38% days of any drinking (SD = 0.22) and 21% days of heavy drinking (SD = 0.20) during the first 30 days of TAC monitoring. Based on the assumption that these data reflect the pattern of drinking in the Control group during the study, we conservatively estimated an 8-week treatment will reduce the percentage of any drinking days/week from 38% to 20% or lower (i.e., a decrease of 18 percentage points) and the percentage of heavy drinking days/week from 21% to 10% or lower (i.e., a decrease of 11 percentage points). A sample size of 175 per group had 99.9% power to detect the expected differences of 18 percentage points in drinking between groups using a two-sided t-test at α = 0.05 and SD = 0.22. Further, 175 per group achieved 80% power to detect a minimum difference of 6% for the percentage of heavy drinking days (SD = 0.2). To account for an attrition rate of 20%, we initially planned to enroll 220 participants per group. However, the nationwide lockdown due to the COVID-19 pandemic in March 2020 posed distinct challenges to recruitment. Therefore, recruitment was stopped in early April 2020. As a result, 216 participants took part (109 in the CM and 107 in the control group). This obtained sample size achieved 98% power to detect the expected differences of 11 percentage points in the percentage of heavy drinking days between the two groups (21% vs. 10%, SD = 0.20). No interim analysis was planned.
Recruitment
A total of, 384 people with a history of DWI arrest were recruited to participate in the present study. Participants were recruited from the county pretrial supervision department, as well as through Recovery Monitoring Solutions Corporation, the local provider of SCRAM monitoring devices from Alcohol Monitoring Systems (AMS). Initially, we planned to recruit only the participants mandated by court order to wear SCRAM under pretrial supervision pending adjudication for a DWI arrest. The enrollment criteria were planned to include those who (1) were mandated to wear transdermal alcohol monitors (i.e., Mandated) and (2) had at least two heavy drinking events during the first 30-day observation period. However, during the earlier recruitment phase, we found that fewer than one-third of DWI arrestees were mandated with TAC monitoring and that only about 10% of those continued to engage in heavy drinking. Based on a clearer understanding of the study population, we concluded that the initial enrollment criteria were too restrictive and therefore, many participants who would potentially benefit from our CM procedure could be excluded. Thus, we expanded the criteria to include those who were not mandated with TAC monitoring (i.e., Non-Mandated) as well and skipped the 30-day observation period.
Final inclusion criteria included: ≥21 years old with a previous DWI arrest in Bexar County, Texas during the past 12 months and self-reported hazardous drinking for the past 12 months (AUDIT >7 for women or >8 for men; Babor & Higgins-Biddle, 2001). Exclusion criteria were: significant alcohol withdrawal symptoms (CIWA ≥10; Sullivan et al., 1989), a medical condition that would contraindicate participation (e.g., pregnancy, scheduled surgery), or a DSM-5 psychiatric disorder with symptoms of psychosis and/or delirium. Those who were not able to comprehend the informed consent process or study instructions were also excluded based on Mini-Mental State Examination (Folstein et al., 1975), where scores >20 indicated capacity to consent (Kim & Caine, 2002). After the screening, 216 participants were identified to be eligible for the study participation. At study entry, 71 participants were Mandated by the local court to wear SCRAM devices, and 145 participants were not mandated (Non-Mandated) to wear a SCRAM device but wore it to participate in the study (see Figure 1). Of the 216 participants who were eligible for the study, five participants missed all prescheduled weekly visits and failed to provide any TAC data. Per intention to treat, analysis on our primary outcome, we treated the contingency outcomes of these participants as failures so that all 216 participants were included in the analysis predicting the probabilities of meeting contingency as defined by SCRAM results (see below for contingency requirements). However, for the Linear Mixed Effects model predicting the percentage of heavy drinking days, we excluded the five participants without TAC readings (i.e., only 211 participants were included in those analysis), as modeling those participants without any relevant data is not really possible. The local institutional review board approved this study and written informed consent was obtained from all participants prior to enrollment. The study is registered on ClinicalTrial.gov (NCT03638596).
FIGURE 1.
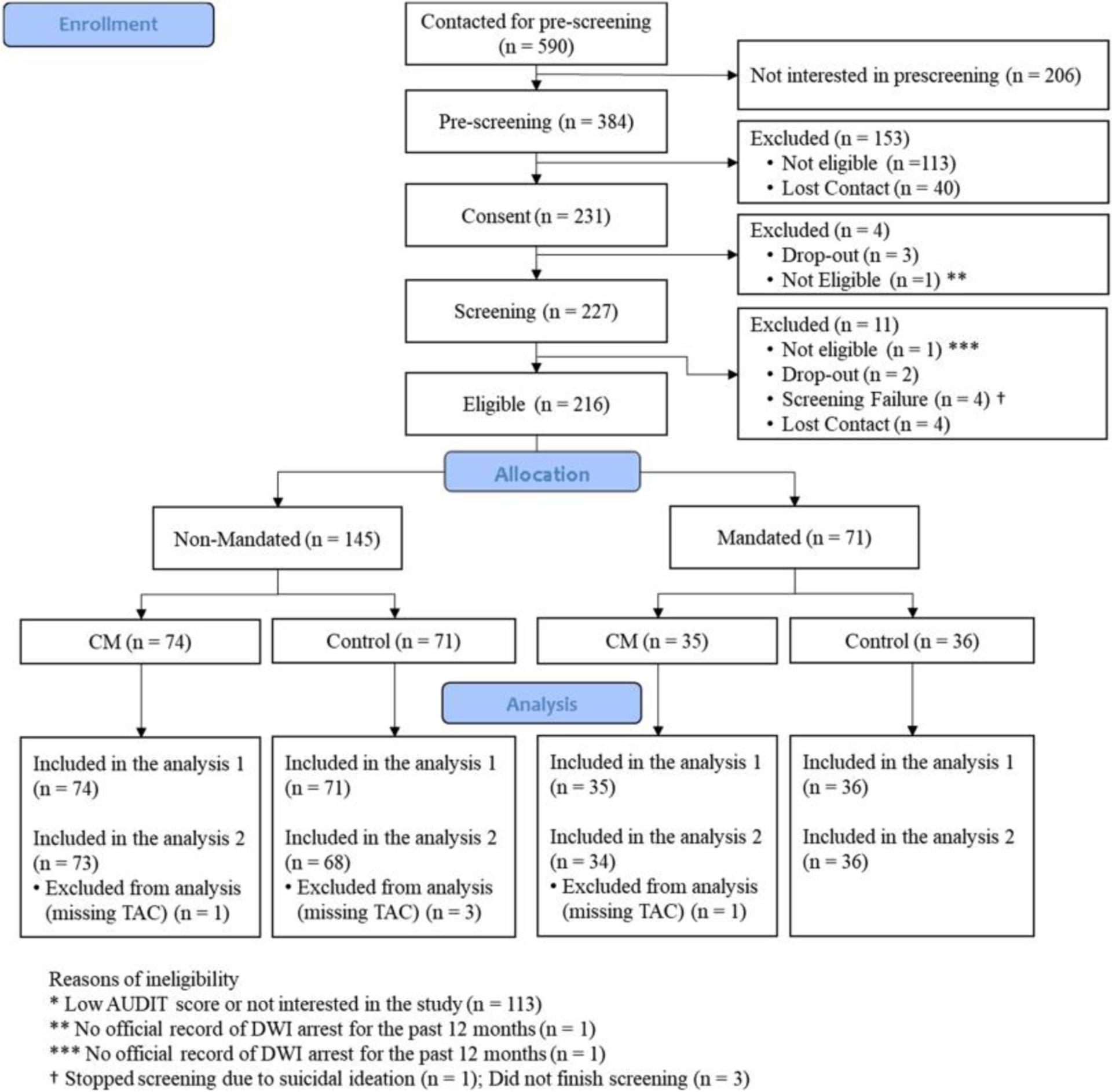
CONSORT diagram.
