Breast cancer is the commonest malignancy in women and comprises 18% of all cancers in women. The United Kingdom has the highest age standardised incidence and mortality from breast cancer in the world.1 Since 1990, death rates from breast cancer have decreased by over 25%, and this is at least in part due to the improved use of adjuvant tamoxifen and chemotherapy. Current research is focused on a greater understanding of the response and resistance to treatment, including the role of apoptosis. Accessibility of the primary tumour makes breast cancer uniquely suitable for such studies. Here we summarise and integrate the data on apoptosis and its role in the development, prognosis, and treatment of breast cancer.
Normal breast development is controlled by a balance between cell proliferation and apoptosis, and there is strong evidence that tumour growth is not just a result of uncontrolled proliferation but also of reduced apoptosis. The balance between proliferation and apoptosis is crucial in determining the overall growth or regression of the tumour2,3 in response to chemotherapy, radiotherapy and, more recently, hormonal treatments. All of these act in part by inducing apoptosis.4–6 Thus it is possible to delineate the biology of individual tumours at the molecular and biochemical level by examining apoptosis and its control and regulation and to exploit these to clinical advantage. Much of this work is still the subject of research. Understanding these relations could allow individually tailored treatments to maximise tumour regression and the efficacy of treatment. It could also help to answer why some tumours fail to respond and thereby indicate new routes of drug development.
Summary points
Increased apoptosis with increased proliferation is associated with malignant tumours
Breast tumours with increased apoptosis are more likely to be high grade and negative for oestrogen receptors
High levels of apoptosis in a breast tumour seem to predict worse survival
Measurable increases in apoptosis occur within 24 hours of the start of chemotherapy
Methods
We searched Medline with the keywords “human breast cancer” and “apoptosis.” We used our own collections of relevant articles and texts and our own experience of the specialty.
Detection and quantification of apoptosis in breast tissue
The gold standard for detection and quantification of apoptosis in situ has been by morphological assessment either with electron microscopy or light microscopy.7 Key features include chromatin condensation, nuclear fragmentation, cell shrinkage, and blebbing of the plasma membrane. However, such assessment is often difficult and time consuming, even for trained histopathologists, and therefore a variety of methods have been developed to ease the identification of these cells. The most widely used techniques concern the incorporation of labelled nucleotides on to the end of the large number of DNA fragments that occur in the late stages of apoptosis—for example, the TUNEL (terminal deoxyribonucleotidyl transferase mediated dUTP nick end labelling) assay (fig 1).8 Cell morphology can be examined simultaneously and apoptotic cells more readily identified. Although labelling of free DNA ends also stains areas of cell necrosis (fig 1), inspection readily differentiates these from apoptotic cells. This technique can be applied to clinical tissue that has been fixed in formalin and embedded in paraffin. Apoptosis can also be assessed by characterising the membrane changes that occur during apoptosis or by detecting the release of enzymes (tissue transglutaminases), but these are not applied to clinical material. However, these methods only provide a snapshot of a dynamic process, as apoptotic bodies are rapidly removed by surrounding macrophages.
Why look at apoptosis in breast cancer?
Apoptosis plays a key part in the development of the normal breast
Dysregulation overrides many of the normal checkpoint pathways and leads to expansion of neoplastic cells
Rates of apoptosis are related to tumour grade, and more aggressive tumours have higher rates of apoptosis and proliferation
Apoptosis is induced by chemotherapy, endocrine treatment, and radiotherapy; when these treatments fail, dysregulation of apoptosis may be a cause
Genes and proteins that control apoptosis may become targets of manipulation to enhance the death of cancer cells
Breast cancer treated with neoadjuvant regimens (chemotherapy or endocrine treatment before surgery) provides ready access to tumours before and during treatment, enabling changes in apoptosis during treatment to be studied and correlated with response
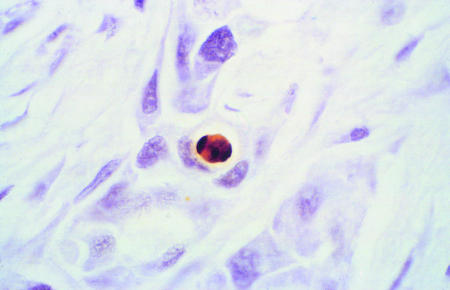
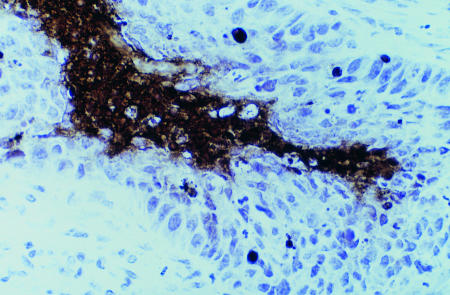
Figure 1.
