Abstract
Background:
Lesion-related factors are associated with severity of language impairment in persons with aphasia. The extent to which demographic and health factors predict language impairment beyond traditional cortical measures remains unknown. Identifying and understanding the contributions of factors to predictive models of severity constitutes critical knowledge for clinicians interested in charting the likely course of aphasia in their patients and designing effective treatment approaches in light of those predictions.
Methods:
Utilizing neuroimaging and language testing from our cohort of 224 individuals in the chronic stage of recovery from a left-hemisphere stroke in a cross-sectional study, we first conducted a lesion symptom mapping (LSM) analysis to identify regions associated with aphasia severity scores. After controlling for lesion volume and damage to pre-identified areas, three models were created to predict severity scores: 1) Demographic Model (N = 147); 2) Health Model (N = 106); and 3) Overall Model (N = 106). Finally, all identified factors were entered into a Final Model to predict raw severity scores.
Results:
Two areas were associated with aphasia severity—left posterior insula and left arcuate fasciculus. The results from the Demographic Model revealed non-linguistic cognitive ability, age at stroke, and time post-stroke as significant predictors of severity (P = .005; P = .02; P = .001, respectively), and results from the Health Model suggested the extent of leukoaraiosis is associated with severity (P = .0004). The Overall Model showed a relationship between aphasia severity and cognitive ability (P = .01), time post-stroke (P = .002), and leukoaraiosis (P = .01). In the Final Model, which aimed to predict raw severity scores, demographic, health, and lesion factors explained 55% of the variance in severity, with health and demographic factors uniquely explaining nearly half of performance variance.
Conclusions:
Results from this study add to the literature suggesting patient-specific variables can shed light on individual differences in severity beyond lesion factors. Additionally, our results emphasize the importance of non-linguistic cognitive ability and brain health in aphasia recovery.
Keywords: Aphasia, Stroke, Recovery, Leukoaraiosis, Aging
1. Introduction
Considerable research has focused on identifying factors that impact aphasia prognosis and treatment response, including lesion-related and non-lesion-related measures. While lesion size and location have consistently been associated with symptom profiles of PWA, the extent to which demographic and health characteristics are related to aphasia severity remains unclear. For example, although stroke incidence has been found to be more prevalent for men (Appelros et al., 2009), the rate of post-stroke aphasia has been found to be higher among women (Berglund et al., 2017). Other studies, however, have reported the opposite, suggesting a higher incidence of post-stroke aphasia in men (Basso, 1992; Kertesz & Sheppard, 1981). Similarly, the impact of sex on diagnosis of aphasia type and severity is unclear in the literature (Godefroy et al., 2002; Hier et al., 1994). The inconsistency in the literature regarding demographic factors’ impact on aphasia severity and prognosis extends to other factors as well, including education level, socio-economic status (SES), pre-morbid handedness, and non-linguistic cognitive ability (Connor et al., 2001; González-Fernández et al., 2011; Holland et al., 2017; Johnson, Basilakos, Yourganov, & et al., 2019; Kim et al., 2019; Laska et al., 2001; Plowman et al., 2012; Watila & Balarabe, 2015). One possible explanation for conflicting reports may be due to the interrelationship between these factors. For example, SES, education, and non-linguistic cognitive ability are likely closely linked to realted factors such as pre-morbid intelligence, literacy level, insurance status, and existence of learning disabilities pre-stroke. It is also important to note that studies that have observed a relationship between SES, education, and severity have been in acute cases, while studies of chronic severity do not observe an effect.
Some studies suggest that the relationship between demographic factors and aphasia severity is rather weak compared to lesion-related factors (i.e., lesion size and location) (Plowman et al., 2012; Watila & Balarabe, 2015). However, more recently, studies have presented evidence that some non-lesion-related factors such as age at stroke, time post-stroke and overall health are associated with aphasia severity in the chronic stage (Basilakos, Stark, Johnson, & et al., 2019; Harnish et al., 2018; Johnson et al., 2019; Wilmskoetter et al., 2019). Rather, age at injury, time post-stroke, and baseline severity (Osa Garcia et al., 2020) have been the most consistent demographic predictors reported in the literature as being associated with chronic severity and recovery (Johnson et al., 2019). Recently, our group found that the older an individual is at the time of their stroke, the more likely they will show a greater degree of language decline as they progress in the chronic stage of recovery (Johnson et al., 2019). This finding is consistent with the literature that reports older age predicts poorer aphasia prognosis post stroke (Laska et al., 2001; Lendrem & Lincoln, 1985).
Patient-specific health factors such as comorbid diabetes, rate of exercise, body mass index (BMI), and extent of white matter hyperintensities (WMH) have also been shown to add to predictive models of aphasia severity, recovery, and treatment outcome. Harnish and colleagues (2012) found that physical exercise done in tandem with traditional language therapy was associated with greater rates of improvement compared to a group who received only language therapy in isolation. In a recent publication from our group, the rate of exercise was found to be a predictor of chronic aphasia improvement in individuals with a comorbid diabetes, suggesting that maintenance of health when diagnosed with comorbid diseases via regular physical exercise may elicit a neuroprotective response (Johnson et al., 2019). BMI, a measure of obesity, has been implicated in the stroke literature for its paradoxical effect on survival and functional recovery. This paradox indicates a “survival benefit” and greater likelihood of functional outcomes in obese patients who have a stroke (Chiquete et al., 2010; Zhao et al., 2014; Kim et al., 2019). However, no study to our knowledge has investigated BMI in chronic aphasia in addition to other health-related measures to determine the significance of the obesity effect. Indicators of poor health (i.e., comorbidity presence/poor management, obesity, substance abuse) have all been associated with a measure of overall brain health as indicated by presence and extent of WMH (Vangberg et al., 2019; Fazekas, 1987; Lin et al., 2017). Further, WMH have been linked to cognitive decline and depression (Ladis, 2011). Recently, WMH ratings have been reported to be associated with aphasia severity and recovery in both the acute and chronic stages of stroke. Basilakos et al. (2019) presented evidence that the severity of leukoaraiosis, as measured by the extent of WMH in the intact hemisphere, was associated with poorer aphasia recovery. Similarly, Wilmskoetter et al., 2019 found that the extent of WMH is an indicator of greater aphasia severity. These findings provide a novel perspective in identifying factors that may impact aphasia severity, suggesting that individual factors, including brain health, may shed light on individual variability in performance, prognosis, and perhaps may provide some insight into aphasia treatment response.
Including lesion-related measures (such as size and location) in predictive models of aphasia progression often explains a sizeable amount of variance in individual performance (Forkel et al., 2014; Marebwa et al., 2017; Plowman et al., 2012; Thye & Mirman, 2018). It is generally accepted that larger lesions are associated with more severe impairments and poor aphasia recovery. Lesion size also negatively influences overall prognosis, with smaller lesions being associated with more recovery and larger lesions being associated with less improvement and an overall lower likelihood of recovery (Goldenberg & Spatt, 1994). Clinical presentation across individuals with aphasia is highly variable and studies investigating individual lesion profiles have attempted to explain this variability. In a study investigating 45 persons with aphasia (PWA), Mazzoni and colleagues (Mazzoni et al., 1992) documented recovery patterns based on lesion size and described that those with small lesions improved in both receptive and expressive language domains, whereas individuals with larger lesions showed improvement only in the receptive domain. Similarly, in a study investigating the prognosis of 669 PWA, Maas and colleagues (Mass et al., 2012) found that individuals with smaller strokes are more likely to have an overall better prognosis.
In addition to lesion size, lesion location has also been shown to significantly predict variance in aphasia severity. In a comprehensive review of non-lesion- and lesion-related factors associated with language impairment due to cerebral insult, Plowman and colleagues (Plowman et al., 2012) concluded that lesion size and location are the most important clinical predictors of aphasia type and severity. Specifically, damage to opercular and insular regions in conjunction with inferior frontal damage has been associated with more severe expressive deficits (Hart & Gordon, 1990), whereas damage to the superior temporal gyrus has been associated with poor language recovery and global deficits (Hanlon et al., 1999). More recently, investigations of white matter connections and their association with overall aphasia severity have found that anterior-posterior language connections are associated with performance on language assessments. Specific to overall aphasia severity, the integrity of the superior longitudinal (SLF) and arcuate fasciculus has been linked to expressive and receptive language as well as overall aphasia severity and overall language performance (Kümmerer et al., 2013; Lee et al., 2021; Rosso et al., 2015). The question remains, however, what extent patient-specific demographic variables add to predictive modeling of longitudinal aphasia progression that lesion data, alone, cannot address.
Taken together, previous work does provide evidence that overall aphasia severity is associated with some demographic and health factors, however, relationships appear to be inconsistent in the present literature. Further, it is also unclear the extent to which residual variability in aphasia severity is explained by non-lesion-related variables after accounting for damage to critical language regions (measure of lesion location) and lesion size. The aim of the present study was to investigate the impact of demographic and health factors that are often implicated to predict severity to identify those that explain the variance in behavior above and beyond lesion profile. Using a data-driven approach to address this aim, we will first identify critical language regions for aphasia severity and regress out damage to those regions as well as total lesion volume from aphasia severity scores. Demographic and health factors will then be added into models to predict the remaining variance in performance to identify factors which explain unique variance in behavior. As discussed above, insular regions and white matter tracts connecting anterior-posterior language regions have been implicated in the literature as being regions most associated with measures of overall aphasia severity. It is for this reason that we hypothesized those regions and white matter connections (i.e., insular cortex, AF, and SLF) as being identified as critical language regions which can give an indication of lesion location. Furthermore, we hypothesize that: (i) demographic factors which show the most consistency in the literature (i.e., age at stroke, time post-onset) and non-linguistic cognitive ability will be associated with severity and (ii) health factors, such as extent of leukoaraiosis and the presence/maintenance of additional comorbidities (i.e., diabetes and obesity) will be associated with severity.
2. Materials and methods
2.1. Participants
This was a retrospective study of prospectively collected data on individuals who previously experienced (≥6 months post-stroke) a stroke in the left hemisphere. We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Data were obtained from a database of individuals who participated in aphasia treatment studies at the University of South Carolina (UofSC) or Medical University of South Carolina (MUSC) from 2005 to 2020. No part of the study procedures or analyses was pre-registered prior to the research being conducted.
