Abstract
Cytotoxic T-lymphocyte (CTL) activity plays a central role in control of viral replication and in determining outcome in cases of human immunodeficiency virus type 1 (HIV-1) infection. Incorporation of important CTL epitope sequences into candidate vaccines is, therefore, vital. Most CTL studies have focused upon small numbers of adult Caucasoid subjects infected with clade-B virus, whereas the global epidemic is most severe in sub-Saharan African populations and predominantly involves clade-C infection in both adults and children. In this study, sensitive enzyme-linked immunospot (elispot) assays have been utilized to identify the dominant Gag-specific CTL epitopes targeted by adults and children infected with clade-B or -C virus. Cohorts evaluated included 44 B-clade-infected Caucasoid American and African American adults and children and 37 C-clade-infected African adults and children from Durban, South Africa. The results show that 3 out of 46 peptides spanning p17Gag and p24Gag sequences tested contain two-thirds of the dominant Gag-specific epitopes, irrespective of the clade, ethnicity, or age group studied. However, there were distinctive differences between the dominant responses made by Caucasoids and Africans. Dominant responses in Caucasoids were more often within p17Gag peptide residues 16 to 30 (38 versus 12%; P < 0.01), while p24Gag peptide residues 41 to 60 contained the dominant Gag epitope more often in the African subjects tested (39 versus 4%; P < 0.005). Within this 20-mer p24Gag, an epitope presented by both B42 and B81 is defined which represents the dominant Gag response in >30% of the total infected population in Durban. This epitope is closely homologous with dominant HIV-2 and simian immunodeficiency virus Gag-specific CTL epitopes. The fine focusing of dominant CTL responses to these few regions of high immunogenicity is of significance to vaccine design.
Evidence has accumulated over the past 13 years which argues that human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes (CTL) are central to the control of viremia and thus to long-term AIDS-free survival from the infection (7, 8, 18, 23, 38–40, 45, 49, 51, 64, 68). High levels of CTL are typically observed in asymptomatic infected adults, whereas CTL numbers decline in association with progression to AIDS (38, 64). In acute infection, the appearance of CTL is temporally linked with the reduction in viremia which occurs both in HIV and simian immunodeficiency virus (SIV) infection (7, 40, 68). Failure to control viremia, in either primary infection or chronic infection, may be associated with escape mutations arising within immunodominant epitopes (8, 18, 23, 39, 49, 50). Chronic HIV-infected adults naive to antiretroviral therapy also show a strong negative association between CTL numbers and viral load (47). More recently, experiments using anti-CD8 monoclonal antibody (MAb) infusions in SIV-infected macaques have demonstrated more directly that plasma virus levels show strong negative associations with CTL numbers, in both acute and chronic infection (30, 53). Macaques whose CTL response was delayed or abrogated altogether progressed significantly more rapidly to disease and death. Together, these data indicate that HIV-specific CTL responses constitute an essential component of an effective HIV vaccine.
Vaccine development urgently needs to target the populations and the clades of virus most relevant to the worldwide epidemic. Sub-Saharan Africa is estimated to have borne two-thirds of the global burden of HIV infection (5). C-clade virus is the most prevalent clade of HIV infection worldwide (4). The scale of the clade-C epidemic is illustrated by the antenatal prevalence rates in KwaZuluNatal, the most densely populated province within South Africa: 10 years ago less than 1% of antenatal mothers were infected; today the figure is greater than 40% (56, 65). The demographic groups most affected are young (15- to 30-year-old) women (annual incidence of new infection, 11% [A. Karim, unpublished data]) and infants (incidence of new infection, 10 to 12% [15, 56]). Thus, whereas published studies have focused upon B-clade-infected Caucasoid adults, vaccine-directed research needs to be applied also to C-clade-infected African mothers and infants.
These studies were therefore designed to take the initial steps in identifying the epitopes which dominate the CTL response in the ethnic groups and age groups worst hit by the global epidemic. We focused first upon Gag-specific responses since Gag-specific responses have been shown to be associated with protection in HIV and SIV infection (30, 38, 47, 52, 53). We used an approach based on enzyme-linked immunospot assays (elispots [41]) to identify the dominant epitopes targeted in persons who generated Gag-specific responses. The main advantage of elispot assays is that the epitope-specific CTL response in large numbers of infected persons can be characterized rapidly and effectively; previous epitope-specific studies which have relied upon more labor-intensive methods have necessarily involved no more than a few subjects. In addition, the high sensitivity of the elispot assays also facilitates study of pediatric CTL responses.
Eighty-one children and adults infected with HIV were studied in order to determine the immunodominant CTL epitopes presented by HLA class I molecules which are prevalent in a variety of ethnic groups. The ethnic groups best represented in these studies were African (62% of the subjects studied) and Caucasoid (30%) (plus 6% Haitian). We show that the immunodominant Gag-specific CTL responses are tightly focused on three highly immunogenic regions which together span 16% of the total length of p17Gag and p24Gag, but which represent two-thirds of the dominant Gag-specific CTL responses detected. Although there were no differences observed in the immunodominant epitopes targeted by infected adults and children, distinctive patterns of immunodominance were apparent when the Caucasoid subjects were compared with the Africans studied. These data are relevant to the development of vaccines designed to develop or boost anti-HIV cellular immunity in the face of the potential obstacle posed by high major histocompatibility complex (MHC) diversity.
MATERIALS AND METHODS
Subjects.
The 36 children studied attended clinics at the Boston Children's Hospital, Boston Medical Center, and Cato Manor Clinic, Durban, South Africa. All were infected via mother-to-child transmission. The mean age of the children studied was 7.5 years. The 45 adults studied attended clinics at the Massachusetts General Hospital, Boston, and Cato Manor Clinic and King Edward VIII Hospital, Durban, South Africa. All of the adults studied except the two hemophiliacs were infected via sexual transmission. Of the adults studied in the Boston cohorts, seven subjects were infected less than 24 months prior to the assay being performed.
All of the children in the Boston cohorts were receiving antiretroviral therapy, which included nucleoside and nonnucleoside reverse transcriptase inhibitors but not protease inhibitors. Five of the 16 adults in the Boston cohort had been previously treated with a triple therapy regimen consisting of one protease inhibitor and two reverse transcriptase inhibitors, but at the time of evaluation none of the 16 adults were receiving antiretroviral therapy. All of the children and adults in the Durban cohorts were antiretroviral therapy-naive.
Peptides.
Lymphocytes from the Boston cohorts of adults and children studied were tested for recognition of a panel of 46 overlapping peptides, 15 to 20 amino acids in length, spanning p17Gag and p24Gag B-clade SF2 sequence (all provided by the National Institute for Biological Standards and Control Centralized Facility for AIDS Reagents, supported by European Union Program EVA and the United Kingdom Medical Research Council, except for 12 overlapping p17Gag peptides, which were synthesized commercially [Research Genetics, Huntsville, Ala.]). Lymphocytes from the Durban cohort of adults and children studied were tested for recognition of a similar panel of 46 overlapping C-clade p17Gag and p24Gag peptides, 15 to 20 amino acids in length. These C-clade sequences were designed to represent a consensus sequence, derived from the published sequences available at the HIV website (www.hiv-lanl.gov). The peptides in both B- and C-clade panels overlapped by 10 to 11 amino acids, and thus there were no regions within p17Gag and p24Gag which were not fully represented by these peptides. The rationale for using B-clade-based sequences for study of the Boston cohorts and C-clade-based sequences for the Durban cohorts was that these are the respective clades of virus overwhelmingly dominant in HIV-infected persons in North America and Southern Africa, respectively (4).
Elispot assays.
