Abstract
Borna disease virus (BDV), the causative agent of severe meningoencephalitis in a wide variety of animal species, has been considered to be genetically invariable and to form a single type within the genus Bornavirus of the family Bornaviridae. BDV infections are of particular interest, because for the first time a virus infection appears to be linked to human psychiatric disorders. We now describe a new subtype of BDV isolated from a horse which was euthanatized due to severe, incurable neurological disease. The nucleotide sequence of this new strain, named No/98, differs from the reference strains by more than 15%, and the subtype is difficult to detect by standard reverse transcriptase PCR protocols. The nucleotide exchanges of the novel BDV isolate have surprisingly little effect on the primary structures of most viral proteins, with the notable exception of the X protein (p10), which is only 81% identical to its counterpart in reference strains. Our data indicate that the genome of BDV is far more variable than previously assumed and that naturally occurring subtypes may escape detection by currently used diagnostic assays.
Borna disease virus (BDV) is the causative agent of severe meningoencephalitis in horses, sheep, and other animal species in central Europe (7, 13, 21, 22), and it is suspected to contribute to human psychiatric disorders worldwide (1, 2, 6, 8–12, 14, 15, 20). The pathogenesis of Borna disease is mediated by a T-cell-dependent immune mechanism. All natural isolates of BDV that have been available to date, independent of species (humans, horses, sheep, cats, dogs, etc.), area (Europe, the United States, Japan), and year of isolation (1929 to 1998), show a remarkably high sequence conservation of the 8.9-kb RNA genome (5, 11, 16, 17). Human BDV isolates are 95 to 100% identical to animal-derived BDV at the nucleotide level and are 97 to 100% identical at the amino acid level (5, 11, 16). BDV is therefore considered a potential zoonotic agent. Because serum antibody titers are frequently low in naturally infected individuals and BDV serology has several other limitations, reverse transcriptase PCR (RT-PCR) technology using primers that match sequences of the viral N or P genes (1, 2, 5, 6, 8, 9, 12, 15, 16, 17, 19) is now widely used for the diagnosis of BDV infection. In this report, we describe a novel subtype of BDV which escapes detection by currently used diagnostic RT-PCR protocols.
MATERIALS AND METHODS
Immunohistochemistry.
Paraffin-embedded brain sections were stained with the monoclonal antibody BO18, directed against the N protein, and a polyclonal mouse antiserum, directed against the X protein of BDV (3), as described previously (21, 22). Staining of cultured cells grown on glass coverslips was carried out according to standard procedures, by using polyclonal antisera raised against purified recombinant X and P proteins of BDV He/80.
Sample preparation and RNA extraction.
Samples from hippocampus and rhinencephalon were homogenized by using liquid nitrogen and were resuspended in diethyl pyrocarbonate-treated water. Following low-speed centrifugation, 140 μl of supernatant was used for RNA extraction, employing the QIAamp viral RNA kit (QIAGEN).
PCR assays.
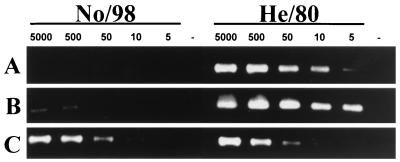
In order to amplify overlapping genome fragments of the causative BDV strain, named No/98, RT-PCR was performed with a large number of different oligonucleotide primer pairs. cDNA synthesis and PCR were carried out in a single step by using the Titan One Tube RT-PCR Kit (Boehringer Mannheim/Roche). A 5-μl volume of extract, containing 50 pmol of RNA (approximately 0.2 μg of RNA), was used in each RT-PCR. For the experiments shown below (see Fig. 4A and B), the primer pairs described by Sorg and Metzler (19) were employed, because PCR assays using these oligonucleotide primers proved best for the detection of classical BDV in a recent multicenter study. Nested PCR (see Fig. 4B) was carried out by using 3 μl of the RT-PCR product, Taq polymerase from Promega, and a MgCl2 concentration of 1.5 mM. For the RT-PCR experiment shown below (see Fig. 4C), the forward primer was 5′-CCTGGCATCCTGTGACTATT-3′, and the reverse primer was 5′-ATCTGCTCTTGGCTGTGTCT-3′ (nucleotide positions 3863 to 3882 and 4254 to 4235 of strain V, respectively).
