Abstract
Patients with chronic obstructive pulmonary disease (COPD) infected with SARS-CoV-2 indicate a higher risk of severe COVID-19 course, which is defined as the need for hospitalization in the intensive care unit, mechanical ventilation, or death. However, simple tools to stratify the risk in patients with COPD suffering from COVID-19 are lacking. The current study aimed to evaluate the predictive value of the C2HEST score in patients with COPD. A retrospective analysis of medical records from 2184 patients hospitalized with COVID-19 at the University Hospital in Wroclaw from February 2020 to June 2021, which was previously used in earlier studies, assessed outcomes such as mortality during hospitalization, all-cause mortality at 3 and 6 months, non-fatal discharge, as well as adverse clinical incidents. This re-analysis specifically examines the outcomes using a COPD split. In the COPD group, 42 deaths were recorded, including 18 in-hospital deaths. In-hospital mortality rates at 3 and 6 months did not significantly differ among C2HEST strata, nor did their impact on subsequent treatment. However, a notable association between the C2HEST score and prognosis was observed in the non-COPD cohort comprising 2109 patients. The C2HEST score’s predictive ability is notably lower in COPD patients compared to non-COPD subjects, with COPD itself indicating a high mortality risk. However, C2HEST effectively identifies patients at high risk of cardiac complications during COVID-19, especially in non-COPD cases.
Keywords: COVID-19, SARS-CoV-2, COPD, prognosis, C2HEST score
1. Introduction
Despite restrictions, vaccination, and increasing knowledge regarding SARS-CoV-2 infection, COVID-19 remains one of the leading causes of hospitalization and patient deaths worldwide within the last three years. According to the data from Johns Hopkins University of Medicine on 3 October 2023, there have been more than 676 million infections since the beginning of the pandemic, of which almost 6.9 million have ended in death [1]. As the course of the disease differs significantly depending on general health, age, and comorbidities, it seems necessary to create or adapt already existing validated scales to implement them to precisely identify individuals at risk of developing severe SARS-CoV-2 infection. Besides the respiratory failure observed during the initial phase of the pandemic, we now know that SARS-CoV-2 infection increases the risk of developing cardiovascular, gastrointestinal, neurological, and rheumatic diseases, which are collectively referred to as “long COVID” [2].
Patients with chronic respiratory diseases, in particular chronic obstructive pulmonary disease (COPD), are at higher risk of developing a severe course of COVID-19. This higher risk may be related to decreased respiratory reserve, increased expression of angiotensin-converting enzyme-2 (ACE-2) receptor in the lower respiratory tract, which facilitates viral endocytosis, or impaired innate immunity mechanisms associated with prolonged exposure to the pathogen [3,4]. Meta-analyses indicate a higher risk of severe disease, which is defined as the need for hospitalization in the intensive care unit, mechanical ventilation, or death in COPD patients infected with SARS-CoV-2 [5,6]. Despite that, the prevalence of patients with COPD among hospitalizations due to SARS-CoV-2 infection ranges, according to available data, from 0.8% to 14.4%; however, among people hospitalized in the ICU, the rate is only 4% with approximately 12% prevalence in the general population [4,7].
The C2HEST score was initially designed to predict the risk of atrial fibrillation (AF); due to its simplicity, it offers an interesting value as a helpful tool for identifying the risk of the severe clinical course of COVID-19. Its components are based on the comorbidities that have been previously shown to have a major impact on the severity of the disease and prediction of critical illness [8]. Hence, the C2HEST score might be a useful tool in predicting the prognosis of patients with concomitant COPD suffering from SARS-CoV-2 infection.
As the usefulness of this score has been previously shown to predict COVID-19 outcomes (death, non-fatal clinical events, ICU hospitalizations) in the diabetic population and people with heart failure in our previous studies, this study verifies its prognostic efficacy in the COPD and non-COPD cohorts [9,10].
2. Materials and Methods
2.1. Study Design and Participants
A retrospective analysis of the hospitalization records of COVID-19 patients at the Wroclaw Medical University Center from February 2020 to June 2021 was performed. The study design and overall study group were published by Rola et al. (2022); the work presented here is a re-analysis of their published data [9]. For the purposes of this research, a subpopulation of individuals with COPD was selected from the given cohort. The COLOS retrospective study protocol received approval from the Institutional Review Board and Ethics Committee of Wroclaw Medical University, Wroclaw, Poland (No: KB-444/2021). The records used for the analyses were taken from the routine retrospective results; therefore, informed consent was not necessary to be obtained from the participants. All study participants had a confirmed SARS-CoV-2 infection determined by nasopharyngeal swab reverse transcription polymerase chain reaction test (RT-PCR). The diagnosis of COPD was confirmed based on their medical history according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 criteria [11]. The database used for analyses included demographic information; the need for oxygen support therapy and its type; tobacco smoking history; concomitant diseases; medical pharmacological treatment before admission; laboratory findings; and the adverse events occurring during hospitalization including septic shock, venous thromboembolism (deep vein thrombosis or pulmonary embolism), acute heart failure, acute kidney injury, acute liver dysfunction, ARDS, and bleedings.
2.2. Follow-Up and Outcomes
The follow-up period started on the day of in-hospital admission and ended on the day of the patient’s discharge or death. Further information regarding the patients’ deaths was collected 3 and 6 months after admission. The individual clinical records were used to obtain patients’ characteristics. The collected details included the in-hospital death rate; mortality at 3 and 6 months regardless of cause; and the nature of hospital discharge not involving death (home discharge, relocation to a different hospital, or need for stationary rehabilitation).
2.3. C2HEST Score Stratification
In this study, a total of 75 consecutive patients with COPD and 2109 patients without a diagnosis of COPD assigned to the control group were included. The baseline characteristics of participating subjects were taken from the database and used to calculate the C2HEST score with a total of 6 individual components Patients could receive 1 point each for the presence of coronary artery disease, COPD in the medical history, arterial hypertension, and prior thyroid diseases. Characteristics such as age over 75 years and systolic cardiac failure earned the participant 2 points on the scale. Significantly, the requirement for coronary artery disease was fulfilled by a past occurrence of either myocardial infarction or coronary revascularization (MI, counted as 1 point). Furthermore, in subsequent sensitivity assessments, the term “thyroid disease” was substituted with greater specificity, delineating between “hyperthyroidism” and “hypothyroidism”. These risk elements were established through a blend of examining medical records and conducting interviews during initial visits. Afterward, according to the scale creators, the patients were divided into low (0–1 points), medium (2–3 points), and high (4 points and more) primary risk categories.
2.4. Statistical Analysis
The presentation of descriptive data includes categorical values expressed in percentages, while numerical variables are represented by the mean, standard deviation, and distribution of results (minimum to maximum) along with the count of available data points. For comprehensive testing, a chi-square test was employed for categorical variables having over 5 anticipated instances in every group, whereas Fisher’s exact test was utilized for fewer instances. Welch’s ANOVA was conducted for continuous variables due to unequal variances between the risk strata and a sufficiently large sample to obtain adequate asymptotic results. The Games–Howell test with Tukey correction was used for post hoc analysis for continuous variables. For categorical variables, a follow-up test mirrored the omnibus test, albeit conducted within subgroups and adjusted with Bonferroni correction. Hospital death and mortality from all causes were presented as data that were censored from the right, prompting the utilization of time-dependent ROC analysis coupled with an inverse probability of censoring weighting (IPCW) estimation for these variables. The C2HEST score was acquired through the utilization of the time-varying area under the curve (AUC). A log-rank test was used to verify the differences in survival curves among the various risk groups. The Grambsch–Therneau test was employed to confirm the assumption of proportional hazards. A Cox proportional hazards model was applied to examine the hazard ratio (HR) for the overall C2HEST score, its individual components, and the various risk categories. For the secondary outcomes, given their binary nature, a logistic regression model was applied. For assessing the predictive capabilities, the classical receiver operating characteristic (ROC) analysis and the AUC measure were used. An odds ratio (OR) was reported as the measure of effect for the influence of the C2HEST score, its individual components, and the different risk categories. All statistical analyses were conducted using R version 4.0.4, with the packages timeROC, pROC, survival, coin, and odds ratio [12,13,14,15]. A threshold of 0.05 was chosen for determining statistical significance in all analyses.
3. Results
3.1. Initial Characteristics and Comorbidities of the Study Population
The study and control group baseline characteristics are presented in Table 1. In the control group, a higher C2HEST score was associated with more advanced age, male sex, cigarette smoking, and the number of comorbidities. Moreover, the prevalence of almost all considered comorbidities (except for asthma) and tobacco smoking was significantly elevated in the group with the higher risk category according to the C2HEST scale. In the study group, the C2HEST score was related to higher average age, incidence of hypertension, atrial arrhythmia, heart failure, and history of myocardial ischemia.
Table 1.