Inclusion criteria for this analysis were defined prior to data analysis and were as follows: availability of aphasia severity scores as indicated by a Western Aphasia Battery–Revised (Kertesz, 2007) (WAB-R) Aphasia Quotient (WAB-AQ), structural neuroimaging data obtained within 6 months of language testing (M = 6.0 ± 19.4 days; range = 0–163 days), 21–85 years old, pre-morbidly right-handed, and in the chronic stage of recovery (≥6 months post-onset) following a left hemisphere stroke (M = 38.5 ± 45.9; range = 6–212). Participants with multiple strokes (regardless of stroke type) were admitted into this study if all stroke events were in the left hemisphere. Participants were excluded if they had history of speech/language impairment or other neurologic deficits affecting the brain. A total of 224 participants met inclusion criteria and their data were included in the lesion symptom mapping (LSM) analysis. Participant demographic information is presented in Table 2. Of the 224 participants, 147 (56 female; age at stroke: M = 56.0 ± 12.1, range = 27–80) had completed a detailed case history form of demographic information. All demographic factors were collected from a questionnaire completed by the participant or participant’s caretaker. Median household income from the US Census Bureau was calculated using participant-provided zip-codes as a proxy for SES (González-Fernández et al., 2011). Non-linguistic cognitive ability, as assessed by the Wechsler Adult Intelligence Scale (Wechsler,1955) (WAIS) Matrices sub-test, and demographic variables of interest (sex, years of education, age at stroke, time post-stroke, and SES) were entered into the Demographic Model. The WAIS Matrices sub-test was selected to attain a measure of nonverbal abstract problem solving and inductive reasoning. In this subtest, participants are shown a series of patterns with one missing element and are subsequently asked to identify (using any communication strategy: gesturing, pointing, speaking, etc.) the pattern that completes the series from four options. Because this subtest does not require any overt, verbal response, it is easily administrable for all aphasia types and severities. The Matrices test was administered and scored in a standard manner and raw scores were normed according to the age-normed scores in the study manual, as this approach is preferred when relating behavioral scores within clinical populations (Wechsler,1955).
Table 2 –
Group Descriptive Statistics.
| Demographic Model (N = 147) | Health Model (N = 106) | |
|---|---|---|
|
| ||
| Age at stroke (years) | ||
| M(SD) | 56.0 (12.1) | 56.0 (12.0) |
| Range | 27.0–80.0 | 27.0–79.0 |
| Sex, n (%) | ||
| Female | 56 (38%) | 37 (35%) |
| Male | 91 (62%) | 69 (65%) |
| Education (years) | ||
| M(SD) | 15.2 (2.3) | 15.6 (2.2) |
| Range | 10.0–20.0 | 12.0–20.0 |
| SES (USD) | ||
| M(SD) | $60,511.22 ($20,038.43) | $58,814.29 ($19,314.50) |
| Range | $23,086-$113,802 | $23,086-$111,250 |
| WAB-AQ | ||
| M(SD) | 62.5 (26.0) | 62.2 (24.7) |
| Range | 5.6–99.6 | 5.6–99.6) |
| WAIS Matrices | ||
| M (SD) | 10.1 (3.7) | 10.3 (3.6) |
| Range | .0–23.0 | .0–23.0 |
| Lesion Volume | ||
| M (SD) | 123,590 (96,471) | 120,588 (93,177) |
| Range | 117–467,458 | 117–467,458 |
| Prop. Damage ROIs | ||
| M (SD) | .53 (.3) | .52 (.3) |
| Range | .0–1.0 | .0–1.0 |
| Exercise >30 min/week | ||
| M (SD) | NA | 3.6 (2.5) |
| Range | .0–14.0 | |
| Body Mass Index | ||
| M (SD) | NA | 27.3 (5.4) |
| Range | 17.4–47.3 | |
| Fazekas Rating | ||
| M (SD) | NA | 3.3 (1.6) |
| Range | .0–6.0 | |
| Diabetes, n (%) | ||
| Present | NA | 21 (20%) |
Health variables of interest including presence of diabetes, exercise rate, extent of WMH, and BMI were selected based on previous studies demonstrating an association between these factors and treatment outcomes and/or aphasia progression (Basilakos et al., 2019; Harnish et al., 2018; Johnson et al., 2019; Wilmskoetter et al., 2019; Zhao et al., 2014). These data were available for a subset of our participants (N = 106), therefore a second prediction model was constructed, which included available health factors (presence of diabetes, exercise rate, BMI, and WMH ratings). Diabetes presence was determined by using a self-reported questionnaire. Participants’ rate of exercise was self-reported and indicates the average number of exercise sessions per week (>30min, regardless of type intensity). Finally, WMH ratings were calculated using a visualmanual rating scale, which is described below. Participants with complete data available for demographic and health variables of interest were included in the Demographic, Health, Overall, and Final Models. Supplemental Fig. 1 provides a flowchart detailing participants included in each model.
Because the models included a different subset of participants from the initial cohort of 224 participants, two, one-way ANOVAs were conducted to address potential sampling bias. No significant group differences (P > .05) were revealed across those included and excluded between the initial cohort and the participants in the Demographic Model, nor were any significant differences revealed between the participants included in the Health Model compared to those not included in the Health Model. Supplemental Tables 1 and 2 provide the results from both ANOVAs.
All participant testing took place at research laboratories at UofSC or MUSC and all assessments were administered by, or under the supervision of, an American Speech-Language-Hearing Association certified speech-language pathologist with extensive experience working with individuals with aphasia. Institutional Review Boards at each university approved the studies in which the data were obtained, and all participants completed written informed consent when admitted into initial and subsequent studies.
2.2. Neuroimaging
T1-weighted images utilized an MP-RAGE sequence with the following parameters: isotropic voxel size = 1 mm (Kertesz & Sheppard, 1981), FOV = 256 × 256mm, 192 sagittal slices, 9-degree flip angle using parallel imaging, TR = 2250 ms, TI = 925 ms, and TE = 4.11 ms. T2-weighted images utilized a sampling perfection with application optimized contrasts using a varying flip angle evolution (3DSPACE) sequence protocol with the following parameters: voxel size = 1 mm (Kertesz & Sheppard, 1981), FOV = 256 × 256mm, 160 sagittal slices, TR = 3200 ms, TE = 212 ms, and no slice acceleration. Lesion masks were drawn by a collaborating neurologist (author LB) or trained expert (author RNN) in MRIcroGL12 (Rorden et al., 2012) on T2 MRI scans in native space. The clinical toolbox was used to normalize the shape and size of each individual’s brain (Rorden et al., 2012). Specifically, the T2 scan was aligned to the T1 image (with the subsequent transform applied to the lesion). Next, enantiomorphic normalization was computed for the T1 image (Nachev et al., 2007). The normalization parameters generated by the enantiomorphic normalization procedure were used to transform the lesion-masks into standard (MNI) space for lesion symptom mapping analysis.
2.3. Patient-specific factors obtained from neuroimaging
2.3.1. Lesion symptom mapping to identify critical language regions
To identify neural correlates associated with overall aphasia severity (WAB-AQ), we implemented a univariate LSM analysis with the NiiStat toolbox for MATLAB (www.nitrc.org/projects/niistat). Using the Johns Hopkins University (JHU) neuroanatomical atlas a one-tailed general linear model was performed to determine which regions and/or tracts are associated with more severe aphasia (Faria et al., 2012). The JHU atlas is composed of 189 parcellated regions including cortical and subcortical regions, and white matter tracts. Areas that were damaged in at least 10% (N = 22) of participants were included in analysis. For the LSM analysis, our parameters were as follows: P-values<.05, corrected for multiple comparisons using permutation thresholding (5000 permutations), while also controlling for total lesion volume as a nuisance regressor (Winkler et al., 2014). Regions which survived thresholding were considered “critical language areas” which, when damaged, were associated with lower WAB-AQ scores (more severe impairment). A region mask including these critical language areas was then created. The number of total voxels (mm3) in this region mask was calculated and proportion damage (# of damaged voxels/total voxels) was calculated for all participants. The proportion damage to these regions and total lesion volume were then included as independent variables in a linear model to predict overall aphasia severity (WAB-AQ scores). Residuals from this model were then used as the dependent variable in the Demographic, Health, and Overall Models.
2.3.2. Rating White Matter Hyperintensities (WMH)
Ratings addressing overall brain health, as measured by presence of WMH, were available for a subset of participants (N = 106). Severity of WMH was rated by researchers with considerable experience in this area (authors LJ, NH, and AB) using the Fazekas scale (Fazekas et al., 1987) on T2-weighted MRI scans. The Fazekas scale is a visual scale that rates the presence and severity of WMH across two domains, each on a 0 (absent WMH) to 3 (severe WMH) scale: i) periventricular space (PVH); and ii) in the deep white matter (DWMH). With the assumption that WMH are generally symmetric (Pantoni, 2008) and in conjunction with prior research on post-stroke WMH (Basilakos et al., 2019), Fazekas ratings were completed for the intact right hemisphere and a total Fazekas score was calculated for each participant by adding both PVH and DWMH ratings together (scores between 0 and 6). Consensus ratings between raters, who were blind to participant demographic information, were performed to score severity of WMH. Reliability of Fazekas ratings showed excellent reliability (single measure intraclass correlation coefficient = .86). (Basilakos et al., 2019).
2.4. Multiple linear regression models
Comprehensive demographic information was available for 147 participants. Of those 147 participants, 106 had health information available. Therefore, two main stepwise linear regression models with leave-one-out (LOO) cross-validation (a Demographic Model and a Health Model) were created to identify significant predictors to be included in the Overall Model. Demographic factors included sex, education, age at stroke, time post-stroke, SES, and non-linguistic cognitive ability (WAIS Matrices subtest). Health factors included total Fazekas score, exercise frequency, body mass index (BMI), and presence of diabetes. Variables were evaluated for normal distribution to ensure homoscedastic distribution via visual inspection and Shapiro–Wilk test of normality. Time post-stroke onset was positively skewed; therefore, a log transformation was performed in this data and transformed values were subsequently used in all analyses. No other violations of assumptions for regression analyses were present. All continuous variables were then correlated (Pearson) to investigate and address potential multicollinearity among independent variables. Spearman correlations were also conducted for all relationships including Fazekas ratings. Significant (P < .05) correlation results are shown in a correlation matrix (Fig. 1). Significant factors from the Demographic and Health Models were included in an Overall Model. To account for the lesion, the residual values from the univariate linear regression to predict WAB-AQ from proportion damage to critical regions and lesion volume were used as the dependent variable in the three aforementioned models (Demographic, Health, and Overall). Finally, a LOO linear regression was conducted to predict raw WAB-AQ scores from lesion variables (volume and proportion damage to critical ROIs), and significant health and demographic variables identified in the initial models. This model, because it used raw scores rather than residual values which allowed for ease of interpretability of the contributions of each factor to explain WAB-AQ scores. To illustrate the relationship between the significant main effects in each model and their respective outcome measures, scatterplots between the outcome measure and raw main effects are presented in each figure with the significance value in the model.