FIG. 4.
No/98 is poorly recognized by conventional diagnostic RT-PCR assays. To examine at which sensitivity levels a published RT-PCR assay is able to detect the novel BDV subtype compared to the classical subtype, we prepared cell mixtures, each containing 5 × 105 uninfected Vero cells and the indicated numbers of Vero cells infected with either BDV No/98 or He/80 and subjected them to PCR analysis. (A) Conventional RT-PCR (19); (B) conventional nested PCR (19); (C) modified RT-PCR using primer pairs (described above) that amplify both No/98 and He/80 genomes.
Sequence analysis.
PCR products were usually sequenced directly (in both directions) without subcloning into plasmid vectors, by using an automated DNA sequencer (ABI PRISM 310 Genetic Analyzer; Perkin-Elmer); only a 0.65-kb fragment of the BDV isolate No/98 was cloned into pCR2.1 by using the TA-ligation procedure as described by the manufacturer (Invitrogen). The software package DNAStar 3 was used for sequence alignment and for construction of the phylogenetic trees.
Isolation of BDV No/98 from frozen brain material.
Specimens from hippocampus and rhinencephalon were homogenized and added to early-passage cultures of young rabbit brain cells. After foci of infected cells were visualized by indirect immunofluorescence analysis, uninfected monkey Vero cells were added. After six cell passages, most rabbit cells were lost and the virus isolate had infected a high percentage of the Vero cells.
Nucleotide sequence accession number.
The sequence of the genome fragment of the new BDV strain described here was submitted to GenBank under accession no. AF136236.
RESULTS AND DISCUSSION
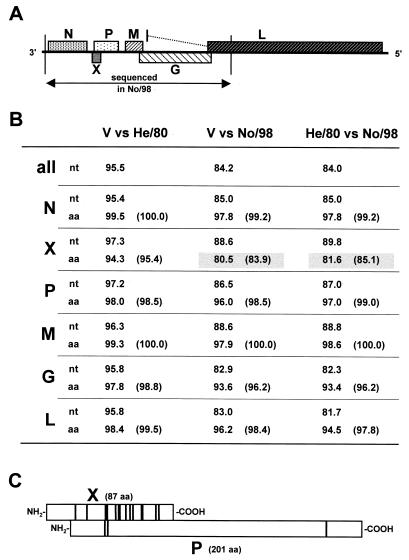
The diseased animal was a 7-year-old pony stallion, originating from the Austrian federal state of Styria, where no cases of Borna disease had been previously recorded. Also, the animal had never been in regions in which BDV is endemic. The animal's clinical and histopathological picture matched that of classical Borna disease; this preliminary diagnosis was confirmed by immunohistochemical detection of BDV antigen in paraffin-embedded brain sections (Fig. 1). Unexpectedly, however, BDV RNA was not detected in frozen brain material by RT-PCR with several primer pairs that routinely give reliable results, indicating major sequence differences from previously isolated BDV strains. To verify this, we performed PCR on cDNA samples from the brain of this horse using a large array of primer pairs, including standard primers for classical BDV as well as primers specifically designed for this novel BDV strain. Using this PCR strategy, we generated overlapping fragments of the viral genome that were sequenced directly. The compiled sequence data yielded information on a large fragment of the genome of the new BDV strain, designated No/98, that corresponds to nucleotide positions 25 to 4234 of reference strain V; this fragment represents almost half of the entire BDV genome, including the complete open reading frames (ORFs) of the viral N, X, P, M and G genes and a small part of the L gene (Fig. 2A). The No/98 sequence was strictly colinear with that of reference strain V, except for a three-nucleotide deletion at positions 90 to 92 that deletes alanine 13 of the N protein, a single nucleotide deletion at position 1170, and a three-nucleotide (GCA) insertion after nucleotide 1204, both located in the first intergenic region between the N and X ORFs. The