The baseline demographic characteristics and concomitant diseases of the study and control groups.
| Variables, Units | All Pts | Low Risk [0–1] | Medium Risk [2–3] | High Risk [≥4] | p-Value | Post Hoc Analysis for Significant p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | No COPD n = 2109 |
COPD n = 75 |
No COPD n = 1412 |
COPD n = 6 |
No COPD n = 467 |
COPD n = 25 |
No COPD n = 230 |
COPD n = 44 |
No COPD | COPD | No COPD | COPD |
| Age, years mean ± SD n, (min.–max.) |
59.6 ± 19.0 2109 (17–100) |
72.0 ± 8.06 75 (54–94) |
51 ± 15.9 1412 (17–74) |
66.7 ± 5.6 6 (57–71) |
76 ± 11.8 467 (29–100) |
67.4 ± 5.7 25 (41–97) |
79.2 ± 9.5 230 (38–100) |
75.3 ± 7.9 44 (59–94) |
<0.0001 | <0.0001 | <0.0001 a,b,c | 0.961 a 0.024 b <0.0001 c |
| Age ≥ 65 years, n (%) | 985 (46.7) |
62 (82.7) |
372 (73.7) | 4 (66.7) |
68 (14.6) | 20 (80.0) |
214 (93.0) | 38 (86.4) |
<0.0001 | 0.3111 | <0.0001 a,b <0.0001 0.0004 c |
N/A |
| Male gender, n (%) | 1033 (49.0) | 49 (65.3) |
730 (51.7) | 5 (83.3) |
192 (41.1) | 16 (64.0) |
111 (48.3) | 28 (63.6) |
0.0003 | 0.8078 | 0.0003 a 1 b 0.26 c |
|
| BMI, kg/m2 mean ± SD, (min.–max.), |
28.4 ± 5.9 (15.4–49.4) 532 |
28.7 ± 4.5 22 (18.6–36.7) |
28.3 ± 5.1 397 (15.4–49.4) |
0 | 29.4 ± 5.7 81 (20.5–47.7) |
28.4 ± 5.6 9 (18.6–36.7) |
27.5 ± 6.3 54 (16.4–48.2) |
28.8 ± 3.7 13 (22.9 –34.9) |
0.07 | 0.8591 | ||
| Normal body weight (BMI = 18.5–24.9 kg/m2), n, n (%) | 136 (25.6) |
5 (22.7) |
100 (25.1) |
0 | 16 (19.6) | 3 (33.3) |
20 (37.0) | 2 (15.4) |
||||
| Underweight (BMI < 18.5 kg/m2), n (%) n = 22 |
5 (1.0) | 0 (0.0) | 3 (0.76) |
0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.7) | 0 (0.0) | ||||
| Overweight (BMI = 25–29.9 kg/m2), n (%), n = 22 | 209 (39.3) | 8 (36.4) |
162 (40.8) |
0 | 31 (38.3) | 2 (22.2) |
16 (29.6) | 6 (46.2) |
||||
| Obesity (BMI ≥ 30 kg/m2), n (%), n = 22 | 182 (34.2) | 9 (40.9) |
132 (33.2) |
0 | 34 (42.0) | 4 (44.4) |
16 (29.6) | 5 (38.5) |
||||
| Tobacco smoking, never/previous/current, n (%), n = 75 | 1953/93/59 (92.8/4.4/2.8) |
34/24/17 (45.3%/32%/22.7%) |
1335/45/32 (94.5/3.2/2.3) |
3/1/2 (50%/16.7%/33.3%) |
421/26/17 (90.7/5.6/3.7) |
10/9/6 (40%/36%/24%) |
197/22/10 (86.0/9.6/4.4) |
21/14/9 (47.7%/31.8%/20.5%) |
<0.0001 | 0.8795 | 0.04 a <0.0001 0.38 c |
|
| Comorbidities | ||||||||||||
| Hypertension, n (%) n = 75 |
962 (45.6) | 60 (80.0) |
416 (29.5) |
0 | 335 (71.7) | 22 (88.0) |
211 (91.7) | 38 (86.4) |
<0.0001 | <0.0001 | <0.0001 a,b,c | <0.0001 a,b 1.0 c |
| Diabetes mellitus, n (%), n = 473 | 486 (34.1) | 30 (40.0) | 234 (16.6) |
1 (16.7) | 144 (30.9) | 10 (40.0) | 108 (47.2) | 19 (43.2) | <0.0001 | 0.9498 | <0.0001 a,b 0.0002 c |
|
| Dyslipidemia, n (%) n = 31 |
574 (73.8) | 15 (48.4) n = 31 |
288 (69.4) |
1 (33.3) n = 3 |
161 (74.2) | 7 (63.6) n = 11 |
125 (85.6) | 7 (41.2) n = 17 |
0.0006 | 0.4939 | 0.001 a 0.72 b 0.04 c |
|
| Atrial fibrillation/flutter, n (%), n = 75 | 260 (12.3) | 30 (40.0) |
49 (3.4) |
0 | 100 (21.4) | 6 (24) |
111 (48.2) | 24 (54.5) |
<0.0001 | <0.0001 | <0.0001 a,b,c | 0.928 a 0.0689 b 0.06698 c |
| Previous coronary revascularization, n (%), n = 75 | 136 (6.4) | 18 (24) |
6 (0.4) | 0 | 36 (7.7) | 1 (4.0) |
94 (40.9) | 17 (38.6) |
<0.0001 | <0.0001 | <0.0001 a,b,c | 1 0.295 b <0.0001 c |
| Previous myocardial infarction, n (%), n = 75 | 170 (8.0) | 21 (28.0) |
11 (0.8) | 0 | 60 (12.8) | 3 (12.0) |
99 (43.0) | 18 (40.9) |
<0.0001 | <0.0001 | <0.0001 a. b. c | 1 a 0.2263 b 0.044 |
| Heart failure, n (%) n = 75 |
226 (10.7) | 29 (38.7) |
0 (0.0) | 0 | 53 (11.34) | 0 | 173 (75.2) | 29 (65.9) |
<0.0001 | <0.0001 | <0.0001 a,b,c | 1 a 0.0102 b <0.0001 c |
| Moderate/severe valvular heart disease or previous valve heart surgery, n (%) n = 75 |
86 (4.1) | 10 (13.3) |
13 (0.9) | 0 | 30 (6.4) | 2 (8.0) |
43 (18.7) | 8 (18.2) |
<0.0001 | 0.4162 | <0.0001 a,b,c | |
| Peripheral artery disease, n (%) n = 75 |
94 (4.5) | 6 (8.0) |
26 (1.8) | 0 | 30 (6.4) | 1 (4.0) |
38 (16.5) | 5 (11.4) |
<0.0001 | 0.6465 | <0.0001 a,b,c | |
| Previous stroke/TIA, n (%), n = 75 | 151 (7.15) | 13 (17.3) |
47 (3.3) | 0 | 57 (12.2) | 2 (8.0) |
47 (20.4) | 11 (25) |
0.0012 | 0.1318 | <0.0001 a,b,c | |
| Chronic kidney disease n = 75 |
212 (10.1) | 19 (25.3) |
69 (4.9) | 1 (16.6) |
66 (14.1) | 4 (16.0) |
77 (33.5) | 14 (31.8) |
<0.0001 | 0.3665 | <0.0001 a,b,c | |
| Hemodialysis, n (%) n = 75 |
53 (2.5) | 5 (6.7) |
19 (1.3 | 0 | 18 (3.9) | 2 (8.0) |
16 (7.0) | 3 (6.8) |
<0.0001 | 1 | 0.004 a <0.0001 b 0.033 c |
|
| Asthma, n (%) n = 75 |
77 (3.7) | 8 (10.7) |
54 (3.8) | 0 | 17 (3.6) | 3 (12.0) |
6 (2.6) | 5 (11.4) |
0.66 | 1 | ||
| Thyroid disease, none/hypothyroidism/hyperthyroidism, n (%) n = 75 |
1890/199/20 (89.6/9.4/1.0) | 65/9/1 (86.7%/12.0%/1.3%) |
1332/76/4 (94.3/5.4/0.3) |
6/0/0 (100.0%/0.0%/0.0%) |
391–66–10 (83.7/14.1/2.2) |
23/2/0 (92.0%/8.0%/0.0%) |
167/57/6 (72.6/24.8/2.6) |
36/7/1 (81.8%/15.9%/2.3%) |
<0.0001 | 0.7322 | <0.0001 a,b 0.0059 c |
|
Presentation of variables: continuous mean ± standard deviation, results distribution (min.–max.), and the number of present values; the values are presented as numbers with a percentage. Information regarding the numbers showing the correct values can be found in the left column. List of abbreviations used: valid measurements—N; the count of patients exceeding the cut-off point—n; standard deviation—SD; body mass index—BMI; transient ischemic attack—TIA; chronic obstructive pulmonary disease—COPD; not applicable—N/A; low- vs. medium-risk groups—a; low- vs. high-risk groups—b; and medium- vs. high-risk groups—c.
From the patient-reported symptoms and vital signs of the non-COPD group, a higher C2HEST score was associated with a greater incidence of cough, dyspnea, and smell dysfunction, and differences in body temperature, heart rate, systolic blood pressure, pulse pressure, and lower baseline blood saturation measured by pulsoxymetry. In the physical examination, the prevalence of crackles, wheezing, pulmonary congestion, and peripheral edema was also higher in this cohort. In the COPD group, there were no variations in the vital signs, symptoms, and physical examination findings upon hospital admission among the strata based on the C2HEST score. Interestingly, except for dyspnea and blood saturation, significant disparities in terms of cough, chest pain, taste impairment, and other assessed vital signs were not detected between the COPD and non-COPD cohorts (Table 2).
Table 2.
