Abstract
Simian immunodeficiency virus (SIV) and human immunodeficiency virus type 1 (HIV-1) Nef proteins are related regulatory proteins that share several functions, including the ability to downregulate class I major histocompatibility complex (MHC) and CD4 expression on the cell surface and to alter T-cell-receptor-initiated signal transduction in T cells. We compared the mechanisms used by SIV mac239 Nef and HIV-1 Nef to downregulate class I MHC and found that the ability of SIV Nef to downregulate class I MHC requires a unique C-terminal region of the SIV mac239 Nef molecule which is not found in HIV-1 Nef. Interestingly, mutation of the PxxP motif in SIV Nef, unlike in HIV-1 Nef, does not affect class I MHC downregulation. We also found that downregulation of class I MHC by SIV Nef requires a conserved tyrosine in the cytoplasmic domain of the class I MHC heavy chain and involves accelerated endocytosis of class I complexes, as previously found with HIV-1 Nef. Thus, while SIV and HIV-1 Nef proteins use a similar mechanism to downregulate class I MHC expression, they have evolved different surfaces for molecular interactions with cell factors that regulate class I MHC traffic. Mutations in the C-terminal domain of SIV mac239 Nef selectively disrupt class I MHC downregulation, having no detectable effect on other functions of Nef, such as the downregulation of CD4 and CD3 surface expression, the stimulation of SIV virion infectivity, and the induction of SIV replication from T cells infected in the absence of stimulation. The resulting mutants will be useful reagents for studying the importance of class I MHC downregulation for SIV replication and AIDS pathogenesis in infected rhesus macaques.
The Nef protein is a regulatory factor of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) that is important for optimal viral replication in the infected host. Viral loads remain low and the development of AIDS is severely attenuated in juvenile rhesus monkeys infected with SIV containing disruptions of the nef coding sequence (27, 49). Similarly, in a subset of humans with nonprogressive HIV type 1 (HIV-1) infection, one can detect HIV-1 containing deletions or inactivating point mutations in nef (9, 18, 28, 40, 53). Notably, SIV strains containing HIV-1 nef in place of SIV nef replicate efficiently in rhesus monkeys, and the infected animals progress to AIDS with a frequency of greater than 50% (3, 29). These observations indicate that, despite the limited sequence similarity between the HIV-1 and SIV Nef proteins, the two proteins are likely to be functional homologues.
The HIV and SIV Nef proteins share several functions, including the ability to downregulate the cell surface expression of CD4 (8, 21, 39) and class I major histocompatibility complex (MHC) molecules (16, 22, 24, 34, 35, 37, 48, 58), the ability to alter the normal function of the T-cell receptor (TCR)–CD3 signaling complex in T lymphocytes (6, 26, 36, 57, 59, 64), the ability to stimulate the infectivity of HIV-1 or SIV virions, and the ability to stimulate the induction of viral replication from HIV-1- or SIV-infected quiescent peripheral blood mononuclear cells (PBMC) (14, 21, 32, 42, 43, 61). Both proteins also share interactions with common elements of the cell protein sorting and signal transduction machineries, including the AP-1 and AP-2 adapter protein complexes and protein serine kinases (24, 26, 32, 34, 35, 45, 51, 54, 55). That the HIV-1 and SIV Nef proteins have similar functions and share molecular interactions with the same cellular proteins further strengthens the possibility that they exploit similar strategies to promote immunodeficiency virus replication in the infected host.
Previous studies showed that both HIV-1 and SIV proteins downregulate steady-state expression of class I MHC complexes assembled with A and B, but not C, heavy chains from the surfaces of T lymphocytes and macrophages. Selective downregulation of class I A and B antigens (34) protects HIV-infected cells from killing by cytotoxic T cells (16) and by natural killer cells (15) in vitro. Such protection likely helps HIV-1 evade the host immune response in vivo (16, 58).
HIV-1 nef increases the rate of endocytosis of class I MHC complexes from the cell surface, and the internalized class I MHC complexes accumulate in the trans-Golgi network (24, 34, 58). This effect of HIV-1 Nef requires an intact PxxP motif as well as other elements in the SH3 domain-binding surface in the structured core of HIV-1 Nef (24, 37). It also requires a conserved tyrosine residue, Y320, in the cytoplasmic domain of the class I MHC B7 heavy chain and a corresponding tyrosine residue in class I A antigens (15, 24, 37). The SH3 domain-binding surface of HIV-1 Nef is known to interact with the SH3 domains of Src family protein tyrosine kinases (4, 5, 25, 33, 50) and is involved in interactions with additional proteins, such as Vav and a p62 serine kinase related to p21-activated kinases (20, 32, 38, 44, 51); these findings imply that a putative interaction of HIV-1 Nef with the signal transduction machinery is important for the regulation of class I MHC traffic (24). While it is known that the Nef protein encoded by SIV mac239 (239-Nef) also downregulates class I MHC surface expression (12, 35, 58), little is known about which regions of the protein are important for class I MHC downregulation.
Here we describe experiments showing that SIV Nef, like HIV-1 Nef, increases the rate of endocytosis of class I MHC molecules and that this function requires conserved tyrosine residue Y320 in class I MHC heavy chains. We also show that, unlike in HIV-1 Nef, the conserved PxxP helix in SIV Nef is dispensable for this function. Instead, the ability of 239-Nef to downregulate class I MHC requires a unique C-terminal region in SIV Nef which is not found in HIV-1 Nef. Finally, we show that mutations in the C-terminal domain that disrupt class I MHC downregulation do not affect other functions of SIV Nef, such as the downregulation of cell surface expression of CD4 and CD3 molecules and the enhancement of SIV replication and virion infectivity. Our observations indicate that HIV-1 Nef and SIV Nef use similar mechanisms to downregulate class I MHC expression but use different surfaces to mediate the molecular interactions that are required for the downregulation of class I MHC expression.
MATERIALS AND METHODS
Plasmids.
The oligonucleotide-directed site-specific mutagenesis of the SIV mac239 nef allele was performed with a single-stranded template DNA as described previously (26, 35). Mutated nef sequences were verified by DNA sequencing and subcloned into a bicistronic pCGCG vector as described previously (24, 35). The pCGCG bicistronic expression vectors contain either the 239-Nef open reading frame or the class I MHC B7 heavy-chain-gene cDNA (19), followed by the green fluorescent protein (GFP) coding sequence under the translational control of the encephalomyocarditis virus internal ribosome entry site (IRES) element isolated from the pCITE2 vector (24, 35) (Novagen). Genes expressing GFP and SIV mac239 Nef were kindly provided by George N. Pavlakis (23, 62) and by Ron C. Desrosiers, respectively. The class I MHC B7 heavy-chain allele was kindly provided by R. D. Salter.
Cell culture and DNA transfections.