Histological sections of biopsy samples of breast tumours under light microscopy stained by TUNEL technique. Apoptotic cell with classic features of DNA condensation giving crescent shaped dense area and retraction of cytoplasm giving halo effect (top). Tumour necrosis showing accumulated nuclear debris and apoptotic bodies adjacent to invasive breast cancer (bottom). ×400
Often apoptosis and cell proliferation are analysed together. The concept of a balance between apoptosis and proliferation has resulted in the exploration of mitotic to apoptotic ratios as indices of overall cell death and proliferation in a tumour.
Apoptosis in the normal breast
The breast is one of the few organs that completes its development after birth in two discrete physiological states: puberty and pregnancy. During these stages there are noticeable alterations in breast proliferation and differentiation.9 Changes in the apoptotic regulatory proteins of the Bcl-2 family occur in part due to the influence of oestrogens and progesterone. Cells in the termini of the developing mammary ducts remain mitotically quiescent until the onset of pregnancy. Then rapid epithelial proliferation occurs, with additional ductal branching and lobuloalveolar growth. After lactation there is massive restructuring and apoptosis leading to involution and a return to the primary structure.9 In the absence of pregnancy there is repeated cycling of the resting state in parallel with menstruation until there follows a gradual process of senile involution with menopause. Most apoptotic changes occur in the lobular unit of the terminal duct,10 with cyclical changes through the menstrual cycle and a peak of apoptosis close to the end of the menstrual cycle. A peak of proliferation occurs a few days earlier. It is clear that a balance between proliferation, differentiation, and death of the cells throughout the mammary gland is critical for normal development and homoeostasis. Situations that can upregulate cell proliferation or downregulate apoptosis may allow accumulation of mutations that result in breast cancer. Defects in the cellular processes that detect substantive damage to DNA and lead to the deletion of the cells can lead to the initial acquisition of these cancer related mutations.
Apoptosis studies in human breast cancer
Apoptosis in the history of breast cancer development
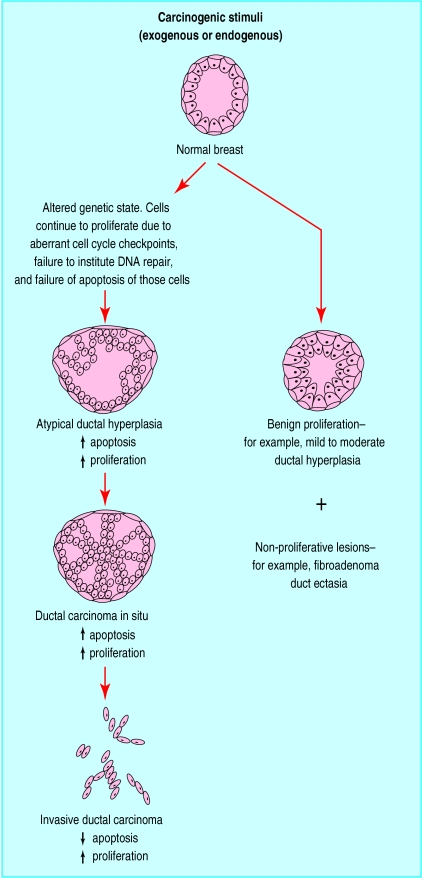
During carcinogenesis in epithelial tissue, genetic mutations accumulate and loss of cellular functions occur. The phenotype of the cells change from normal through a series of malignant lesions to superficial cancers and finally invasive disease (fig 2). In general these processes are protracted and are believed to occur up to 30 years before the clinical appearance of breast cancer.11 In the “premalignant” stages there are major alterations in apoptosis, proliferation, and regulatory biomarkers of the cell cycle.
Figure 2.
