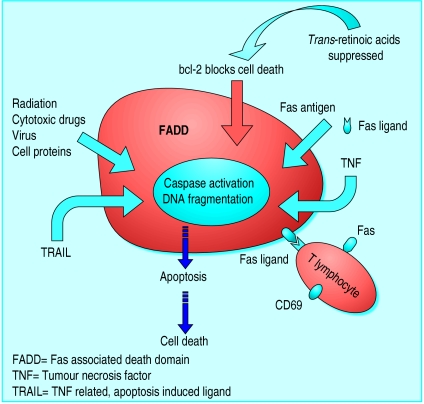
Apoptosis, or programmed cell death, is an active process of self destruction that requires the activation of a genetic programme that may lead to changes in cell morphology, DNA fragmentation, and protein cross linking.1 Apoptosis can be triggered in several ways and involves many cellular functions.2 The mechanism provides protection from the possible consequences of uncontrolled cell proliferation, which could lead to neoplasia. Cell death is a factor in the pathogenesis of several diseases, including autoimmune disorders, cancer, AIDS, and neurodegenerative diseases. Regulation of apoptosis in cells undergoing proliferation may be the key to reversing the natural progression of these disorders.3 Apoptosis involves the sequential activation of a series of caspases, which are proteolytic enzymes that degrade a number of death substrates. Caspase is activated by two pathways—the mitochondrial pathway and the death receptor pathway—and thereby may trigger nuclear enzymes to degrade chromosomal DNA and alter mitochondrial function. Specific pathways and non-specific signals (such as cytotoxic drugs and radiation) may activate caspase. The most common of these pathways involves death receptors that have structures belonging to the tumour necrosis factor (TNF) receptor superfamily of proteins. Interaction of tumour necrosis factor with this receptor can induce cell death by the activation of various kinase enzymes that act as secondary messengers within the cell.4 Another member of this family, Fas antigen, and its ligand (FasL), are molecules used by immune effector cells to kill targets.5
Summary points
Thyroid stimulating hormone (TSH) inhibits Fas expression
Antibodies to TSH receptors inhibit Fas mediated apoptosis
Soluble Fas increases in untreated Graves' disease
Interleukin 1β and TNF-α induce the production of soluble Fas
Fas, DR4, and DR5 are expressed in thyroid carcinoma cell lines
Recombinant TRAIL (TNF related, apoptosis induced ligand) induced apoptosis occurs in 10 thyroid carcinoma cell lines
FasL is strongly reduced in aggressive thyroid carcinomas
mdm2 gene promotes apoptosis in human medullary thyroid carcinoma cells that are deficient in p53
Apoptosis is involved in the homoeostasis of follicular cells of the thyroid gland as well as in the destructive mechanisms of autoimmune thyroiditis and cell death in thyroid cancer. Autoimmune thyroid disorders and carcinomas of thyroid epithelial origin are not uncommon diseases.6,7 Autoimmune thyroid disorders are characterised by the stimulation or inhibition (or both) of autoantibodies.8 The clinical features of these diseases, including thyroid malfunctions or presentations of goitre, depend on the type and amount of the autoantibodies to thyroid stimulating hormone (TSH) receptors in the serum. Most thyroid cancers are well differentiated and of follicular epithelial origin; about 85% are papillary and follicular thyroid cancers.9 The influence of apoptotic markers in these thyroid cancer cells and antiproliferative or anti-apoptotic effects of various cytokines have been reported.10
Methods
I obtained the information about the role of apoptosis in autoimmune thyroid disorders and thyroid cancers by searching Medline using the key words “apoptosis,” “autoimmune thyroid disorder,” and “thyroid cancer.” I focused mainly on well differentiated human thyroid cancers and redifferentiated therapy.