Fresh peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Ficoll-Hypaque (Sigma, St. Louis, Mo.) density gradient centrifugation and were plated out in 96-well polyvinylidene plates (Millipore, Bedford, Mass.) which had been precoated with 0.5-μg/ml anti-gamma interferon (IFN-γ) MAb 1-DIK (Mabtech, Stockholm, Sweden). The peptides were added in a volume of 20 μl and then PBMCs were added at 50,000 cells per well in a volume of 180 μl. The final concentration of the peptides was 10 μM. The plates were incubated overnight at 37°C in 5% CO2 and were washed with phosphate-buffered saline before the addition of the second, biotinylated anti-IFN-γ MAb, 7-B6-1 biotin (Mabtech), at 0.5 μg/ml and were incubated at room temperature for 100 min. Following washing, streptavidin-conjugated alkaline phosphatase (Mabtech) was added at room temperature for 40 min. Individual cytokine-producing cells were detected as dark spots after a 20-min reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium by using an alkaline phosphatase-conjugate substrate (Bio-Rad, Richmond, Calif.). The number of specific T cells was calculated by subtracting the negative control values, unless the background was above 40 spot-forming cells (sfcs) per one million PBMCs (two immunospots/well at 50,000 PBMCs/well), in which case the assay was repeated. Significant Gag-specific responses were defined as responses at a frequency of 100 per one million PBMCs or higher (represented by five immunospots/well or more, at 50,000 PBMCs/well).
In all cases, positive responses were additionally verified by at least one of five methods: by demonstration of recognition of the same overlapping peptide in PBMCs from more than one time point; by recognition in repeat elispot assays of shorter 8- to 11-mer peptides contained within longer peptides that were recognized; by demonstration in repeat elispot assays of the same peptide-specific response in CD8+ positively sorted lymphocyte populations or CD4+-depleted lymphocyte populations, but not in CD8+-depleted or CD4 positively sorted lymphocyte populations; by staining of cells with the appropriate peptide-MHC tetramer; or by chromium release assays following the generation of CTL clones by using MHC class I-matched target cells. False-positive responses due to peptide contamination were further excluded by use of the same peptides in elispot assays using negative control PBMCs from nonresponsive subjects.
Immunodominance of the CTL response.
The immunodominant response was defined as the strongest response detected in the elispot assay. If more than 50 spots were counted in a well, the frequency was determined to be >1,000 per one million PBMCs for an input number of 50,000 PBMCs/well. In some cases, where more than one response was present at >1,000 per one million PBMCs, the dominance of the response was determined by repeat assays by using lower input numbers of PBMCs/well, down to 4,000 PBMCs/well. Where repeat assays could not be undertaken because of lack of cell availability, the responses present at >1,000 per one million PBMCs were considered codominant.
Cell sorting.
Sorting of cells into CD4+ and CD8+ T-cell-enriched and -depleted populations was done by using magnetic microbeads (Miltenyi Biotech). Greater than 99% purity was achieved in the positively sorted populations.
MHC-tetramer staining of lymphocytes.
Staining of lymphocytes was carried out by using the A*0201-SLYNTVATL tetrameric complex and the B42-TPQDLNTML complex, as appropriate. The phycoerythrin-labelled complexes were prepared as previously described (2). PBMCs or effector cells (500,000) were incubated for 15 min at 37°C with 0.5 mg of the appropriate tetramer and then for an additional 10 min with saturating amounts of PerCP-conjugated anti-CD8 monoclonal antibody and fluorescein isothiocyanate-conjugated anti-CD4 MAb (Becton Dickinson). Stained samples were analyzed on a FACSCalibur flow cytometer using CellQuest software. Control samples for the tetramer staining were PBMCs from HLA-mismatched HIV-infected persons. Quadrant boundaries for tetramer staining were established by exclusion of >99.97% of control CD8+ T cells.
Generation of CTL clones and precursor frequency assays.
CTL clones were generated by using methods previously described (63). In brief, PBMCs were plated out in 96-well plates at limiting dilution (100 to 10 cells/well) and were cultured with irradiated allogeneic feeder PBMCs at 50,000 cells/well in a final volume per well of 200 μl of R10 medium (RPMI 1640 medium [Sigma]), 10% fetal calf serum (Sigma), and 10 mM HEPES buffer (M-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid; Sigma) with antibiotics (2 mM l-glutamine and 50 U of penicillin-streptomycin per ml). The anti-CD3 MAb, 12F6, was added at 100 μg/ml. On day 5 and once weekly thereafter, the medium was changed with R10 medium containing 50 U of recombinant interleukin 2. Wells were screened for specific recognition of HLA-matched, peptide-pulsed, 51Cr (New England Nuclear, North Billerica, Mass.)-labelled Epstein-Barr virus-transformed B-lymphoblastoid cell line (BCL) target cells after 21 to 28 days in culture. Wells showing high specific recognition of the relevant peptide were then transferred to 24-well plates and were restimulated as above, except 106 feeders were added to each well and recombinant interleukin 2 was added on day 0. Expanded wells were then retested for lytic activity from day 14 of culture onwards and were maintained in culture by monthly restimulations as described.
Precursor frequency assays were set up in the same way, except that dilutions of PBMCs from 16,000 to 100 cells per well were plated out in 24 replicate wells. Chromium release assays (62) using HLA-matched BCL targets were performed after 14 to 21 days of culture.
Chromium release assays.
BCL target cells were labelled with chromium-51 by incubation of pelleted BCL with 50 μCi of Na2CrO4 (New England Nuclear) for 1 h at 37°C in 5% CO2. Targets were washed thrice and were then incubated with peptide dilutions (in the peptide titration assays) for a further 90 min prior to addition of effectors. The supernatants were harvested following 4 to 6 h of further incubation at 37°C in 5% CO2 (62).
HLA typing.
HLA typing was performed by sequence-specific primer PCR (SSP-PCR) (11).
RESULTS
Dominant Gag-specific CTL responses in B-clade-infected persons.
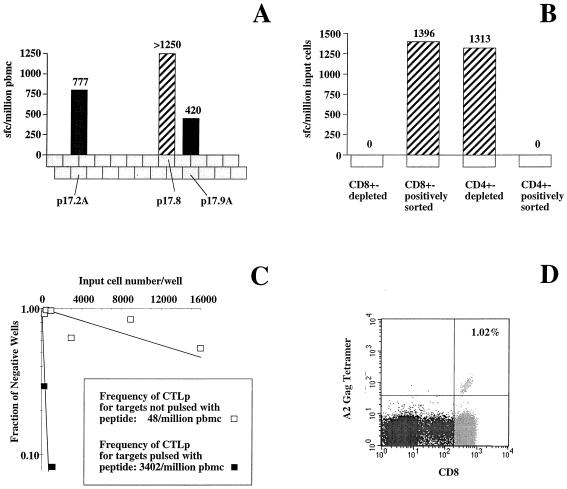
The method by which the dominant Gag-specific response was determined by using the elispot assays described and the methods by which the CD8 T-cell dependence of these responses were confirmed are illustrated for one infected individual in Fig. 1. Three 15-mer peptides out of 24 overlapping p17Gag peptides stimulated responses (Fig. 1A), the best response of which was to peptide p17.8 (GSEELRSLYNTVATL, residues 71 to 85). Following positive and negative cell sorting using CD4- and CD8-conjugated magnetic microbeads, respectively, the response to the 15-mer GSEELRSLYNTVATL was retested, and IFN-γ-producing cells were found only within CD4 T-cell-depleted populations and positively sorted CD8 T-cell populations (Fig. 1B). The precursor frequency assay, using A*0201-matched target cells pulsed with the optimal epitope peptide SLYNTVATL (31, 59), showed specific recognition of this peptide at a frequency of 3,354 per one million PBMCs (Fig. 1C). This was comparable with the level of tetramer staining of PBMCs, which was 1.02% or 10,200 per one million PBMCs (Fig. 1D).
FIG. 1.
(A) Elispot assay testing recognition by PBMCs from donor 161j (HLA class I type; HLA-A*0201/3 B7/60 Cw3/7) of the panel of 24 overlapping p17Gag peptides. A sample of 36,000 PBMCs per well was used (i.e., one spot represented 28 spot-forming cells [SFC]). More than 45 spots were present in the well containing peptide p17.8 (sequence GSEELRSLYNTVATL); thus the frequency of SFC specific for this peptide was >1,250 SFC per one million. (B) Elispot assay in panel A repeated using sorted cells and testing recognition only of peptide p17.8. (C) Precursor frequency assay using PBMCs from donor 161j, tested against the optimal HLA-A*0201-restricted epitope, SLYNTVATL, using A*0201-matched target cells. (D) Staining of PBMCs from donor 161j with the A*0201-SLYNTVATL MHC-peptide tetrameric complex, showing 1% of PBMCs staining with the tetramer.