Jurkat T cells expressing human CD4 at a high level were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 20 mM HEPES (pH 7.4) (complete RPMI 1640 medium), and cultures were periodically diluted 10-fold when they reached densities of 2 × 105 to 3 × 105 cells/ml. CV1 cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS. Aliquots of 107 Jurkat T cells and CV1 cells from exponentially growing cultures were electroporated at 200 V and 960 μF with a total of 10 to 20 μg of plasmid DNA containing the desired amounts of expression vectors and empty pCG plasmid DNA as a carrier as described previously (23, 26, 39). Class I MHC expression on CV1 cells was analyzed as previously described (35). Cells were harvested for flow cytometry and biochemical analyses 16 to 24 h after transfection.
Flow cytometric analyses of CD3, CD4, class I MHC, and GFP expression.
Flow cytometric analyses of CD3, CD4, class I MHC, and GFP expression in cells transfected with a bicistronic transcription unit expressing 239-Nef and GFP or class I MHC B7 heavy chain and GFP were performed on an Epics Elite flow cytometer as described previously (24, 26, 35). Briefly, aliquots of 2 × 105 cells were reacted with saturating amounts of phycoerythrin (PE) (Becton Dickinson)-conjugated monoclonal antibody (MAb) Leu3A, specific for human CD4; biotinylated MAb W6/32, specific for the assembled class I MHC heavy chain–β2-microglobulin complex (Immunotech), followed by PE-conjugated streptavidin (Caltag); or PE-conjugated MAb HIT3A, specific for the CD3 complex (Pharmingen). Reaction mixtures contained phosphate-buffered saline with 1% FBS and 0.1% sodium azide.
Endocytosis assays.
Jurkat T cells were transfected with 20 μg of pCGCG plasmids expressing GFP alone or coexpressing HIV-1 NA7 Nef (or SIV 239-Nef) and GFP from the same bicistronic message (24, 35). At 16 to 24 h after transfection, aliquots of 107 cells were reacted with MAb W6/32 conjugated to PE for 30 min at 4°C in complete RPMI 1640 medium. The cells were then washed in ice-cold RPMI 1640 medium, and aliquots of 106 cells were incubated at 37°C to allow endocytosis of class I MHC-MAb W6/32 complexes for various amounts of time. Incubations were terminated by transferring the tubes containing aliquots of cells to an ice bath. Each sample was then divided into two aliquots. One aliquot was diluted fivefold with ice-cold phosphate-buffered saline, and the other was diluted with ice-cold RPMI 1640 medium adjusted to pH 2 to release MAb W6/32 from antigen-antibody complexes that were not internalized from the cell surface. Total class I MHC (surface plus internalized) and internalized class I MHC levels were determined by flow cytometry for cells showing identical levels of GFP expression as described previously (23, 24). The fraction of internalized class I MHC was calculated as described previously (23).
Recombinant proteins, in vitro binding assays, and detection of AP-1 and AP-2 adapter complexes.
Genes expressing 239-Nef–glutathione S-transferase (GST) fusion proteins were constructed using PCR and subcloned into the pSBET Escherichia coli expression vector as described previously (35, 56). The fusion proteins were expressed in E. coli strain BL21 (DE3) (63) following induction with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 18°C and purified on glutathione-Sepharose beads (Pharmacia) according to the manufacturer's instructions. The purified 239-Nef–GST proteins were quantitated by comparison of serially diluted aliquots with known amounts of bovine serum albumin (BSA) standards, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and visualized by staining with Coomassie brilliant blue.
AP-1 and AP-2 adapter complexes were partially purified from calf brains by several rounds of low-speed and high-speed centrifugation as described previously (11, 41). Extracts were quantitated by use of bicinchoninic acid reagent (Pierce). Aliquots (100 μg) of 239-Nef–GST fusion proteins were inoculated with 200-μg aliquots of partially purified adapter protein complexes in a total volume of 200 μl of HEMGN buffer (35). After 4 h at room temperature, beads were washed extensively with 2× HEMGN buffer, and the bound proteins were eluted by boiling in reducing sample buffer (35). AP-1 and AP-2 complexes in the partially purified preparations and those bound to beads were quantitated by immunoblot analysis with MAb 100/3, reacting with the γ-adaptin subunit, specific for the AP-1 complex (1) (Sigma) and with MAb 100/2, reacting with the α-adaptin subunit, specific for the AP-2 complex (1) (Sigma), as described below for the immunoblot detection of 239-Nef.
Immunoblot analysis of 239-Nef expression.
Cytoplasmic extracts from transiently transfected Jurkat T cells were prepared approximately 16 to 20 h posttransfection. Cells were lysed for 40 min on ice in buffer containing 1% Triton X-100, 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 10% glycerol, 2 μg of aprotinin per ml, 2 μg of pepstatin per ml, 2 μg of leupeptin per ml, 0.1 mM sodium vanadate, and 0.5 mM sodium fluoride. Nuclei and cell debris were removed by centrifugation at 10,000 × g for 10 min at 4°C. Aliquots of extracts containing 50 μg of protein were denatured in reducing sample buffer, resolved on 14% polyacrylamide gels, and electroblotted onto polyvinylidene difluoride membranes (Immobilon; Millipore) as described previously (52). Membranes were incubated for 1 h in blocking solution containing NT buffer (150 mM NaCl–50 mM Tris-HCl [pH 7.5] with 0.1% Tween 20, 1% BSA, and 5% nonfat dried milk [Carnation]) and then exposed to anti–239-Nef serum (1:1,000 dilution) in blocking solution. Membranes were washed three times with NT buffer, incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibodies, and developed using an enhanced chemiluminescence detection system under the conditions recommended by the manufacturer (Amersham).
Production of SIV stocks and replication and infectivity assays.
Generation of virus stocks was performed as described earlier (12, 46). Briefly, 293T cells grown in DMEM supplemented with 10% FBS were transfected by the calcium phosphate method with 5 μg of plasmid containing the full-length SIV mac239 proviral constructs differing only in the nef gene. After overnight culturing of the transfected cells, the medium was changed; cell-free supernatants containing virus were harvested 24 h later and passed through a 0.45-μm-pore-size filter. Viral stocks were divided into aliquots and frozen at −80°C. The p27 antigen concentration in viral stocks was then quantitated with a commercial HIV-1 and HIV-2 enzyme-linked immunosorbent assay (Immunogenetics, Zwijndrecht, Belgium).
sMAGI cells were grown in DMEM supplemented with 10% FBS (13). Infection of sMAGI cells was performed as described previously (30, 35). Viral infectivity was quantitated with a Galacto-Light Plus Chemiluminescence Reporter Assay Kit (Tropix, Bedford, Mass.) as recommended by the manufacturer.
The herpesvirus saimiri-transformed T-cell line 221 (2) was maintained in the presence of 100 U of interleukin 2 (IL-2) (Boehringer, Heidelberg, Germany) per ml and 20% FBS. Infections were performed with viral stocks containing 10 ng of p27 antigen, and cells were cultured in the absence of IL-2 and with 5% FBS as described previously (2).