overall sequence identities between No/98 and the two reference strains V and He/80 were 84.2 and 84.0%, respectively (Fig. 2B). The nucleotide exchanges were distributed fairly evenly over the entire region that we have sequenced. Within the coding sequences of the N, P, M, G, and L genes, most nucleotide exchanges were silent, because they frequently affected the third positions of the codons. Consequently, the amino acid sequences were more than 93% identical to their counterparts in strains V and He/80. When conservative amino acid substitutions were taken into account, similarities were more than 96% (Fig. 2B). A different situation emerged for the X protein that is encoded by an ORF which overlaps the P ORF. Here, most nucleotide exchanges were not silent, and the X protein of No/98 exhibited only about 81% identity to its counterparts in strains V and He/80 (Fig. 2B). More careful inspection (Fig. 2C) demonstrated a highly biased distribution of nucleotide exchanges which strongly favored conservation of the P ORF over the X ORF, indicating that the latter protein can tolerate more variation. It is of interest to note, however, that the N-terminal region of the X protein, which harbors a nuclear export sequence (M. Salvatore, R. E. O'Neill, M. Schwemmle, P. Palese, and W. I. Lipkin, Abstr. Bornavir. Meet. 1998, abstr. V3, 1998) and a domain that mediates interactions with the P protein (Salvatore et al., Abstr. Bornavir. Meet. 1998), was completely invariant. A more variable region within the second transcription unit was also described in highly conserved BDV isolates (11, 17).
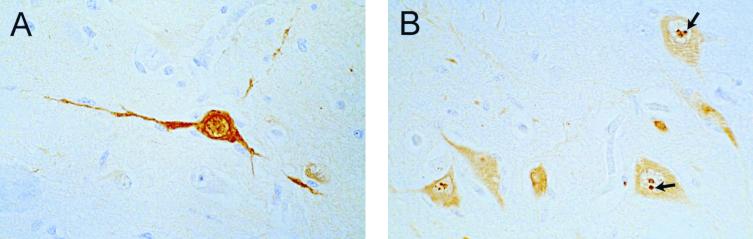
FIG. 1.
Immunohistochemical staining of paraffin-embedded brain tissue by using the avidin-biotin complex technique and the monoclonal antibody BO18. (A) Specifically labeled neuron within the hippocampus region (magnification, ×335). (B) Positive immunostaining of hippocampal neurons. Note the numerous intranuclear Joest-Degen inclusion bodies (arrows), which are considered to be characteristic of BDV infection (magnification, ×335).
FIG. 2.
Comparison of nucleotide and amino acid sequences of BDV No/98 and common laboratory strains. (A) Gene order and coding strategy of BDV. Overlapping RT-PCR products of No/98 corresponding to nucleotide positions 25 to 4234 of reference strain V (3) were sequenced. This fragment includes the complete coding sequences for the viral proteins N, X, P, M, and G and part of the L gene, as indicated. (B) Comparison of nucleotide (nt) and amino acid (aa) sequences of No/98 and laboratory strains V (3) and He/80 (4). Percentage of overall sequence identity (all) or sequence identities of the particular gene products at nucleotide and amino acid levels are indicated. Numbers in parentheses show percentage of sequence similarity when conserved amino acid exchanges are taken into account. Amino acid exchanges were rated conservative when the affected residues had similar biochemical properties. Any exchanges within the following groups of amino acids were considered conservative (single letter code): AILVM, STC, FY, NQ, WF, DE, and KR. Numbers boxed with gray highlight the striking dissimilarity of the X gene product. (C) Strongly biased conservation of the P protein sequence in the X-P gene overlap region. Positions of nonconservative amino acid exchanges in the X (n = 14) and P (n = 3) proteins are indicated by vertical bars. The comparison of strains V and No/98 is shown.