Fig. 1 –
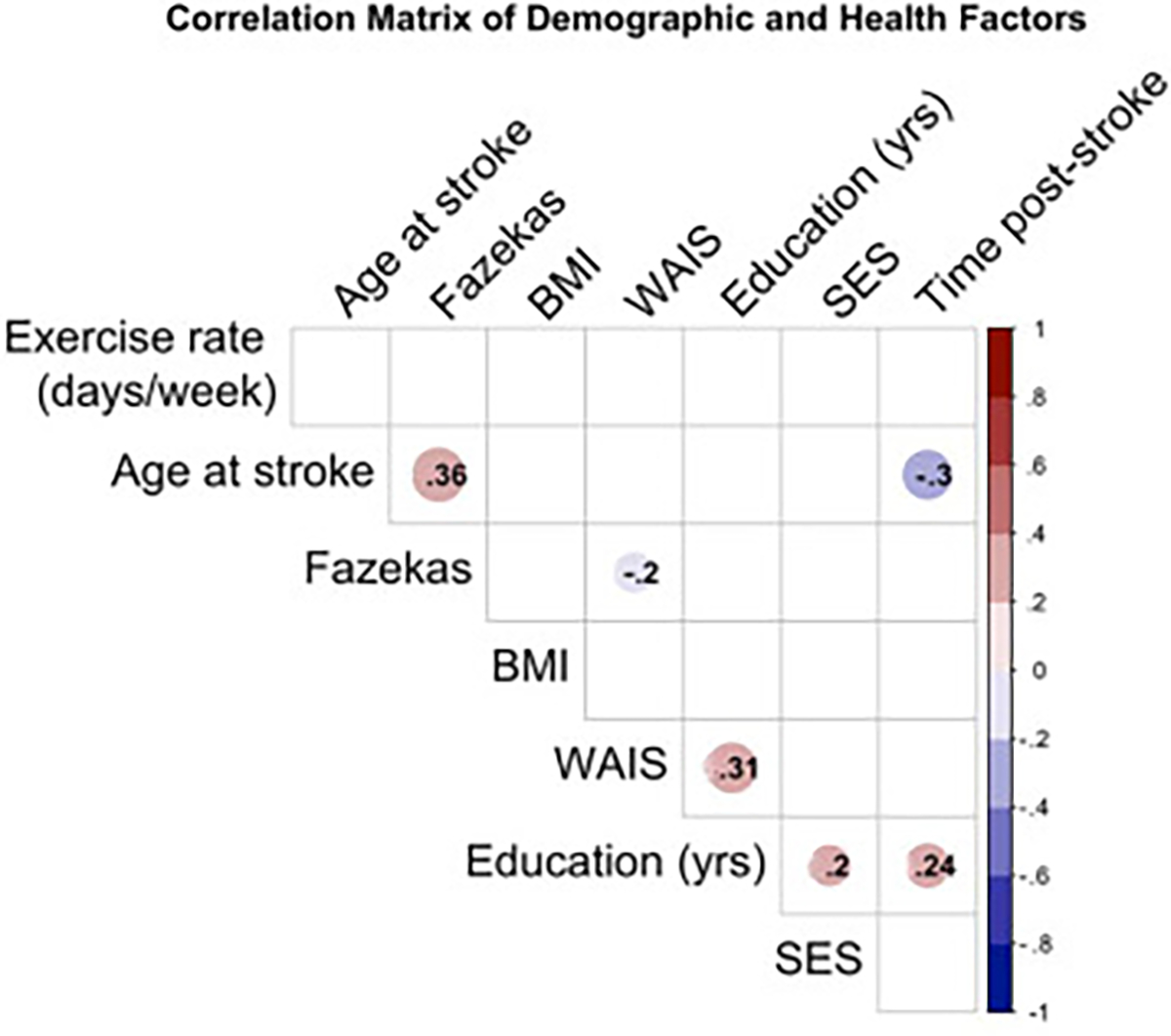
Correlation matrix between all continuous independent variables included in the models. Correlations between continuous factors utilized Pearson correlation, whereas correlations including a variable measured on an ordinal scale (Fazekas) utilized Spearman correlation. Significant correlations (p < .05) are indicated by presence of color (where the lower p-value is indicated by deeper color) around the correlation coefficient between two variables.
All statistical analyses were done using the MATLAB Leave-One-Out toolbox (https://github.com/grigori-yourganov/leave_one_out), and all figures were made using package GGPLOT2 (Wickham, 2016) in statistical software R (Team, 2018). Variables included in at least 5% of iterations with P < .05 were considered statistically significant and are reported. Model outputs from the toolbox provides results from both the stepwise linear model and results when using the LOO cross-validation, therefore t-statistics for significant variables are provided from both outputs.
2.5. Data availability
The conditions of our ethics approval do not permit sharing of the raw MRI data supporting this study with any individual outside the author team under any circumstances. However, de-identified neuroimaging and clinical data are made available in addition to scripts used for figure-making in a GitHub repository (https://github.com/lajohn25/PredictorsBeyondtheLesion_Cortex).
3. Results
3.1. LSM analysis to identify critical regions associated with aphasia severity
The lesion overlay map of the participants included in the atlas-based LSM analysis to identify critical language regions associated with aphasia severity is presented in Fig. 2A. The LSM analysis to predict WAB-AQ revealed one white matter tract and one cortical region, which, when damaged, were associated with more severe aphasia: the superior longitudinal fasciculus (SLF; z = 3.64) and posterior insula (z = 3.15; Fig. 2B and C). It is important to note that the atlas used in the LSM analysis (JHU) describes the SLF as a tract containing connections between the frontal, parietal, occipital, and temporal lobes including language-related areas (Broca’s, Geschwind’s, and Wernicke’s territories). (Description about JHU Multi) Given this description, the high degree of overlap between this region’s coordinates and the arcuate fasciculus (Catani & Thiebaut de Schotten, 2008), and the fact that the JHU atlas does not have a tract dedicated to the arcuate, we will henceforth refer to this tract as the superior longitudinal fasciculus-arcuate branch (SLF-A).
Fig. 2 –
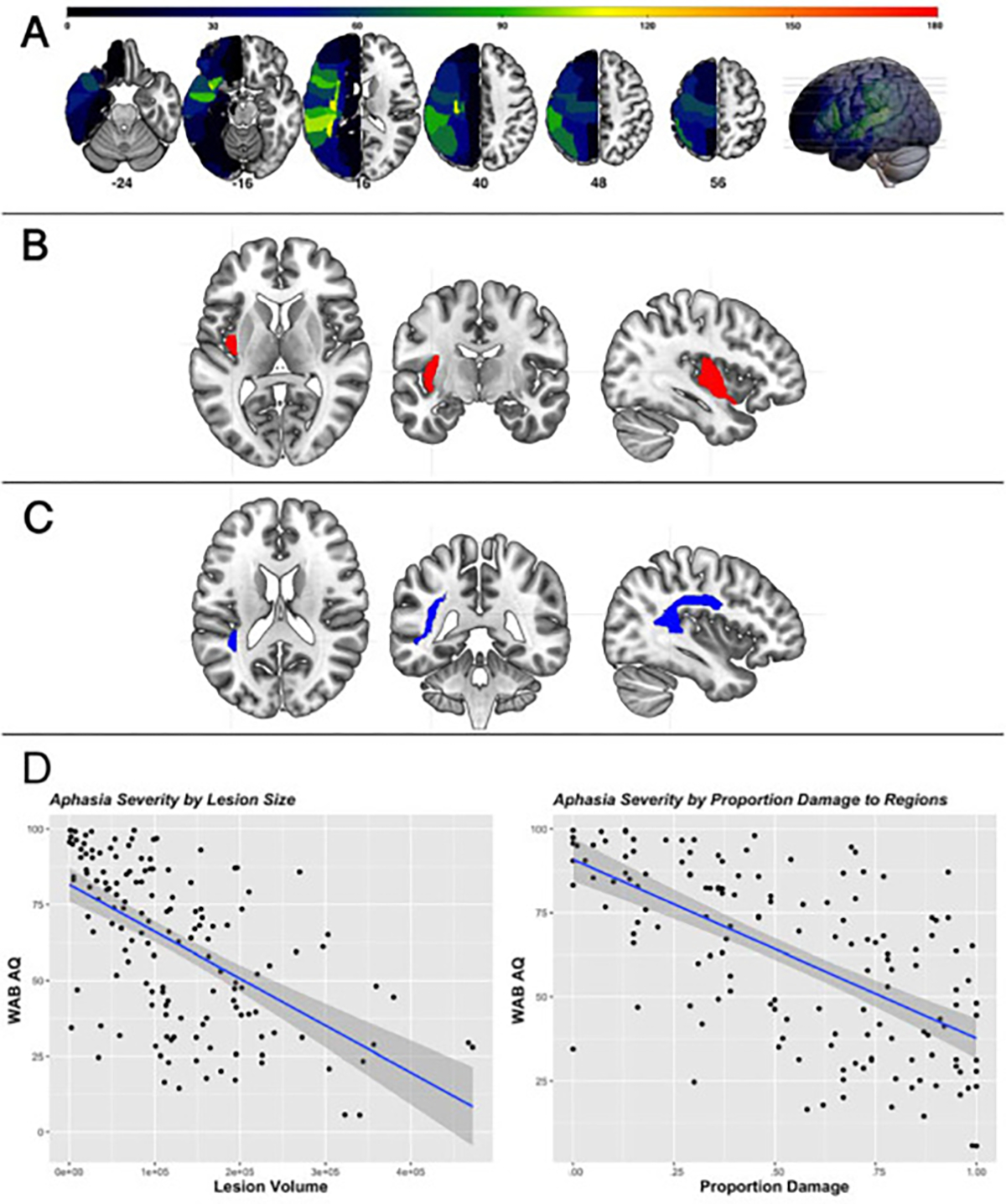
(A) Lesion overlay map of all participants (N = 224). Red indicates ~80% overlap; critical regions segmented in the JHU atlas which predict aphasia severity, (B) Posterior insula (red), (C) Superior longitudinal fasciculus (blue), and (D) Illustration of main effects between lesion size (left) and regional proportion damage (right) and aphasia severity (WAB-AQ) (P = .03;P < .00001, respectively).