Rhesus monkey PBMC were isolated using lymphocyte separation medium (Organon Teknika Corporation, Durham, N.C.). Isolated PBMC were immediately infected with virus aliquots containing 5 ng of p27 and kept in RPMI 1640 medium with 10% FBS. Residual nonadsorbed virus was removed by washing the cells 16 to 18 h after infection. Six days postinfection, the cells were stimulated with phytohemagglutinin (4.5 μg/ml; Sigma) for 3 days, washed, and maintained in RPMI 1640 medium supplemented with 20% FBS and 100 U of IL-2 per ml. Supernatants were collected at 2- to 4-day intervals, and virus production was measured by a reverse transcriptase (RT) assay as described previously (47).
RESULTS
SIV and HIV-1 Nef proteins use a similar mechanism to downregulate surface class I MHC complexes.
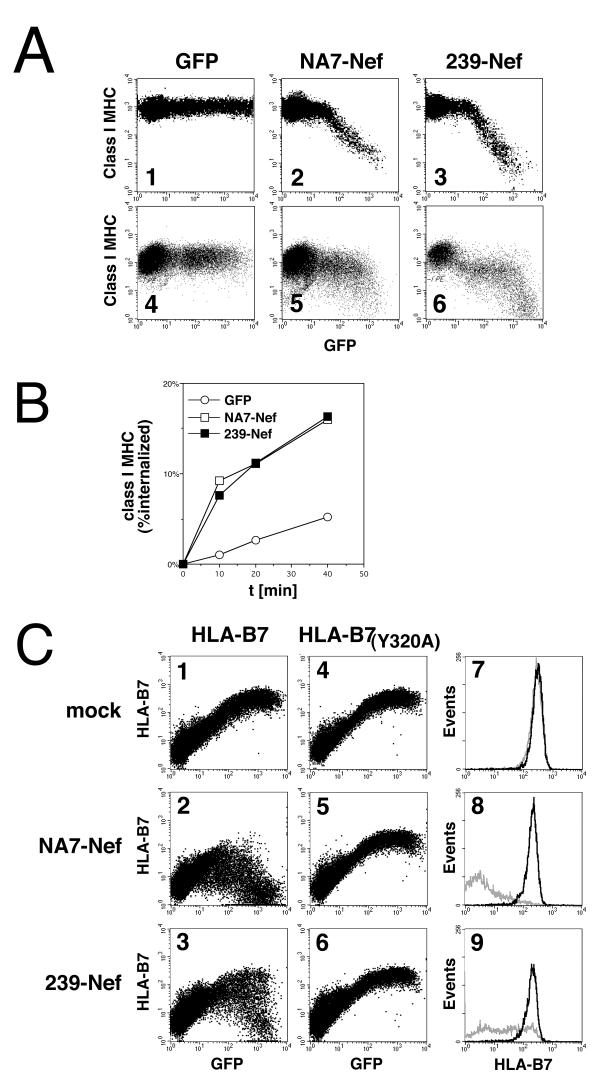
We studied the activities of SIV mac239 Nef and HIV-1 NA7 Nef using a transient class I MHC downregulation assay with human Jurkat T cells (24). This assay takes advantage of a previously constructed vector expressing Nef and GFP from the same bicistronic transcription unit. Since the level of Nef protein expressed is proportional to GFP fluorescence intensity, this design allows a relatively reliable comparison of activities of different nef alleles (24, 35). As shown in Fig. 1A, transient transfection of the bicistronic vectors expressing the NA7-Nef and 239-Nef proteins resulted in up to 100-fold downregulation of steady-state surface class I MHC complexes, which correlated with increasing intensity of GFP fluorescence (panels 2 and 3). Notably, the slopes characterizing the decrease in class I MHC expression on cells with increasing GFP fluorescence were similar for 239-Nef and NA7-Nef.
FIG. 1.
Analysis of the downregulation of class I MHC complexes by SIV and HIV-1 Nef proteins. (A) Dose-response analysis of the downregulation of surface class I MHC complexes in Jurkat T cells by SIV Nef and HIV-1 Nef. Jurkat T cells (panels 1 to 3) and simian CV1 cells (panels 4 to 6) were transfected with 10 μg of the bicistronic vectors expressing HIV-1 NA7-Nef and a GFP reporter (panels 2 and 5) and SIV 239-Nef and a GFP reporter (panels 3 and 6) or a control vector expressing GFP alone (panels 1 and 4). Surface class I MHC complexes and GFP were detected simultaneously by two-color flow cytometry. Class I MHC fluorescence and GFP fluorescence are shown on a logarithmic scale on the ordinate and the abscissa, respectively. The frequency of GFP-positive cells was between 40 and 60%, and 50,000 events were analyzed in each experiment. (B) Kinetics of internalization of class I MHC complexes in Jurkat T cells expressing SIV or HIV-1 Nef proteins are similar. Data represent the percent fraction of class I MHC complexes internalized in Jurkat T cells expressing NA7-Nef, 239-Nef and GFP, or GFP alone as a function of time (t). (C) SIV Nef, like HIV-1 Nef, requires tyrosine Y320 in the class I MHC heavy chain cytoplasmic domain to downregulate the surface expression of class I MHC complexes. JJK T cells were cotransfected with 2 μg of vectors expressing the wild-type B7 (panels 1 to 3) or the Y320A-mutated B7 (panels 4 to 6) class I heavy chain and the GFP marker from the same bicistronic transcription unit and with 10 μg of NA7-Nef expression vector (panels 2 and 5), of 239-Nef expression vector (panels 3 and 6), or of a control empty vector (panels 1 and 4). Histograms of B7 heavy-chain expression on the surfaces of cells expressing the wild-type B7 (black line) and the Y320A-mutated B7 (grey line) are shown for populations of cells with identical levels of GFP expression (panels 7 to 9) and therefore comparable amounts of total cellular B7 protein.
To demonstrate that SIV Nef can downregulate class I MHC surface expression in a simian cell line, plasmids coexpressing NA7-Nef or 239-Nef and GFP or control plasmids were transfected into a CV1 cell line. As shown in Fig. 1A, transient transfection of NA7-Nef or 239-Nef expression plasmids resulted in a readily detectable decrease in class I MHC expression on the surfaces of positively transfected GFP-positive cells (panels 5 and 6). These data indicate that SIV 239-Nef as well as HIV-1 NA7 Nef proteins downregulate the expression of simian homologues of class I MHC in simian cells.
It was previously shown that class I MHC downregulation by HIV-1 Nef is associated with increased rates of internalization of class I MHC complexes from the cell surface (24, 58). Therefore, we measured the effect of 239-Nef on the rate of class I MHC internalization and compared it to that observed with NA7-Nef, again using plasmids expressing 239-Nef or NA7-Nef and GFP from the same bicistronic transcription unit. We measured the rates of class I MHC internalization in transfected cells that showed similar levels of GFP fluorescence and thus similar levels of Nef protein expression. As shown in Fig. 1B, SIV Nef increased the rate of class I MHC internalization, and this increase was similar to that observed with HIV-1 Nef.
Downregulation of class I MHC by 239-Nef requires the tyrosine residue Y320 in class MHC I heavy chains.