The symptoms reported by patients, measured vital signs, and baseline physical examination findings in the study and control groups.
| Variables, Units | All Pts | Low Risk [0–1] | Medium [2–3] | High Risk [≥4] | p-Value | Post Hoc Analysis for Significant p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-Reported Symptoms | No COPD n = 2109 |
COPD n = 75 |
No COPD n = 1412 |
COPD n = 6 |
No COPD n = 467 |
COPD n = 25 |
No COPD n = 230 |
COPD n = 44 |
No COPD | COPD | No COPD | COPD |
| Cough, n (%) n = 75 |
628 (29.8) | 20 (26.7) |
455 (32.2) | 0 | 116 (24.8) | 8 (32.0) |
57 (24.8) | 12 (27.3) |
0.0022 | 0.3753 | 0.01 a 0.09 b 1 c |
|
| Dyspnea, n (%) n = 75 |
869 (41.2) | 52 (69.3) |
567 (40.2) | 2 (33.3) |
190 (40.7) | 16 (64.0) |
112 (48.7) | 34 (77.3) |
0.0492 | 0.0606 | 1 a 0.053 b 0.16 c |
|
| Chest pain, n (%) n = 75 |
153 (7.3) | 10 (13.3) |
101 (7.2) | 1 (16.7) |
30 (6.4) | 4 (16.0) |
22 (9.6) | 5 (11.4) |
0.31 | 0.6684 | ||
| Hemoptysis, n (%) n = 75 |
15 (0.7) | 0 | 9 (0.6) | 0 | 2 (0.4) | 0 | 4 (1.7) | 0 | 0.13 | <0.0001 | 0.002 a <0.0001 b 0.07 c |
|
| Smell dysfunction, n (%), n = 75 | 75 (3.6) | 1 (1.3) |
61 (4.3) | 0 | 10 (2.1) | 0 | 4 (1.7) | 1 (1.3) |
0.025 | 1 | 0.13 a 0.18 b 1 c |
|
| Taste dysfunction, n (%), n = 75 | 64 (3.0) | 2 (2.7) |
49 (3.5) | 0 | 10 (2.1) | 0 | 5 (2.2) | 2 4.5 |
0.25 | 0.6036 | ||
| Abdominal pain, n (%), n = 75 | 142 (6.7) | 5 (6.7) |
103 (7.3) | 1 (16.7) |
26 (5.6) | 0 | 13 (5.7) | 4 (9.1) |
0.34 | 0.1704 | ||
| Diarrhea, n (%) n = 75 |
120 (5.7) | 7 (9.3) |
74 (5.2) | 1 (16.7) |
32 (6.9) | 1 (4.0) |
14 (6.1) | 5 (11.4) |
0.41 | 0.2900 | ||
| Nausea/Vomiting, n (%), n = 75 | 97 (4.6) | 1 (1.3) |
57 (4.0) | 0 | 27 (5.8) | 0 | 13 (5.7) | 1 (2.3) |
0.21 | 1 | ||
| Measured vital signs | ||||||||||||
| Body temperature, °C, mean ± SD (min.–max.), n = 40 |
37.0 ± 0.88 (34.3–40.5) |
37.1 ± 0.97 (35.2–39.0) n = 40 |
37.1 ± 0.9 (34.4–40.5) |
37.3 ± 1.58 (35.9–39.0) n = 3 |
36.9 ± 0.9 (35–40) |
37.0 ± 0.85 (36.0–38.5) n = 13 |
36.9 ± 0.8 (35.5–40) |
37.1 ± 0.99 (35.2–39.0) n = 24 |
0.032 | 0.9644 | 0.1 a 0.13 b 0.98 c |
|
| Heart rate, beats/minute mean ± SD (min.–max.), n = 66 |
85.6 ± 16.2 (36–160) |
86.1 ± 19.69 (60–170) n = 66 |
86.4 ± 15.6 (48–160) |
87.5 ± 23.63 (70–120) n = 4 |
83.9 ± 16.5 (50–160) |
88.5 ± 16.02 (69–121) n = 20 |
84.7 ± 18.3 (36–150) |
84.9 ± 21.22 (60–170) n = 42 |
0.03 | 0.7849 | 0.03 a 0.45 b 0.85 c |
|
| Respiratory rate, breaths/minute mean ± SD (min.–max.), n = 15 |
18.4 ± 5.6 (12–50) |
21.4 ± 8.26 (16–50) n = 15 |
18.4 ± 5.8 (12–50) |
18.5 ± 5.5 (12–45) |
21.8 ± 2.36 (20–25) n = 4 |
18.7 ± 4.5 (12–30) |
21.3 ± 9.69 (16–50) n = 11 |
0.92 | 0.882 | |||
| Systolic blood pressure, mmHg mean ± SD (min.–max.), n = 65 |
132.0 ± 22.9 (50–270) |
134.2 ± 22.3 (85–184) n = 65 |
130.7 ± 21.3 (60–240) |
131.0 ± 25.1 (105–155) n = 3 |
134 ± 25.6 (50–270) |
138.6 ± 19.73 (90–167) n = 20 |
134.9 ± 25.0 (70–210) |
132.4 ± 23.48 (85–184) n = 42 |
0.014 | 0.5991 | 0.07 a,b 0.9 c |
|
| Systolic blood pressure <100 mmHg, n (%), n = 65 | 73 (4.6) | 5 (7.69) n = 65 |
45 (4.3) | 0 (0.0) n = 3 |
17 (4.7) | 1 (5.0) n = 20 |
11 (5.4) | 4 (9.5) n = 42 |
0.78 | 0.9999 | ||
| Diastolic blood pressure, mmHg mean ± SD (min.–max.), n = 65 |
78.1 ± 13.4 (40–157) |
76.9 ± 13.1 (45–110) n = 65 |
78.5 ± 12.7 (40–150) |
80.0 ± 17.32 (70–100) n = 3 |
78.0 ± 13.8 (40–157) |
79.0 ± 11.0 (50–95) n = 20 |
75.8 ± 15.6 (40–143) |
75.7 ± 13.9 (45–110) n = 42 |
0.06 | 0.6501 | ||
| Mean blood pressure, mmHg MAP mean ± SD (min.–max.), n = 65 |
96.2 ± 14.9 (46.7–190) |
96.0 ± 14.6 (58.3–125) n = 65 |
96.0 ± 14.2 (46.7–179) |
97.0 ± 19.06 (81.7–118.3) n = 3 |
97.1 ± 15.6 (59.7–190) |
98.8 ± 13.2 (63.3–115.7) n = 20 |
95.5 ± 17.1 (50–165.3) |
94.6 ± 15.11 (58.3–125) n = 42 |
0.43 | 0.6001 | ||
| Pulse pressure mean ± SD (min.–max.), n = 65 |
54.3 ± 16.8 (11–136) |
57.3 ± 17.3 (24–99) n = 65 |
52.3 ± 15.3 (11–136) |
51.0 ± 14.4 (35–63) n = 3 |
57.1 ± 18.7 (50–100) |
59.7 ± 12.8 (40–80) n = 20 |
59.2 ± 8.5 (20–130) |
56.6 ± 19.4 (24–99) n = 42 |
<0.0001 | 0.6016 | <0.0001 a,b 0.412 c |
|
| SpO2 on room air, % (FiO2 = 21%) mean ± SD (min.–max.), n = 52 |
91.2 ± 8.0 (48–100) |
87.8 ± 9.18 (56–99) n = 52 |
92.9 ± 7.1 (48–100) |
91.5 ± 5.69 (85–98) n = 4 |
89.9 ± 9.5 (50–100) |
85.0 ± 11.29 (56–99) n = 14 |
90.6 ± 8.5 (50–99) |
88.6 ± 8.45 (65–99) n = 34 |
<0.0001 | 0.3581 | <0.0001 a 0.012 b 0.772 c |
|
| Sp O2 < 90%, n (%) n = 52 |
316 (15.0) | 26 (50.0) n = 52 |
181 (22.3) | 2 (50.0) n = 4 |
92 (34.5) | 10 (71.4) n = 14 |
43 (32.3) | 14 (41.2) n = 34 |
0.0001 | 0.2119 | 0.0003 a 0.048 b 1 c |
|
| GCS, points, n = 18 | 14.5 ± 1.9 (1–15) |
14.8 ± 0.51 (13–15) n = 18 |
14.6 ± 1.8 (1–15) |
0 | 14.5 ± 1.7 (3–15) |
15.0 ± 0.0 (15–15) n = 6 |
14.1 ± 2.5 (3–15) | 14.75 ± 0.62 (13–15) n = 12 |
0.049 | NaN | 0.38 a 0.07 b 0.34 c |
|
| Abnormalities detected during physical examination | ||||||||||||
| Crackles, n (%) n = 75 validated |
304 (14.4) | 15 (20.0) |
153 (10.8) | 1 (16.7) |
96 (20.6) | 3 (12) |
55 (23.9) | 11 (25.0) |
<0.0001 | 0.4534 | <0.0001 a,b 1 c |
|
| Wheezing, n (%) n = 75 |
187 (8.9) | 32 (42.7) |
92 (6.5) | 2 (33.3) |
49 (10.5) | 7 (28.0) |
46 (20.0) | 23 (52.3) |
<0.0001 | 0.1553 | 0.02 a <0.0001 b 0.003 |
|
| Pulmonary congestion, n (%) n = 75 |
343 (16.3) | 24 (32) |
183 (13.0) | 1 (16.7) |
98 (21.0) | 7 (28.0) |
62 (27.9) | 16 (36.4) |
<0.0001 | 0.6894 | 0.0001 a,b 0.29 c |
|
| Peripheral edema, n (%) n = 75 | 177 (8.4) | 12 (16.0) |
75 (5.3) | 1 (16.7) |
56 (12.0) | 4 (16.0) |
46 (20.0) | 7 (15.9) |
<0.0001 | 1 | <0.0001 a,b 0.02 c |
|
| Hemiplegia/ hemiparesis, n (%) n = 75 |
70 (3.3) | 3 (4.0) |
31 (2.2) | 0 | 24 (5.1) | 0 | 15 (6.5) | 3 (6.8) |
0.0001 | 0.6498 | 0.006 a 0.002 b 1 c |
|
| VES–13, points n = 9 |
5.4 ± 3.2 (1–13) |
5.1 ± 3.1 (1–10) n = 9 |
4.1 ± 2.9 (1–9) |
0 | 5.8 ± 3.3 (1–12_ |
4 ± 4.36 (1–9) n = 3 |
6.7 ± 3.0 (3–13) |
5.7 ± 2.58 (3–10) n = 6 |
0.13 | 0.5884 | 0.094 a 0.014 b 0.542 c |
|
Presentation of variables: continuous mean ± standard deviation, results distribution (min.–max.), and the number of present values; the values are presented as numbers with a percentage. Information regarding the numbers showing the correct values can be found in the left column. Abbreviation list: valid measures—N; count of patients exceeding cut-off point—n; standard deviation—SD; body mass index—BMI; transient ischemic attack—TIA; chronic obstructive pulmonary disease—COPD; low- vs. medium-risk groups—a; low- vs. high-risk groups—b; and medium- vs. high-risk groups—c.