Phenotypic changes from normal breast, atypical ductal hyperplasia (intraepithelial neoplasia) through to intraductal carcinoma in situ and invasive ductal or lobular carcinoma
Apoptosis is increased in ductal carcinoma in situ and invasive breast cancer.12,13 Apoptosis seems to be reduced relative to proliferation in “normal” breast epithelium around invasive breast cancer.14 Mommers et al have shown differences in mitotic:apoptotic indices in preinvasive ductal lesions compared with invasive carcinoma.15 They propose an increase in mitotic index and apoptosis in poorly differentiated hyperplastic breast lesions compared with poorly differentiated ductal carcinoma in situ and a further increase in mitotic index but relative decrease in apoptosis in poorly differentiated invasive breast cancers. They suggest an alternative mechanism of increased proliferation in well differentiated cancers with a better prognosis.16
Apoptosis as a marker of prognosis
It would be rational to suppose that low levels of apoptosis are a poor prognostic feature, yet apoptosis is often increased in malignant tumours. This is more noticeable in high grade tumours where it is accompanied by high levels of proliferation.13 This suggests that the controls that integrate proliferation and apoptosis in normal tissues persist, at least in part, during malignancy. High levels of apoptosis in tumours have been correlated with worse survival13,17,18 and have been reported by others to be an independent variable when all other prognostic indicators are considered.19,20 Two large studies have also related high levels of apoptosis to the absence of oestrogen receptors,13,17 a known poor prognostic indicator.
Changes in apoptosis with chemotherapy
In model systems the established role of apoptosis in response to chemotherapy suggests that it is likely to be a good predictive or intermediate marker of breast cancer. Preoperative chemotherapy in large early breast cancer allows a unique in vivo human model in which to study the changes in apoptosis that accompany and may determine the response to treatment.21,22 Biopsy samples (or fine needle aspirates) taken before, during, and after preoperative chemotherapy can be examined for biomarkers or changes in biomarkers that may predict response, relapse, or survival. If a biomarker has a strong and persistent correlation with outcome then a clinician could see how effective a treatment has been and be guided to additional or alternative treatments.
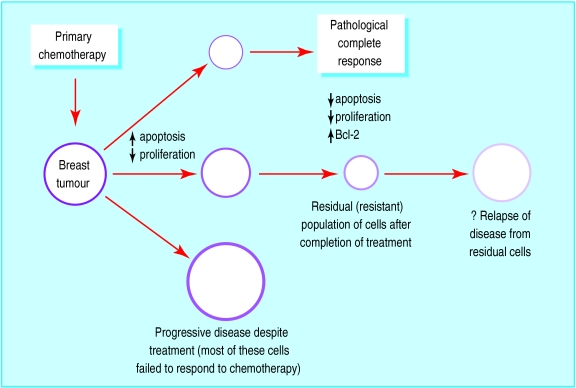
Measurable increases in apoptosis occur in breast tumours within 24 hours of starting chemotherapy.23 This is also associated with a decrease in proliferation.24 A wide variation in changes was found between different tumours, but there was no correlation with clinical response to treatment. These studies were, however, relatively small. At the end of chemotherapy both apoptosis and proliferation are much reduced in residual tissue,25 and the levels of the antiapoptotic protein Bcl-2 are increased. Further characterisation of these residual, chemoresistant cells is important because they may be the source of future relapse and death (fig 3).
Figure 3.
Schematic representation of tumours responding or resistant to chemotherapy and associated changes in growth control
Apoptosis related proteins in breast cancer and treatment
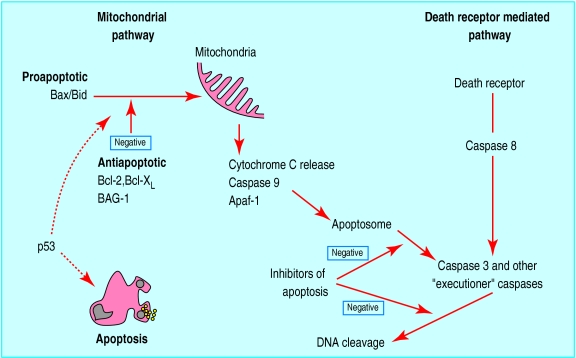
The core machinery of the pathway to cell death can be reduced to a few critical proteins that have been largely conserved throughout species. In humans these regulators exist as multigene families with many homologues that are individually expressed in various tissues.2 The Bcl-2 family (containing inhibitors and promoters of apoptosis) and the p53 tumour suppressor gene have been extensively studied in breast cancer. Figure 4 shows the relation between these oncogenes and proteins in the apoptotic pathways.