Mandate
Mandate in this study refers to the court-ordered alcohol monitoring using the SCRAM ankle monitor. The typical sequence of events after a DWI arrest in our county (similar to procedures nationally) is as follows. After a DWI arrest, a magistrate assigns the defendant to a court docket (DWI defendants are equally distributed across courts) and implements pretrial protocols and conditions required by the judge. All DWI defendants are mandated to abstain from alcohol. Once released from jail on bond, defendants experience pretrial supervision, typically until their case is adjudicated. In Bexar County, TX this pretrial supervision period lasts about 1 year. Some judges, but not all, include standard TAC monitoring (usually for 60–90 days) as a condition of pretrial release. For those who were mandated, the pretrial supervision procedure for monitoring TAC is in conjunction with the manufacturer (event monitoring by Alcohol Monitoring Systems, Inc.[AMS]), who reports detected drinking events to the Pretrial Services. Once a pretrial officer receives a report of an AMS-confirmed drinking event, the pretrial response can be determined at several levels (e.g., pre-trial officer, parole officer, or court). Possible consequences range from counseling or changes in bond conditions to revocation of bond release.
Non-Mandate
Non-Mandate is the term we used to refer to DWI arrestees who were not mandated to wear the SCRAM device, but who wear it in order to participate in the experimental study. The TAC data for Non-Mandated participants were obtained only by the experimenter and were not shared or made available to Bexar County Pre-Trial services or any judicial court.
Study procedure
Study design
Eligible participants were randomized within each group of mandate status (Mandated vs. Non-Mandated) to either a contingency management (CM) group or a Control group using stratified, blocked randomization, to assure balance on gender (Male vs. Female), and percentage of days with a drinking episode in the past 28 days assessed at baseline using timeline follow back (TLFB) methods (see below). Stratification on drinking frequency divided high versus low using a median split of all previously randomized participants, so that balance between groups would be maintained as the study progressed. After randomization, 109 participants were assigned to the CM group (35 Mandated and 74 Non-Mandated), and 107 participants were assigned to the Control group (36 Mandated and 71 Non-Mandated; see Figure 1). Then, participants in both the CM and the Control groups began the 56-day intervention with 8 weekly assessments. At each weekly visit, participants visited the laboratory to complete various self-report data and to have data downloaded from their TAC monitoring devices, and then received financial compensation determined by their CM versus Control payment contingencies (described below). In addition to the contingency payment, both Mandated and Non-Mandated participants were paid $20 for each weekly visit.
Transdermal alcohol monitoring
Once randomized, all participants were required to wear the secure continuous remote alcohol monitors (SCRAM: AMS) to measure TAC for the 8-week intervention period. Mandated participants were already wearing these monitors as a condition of pretrial service prior to randomization, but Non-Mandated participants were strapped with SCRAM monitors by study staff. SCRAM measured ethanol emitted through the skin every 30 min, 24 h/day. Deception and tampering were minimized by a locking mechanism and sensors that detect proximity to the skin using infrared reflectivity and body temperature. These prevented the removal of the device and detected efforts to impede alcohol use detection. TAC data was stored on web-based AMS servers which were accessed directly from AMS in a manner approved by our institutional review board. All study-related readings and interpretation of SCRAM TAC data were conducted independently, and no feedback was provided to pretrial supervision services. TAC data was used to determine whether incentives should be delivered during the 8-week intervention period as previously described (Dougherty, Lake, et al., 2015). For the Mandated participants, researchers paid the SCRAM monitoring fees directly to AMS on their behalf. The Non-Mandated participants were paid $10/day by study staff as compensation for wearing the SCRAM at no cost to themselves.
Intervention (contingency procedure)
On each weekly visit of the 8-week intervention phase, TAC data were collected from SCRAM monitors and used to objectively measure days of drinking and whether those were heavy drinking days. At each weekly visit, the CM group received a $50 incentive for not exceeding the TAC-based criterion set to identify heavy drinking on any day in the past week. TAC data were automatically collected every 30 min throughout the study and were processed to identify drinking events according to the research rules as described previously (Roache et al., 2019). Notably, these research rules were more sensitive to identifying drinking events than those used by the manufacturer and more sensitive to detecting heavy drinking (Karns-Wright et al., 2018; Roache et al., 2015). Briefly, drinking events were identified as a day on which non-zero TAC readings were obtained, but not removed as spurious or artifactual due to the application of the rules, and heavy drinking was defined as any non-removed TAC event that had two or more TAC readings ≥0.02 g/dL. Participants in the CM group whose TAC data indicated any day of heavy drinking in the past week received no payment and were told it was because of drinking too much on those days designated by the SCRAM monitors.
The Control group experienced these same monitoring and weekly visit procedures but received $50 yoked to the CM group’s payments—that is, incentives were not based on their TAC results, but upon the TAC results of the pre-matched participants in the CM group. Yoked payments control the rate and pattern of payments and have been used in previous CM studies (Higgins et al., 2000; Silverman et al., 1996). Specifically, payments for the first 18 control participants were matched to those of 18 randomly selected (without replacement) participants from our previous study (Dougherty, Karns, et al., 2015; Dougherty, Lake, et al., 2015). After this, subsequent control participants received payments that were matched to a randomly selected (without replacement) CM participant who had completed the study. Thus, about 90% of control participants were yoked directly to participants in the CM group, which resulted in the adequate matching of the rate and pattern of incentive delivery and still allowed random assignment of all participants.
Contingency and study compensation (i.e., visit, wearing SCRAM) were delivered using the ClinCard system (Greenphire Clincard, 2021) by which payments were loaded electronically onto the participant’s study card every week. Before delivering the contingency and study compensation, we thoroughly explained the itemized payment (i.e., contingency payment, visit payment, compensation for wearing a monitor for the Non-Mandated participants) every visit with a written payment form, which participants signed.
Measures
Outcome variables
The primary outcomes of this study included (1) the percentage meeting contingency each week and (2) the percentage of heavy drinking days. Both measures were calculated for each week during the 8-week intervention period using TAC readings. All TAC readings were analyzed based on a 24-h (noon-to-noon) assessment period rather than a midnight-to-midnight calendar day as previously described (Roache et al., 2019) to account for positive TAC readings commonly detected past midnight after an evening of drinking.
Meeting contingency
Once a week, TAC readings were reviewed to confirm if participants met the contingency criteria or not. If days of TAC readings were missing due to monitor malfunction, or participant incarceration or hospitalization, those days were coded differently to not penalize as if those days failed contingency. All remaining days were classified as to whether the participant met the contingency (i.e., success = 1; failure = 0) each week for all 216 participants. Thus, we were able to count the number of days in which participants successfully met the contingency and the number of weeks on which no day failed the criteria.
Percentage of heavy drinking days
The percentage of heavy drinking days was calculated by dividing the number of heavy drinking days per week by the number of days with valid monitoring per week.