Downregulation of surface class I MHC complexes by HIV-1 Nef requires a single tyrosine residue, Y320, in the cytoplasmic domain of class I heavy chains (15, 24, 34). To test whether SIV Nef uses a similar mechanism to downregulate class I MHC, we asked whether it also requires the tyrosine residue Y320 in class I heavy chains. Jurkat T cells, which do not express the class I heavy-chain B7 allele, were transiently cotransfected with a bicistronic plasmid expressing both B7 and the GFP marker, together with a plasmid expressing NA7-Nef or 239-Nef or a control empty vector. The effect of Nef on the expression of B7 on the cell surface was measured by flow cytometry. As shown in Fig. 1C (panels 2 and 3), both Nef proteins efficiently downregulated the expression of the wild-type B7 molecule from the surfaces of positively transfected cells. In contrast, tyrosine-mutated B7 molecules [B7(Y320A)] were not downregulated by either of the Nef proteins (Fig. 1C, panels 5 and 6). The observations that the downregulation of class I MHC complexes from the cell surface by both HIV-1 Nef and SIV Nef proteins involves accelerated endocytosis of class I complexes and that downregulation by both proteins requires tyrosine residue Y320 in class I heavy chains suggest that the two proteins use a similar mechanism to downregulate class I MHC expression.
The ability of SIV Nef to downregulate class I MHC expression does not require the PxxP motif in the SIV Nef core.
Next we tested the effect of mutations in prolines P104 and P107 of the PxxP motif in 239-Nef on class I MHC downregulation because this sequence is conserved between SIV and HIV-1 Nef proteins and because the prolines of the PxxP motif in HIV-1 Nef are required for its ability to downregulate class I MHC (24, 37). Surprisingly, such mutations in SIV Nef had no detectable effect on its ability to downregulate class I MHC (Fig. 2A, panel 3). The different phenotypes of PxxP mutations in the SIV and HIV-1 Nef proteins suggested that interactions with cellular factors important for the downregulation of class I MHC expression are mediated by different surfaces on the SIV and HIV-1 Nef molecules.
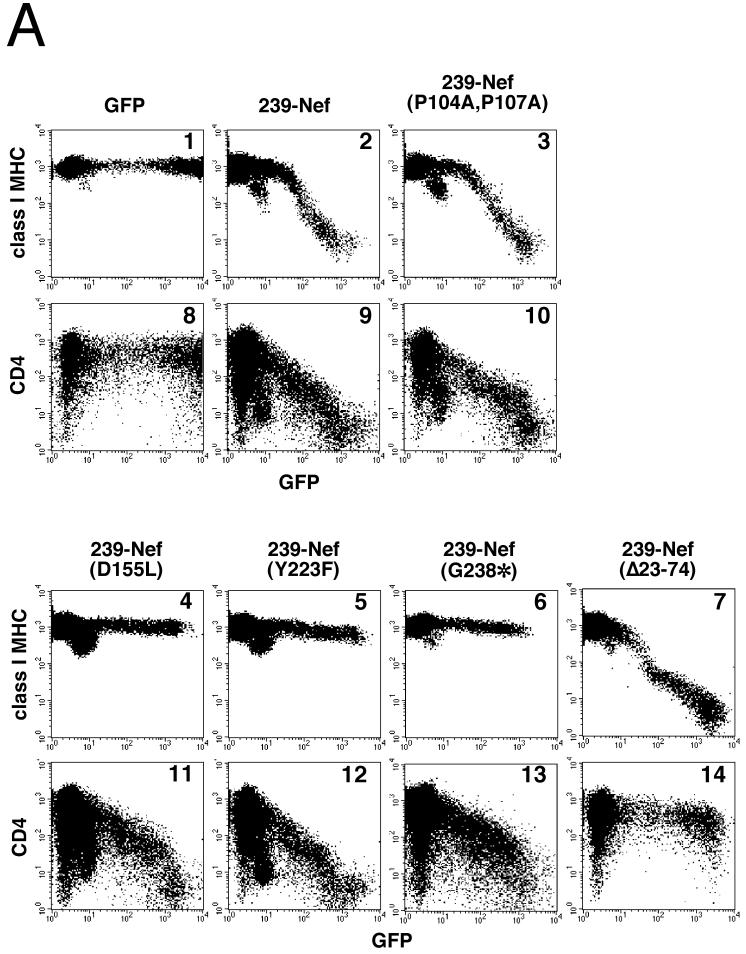
FIG. 2.
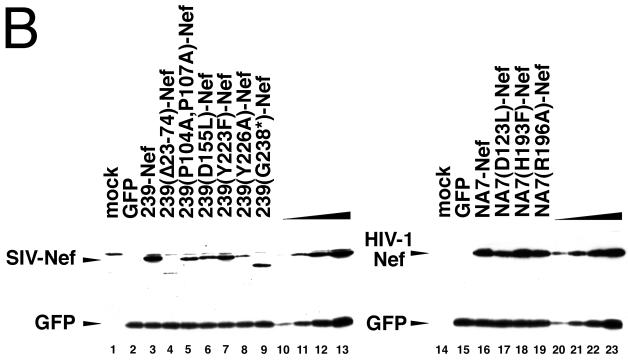
The ability of 239-Nef to downregulate class I MHC requires the unique C-terminal domain in 239-Nef. (A) Flow cytometric analysis of the effect of mutations in 239-Nef on steady-state class I MHC expression (panels 1 to 7) and, as a control, on CD4 expression (panels 8 to 14) on the cell surface. Jurkat T cells were transfected with 10 μg of the bicistronic vectors expressing wild-type or mutant 239-Nef and a GFP reporter or with a control vector expressing GFP alone. Surface class I MHC and GFP or CD4 and GFP were detected simultaneously by two-color flow cytometry. Class I MHC or CD4 fluorescence and GFP fluorescence are shown on a logarithmic scale on the ordinate and the abscissa, respectively. (B) Immunoblot analysis of mutant 239-Nef and NA7-Nef proteins. Aliquots of cytoplasmic extracts prepared from Jurkat T cells transfected with 10 μg of bicistronic plasmids encoding GFP and either wild-type or mutant 239-Nef or NA7-Nef proteins and normalized for GFP expression were immunoblotted with rabbit antiserum to 239-Nef (left panel) or NA7-Nef (right panel). Serial twofold dilutions of extracts prepared from cells expressing wild-type Nef proteins were used as standards for comparison (lanes 10 to 13 and 20 to 23).
The ability of SIV Nef to downregulate class I MHC expression requires the unique C-terminal region of the Nef protein.