The HLA types and dominant Gag-specific responses in the pediatric and adult Boston cohorts studied (n = 44) are shown in Table 1. The ethnic groups represented in the Boston cohorts were African (41%), Hispanic (3%), and Caucasoid (57%), all presumed to be infected with B-clade virus (4). All 16 adults tested and all but four of the children tested (28 of 32 [88%]) made Gag-specific responses at frequencies of >100 per one million PBMCs. Irrespective of the HLA types of the persons in the Boston cohorts studied, a minority of the 46 15- to 20-mer overlapping clade-B peptides spanning p17Gag and p24Gag were disproportionately targeted by the dominant Gag-specific CTL. Overall, three peptides, WEKIRLRPGGKKKYKLK (p17 residues 16 to 30), GSEELRSLYNTVATL (p17Gag residues 71 to 85), and SALSEGATPQDLNTMLNTVG (p24Gag residues 41 to 60), together representing 15% of the length of sequence spanned by the 46 overlapping peptides, contained the dominant Gag-specific epitope in 31 of 44 (70%) persons tested who showed Gag-specific responses (Table 1).
TABLE 1.
Gag-specific CTL responses in Boston cohorts studied
| Best-recognized peptide | Subject | HLA class I type | Racial group | CTL (per one million PBMCs) | Defined dominant epitope [HLA restriction]a |
|---|---|---|---|---|---|
| p17.2A | 014-TCH | A*0201/3 B35/44 Cw1/4 | African American Caucasoid | 600 | RLRPGGKKK [A3] |
| 019-TCH | A3/*3001 B51/60 CW15/16 | Caucasoid | 723 | KIRLRPGGK [A3] | |
| 027-TCH | A1/*0201 B18/57 Cw6/7 | Caucasoid | 472 | WEKIRLRPGGKKKYK [NDb] | |
| 048-TCH | A1/*0201 B27/37 Cw2/6 | Caucasoid | 780 | IRLRPGGKK [B27] | |
| 049-TCH | A1/24 B27/37 Cw1/6 | Caucasoid | 170 | IRLRPGGKK [B27] | |
| 004-BMC | A3/*3001 B7/35 Cw4/7 | Haitian | 273 | KIRLRPGGK [A3] | |
| 011-BMC | A24/29 B7/45 Cw6/7 | African American | 170 | RLRPGGKKKYK [ND] | |
| 96/00600 | A3/11 B44/62 Cw3/5 | Caucasoid | 225 | WEKIRLRPGGKKKYK [ND] | |
| 96/01114 | A2/3 B35/- Cw4/- | Caucasoid | >1,000 | RLRPGGKKK [A3] | |
| 85/00003 | A*0201/3 B7/51 Cw7/- | Caucasoid | >1,000 | RLRPGGKKK [A3] | |
| JC | A*0201/3 B35/55 Cw-/- | Caucasoid | 880 | RLRPGGKKK [A3] | |
| GV | A*0201/3 B8/62 Cw7/10 | Caucasoid | 280 | RLRPGGKKK [A3] | |
| TD | A3/68 B35/44 Cw4/7 | Caucasoid | 1,280 | RLRPGGKKK [A3] | |
| p17.4A | 042-TCH | A3/33 B35/53 Cw4/- | African American | >1,000 | WASRELERF [ND] |
| p17.8 | 001-UNC | A*0201/74 B7/35 Cw7/16 | African American | 2,800 | SLYNTVATL [A*0201] |
| 004-TCH | A*0202/29 B7/44 Cw7/16 | Caucasoid Hispanic | 1,400 | SLYNTVATL [A*0202] | |
| 032-TCH | A*0201/23 B44/61 Cw2/4 | Caucasoid | 160 | SLYNTVATL [A*0201] | |
| 034-TCH | A*0201/68 B35/44 Cw4/7 | Caucasoid | 120 | SLYNTVATL [A*0201] | |
| 036-TCH | A*0201/68 B18/51 Cw5/16 | Haitian | 213 | SLYNTVATL [A*0201] | |
| 040-TCH | A*0201/34 B7/52 Cw7/16 | Haitian | 400 | SLYNTVATL [A*0201] | |
| 007-BMC | A*0201/23 B14/45 Cw6/8 | African American | 520 | SLYNTVATL [A*0201] | |
| 010-161j | A*0201/3 B7/60 Cw3/7 | Caucasoid | >2,000 | SLYNTVATL [A*0201] | |
| 9354 | A*0201/3 B7/35 Cw4/7 | Caucasoid | 5,600 | SLYNTVATL [A*0201] | |
| 013-661 | A1/*0201 B44/52 Cw-/- | Caucasoid | 920 | SLYNTVATL [A*0201] | |
| 85/00003 | A*0201/3 B7/51 Cw7/- | Caucasoid | >1,000 | SLYNTVATL [A*0201] | |
| p17.8A | 013-199 | A*0201/*3002 B44/51 Cw-/- | Caucasoid | 5,650 | RSLYNTVATLY [A*3002] |
| p17.9A | 028-BMC | A1/*0201 B8/63 Cw7/- | Caucasoid | >1,000 | DVKDTKEAL [ND] |
| 045-TCH | A23/68 B45/72 Cw2/16 | Haitian | 133 | RIDVKDTKEAL [ND] | |
| p24.2 | 033-BMC | A34/68 B57/71 Cw3/7 | Haitian | 500 | VQHAISPRTLNAWV [ND] |
| p24.3 | GS | A*0201/30 B44/57 Cw-/- | Caucasoid | 770 | KAFSPEVIPMF [B57] |
| p24.5 | 015-TCH | A*3002/68 B14/*5802 Cw6/8 | African American | 462 | GATPQDLNTMLNTV [ND] |
| 016-TCH | A*3001/- B42/- Cw17/- | African American | 507 | TPQDLNTML [B42] | |
| 044-TCH | A*0201/30 B42/52 Cw16/17 | African American | 1,450 | TPQDLNTML [B42] | |
| 009-BMC | A*0201/*3002 B14/27 Cw1/8 | African American | 360 | TPQDLNTML [B14/Cw8] | |
| 021-BMC | A3/*3001 B42/- Cw17/- | African American | >1,000 | TPQDLNTML [B42] | |
| 026-BMC | A3/- B42/*5703 Cw7/17 | Hispanic | 2,460 | TPQDLNTML [B42] | |
| 032-BMC | A1/80 B42/45 Cw16/17 | African American | 1,810 | TPQDLNTML [B42] | |
| 94/00181 | A11/24 B14/44 Cw8/16 | Caucasoid | >1,000 | TPQDLNTML [ND] | |
| p24.13 | 029-TCH | A*0201/32 B7/8 Cw7/15 | Caucasoid | 433 | EIYKRWII [B8] |
| p24.14 | 023-TCH | A23/26 B62/72 Cw2/3 | African American | 660 | GLNKIVRMY [B62] |
| 043-TCH | ND | African American | 1,500 | KRWILGLNKIVRMY [ND] | |
| p24.17 | KM | A3/- B14/60 Cw3/8 | Caucasoid | 1,600 | DRFYKTLRA [B14] |
| JFM | A*0201/68 B14/44 Cw5/8 | Caucasoid | 120 | DRFYKTLRA [B14] | |
| p24.22 | KS | A3/- B7/- Cw7/- | Caucasoid | 1,020 | GPSHKARVL [B7] |
| DK | A*0201/11 B18/44 Cw-/- | Caucasoid | 1,300 | ACQGVGGPGHK [A11] |
In each case the defined epitopes shown represent the HLA restriction, and epitope optimization was determined for each subject. Optimized epitopes described elsewhere (for example, WASRELERF as a B35-restricted epitope [10]) are not shown unless the response was demonstrated in the subjects studied.
ND, not determined.