Apoptosis and autoimmune thyroid disorders
Although both genetic and environmental factors have important roles in autoimmune thyroid disease, the underlying pathogenesis of these disorders remains unclear. The percentage of in situ apoptotic thyrocytes increases in Hashimoto's thyroiditis but decreases in Graves' disease, suggesting that apoptosis has a role in function regulation and cell proliferation. Most of the apoptotic cells are detected in disrupted follicles, on the periphery of infiltrating lymphoid cells.11 In the thyroid gland of patients with Graves' disease, CD4 T cells express FasL and kill Fas target cells. The cytotoxicity of the activated FasL CD4 T cells may be suppressed by thyroid stimulating antibody, promoting hyperplasia of thyrocytes.12 In thyroid cells, thyroid stimulating antibody mediated through cyclic adenosine monophosphate (cAMP) can have the role of signal inhibition on Fas expression, thus acting as an anti-apoptotic factor. TSH receptor antibodies from patients with Graves' disease can also inhibit Fas mediated apoptosis, promoting the induction of the goitre.13 In thyrocytes, IgG from patients with Graves' disease and thyroid stimulating hormone downregulate Fas expression.12 By contrast, IgG from patients with idiopathic myxoedema abrogated the effect of thyroid stimulating hormone on both cAMP production and the inhibition of Fas expression in thyrocytes. Soluble Fas is a spliced form of Fas that has been reported to suppress Fas-mediated apoptosis by competitively binding with FasL and altering lymphocyte development and proliferation in response to self antigens.14,15 In addition, soluble Fas has a key role in inhibition of the Fas and FasL system in patients with Graves' disease. Serum concentration of soluble Fas in untreated patients with the disease was significantly increased compared with age matched control subjects. The levels of soluble Fas decreased after the patients were treated with antithyroid drugs for six to eight weeks. Soluble Fas concentration was correlated with anti-TSH receptor antibodies but not correlated with other factors, such as free triiodothyronine, free thyroxine, thyroid stimulating hormone, antimicrosomal antibody (AMA), and antithyroglobulin antibody (ATA). In cultured thyrocytes from the patients with Graves' disease, soluble Fas could be detected in the supernatant, and its production could be induced by interleukin 1β and TNF-α. From these results, it is clear that soluble Fas has an important role in the pathogenesis of Graves' disease.
The bcl-2 proto-oncogene has been reported as conferring resistance to apoptosis in the thyroid. The immunoreactivity with bcl-2 significantly decreased (with a corresponding decrease in goitre weight) two days after rats were switched to a normal diet.16 In the study, using an antiserum to apoptosis specific protein, immunoreactivity during the production of goitre (goitrogenesis) was shown to increase, and then after two days of involution, to localise predominantly in stromal and vascular tissue. Fas expression, after another goitrogenesis pathway, also increased during goitrogenesis.17
Hashimoto's disease is characterised by the loss of thyroid epithelial cells, which are gradually replaced by mononuclear cells. Recent evidence shows that T cells probably have a role in the initiation and amplification of the autoimmune response against thyroid cells, although there is no direct evidence of cytotoxic T cell involvement in the destruction of thyrocytes.18 Infiltrating T lymphocytes express high levels of Fas and CD69. In contrast to thyrocytes, T lymphocytes do not express substantial amounts of FasL, suggesting that they are not directly involved in the destruction of thyrocytes.19 Autocrine or paracrine interaction between Fas and FasL is a major mechanism in the autoimmune destruction of thyrocytes. The expression of the Fas antigen has been reported to increase after treatment with inflammatory cytokines. Production of interleukin 1β during thyroid inflammation induced upregulation of Fas expression and apoptosis in thyrocytes.20 In in vivo studies of BioBreeding/Worcester rats, Fas and FasL gene expression significantly increased in lymphocytic thyroiditis.21 Further animal studies showed that administration of plasmid DNA coding for FasL in thyroid follicular cells inhibited development of lymphocytic infiltration of the thyroid and induced the death of infiltrating T cells.22 This experimental autoimmune thyroiditis animal model shows a total abrogation of the thyroglobulin specific cytotoxic T cell response.