Analytic procedure
Data were analyzed by accounting for the mixed between-groups effects of the mandated status of participants (Mandated vs. Non-Mandated) and the randomized treatment intervention (CM vs. Control), and the within-participant effects of visits (8 weekly visits). The main outcomes were: (1) meeting contingency each week (Yes/No); (2) the percent of heavy drinking days each week. Possible predictor variables including age, gender, and baseline drinking frequency, were considered as covariates in mixed-effects logistic models. Participant characteristics were summarized using descriptive statistics and compared among the four treatment/mandate groups using Kruskal-Wallis H test for continuous variables and Chi-square or Fisher’s exact test for categorical variables as appropriate.
Covariates
Age, gender, and baseline drinking characteristics were included as covariates in the analysis. Baseline drinking characteristics were measured by timeline follow-back (Sobell & Sobell, 1992) for the 28 days before study entry. We calculated the percentage of days with drinking events recorded by the timeline follow-back and then categorized participants into three groups based on the percentage: no-drinking (0% of days with drinking episodes), occasional drinking (0%–30% of days with drinking episodes), and frequent drinking (>30% of days with drinking episodes). The 30% of days with drinking episodes was chosen as a logical cutting point between occasional and frequent drinking based on three reasons: (1) Weekend-only drinkers (i.e., 2 days/week) would have less than 30% of days with drinking episodes, while someone who drank at least 1 day during the week in addition to weekends would have greater than 30% of days with drinking episodes; (2) Previous studies of treatment for AUD have often reported similar distribution of the percentages of days with drinking episodes in their samples, that is 30% of drinking days are not uncommon in either population studies (Hasin et al., 2019) or clinical trials (Petrakis et al., 2018); (3) 30% of days with drinking episodes would leave room for clinically-meaningful increases or decreases to occur. For the weekly measured binary outcome of meeting contingency, mixed-effects logistic regression was used to examine the effect of treatment while taking into account the correlations among repeated measures from the same participant over time and adjusting for covariates (age, gender, and baseline drinking frequency categories). In the mixed-effects logistic regression model, the three-way interaction among treatment conditions (CM vs. Control), mandate (Mandated vs. Non-Mandated), and weekly visits (treated as a continuous variable) was included, along with all lower-order interactions (i.e., treatment × mandate, treatment × visit, and mandate × visit) and the main effects. Model-based marginal probability of meeting contingency was estimated for each treatment/mandate group at each weekly visit with corresponding 95% confidence intervals. Based on the mixed-effects model, the treatment effect among Mandated and Non-Mandated participants at each weekly visit was calculated with Scheffe-adjusted 95% confidence intervals for multiple comparison adjustments as needed.
For the percentage of heavy drinking days per week, a linear mixed-effects model with treatment condition, mandate status of participants, and visits as factors was used including age, gender, and baseline drinking characteristics as covariates. Model-based marginal mean percentage of heavy drinking days per week was estimated for each treatment/mandate group at each weekly visit with corresponding 95% confidence intervals. Similarly, the treatment effect among both Mandated and Non-Mandated participants at each weekly visit was calculated with Scheffe’s method as needed. In the mixed-effects models for both outcomes, all available data were utilized. Data were assumed to be missing at random. No data imputation or model/variable selection was implemented. All analyses were performed using Stata/SE (version 17).
RESULTS
Participant characteristics
Participants’ characteristics are shown in Table 1. Participants were mean aged = 38.7 years (SD = 10.9), mostly male (77%) and Hispanic (73%), with no significant differences in age, gender, ethnicity, and race across the four study conditions. Statistically significant differences existed in the baseline drinking between the Mandated and the Non-Mandated participants. The Non-Mandated participants reported significantly more frequent drinking for the 28 days prior to the study entry (p < 0.001) than the Mandated counterparts. Specifically, the baseline frequency of any drinking in the Non-Mandated participants (27.7% of the past 28 days) was more than three times greater than that of the Mandated participants (8.3%). In addition, while more than half of the Mandated participants (57.7%) reported no drinking in the past 28 days, only 11.5% of the Non-Mandated participants reported no drinking (see Table 1).
Table 1.
Characteristics of the sample (N = 216)
| Variable | Mandated | Non-Mandated | Total | P-value | ||
|---|---|---|---|---|---|---|
| CM (N = 35) | Control (N = 36) | CM (N = 74) | Control (N = 71) | |||
| Age, mean ± SD | 40.7 ± 11.9 | 40.3 ± 11.0 | 37.2 ± 10.9 | 37.1 ± 10.4 | 38.7 ± 10.9 | 0.191 |
| Gender, n (%) | 0.972 | |||||
| Female | 7 (20) | 9 (25) | 17 (23) | 16 (23) | 49 (23) | |
| Male | 28 (80) | 27 (75) | 57 (77) | 55 (77) | 167 (77) | |
| Ethnicity, n (%) | 0.322 | |||||
| Hispanic or Latino | 22 (63) | 33 (92) | 53 (72) | 50 (70) | 158 (73%) | |
| Non-Hispanic/Non-Latino | 13 (37) | 3 (8) | 21 (28) | 21 (30) | 58 (24%) | |
| Race, n (%) | 0.442 | |||||
| White | 21 (60) | 16 (44) | 40 (54) | 27 (38) | 104 (48) | |
| Non-White | 14 (40) | 20 (55) | 34 (46) | 44 (62) | 112 (52) | |
| Percentage of drinking for 28 days, mean ± SD | 11.1 ± 15.4 | 5.4 ± 10.9 | 25.6 ± 26.2 | 29.9 ± 27.7 | 19.6 ± 24.7 | < 0.0011 |
| Drinking frequency 4 , n (%) | < 0.0013 | |||||
| None (0%) | 16 (46) | 25 (69) | 17 (23) | 14 (20) | 72 (33) | |
| Occasional (0% – 30%) | 14 (40) | 9 (25) | 30 (40) | 36 (51) | 89 (41) | |
| Frequent (> 30%) | 5 (14) | 2 (6) | 27 (37) | 21 (30) | 55 (26) | |
Kruskal–Wallis H test.
Chi-square test.
Fisher’s exact test
Drinking frequency was calculated using the baseline percentage of drinking days for the past 28 days based on the Timeline Follow-back assessment.
Participants received an average of $236.81 (Control: M = 237.71, SD = 135.30; CM: M = 240.83, SD = 136.45) as contingency payment during the 8-week study period (59.2% of the maximum amount; range 0%–100%). Participants remained (i.e., not dropped out or lost contact) in the study for 7.49 weeks (SD = 1.64) on average (Mandated-CM: M = 7.26, SD = 1.83; Mandated-Control: M = 7.81 weeks, SD = 0.67; Non-Mandated CM: M = 7.49 weeks, SD = 1.68; Non-Mandated Control: M = 7.46, SD = 1.75) and no significant differences were found between groups (F = 0.70, p = 0.55). On average, 88.4% of participants completed all 8 weeks.
Probability of meeting contingency
Figure 2 shows the weekly percentage of participants in each treatment and alcohol-monitoring-mandated group meeting the drinking contingency. The results for the Non-Mandated participants are shown in Figure 2A and the results for the Mandated participants are in Figure 2B. Results for participants assigned to the Control condition are represented by triangles and results for those assigned to the CM condition are represented by circles. The model-based estimates for the Control condition are shown by the dashed lines and for the CM condition by the solid line. As demonstrated by Figure 2 and the three-way interaction between Mandate/Non-Mandate × Control/CM × Visit (Table 2: OR = 0.73, p = 0.01), the effect of treatment assignment over time depended upon whether one had been mandated to wear the SCRAM monitor or not. Thus, we analyzed the effects of CM for the Non-Mandated and the Mandated group separately.