Dominant Gag-specific CTL responses in C-clade-infected persons.
The HLA types and dominant Gag-specific responses in the pediatric and adult Durban cohorts studied (n = 37) are shown in Table 2. The ethnic group studied within the Durban was uniformly African, subjects coming from the Zulu and Xhosa tribal groups. Thirty-seven of 44 persons tested (84%) showed Gag-specific responses at a frequency of >100 per one million PBMCs. Once again, a minority of the 46 15- to 20-mer C-clade-based overlapping peptides spanning p17Gag and p24Gag were disproportionately targeted by the dominant Gag-specific CTL in the 37 persons studied who showed responses at a CTL frequency of >100 per one million PBMCs (Table 2). Overall, three peptides, representing 16% of the length of sequence spanned by the 46 overlapping peptides, contained the dominant Gag-specific epitope in 23 of 37 (63%) persons tested. These C-clade peptides, RLRPGGKKHYMIKHLVW (p17Gag residues 20 to 36), ELRSLYNTVATLYCV (p17Gag residues 74 to 88), and SALSEGATPQDLNTMLNTVG (p24Gag residues 41 to 60), either closely or exactly overlap the three most immunogenic B-clade peptides identified. Two additional p24Gag peptides, FRDYVDRFFKTLRAEQA (residues 161 to 177) and SILDIKQGKEPFRDY (residues 149 to 164), together with the three listed above, account for the dominant or codominant Gag-specific response in 32 of 37 (86%) of the clade-C-infected subjects studied.
TABLE 2.
Gag-specific CTL responses in the Durban cohorts studied
| Best-recognized peptide | Subjecta | HLA class I type | CTL (per one million PBMCs) | Defined dominant epitope [HLA restriction] |
|---|---|---|---|---|
| p17c.4 | RCC-001 | A3/23 B49/5802 Cw6/- | 1,000 | RLRPGGKKK [A3] |
| RCC-002 | A*3001/66 B42/81 Cw4/17 | >1,000 | RLRPGGKKHYMIKHLVW [NDb] | |
| 338-M | A2/69 B7/42 Cw7/17 | >1,000 | RLRPGGKKHYMIKHLVW [ND] | |
| KEH04-M | A30/- B42/72 Cw2/17 | 354 | RLRPGGKKHYMIKHLVW [ND] | |
| p17c.7 | RCC-006 | A6801/- B62/71 Cw-/- | 600 | LVWASRELERFALNPGL [ND] |
| RCC-007 | ND | 1,000 | LVWASRELERFALNPGL [ND] | |
| p17c.11 | 338-M | A2/69 B7/42 Cw7/17 | >1,000 | IIKQLQPALQTGTEEL [ND] |
| p17c.13 | 338-M | A2/69 B7/42 Cw7/17 | >1,000 | SLYNTVATL [A2] |
| 325-M | A3/30 B*5802/72 Cw2/6 | >1,000 | ELRSLYNTVATLYCV [ND] | |
| 200-M | ND | 1,500 | ELRSLYNTVATLYCV [ND] | |
| KEH18-M | A*6802/- B44/*5802 Cw4/6 | 320 | ELRSLYNTVATLYCV [ND] | |
| p24c.3 | 994-M | A3/74 B57/62 Cw04/- | 760 | NAWVKVIEEKAFSPEVIPF [ND] |
| p24.5 | 1020-C5Y | A2/*3002 B14/*5802 Cw6/8 | 640 | TPQDLNTML [ND] |
| 1023-C6W | A24/30 B8/72 Cw2/7 | 540 | TPQDLNTML [ND] | |
| 021-M | A1/34 B72/81 Cw2/18 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 023-M | A29/3002 B7/42 Cw7/17 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 252-M | A29/*6802 B18/*5802 Cw5/6 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 270-M | A23/*6802 B41/71 Cw3/17 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 272-M | A30/34 B*5802/81 Cw6/18 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 298-M | A1/*6801 B*5802/81 Cw6/18 | >1,500 | SALSEGATPQDLNTMLNTVG [ND] | |
| 982-M | ND | >1,000 | TPQDLNTML [ND] | |
| 987-M | A2/*3002 B*5801/81 Cw04/- | >1,000 | TPQDLNTML [ND] | |
| 991-M | A1/*3002 B8/81 Cw7/18 | 980 | TPQDLNTML [ND] | |
| 1023-M | A2/24 B8/*5802 Cw6/7 | >1,000 | SALSEGATPQDLNTMLNTVG [ND] | |
| 1031-M | ND | >1,000 | TPQDLNTML [ND] | |
| KEH01-M | A30/68 B14/42 Cw8/17 | 380 | SALSEGATPQDLNTMLNTVG [ND] | |
| KEH02-M | A30/- B42/72 Cw2/17 | 900 | SALSEGATPQDLNTMLNTVG [ND] | |
| KEH19-M | A*3002/66 B42/*5802 Cw6/17 | 920 | TPQDLNTML [B42] | |
| p24c.16 | RC-005 | ND | 520 | NPPIPVDIYKRWII [ND] |
| 973-M | A1/3 B8/- Cw7/- | 1,860 | DIYKRWII [B8] | |
| p24c.17 | 1020-M | A2/- B*5802/62 Cw4/6 | 900 | GLNKIVRMY [ND] |
| p24c.20 | RCC-007 | ND | 1,000 | SILDIKQGPKEPFRDY [ND] |
| 109-M | A2/23 B72/- Cw2/- | 420 | SILDIKQGPKEPFRDY [ND] | |
| 188-M | A2/*6801 B18/*5802 Cw5/6 | 720 | SILDIKQGPKEPFRDY [ND] | |
| 260-M | ND | 173 | SILDIKQGPKEPFRDY [ND] | |
| p24c.22 | RCC-004 | A23/69 B-/- Cw3/18 | >1,000 | FRDYVDRFFKTLRAEQA [ND] |
| 022-M | A*3001/*3002 B42/45 Cw3/17 | 380 | FRDYVDRFFKTLRAEQA [ND] | |
| 270-M | A23/*6802 B41/71 Cw3/17 | >1,000 | FRDYVDRFFKTLRAEQA [ND] | |
| 971-M | A26/68 B8/71 Cw3/7 | 1,080 | FRDYVDRFFKTLRAEQA [ND] | |
| 1011-M | A*6802/74 B71/- Cw3/4 | 840 | FRDYVDRFFKTLRAEQA [ND] | |
| p24c.24 | RCC-004 | A23/69 B-/- Cw3/18 | >1,000 | QATQDVKNWMTDTLLV [ND] |
| p24c.25 | RC-007 | ND | 520 | WMTDTLLVQNANPDCKTI [ND] |
| p24c.28 | KEH14-M | A3/33 B71/72 Cw-/- | 340 | EEMMTACQGVGGPSHKARVL [ND] |
Subjects coded by a number followed by “M” represent adults in the cohort. All subjects were of the African racial group.
ND, not determined.
Comparison between Caucasoid and African responses.