Factors promoting apoptosis
Caspase
Tumour necrosis factor (TNF)
Fas antigen
Fas ligand (FasL)
Interleukin 1β
p53 protein
TNF related, apoptosis induced ligand
bcl-2 associated anti-death gene 1 (BAG-1)
Apoptosis and thyroid cancers
Regulation of apoptosis is an effective way to improve antitumour therapy. This could be achieved by inhibiting the apoptotic response in normal tissues or increasing the apoptotic response to cancerous tissues.23 In papillary thyroid carcinoma the apoptotic index (the percentage of TUNEL (terminal deoxynucleotidyl transferase mediated dUTP digoxigenin nick end labelling) positive neoplastic cells) correlated inversely with bcl-2 and directly with p53 protein.24 The percentage of proliferating cells was significantly different among the histotypes, increasing with tumour aggressiveness, from papillary thyroid carcinoma to poorly differentiated and undifferentiated thyroid carcinoma.
A recent study found Fas, DR4, and DR5 expressed in thyroid carcinoma cell lines and in 31 thyroid carcinoma specimens as determined by western blot analysis and immunohistochemistry respectively. This study tested the sensitivity of thyroid carcinoma cell lines to apoptosis induced by Fas and TRAIL (TNF related, apoptosis induced ligand).25 Fas was expressed in 12 thyroid cancer cell lines, including papillary, follicular, medullary, poorly differentiated, and anaplastic cell lines. Recombinant TRAIL induced apoptosis, in 10 of the 12 thyroid carcinoma cell lines tested, activated caspase-10 at the receptor level and triggered an apoptotic cascade mediated by caspase. In contrast, the medullary carcinoma cell line was resistant to Fas and TRAIL induced apoptosis, even in the presence of cycloheximide. This observation shows that TRAIL effectively kills carcinoma originating from the follicular epithelium of the thyroid gland by inducing caspase mediated apoptosis. These findings provide a potentially potent therapeutic reagent against thyroid cancer. According to immunohistochemical and mRNA studies, different thyroid tissue expressions of Fas and FasL are substantially upregulated in adenoma and in well differentiated papillary and follicular carcinomas. In contrast, Fas is suppressed, and FasL is strongly reduced in the most aggressive histological variants (poorly differentiated and undifferentiated carcinoma).26 bcl-2, Fas, and FasL may be used as differentiation markers of thyroid carcinoma. In the same histopathological thyroid carcinoma these markers could be used as prognostic factors and pre-empt the need for further investigation.
Factors inhibiting apoptosis
Thyroid stimulating hormone
Thyroid stimulating antibody
bcl-2 proto-oncogene
Trans-retinoic acids (trans-RA) have been used in vitro and in vivo as redifferentiation agents in well differentiated thyroid carcinomas.27 The anticancer effects of retinoic acids are mainly due to their inhibition of cell proliferation, induction of cell differentiation, and promotion of apoptosis. In a recent study, RA-induced suppression of Bcl-2 expression was abrogated by overexpression of the BAG-1 gene (bcl-2 associated anti-death gene 1).28 The BAG-1 was cloned from a murine embryo cDNA library using a protein-protein interaction technique. Overexpression of BAG-1 inhibits trans-RA induced apoptosis and prevents trans-RA downregulation of bcl-2 expression.
Another recent study found that the mdm2 gene product promoted apoptosis in human medullary thyroid carcinoma cells deficient in p53 (specifically, in the MTC cell line).29 After transfection of mdm2 gene cDNA, apoptotic cells were identified by TUNEL staining. Protein levels of caspase-2 were also raised in mdm2-transfected cells. With the use of anaplastic human thyroid cancer cell lines (ARO, C643, DRO, Hth-74, KAT-4, and KAT-18), manumycin and apigenin have been shown to induce apoptosis in vitro.30 In the near future we may find the way to treat this very aggressive solid tumour.