Figure 4.
Relation between some major controlling factors involved in apoptosis. Two major pathways of caspase activation have been described in mammalian cells, resulting in apoptosis. In the mitochondrial pathway Bax and Bid are cell death factors, which increase mitochondrial permeability and release of cytochrome C. Cell survival factors Bcl-2, Bcl-XL, and BAG-1 inhibit actions of Bax and Bid on mitochondria. Apaf-1, a downstream mediator of apoptosis, along with cytochrome C, associates with caspase 9 in cytoplasm and leads to its activation. The resultant apoptosome initiates a cascade of effector caspases, which include caspases 3, 6, and 7. Active caspase 3 activates DNA fragmentation factor and promotes internucleosomal cleavage of DNA. The death receptor pathway is triggered by members of the death receptor superfamily such as CD95 and tumour necrosis factor receptor. Formation of a death inducing signalling complex induces caspase 8 activation and thereby the downstream caspase cascade. Further control of the apoptotic process is provided by inhibitors of apoptosis in the cytoplasm that abrogate caspase activity.Interaction between the two pathways exist. P53 is known to induce apoptosis, but exact mechanisms are not clear. The mitochondrial pathway seems to dominate in breast cancer
The Bcl-2 family of genes encodes proteins that may promote or inhibit apoptosis. Proapoptotic proteins include Bax, Bak, Bad, and Bcl-xs whereas Bcl-2 and Bcl-xL are antiapoptotic. In humans Bcl-2 is expressed in about 80% of breast cancers26 and is correlated with the expression of oestrogen and progesterone receptors—good prognostic features in breast cancer. This surprising association between an apoptosis inhibitor and good prognostic features is confirmed by the improved survival of patients with tumours that are Bcl-2 positive compared with those that are negative. Major correlations between Bax protein and outcome have not been observed, although studies have shown reductions in Bax to be associated with a poor response to chemotherapy in metastatic breast cancer.2,26 More recently BAG-1, a multifunctional protein that blocks apoptosis, has been correlated with improved survival in early stage breast cancer.27 Again, this seemingly contradictory finding in an inhibitor protein reflects the complex mechanisms in which these proteins work: BAG-1 is known to interact with other members of the Bcl-2 family as well as heat shock proteins and oestrogen receptors.
The protein product of p53 controls cellular functions involved in apoptosis, the cell cycle, and the repair of DNA.28 Mutations in this gene are the most common mutational event in cancer. Mutations in the gene or increased expression of the p53 protein (an indirect marker of mutation as this often results in stabilisation of the protein), has been associated with a poor prognosis to breast cancer in some studies.29 Chemotherapy, tamoxifen, and radiotherapy are known to induce apoptosis through p53 dependent and p53 independent pathways. Those studies that have measured mutation as opposed to protein over expression have consistently shown that mutated p53 is related to a poor response to chemotherapy.30,31 Response to tamoxifen does not seem to be consistently affected by the presence of mutant p53.
New biomarkers related to apoptosis that are being investigated include the caspases, proteolytic enzymes that form the principal intracellular effectors of apoptosis (the main “executioners of cell death”). So far 11 caspases have been identified in humans. Because caspases are synthesised as inactive zymogens that become activated by cleavage through an intracellular proteolytic cascade they can act as both initiators and effectors of the apoptotic pathway. Regulation of activation and activity occurs at several levels and in some cases involves antiapoptotic members of the Bcl-2 family, which block procaspases.32 Limited work has been carried out on these proteins in cancerous tissue from human breasts, but there is indication that caspases 3, 6, and 8 are associated with higher levels of apoptosis and histological grade.33 The recently identified family of genes that are inhibitors of apoptosis may also have major potential as predictive or prognostic biomarkers. These genes encode a group of proteins (XIAP, cIAP1, cIAP2, NIAP, and survivin in humans) that prevent apoptosis, in many cases by directly binding and inhibiting caspases. XIAP has emerged as one of the most powerful inhibitors and has been shown to be an adverse prognostic marker in acute myeloid leukaemia.3
Problems with studies in apoptosis in breast cancer in humans
Current studies in apoptosis take a snapshot of a dynamic process; the half life of apoptotic cells in breast cancer is not known
Apoptotic bodies in tissue are the final product of a complex mechanism of cell death—many regulatory proteins are involved, and these may provide the key to control of induced cell death by chemotherapy
Many different methods are used to detect these biomarkers, and results are not always comparable
Only small numbers of patients in most of the studies are assessed, making the detection of the variable response to chemotherapy according to variation of apoptosis difficult
Prospective studies in which investigation of apoptotic changes is a primary or even a secondary end point are lacking
Monitoring of apoptosis during treatment currently involves serial biopsies of the breast cancer, an invasive procedure
Future developments
A major goal of contemporary research into clinical breast cancer is to develop treatment regimens that are tailored to individual tumours and thereby maximise survival. Given that apoptosis is intimately involved in the response process, apoptosis and its controls are strong candidates for predictors and indices of response. So far the area is in its infancy as new biomarkers with greater potential are discovered and the complex interactions of the existing factors make interpretation of results difficult. The application of new microarray based approaches is likely to increase noticeably the number of candidate factors. The end result may well be a panel of biomarkers related to apoptosis that will profile the biology of an individual tumour and will enable clinicians to provide optimum treatment.