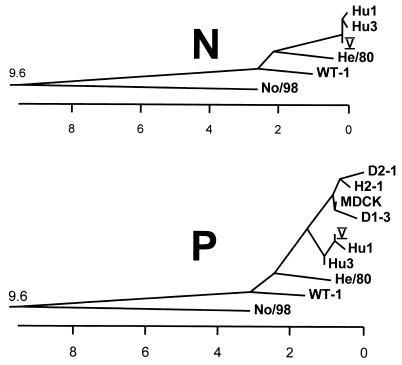
To elucidate the relationship between No/98 and previously known BDV strains, we constructed phylogenetic trees based on published nucleotide sequences of N and P gene fragments (Fig. 3). This comparison included the prototype strains V (3), He/80 (4), WT-1 (17), and MDCK (9) and various BDV-derived nucleotide sequences found in blood samples of humans from Germany (1, 5) and Japan (9). As previously noted by others (5, 11, 16, 17), all BDV strains that had been previously recognized had shown highly similar nucleotide sequences. In sharp contrast, No/98 occupies a unique position in both phylogenetic trees, indicating that it may represent the first member of a novel BDV subtype.
FIG. 3.
Phylogenetic trees of BDV strains. Nucleotide sequence comparisons of fragments from the N gene coding region (corresponding to nucleotides 262 to 829 of reference strain V) and from the P gene coding region (corresponding to nucleotides 1482 to 1814 of reference strain V) were performed. Distances between strains indicate percentage of sequence divergence. Sequence information used to construct these trees was taken from the following sources: No/98, this report and GenBank no. AF136236; V, reference 3 and GenBank no. U04608; He/80, reference 4 and GenBank no. L27077; WT-1 N gene, reference 17 and GenBank no. S67502; WT-1 P gene, reference 17 and GenBank no. S67507; Hu1 N gene, reference 1 and GenBank no. U58594; Hu1 P gene, reference 5 and GenBank no. L76234; Hu3 N gene, reference 1 and GenBank no. U58596; Hu3 P gene, reference 5 and GenBank no. L76236. Sequences designated MDCK, D1-3, D2-1, and H2-1 were taken from the work of Iwata et al. (9).
To study the biological properties of No/98, we isolated this virus from frozen brain material by using cultures of primary young rabbit brain cells. Viral replication was monitored by indirect immunofluorescence analysis. The newly isolated virus could be transmitted to cultures of Vero monkey cells, in which it spread quickly without inducing a cytopathic effect. Vero cells infected with No/98 showed strong nuclear staining with monospecific antiserum raised against either X or P of strain He/80. To verify that the Vero cells were indeed infected with No/98, we performed RT-PCR on RNA from the persistently infected Vero cell culture. The amplified 0.65-kb fragment (corresponding to nucleotide positions 1202 to 1856) included the complete X ORF and part of the intergenic region between the N and X genes. Its sequence precisely matched the No/98 sequence previously established by direct analysis of horse brain material. All nucleotide exchanges and the three-base insertion after position 1204 were also present in the virus isolate, indicating that the genome of No/98 did not rapidly acquire major alterations during replication in Vero cells.
Sequence comparisons revealed that currently used diagnostic RT-PCR assays employ primer pairs that only poorly match the No/98 sequence. To test if they would still detect this virus subtype, we prepared mixtures of 5 × 105 uninfected Vero cells and various numbers of cells infected with either BDV strain He/80 or No/98, extracted RNA from these cell mixtures, and used samples for reverse transcription. Standard (nonnested) PCR for detection of transcripts from the BDV N gene yielded the expected amplification products with all cell mixtures containing between 5,000 and 5 He/80-infected cells (Fig. 4A). By contrast, no amplification products were observed with cell mixtures containing high or low numbers of No/98-infected cells (Fig. 4A). Only when nested PCR was performed (Fig. 4B) did the samples containing 5,000 and 500 No/98-infected cells become weakly positive. When other primer pairs that better match both viral genomes were used, No/98- and He/80-infected cells were detected at similar sensitivities (Fig. 4C). The above experiment was repeated with other published RT-PCR protocols and showed results very similar to those presented in Fig. 4A and B; they either failed completely to amplify BDV No/98 sequences or exhibited significantly lower sensitivities in detecting this novel BDV subtype, even when altering the PCR conditions.