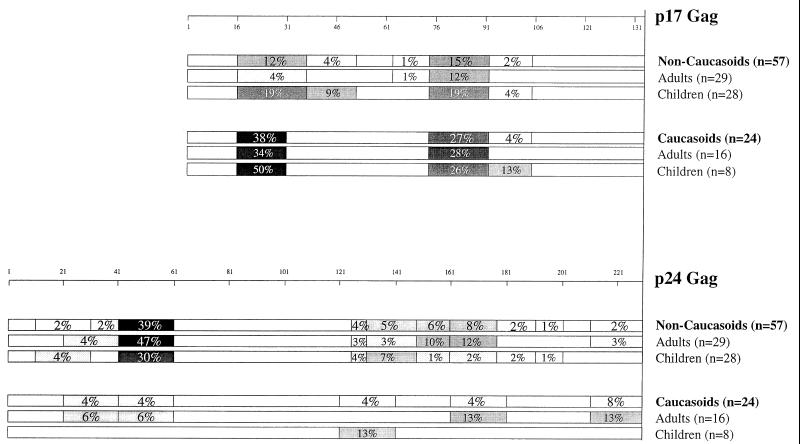
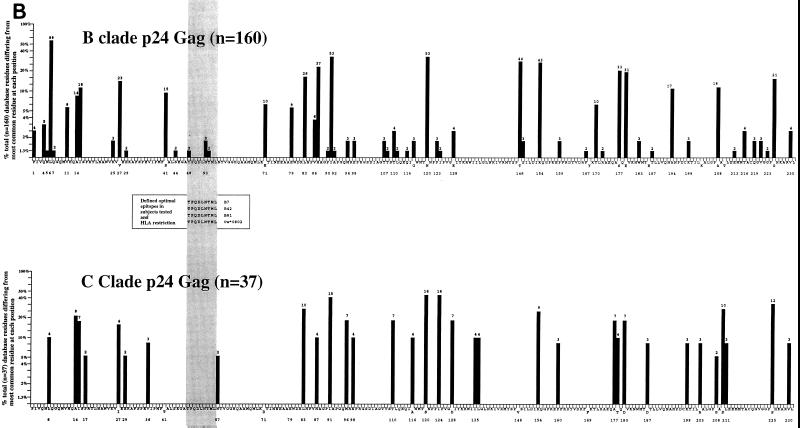
The Gag regions containing the dominant CTL responses which were detected in the Caucasoid subjects studied (n = 24) were compared with those targeted by dominant responses in the non-Caucasoids studied (n = 57), all but two of whom were ethnic Africans (Fig. 2). The region predominantly targeted by Caucasoids was in p17 (peptide p17 residues 16 to 30), which contained the immunodominant epitope in 38% of persons studied. In comparison, this region only accounted for 12% of the dominant responses in the non-Caucasoids studied (χ2 = 7.0; P < 0.01, Fisher's exact test). In contrast, one peptide in p24Gag (residues 41 to 60) accounted for 39% of dominant Gag responses in the Africans studied, compared with 4% in Caucasoids studied (χ2 = 10.3; P < 0.005, chi-square test). Comparisons of the epitopes targeted by pediatric and adult subjects showed no significant differences (data not shown).
FIG. 2.
Frequency with which each Gag peptide tested contained the dominant CTL response in 81 subjects studied.
Fine specificity of the immunodominant p24Gag CTL response.
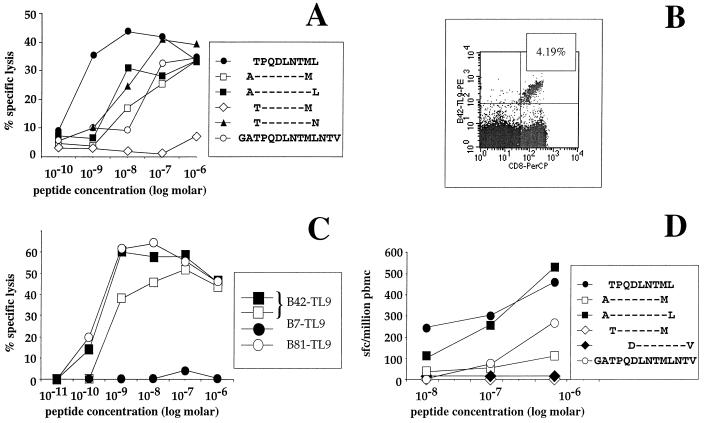
Having identified a single peptide, SALSEGATPQDLNTMLNTVG (SG20) (p24Gag residues 41 to 60), out of the 46 tested which contained the dominant Gag epitope in 39% of 55 ethnic Africans studied, we proceeded to define precisely the optimal epitopes which were targeted. The optimal epitope identified in each of the subjects with HLA-B42 which was characterized was the peptide TPQDLNTML (TL9) (p24Gag residues 48 to 56 [Fig. 3A and B]). This B42-restricted epitope was also recognized by B42-positive effectors when presented by B81-positive target cells (Fig. 3C), reflecting the very close sequence similarity between HLA-B42 and -B81 (6, 61). The same peptide TL9 is thus almost certainly also an optimal HLA-B81-restricted epitope (58), although this has not been definitively established; however, previous studies have demonstrated that an HLA-B81-restricted epitope exists within the same 20-mer SG20 (17).
FIG. 3.
(A) Optimization of the HLA-B42-restricted epitope, TPQDLNTML (TL9). Effectors shown from donor 016-TCH (HLA-A*3001/- B42/- Cw17/-) against HLA-B42-matched target cells. (B) Staining of PBMCs from 016-TCH with the B42-TL9 MHC-peptide complex, showing 4.19% of CD8+ T cells staining with the tetramer. (C) Recognition of TL9-pulsed target cells by 016-TCH clones (as in panel A): target cells included B42-matched targets (square symbols) (autologous targets are represented by closed squares); HLA-B81-positive, HLA-B42-negative targets (open circles); and HLA-B7-positive, HLA-B42-negative targets (closed circles). (D) Optimization of the HLA-B14-Cw*0802-restricted epitope TPQDLNTML by elispot assay of PBMCs from donor 009-BMC (A*0201/*3002 B14/27 Cw1/8). HLA restriction of the response was previously determined by precursor frequency assay by using targets matched through B14 and Cw8 (data not shown).
In addition to the B42- and B81-restricted TL9-specific responses, which accounted for the dominant CTL responses in 14 of 22 of the HLA-typed subjects targeting the SG20, 5 of 22 of these subjects expressed HLA-B14 and HLA-Cw*0802, which are in tight linkage disequilibrium (14) and are almost invariably coexpressed. Although there is a described HLA-Cw*0802-restricted epitope in SG20 (10), in the subjects studied here with B14-Cw*0802 whose SG20 response was characterized, the optimal epitope again proved to be TL9 (Fig. 3D), with no recognition of the described epitope DLNTMLNTV (10). The HLA restriction of this response was confirmed as HLA-B14 and HLA-Cw*0802 (data not shown). Distinction between B14 and Cw8 as the restriction element is complicated by the linkage disequilibrium, but on purely theoretical grounds this epitope would precisely fit the Cw8 peptide binding motif (Pro, Val, or Leu at position 2, Val or Leu at position 9) and would not fit the B14 motif (Arg at position 2, Phe or Tyr at position 3, and Ala or Val at position 9) (52). In the three remaining HLA-typed subjects who targeted SG20 as their dominant Gag-specific CTL response but who did not have either HLA-B42, HLA-B81, or HLA-B14-Cw8, these responses have yet to be precisely defined.
The significance of the TL9 epitope as an important component of the anti-HIV CTL response generated by infected Zulu and Xhosa in South Africa is that either or both HLA-B42 and -B81 are expressed in 40 to 45% of infected persons (M. G. Hammond and P. J. R. Goulder, unpublished data) and that in 14 of 18 (78%) persons studied who have B42 and/or B81, this is the dominant Gag-specific epitope targeted. Thus, approximately 30% of all Africans expressing HLA class I molecules in similar frequencies to the subjects studied here in Durban would be expected to use TL9 as the dominant Gag-specific CTL epitope.
Fine specificity of the immunodominant p17Gag CTL responses.
Two regions within p17Gag (p17Gag residues 16 to 34 and 71 to 88) were predominantly targeted (Fig. 2). The first, represented in the B-clade panel of peptides by the 15-mer WEKIRLRPGGKKKYK (WK15) (residues 16 to 30), as stated above, contained significantly more dominant epitopes in the Caucasoids than the non-Caucasoids tested by using the appropriate B- or C-clade peptides. Of the 10 HLA-typed Caucasoids whose dominant Gag response was targeted at this region, seven have HLA-A3. In all of these, the optimal epitopes were either the previously defined sequences (10, 22), RLRPGGKKK (in six of seven subjects) or KIRLRPGGK (in one of seven subjects). Although several epitopes restricted by HLA alleles other than HLA-A3 are clustered within this region, such as IRLRPGGKK by HLA-B27 (10), the specific focusing of dominant CTL responses in the region of peptide WK15 in this study is largely explained by the high phenotypic frequency of HLA-A3 in Caucasoids (20 to 25%) compared to the frequency in Africans (5 to 10%) (14; M. G. Hammond and P. J. R. Goulder, unpublished data).