To account for the variance explained by damage to critical language regions and total lesion volume, total lesion size (measured in mm3) and proportion damage to the aforementioned region and tract for each participant were calculated included as independent variables in a linear regression model to predict WAB-AQ. This lesion model accounted for 47% of the variance (Adj. R2 = .47, F (2,224) = 101.9), P < .00001), and results are presented in Table 1. To illustrate the significant main effects between WAB-AQ and lesion variables, scatterplots between lesion volume (left) and proportion damage to critical regions (right) and WAB-AQ are presented with their respective p-value from the regression model (Fig. 2D).
Table 1 –
Linear regression results for Lesion Model.
| Linear Regression Model Results |
||||||
|---|---|---|---|---|---|---|
| Est. | Std. Error | tStat | pValue | Adj. R2 (P-value) | ||
|
| ||||||
| Lesion | (Intercept) | 91.1 | 2.5 | 36.3 | <.00001 | |
| Model | Total Lesion Volume(mm3) | −37.6 | 17.1 | −2.2 | .03* | .47 (<.00001) |
| Prop. Damage to Critical ROIs | −46.3 | 6.9 | −6.7 | <.00001** | ||
p < .05;
p < .01.
3.2. Stepwise regression with leave-one-out cross validation results: Demographic Model
Table 2 provides summary statistics of demographic information (N = 147) and health data (N = 106) for the participants included in the regression models. The independent variables included in the Demographic Model included: age-corrected WAIS Matrices scores, time post-stroke (in months), age at stroke, sex, education (in years), and SES (median household income). No interaction effects were included in the model. The dependent variable in this model was aphasia severity after accounting for proportion damage to critical language areas and lesion volume (i.e., residuals from the univariate linear regression described above). Three statistically significant predictors (WAIS, age at stroke, and time post-stroke), illustrated in Fig. 3, were revealed from our Demographic Model, indicating a relationship between non-linguistic cognitive ability (P = .005), age at stroke (P = .02), and time post stroke (P = .001) and aphasia severity even when the effect of lesion size (measured in mm3) and location had been factored out (Fig. 3). The Demographic Model explained 15% of the variance in residual WAB AQ scores unexplained by the lesion (Adj. R2 = .15, F (18.2,147) = 9.55), P < .00001). The correlation between actual and predicted scores from this model was statistically significant (r = .35, P < .00001), and the following features were selected in at least 5% of iterations: WAIS scores (t = 2.9, 100% of iterations), age at stroke (t = −2.3, 99.32% of iterations), and time post-stroke (t = 3.3, 100% of iterations). Table 3 provides the summary statistics for the Demographic Model.
Fig. 3 –
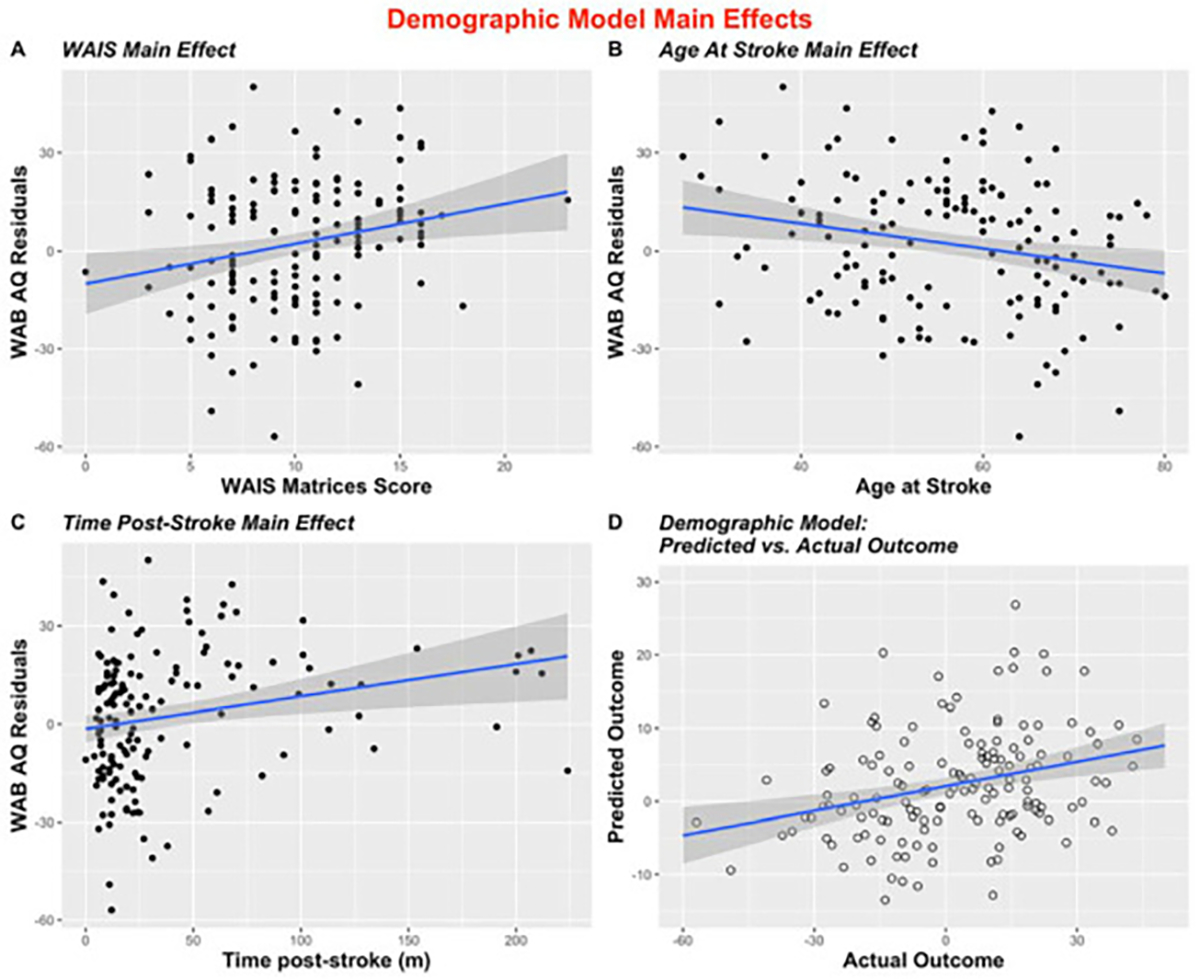
Scatterplots to illustrate all significant (entered into >5% of iterations) main effects in the stepwise LOOCV Demographic Model (A) Illustration of main effect of WAIS by WAB-AQ residual values (p = .004); (B) Illustration of main effect of age at stroke and WAB-AQ residuals (p = .02); (C) Main effect of time post-stroke by WAB AQ residual values (p = .03); (D) Predicted vs. actual outcomes of stepwise regression (r = .29, p = .0003).
Table 3 –
Stepwise Regression Model Results from Demographic, Health, and Overall Models with Leave-One-Out Cross Validation. NA indicates independent factors not selected in >5% of iterations in leave-one-out procedure.
| Stepwise Regression Model Results |
LOO Stepwise Regression Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Est. | Std. Error | tStat | pValue | Adj. R2 (P-value) | T | % iterations | ||
|
| ||||||||
| Demographic Model | (Intercept) | −10.2 | 11.0 | −0.9 | .36 | .15 (<.00001) | ||
| WAIS Matrices score | 1.2 | 0.4 | 2.9 | .005** | 2.9 | 100% | ||
| Time Post-Stroke(mos) | 5.4 | 1.6 | 3.3 | .001** | 3.3 | 100% | ||
| Age at Stroke(yrs) | −0.3 | 0.1 | −2.3 | .02* | −2.3 | 99.3% | ||
| Health | (Intercept) | 14.9 | 4.1 | 3.6 | .0004 | .11 (.0004) | ||
| Model | Total Fazekas | −4.2 | 1.1 | −3.7 | .0004** | −3.7 | 100% | |
| Overall Model | (Intercept) | −3.8 | 12.6 | −0.3 | .76 | .27 (<.00001) | ||
| WAIS Matrices score | 1.1 | 0.5 | 2.5 | .01* | 2.5 | 100% | ||
| Time Post-Stroke(mos) | 6.1 | 1.9 | 3.2 | .001** | 3.2 | 100% | ||
| Age at Stroke(yrs) | −0.3 | 0.2 | −1.9 | .057 | −2.1 | 96.2% | ||
| Total Fazekas | −3.0 | 1.1 | −2.6 | .01* | −2.6 | 100% | ||
p < .05;
p < .01.
3.3. Stepwise regression with leave-one-out cross validation results: Health Model
In the subset of participants with health data, a second model including BMI, presence of diabetes, exercise rate post-stroke, and WMH ratings as measured using the Fazekas rating scale (Fazekas et al., 1987) was constructed (N = 106). As before, the dependent variable was aphasia severity after accounting for lesion factors. A statistically significant effect of total Fazekas scores (P = .0004) was found in our model (Fig. 4). The Health Model explained 11% of the variance in residual WAB AQ scores unexplained by lesion factors (Adj. R2 = .11, F (18.4,104) = 13.6), P = .0004). The correlation between actual and predicted scores from this model was significant (r = .29, P = .003), and one feature was selected to be included in at least 5% of iterations: Fazekas rating (t = −3.7, 100% of iterations). Table 3 provides summary statistics from the Health Model.