FIGURE 2.
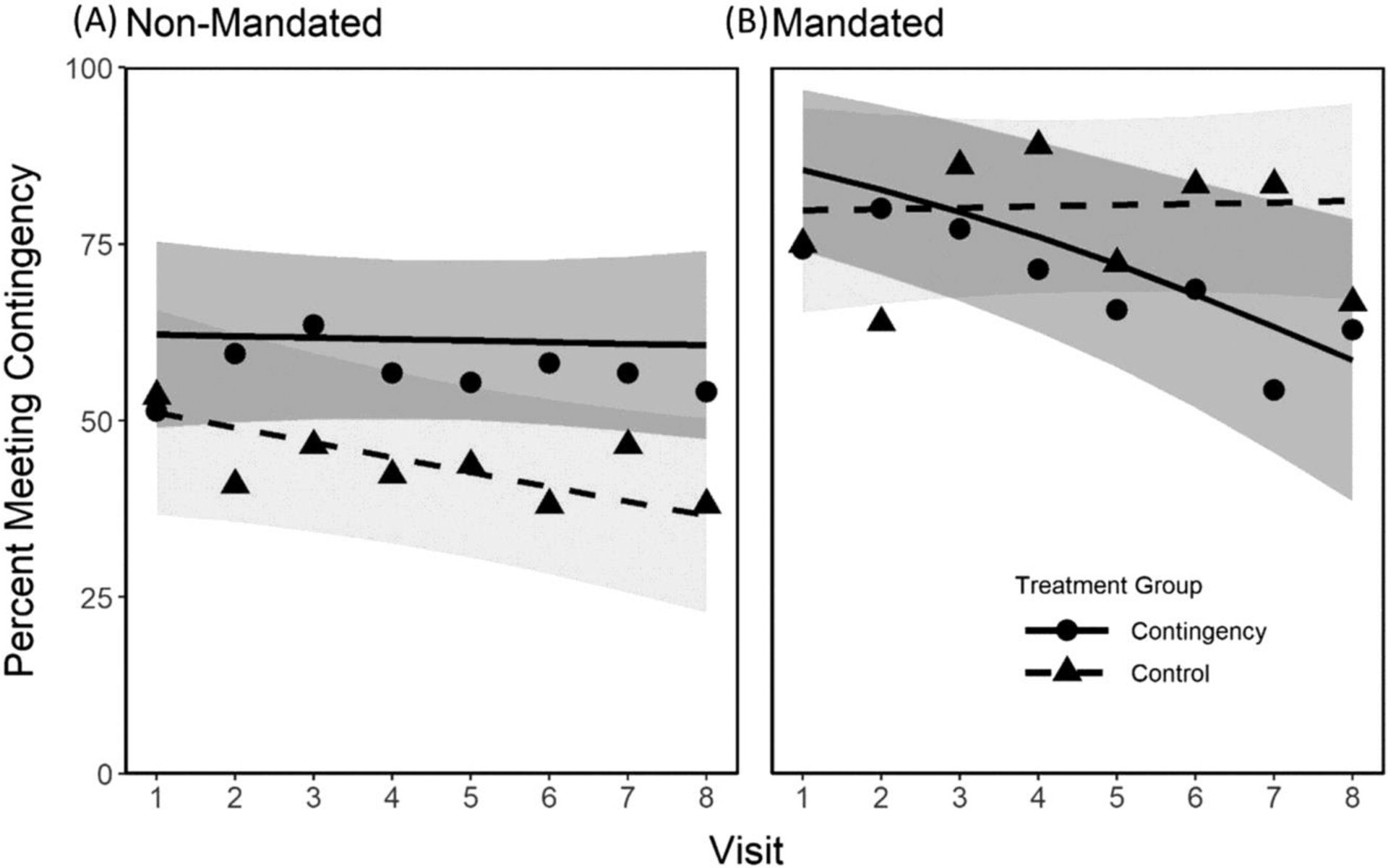
Estimated probabilities of meeting contingencies for each treatment and alcohol-monitoring mandating condition by week. The results for Non-Mandated participants are shown on panel (A), and the results for the Mandated participants are on panel (B). Results for participants assigned to the Control condition are represented by triangles and results for those assigned to the CM condition are represented by circles. The solid and dotted lines represent estimates for the CM and the Control group, respectively. Gray shades (dark gray: CM group; light gray: control group) indicate 95% confidence intervals for each condition.
Table 2.
Predicting the odds of meeting contingency (N = 216)
| OR [95% CI] | P value | ||
|---|---|---|---|
| Covariates | |||
| Age (year) | 1.03 | [0.99, 1.06] | 0.06 |
| Gender (female vs. male) | 0.53 | [0.26, 1.09] | 0.09 |
| Drinking frequency - Past 28 daysa | |||
| Occasional vs. None | 0.48 | [0.23, 1.03] | 0.14 |
| Frequent vs. None | 0.53 | [0.22, 1.23] | 0.67 |
| Main effects | |||
| Treatment condition (CM vs. Control) | 1.49 | [0.58, 3.83] | 0.40 |
| Mandate (Mandated vs. Non-Mandated) | 3.69 | [1.08, 12,66] | 0.04 |
| Visit (week) | 0.91 | [0.83, 1.01] | 0.07 |
| Interactions | |||
| Treatment × Mandate | 1.28 | [0.23, 7.03] | 0.78 |
| Treatment × Visit | 1.08 | [0.95, 1,24] | 0.23 |
| Mandate × Visit | 1.11 | [0.94, 1.32] | 0.23 |
| Treatment × Mandate × Visit | 0.73 | [0.58, 0.93] | 0.01 |
Note.
Self-reported drinking frequency: None: 0% of drinking days for the past 28 days; Occasional drinking: > 0% to <= 30%; Frequent drinking: >30%.
As presented in Figure 2A, at the first visit about half to two-thirds Non-Mandated participants met the contingencies for the week and the difference between the two treatment groups was not statistically significant (p = 0.27) when adjusting for age, gender, and self-reported drinking frequency in the 4 weeks preceding study entry. The percentage of Non-Mandated participants assigned to the CM condition meeting the contingency in the week preceding a visit stayed relatively constant across the eight visits (slope in the log odds scale [95% CI] = −0.01 [−0.10–0.08], p = 0.83). Participants assigned to the Control condition, in contrast, were less likely to meet the contingency as the study progressed (slope [95% CI] = −0.09 [−0.19–0.01], p = 0.07). However, the difference in the slope between the two groups was not significantly different (p = 0.23). Still, the average CM treatment effect, when all the covariates were held at their mean values between the two groups, was significantly different (p = 0.03). Significant (p = 0.01) CM treatment effects were also seen in that only 37% of the individuals assigned to the Control condition were estimated to meet the contingency in the week preceding visit 8 compared to 61% of those assigned to CM.