The second region of major immunogenicity identified in p17Gag is represented by the 15-mer ELRSLYNTVATLYCV (EV15) (residues 74 to 78). Thirteen of fifteen subjects targeting this region expressed HLA-A2, and the majority of these responses are accounted for by the immunodominant HLA-A*0201-restricted epitope, SLYNTVATL (residues 77 to 85) (9, 22, 24). This region tended not to feature as prominently in the Gag-specific CTL responses of the African subjects studied (χ2 = 1.50; P > 0.25, Fisher's exact test), especially in Zulu subjects studied, where the frequency of the A*0201 subtype of A2 is very low (∼1% of the population) (25). Approximately 45% of the Caucasoid population studied in Boston express HLA-A*0201 (14). However, this region also contains the undescribed A*3002-restricted epitope, RSLYNTVATLY (P. J. R. Goulder, Y. Tang, M. Bunce, E. S. Rosenberg, and B. D. Walker, unpublished data). Since HLA-A*3002 is present at high frequency (25% [M. G. Hammond and P. J. R. Goulder, unpublished data]) in African populations and low frequency (<4% [14]) in Caucasoid populations, this region of p17 Gag is likely to feature strongly in the CTL responses of both groups.
DISCUSSION
These studies show that, irrespective of a wide diversity in HLA class I expression between the subjects investigated, dominant C- and B-clade Gag-specific CTL responses are focused upon a small number of highly immunogenic regions. However, characteristic differences in epitope specificity were evident when the different ethnic groups studied were compared. The regions of Gag which contained the dominant epitope in the majority (64%) of the 24 Caucasoids studied were in p17Gag. These regions include epitopes restricted by HLA-A*0201 and -A3, two alleles occurring frequently in Caucasoids. The region of Gag most commonly (39%) containing the dominant epitope in the 55 ethnic Africans studied was in p24Gag. The immunogenicity of this p24Gag peptide principally reflects the dominant CTL responses made through HLA-B42 and -B81, alleles almost exclusively found in Africans (14). Finally, the major Gag responses seen in infected adults (n = 45) did not differ significantly from those which were observed in the infected children studied (n = 36).
This is by far the largest epitope-specific study of CTL activity to have been undertaken, in which 81 adults and children were evaluated. The phenomenon of epitopes clustering into regions of high immunogenicity within proteins is not new (10, 16, 47), but the extent of epitope clustering that evidently exists from the data described above was not known. Prior to the advent of elispot assays, epitope-specific CTL studies were labor intensive and were necessarily only carried out on very small numbers of persons at a time. Since only novel epitopes will be published, a database understates the true degree of epitope clustering.
These results are potentially of significance if CTL epitope sequences are to be incorporated into candidate vaccines. A theoretical problem in the approach using CTL epitope sequences within polyepitope vaccines would be that individual vaccines would need to be tailored to the HLA type of each individual vaccinee. However, if the dominant CTL epitopes are clustered within very small regions of viral proteins irrespective of the HLA type of the potential vaccinee, the same vaccine may be given to all persons and effective CTL responses will be induced. Although a proportion of the subjects studied did show strong responses to epitopes outside the highly immunogenic regions noted, the degree of immunodominant epitope clustering observed in the majority of subjects is striking. However, these data taken in isolation do not demonstrate that these particular CTL responses are protective or beneficial in the control of HIV replication. In fact, study of acutely infected subjects shows that epitopes targeted in acute infection differ significantly from those targeted in chronic infection (P. J. R. Goulder, M. A. Altfeld, E. S. Rosenberg, Y. Tang, B. Eldridge, B. D. Walker, and C. Brander, unpublished data). Further investigation of the effectiveness of the CTL responses that are detectable in chronic infection is warranted.
The advantage of an approach using polyepitope vaccines, rather than full-length gene products, to induce stronger CTL responses has not been definitively established. However, an enormous increase in peptide-MHC class I formation resulting from minigene products has been demonstrated (5, 69). The subdominance of certain epitopes may partly reflect inefficient processing (67). Polyepitope vaccination in mice has recently been utilized to induce CTL responses which protect against virus challenge (57), and, more recently, strong CTL SIV-specific responses have been demonstrated in macaques following polyepitope vaccination (26).
A second, more theoretical, advantage of a polyepitope vaccine approach would be that differences clearly exist between CTL (19, 27, 29, 33, 34, 70), such that subdominant CTL responses may in some cases be more effective in controlling viremia than dominant responses (19). Simple use of full-length gene products to induce CTL would not allow the flexibility available in a polyepitope vaccine to exclude immunogenic regions which might induce dominant but ineffective CTL responses. Although many studies have shown associations of particular HLA class I molecules with differences in speed of progression to disease in HIV and other infections (12, 21, 28, 36, 37), the mechanism accounting for these differences (which presumably operates through the HLA class I-restricted CTL activity) has not been elucidated.
The specificity of the HIV-specific CTL responses in infected Africans is clearly fundamental to understanding which epitopes may be important in controlling viremia in the populations worst hit by the global epidemic. It is somewhat surprising to discover from the data described here that as much as one-third of infected Africans target their immunodominant Gag-specific CTL response to a single previously undescribed epitope peptide, whether presented by HLA-B42, -B81 or -Cw*0802. Strong HLA-B53-restricted CTL responses have been described (20) towards the exact HIV type 2 homologue of TL9 (TPYDINQML) and, notably, much of what has been learned from CTL responses in SIV-infected macaques has come from study of the single Mamu-A*01-restricted epitope CTPYDINQM (CM9) (1), which is almost exactly homologous in sequence to TL9. If the B42- and B81-restricted TL9 response is analogous to the Mamu-A*01 CM9 response, then this TL9 epitope can be expected to play a vital part in control of HIV in infected Africans (13, 54).
The subjects studied represent an extremely heterogeneous group, not only with regard to the ethnicity and age of the persons studied, but also with regard to the clade of virus responsible for infection and the use of antiretroviral therapy. In terms of the effect of antiretroviral therapy on the hierarchy of HIV-specific CTL responses detectable, the detailed studies that have been carried out indicate that the magnitude of each specificity is diminished, but that the responses persist and the hierarchy remains the same even years after effective highly active antiretroviral therapy (34).
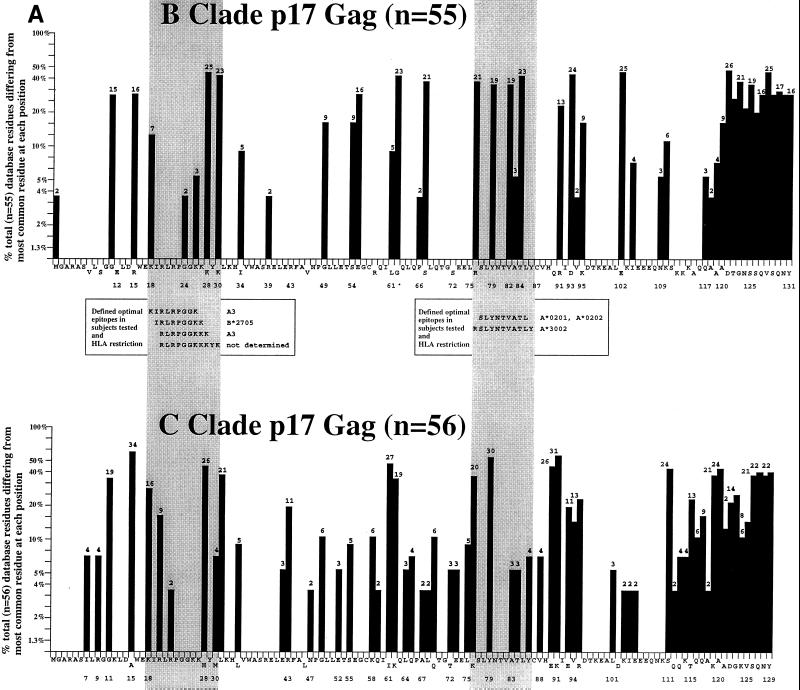
The effect of the clade of virus on epitopes targeted may be gauged by comparing the responses detected in B-clade-infected African Americans and C-clade-infected African Zulu and Xhosa from KwaZuluNatal. In both groups, the immunodominance of the HLA-B42-restricted TL9-specific response is striking. Although the clade of virus causing infection was not determined in the specific African subjects in KwaZuluNatal studied, 84 of 90 (93%) infected persons in KwaZuluNatal for whom the clade of infecting virus has recently been determined were infected with clade-C virus (46, 60; D. York, unpublished data). A measure of the level of variability of the respective B- and C-clade Gag proteins (Fig. 4) shows that a similar degree of sequence variation exists in these proteins in B- and C-clade-infected persons. The data in Fig. 4, however, do not distinguish between conservative and nonconservative sequence changes. Nonconservative amino acid substitutions are more likely to be significant in terms of CTL recognition. Despite the high degree of variability of p17Gag, this protein nonetheless contained a high frequency of immunodominant Gag epitopes in the B-clade-infected persons. Thus, the differences observed in epitopes targeted by infected Africans and infected Caucasoids do not appear to have been the result of an artifact due, for example, to greater conservation of p24Gag in C-clade-infected persons. Clearly, the ideal approach, but an impractical one, to determining the CTL activity of each HIV-infected subject would be to determine the autologous virus sequence beforehand. The best practical approach continues to be screening for CTL responses using peptides based on the most likely appropriate clade of infecting virus.