3.2. Characteristics of the In-Hospital Laboratory Tests and Treatment Applied
3.2.1. Laboratory Assays
The full available characteristics of the laboratory results from the period of hospitalization for the COPD and non-COPD groups are summarized in the Supplementary Materials, Table S1. The measured parameters include routine blood count, blood gas analysis, selected biochemical parameters (including IL-6, vitamin B12, and iron metabolism), blood coagulation, and concentration of selected hormones (TSH, cortisol, and parathormone). Both groups are characterized by lower hemoglobin levels in the high-risk C2HEST strata. In the control group, there was a noticeable difference in the distribution of leukocytes (lymphopenia and neutropenia) and the prevalence of thrombocytopenia at the end of hospitalization among the C2HEST strata. During the whole hospitalization period, the high-risk category was characterized by higher lactate, ALAT, and TSH levels on admission and lower CRP levels upon discharge. The D-dimers, INR, APTT, glucose, urea, creatinine, albumin/protein, bilirubin, troponin, LDL, TG, and BNP values remained elevated throughout the whole hospitalization period. Among patients diagnosed with COPD, with the exception of a higher eosinophil count, lower procalcitonin concentration at discharge, and higher APTT, INR, TSH, glucose, urea, and creatinine levels on admission, there were no significant differences among the C2HEST strata.
3.2.2. Treatment Applied during the Hospitalization Period
There were no differences in the administration of COVID-19-specific treatment, including corticoids, remdesivir, tocilizumab, nor convalescent plasma among the C2HEST strata in both studied cohorts. Only the use of antimicrobial agents was higher in the high-risk stratum from the control group.
As for supportive treatment, in the control group, with increasing C2HEST score, there was an extended need for oxygen therapy; in addition, coronary artery revascularization and the use of catecholamines were more common among high-risk patients. Interestingly, in the COPD cohort, no differences regarding the above-mentioned parameters were found in the C2HEST strata (Table 3).
Table 3.
Applied treatment and necessary procedures in the study and control groups after C2HEST risk stratification.
| Variables, Units | All Pts | Low Risk [0–1] | Medium [2–3] | High Risk [≥4] | p-Value | Post Hoc Analysis for Significant p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No COPD | COPD n = 75 |
No COPD | COPD n = 6 |
No COPD | COPD n = 25 |
No COPD | COPD n = 44 |
No COPD | COPD | No COPD | COPD | |
| Treatment and procedures administered | ||||||||||||
| The highest level of respiratory assistance provided throughout the hospital stay | ||||||||||||
| No oxygen N, n (%) | 1014 (48.1) | 19 (25.3) | 739 (52.3) | 3 (50.0) |
196 (42.0) | 6 (24.0) | 79 (34.3) | 10 (22.7) | <0.0001 | 0.32 | <0.0001 a 0.0008 b 0.53 c |
|
| Low flow oxygen support N, n (%) | 724 (34.3) | 39 (52.0) | 448 (31.8) | 3 (50.0) |
178 (38.2) | 10 (40.0) | 98 (42.6) | 26 (59.1) | ||||
| High-flow nasal cannula, Non-invasive ventilation N, n (%) |
161 (7.6) | 13 (15.0) | 82 (5.8) | 0 (0.0) |
47 (11.8) | 5 (20.0) | 32 (13.9) | 7 (15.9) | ||||
| Invasive ventilation N, n (%) | 207 (9.8) | 5 (6.7) | 141 (10.0) | 0 (0.0) | 45 (9.7) | 4 (16.0) | 21 (9.1) | 1 (2.3) | ||||
| Oxygenation parameters from the period of qualification for advanced respiratory support: PaO2, mmHg Mean ± SD (min.–max.) |
68.07 ± 25.32 (29–168) N = 150 |
104 ± 22.6 (74–125) n = 23 |
66.1 ± 25.1 (34–168) |
N/A | 69.7 ± 24.9 (29–130) |
105.3 ± 827.4 (74–125) n = 11 |
76.5 ± 25.5 (38–137) |
100 | 0.26 | N/A | ||
| Therapy with catecholamines, N, n (%) |
207 (9.8) | 11 (14.7) | 131 (9.3) | 0 (0.0) | 41 (8.8) | 4 (16.0) | 35 (15.2) | 7 (15.9) | 0.014 | 0.78 | 1 a 0.024 b 0.045 c |
|
| Coronary revascularization or/and an indication for coronary revascularization, N, n (%) | 22 (1.0) | 4 (5.3) | 8 (0.6) | 0 (0.0) | 8 (1.7) | 3 (12.0) | 6 (2.6) | 2 (4.5) | 0.006 | 0.27 | 0.048 a 0.0499 b 1 c |
|
| Hemodialysis, N, n (%) | 70 (3.3) | 2 (2.7) | 47 (3.3) | 0 (0.0) | 12 (2.6) | 1 (4.0) | 11 (4.8) | 1 (2.3) | 0.31 | 0.99 | ||
| Systemic corticosteroids N, n (%) | 1047 (49.6) | 49 (65.3) | 704 (49.9) | 4 (66.7) | 229 (49.0) | 17 (68.0) | 114 (49.6) | 28 (63.6) | 0.95 | 0.93 | ||
| Plasma of the recovered, N, n (%) | 231 (11.0) | 8 (10.7) | 166 (11.8) | 1 (16.7) | 37 (7.9) | 4 (16.0) | 28 (12.2) | 3 (6.8) | 0.058 | 0.33 | ||
| Remdesivir, N, n (%) | 327 (15.5) | 16 (21.3) | 235 (16.6) | 1 (16.7) | 65 (13.9) | 7 (28.0) | 27 (11.7) | 8 (18.2) | 0.09 | 0.68 | ||
| Antibiotics, N, n (%) | 1183 (56.1) | 58 (77.3) | 743 (52.6) | 4 (66.7) | 284 (60.8) | 19 (76.0) | 156 (67.8) | 35 (79.5) | <0.0001 | 0.69 | 0.007 a <0.0001 b 0.26 c |
|
Presentation of variables: continuous mean ± standard deviation, results distribution (min.–max.), and the number of present values; the values are presented as numbers with a percentage. Information regarding the numbers showing the correct values can be found in the left column. List of used abbreviations: valid measurements—N; the number of patients with a parameter above the cut-off point—n; standard deviation—SD; body mass index—BMI; transient ischemic attack—TIA; chronic obstructive pulmonary disease—COPD; non-applicable—N/A; low- vs. medium-risk stratum—a; low- vs. high-risk stratum—b; and medium- vs. high-risk stratum—c.
3.3. Associations of the C2HEST Score with Fatal Outcomes
3.3.1. C2HEST Score Results and Mortality
A total of 42 deaths (73.7%) from 75 patients were reported in the COPD cohort with 18 (24%) in-hospital events. The mortality rates during hospitalization at 3 and 6 months among the C2HEST strata as well as its influence on the type and destination of further treatment did not significantly differ. On the contrary, in the non-COPD cohort, a strong, significant association between the C2HEST score and the prognosis was observed (Table 4).
Table 4.
Total, in-hospital, and after-discharge (until 20 September 2021) all-cause mortality in the study and control groups according to C2HEST risk strata.
| Variables, Units | All Pts n = 75 |
Low Risk [0–1] n = 6 |
Medium [2–3] n = 25 |
High Risk [≥4] n = 44 |
p-Value | Post Hoc Analysis for Significant p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No COPD | COPD n = 75 |
No COPD | COPD n = 6 |
No COPD | COPD n = 25 |
No COPD | COPD n = 44 |
No COPD | COPD | No COPD | COPD | |
| All-cause mortality rate | ||||||||||||
| In-hospital mortality, n (%) n = 75 |
308 (14.6) | 18 (24.0) |
118 (8.4) | 1 (16.7) |
104 (22.3) | 6 (24.0) |
86 (37.4) | 11 (25.0) |
<0.0001 | 1 | <0.0001 a,b,c | |
| 3-month mortality, n (%) | 507 (24.0) | 39 (54.2) |
198 (14.0) | 3 (60.0) |
187 (40.0) | 11 (44.0) |
122 (53.0) | 25 (59.5) |
<0.0001 | 0.4578 | <0.0001 a,b 0.012 c |
|
| 6-month mortality, n (%) | 536 (25.4) | 42 (73.7) |
211 (14.9) | 3 (50.0) |
196 (42.0) | 12 (48.0) |
129 (56.1) | 27 (61.4) |
<0.0001 | 0.3928 | <0.0001 a,b 0.17 c |
|
| Mortality until 20.09.2021 | 556 (26.4) | 42 (56.0) |
216 (15.3) | 3 (50.0) |
204 (43.7) | 12 (48.0) |
136 (59.1) | 27 (61.4) |
<0.0001 | 0.5886 | <0.0001 a,b 0.0005 c |
|
| Hospitalization | ||||||||||||
| Duration of hospitalization, days n = 75 |
12.4 ± 14.2 1–131 |
14.3 ± 13.6 1–72 |
11.6 ± 14.0 1–131 |
10.8 ± 19.5 1–50 |
13.0 ± 13.5 1–124 |
15.8 ± 15.2 1–72 |
16.5 ± 16.5 1–121 |
14.0 ± 11.9 1–46 |
<0.0001 | 0.8002 | 0.109 a <0.0001 b 0.017 c |
|
| End of hospitalization, n (%) Death |
308 (14.6) | 18 (24) |
118 (8.4) |
1 (16.7) |
104 (22.3) |
6 (24.0) |
86 (37.4) |
11 (25.0) |
<0.0001 | 0.9476 | <0.0001 a <0.0001 b 0.0013 c |
|
| Discharge to home—full recovery | 1289 (61.1) | 27 (36.0) |
991 (70.2) |
2 (33.3) |
212 (45.4) |
8 (32.0) |
86 (37.4) |
17 (38.6) |
||||
| Transfer to another hospital—worsening) | 267 (12.7) | 13 (17.3) |
137 (9.7) |
2 (33.3) |
93 (19.9) |
4 (16.0) |
37 (16.1) |
7 (15.9) |
||||
| Transfer to another hospital—in recovery | 245 (11.6) | 17 (22.7) |
166 (11.8) |
1 (16.7) |
58 (12.4) |
7 (28.0) |
21 (9.1) |
9 (20.5) |
||||
Presentation of variables: continuous mean ± standard deviation, results distribution (min.–max.), and the number of present values; the values are presented as numbers with a percentage. Information regarding the numbers showing the correct values can be found in the left column. Abbreviation list: valid measures—N; patients exceeding cut-off point—n; standard deviation—SD; body mass index—BMI; transient ischemic attack—TIA; chronic obstructive pulmonary disease—COPD; low- vs. medium-risk groups—a; low- vs. high-risk groups—b; and medium- vs. high-risk groups—c.