As shown in Figure 2B, participants mandated to wear the SCRAM monitor met the contingency 75%–85% of the time in the week preceding visit 1, and this frequency was similar between the two groups and not significantly different (p = 0.53). Among the participants in the Mandated Control group, the frequency of meeting the contingency was stable across visits (slope = 0.01 [−0.13–0.15], p = 0.86), while the frequency decreased across time (slope = −0.22 [−0.36 to −0.07], p = 0.004) among those in the Mandated CM group; and the difference in slopes was significantly different (p = 0.03). In contrast to the Non-Mandated participants who showed significant CM treatment effects (see Figure 2A), there were no significant effects seen in the average treatment effect (p = 0.42) or the treatment effect at visit 8 (p = 0.06) among the Mandated group.
Percentage of heavy drinking days per week
The second analysis examined the impact of contingency management on heavy drinking. Figure 3 shows the mean percent of heavy drinking days observed for each treatment across weeks in the two groups. The results for Non-Mandated participants are shown in Figure 3A, and the results for the Mandated participants are in Figure 3B. Results for participants assigned to the Control condition are represented by triangles and results for those assigned to the CM condition are represented by circles. The model-based estimates for the Control condition are shown by the dashed lines and for the CM condition by the solid line. The effect of treatment assignment over time tended to depend upon whether one had been Mandated for TAC monitoring or not (see Table 3 for three-way interaction between Mandate condition × treatment × Visit Coefficient = 1.24, p = 0.06). Therefore, and to be consistent with our analysis of meeting the contingency, we analyzed the effects of CM for the Non-Mandate and the Mandate groups separately. As shown in Figure 3A, the Non-Mandated participants assigned to the CM group drank heavily less often than those in the Control condition (10% vs. 17%, respectively, p = 0.03). The percentage of days with heavy drinking in Non-Mandated participants in the CM condition stayed relatively constant across the eight visits (slope [95% CI] = 0.03, [−0.48–0.54], p = 0.90). In contrast, the percentage of days of heavy drinking increased for the Non-Mandated participants in the Control condition across the study (slope [95% CI] = 1.03 [0.50–1.55], p < 0.001), and the difference in the slope between the two groups was significantly different (p = 0.008). Further, the average treatment effect was significantly different (p < 0.001) when all the covariates were held at their mean values between the two groups. Participants assigned to the Control condition drank heavily on an estimated 24% of the days in the last week of the study compared to 10% for those assigned to the CM condition (p < 0.001).
FIGURE 3.
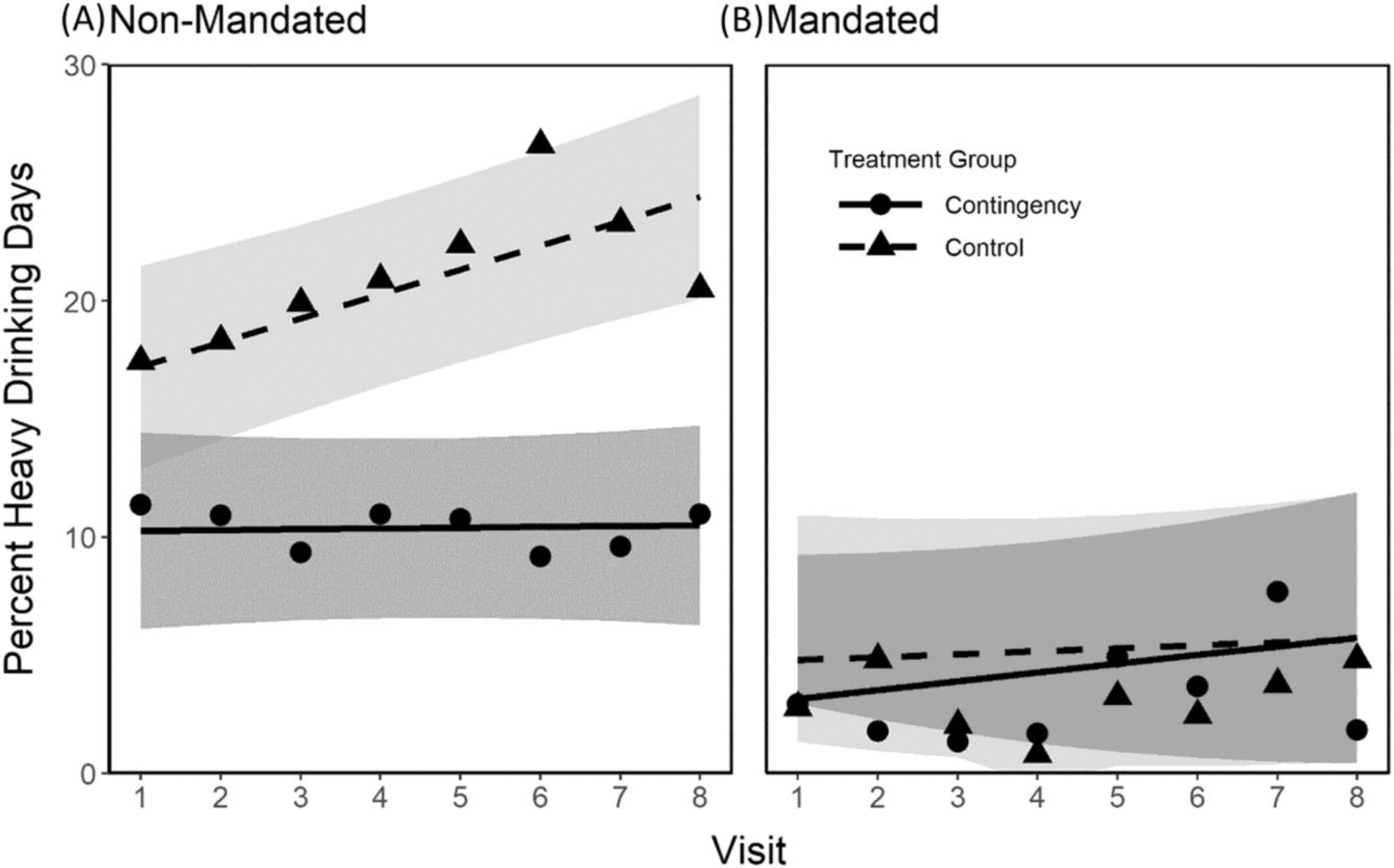
Estimated mean of the percentage of heavy drinking days per week for each treatment and alcohol-monitoring mandating condition by week. The results for Non-Mandated participants are shown on panel (A), and the results for the Mandated participants are on panel (B). Results for participants assigned to the Control condition are represented by triangles and results for those assigned to the CM condition are represented by circles. The solid and dotted lines represent estimates for the CM and the Control group, respectively. Gray shades (dark gray: CM group; light gray: control group) indicate 95% confidence intervals for each condition.
Table 3.
Predicting the percentage of heavy drinking days per week (N = 211)
| Coefficient [95% CI] | P value | ||
|---|---|---|---|
| Covariates | |||
| Age (year) | −0.09 | [−0.29, 0.12] | 0.39 |
| Gender (female vs. male) | 3.25 | [−2.04, 8.54] | 0.24 |
| Drinking frequency - Past 28 daysa | |||
| Occasional vs. None | 3.5 | [−2.01, 9.01] | 0.21 |
| Frequent vs. None | 7.42 | [1.78, 13.67] | 0.02 |
| Main effects | |||
| Treatment condition (CM vs. Control) | −5.94 | [−12.19, 0.32] | 0.06 |
| Mandate (Mandated vs. Non-Mandated) | −11.49 | [−19.56, −3.44] | 0.01 |
| Visit (week) | 1.03 | [0.51, 1.55] | 0.00 |
| Interactions | |||
| Treatment × Mandate | 4.04 | [−6.90, 14.98] | 0.47 |
| Treatment × Visit | −0.99 | [−1.73, −0.26] | 0.01 |
| Mandate × Visit | −0.9 | [−1.79, −0.02] | 0.04 |
| Treatment × Mandate × Visit | 1.24 | [−0.03, 2.51] | 0.06 |
Note.