Fig. 4 –
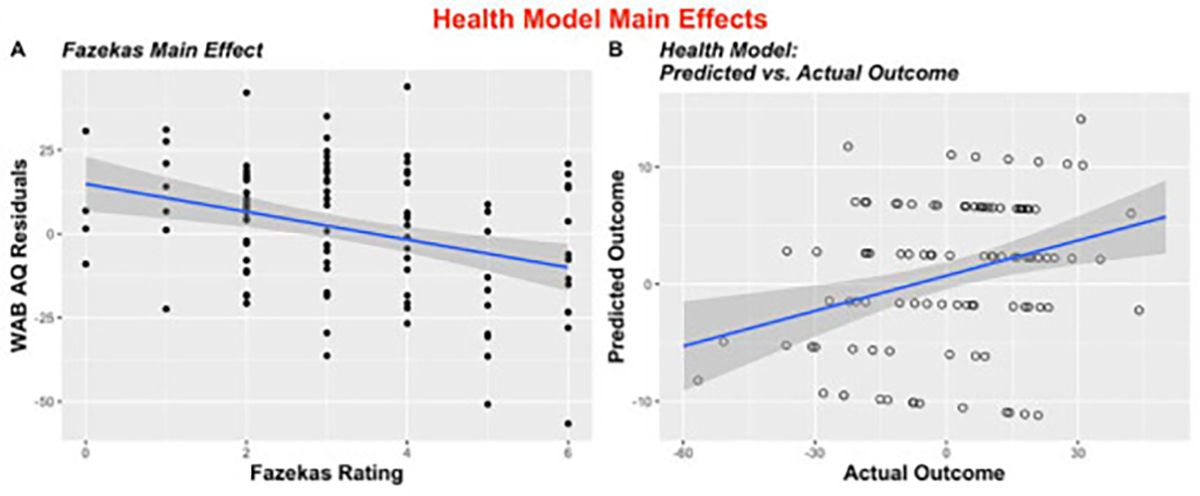
Scatterplots to illustrate all significant (entered into >5% of iterations) main effects in the stepwise LOOCV Health Model (A) Illustration of main effect of Fazekas rating by WAB AQ residual values (p = .0004); (B) Predicted vs. actual outcomes of stepwise regression (r = −.29, p = .003).
3.4. Stepwise regression with leave-one-out cross validation results: Overall Model
A total of 106 participants had all data available and were included in the Overall Model. The independent variables included in the model were: WAIS score, time post-stroke, age at stroke, and the extent of WMH. The dependent variable was aphasia severity after accounting for proportion damage to critical language areas and lesion volume, consistent with the two previous models. All four effects were entered into at least 5% of iterations: WAIS score (t = 2.5, 100% of iterations, P = .01), time post-stroke (t = 3.2, 100% of iterations, P = .001), total Fazekas (t = −2.6, 100% of iterations, P = .01), and age at stroke (t = −1.9, 96.2% of iterations, P = .057). The Overall Model explained 27% of the variance in residual WAB AQ scores controlled for lesion to critical language regions (Adj. R2 = .27, F (16.6,101) = 10.8), P < .00001). The correlation between actual and predicted values for this model was statistically significant (r = .45, P < .00001). Table 3 provides the summary statistics for the Overall Model.
3.5. Linear regression with leave-one-out cross validation results: Final Model
A Final Model was created to predict raw WAB-AQ scores from identified demographic, health, and lesion factors to aid in interpretability in factor contributions to severity scores. Therefore, the independent variables included in the model were: WAIS Matrices score, time post-stroke (transformed to address heteroskedastic distribution), age at stroke, total Fazekas rating, total lesion volume, and proportion damage to critical areas (insula and SLF-A tract). Data from 106 participants were available for this model. This Final Model accounted for 55% of the variance in aphasia severity (Adj. R2 = .55) and the correlation between actual and predicted values for this model was statistically significant (r = .72, P < .00001). The following variables were significant (P < .05) and were entered into at least 5% of iterations: Proportion damage to critical areas (t = −6.0, 100% of iterations, P < .00001), time post-stroke (t = 3.0, 100% of iterations, P = .003), WAIS Matrices score (t = 2.7, 100% of iterations, P = .007), and total Fazekas rating (t = −2.5, 100% of iterations, P = .02). Total lesion volume and age at stroke did not contribute significantly to the model nor were they selected into >5% of iterations (P = .52, P = .09, respectively). Partial R2 was calculated for each factor entered in the model to measure relative contribution of each factor to explaining aphasia severity using the following equation: R2 = t-statistic (Berglund et al., 2017)/(t-statistic (Berglund et al., 2017) + degrees of freedom). Table 4 provides model statistics for the Final Model and Fig. 5 shows the relative contribution of each factor.
Table 4 –
Linear Regression Model Results for the Final Model with Leave-One-Out Cross Validation. NA indicates independent factors not selected in >5% of iterations in leave-one-out procedure.
|
|
Linear Regression Model Results |
LOO Regression Model |
||||||
|---|---|---|---|---|---|---|---|---|
| Est. | Std. Error | tStat | pValue | Adj. R2 (P-value) | T | % iterations | ||
|
| ||||||||
| Final | (Intercept) | 82.0 | 13.7 | 6.0 | <.00001 | .55 (<.00001) | ||
| Model | WAIS Matrices score | 1.3 | 0.5 | 2.8 | .007** | 2.7 | 100% | |
| Time Post-Stroke(mos) | 5.8 | 1.9 | 3.1 | .003** | 3.0 | 100% | ||
| Age at Stroke(yrs) | −0.3 | 0.2 | −1.7 | .09 | NA | NA | ||
| Total Fazekas | −2.8 | 1.1 | −2.5 | .02* | −2.5 | 100% | ||
| Total Lesion Volume(mm3) | 1.8E-5 | 2.7E-5 | −0.7 | .52 | NA | NA | ||
| Prop. Damage to Critical ROIs | −49.0 | 8.2 | −6.0 | <.00001** | −6.0 | 100% | ||
p < .05;
p < .01.
Fig. 5 –
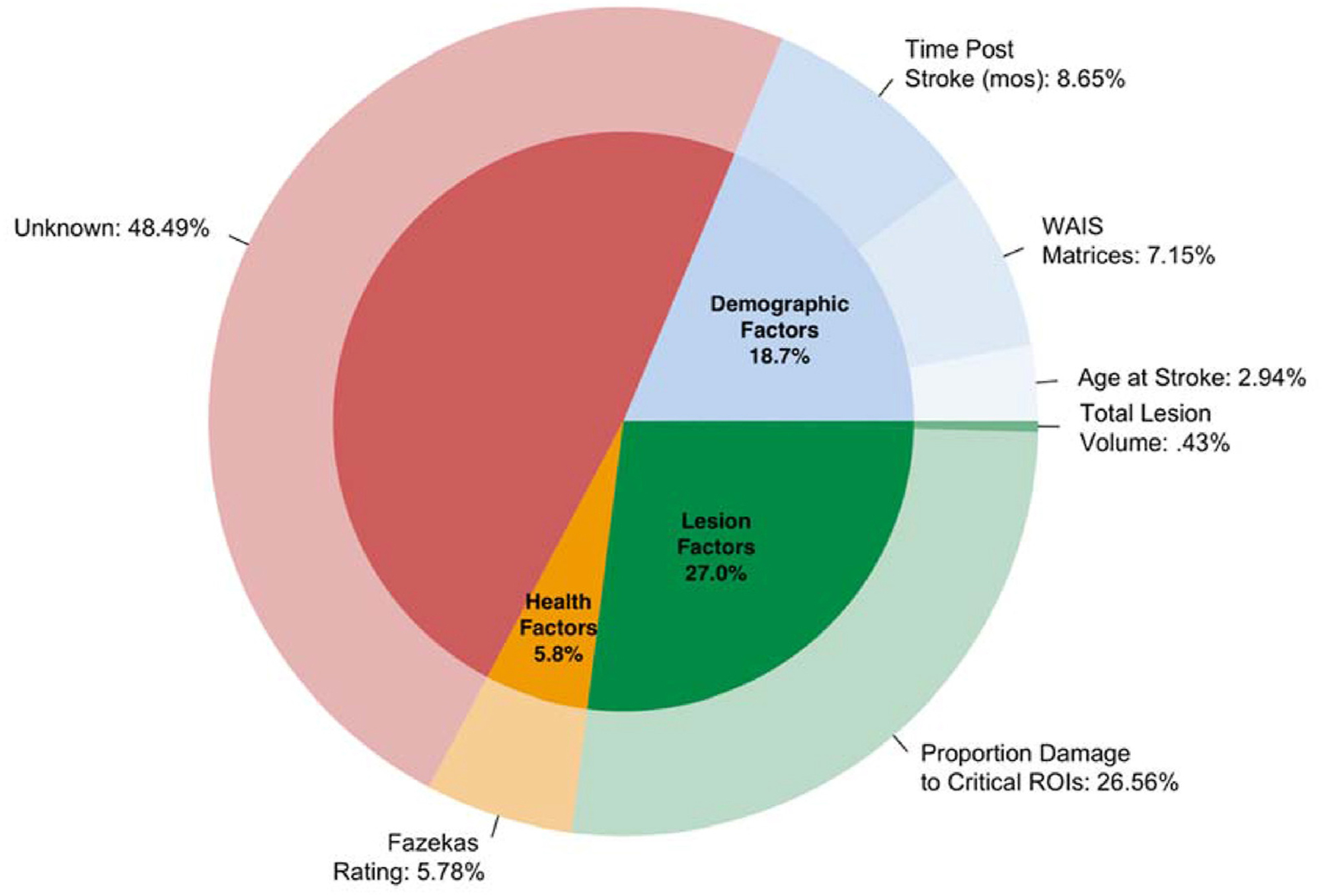
Contribution of individual independent variables entered into the Final LOO linear regression model.
4. Discussion
The present study sought to identify non-lesion-related characteristics that can explain the variance in aphasia severity after accounting for damage to critical language areas. Two models (Demographic Model (N = 147), and Health Model (N = 106)) investigated the following non-lesion-related characteristics and their association with aphasia severity: sex, education, age at stroke, time post-stroke, non-linguistic cognitive ability, exercise frequency post-stroke, presence of diabetes, BMI, and WMH. After identifying factors associated with residual WAB-AQ values from the Demographic and Health Models, an Overall Model was created (N = 106) including critical health and demographic variables identified from the previous models. Finally, raw WAB-AQ scores were used as the dependent factor in a Final Model to explore the relative contribution of each variable (lesion, demographic, and health variables) to aphasia severity.
4.1. Damage to the insula and SLF-A predict aphasia severity
The present study utilized data from 224 participants with aphasia to identify critical language regions and/or tracts which, when damaged, were associated with greater aphasia severity as per the WAB-R. One region and one white matter tract within the dorsal stream were revealed in this analysis: the posterior insula and the SLF-A.
Given its structural connections with the auditory, motor, parietal, and cingulate cortices, the insula has been implicated in a range of functions including auditory processing (Augustine, 1996; Fifer, 1993), cognition (Seeley et al., 2007; Uddin, 2015), and speech production (Ackermann & Riecker, 2010; Baier et al., 2011). Though focal lesions of the insula can result in heterogeneous behavioral deficits, damage to the insula typically results in deficits in multiple language processes rather than impacting a single process (Di Stefano et al., 2021; Fridriksson et al., 2018). Given its involvement within both speech production and auditory processing, damage to insular structures should result in a more global deficit, thus a more severe WAB-AQ.