Preliminary work with targeted therapy with antisense oligonucleotides against the apoptosis inhibitors Bcl-2 and BclxL have been carried out in vitro.34,35 New non-invasive techniques to measure apoptosis in the whole tumour are being developed using positron electron tomography and magnetic resonance spectroscopy. These may allow a more dynamic method of assessment as opposed to the snapshots afforded by biopsy.
Acknowledgments
Figure 2 is adapted with permission from original by Dr C Harper-Wynne.
Footnotes
Competing interests: None declared.
References
- 1.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 3.Tamm I, Schriever F, Dorken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol. 2001;2:33–42. doi: 10.1016/S1470-2045(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 4.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 5.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301:133–142. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 6.Ellis PA, Saccani-Jotti G, Clarke R, Johnston SR, Anderson E, Howell A, et al. Induction of apoptosis by tamoxifen and ICI 182780 in primary breast cancer. Int J Cancer. 1997;72:608–613. doi: 10.1002/(sici)1097-0215(19970807)72:4<608::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. . [Published erratum appears in Cancer 1994;73:3108.] [DOI] [PubMed] [Google Scholar]
- 8.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar R, Vadlamudi RK, Adam L. Apoptosis in mammary gland and cancer. Endocr Relat Cancer. 2000;7:257–269. doi: 10.1677/erc.0.0070257. [DOI] [PubMed] [Google Scholar]
- 10.Anderson TJ. Pathological studies of apoptosis in the normal breast. Endocr Relat Cancer. 1999;6:9–12. doi: 10.1677/erc.0.0060009. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ, Sigman CC, Johnson KM, Boone CW, Greenwald P, Crowell JA, et al. Perspectives on surrogate end points in the development of drugs that reduce the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:127–137. [PubMed] [Google Scholar]
- 12.Gandhi A, Holland PA, Knox WF, Potten CS, Bundred NJ. Evidence of significant apoptosis in poorly differentiated ductal carcinoma in situ of the breast. Br J Cancer. 1998;78:788–794. doi: 10.1038/bjc.1998.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipponen P, Aaltomaa S, Kosma VM, Syrjanen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;14:2068–2073. doi: 10.1016/0959-8049(94)00342-3. [DOI] [PubMed] [Google Scholar]
- 14.Allan DJ, Howell A, Roberts SA, Williams GT, Watson RJ, Coyne JD, et al. Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human breast. J Pathol. 1992;167:25–32. doi: 10.1002/path.1711670106. [DOI] [PubMed] [Google Scholar]
- 15.Mommers EC, van Diest PJ, Leonhart AM, Meijer CJ, Baak JP. Balance of cell proliferation and apoptosis in breast carcinogenesis. Breast Cancer Res Treat. 1999;58:163–169. doi: 10.1023/a:1006396103777. [DOI] [PubMed] [Google Scholar]
- 16.Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berardo MD, Elledge RM, de Moor C, Clark GM, Osborne CK, Allred DC. bcl-2 and apoptosis in lymph node positive breast carcinoma. Cancer. 1998;82:1296–1302. [PubMed] [Google Scholar]
- 18.Zhang GJ, Kimijima I, Abe R, Watanabe T, Kanno M, Hara K, et al. Apoptotic index correlates to bcl-2 and p53 protein expression, histological grade and prognosis in invasive breast cancers. Anticancer Res. 1998;18(3B):1989–1998. [PubMed] [Google Scholar]