Self-reported drinking frequency: None: 0% of drinking days for the past 28 days; Occasional drinking: > 0% to <= 30%; Frequent drinking: >30%.
Figure 3B shows that participants mandated for TAC monitoring drank heavily only an average on 3%–5% of the days in the week preceding visit 1, and this frequency was similar for both the CM and the Control groups (p = 0.70). Among the participants in the Control group, this frequency was stable across visits (slope [95% CI] = 0.12 [−0.59–0.83], p = 0.73), which was not significantly different (p = 0.64) from that of the CM participants (slope [95% CI] = 0.37 [−0.37–1.12], p = 0.33). Further, neither the average treatment effect (p = 0.83) nor the treatment effect at visit 8 (p = 0.99) was significant between groups with heavy drinking averaging on only 6% of the days in the last week of the study.
DISCUSSION
The effects of the CM treatment depended upon whether or not individuals had been mandated to TAC monitoring. Among those not mandated to TAC monitoring, CM prevented a return to more frequent heavy drinking in a sample who had initially reduced their heavy drinking. Among those who were mandated to TAC monitoring, our CM treatment provided little additional benefit, likely because most mandated individuals had already substantially reduced their heavy drinking and maintained this reduction throughout the study period resulting in a floor effect with no room for further improvement.
We had originally planned to evaluate CM treatment effectiveness in persons mandated to TAC monitoring who continued to drink heavily. However, when we found so few mandated individuals engaged in heavy drinking, we expanded the enrollment criteria to include those not mandated to TAC monitoring. Within the Non-Mandated group, about half to two-thirds of the participants met our TAC criterion for not drinking heavily in the initial study week, but this proportion declined steadily in those assigned to the Control condition, whereas participants in the CM condition maintained their success over the study period. Thus, the success of our CM intervention was to prevent a return to heavy drinking in those who had initially moderated their heavy drinking among DWI arrestees. This further suggests that the benefits of judicially-mandated alcohol monitoring appear to be to provide a similar motivation to not return to heavy drinking. Within the Mandated DWI arrestees, we also must conclude that CM did not motivate individuals to initiate an episode of reduced heavy drinking. Nonetheless, we have previously reported that the same CM intervention did in fact initiate episodes of reduced heavy drinking in a general community sample of heavy drinkers (Dougherty et al., 2014; Dougherty, Karns, et al., 2015).
These findings are consistent with the notion that CM has two functions. First, reinforcing the desired behavior, in this case, an absence of heavy drinking. As reinforcement works by increasing the probability of the behavior that precedes it, only if an absence of heavy drinking occurred can it be reinforced by the delivery of the incentive (for discussion, see Lamb et al., 2004, 2010). Put more simply, only in those who initially reduce their heavy drinking to a level that meets our criterion, our CM program would be expected to reinforce their absence of heavy drinking and promote this continued reduction in heavy drinking. Thus, if our CM program effectively reinforced behavior, the expected result would be the prevention of a return to heavy drinking, which was what was observed. Another therapeutic function CM can have is promoting the initiation of behavior, a motivating or discriminative function. In this case, more people in the CM group would be expected to initiate a reduction in heavy drinking, an effect that was not observed in the DWI population (current study), though it did occur in community samples who had not already initiated these reductions (Dougherty, Karns, et al., 2015; Dougherty, Lake, et al., 2015).
There are several possible ways to enhance the effects of CM in a DWI population. One way is to offer larger incentives (for examples in other substances, see Kirby et al., 1998, Silverman et al., 1999, Stitzer & Bigelow, 1984, and for a discussion, see Lamb et al., 2004). Whether or not higher incentive amounts would prove practical, acceptable, or cost-effective for DWI offenders remains to be examined. In this study, we provided payments contingent on an entire week without heavy drinking, a unit of behavior that tended to occur infrequently for most of our Non-Mandated participants. However, days without heavy drinking occurred in all our participants and would provide a unit of behavior that could have been shaped into more sustained periods without any heavy drinking at all (for discussions of shaping, see Galbicka, 1994, Lamb et al., 2004, 2010). Such shaping procedures also would be expected to benefit from the use of escalating payment schedules (Romanowich & Lamb, 2015), which appear to differentially reinforce extended sequences of the target behavior (Roll et al., 1996; Roll & Higgins, 2000). While the current study used relatively modest fixed incentives once a week and achieved statistically significant reductions in recidivism for many Non-Mandated participants, daily reinforcements using an escalating payment could certainly have achieved superior results—perhaps even within the Mandated group.
These findings extend the previous literature using CM to reduce heavy drinking. For instance, in a study of 30 community-recruited heavy drinkers, Dougherty, Lake, et al. (2015) found that CM significantly increased the proportion of participants meeting a TAC-based contingency targeting heavy drinking from 9.6% to 44.2% during CM. Barnett et al. (2011) also reported that a 2-week CM intervention using a TAC-based contingency among 13 community-sampled heavy drinkers produced an average 72% reduction in TAC.
Other studies have used other alcohol monitoring technologies to implement CM to reduce alcohol consumption among community-recruited participants. Koffarnus et al. (2018) showed the efficacy of CM based on remote BrAC monitoring for 40 treatment-seeking community-recruited drinkers: the contingency group showed 47 percentage points higher rates of abstinence than the control group during a 21-day intervention. Similarly, Oluwoye, Reneau, et al. (2020) showed that a 4-week CM intervention based on remote BrAC monitoring was effective to increase the likelihood of submitting alcohol-negative BrAC samples. A BrAC-based CM was also used by Averill et al. (2018) in a pilot trial in 37 community-recruited DWI offenders. Although those investigators did not report whether any of their subjects were court-mandated to wear SCRAM, they did have subjects who wore the device and reported a significant reduction in weekly peak TAC from 0.15 g/dL at week 1 to 0.09 g/dL at week 6. Though our findings cannot be directly compared to most of these other studies of CM for alcohol because of differences in outcome measures, type, and length of intervention, or types of monitoring technologies used for CM reinforcement, we can conclude that CM can be effective in facilitating reduced drinking among community samples of heavy drinkers including DWI arrestees.
In those mandated to TAC monitoring, recidivism was minimal and our CM program was of little additional benefit. This finding was unexpected in at least two ways. First, as already alluded to, we expected much higher rates of recidivism in our sample of DWI arrestees, yet objectively, the Mandated participant maintained low levels of heavy drinking throughout the intervention. These low rates of recidivism may also be true in other jurisdictions. For instance, Long et al. (2009) in South Dakota reported that 75% were fully compliant and 95% had two or fewer drinking episodes—though no objective alcohol-monitoring devices were used to verify these rates. In contrast, McKelvie (2005) found that 56% of 319 individuals in Alaska who were mandated with TAC monitoring were fully compliant according to court-appointed officials. One question that can be raised from the low levels of heavy drinking among the Mandated participants is whether this indicates that everyone should be mandated to TAC monitoring. Unfortunately, those mandated to TAC monitoring were determined by judicial preference, and not randomization. Though anecdotal evidence suggests judges make these decisions based on personal preference, we do not know what other factors may have introduced confounding factors that would prevent direct comparisons between mandated and non-mandated populations. A randomized control trial of mandated TAC monitoring would be necessary to understand the effectiveness and cost-effectiveness of this procedure to reduce heavy drinking in those arrested for DWI offenses.