White matter tracts which serve to connect anterior and posterior language regions have long been associated with measures of gross language performance (Catani & Mesulam, 2008; Fridriksson et al., 2018; Lee et al., 2021; Rosso et al., 2015; Tak & Jang, 2014). In the present paper, we found that the integrity of the SLF (area 155 in the JHU atlas), a tract described as a connection between frontal, temporal, and parietal cortices, is associated with aphasia severity. Historically, disentangling the branches of the SLF has long been debated and the relative dissociation between branches is even more controversial. There is some agreement, however, that a significant component of the SLF, particularly that which connects the superior temporal cortices to ventrolateral prefrontal cortex, is often referred to as the arcuate fasciculus (AF). Due to the description of area 155 and the neuroanatomical location, we refer to the identified region as the SLF-A. Evidence of the SLF-A’s contribution to language performance has been observed in both healthy older adults and clinical populations (Madhavan et al., 2014). Evidence of its involvement in the language network is particularly evident in the aphasia literature. In a study using tractography to predict aphasia severity, damage to the SLF and arcuate fasciculi showed the best accuracy at predicting aphasia severity (Nachev et al., 2007). The relationship between SLF-A integrity and aphasia severity is likely because of its involvement in overall language function (Glasser & Rilling, 2008). This relationship is further evidenced in studies investigating age-related changes of the SLF in healthy controls, of which is associated with decreased language functioning as measured by vocabulary, naming, word association, and semantic fluency tasks (Madhavan et al., 2014).
Together, integrity of the SLF-A provides inter-regional connectivity of dorsal stream cortical regions, including the insula, with neighboring language regions. That the two are the only surviving areas/tracts in a lesion symptom analysis of overall aphasia severity emphasizes the language network’s dependency on the insular cortex and the degree of functional and/or structural connectivity between it and other well-documented language areas such as inferior frontal cortex and posterior temporal regions.
4.2. Age at stroke impacts aphasia severity
Many studies have observed an effect of age on overall aphasia severity and treatment response (Gilmore et al., 2019; Holland et al., 2017; Smith, 1971). Increased age may lead to mild cognitive declines, which has been shown to impact performance on speech production and comprehension (Shafto & Tyler, 2014; Messer, 2017). For example, speech production in older adults is often described as more vague and simplified, and is produced at a slower rate compared to speech produced by younger adults (Kemper & Sumner, 2001; Neumann-Werth et al., 2009). Healthy older adults also report greater difficulty with word finding and this manifests in speech production as an increase in pauses (Schmitter-Edgecombe et al., 2000). Age-related declines in comprehension at the word, sentence, and discourse level have also been frequently documented showing that not only do older adults present with more errors on comprehension tasks, but their recognition is significantly slower than younger adults. (Messer, 2017; Kliegl et al., 2004). Although age-related structural and functional brain changes may underlie some of these findings (i.e., some studies showing that the recruitment of insula-frontostriatal structures in older adults is associated with greater “tip of the tongue” states (Shafto & Tyler, 2014)), little is known about the interaction of such changes with health and demographic factors. Results from our study highlight the importance of including age in predictive models of aphasia severity, particularly when investigating demographic predictors of severity. Interestingly, when included with health factors in predictive models of severity, age at stroke is no longer significant. This may be due to a possible interaction between age at stroke and health factors such as WMH, but future studies must be done to tease apart this effect.
4.3. Performance improves beyond stroke incident
Recent studies have provided evidence that the chronic stage of recovery is quite dynamic, with about half of stroke survivors improving on language performance years beyond stroke incidence (Holland et al., 2017; Hope et al., 2017; Johnson et al., 2019). Though our study did not evaluate individuals’ aphasia progression longitudinally, results from our study do suggest that individuals who are in the later stages of recovery present with less severe aphasia. Studies investigating the dynamics of brain connectivity have shown that, as time post-stroke increases, stroke survivors can reach the same level of connectivity as healthy participants even after initial reduction of overall connectivity (see Desowska & Turner (Baldo et al., 2015) for a review) (Desowska & Turner, 2019). Further, such connectivity increase is positively correlated with behavioral gains. This review further detailed that these behavioral and connectivity improvements are moderated by therapy or can be observed without a direct intervention. Of note, this systematic review by Desowska & Turner (Desowska & Turner, 2019) found evidence of dynamic connectivity patterns in the motor network, specifically, which aligns with prior studies showing individuals with more anterior lesions have a greater likelihood of recovery (Hanlon et al., 1999; Naeser et al., 1989; Sul et al., 2019). It is unclear if posterior language regions show a similar pattern. Additionally, given the participants in our cohort indicate their interest in participating in continued research (treatment studies and studies that do not provide treatment), it is evident that motivation among these more “seasoned” stroke survivors is high. Therefore, it is possible that these participants continued to seek practice activities, clinical treatment, and could, perhaps, have had more opportunities to develop communication strategies during their recovery. Results from this study emphasize the need to evaluate other neural changes that could provide an explanation for less severe aphasic deficits as time post-stroke increases.
4.4. Cognitive reserve is associated with aphasia severity
The relationship between cognition and language ability is a challenging area of study given the inter-dependence of language on higher-level cognitive domains. Given this challenge, few studies have attempted to address the degree of importance of cognitive ability on aphasia severity and recovery patterns. Conventional descriptions of aphasia indicate that it exclusively impacts language while leaving overall cognition relatively spared. However, a number of studies have presented evidence that individuals with aphasia do present with deficits in reasoning, problem-solving, and more general cognitive domains (Baldo et al., 2005, 2010, 2015; González-Fernández et al., 2011; Helm-Estabrooks, 2002). In a cross-sectional study, Gonzalez-Fernandez and colleagues (González-Fernández et al., 2011) found that cognitive reserve (as indicated by participant education level) was significantly associated with acute aphasia severity after controlling for age, sex, lesion volume and socio-economic status (SES). This study also found a relationship between errors on language tasks and poorer performance on cognitive assessments. Baldo and colleagues (Baldo et al., 2015) found a similar relationship between chronic aphasia severity and cognition, in that persons with aphasia performed worse, overall, on evaluations of cognition compared to neurotypical controls. However, the impact of aphasia on cognition appeared to particularly impair reasoning tasks, leaving all other non-linguistic cognitive tasks comparable in performance to that of controls. The stroke literature, more broadly, also points to a relationship between stroke-related impairment and cognitive reserve. In a review by Rosenich and colleagues (Kertesz, 2007), the authors discussed previous literature which provide evidence of the significance of cognitive reserve on stroke recovery, disability burden, language deficits, and psychological well-being (Rosenich et al., 2020).
Aphasia has been associated with impairments in attention (Villard & Kiran, 2015), executive function (Purdy, 2002), and short-term memory (Lang & Quitz, 2012) which suggests that the neural networks responsible for language and cognition are not mutually exclusive. To our knowledge, no study has investigated the relationship between cognitive ability and aphasia severity after controlling for lesion damage. As the present study was retrospective and precluded examination of specific cognitive domains, our results suggest that non-linguistic cognitive reserve is related to aphasia severity even after controlling for the influence of lesion size and location. Previous literature has shown evidence that Subjective Cognitive Decline (SCD), a self-reported measure of worsening or more frequent cognitive difficulty, is twice as prevalent in populations who have coronary heart disease or suffered a stroke compared to healthy adults (van Rijsbergen et al., 2014). Linguistic tasks rely heavily upon the integration of non-linguistic cognitive abilities such as memory, attention, and executive function (Peach, 2017). A review by Fonseca et al., (Fonseca et al., 1515) revealed contradictory findings regarding the association between cognition and aphasia severity, comprehension, and speech fluency. For example, while some studies report an association between aphasia and cognitive deficits, others report that cognitive ability in individuals with aphasia is comparable to that of controls. Studies investigating treatment response in post-stroke aphasia have also presented evidence that pretreatment cognitive reserve (both linguistic and non-linguistic) explains some of the variance in response variability across PWA (Gilmore et al., 2019; Bonini & Radanovic, 2015; el Hachioui et al., 2012; Lambon Ralph et al., 2010). Results from the present study indicate that WAIS scores can explain aphasia severity beyond what is accounted for by the lesion and lesion location. Understanding the relationship between these three factors could explain why previous literature has been inconsistent regarding the association with cognition and language ability.
4.5. Maintaining healthy contralesional white matter is associated with less severe aphasia
Results from the present study indicate that less severe WMH, as measured by Fazekas ratings, is an important prognostic of aphasia severity. More severe WMH have been associated with cardiovascular risk factors such as atherosclerotic disease, hypertension, obesity, and diabetes, and has been identified as an indicator of general cognitive decline (Fazekas et al., 1987; Novak et al., 2006). Those with severe WMH often present with depression and loss of functional ability (Pantoni, 2008), which are also associated with post-stroke deficits. The relationship between cognitive decline and WMH is further evidenced by the results observed in this study (Fig. 1) showing a correlation between age-normed WAIS scores and Fazekas (Spearman rho = −.20, P = .04). Further investigation of the interaction between WMH and cognition is necessary to better our understanding of the aging brain and recovery patterns in stroke survivors. Additionally, other health factors investigated in the present study often co-occur with the presence of WMH. For instance, some studies have reported a relationship between exercise rate and extent of WMH, while others have found no association (Torres et al., 2015). In the present study, we found no association between exercise rate and WMH.
The investigation of WMH’s influence on chronic aphasia severity and recovery is a relatively recent endeavor. Basilakos and colleagues (Basilakos et al., 2019) found that the extent of WMH in persons with aphasia is a significant predictor of declining language abilities in the chronic stage of recovery. Additionally, Wilmskoetter and colleagues (Wilmskoetter et al., 2019) found that those with more WMH in the periventricular space presented with greater aphasia severity. Given the relationship between age, cognition, and contralesional white matter integrity (as indicated by WMH ratings), it is important to understand how these factors impact each other and perhaps influence a clinician’s intervention approaches and patient expectations on performance.
4.6. Limitations
Due to the retrospective nature of the present study, there are several limitations that should be considered when interpreting the results. First, each Model included a subset of participants from a larger cohort (LSM cohort; N = 224). To address potential bias of sample inclusion, one-way ANOVAs were conducted between those included and excluded in each model (Demographic and Health/Overall Models). Though there were no statistically significant differences between dependent and independent variables (Supplementary Tables 1 and 2), it is possible that the samples of each model were unrepresentative of the population sample. For this reason, we encourage future work investigating predictors of severity to utilize large, diverse samples that are representative of the population of persons with aphasia and avoid missing data, particularly for health factors.