- 19.De Jong JS, van Diest PJ, Baak JP. Number of apoptotic cells as a prognostic marker in invasive breast cancer. Br J Cancer. 2000;82:368–373. doi: 10.1054/bjoc.1999.0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Campora R, Galera Ruiz MR, Vazquez Ramirez F, Rios Martin JJ, Fernandez Santos JM, Ramos Martos MM, et al. Apoptosis in breast carcinoma. Pathol Res Pract. 2000;196:167–174. doi: 10.1016/s0344-0338(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 21.Forrest AP, Levack PA, Chetty U, Hawkins RA, Miller WR, Smyth JF, et al. A human tumour model. Lancet. 1986;2:840–842. doi: 10.1016/s0140-6736(86)92872-2. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol. 2000;18:1558–1569. doi: 10.1200/JCO.2000.18.7.1558. [DOI] [PubMed] [Google Scholar]
- 23.Ellis PA, Smith IE, McCarthy K, Detre S, Salter J, Dowsett M. Preoperative chemotherapy induces apoptosis in early breast cancer [letter] Lancet. 1997;349:849. doi: 10.1016/s0140-6736(05)61752-7. [DOI] [PubMed] [Google Scholar]
- 24.Archer CD, Ellis PA, Salter J, Hills M, Dowsett M, Smith IE. Induction of apoptosis and reduction in proliferation 24 hours after chemotherapy in early breast cancer [abstract.]. Proceedings of the American Society of Clinical Oncology, Atlanta, 1999. Baltimore, MD: Lippincott-Williams and Wilkins.
- 25.Ellis PA, Smith IE, Detre S, Burton SA, Salter J, A'Hern R, et al. Reduced apoptosis and proliferation and increased Bcl-2 in residual breast cancer following preoperative chemotherapy. Breast Cancer Res Treat. 1998;48:107–116. doi: 10.1023/a:1005933815809. [DOI] [PubMed] [Google Scholar]
- 26.Krajewski S, Krajewska M, Turner BC, Pratt C, Howard B, Zapata JM, et al. Prognostic significance of apoptosis regulators in breast cancer. Endocr Relat Cancer. 1999;6:29–40. doi: 10.1677/erc.0.0060029. [DOI] [PubMed] [Google Scholar]
- 27.Turner BC, Krajewski S, Krajewska M, Takayama S, Gumbs AA, Carter D, et al. BAG-1: a novel biomarker predicting long-term survival in early-stage breast cancer. J Clin Oncol. 2001;19:992–1000. doi: 10.1200/JCO.2001.19.4.992. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira CG, Tolis C, Giaccone G. p53 and chemosensitivity. Ann Oncol. 1999;10:1011–1021. doi: 10.1023/a:1008361818480. [DOI] [PubMed] [Google Scholar]
- 29.Bergh J. Clinical studies of p53 in treatment and benefit of breast cancer patients. Endocr Relat Cancer. 1999;6:51–59. doi: 10.1677/erc.0.0060051. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton A, Piccart M. The contribution of molecular markers to the prediction of response in the treatment of breast cancer: a review of the literature on HER-2, p53 and BCL-2. Ann Oncol. 2000;11:647–663. doi: 10.1023/a:1008390429428. [DOI] [PubMed] [Google Scholar]
- 31.Aas T, Borresen AL, Geisler S, Smith-Sorensen B, Johnsen H, Varhaug JE, et al. Specific p53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811–814. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S. Mechanisms mediating caspase activation in cell death. Cell Death Differ. 1999;6:1060–1066. doi: 10.1038/sj.cdd.4400600. [DOI] [PubMed] [Google Scholar]
- 33.Vakkala M, Paakko P, Soini Y. Expression of caspases 3, 6 and 8 is increased in parallel with apoptosis and histological aggressiveness of the breast lesion. Br J Cancer. 1999;81:592–599. doi: 10.1038/sj.bjc.6690735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- 35.Simoes-Wust AP, Olie RA, Gautschi O, Leech SH, Haner R, Hall J, et al. Bcl-xl antisense treatment induces apoptosis in breast carcinoma cells. Int J Cancer. 2000;87:582–590. doi: 10.1002/1097-0215(20000815)87:4<582::aid-ijc19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]