Apoptosis of the thyroid follicular cells is an important mechanism in the pathogenesis of human autoimmune thyroid disorders and thyroid cancer. More information is needed before apoptosis can be clinically applied to treat these disorders. Further determination of the role of apoptosis in thyroid follicular cell may enable development of treatments—for example, gene therapy for thyroid disorders—to control these disorders.
Figure.
Mechanism behind apoptosis
Footnotes
Competing interests: J-DL has been reimbursed by Takeda Chemical Industries (Taiwan) for attending a symposium on peroxisome proliferator activated receptors in Florence, Italy, and has received a research grant (NSC 89-2314-B-182A-205) from the National Science Council in Taiwan and research funding from Chang Gung Medical Center.
References
- 1.Wyllie AH, Morris RG, Smith AL, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- 2.Arscott PL, Baker JR. Apoptosis and thyroiditis. Clin Immunol Immunopath. 1998;87:207–217. doi: 10.1006/clin.1998.4526. [DOI] [PubMed] [Google Scholar]
- 3.Kiess W, Gallaher B. Hormonal control of programmed cell death/apoptosis. Eur J Endocrinol. 1998;138:482–491. doi: 10.1530/eje.0.1380482. [DOI] [PubMed] [Google Scholar]
- 4.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 5.Benoist C, Mathis D. Cell death mediators in autoimmune diabetes—no shortage of suspects. Cell. 1997;89:1–4. doi: 10.1016/s0092-8674(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJB, Phillips DIW. Current incidence of thyrotoxicosis and past prevalence of goitre in 12 British towns. Lancet. 1984;ii:567–570. doi: 10.1016/s0140-6736(84)90776-1. [DOI] [PubMed] [Google Scholar]
- 7.Lin JD, Huang MJ, Chao TC, Weng HF, Hsueh C. Prevalence of thyroid cancer in 1894 patients with surgically treated thyroid nodules. Cancer J. 1997;10:217–221. [Google Scholar]
- 8.DeGroot LJ, Quintans J. The causes of autoimmune thyroid disease. Endocr Rev. 1989;10:537–562. doi: 10.1210/edrv-10-4-537. [DOI] [PubMed] [Google Scholar]
- 9.Pettersson B, Adami HO, Wilander E, Coleman MP. Trends in thyroid cancer incidence in Sweden, 1958-1981, by histopathologic type. Int J Cancer. 1991;48:28–33. doi: 10.1002/ijc.2910480106. [DOI] [PubMed] [Google Scholar]
- 10.Bretz JD, Arscott PL, Myc A, Baker JR., Jr Inflammatory cytokine regulation of Fas-mediated apoptosis in thyroid follicular cells. J Biol Chem. 1999;274:25433–25438. doi: 10.1074/jbc.274.36.25433. [DOI] [PubMed] [Google Scholar]
- 11.Hammond LJ, Lowdell MW, Cerrano PG, Goode AW, Bottazzo GF, Mirakian R. Analysis of apoptosis in relation to tissue destruction associated with Hashomoto's autoimmune thyroiditis. J Pathol. 1997;182:138–144. doi: 10.1002/(SICI)1096-9896(199706)182:2<138::AID-PATH810>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Urayama S, Kawabe Y, et al. Modulation of Fas-mediated apoptosis of human thyroid epithelial cell by IgG from patients with Graves' disease (GD) and idiopathic myxedema. Clin Exp Immunol. 1997;110:434–439. doi: 10.1046/j.1365-2249.1997.4301447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107. doi: 10.1056/NEJM199607113350206. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 15.Hiromatsu Y, Bednarczuk T, Soyejima E, Miyake I, Yang D, Fukazawa H, et al. Increased serum soluble Fas in patients with Graves' disease. Thyroid. 1999;9:341–345. doi: 10.1089/thy.1999.9.341. [DOI] [PubMed] [Google Scholar]