It is important to note that our approach of using residual values from the linear regression predicting aphasia severity from damage as the dependent variables in the three models yields results that compromise ease of interpretability. Though a standard approach for controlling for a variable, we emphasize to the readers to exercise caution in interpretation of model results. For this reason, we have included a Final Model which used raw WAB-AQ scores as the dependent variable and we refer readers to this model to better interpret relative contribution of each factor.
Only WAB-R assessments collected within 6 months of the completion of a patient’s case history form were included in the analysis, however, it is possible exercise rate and/or the status of pertinent health factors may have changed within this time frame. Additionally, we did not collect information on the type of physical exercise one performed, type of diabetes, or age at diabetes onset. Having more detailed information could shed light on possible interactions between these factors.
The nature of WMH, aging, diabetes, exercise, cognitive decline, and time post-stroke all co-exist and may be influenced by one another. It is unclear the effect one factor may have on another. For example, WMH may have been present in individuals prior to stroke due to poor general health or are, perhaps, a direct cause of poor vasculature related to the stroke event. In the same vein, it is difficult to ascertain the effect of time post-stroke as an indicator of less severe aphasia. It is likely that time post-stroke is a proxy measure for number of treatment hours, or perhaps this effect is driven by the fact that individuals adopt successful coping strategies during the course of their recovery (i.e., the longer an individual is living with aphasia, it is likely coping mechanisms are developed to avoid a potential communication breakdown). Data capturing treatment obtained or functional communication was not available for this cohort, but future studies would benefit from disentangling these potential confounds with time post-injury.
Finally, we did not have access to the health status of the participants prior to their stroke, nor were we able to explore other measures of health such as medication use, hypertension, history of smoking, and depression which have been less explored in the aphasia literature. It is likely that maintenance of one’s health prior to and after their stroke may explain some of the variability in patient severity, but the extent is unknown.
5. Conclusions
We conducted a large retrospective analysis of a multi-modal chronic stroke dataset in order to investigate the contributions of demographic and health factors to predictive models of aphasia severity (above and beyond lesion size and location, traditional stalwarts in the field). A number of the factors we investigated, including sex, education, and socioeconomic status, are still subject to considerable debate, and require further investigation before sensible mechanistic explanations can be formulated. Factors related to cognitive ability, overall brain health, physical health, and comorbid cardiovascular risk factors are emerging as important considerations for clinicians and researchers interested in maximizing their ability to predict the consequences and progression of post-stroke aphasia. While more research is required to fully understand all the possible interactions between factors, our results make a strong case that the maintenance of one’s overall health is a prognostic of less severe aphasia.
Supplementary Material
Acknowledgements
The authors thank Audrey Holland for her careful review and added insight to this manuscript.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders (Fridriksson: R03 DC005915, R01 DC008355, R01 DC009571, R03 DC010262, R01 DC011739, R21 DC014170, P50 DC014664; Basilakos: T32 DC014435).
Footnotes
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2022.06.013.
REFERENCES
- Ackermann H, & Riecker A (2010). The contribution(s) of the insula to speech production: A review of the clinical and functional imaging literature. Brain Structure & Function, 214(5–6), 419–433. 10.1007/s00429-010-0257-x [DOI] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, & Terent A (2009). Sex differences in stroke epidemiology: A systematic review. Stroke, 40(4), 1082–1090. 10.1161/STROKEAHA.108.540781 [DOI] [PubMed] [Google Scholar]
- Augustine JR (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev, 22(3), 229–244. 10.1016/s0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Baier B, zu Eulenburg P, Glassl O, & Dieterich M (2011). Lesions to the posterior insular cortex cause dysarthria. European Journal of Neurology: the Official Journal of the European Federation of Neurological Societies, 18(12), 1429–1431. 10.1111/j.1468-1331.2011.03473.x [DOI] [PubMed] [Google Scholar]
- Baldo J, Bunge S, Wilson SM, & Dronkers NF (2010). Is relational reasoning dependent on language? A voxel- based lesion symptom mapping study. Brain and Language, 113(2), 59–64. 10.1016/j.bandl.2010.01.004.Is [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo J v, Dronkers NF, Wilkins D, Ludy C, Raskin P, & Kim J. (2005). Is problem solving dependent on language? Brain and Language, 92(3), 240–250. 10.1016/j.bandl.2004.06.103 [DOI] [PubMed] [Google Scholar]
- Baldo J.v., Paulraj SR, Curran BC, & Dronkers NF (2015). Impaired reasoning and problem-solving in individuals with language impairment due to aphasia or language delay. Frontiers in Psychology, 6(OCT), 1–14. 10.3389/fpsyg.2015.01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos A, Stark BC, Johnson L, Rorden C, Yourganov G, Bonilha L, & Fridriksson J (2019). Leukoaraiosis is associated with a decline in language abilities in chronic aphasia. Neurorehabilitation and Neural Repair, 33(9). 10.1177/1545968319862561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A (1992). Prognostic factors in aphasia. Aphasiology, 6(4), 337–348. 10.1080/02687039208248605 [DOI] [Google Scholar]
- Berglund A, Schenck-Gustafsson K, & von Euler M (2017). Sex differences in the presentation of stroke. Maturitas, 99, 47–50. 10.1016/j.maturitas.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Bonini M v, & Radanovic M. (2015). Cognitive deficits in post-stroke aphasia. Arquivos de neuro-psiquiatria, 73(10), 840–847. 10.1590/0004-282X20150133 [DOI] [PubMed] [Google Scholar]
- Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. 10.1016/j.cortex.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, & Thiebaut de Schotten M (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex, 44(8), 1105–1132. 10.1016/j.cortex.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Connor LT, Obler LK, Tocco M, Fitzpatrick PM, & Albert ML (2001). Effect of socioeconomic status on aphasia severity and recovery. Brain and Language, 78(2), 254–257. 10.1006/brln.2001.2459 [DOI] [PubMed] [Google Scholar]
- Description about JHU multi-atlas library. https://braingps.mricloud.org/docs/atlasrepodocs/AtlasDescriptionAndProtocolV7a.pdf.
- Desowska A, & Turner DL (2019). Dynamics of brain connectivity after stroke. Reviews in the Neurosciences, 30(6), 605–623. 10.1515/revneuro-2018-0082 [DOI] [PubMed] [Google Scholar]
- Di Stefano V, De Angelis MV, Montemitro C, Russo M, Carrarini C, Di Giannantonio M, … Simister R (2021). Clinical presentation of strokes confined to the insula: A systematic review of literature. Neurological Sciences, 42(5), 1697–1704. 10.1007/s10072-021-05109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Hachioui H, Lingsma H, van de Sandt-Koenderman M, Dippel D, J Koudstaal P, & Visch-Brink E (2012). Long-term prognosis of aphasia after stroke (Vol. 84). 10.1136/jnnp-2012-302596 [DOI] [PubMed] [Google Scholar]
- Faria A v, Joel SE, Zhang Y, Oishi K, Van Zjil P, Miller M, … Mori S. (2012). Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage, 61(3), 613–621. 10.1016/j.neuroimage.2012.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, & Alavi A (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR. American Journal of Neuroradiology, 8(3), 421–426. [DOI] [PubMed] [Google Scholar]
- Fifer RC (1993). Insular stroke causing unilateral auditory processing disorder: Case report. Journal of the American Academy of Audiology, 4(6), 364–369. [PubMed] [Google Scholar]
- Fonseca J, Ferreira JJ, Pavão Martins I. Cognitive performance in aphasia due to stroke: A systematic review. International Journal on Disability and Human Development. 16(2):127–139. doi: 10.1515/ijdhd-2016-0011. [DOI] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, Kalra L, Murphy D, Williams S, & Catani M (2014). Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain, 137(7), 2027–2039. 10.1093/brain/awu113 [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, … Bonilha L (2018). Anatomy of aphasia revisited. Published online, 848–862. 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, & Kiran S (2019). Nonlinguistic cognitive factors predict treatment-induced recovery in chronic poststroke aphasia. Archives of Physical Medicine and Rehabilitation, 100(7), 1251–1258. 10.1016/j.apmr.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, & Rilling JK (2008). DTI tractography of the human brain’s language pathways. Cerebral Cortex, 18(11), 2471–2482. 10.1093/cercor/bhn011 [DOI] [PubMed] [Google Scholar]
- Godefroy O, Dubois C, Debachy B, Leclerc M, & Kreisler A (2002). Vascular aphasias: Main characteristics of patients hospitalized in acute stroke units. Stroke, 33(3), 702–705. 10.1161/hs0302.103653 [DOI] [PubMed] [Google Scholar]
- Goldenberg G, & Spatt J (1994). Influence of size and site of cerebral lesions on spontaneous recovery of aphasia and on success of language therapy. Brain and Language, 47(4), 684–698. 10.1006/brln.1994.1063 [DOI] [PubMed] [Google Scholar]
- González-Fernández M, Davis C, Molitoris JJ, Newhart M, Leigh R, & Hillis AE (2011). Formal education, socioeconomic status, and the severity of aphasia after stroke. Archives of Physical Medicine and Rehabilitation, 92(11), 1809–1813. 10.1016/j.apmr.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon RE, Lux WE, & Dromerick AW (1999). Global aphasia without hemiparesis: Language profiles and lesion distribution. Neurologia I Neurochirurgia Polska, 66(3), 365–369. 10.1136/jnnp.66.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish SM, Rodriguez AD, Blackett DS, Gregory C, Seeds L, Boatright J, & Crosson B (2018). Aerobic exercise as an adjuvant to aphasia therapy: Theory, preliminary findings, and future directions. Clinical Therapeutics, 40(1), 35–48. 10.1016/j.clinthera.2017.12.002.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J Jr., & Gordon B (1990). Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Annals of Neurology, 27(3), 226–231. 10.1002/ana.410270303 [DOI] [PubMed] [Google Scholar]
- Helm-Estabrooks N (2002). Cognition and aphasia: A discussion and a study. J Commun Disord, 35(2), 171–186. 10.1016/s0021-9924(02)00063-1 [DOI] [PubMed] [Google Scholar]
