Abstract
Enumeration and characterization of herpes simplex virus (HSV)-specific CD8+ T lymphocytes are tedious and indirect. We quantitated antigen-specific CD8+ T cells during acute and secondary stages of HSV infection using intracellular gamma interferon production upon stimulation with virus or immunodominant peptide. Results show a substantial increase in the number of CD8+ T cells which was otherwise underestimated with the conventional limiting dilution analysis.
Quantifying virus-specific cytotoxic T lymphocyte (CTL) responses by the tedious limiting dilution analysis (LDA) technique indicated that the immune response to viruses involves surprisingly few cells. This was especially notable with herpes simplex virus (HSV), with which, even at its peak, the number of CTL precursors detectable by LDA was in the 1/1,000 to 1/2,000 range (6). The advent of major histocompatibility complex tetramers as well as the detection by fluorescence-activated cell sorting (FACS) or ELISPOT of peptide-stimulated CD8+ T cells that produce intracellular gamma interferon (IFN-γ) has rewritten the numbers book (3–5). Remarkably, in fact, with lymphocytic choriomeningitis virus during the acute phase, lymphocytic choriomeningitis virus-specific CD8+ T cells reach 70% of the total CD8+ population and represent as many as 50% long into the memory phase. In this report, we have applied the antigen-driven intracellular IFN-γ (ICG) assay to measure the CD8+ T-cell response of C57BL/6 and BALB/c mice to HSV infection and have compared it to LDA. Our results show far greater numbers of cells detected by the ICG assay (around 100-fold), but the maximum percentage of cells detectable in virus-stimulated splenic CD8 T cells was less than 4% and by 90 days was less than 1%.
C57BL/6 and BALB/c mice were infected with 5 × 106 PFU of HSV(kos) in the footpad. The spleens were removed from these animals at different time points after infection for LDA and ICG analyses to assess the frequency of HSV-specific CD8+ T cells as CTL precursors (CTL-p) and effector CTLs (eCTL), respectively. C57BL/6 and BALB/c splenocytes were stimulated with HSV(kos)-infected (at a multiplicity of infection of 5) and X-ray-irradiated syngeneic cells (MC38 and EMT6, respectively) for 10 to 12 h. A portion of C57BL/6 splenocytes was also stimulated with SSIEFARL (HSVgB498–505[synthesized at Genemed Synthesis, Inc., San Francisco, Calif.]) peptide (1 mg/ml) for 6 h, in a 96-well flat-bottomed plate at a concentration of 106 cells/well in a volume of 0.2 ml of complete medium (RPMI 1640–10% fetal bovine serum) supplemented with 50 U of human recombinant interleukin 2. Brefeldin A was added at the beginning of the peptide-stimulated splenocyte cultures, while virus-stimulated cultures received brefeldin A during the last 5 h of the culture period. The period of stimulation and the doses of peptide and virus used were optimal based on the results of initial experiments. The cells were harvested after the incubation period, washed with RPMI 1640, and resuspended in FACS buffer (phosphate-buffered saline containing 3% fetal bovine serum and 1 mM NaN3). The cells were surface stained for CD8 (fluorescein isothiocyanate [FITC]-labeled anti-CD8a) or CD69 (FITC-labeled anti-CD69 early activation marker) or CD44 (FITC-labeled anti-CD44) or CD62L (FITC-labeled anti-CD62L) for 30 min. The unbound antibodies were washed, and the cells were analyzed for intracellular IFN-γ per the protocol described for intracellular staining in the kit purchased from Pharmingen (Becton Dickinson). The phycoerythrin-labeled anti-IFN-γ and its isotype antibody control and other antibodies used in the staining were all purchased from Pharmingen. The stained cells were immediately analyzed using the FACScan (Becton Dickinson) and CellQuest software. As a positive control for the intracellular staining procedure, polyclonal stimulators phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml) were used (data not shown).
The LDA was performed as previously described (6). Frequency estimates of CTL-p were made using the minimal χ2 method described by Taswell (7).
With HSV, few peptides have been identified which act as CD8+-recognized epitopes. The notable exception is SSIEFARL (amino acids 498 to 505), which acts as an immunodominant epitope in C57BL/6 mice (2). Previous reports with other viruses (3–5, 9) using the ICG assay have all used peptide stimulation to drive intracellular IFN-γ expression.
Lacking such peptides to stimulate BALB/c HSV-immune CD8 cells to produce intracellular IFN-γ, virus stimulation was used. As is evident in Table 1, when the same population of splenocytes from HSV-immune BALB/c mice was analyzed for CTL-p by LDA and for eCTL by the ICG assay, the latter assay detected around 100-fold greater frequencies. In the day 14 sample, for example, the CD8+ eCTL accounted for 2.8% (range, 2.6 to 3.3% in three experiments) of the total CD8 population and had fallen to <2% by day 60. However, by day 60, CTL-p frequencies had decreased even more and were on the borderline of being too low to accurately compute.
TABLE 1.