Additional mutations in the conserved core of 239-Nef and in the unique C-terminal region of 239-Nef, which is not present in the HIV-1 Nef protein, were also tested. These mutations were identified from a broad mutagenesis scan of the SIV Nef core region for residues that disrupted class I MHC downregulation but not CD4 downregulation. A summary of these mutations and their effects on class I MHC and CD4 downregulation is shown in Fig. 3, and selected results are shown in Fig. 2. We found that substitution of leucine for aspartic acid D155 (D155L) in the core of 239-Nef selectively disrupted its ability to downregulate class I MHC expression, having no effect on the downregulation of CD4 expression. We also found that substitutions for tyrosines Y223 and Y226 (Y223F and Y226A) in 239-Nef each similarly disrupted class I MHC downregulation. Furthermore, a point mutation introducing a premature stop codon at position 238 (G238*), which deletes the 27 C-terminal amino acid residues of 239-Nef, also selectively disrupted class I MHC downregulation. This 27-amino-acid sequence is not found in HIV-1 Nef, suggesting that this unique domain of SIV Nef plays an important role in class I MHC downregulation. Since the mutations had little effect on the ability of Nef to downregulate the expression of the CD4 molecule, they likely did not affect the general folding of the Nef molecule. Therefore, we conclude that these mutations disrupted locally the surfaces of the 239-Nef molecule that are important for molecular interactions required for class I MHC downregulation.
FIG. 3.
Effect of mutations in the C-terminal regions of SIV 239-Nef and HIV-1 NA7-Nef on class I MHC, CD3, and CD4 expression. Amino acid sequences of two sets (A and B) of mutant 239-Nef and NA7-Nef proteins are aligned, and the relative ability of the proteins to downregulate CD4, class I MHC, and CD3 expression is shown. This ability is quantitated as follows: −, less than 20% of wild-type 239-Nef activity in downregulating class I MHC (or CD4) expression on the surfaces of cells expressing the appropriate mutant 239-Nef; +, 20 to 50%; ++, 50 to 90%; and +++, activity greater than 90% the activity seen with wild-type 239-Nef. All determinations were performed with the bicistronic pCGCG vectors expressing 239-Nef and GFP or NA7-Nef and GFP (see Fig. 2). Dots identify amino acid identities, letters identify amino acid substitutions in the single-letter code, and asterisks reflect C termini. Since NA7-Nef does not downregulate CD3 surface expression (7, 60), the effect of mutations in NA7-Nef on CD3 expression is not applicable (N/A). The vertical lines and colons indicate amino acid identities and similarities between the aligned 239-Nef and NA7-Nef sequences.
As shown in Fig. 3, the 239-Nef region containing these tyrosines has limited similarity to the C-terminal sequence of HIV-1 Nef. Nevertheless, to assess whether this region in HIV-1 Nef is also involved in class I MHC downregulation, we tested the effects of amino acid substitutions for amino acids H193 and R196, which correspond to the positions occupied by Y223 and Y226 in 239-Nef. As shown in Fig. 3, neither mutation had a detectable effect on the ability of NA7-Nef to downregulate class I MHC, in contrast to the selective effects of similar mutations in 239-Nef on class I MHC downregulation. These observations provide more evidence that the HIV-1 and SIV Nef proteins use different surfaces for the molecular interactions that are required for the downregulation of class I MHC expression.
The 239-Nef surface required for class I MHC downregulation is not important for the downregulation of surface CD3 expression.
In HIV-1 Nef, the PxxP motif on the SH3 domain-binding surface of the Nef core is required both for class I MHC downregulation and for the ability of HIV-1 Nef to perturb signal transduction by the TCR-CD3 complex in the Jurkat T-cell line (24, 26). In contrast, the PxxP motif in the 239-Nef molecule is not important for either of these two functions (26). To test whether, by analogy to HIV-1 Nef, the surface of the unique C-terminal region of 239-Nef is required for both functions, we tested the role of this surface in the downregulation of surface CD3 expression (7), a phenomenon which is known to underlie the 239-Nef-induced perturbation of TCR-CD3 signaling (reference 60 and data not shown).
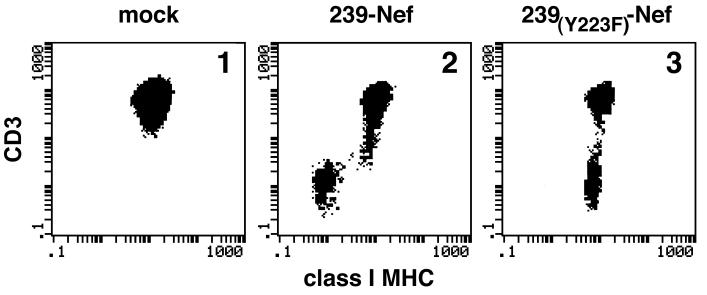
Plasmids encoding 239-Nef molecules with mutations disrupting class I MHC downregulation were transiently expressed in Jurkat T cells, and CD3 expression, along with class I MHC expression, on the cells was analyzed by two-color flow cytometry. The results of these experiments are summarized in Fig. 3, and selected data are shown in Fig. 4. We found that the D155L and Y223F substitutions did not have significant effects on the ability of Nef to downregulate CD3 expression. Similar results were observed with the 239-Nef variant with a premature stop codon introduced for G238 (G238*), which deleted the C-terminal region. All of these mutant 239-Nef proteins retained full ability to downregulate CD4 expression and therefore were not grossly misfolded. We conclude that, unlike in HIV-1 Nef, the surface of 239-Nef required for class I MHC downregulation is not important for the disruption of CD3 signaling.
FIG. 4.
Tyrosine Y223 in 239-Nef is required selectively for the downregulation of class I MHC but not CD3 expression. Class I MHC and CD3 expression in Jurkat T cells transiently transfected with plasmids expressing wild-type (panel 2) or Y223F-mutated (panel 3) 239-Nef or a control empty vector (panel 1) was analyzed by two-color flow cytometry.
Downregulation of class I MHC expression and binding of the AP-1 and AP-2 clathrin adapters are separate functions of SIV Nef.
The induction of class I MHC downregulation by 239-Nef reflects accelerated endocytosis of class I MHC complexes. We previously described an interaction between the endocytic machinery, namely, the AP-1 and AP-2 adapter complexes, and two elements in the N-terminal region of 239-Nef (35). A large deletion removing amino acids 23 to 74 in this N-terminal region, thus abolishing the interaction of SIV Nef with the AP-2 clathrin adapter complex in vivo and in vitro, did not affect the ability of 239-Nef to downregulate class I MHC expression, even though it drastically disrupted CD4 downregulation (Fig. 2A, panel 7). This separation of class I MHC downregulation from the AP-1 and AP-2 binding function of 239-Nef is similar to previous observations for HIV-1 Nef, which showed that mutations disrupting the interaction with AP-2 have no effect on class I MHC downregulation (22, 24, 48). It remains a possibility, however, that the C-terminal region of 239-Nef, including Y223 and Y226, is involved in this interaction and thereby mediates class I MHC endocytosis. To address this possibility, we tested whether a deletion that removes the C-terminal end of the 239-Nef molecule and disrupts class I MHC downregulation (G238*) affects 239-Nef AP-1 and AP-2 adapter interactions in vitro.