3.3.2. Differentiating Ability of the C2HEST Score in Predicting Overall Mortality
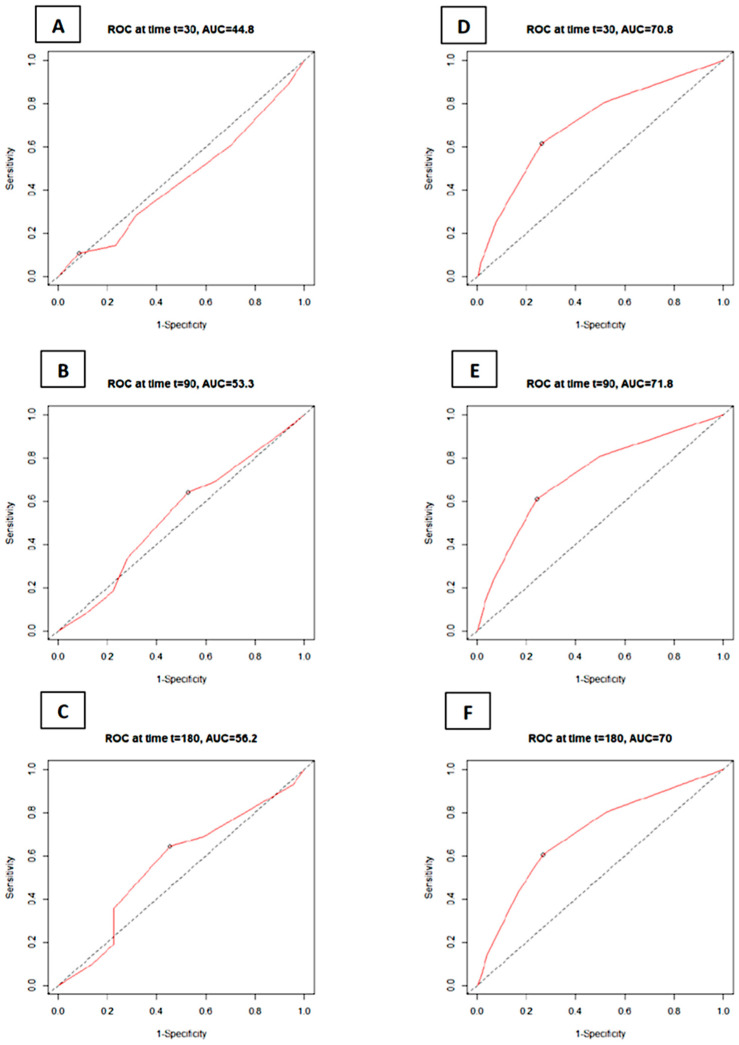
The receiver operating characteristic (ROC) analysis revealed that C2HEST failed to predict the 1-, 3-, and 6-month mortality in the COPD cohort. On the other hand, it was revealed to be a fair indicator of death prevalence in the non-COPD group. The C2HEST predicting values assessed by the AUC analysis in the COPD vs. non-COPD cohorts are as follows: the 1-month AUC30 = 44.8% vs. 70.8%; 3-month AUC90 = 53.3% vs. 71.8%; and 6-month AUC180 = 56.1% vs. 70.0%. All data were computed considering all-cause mortality without competing risks (Figure 1).
Figure 1.
ROC curves for the C2HEST score in predicting total mortality in the study groups: COPD (A–C) and control non-COPD groups (D–F) at selected time points (30 days; 90 days; and 180 days) after the positive RT-PCR test. Abbreviations: area under the curve—AUC; receiver operating characteristic—ROC.
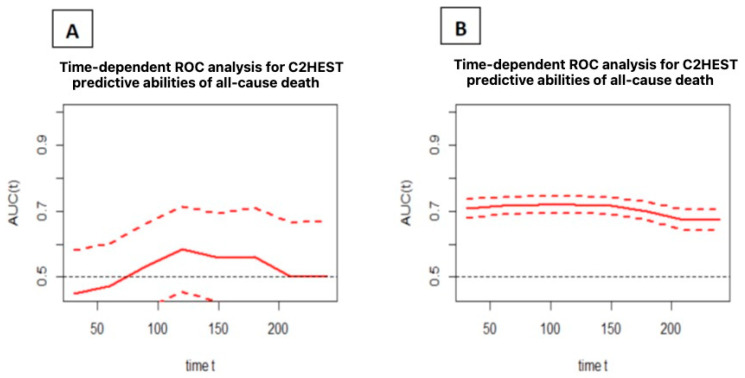
In addition, a timeROC analysis was performed to assess the predictive ability of the C2HEST score for deaths after a specified time “t” from hospital admission. All causes of death were considered in the analysis. Figure 2 shows the predictive ability expressed as the area under the ROC curve as a function of time, together with the confidence intervals.
Figure 2.
Time-dependent ROC analysis for the C2HEST predictive values of all-cause death in the study (A) and control (B) groups (mean with CI). Abbreviations: area under the curve—AUC; receiver operating characteristic—ROC.
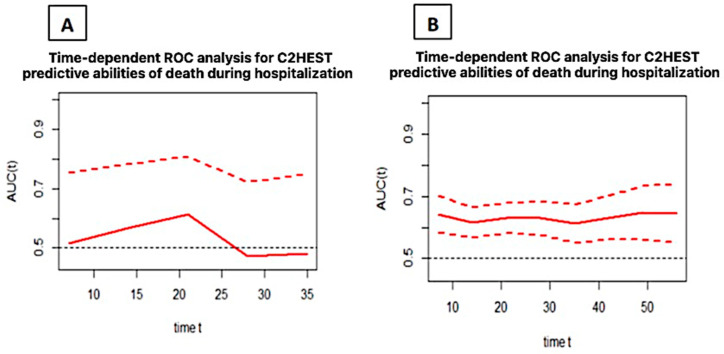
3.3.3. Discriminatory Performance of the C2HEST Score on the In-Hospital All-Cause Mortality–Time–ROC
An analysis presented in Figure 3 shows the time-dependent AUC for the C2HEST score in predicting the in-hospital deaths in both cohorts. The predicting abilities, regardless of the time of hospitalization, were poor in the control group while it failed in the COPD cohort.
Figure 3.
Time-dependent ROC analysis for the C2HEST predictive values of in-hospital all-cause death in the study (A) and control (B) groups (mean with CI). Abbreviations: area under the curve—AUC; receiver operating characteristic—ROC.
3.3.4. The Probability of Survival in Hospitalized COVID-19 Patients
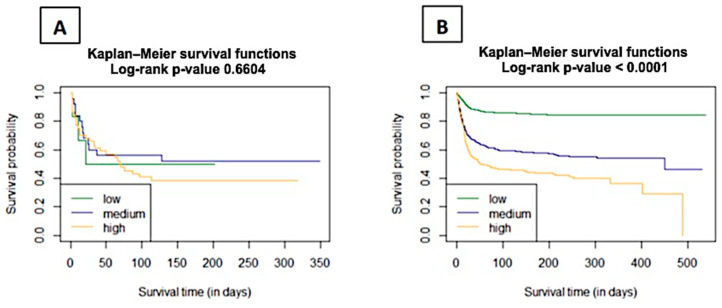
The Kaplan–Meier functions were used to estimate the survival curves for C2HEST groups according to the first categorization of low/medium/high for 0–1/2–3/≥4 points, respectively. The curves were compared using the Log-rank test—a p-value of 0.6604 indicates that the probability of survival in the risk strata for the COPD cohort is not statistically significant (Figure 4). On the contrary, in the non-COPD cohort, the p-value was <0.0001.
Figure 4.
Analysis of 6-month survival across low, medium, and high C2HEST risk categories in the study (A) and control (B) groups (mean with CI). Abbreviation: area under the curve—AUC.
3.3.5. Risk Strata Matching for Analysis
To determine whether the original layout, which includes the low/medium/high-risk categories for 0–1/2–3/≥4 points, respectively, of the C2HEST scale is optimal for patients with COPD, an analysis was performed taking into consideration all the possible C2HEST intervals with the performance of the log-rank statistics test for each one (Supplementary Materials, Table S2).
The highest value of the log-rank statistics for the COPD cohort corresponded with the C2HEST strata, which were estimated as follows:
-
●
0–4—low;
-
●
5–5—medium;
-
●
6–8—high.