Pretrial monitoring of TAC is not without costs and complications. First, it costs offenders the expense of monitoring on top of other legal expenses they incur. Second, it costs the courts and pretrial services the expense of monitoring results and acting upon offenders who do not respond appropriately. It may be that the most effective approach is some hybrid of CM and judicial monitoring. For example, DWI offenders may be required initially to enter into a deposit contract that would pay the costs of TAC monitoring, but then could automatically earn back their expense as an even more effective reinforcement contingency requiring less judicial oversight. Only if they continually fail to refrain from heavy drinking, would enter into judicially monitored TAC. This might minimize both the costs to the judicial system of monitoring individuals arrested for DWI offenses and a return to heavy drinking by these individuals.
It is unclear, to what extent a return to heavy drinking represents a return to DWI reoffending or to what extent its absence predicts a future absence of reoffence. Most individuals in all groups of this study were required to have interlock devices installed on their cars, which should reduce their ability to drive while intoxicated (Beck et al., 2015; Marques et al., 1999). Thus, heavy drinking may represent drinking at home or the use of transportation other than their own vehicle rather than re-offense per se. Even though those who do not drink heavily also would be less likely to drive with blood alcohol levels above the legal limit, it is also reasonable to suppose that the absence of heavy drinking is a better prognostic indicator of the absence of future DWI than the presence of heavy drinking.
There are several limitations to our study. First, our a priori plan included a 30-day pre-intervention TAC monitoring period to identify eligible participants among the mandated population who had at least two heavy drinking events prior to the intervention period. However, the observed lack of heavy drinking led us to rely on self-reported alcohol use to characterize baseline drinking patterns and to open up enrollment to DWI offenders whose judge did not mandate TAC monitoring. As shown in previous studies (Dougherty et al., 2014; Mathias et al., 2018), a pre-intervention TAC monitoring period would have added confidence in drinking verification and shown within-subject evidence of an intervention effect. Second, we used SCRAM ankle monitors for TAC monitoring to define whether or not heavy drinking occurred according to procedures previously published (Karns-Wright et al., 2018; Roache et al., 2015) Despite the strengths of this device such as passive and near-continuous monitoring and relatively extensive validation, the SCRAM device has serious limitations such as physical discomfort, stigma, and embarrassment (Averill et al., 2018; Barnett et al., 2017) that dimmish its acceptability for more widespread use without a court order. Our study could have used home-based cellular technology to upload TAC data, but we did not, resulting in the requirement for participants to visit our clinic weekly to upload their TAC monitoring data. Given the increasing availability of more convenient and less intrusive alcohol monitoring technologies equipped with advanced mobile connectivity, future research needs to validate those technologies for clinical use and examine how to best use those emerging technologies to deliver interventions like our study to reduce heavy alcohol use.
In summary, in those mandated to TAC monitoring, heavy drinking was infrequent during our study, and being assigned to the CM group had little effect on their drinking behaviors. In contrast, in individuals not mandated to TAC monitoring, heavy drinking was infrequent in most participants initially but increased over time in the control group. Fortunately, this increase in heavy drinking was prevented by our CM intervention. These results suggest that randomized trials of the effectiveness of mandated TAC monitoring are merited, or that some combination of CM and mandated TAC monitoring be evaluated to determine the most cost-effective means of reducing DWI recidivism, at least in the shorter term.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institutes of Health [award numbers R01AA014988; R01 AA014988-15-S1]. Authors are solely responsible for the manuscript, which does not necessarily represent the official views of the National Institutes of Health. Funders had no role in the design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Funding information
National Institutes of Health, Grant/Award Number: 3R01 AA014988-15-S1 and R01AA014988
Footnotes
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare.
REFERENCES
- Alessi SM & Petry NM (2013) A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction, 108, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill F, Brown TG, Robertson RD, Tchomgang A, Berbiche D, Nadeau L et al. (2018) Transdermal alcohol monitoring combined with contingency management for driving while impaired offenders: a pilot randomized controlled study. Traffic Injury Prevention, 19, 455–461. [DOI] [PubMed] [Google Scholar]
- Babor TF & Higgins-Biddle JC (2001) Brief intervention for hazardous and harmful drinking: A manual for use in primary care, World Health Organization. [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM & Swift RM (2017) A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction, 112, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R & Colby SM (2011) Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug and Alcohol Dependence, 118, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KH, Kelley-Baker T & Voas RB (2015) DUI offenders’ experience with an ignition interlock program: comparing those who have and have not adapted from their primary drinking location. Traffic Injury Prevention, 16, 329–335. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR & Badger GJ (2008) Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Experimental and Clinical Psychopharmacology, 16, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann B, Beike J, Köhler H, Heinecke A & Bajanowski T (2002) Incidence of alcohol dependence among drunken drivers. Drug and Alcohol Dependence, 66, 7–10. [DOI] [PubMed] [Google Scholar]
- Brown TG, Dongier M, Ouimet MC, Tremblay J, Chanut F, Legault L et al. (2010) Brief motivational interviewing for DWI recidivists who abuse alcohol and are not participating in DWI intervention: a randomized controlled trial. Alcoholism, Clinical and Experimental Research, 34, 292–301. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T & Rounsaville BJ (2002) Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology, 10, 54–63. [DOI] [PubMed] [Google Scholar]
- Cavaiola AA, Strohmetz DB & Abreo SD (2007) Characteristics of DUI recidivists: a 12-year follow-up study of first time DUI offenders. Addictive Behaviors, 32, 855–861. [DOI] [PubMed] [Google Scholar]
- Cavaiola AA & Wuth C (2016) Assessment and treatment of the DWI offender. New York, NY: Routledge. [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ & Marsch LA (2019) Technology-based contingency management in the treatment of substance-use disorders. Perspectives on Behavior Science, 42, 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL et al. (2014) Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug and Alcohol Dependence, 142, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, Liang Y, Lake SL, Roache JD et al. (2015) Transdermal alcohol concentration data collected during a contingency management program to reduce at-risk drinking. Drug and Alcohol Dependence, 148, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns TE, Mullen J et al. (2015) Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcoholism, Clinical and Experimental Research, 39, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn CE & Kang D (2021) Transdermal alcohol monitors: research, applications, and future directions. In: Frings D & Albery IP (Eds.) The handbook of alcohol use, the handbook of alcohol use. London, UK: Academic Press, pp. 551–562. [Google Scholar]
- Federal Bureau of Investigation. (2020) Table 29: Estimated number of arrests, United States 2019 Department of Justice, Washington, DC. [Google Scholar]
- Fell JC (2014) Update: repeat DWI offenders involvement in fatal crashes in 2010. Traffic Injury Prevention, 15, 431–433. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Dugosh KL, Kirby KC & Seymour BL (2014) Contingency management for cocaine treatment: cash vs. vouchers. Journal of Substance Abuse Treatment, 47, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE & McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Galbicka G (1994) Shaping in the 21st century: moving percentile schedules into applied settings. Journal of Applied Behavior Analysis, 27, 739–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenphire Clincard. (2021). https://greenphire.com/clincard/