FIG. 4.
Variability of p17Gag (A) and p24Gag (B) B- and C-clade sequences, with reference to an amino acid sequence showing the most common residue at each position (HIV website [www.hiv-lanl.gov]). All database sequences available were used for the analysis except for B-clade p17Gag sequences; in this case, every fifth of 270 sequences derived in the United States was analyzed in order to compare variability in an equivalent number of B- and C-clade sequences.
Understanding the role of CTL in pediatric infection has been a particularly neglected area of investigation. The largest previous epitope-specific study of infected children described no more than three children (66). The data described here show that in the 36 children studied (mean age, 7.5 years), similar Gag epitopes are targeted in both infected children and adults. Earlier studies of infected children have shown that, particularly in subjects' first year of life, CTL responses are hard to detect in comparison with those of adults at a similar stage of infection (42, 43, 50). These low levels of CTL responses early in pediatric infection are seen in association with a failure to control viremia in the first 2 to 3 years of life (44, 55), in contrast to the rapid control of viremia observed in association with the early appearance of CTL in adult HIV infection and in SIV infection (7, 30, 40, 45, 53, 68). Further studies in children younger than those described in this work are therefore needed to better define the role of HIV-specific CTL in controlling pediatric infection.
In conclusion, Gag-specific CTL responses in B- and C-clade-infected African and Caucasoid children and adults are focused on a small number of highly immunogenic regions. The immunogenicity of these regions is partly explained by the clustering together of similar or identical epitopes, but generally reflects the predominance of HLA-A2- and -A3-restricted p17Gag-specific responses in Caucasoids and HLA-B42- and -B81-restricted p24Gag-specific responses in Africans. Comparison of epitope-specific responses in infected adults and children shows that high-level CTL responses targeting the same immunogenic regions were observed in children and adults. These data also support the value of polyepitope vaccine design, since the theoretical obstacle posed by the degree of MHC diversity is largely overcome by the tight clustering of dominant epitopes which is observed. It will also be important to define the immunodominant epitopes within the other major immunogenic proteins (10), Nef, reverse transcriptase, and envelope; to determine the effectiveness of CTL of these different specificities; and to further address the potential importance of clade-specific CTL activity in controlling HIV. Although potential vaccine constructs might ideally match the clade of virus to which vaccinees are likely to be exposed, the cross-clade conservation of the region showing the highest immunogenicity of the Gag peptides tested in these studies (SG20) (p24Gag residues 41 to 60) suggests that B-clade-based vaccines would also be effective to a degree in African populations exposed to C-clade virus.
ACKNOWLEDGMENTS
We are greatly indebted to Nancy Karthas, Lynne Lewis, Rosemary Galvin, Catherine Kneut, Eileen Macnamara, Graz Luzzi, Andrew Tippett, and Megan Valentine for the collection of blood samples and painstaking provision of clinical data in order to study the CTL responses described above, and their input is gratefully acknowledged.
This work was supported by grants to P.J.R.G. from the Elizabeth Glaser Pediatric AIDS Foundation, the Medical Research Foundation (United Kingdom) (grant G108/274), and the National Institutes of Health (AI46995) and to B.D.W. through the National Institutes of Health (AI28568, AI30914) and the Doris Duke Charitable Foundation. P.J.R.G. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. B.D.W. is a Doris Duke Distinguished Clinical Science Professor.
REFERENCES
- 1.Allen T M, Sidney J, del Guerico M-F, Glickman R L, Lensmeye G L, Wiebe F A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide-binding motif of a Rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Altman J, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 3.Anonymous. The current global situation of the HIV/AIDS pandemic. Wkly Epidemiol Rec. 1997;72:359–360. [PubMed] [Google Scholar]
- 4.Anonymous. HIV-1 subtypes: implications for epidemiology, pathogenicity, vaccines and diagnostics. Workshop report from the European Commission and the Joint United Nations Programme on HIV/AIDS. AIDS. 1997;11:17–36. [PubMed] [Google Scholar]
- 5.Anton L C, Yewdell J W, Bennink J R. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535. [PubMed] [Google Scholar]
- 6.Arnett K L, Parham P. HLA class I nucleotide sequences. Tissue Antigens. 1995;46:217–257. doi: 10.1111/j.1399-0039.1995.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 9.Brander C, Hartman K E, Trocha A K, Jones N G, Johnson R P, Korber B, Wentworth P, Buchbinder S P, Wolinsky S, Walker B D, Kalams S A. Lack of strong immune selection pressure by the immunodominant HLA-A*0201 restricted CTL response in chronic HIV-1 infection. J Clin Investig. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brander C, Goulder P J R. Recent advances in the optimization of HIV-specific CTL epitopes. In: Korber B T M, Brander C, Walker B D, Koup R A, Moore J, Haynes B, Meyers G, editors. HIV molecular immunology database. Los Alamos, N.Mex: Los Alamos National Laboratory of Theoretical Biology and Biophysics; 1999. [Google Scholar]
- 11.Bunce M, O'Neill C M, Barnardo M C, Krausa P, Browning M J, Morris P J, Welsh K I. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 12.Carrington M, Nelson G W, Martin M P, Kissner T, Vlahov D, Goedert J J, Kaslow R A, Buchbinder S, Hoots K, O'Brien S J. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z W, Shen L, Miller M D, Ghim S H, Hughes A L, Letvin N L. Cytotoxic T lymphocytes do not appear to select for mutations in an immunodominant epitope of simian immunodeficiency virus Gag. J Immunol. 1992;149:4060–4066. [PubMed] [Google Scholar]
- 14.Clayton J, Lonjou C. Allele and haplotype frequencies for HLA loci in various ethnic groups. In: Charron D, editor. HLA: genetic diversity of HLA: functional and medical implication. Proceedings of the Twelfth International Histocompatibility Workshop and Conference. Paris, France: EDK; 1997. pp. 665–820. [Google Scholar]
- 15.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia H M. Influence of infant feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 16.Culmann B, Gomard E, Kieny M P, Guy B, Dreyfus F, Saimot A G, Sereni D, Sicard D, Levy J P. Six epitopes reacting with human cytotoxic CD8+ T cells in the central region of the HIV-1 Nef protein. J Immunol. 1991;146:1560–1565. [PubMed] [Google Scholar]
- 17.Dorrell L, Dong T, Ogg G S, Lister S, McAdam S, Rostron T, Conlon C, McMichael A J, Rowland-Jones S. Distinct recognition of non-clade B human immunodeficiency virus type 1 epitopes by cytotoxic T lymphocytes generated from donors infected in Africa. J Virol. 1999;73:1708–1714. doi: 10.1128/jvi.73.2.1708-1714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 19.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H-G. Protective immunity does not correlate with the hierarchy of virus-specific CTL responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotch F M, McAdam S N, Allsopp K E, Gallimore A, Elvin J, Kieny M P, Hill A V S, McMichael A J, Whittle H C. Cytotoxic T cells in HIV-2 seropositive Gambians: identification of a virus-specific MHC-restricted peptide epitope. J Immunol. 1993;151:3361. [PubMed] [Google Scholar]