Chimeric proteins comprising either wild-type or mutant 239-Nef fused to GST or GST alone were immobilized on glutathione-Sepharose and incubated with adapter protein complexes prepared from calf brains. The beads were washed, and bound proteins were eluted from the glutathione-Sepharose. AP-1 and AP-2 complexes were detected by immunoblotting with AP-2-specific MAb 100/2, reacting with the α-adaptin subunit, and with AP-1-specific MAb 100/3, reacting with the γ-adaptin subunit. As shown in Fig. 5, lane 2, the 239-Nef–GST fusion protein bound both the AP-1 and the AP-2 adapter complexes. As expected, the 239-Nef–GST fusion protein containing the Δ23-74 deletion did not precipitate detectable amounts of the AP-1 and AP-2 adapters (Fig. 5, lane 3). Interestingly, the 239-Nef–GST fusion with the G238* deletion precipitated more AP-1 and AP-2 complexes than the 239-Nef–GST fusion containing wild-type 239-Nef (Fig. 5, lane 4). These observations indicate that the ability of 239-Nef to associate with AP-1 and AP-2 complexes is not required for class I MHC downregulation and that the C-terminal region of 239-Nef that is required for class I MHC downregulation is dispensable for the interaction of 239-Nef with clathrin adapters.
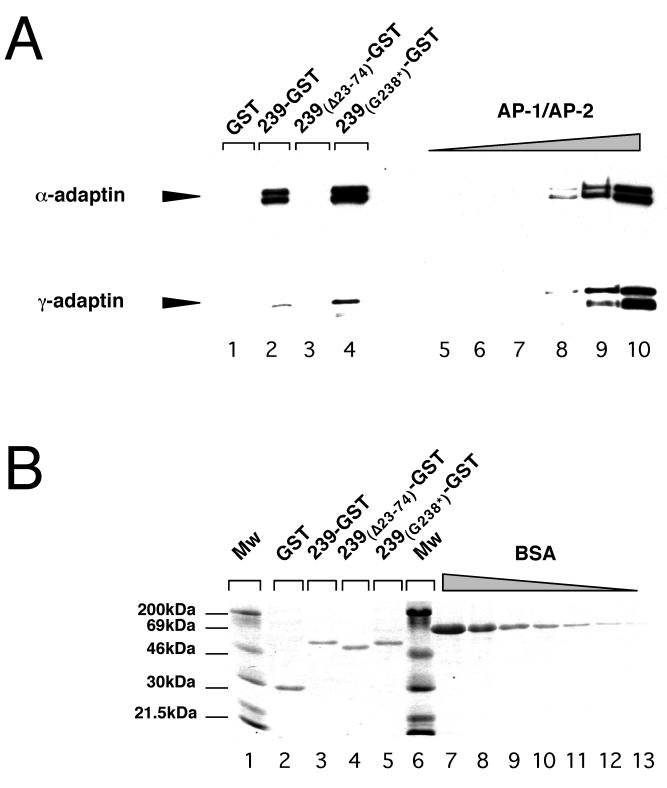
FIG. 5.
The ability of 239-Nef to associate with AP-1 and AP-2 adapter protein complexes is not required for class I MHC downregulation. (A) Aliquots (100 μg) of 239-Nef–GST proteins containing wild-type or mutant 239-Nef moieties were incubated with partially purified AP-1 and AP-2 adapter protein complexes. The bound AP-2 and AP-1 complexes were quantitated by immunoblot analysis (lanes 1 to 4) with the α-adaptin-specific MAb 100/2 (α-adaptin) and the γ-adaptin-specific MAb 100/3 (γ-adaptin). Twofold dilutions of AP-1 and AP-2 preparations were analyzed to provide standards for quantitations (lanes 5 to 10). (B) Two percent aliquots of the reactions used for the α-adaptin immunoblot shown in panel A were resolved by electrophoresis on SDS-polyacrylamide gels and stained with Coomassie brilliant blue to demonstrate that equivalent amounts of various 239-Nef–GST chimeric proteins were used in the binding reactions (lanes 2 to 5). Twofold dilutions of BSA (from 2 to 0.25 μg of protein) were used as standards for quantitations (lanes 7 to 13). The difference in the predicted molecular masses of 239-Nef–GST and 239-Nef–GST with G238* is approximately 5%. Therefore, the migration of the two proteins is similar.
Downregulation of class I MHC is not required for the stimulation of SIV infectivity and replication by 239-Nef.
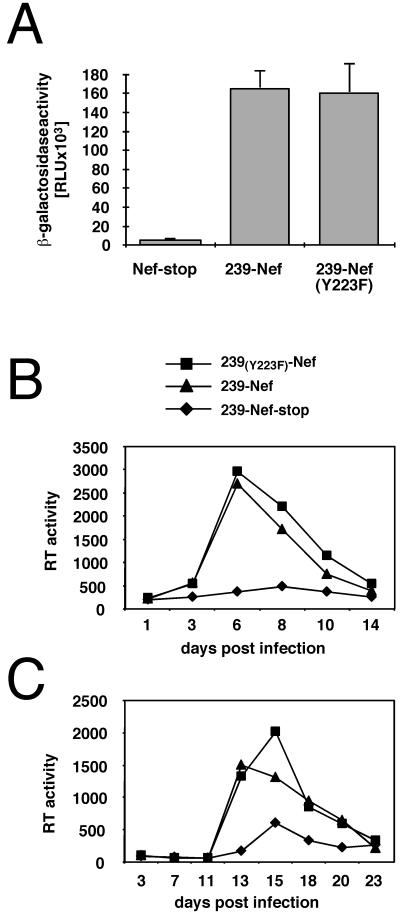
Previous observations suggested that the interaction of Nef with clathrin adapters is important both for the downregulation of CD4 expression and for the stimulation of SIV or HIV replication (17, 35). To test whether the molecular interactions of 239-Nef required for class I MHC downregulation are important for SIV replication, we analyzed the effect of the Y223F mutation on SIV virion infectivity and on the induction of SIV replication in quiescent rhesus monkey PBMC and in an IL-2-dependent T-cell line. The nef allele containing the Y223F mutation was introduced into SIV, and viral stocks were generated. The infectivity of viral particles was determined with the sMAGI indicator cell line (13, 32). As shown in Fig. 6A, the infectivity of SIV encoding functional 239-Nef was more than 10-fold higher than that of SIV containing the nef allele inactivated by a stop codon at position 93. Notably, the infectivity of SIV containing nef with the Y223F mutation was similar to that of SIV with a functional nef open reading frame.
FIG. 6.
The Y223F mutation in 239-Nef does not affect SIV replication and virion infectivity. (A) The infectivity of SIV mac239 containing wild-type and Y223F-mutated Nef in the sMAGI indicator cell line is shown. sMAGI cells were infected with aliquots of virus stocks containing 10 or 100 ng of p27 capsid. The values are shown as a percentage of wild-type SIV mac239 activity and are the average of 12 independent measurements of four different virus stocks. RLU, relative light units. (B) Replication of SIV mac239 containing wild-type or mutated 239-Nef in rhesus monkey PBMC. Unstimulated PBMC were infected immediately after isolation with virus stocks containing 5 ng of p27 antigen and stimulated with phytohemagglutinin 6 days postinfection. (C) Growth of variant SIV mac239 in herpesvirus saimiri-transformed rhesus monkey T-cell line 221 in the absence of IL-2. RT activity was assayed as described in Materials and Methods.