This calculation results in better risk stratification than the generally accepted one; however, the original subdivision method is used in the following sections of this study. Similar statistical analysis regarding total and in-hospital mortality revealed that, for the non-COPD cohort, the main risk categories (0–1 = low; 2–3 = medium; and >4 = high) most accurately represent the mortality trends (Supplementary Materials, Table S3).
3.3.6. Effect of the C2HEST Risk Stratification Result on COVID-19 Survival
A power analysis of the effect of scale results on survival was performed using the Cox model. It tested how increasing the scale score by 1 and how changing the risk group affects mortality. In the COPD and non-COPD groups, an increase in one point resulted in a death rate increase of 4.1% and 42.4%, respectively. Upgrading the risk category from low to medium changed the death likelihood by 0.84 and 3.41 as well as 1.15 and 5.11 between the low-risk and high-risk groups in the COPD and non-COPD cohorts (Table 5). Importantly, the results in the group of subjects with a diagnosis of COPD are not statistically significant (p > 0.05) in opposition to people without such a diagnosis.
Table 5.
The hazard ratios for overall mortality across C2HEST risk stratification in both COPD and non-COPD groups.
| COPD | Non-COPD | |||||
|---|---|---|---|---|---|---|
| Total Deaths | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Overall | 1.04 | 0.88–1.23 | 0.64 | 1.42 | 1.37–1.48 | <0.0001 |
| Risk strata | ||||||
| Medium- vs. low-risk | 0.84 | 024–2.99 | 0.79 | 3.44 | 2.84–4.16 | <0.0001 |
| High- vs. low-risk | 1.15 | 0.35–3.80 | 0.81 | 5.11 | 4.12–6.34 | <0.0001 |
A similar analysis was performed for in-hospital deaths. For the COPD cohort, a one-point increase in C2HEST score was related to an 11.6% increase in in-hospital death. This risk does not significantly change between the medium- vs. low-risk and high- vs. low-risk strata. The same statistical model for the non-COPD cohort revealed a 28.3% increase in in-hospital mortality for each C2HEST point and a hazard ratio of 2.34 after the change from low- to medium-risk compartment, and 3.05 after the change from low- to high-risk, respectively (Table 6).
Table 6.
The hazard ratios for all-cause mortality during hospitalization across C2HEST risk stratification in both COPD and non-COPD groups (N/A—not applicable).
| COPD | Non-COPD | |||||
|---|---|---|---|---|---|---|
| In-Hospital Deaths | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Overall | 1.12 | 0.85–1.47 | 0.43 | 1.28 | 1.21–1.36 | <0.0001 |
| Risk strata | ||||||
| Medium- vs. low-risk | 0.96 | 0.11–8.28 | 0.97 | 2.34 | N/A | N/A |
| High- vs. low-risk | 1.02 | 0.13–8.14 | 0.98 | 3.05 | N/A | N/A |
The associations of individual C2HEST score components with mortality and other selected endpoints are included in the Supplementary Materials, Table S4a,b. Cox model analysis showed the greatest effect on in-hospital mortality of age and diagnosis of heart failure with reduced ejection fraction for the group with COPD and age and diagnosis of coronary artery disease for the non-COPD cohort, respectively.
3.3.7. Associations of the C2HEST Score with Other, Non-Fatal Events
The receiver operating characteristic (ROC) analysis performed on the COPD cohort revealed that the C2HEST predicts hypovolemic shock (AUC = 0.715), deep vein thrombosis (AUC = 0.959), acute heart failure (AUC = 0.847), multi-organ dysfunction syndrome (MODS) (AUC = 0.801), and all bleedings (AUC 0.701) including respiratory tract (AUC = 0.959), upper gastrointestinal tract (AUC = 0.808), and urinary tract (AUC = 0.797) bleeding. The Cox model showed that a 1-point change in this scale significantly predicts only the risk of acute heart failure (2.5-fold increase, 95% CI = 1.24–7.32, p = 0.0312). For a group without COPD, the ROC showed that C2HEST predicts cardiogenic shock (AUC = 0.773), acute heart failure (AUC = 0.869), and new cognitive signs and symptoms (AUC = 0.723). Each additional point in the C2HEST score increased the risk of cardiogenic shock by 72% with 4.6-fold (95% CI 1.65–13.80, p < 0.0001) and 12.8-fold (95% CI 4.96–13.80, p = 0.0039) increases for strata change from low- to medium-risk and low- to high-risk, respectively. For the acute heart failure, the risk increases by 112% with each C2HEST point (OR low vs. medium: 8.67, 95% CI = 3.99–20.90, p < 0.0001; OR low vs. high: 39.20, 95% CI = 19.12–91.26, p < 0.0001) and by 48% (OR low vs. medium: 4.56, 95% CI = 2.95–7.10, p < 0.0001; OR low vs. high: 5.36, 95% CI = 3.20–8.89, p < 0.0001) for the new cognitive signs and symptoms. The detailed results of the analyses can be found in Table 7.
Table 7.
Clinical non-fatal events and hospitalization outcomes in the C2HEST risk strata in the COPD and non-COPD cohorts.
| Variables, Units | All Pts n = 75 |
Low Risk [0–1] n = 6 |
Medium [2–3] n = 25 |
High Risk [≥4] n = 44 |
p-Value | Post Hoc Analysis for Significant p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selected Comorbidities | No COPD | COPD n = 75 |
No COPD | COPD n = 6 |
No COPD | COPD n = 25 |
No COPD | COPD n = 44 |
No COPD | COPD | No COPD | COPD |
| Aborted cardiac arrest, n (%) n = 75 |
23 (1.1) | 1 (1.3) | 15 (1.1) | 0 (0.0) | 3 (0.6) | 0 (0.0) | 5 (2.2) | 1 (2.3) | 0.19 | 1 | ||
| Shock, n (%) n = 75 |
181 (8.6) | 7 (9.3) | 108 (7.6) | 1 (16.7) | 42 (9.0) | 4 (16.0) | 31 (13.5) | 2 (4.5) | 0.013 | 0.207961 | 1 a 0.015 b 0.28 c |
|
| Hypovolemic shock, n (%) n = 75 |
33 (1.6) | 2 (2.7) | 22 (1.6) | 0 (0.0) | 5 (1.1) | 2 (8.0) | 6 (2.6) | 0 | 0.29 | 0.262703 | ||
| Cardiogenic shock, n (%) n = 75 |
27 (1.3) | 5 (6.7) | 6 (0.4) | 1 (16.7) | 9 (1.9) | 2 (8.0) | 12 (5.2) | 2 (4.5) | <0.0001 | 0.331994 | 0.012 a <0.0001 b 0.09 c |
|
| Septic shock, n (%) n = 75 |
137 (6.5) | 4 (5.3) | 89 (6.3) | 0 | 28 (6.0) | 2 (8.0) | 20 (8.7) | 2 (4.5) | 0.348 | 0.727591 | ||
| Venous thromboembolic disease, n (%) n = 75 |
67 (3.1) | 2 (2.7) | 47 (3.3) | 0 (0.0) | 13 (2.8) | 0 (0.0) | 7 (3.0) | 2 (4.5) |
0.83 | 0.603604 | ||
| Pulmonary embolism, n (%) n = 75 |
47 (2.2) | 1 (1.3) | 39 (2.7) | 0 (0.0) | 11 (2.3) | 0 (0.0) | 6 (2.6) | 1 (2.3) | 0.98 | |||
| Deep vein thrombosis, n (%) n = 75 |
20 (0.9) | 1 (1.3) | 15 (1.1) | 0 (0.0) | 4 (0.9) | 0 (0.0) | 1 (0.4) | 1 (2.3) | ||||
| Myocardial infarction, n (%) n = 75 |
26 (1.2) | 0 (0.0) | 8 (0.6) | 0 (0.0) | 10 (2.1) | 0 (0.0) | 8 (3.5) | 0 (0.0) | 0.0001 | <0.0001 | 0.015 a 0.0018 b 0.95 c |
<0.0001 a,b 0.0665 c |
| Myocardial injury, n (%) 3x n = 53 |
276 (24.6) | 22 (41.5) n = 53 |
112 (16.5) | 1 (33.3) n = 3 |
91 (31.6) | 7 (41.2) n = 17 |
73 (46.2) | 14 (42.4) n = 33 |
<0.0001 | 0.999999 | <0.0001 a <0.0001 b 0.009 c |
|
| Myocardial injury, n (%) 5x n = 53 |
207 (18.5) | 18 (34.0) n = 53 |
89 (13.2) | 1 (33.3) n = 3 |
66 (22.9) | 7 (41.2) n = 89 |
52 (32.9) | 10 (30.3) n = 87 |
<0.0001 | 0.786862 | 0.0007 a <0.0001 b 0.09 c |
|
| Acute heart failure, n (%) n = 75 |
72 (3.4) | 4 (5.3) | 8 (0.6) | 0 (0.0) | 22 (4.7) | 0 (0.0) | 42 (18.3) | 4 (9.1) | <0.0001 | 0.37735 | <0.0001 a,b,c | |
| Stroke/TIA, n (%) n = 75 |
43 (2.0) | 1 (1.3) |
18 (1.3) | 0 (0.0) |
19 (4.1) | 0 (0.0) |
6 (2.6) | 1 (2.3) |
0.0012 | 1 | 0.002 a 0.4 b 1 c |
|
| New cognitive signs and symptoms, n (%) n = 75 |
117 (5.5) | 4 (5.3) | 37 (2.6) | 1 (16.7) | 51 (10.9) | 0 (0.0) | 29 (12.6) | 3 (6.8) | <0.0001 | 0.182401 | <0.0001 a,b 1 |
|
| Pneumonia, n (%) n = 75 |
1009 (47.8) | 52 (69.3) | 602 (42.6) | 4 (66.7) |
265 (56.7) | 14 (56.0) |
142 (61.7) | 34 (77.3) |
<0.0001 | 0.1817010 | <0.0001 a,b 0.72 c |
|
| Complete respiratory failure, n (%) n = 20 |
134 (6.3) | 12 (60.0) n = 20 |
56 (4.0) | 1 (100.0) n = 1 |
42 (9.0) | 4 (66.7) n = 6 |
36 (15.7) | 7 (53.8) n = 13 |
0.049 | 0.99999 | 1 a 0.068 b 0.33 c |
|
| SIRS, n (%) n = 75 |
210 (10.3) | 10 (13.3) |
140 (10.4) | 2 (33.3) |
40 (8.6) | 2 (8.0) |
30 (13.1) | 6 (13.6) |
0.18 | 0.254624 | ||
| Sepsis, n (%) n = 24 |
21 (2.4) | 2 (8.3) n = 24 |
9 (1.6) | 7 (1.5) | 0 n = 6 |
5 (2.1) | 2 (11.1) n = 18 |
0.037 | 0.254624 | 0.2119 a 0.16 b 1 c |
||
| Acute kidney injury, n (%) n = 75 |
223 (10.5) | 14 (18.7) | 111 (7.9) | 0 (0.0) | 62 (13.3) | 5 (20.0) | 50 (21.7) | 9 (20.5) | <0.0001 | 0.730629 | 0.002 a <0.0001 b 0.018 c |
|
| Acute liver dysfunction, n (%) n = 69 |
65 (3.4) | 1 (1.4) n = 69 |
30 (2.4) | 0 (0.0) n = 6 |
22 (5.0) | 0 (0.0) n = 25 |
13 (6.0) | 1 (2.63) n = 38 |
0.0027 | 0.999999 | 0.03 a 0.02 b 1 c |
|
| Multiple organ dysfunction syndrome, n (%) n = 75 |
35 (1.7) | 2 (2.7) | 20 (1.4) | 1 (16.7) | 7 (1.5) | 1 (4.0) | 8 (3.5) | 0 (0.0) | 0.09 | 0.059459 | ||
| Lactic acidosis (on admission) n = 17 |
20 (8.7) | 2 (11.8) n = 17 |
9 (8.7) | 0 (0.0) n = 1 |