- 21.Goulder P J R, Crowley S, Krausa P, Morgan B, Edwards A, Giangrande P, McIntyre K, McMichael A J. Novel, cross-restricted, conserved and immunodominant CTL epitopes in long-term slow progressors in HIV-1 infection. AIDS Res Hum Retrovir. 1996;12:1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 22.Goulder P J R, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two HLA-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 24.Gray C, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, David M M, Merigan T C. Frequency of class I restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy. J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 25.Hammond M G, du Toit E D, Sanchez-Mazas A, Andrien M, Coluzzi M, de Pablo M R, de Stefano G, Kaplan C, Kennedy L J, Louie L, Migot F. HLA in sub-Saharan Africa: 12th International Histocompatibility Workshop SSAF report. In: Charron D, editor. Proceedings of the Twelfth International Histocompatibility Workshop and Conference. Paris, France: EDK; 1997. pp. 345–353. [Google Scholar]
- 26.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hay C M, Ruhl D J, Basgoz N, Wilson C C, Billingsley J M, DePasquale M P, D'Aquila R, Wolinsky S, Crawford J M, Montefiori D, Walker B D. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J Virol. 1999;73:5509–5519. doi: 10.1128/jvi.73.7.5509-5519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill A V S. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey J M, Usuku K, Hall S E, Matsumoto W, Taylor G M, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, Lloyd A L, Nowak M A, Nagai M, Kodama D, Izumo S, Osame M, Bangham C R M. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-1-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin X, Bauer D E, Tuttleton S E, Gettie A, Blanchard J, Irwin C E, Safrit J T, Lewin S, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in SIV-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R P, Trocha A K, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 32.Kalams S A, Johnson R P, Dynan M, Hartman K E, Harrer T, Harrer E, Trocha A K, Blattner W A, Buchbinder S P, Walker B D. T cell receptor usage and fine specificity of human immunodeficiency virus 1-specific cytotoxic T lymphocyte clones: analysis of quasispecies recognition reveals a dominant response directed against a minor in vivo variant. J Exp Med. 1996;183:1669–1679. doi: 10.1084/jem.183.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalams S A, Goulder P J R, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaslow R A, Carrington M, Apple R, Park L, Munoz A, Saah A J, Goedert J J, Winkler C, O'Brien S J, Rinaldo C R, Detels R, Blattner W, Phair J, Ehrlich H, Mann D. Influence of human MHC genes on the course of HIV infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 37.Keet I P, Tang J, Klein M R, LeBlanc S, Enger C, Rivers C, Apple R, Mann D, Goedert J J, Miedema F, Kaslow R A. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J Infect Dis. 1999;180:299–309. doi: 10.1086/314862. [DOI] [PubMed] [Google Scholar]
- 38.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific CTL responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig S, Conley A J, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C, et al. Transfer of HIV-1 specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 40.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luzuriaga K, Koup R A, Pikora C A, Brettler D B, Sullivan J L. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 43.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali D, Sullivan J L. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 44.McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, Burchett S K. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J. 1996;15:1087–1091. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Mellors J W, Rinaldo C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 46.Moodley D, Smith T-L, Van Rensburg E J, Moodley J, Engelbrecht S. HIV type 1 V3 region subtyping in KwaZulu-Natal, a high-seroprevalence South African Region. AIDS Res Hum Retrovir. 1998;14:1015–1018. doi: 10.1089/aid.1998.14.1015. [DOI] [PubMed] [Google Scholar]
- 47.Nixon D F, McMichael A J. Cytotoxic T cell recognition of HIV proteins and peptides. AIDS. 1991;5:1049–1059. [PubMed] [Google Scholar]
- 48.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon N F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 49.Phillips R E, Rowland-Jones S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R M, Rizza C R, Townsend A R M, McMichael A J. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 50.Pikora C A, Sullivan J L, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–1161. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price D A, Goulder P J R, Klenerman P, Sewell A K, Easterbrook P J, Bangham C R M, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rammensee H G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 53.Riviere Y, McChesney M B, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retrovir. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz J E, Kuroda M, Santra S, Sasseville V, Simon M, Lifton M, Racz P, Tenner-Racz K, Dalesandro M, Scallon B, Ghrayeb J, Forman M, Montefiori D, Rieber E, Letvin N, Reimann K. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 55.Shearer W T, Quinn T C, LaRussa P, Lew J F, Mofenson L, Almy S, Rich K, Handelsman E, Diaz C, Pagano M, Smeriglio V, Kalish L A. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N Engl J Med. 1997;336:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 56.South African Department of Health. Ninth annual national HIV sero-prevalence survey of women attending antenatal clinics in South Africa, 1998. Pretoria, South Africa: Health Systems Research and Epidemiology, Department of Health; 1999. [Google Scholar]
- 57.Thomson S A, Sherritt M A, Medveczky J, Elliott S L, Moss D J, Fernando G J, Brown L E, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717. [PubMed] [Google Scholar]
- 58.Threlkeld S C, Wentworth P A, Kalams S A, Wilkes B M, Ruhl D J, Keogh E, Sidney J, Southwood S, Walker B D, Sette A. Degenerate and promiscuous recognition by CTL of peptides presented by the MHC class I A3-like superfamily. J Immunol. 1997;159:1648–1657. [PubMed] [Google Scholar]
- 59.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Harmelen J H, van der Ryst E, Loubser A S, York D, Madural S, Lyons S, Wood R, Williamson C. A predominantly HIV type 1 subtype C-restricted epidemic in South African urban populations. AIDS Res Hum Retrovir. 1999;15:395–398. doi: 10.1089/088922299311376. [DOI] [PubMed] [Google Scholar]
- 61.Vilches C, Sanz L, de Pablo R, Moreno M E, Puente S, Kreisler M. Molecular characterization of the new alleles HLA-B*8101 and B*4407. Tissue Antigen. 1996;47:139–142. doi: 10.1111/j.1399-0039.1996.tb02527.x. [DOI] [PubMed] [Google Scholar]
- 62.Walker B D. HIV-1-specific cytotoxic T lymphocytes. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. p. 201. [Google Scholar]
- 63.Walker B D, Flexner C, Birch-Limberger K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker B D, Chakrabati S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. HIV-specific T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 65.Wilkinson D, Connolly C, Rotchford K. Continued explosive rise in HIV prevalence among pregnant women in rural South Africa. AIDS. 1999;13:740. doi: 10.1097/00002030-199904160-00023. [DOI] [PubMed] [Google Scholar]
- 66.Wilson C C, Brown R C, Korber B T, Wilkes B M, Ruhl D J, Sakamoto D, Kunstman K, Luzuriaga K, Hanson I C, Widmayer S, Wiznia A, Clapp S, Ammann A J, Koup R A, Wolinsky S M, Walker B D The Ariel Project Investigators. Frequent detection of escape from cytotoxic T-lymphocyte recognition in perinatal human immunodeficiency virus (HIV) type 1 transmission: the Ariel Project for the prevention of transmission of HIV from mother to infant. J Virol. 1999;73:3975–3985. doi: 10.1128/jvi.73.5.3975-3985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodberry T, Gardner J, Mateo L, Eisen D, Medveczky J, Ramshaw I A, Thomson S A, Ffrench R A, Elliott S L, Firat H, Lemonnier F A, Suhrbier A. Immunogenicity of a human immunodeficiency virus (HIV) polytope vaccine containing multiple HLA A2 HIV CD8+ cytotoxic T-cell epitopes. J Virol. 1999;73:5320–5325. doi: 10.1128/jvi.73.7.5320-5325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yewdell J W, Bennink J R. Immunodominance in MHC class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 70.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J D, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]