The identification of a new BDV field isolate that escapes detection by currently used diagnostic assays has far-reaching implications for proper diagnosis of BDV in, for example, human neuropsychiatric disorders. The new findings imply that previous studies, which relied on RT-PCR technology, might have underestimated the true prevalence rates of human BDV infections and might also have missed etiological correlations between BDV infection and certain neuropsychiatric disorders. The isolation of a BDV with a highly variant genome disproves the general opinion of high sequence conservation of all BDV genomes; the possibility that further, yet unidentified, BDV subtypes do exist should be considered seriously.
ACKNOWLEDGMENTS
This work was supported by grants from the Austrian federal state of Vorarlberg, the state of Baden-Württemberg, and the Deutsche Forschungsgemeinschaft.
We thank Otto Haller for critical reading of the manuscript.
REFERENCES
- 1.Bode L, Dürrwald R, Rantam F A, Ferszt R, Ludwig H. First isolates of infectious human Borna disease virus from patients with mood disorders. Mol Psychiatry. 1996;1:200–212. [PubMed] [Google Scholar]
- 2.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 3.Briese T, Schneemann A, Lewis A J, Park Y-S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Torre J C, Bode L, Dürrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 6.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grässer F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 7.Dürrwald R, Ludwig H. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. J Vet Med Ser B. 1997;44:147–184. doi: 10.1111/j.1439-0450.1997.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 8.Haga S, Motoi Y, Ikeda K the Japan Bornavirus Study Group. Borna disease virus and neuropsychiatric disorders. Lancet. 1997;350:592–593. doi: 10.1016/s0140-6736(05)63183-2. [DOI] [PubMed] [Google Scholar]
- 9.Iwata Y, Takahashi K, Peng X, Fukuda K, Ohno K, Ogawa T, Gonda K, Mori N, Niwa S, Shigeta S. Detection and sequence analysis of Borna disease virus p24 RNA from peripheral blood mononuclear cells of patients with mood disorders or schizophrenia and of blood donors. J Virol. 1998;72:10044–10049. doi: 10.1128/jvi.72.12.10044-10049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowotny N, Windhaber J. Borna disease virus and neuropsychiatric disorders. Lancet. 1997;350:593. doi: 10.1016/s0140-6736(05)63184-4. [DOI] [PubMed] [Google Scholar]
- 11.Planz O, Rentzsch C, Batra A, Batra A, Winkler T, Büttner M, Rziha H-J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planz O, Rentzsch C, Batra A, Rziha H-J, Stitz L. Persistence of Borna disease virus-specific nucleic acid in blood of psychiatric patient. Lancet. 1998;352:623. doi: 10.1016/S0140-6736(05)79577-5. [DOI] [PubMed] [Google Scholar]
- 13.Richt J A, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 15.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I the Bornavirus Study Group. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 16.Sauder C, Müller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, de la Torre J C, Grässer F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider P A, Briese T, Zimmermann W, Ludwig H, Lipkin W I. Sequence conservation in field and experimental isolates of Borna disease virus. J Virol. 1994;68:63–68. doi: 10.1128/jvi.68.1.63-68.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwemmle M, Salvatore M, Shi L, Richt J, Lee C H, Lipkin W I. Interactions of the Borna disease virus P, N, and X proteins and their functional implications. J Biol Chem. 1998;273:9007–9012. doi: 10.1074/jbc.273.15.9007. [DOI] [PubMed] [Google Scholar]
- 19.Sorg I, Metzler A. Detection of Borna disease virus RNA in formalin-fixed, paraffin-embedded brain tissues by nested PCR. J Clin Microbiol. 1995;33:821–823. doi: 10.1128/jcm.33.4.821-823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VandeWoude S, Richt J A, Zink M C, Rott R, Narayan O, Clements J E. A Borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990;250:1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- 21.Weissenböck H, Nowotny N, Caplazi P, Kolodziejek J, Ehrensperger F. Borna disease in a dog with lethal meningoencephalitis. J Clin Microbiol. 1998;36:2127–2130. doi: 10.1128/jcm.36.7.2127-2130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissenböck H, Suchy A, Caplazi P, Herzog S, Nowotny N. Borna disease in Austrian horses. Vet Rec. 1998;143:21–22. doi: 10.1136/vr.143.1.21. [DOI] [PubMed] [Google Scholar]