5 (6.8) | 0 (0.0) n = 5 |
6 (12.0) | 2 (18.2) n = 11 |
0.59 | 1 | ||
| Hyperlactatemia (on admission) n = 17 |
158 (69.3) | 9 (52.9) n = 17 |
77 (74.0) | 1 (100.0) n = 1 |
49 (66.2) | 3 (60.0) n = 5 |
32 (64.0) | 5 (45.5) n = 11 |
0.35 | 0.999999 | ||
| Bleedings, n (%) n = 75 |
110 (5.2) | 4 (5.3) |
64 (4.5) | 0 (0.0) | 24 (5.1) | 1 (4.0) | 22 (9.6) | 3 (6.8) | 0.006 | 0.999999 | 1 a 0.008 b 0.12 c |
|
| Intracranial bleeding, n (%) n = 75 |
21 (1.0) | 0 (0.0) | 12 (0.8) | 0 (0.0) | 8 (1.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0.205 | <0.0001 | <0.0001 a,b 0.0665 c |
|
| Respiratory tract bleeding, n (%) n = 75 |
33 (1.6) | 1 (1.3) | 23 (1.6) | 0 (0.0) | 4 (0.9) | 0 (0.0) |
6 (2.6) | 1 (2.3) |
0.2 | 1 | ||
| Gastrointestinal tract bleeding, n (%) n = 75 |
39 (1.8) | 2 (2.7) | 20 (1.4) | 0 (0.0) | 9 (1.9) | 0 (0.0) | 10 (4.3) | 2 (4.5) | 0.029 | 0.603604 | 1 a 0.02 b 0.4 c |
|
| Urinary tract bleeding, n (%) n = 75 |
17 (0.8) | 1 (1.3) | 9 (0.6) | 0 (0.0) | 3 (0.6) | 1 (4.0) | 5 (2.2) | 0 (0.0) | 0.08 | 0.413333 | ||
Presentation of variables: continuous mean ± standard deviation, results distribution (min.–max.), and the number of present values; the values are presented as numbers with a percentage. Information regarding the numbers showing the correct values can be found in the left column. Abbreviation list: valid measurements—N; number of patients exceeding the cut-off point—n; standard deviation—SD; body mass index—BMI; transient ischemic attack—TIA; chronic obstructive pulmonary disease—COPD; low- vs. medium-risk category—a; low- vs. high-risk category—b; and medium- vs. high-risk category—c.
For the non-COPD cohort, it is noteworthy that the C2HEST score can predict the occurrence of myocardial infarction and injury, stroke/TIA, acute kidney and liver dysfunction, and pneumonia. Surprisingly, there was no significant prognostic value for an occurrence of deep vein thrombosis, pulmonary embolism, and septic shock. With the exception of acute heart failure, this scale had no predictive value for adverse clinical outcomes in the COPD cohort.
3.3.8. Sensitivity Analysis
It is noteworthy that when calculating the C2HEST score, taking into account “hypothyroidism” in the place of “thyroid disease” and altering the cut-off point for age from above 75 years to above 65 years led to a notable enhancement in the predictive efficacy of the tested scale in the control group, but it did not have an impact on its parameters in the COPD group regarding the primary study endpoints and most of the adverse clinical events, with the exception of worsening the predictive value for acute heart failure in study group. The findings from the sensitivity analysis are outlined in the Supplementary Materials, Tables S5a,b and S6a,b.
4. Discussion
To the best of our knowledge, this study is the initial examination of the prognostic utility of the C2HEST scale in predicting mortality risk in COPD patients with COVID-19. The usefulness of this scale in assessing the risk of death has been demonstrated in other cohorts selected from the COLOS study population, including subjects with type 2 diabetes or heart failure [9,10]. Reports from the beginning of the pandemic indicated the diagnosis of chronic respiratory diseases, in particular COPD, which together with active smoking significantly increased the risk of a severe course of SARS-CoV-2 infection, which is defined as the need for mechanical ventilation or death. According to a meta-analysis performed by Zhao et al., the risk of a severe course of COVID-19 among patients with COPD increased by 4.38 fold, with the prevalence of severe course of COVID-19 widely differing among the component studies (6.10–28%) [6]. In our study, which takes into account a longer period (17 months vs. 4 months), the mortality rate in the COPD cohort reached 24%, which is in the upper range of the initially analyzed studies [6]. Due to the substantial heterogeneity in the population diagnosed with COPD—from those requiring only low doses of inhalation drugs to those requiring continuous oxygen therapy at home—it appears necessary to find a tool to anticipate the patient’s outcome and to identify the group of patients requiring special attention.
The initial purpose of the C2HEST scale was to forecast the likelihood of atrial fibrillation (AF) occurrence [16]. Its predictive ability has been demonstrated in large population-based studies [17,18,19]. Some authors have proven its efficacy not only in AF prediction but also in anticipation of other clinical outcomes such as death or requirement for hospitalization among patients diagnosed with heart failure and preserved ejection fraction (HFpEF) [20]. Among the COLOS study population, this scale also showed a potent predictive value, not only for mortality and the risk for a severe course of COVID-19, but also for other endpoints such as the development of acute liver or renal failure, the incidence of shock, or the need for more aggressive oxygen therapy (unpublished data). Interestingly, when considering only the population with a diagnosis of COPD, statistically significant differences were only found regarding cardiac complications—the occurrence of a new myocardial infarction or increased risk of acute heart failure and cardiogenic shock for each additional point in the C2HEST score. The lack of association, e.g., with mortality, may be due to the fact that the diagnosis of COPD itself is a factor in the significantly increased risk of death in COVID-19, though, among other aspects, such as reduced respiratory reserve, bronchial hyperresponsiveness, and impaired non-specific immunity resulting in the development of severe pneumonia [5,21].
Additionally, the predictive value of this scale may be reduced by the fact that one of its components is a diagnosis of COPD. Therefore, the cut-off points would need to be modified as proposed in this study. However, such a change would affect the clarity and convenience of the scale, so statistical calculations were performed for its original version. Despite these limitations, a statistically significant association of the C2HEST scale with acute heart failure was observed. Both heart failure and COPD have common risk factors for their development [22]. Furthermore, in COPD patients, there is a 20–32% prevalence of HF, while in the HF population, the comorbidity with COPD reaches 10% [23]. These two conditions in part also share the pathogenesis including endothelial dysfunction, oxidative stress, and aforementioned chronic inflammation [24,25]. The C2HEST score may help to identify patients at higher risk of developing not only acute heart failure but also myocardial infarction during hospitalization due to COVID-19. The diagnosis of COPD itself is associated with a higher incidence of myocardial infarction compared to the general population [26]. In addition, this risk increases during further exacerbations [27,28]. As COVID-19 symptoms depend not only on the activity of the virus itself but also on an enhanced inflammatory response, SARS-CoV-2 pneumonia, itself an exacerbation of COPD, is a risk factor for myocardial infarction [29]. In the case of rapidly developing acute respiratory failure, especially during a pandemic with a significantly overloaded healthcare system, the traditional risk assessment is difficult to perform [30]. Our study showed that the C2HEST score, due to its simplicity, enables easy identification of patients with a particularly high risk for adverse outcomes.
5. Limitations
Our study has several limitations. Firstly, in the study cohort, the number of patients with COPD was relatively low, accounting for less than 5% of the entire study population. Moreover, our results are based on a retrospective analysis of cases of patients hospitalized in a single center, which could affect the validity of our conclusions. Furthermore, all patients included in this study were from Central and Eastern Europe, which may limit relating the obtained conclusions to the world population. Furthermore, the data of the presented study group were from the years 2020–2021, which is the early period of the pandemic when vaccines were either not yet available or accessible to only a small portion of the population. This means that the presented database does not include vaccination information, which could have impacted the results related to incidence, disease severity, and mortality due to COVID-19. Finally, this study pertains to the early years of the pandemic; new variants of the virus began to emerge in the subsequent years. Consequently, the results concerning the C2HEST scale from this study may not be replicable for new groups infected with different variants of the virus.