Rhesus monkey PBMC were infected at a low multiplicity with mutant SIV and control SIV and stimulated with phytohemagglutinin 6 days later, and the RT activity in the culture supernatants was determined at various times following stimulation. As shown in Fig. 6B, wild-type SIV displayed higher RT activity than SIV with a premature stop codon in nef, with a maximal 10-fold difference at 13 days postinfection. Notably, SIV containing the Y223F mutation in Nef replicated as well as wild-type SIV. Similar results were obtained with unstimulated 221 T cells, where SIV expressing either wild-type 239-Nef or 239-Nef with the Y223F mutation displayed higher RT activity than SIV with a premature stop codon in nef, with a maximal sevenfold difference at 6 days postinfection (Fig. 6C). These observations indicate that the C-terminal domain of SIV Nef which is essential for the downregulation of class I MHC is not required for 239-Nef to enhance SIV replication in cultured cells or to enhance SIV virion infectivity.
DISCUSSION
We have identified a functional element in the C-terminal region of 239-Nef that is required for its ability to downregulate the surface expression of class I MHC. As shown by the mutation Y223F, this element is dispensable for other known functions of 239-Nef, including its ability to downregulate CD4 and CD3 surface expression and to enhance SIV virion infectivity. In addition, the Y223F mutation does not affect the ability of 239-Nef to colocalize with the AP-2 clathrin adapter in vivo and to precipitate AP-1 and AP-2 adapter complexes in vitro in GST pulldown experiments (data not shown). Also, other mutations in this region, such as D155L, G238*, and Y226A, likely disrupt local surfaces on the 239-Nef molecule important for the molecular interactions of Nef with cellular factors that regulate the traffic of class I MHC complexes or perhaps with the class I MHC heavy chain itself. These mutants will be useful reagents for studying the role of class I MHC downregulation in SIV replication and in AIDS progression in experimentally infected rhesus macaques.
While the downregulation of class I MHC expression is a conserved function of HIV-1 and SIV Nef proteins, our observations indicate that each of these two proteins uses different surfaces to interact with cellular factors important for class I MHC downregulation. The ability of SIV Nef to downregulate class I MHC requires the C-terminal sequence, which is not found in HIV-1 Nef, but not prolines P104 and P107 of the conserved PxxP motif. In contrast, in HIV-1 Nef, an intact PxxP motif is essential for class I MHC downregulation (24, 37). Despite these differences, class I MHC downregulation by both HIV-1 and SIV Nef proteins requires the conserved tyrosine residue Y320 in class I heavy chains and involves the accelerated endocytosis of the class I MHC complex from the cell surface. Therefore, both proteins likely use a similar strategy to downregulate class I MHC expression, and this strategy could involve modulation of the phosphorylation status of the conserved tyrosine residue Y320.
Notably, Nef proteins encoded by SIV strains isolated from other simian species, such as SIV agm, SIV lhoest, and SIV syk, resemble HIV-1 Nef in that they are shorter than 239-Nef and do not contain a sequence corresponding to the C-terminal region of 239-Nef distal to G238 (24, 37). Interestingly, the amino acid sequences of the N-terminal regions of these Nef proteins do not contain the N-distal and/or N-proximal elements found in 239-Nef that mediate interactions with the AP-2 clathrin adapter complex. Therefore, the SIV agm, SIV lhoest, and SIV syk Nef proteins are more similar to HIV-1 Nef than to 239-Nef, and they likely share the functional organization of surfaces that mediate class I MHC downregulation with HIV-1 Nef.
The C-terminal sequence in 239-Nef that is required for class I MHC downregulation contains a dileucine sequence, L253-L254. However, these leucines are not likely to function as a bona fide sorting signal because substitution of these leucines with the isoleucine (253) and proline (254) sequence which is found at the corresponding positions in HIV-2 Nef has no detectable effect on the ability of 239-Nef to downregulate class I MHC (data not shown).
We have previously shown that the HIV-1 and SIV Nef proteins use different surfaces and molecular interactions to perform conserved functions other than class I MHC downregulation, notably, CD4 downregulation. Both HIV-1 and SIV Nef proteins interact in vivo and in vitro with the AP-2 clathrin adapter, and this interaction is critical for the ability of Nef proteins to induce CD4 endocytosis (17, 22, 34, 35, 45). However, HIV-1 Nef likely interacts with the AP-2 complex via a dileucine-based sorting signal located in the C-terminal disordered loop of the protein (10, 17, 22), while in SIV Nef, an element located within the N-terminal disordered region of the protein is sufficient for this interaction (35).
Perhaps the most striking difference in the functional organization and strategies used by the SIV and HIV-1 Nef proteins to perform the same end functions is illustrated by the different ways in which the two proteins have evolved to disrupt TCR-CD3-initiated signal transduction. Both SIV and HIV-1 Nef proteins block induction of the very early activation antigen CD69 in T cells, which follows the TCR stimulation of Jurkat T cells (26). However, SIV Nef blocks the induction of CD69 by downregulating the surface expression of the TCR-CD3 complex at the cell surface (7, 60), and this effect does not require the PxxP motif. In contrast, HIV-1 Nef does not downregulate the TCR-CD3 complex but instead blocks a receptor-proximal event in CD3-initiated signaling, and this effect requires an intact PxxP motif and other elements of the SH3 domain-binding surface in the Nef core (26).
At present, it is unclear why the HIV-1 and SIV Nef proteins have evolved different surfaces to carry out interactions with the same cellular factors and why they have evolved different mechanisms to achieve similar perturbations of target cell biology. It can be speculated that these differences reflect genomic constraints imposed by the coevolution of Nef with other SIV or HIV-1 proteins or reflect different strategies evolved by these viruses during adaptation to their respective monkey or human hosts. One important consequence of the difference in the functional organization of HIV-1 and SIV Nef proteins is that results from studies of HIV-1 or SIV Nef proteins cannot be universally applied to each other. Importantly, our observations which illustrate that genetic selection maintains similar functions and molecular interactions via different surfaces indicate that these functions and interactions must have critical roles for the viral life cycle in vivo.
It has been suggested that the downregulation of class I MHC by Nef may protect infected T cells from recognition and destruction by the host immune system (16, 58). Therefore, it is only in the context of the immune response that the downregulation of class I MHC should enhance viral propagation. Accordingly, we observed that the Y223F mutation in the C-terminal region of 239-Nef, which disrupts class I MHC downregulation, does not diminish the infectivity of SIV particles or diminish viral replication in unstimulated T-cell cultures. Such mutations, however, are likely to diminish viral persistence in vivo and will be very useful for studying the role of class I MHC downregulation by Nef in SIV replication in vivo and in the pathogenesis of AIDS. A recent report that the C-terminal domain of SIV macBK-41 Nef is important for SIV pathogenesis is consistent with the key role of class I MHC downregulation by Nef in SIV virulence (31).