- 16.Patel VA, Hill DJ, Sheppard MC, Wang JF, Logan A, Eggo MC. Apoptosis during goiter involution—the role of Bcl-2. J Endocrinol. 2000;164:323–330. doi: 10.1677/joe.0.1640323. [DOI] [PubMed] [Google Scholar]
- 17.Tamura M, Kimura H, Koji T, Tominaga T, Ashizawa K, Kiriyama T, et al. Role of apoptosis of thyrocytes in a rat model of goiter. Endocrinology. 1998;139:3646–3653. doi: 10.1210/endo.139.8.6140. [DOI] [PubMed] [Google Scholar]
- 18.Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 19.Stassi G, Todaro M, Bucchieri F, Stoppacciaro A, Farina F, Zummo G, et al. Fas/Fas ligand-driven T cell apoptosis as a consequence of ineffective thyroid immunoprivilege in Hashimoto's thyroiditis. J Immunol. 1999;162:263–267. [PubMed] [Google Scholar]
- 20.Giordano C, Stassi R, De Maria M, Todaro P, Richiusa G, Papoff G, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science. 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 21.Bluher M, Krohn K, Wallaschofski H, Braverman LE, Paschke R. Fas and Fas ligand gene expression in autoimmune thyroiditis in BB/W rats. Eur J Endocrinol. 1999;141:506–511. doi: 10.1530/eje.0.1410506. [DOI] [PubMed] [Google Scholar]
- 22.Batteux F, Tourneur L, Trebeden H, Charreire J, Chiocchia G. Gene therapy of experimental autoimmune thyroiditis by in vivo administration of plasmid DNA coding for Fas ligand. J Immunol. 1999;162:603–608. [PubMed] [Google Scholar]
- 23.Milas L, Stephens LS, Meyn RE. Relation of apoptosis to cancer therapy. In Vivo. 1994;8:665–673. [PubMed] [Google Scholar]
- 24.Basolo F, Pollina L, Fontanini G, Fiore L, Pacini F, Baldanzi A. Apoptosis and proliferation in thyroid carcinoma: correlation with bcl-2 and p53 protein expression. Br J Cancer. 1997;75:537–541. doi: 10.1038/bjc.1997.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsiades N, Poulaki V, Tseleni-Balafouta S, Koutras DA, Stamenkovic I. Thyroid carcinoma cells are resistant to Fas-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:4122–4129. [PubMed] [Google Scholar]
- 26.Basolo F, Fiore L, Baldanzi A, Giannini R, Dell'Omodarme M, Fontanini G, et al. Suppression of Fas expression and down-regulation of Fas ligand in highly aggressive human thyroid carcinoma. Lab Invest. 2000;80:1413–1419. doi: 10.1038/labinvest.3780148. [DOI] [PubMed] [Google Scholar]
- 27.Kurebayashi J, Tanaka K, Otsuki T, Moriya T, Kunisue H, Uno M, et al. All-trans-retinoic acid modulates expression levels of thyroglobulin and cytokines in a new human poorly differentiated papillary thyroid carcinoma cell line, KTC-1. J Clin Endocrinol Metab. 2000;85:2889–2896. doi: 10.1210/jcem.85.8.6732. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Takayama S, Zheng Y, Froesch B, Chen G, Zhang X, et al. Interaction of BAG-1 with retinoic acid receptor and its inhibition of retinoic acid-induced apoptosis in cancer cells. J Biol Chem. 1998;273:16985–16992. doi: 10.1074/jbc.273.27.16985. [DOI] [PubMed] [Google Scholar]
- 29.Dilla T, Velasco JA, Medina DL, Gonzalez-Palacios F, Santisteban P. The MDM2 oncoprotein promotes apoptosis in p53-deficient human medullary thyroid carcinoma cells. Endocrinology. 2000;141:420–429. doi: 10.1210/endo.141.1.7265. [DOI] [PubMed] [Google Scholar]
- 30.Yin F, Giuliano AE, VanHerle AJ. Signal pathway involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]