6. Conclusions
In conclusion, the C2HEST score enables the prediction of the risk of death and many other complications in the course of SARS-CoV-2 infection. Within the population of patients with COPD, the predictive ability of this scale is much lower; however, the C2HEST score easily identifies patients with a particularly high risk of cardiac complications in the course of COVID-19.
Acknowledgments
The authors thank the following researchers from the Faculty of Medicine, Wroclaw Medical University, for their help in creating the database: Arkadiusz Derkacz, Barbara Adamik, Krzysztof Kaliszewski, Katarzyna Kiliś-Pstrusińska, Agnieszka Matera-Witkiewicz, Michal Pomorski, and Marcin Protasiewicz.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12061238/s1, Table S1. Summary of the results of the laboratory parameters measured during hospitalization for the COPD and non-COPD cohorts. Table S2. The Log-rank statistics for matching the C2HEST risk strata for total mortality in the COPD cohort. Table S3. The Log-rank statistics for matching the C2HEST risk strata for in-hospital mortality in the COPD cohort. Table S4a. Components of the C2HEST score and primary endpoints in the univariate Cox proportional hazard model in the COPD cohort. Table S4b. Components of the C2HEST score and selected secondary endpoints and concomitant diseases in the univariate Cox proportional hazard model in the COPD cohort. Table S5a. Impact of replacement of the general definition of “thyroid disease” with the more precise term “hypothyroidism” on the C2HEST score sensitivity to primary endpoints in the COPD cohort. Table S5b. Impact of replacement of the general definition of “thyroid disease” with the more precise term “hypothyroidism” on the C2HEST score sensitivity to selected secondary endpoints and concomitant diseases in the COPD cohort. Table S6a. Impact of changing the cut-off point for age to “>65 years” instead of “>75 years” on the C2HEST score sensitivity to primary endpoints in the COPD cohort. Table S6b. Impact of changing the cut-off point of age to “>65 years” instead of “>75 years” on the C2HEST score sensitivity to selected secondary endpoints and concomitant diseases in the COPD cohort.
Author Contributions
Conceptualization, J.G., A.D. and K.M.; data curation, K.K. and A.B.-S.; formal analysis, M.T., D.G., K.G., E.S.-K. and M.R.; investigation, P.R. and A.S.; methodology, K.K. and A.B.-S.; project administration, O.D. and K.M.; resources, J.S. and K.M.; software, K.K. and A.B.-S.; supervision, K.M.; validation, K.K. and K.M.; visualization, J.G., A.D., O.D. and K.M.; writing—original draft, J.G., A.D., O.D., M.T., D.G., K.G., E.S.-K., M.R., P.R., A.S., J.S., M.M., E.A.J., D.B.-C. and E.K.; writing—review and editing, J.G., A.D., O.D., M.T., D.G., K.G., E.S.-K., M.R., P.R., A.S., J.S., M.M., E.A.J., D.B.-C., E.K. and K.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets used and/or analyzed during the present study are available in this paper and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Johns Hopkins University & Medicine COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [(accessed on 3 October 2023)]. Available online: https://coronavirus.jhu.edu/map.html.
- 2.Koc H.C., Xiao J., Liu W., Li Y., Chen G. Long COVID and its Management. Int. J. Biol. Sci. 2022;18:4768–4780. doi: 10.7150/ijbs.75056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milne S., Yang C.X., Timens W., Bossé Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir. Med. 2020;8:e50–e51. doi: 10.1016/s2213-2600(20)30224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung J.M., Niikura M., Yang C.W.T., Sin D.D. COVID-19 and COPD. Eur. Respir. J. 2020;56:2002108. doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerayeli F.V., Milne S., Cheung C., Li X., Yang C.W.T., Tam A., Choi L.H., Bae A., Sin D.D. COPD and the risk of poor outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2021;33:100789. doi: 10.1016/j.eclinm.2021.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., Deng Y., Lin S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020;92:1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varmaghani M., Dehghani M., Heidari E., Sharifi F., Moghaddam S.S., Farzadfar F. Global prevalence of chronic obstructive pulmonary disease: Systematic review and meta-analysis. East. Mediterr. Health J. 2018;25:47–57. doi: 10.26719/emhj.18.014. [DOI] [PubMed] [Google Scholar]
- 8.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., Li Y., Guan W., Sang L., Lu J., et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rola P., Doroszko A., Trocha M., Giniewicz K., Kujawa K., Gawryś J., Matys T., Gajecki D., Madziarski M., Zieliński S., et al. Usefulness of C2HEST Score in Predicting Clinical Outcomes of COVID-19 in Heart Failure and Non-Heart-Failure Cohorts. J. Clin. Med. 2022;11:3495. doi: 10.3390/jcm11123495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajecki D., Doroszko A., Trocha M., Giniewicz K., Kujawa K., Skarupski M., Gawryś J., Matys T., Szahidewicz-Krupska E., Rola P., et al. Usefulness of the C2HEST Score in Predicting the Clinical Outcomes of COVID-19 in Diabetic and Non-Diabetic Cohorts. J. Clin. Med. 2022;11:873. doi: 10.3390/jcm11030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report) [(accessed on 2 December 2023)]. Available online: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf.
- 12.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therneau T. A Package for Survival Analysis in R. Version 3.2-11. [(accessed on 2 December 2023)]. Available online: https://CRAN.R-project.org/package=survival.
- 14.Hothorn T., Hornik K., van de Wiel M.A., Zeileis A. A Lego System for Conditional Inference. Am. Stat. 2006;60:257–263. doi: 10.1198/000313006x118430. [DOI] [Google Scholar]
- 15.Muenchow J., Schratz P., Brenning A. RQGIS: Integrating R with QGIS for Statistical Geocomputing. R J. 2017;9:409–428. doi: 10.32614/rj-2017-067. [DOI] [Google Scholar]
- 16.Himmelreich J.C.L., Veelers L., Lucassen W.A.M., Schnabel R.B., Rienstra M., van Weert H.C.P.M., E Harskamp R. Prediction models for atrial fibrillation applicable in the community: A systematic review and meta-analysis. EP Eur. 2020;22:684–694. doi: 10.1093/europace/euaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu W.-S., Lin C.-L. Prediction of new-onset atrial fibrillation for general population in Asia: A comparison of C2HEST and HATCH scores. Int. J. Cardiol. 2020;313:60–63. doi: 10.1016/j.ijcard.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Lip G.Y.H., Skjøth F., Nielsen P.B., Larsen T.B. Evaluation of the C2HEST Risk Score as a Possible Opportunistic Screening Tool for Incident Atrial Fibrillation in a Healthy Population (From a Nationwide Danish Cohort Study) Am. J. Cardiol. 2020;125:48–54. doi: 10.1016/j.amjcard.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Li Y.-G., Pastori D., Farcomeni A., Yang P.-S., Jang E., Joung B., Wang Y.-T., Guo Y.-T., Lip G.Y. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: Derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155:510–518. doi: 10.1016/j.chest.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W., Wu Y., Xue R., Wu Z., Wu D., He J., Dong Y., Lip G.Y.H., Zhu W., Liu C. C2HEST score predicts clinical outcomes in heart failure with preserved ejection fraction: A secondary analysis of the TOPCAT trial. BMC Med. 2021;19:44. doi: 10.1186/s12916-021-01921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herr C., Beisswenger C., Hess C., Kandler K., Suttorp N., Welte T., Schroeder J.-M., Vogelmeier C., R Bals for the CAPNETZ Study Group Group Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144–149. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins N.M., Petrie M.C., Jhund P.S., Chalmers G.W., Dunn F.G., Mcmurray J.J. Heart failure and chronic obstructive pulmonary disease: Diagnostic pitfalls and epidemiology. Eur. J. Hear. Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni H., Nauman D.J., Hershberger R.E. Managed care and outcomes of hospitalization among elderly patients with congestive heart failure. Arch. Intern. Med. 1998;158:1231–1236. doi: 10.1001/archinte.158.11.1231. [DOI] [PubMed] [Google Scholar]
- 24.Mills N.L., Miller J.J., Anand A., Robinson S.D., A Frazer G., Anderson D., Breen L., Wilkinson I.B., McEniery C.M., Donaldson K., et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: A mechanism for increased cardiovascular risk. Thorax. 2008;63:306–311. doi: 10.1136/thx.2007.083493. [DOI] [PubMed] [Google Scholar]
- 25.Eickhoff P., Valipour A., Kiss D., Schreder M., Cekici L., Geyer K., Kohansal R., Burghuber O.C. Determinants of Systemic Vascular Function in Patients with Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412oc. [DOI] [PubMed] [Google Scholar]
- 26.Sin D.D., Man S.F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen M., Dahl M., Lange P., Vestbo J., Nordestgaard B.G. Inflammatory Biomarkers and Comorbidities in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012;186:982–988. doi: 10.1164/rccm.201206-1113oc. [DOI] [PubMed] [Google Scholar]
- 28.Hoiseth A.D., Neukamm A., Karlsson B.D., Omland T., Brekke P.H., Soyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2011;66:775–781. doi: 10.1136/thx.2010.153122. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donoghue M.L., Morrow D.A., Cannon C.P., Jarolim P., Desai N.R., Sherwood M.W., Murphy S.A., Gerszten R.E., Sabatine M.S. Multimarker Risk Stratification in Patients with Acute Myocardial Infarction. J. Am. Hear. Assoc. 2016;5:002586. doi: 10.1161/jaha.115.002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available in this paper and in the Supplementary Materials.