ACKNOWLEDGMENTS
We thank Nadim Shohdy for excellent technical assistance and Klara Velinzon for excellent and imaginative help with flow cytometry. We also thank Michael Greenberg, Martin Lock, and other members of the laboratory for sharing reagents and discussions.
This work was supported by grants from the Public Health Service (AI-42561 to J.S.) and from the Council for Tobacco Research (to J.S.) and by BMSF grant 01Ki9478 (to F.K.).
REFERENCES
- 1.Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander L, Du Z, Howe A Y, Czajak S, Desrosiers R C. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J Virol. 1999;73:5814–5825. doi: 10.1128/jvi.73.7.5814-5825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arold S, Franken P, Strub M P, Hoh F, Benichou S, Benarous R, Dumas C. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure. 1997;5:1361–1372. doi: 10.1016/s0969-2126(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 5.Arold S, O'Brien R, Franken P, Strub M P, Hoh F, Dumas C, Ladbury J E. RT loop flexibility enhances the specificity of Src family SH3 domains for HIV-1 Nef. Biochemistry. 1998;37:14683–14691. doi: 10.1021/bi980989q. [DOI] [PubMed] [Google Scholar]
- 6.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart T A. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J Gen Virol. 1998;79:2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 8.Benson R E, Sanfridson A, Ottinger J S, Doyle C, Cullen B R. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambilla A, Turchetto L, Gatti A, Bovolenta C, Veglia F, Santagostino E, Gringeri A, Clementi M, Poli G, Bagnarelli P, Vicenzi E. Defective nef alleles in a cohort of hemophiliacs with progressing and nonprogressing HIV-1 infection. Virology. 1999;259:349–368. doi: 10.1006/viro.1999.9783. [DOI] [PubMed] [Google Scholar]
- 10.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 11.Campbell C, Squicciarini J, Shia M, Pilch P F, Fine R E. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984;23:4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- 12.Carl S, Iafrate A J, Lang S M, Stahl-Hennig C, Kuhn E M, Fuchs D, Matz-Rensing K, ten Haaft P, Heeney J L, Skowronski J, Kirchhoff F. The acidic region and conserved putative protein kinase C phosphorylation site in Nef are important for SIV replication in rhesus macaques. Virology. 1999;257:138–155. doi: 10.1006/viro.1999.9645. [DOI] [PubMed] [Google Scholar]
- 13.Chackerian B, Haigwood N L, Overbaugh J. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology. 1995;213:386–394. doi: 10.1006/viro.1995.0011. [DOI] [PubMed] [Google Scholar]
- 14.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 16.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 17.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 19.Ennis P D, Zemmour J, Salter R D, Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci USA. 1990;87:2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fackler O T, Luo W, Geyer M, Alberts A S, Peterlin B M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 21.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg M E, Iafrate A J, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 26.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff F, Munch J, Carl S, Stolte N, Matz-Rensing K, Fuchs D, Haaft P T, Heeney J L, Swigut T, Skowronski J, Stahl-Hennig C. The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J Virol. 1999;73:8371–8383. doi: 10.1128/jvi.73.10.8371-8383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korbel B, Kuiken C L, Foley B, Hahn B, McCutchan F, Mellors J W, Sodroski J, editors. Human retroviruses and AIDS: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1988. [Google Scholar]
- 31.Lafont B A, Riviere Y, Gloecker L, Beyer C, Hurtrel B, Kieny M P, Kirn A, Aubertin A M. Implication of the C-terminal domain of Nef in the reversion to pathogenicity of attenuated SIV macBK28-41 in macaques. Virology. 2000;266:286–298. doi: 10.1006/viro.1999.9991. [DOI] [PubMed] [Google Scholar]
- 32.Lang S M, Iafrate A J, Stahl-Hennig C, Kuhn E M, Nisslein T, Kaup F J, Haupt M, Hunsmann G, Skowronski J, Kirchhoff F. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat Med. 1997;3:860–865. doi: 10.1038/nm0897-860. [DOI] [PubMed] [Google Scholar]
- 33.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 34.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J M, Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 35.Lock M, Greenberg M E, Iafrate A J, Swigut T, Muench J, Kirchhoff F, Shohdy N, Skowronski J. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J. 1999;18:2722–2733. doi: 10.1093/emboj/18.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luria S, Chambers I, Berg P. Expression of the type 1 human immunodeficiency virus Nef protein in T cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc Natl Acad Sci USA. 1991;88:5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangasarian A, Piguet V, Wang J K, Chen Y L, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73:1964–1973. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manninen A, Hiipakka M, Vihinen M, Lu W, Mayer B J, Saksela K. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology. 1998;250:273–282. doi: 10.1006/viro.1998.9381. [DOI] [PubMed] [Google Scholar]
- 39.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29:10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- 42.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:579–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunn M F, Marsh J W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piguet V, Chen Y L, Mangasarian A, Foti M, Carpentier J L, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pöhlmann S, Flöss P, Ilyinskii P O, Stamminger T, Kirchhoff F. Sequences just upstream of the simian immunodeficiency virus core enhancer allow efficient replication in the absence of the NF-κB and SP1 binding elements. J Virol. 1998;72:5589–5598. doi: 10.1128/jvi.72.7.5589-5598.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potts B J. “Mini” reverse transcriptase (RT) assay. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton; 1990. pp. 103–106. [Google Scholar]
- 48.Riggs N L, Craig H M, Pandori M W, Guatelli J C. The dileucine based sorting motif in HIV-1 Nef is not required for down-regulation of class I MHC. Virology. 1999;258:203–207. doi: 10.1006/viro.1999.9736. [DOI] [PubMed] [Google Scholar]
- 49.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke B E. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 50.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saksela K. HIV-1 Nef and host cell protein kinases. Front Biosci. 1997;2:d606–d618. doi: 10.2741/a217. [DOI] [PubMed] [Google Scholar]
- 52.Salghetti S, Mariani R, Skowronski J. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc Natl Acad Sci USA. 1995;92:349–353. doi: 10.1073/pnas.92.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvi R, Garbuglia A R, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawai E T, Khan I H, Montbriand P M, Peterlin B M, Cheng-Mayer C, Luciw P A. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6:1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 56.Schenk P M, Baumann S, Mattes R, Steinbiss H H. Improved high-level expression system for eukaryotic genes in Escherichia coli using T7 RNA polymerase and rare ArgtRNAs. BioTechniques. 1995;19:196–198. [PubMed] [Google Scholar]
- 57.Schrager J A, Marsh J W. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc Natl Acad Sci USA. 1999;96:8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 59.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol., in press. [DOI] [PubMed]
- 61.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stauber R H, Horie K, Carney P, Hudson E A, Tarasova N I, Gaitanaris G A, Pavlakis G N. Development and applications of enhanced green fluorescent protein mutants. BioTechniques. 1998;24:462–471. doi: 10.2144/98243rr01. [DOI] [PubMed] [Google Scholar]
- 63.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 64.Xu X N, Laffert B, Screaton G R, Kraft M, Wolf D, Kolanus W, Mongkolsapay J, McMichael A J, Baur A S. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J Exp Med. 1999;189:1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]