- Hasin DS, Shmulewitz D & Keyes K (2019) Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug and Alcohol Dependence, 205, 107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST (1997) Applying learning and conditioning theory to the treatment of alcohol and cocaine abuse. In: Johnson BA & Roache JD (Eds.) Drug addiction and its treatment: nexus of neuroscience and behavior. Philadelphia, PA: Lippincott, Williams, & Wilkins, pp. 367–385. [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DEH & Dantona RL (2000) Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology, 68, 64–72. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hatch JP & Lamb RJ (2005) Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction, 100, 159–167. [DOI] [PubMed] [Google Scholar]
- Karns-Wright TE, Dougherty DM, Hill-Kapturczak N, Mathias CW & Roache JD (2018) The correspondence between transdermal alcohol monitoring and daily self-reported alcohol consumption. Addictive Behaviors, 85, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SYH & Caine ED (2002) Utility and limits of the mini mental state examination in evaluating consent capacity in Alzheimer’s disease. Psychiatric Services, 53, 1322–1324. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Lamb RJ & Platt JJ (1998) Schedule of voucher delivery influences initiation of cocaine abstinence. Journal of Consulting and Clinical Psychology, 66, 761–767. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Bickel WK & Kablinger AS (2018) Remote alcohol monitoring to facilitate incentive-based treatment for alcohol use disorder: a randomized trial. Alcoholism, Clinical and Experimental Research, 42, 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G & Iguchi MY (2004) Improving contingency management programs for addiction. Addictive Behaviors, 29, 507–523. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G & Iguchi MY (2010) Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology, 78, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Galbicka G, Kirby KC & Iguchi MY (2005) Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology, 13, 83–92. [DOI] [PubMed] [Google Scholar]
- Long L, Talpins SK & DuPont RL (2009) The South Dakota 24/7 Sobriety Project: A Summary Report Washington DC. [Google Scholar]
- Marques PR, Voas RB, Tippetts AS & Beirness DJ (1999) Behavioral monitoring of DUI offenders with the alcohol ignition interlock recorder. Addiction, 94, 1861–1870. [DOI] [PubMed] [Google Scholar]
- Mathias CW, Hill-Kapturczak N, Karns-Wright TE, Mullen J, Roache JD, Fell JC et al. (2018) Translating transdermal alcohol monitoring procedures for contingency management among adults recently arrested for DWI. Addictive Behaviors, 83, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon VV, Heath AC, Edenberg HJ, Grucza RA, Hesselbrock VM, Kramer JR et al. (2009) Alcohol criteria endorsement and psychiatric and drug use disorders among DUI offenders: greater severity among women and multiple offenders. Addictive Behaviors, 34(5), 432–439. Available from: 10.1016/j.addbeh.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvie AR (2005) An implementation of remote alcohol monitoring in Alaska [online]. Available from: Alaska Justice Statistical Analysis Center, University of Alaska. [Google Scholar]
- Mullen J, Ryan SR, Mathias CW & Dougherty DM (2015) Treatment needs of driving while intoxicated offenders: the need for a multimodal approach to treatment. Traffic Injury Prevention, 16, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SE, Belkin K, LaPlante DA, Bosworth L & Shaffer HJ (2015) A prospective study of psychiatric comorbidity and recidivism among repeat DUI offenders. Archives of Scientific Psychology, 3(1), 8–17. Available from: 10.1037/arc0000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHTSA. (2020) Traffic safety facts: Overview of motor vehicle crashes in 2019 (DOT HS 813 060), Washington, DC. [Google Scholar]
- Oluwoye O, Kriegel L, Alcover KC, McPherson S, McDonell MG & Roll JM (2020) The dissemination and implementation of contingency management for substance use disorders: a systematic review. Psychology of Addictive Behaviors, 34, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwoye O, Reneau H, Herron J, Alcover KC, McPherson S, Roll J et al. (2020) Pilot study of an integrated smartphone and breathalyzer contingency management intervention for alcohol use. Journal of Addiction Medicine, 14, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Gueorguieva R, O’Malley SS, Arias A, Sevarino KA et al. (2018) Mecamylamine treatment for alcohol dependence: a randomized controlled trial. Addiction, 113, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ & Zajac K (2017) Contingency management treatment for substance use disorders: how far has it come, and where does it need to go? Psychology of Addictive Behaviors, 31, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L & Roll J (2006) Contingency management for treatment of substance use disorders: a meta-analysis. Addiction, 101, 1546–1560. [DOI] [PubMed] [Google Scholar]
- Roache JD, Karns TE, Hill-Kapturczak N, Mullen J, Liang Y, Lamb RJ et al. (2015) Using transdermal alcohol monitoring to detect low-level drinking. Alcoholism, Clinical and Experimental Research, 39, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD, Karns-Wright TE, Goros M, Hill-Kapturczak N, Mathias CW & Dougherty DM (2019) Processing transdermal alcohol concentration (TAC) data to detect low-level drinking. Alcohol, 81, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM & Higgins ST (2000) A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence, 58, 103–109. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST & Badger GJ (1996) An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis, 29, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P & Lamb RJ (2015) The effects of fixed versus escalating reinforcement schedules on smoking abstinence. Journal of Applied Behavior Analysis, 48, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Lamb RJ & Dallery J (2008) Tobacco. In: Contingency management in substance abuse treatment, contingency management in substance abuse treatment. New York, NY: The Guilford Press, pp. 99–119. [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE & Stitzer ML (1999) Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology, 146, 128–138. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR et al. (1996) Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry, 53, 409–415. [DOI] [PubMed] [Google Scholar]
- Sobell LC & Sobell MB (1992) Timeline follow-back. In: Litten RZ & Allen JP (Eds.) Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press, pp. 41–72. [Google Scholar]
- Stitzer ML & Bigelow GE (1984) Contingent reinforcement for carbon monoxide reduction: within-subject effects of pay amount. Journal of Applied Behavior Analysis, 17, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA & Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction, 84, 1353–1357. [DOI] [PubMed] [Google Scholar]
- Swift R (2003) Direct measurement of alcohol and its metabolites. Addiction, 98(Suppl 2), 73–80. [DOI] [PubMed] [Google Scholar]
- Vanlaar W, Robertson R, Schaap D & Vissers J (2010) Understanding behavioural patterns of interlocked offenders to inform the efficient and effective implementation of interlock programs: How offenders on an interlock learn to comply, Traffic Injury Research Foundation, Ottawa, Canada. [Google Scholar]
- Warren-Kigenyi N & Coleman H (2014) DWI Recidivism in the United States: An Examination of State-Level Driver Data and The Effect of Look-Back Periods on Recidivism Prevalence (DOT HS 811 991), National Highway Traffic Safety Administration, Washington, DC. [Google Scholar]
- West MA, Smith A, Helm T, Reicks P, Thorson M & Gipson J (2019) Use of a statewide public health tool to estimate miles driven by intoxicated drivers. JAMA Surgery, 154, 875–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Suchting R, McKay SA, San Miguel GG, Vujanovic AA, Stotts AL et al. (2020) Baseline cocaine demand predicts contingency management treatment outcomes for cocaine-use disorder. Psychology of Addictive Behaviors, 34, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, Miller TR & Blincoe LJ (2013) Costs of alcohol-involved crashes, United States, 2010. Annals of Advances in Automotive Medicine, 57, 3–12. [PMC free article] [PubMed] [Google Scholar]