- Hier DB, Yoon WB, Mohr JP, Price TR, & Wolf PA (1994). Gender and aphasia in the stroke data bank. Brain and Language, 47(1), 155–167. 10.1006/brln.1994.1046 [DOI] [PubMed] [Google Scholar]
- Holland A, Fromm D, Forbes M, & MacWhinney B (2017). Long-term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31(2), 152–165. 10.1080/02687038.2016.1184221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TMH, Leff AP, Prejawa S, Bruce R, Haigh Z, Lim L, … Hope T (2017). Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain: a Journal of Neurology, 140, 1718–1728. 10.1093/brain/awx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Basilakos A, Yourganov G, et al. (2019). Progression of aphasia severity in the chronic stages of stroke. The American Journal of Surgical Pathology, 28(2). 10.1044/2018_AJSLP-18-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper S, & Sumner A (2001). The structure of verbal abilities in young and older adults. Psychology and Aging, 16(2), 312–322. 10.1037/0882-7974.16.2.312 [DOI] [PubMed] [Google Scholar]
- Kertesz A (2007). Western aphasia battery-R. Grune & Stratton. [Google Scholar]
- Kertesz A, & Sheppard A (1981). The epidemiology of aphasic and cognitive impairment in stroke: Age, sex, aphasia type, and laterality differences. Brain, 104(1), 117–128. 10.1093/brain/104.1.117 [DOI] [PubMed] [Google Scholar]
- Kim KA, Lee JS, Chang WH, Kim DY, Shin YI, Kim SY, … Kim YH (2019). Changes in language function and recovery-related prognostic factors in first-ever left hemispheric ischemic stroke. Ann Rehabil Med, 43(6), 625–634. 10.5535/arm.2019.43.6.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R, Grabner E, Rolfs M, & Engbert R (2004). Length, frequency, and predictability effects of words on eye movements in reading. European Journal of Cognitive Psychology, 16(1–2), 262–284. 10.1080/09541440340000213 [DOI] [Google Scholar]
- Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, … Saur D (2013). Damage to ventral and dorsal language pathways in acute aphasia. Brain, 136(2), 619–629. 10.1093/brain/aws354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Snell C, Fillingham JK, Conroy P, & Sage K (2010). Predicting the outcome of anomia therapy for people with aphasia post CVA: Both language and cognitive status are key predictors. Neuropsychological Rehabilitation, 20(2), 289–305. 10.1080/09602010903237875 [DOI] [PubMed] [Google Scholar]
- Lang CJG, & Quitz A (2012). Verbal and nonverbal memory impairment in aphasia. Journal of Neurology, 259(8), 1655–1661. 10.1007/s00415-011-6394-1 [DOI] [PubMed] [Google Scholar]
- Laska AC, Hellblom A, Murray V, Kahan T, & von Arbin M (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249(5), 413–422. 10.1046/j.1365-2796.2001.00812.x [DOI] [PubMed] [Google Scholar]
- Lee JK, Ko MH, Park SH, & Kim GW (2021). Prediction of aphasia severity in patients with stroke using diffusion tensor imaging. Brain Sciences, 11(304). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendrem W, & Lincoln NB (1985). Spontaneous recovery of language in patients with aphasia between 4 and 34 weeks after stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 48(8), 743–748. 10.1136/jnnp.48.8.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan KM, McQueeny T, Howe SR, Shear P, & Szaflarski J (2014). Superior longitudinal fasciculus and language functioning in healthy aging. Brain Research, 1562, 11–22. 10.1016/j.brainres.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer R (2017). Harris Wright H. (Ed.), Cognition, Language, and Aging. Amsterdam/Philadelphia: John Benjamins Publishing, 2016. Pp. 258 ISBN 978–9-027–21232-0. Language and Cognition, 9(4), 741–746. 10.1017/langcog.2017.4 [DOI] [Google Scholar]
- Marebwa BK, Fridriksson J, Yourganov G, Feenaughty L, Rorden C, & Bonilha L (2017). Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Scientific Reports, 7(1), 8188. 10.1038/s41598-017-07607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass MB, Lev MH, Ay H, Singhal A, Greer D, Smith W, … Furie K (2012). The prognosis for aphasia in stroke. Journal of Stroke and Cerebrovascular Diseases, 21(5), 350–357. 10.1016/j.jstrokecerebrovasdis.2010.09.009(The). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni M, Vista M, Pardossi L, Avila L, Bianchi F, & Moretti P (1992). Spontaneous evolution of aphasia after ischaemic stroke. Aphasiology, 6(4), 387–396. 10.1080/02687039208248609 [DOI] [Google Scholar]
- Nachev P, Coulthard E, Jager HR, Kennard C, & Husain M (2007). Enantiomorphic normalization of focally lesioned brains. Neuroimage, 39(3–3), 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-estabrooks N, Stiassnyeder D, & Albert ML (1989). Severe nonfluency in aphasia: Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain, 112(1), 1–38. 10.1093/brain/112.1.1 [DOI] [PubMed] [Google Scholar]
- Neumann-Werth Y, Obler L, Gomes H, & Shafer V (2009). Phonological vs sensory contributions to age effects in naming: An electrophysiological study (Vol. 23). 10.1080/02687030802661630 [DOI] [Google Scholar]
- Novak V, Last D, Alsop DC, Abduljalil A, Hu K, Lepicovsky L, … Lipsitz L (2006). Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care, 29(7), 1529–1534. 10.2337/dc06-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L (2008). Leukoaraiosis: From an ancient term to an actual marker of poor prognosis. Stroke, 39(5), 1401–1403. 10.1161/STROKEAHA.107.505602 [DOI] [PubMed] [Google Scholar]
- Peach RK (2017). Cognitive approaches to aphasia treatment: Application of the cognition of language to aphasia intervention. Seminars in Speech and Language, 38(1), 3–4. 10.1055/s-0036-1597259 [DOI] [PubMed] [Google Scholar]
- Plowman E, Hentz B, & Ellis C (2012). Post-stroke aphasia prognosis: A review of patient-related and stroke-related factors. Journal of Evaluation in Clinical Practice, 18(3). 10.1111/j.1365-2753.2011.01650.x [DOI] [PubMed] [Google Scholar]
- Purdy M (2002). Executive function ability in persons with aphasia. Aphasiology, 16(4–6), 549–557. 10.1080/02687030244000176 [DOI] [Google Scholar]
- van Rijsbergen MWA, Mark RE, de Kort PLM, & Sitskoorn MM (2014). Subjective cognitive complaints after stroke: A systematic review. Journal of Stroke and Cerebrovascular Diseases, 23(3), 408–420. 10.1016/j.jstrokecerebrovasdis.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, & Karnath HO (2012). Age-specific CT and MRI templates for spatial normalization Christopher. Neuroimage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020.Age-specific [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenich E, Hordacre B, Paquet C, Koblar SA, & Hillier SL (2020). Cognitive reserve as an emerging concept in stroke recovery. Neurorehabilitation and Neural Repair, 34(3), 187–199. 10.1177/1545968320907071 [DOI] [PubMed] [Google Scholar]
- Rosso C, Vargas P, Valabregue R, Arbizu C, Henry-Amar F, Leger A, … Samson Y (2015). Aphasia severity in chronic stroke patients: A combined disconnection in the dorsal and ventral language pathways. Neurorehabilitation and Neural Repair, 29(3), 287–295. 10.1177/1545968314543926 [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Vesneski M, & Jones DWR (2000). Aging and word-finding: A comparison of spontaneous and constrained naming tests. Archives of Clinical Neuropsychology, 15(6), 479–493. 10.1016/S0887-6177(99)00039-6 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover G, Kenna H, … Greicius M, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, & Tyler LK (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science (1979), 346(6209), 583–587. http://science.sciencemag.org/content/346/6209/583.abstract. [DOI] [PubMed] [Google Scholar]
- Smith A (1971). Objective indices of severity of chronic aphasia in stroke patients. Journal of Speech and Hearing Disorders, 36(2), 167–207. 10.1044/jshd.3602.167 [DOI] [PubMed] [Google Scholar]
- Sul B, Lee KB, Hong BY, Kim JS, Kim J, Hwang WS, & Lim SH (2019). Association of lesion location with long-term recovery in post-stroke aphasia and language deficits. Frontiers in Neurology, 10, 776. 10.3389/fneur.2019.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak HJ, & Jang SH (2014). Relation between aphasia and arcuate fasciculus in chronic stroke patients. BMC Neurology, 14(1), 46. 10.1186/1471-2377-14-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R. C. R. (2018). A language and environment for statistical computing. Published online. [Google Scholar]
- Thye M, & Mirman D (2018). Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. Neuroimage Clin, 20, 1129–1138. 10.1016/j.nicl.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres ER, Strack EF, Fernandez CE, Tumey TA, & Hitchcock ME (2015). Physical activity and white matter hyperintensities: A systematic review of quantitative studies. Preventive Medicine Reports, 2, 319–325. 10.1016/j.pmedr.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews. Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Vangberg TR, Eikenes L, & Håberg AK (2019). The effect of white matter hyperintensities on regional brain volumes and white matter microstructure, a population-based study in HUNT. Neuroimage, 203(August), Article 116158. 10.1016/j.neuroimage.2019.116158 [DOI] [PubMed] [Google Scholar]
- Villard S, & Kiran S (2015). Between-session intra-individual variability in sustained, selective, and integrational non-linguistic attention in aphasia. Neuropsychologia, 66, 204–212. 10.1016/j.neuropsychologia.2014.11.026 [DOI] [PubMed] [Google Scholar]
- Watila MM, & Balarabe B (2015). Factors predicting post-stroke aphasia recovery. Journal of the Neurological Sciences, 352(1–2), 12–18. 10.1016/j.jns.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Wechsler D Wechsler adult intelligence scale. Archives of Clinical Neuropsychology. Published online; 1955:’. [Google Scholar]
- Wickham H (2016). Ggplot 2: Elegant graphics for data analysis. Springer-Verlag; New York. https://ggplot2.tidyverse.org. [Google Scholar]
- Wilmskoetter J, Marebwa B, Basilakos A, Fridriksson J, Rorden C, Stark B, … Bonilha L (2019). Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain, 142(10). 10.1093/brain/awz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, & Nichols TE (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Du W, Zhao X, Liu L, Wang C, Wang Y, … Xu Y (2014). Favorable functional recovery in overweight ischemic stroke survivors: Findings from the China national stroke registry. Journal of Stroke and Cerebrovascular Diseases, 23(3), e201–e206. 10.1016/j.jstrokecerebrovasdis.2013.10.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The conditions of our ethics approval do not permit sharing of the raw MRI data supporting this study with any individual outside the author team under any circumstances. However, de-identified neuroimaging and clinical data are made available in addition to scripts used for figure-making in a GitHub repository (https://github.com/lajohn25/PredictorsBeyondtheLesion_Cortex).