Frequency of HSV-specific T cells in BALB/c micea
| Days postinfection | Results of LDA for CTL-p
|
Results of ICG assay for eCTL
|
|||
|---|---|---|---|---|---|
| Frequency | No. per 106 cells | Frequency | No. per 106 cells | % Splenic CD8 | |
| 0 | <1/100,000 | <1 | <200 | <0.2 | |
| 14 | 1/15,250 | 66 | 1/154 | 6,493 | 2.8 |
| 60 | 1/81,000 | 12 | 1/173 | 5,780 | 1.7 |
Shown are results for CTL-p and eCTL on the same pool of splenocytes from five mice infected 14 or 60 days previously with HSV(kos) (5 × 106 PFU). Two repeat experiments of like design provided a similar pattern of results.
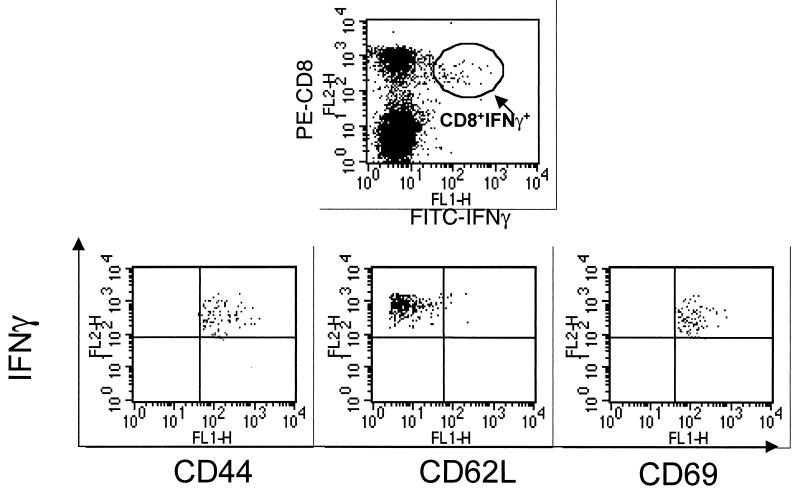
For C57BL/6 mice (Table 2), the ICG assay was used to measure both peptide- and virus-stimulated responses. When the two were compared in parallel, greater eCTL frequencies were always detected by peptide stimulation, although differences were usually greater than twofold. An explanation for this finding is not at hand. However, it could relate both to the fact that SSIEFARL is an overwhelmingly dominant epitope in C57BL/6 mice (8) and the fact that when peptides are used for CTL recognition, a wider affinity spectrum of CTLs is recorded. Comparisons between LDA and the ICG assay with the same cell population in the C57BL/6 system always used peptide to stimulate intracellular IFN-γ expression. In such comparisons, the eCTL approach detected around 80-fold higher frequencies than CTL-p at 14 days and 470-fold at 60 days (Table 2). Several other time periods after HSV infections were tested for peptide-stimulated ICG responses in C57BL/6 mice. At peak times (14 days), responses were between 5 and 6% of the total CD8+ splenocytes and by 90 days had declined to <1%. However, cells detected at 90 days were still considered above background and specific. Finally, additional phenotypes were measured on the IFN-γ-positive cells. After day 14, samples of all IFN-γ+ cells were judged to be of the memory/effector phenotype in additional to being CD44hi and CD62Llo and also expressing CD69 (Fig. 1).
TABLE 2.
Frequency of HSV-specific T cells in C57BL/6 micea
| Days postinfection | Results of LDA for CTL-p
|
Results of ICG assay for eCTL
|
||||
|---|---|---|---|---|---|---|
| Frequency of CTL-p | No. of CTL-p per 106 cells | Peptide specific
|
HSV specific % CD8c | |||
| Frequency of CTL-p | No. of eCTL per 106 cells | % CD8b | ||||
| 0 | <1/100,000 | <1 | <0.1 | |||
| 5 | 0.5 | <0.2 | ||||
| 9 | 2.5 ± 0.4 | 1.1 ± 0.2 | ||||
| 14 | 1/7,020 | 14 | 1/87 | 11,494 | 5.5 | |
| 5.4 ± 0.6 | 3 ± 0.3 | |||||
| 21 | 4.4 ± 0.7 | 2.7 ± 0.4 | ||||
| 30 | 3.5 ± 0.4 | 2.4 ± 0.3 | ||||
| 60 | 1/42,000 | 23 | 1/92 | 10,869 | 3.4 | |
| 2.9 ± 0.4 | 2.2 ± 0.3 | |||||
| 90 | 0.9 | 0.3 | ||||
Shown are results for CTL-p and eCTL on the same pool of splenocytes from five mice infected 14 or 60 days previously with HSV(kos) (5 × 106 PFU) or uninfected. Two repeat experiments of like design provided a similar pattern of results.
Percent total of CD8+ T cells that make IFN-γ on stimulation with the SSIEFARL peptide.
Percent total of CD8+ T cells that make IFN-γ on stimulation with HSV (same splenocyte population as that used for peptide stimulation).
FIG. 1.
Phenotype of the IFN-γ+ cells. Splenocytes were surface stained for CD44, CD62L, or CD69 FITC-labeled antibody and followed by intracellular staining with phycoerythrin-labeled anti-IFN-γ. The same region which was gated to analyze CD8+ IFN-γ+ cells was applied to these analyses.
In conclusion, this note reports for the first time the value of the intracellular IFN-γ assay to quantify HSV-specific CD8+ T-cell responses in mice. We anxiously await major histocompatibility complex tetramers and expect these will soon be available soon for at least the peptide SSIEFARL in C57BL/6 mice (S. S. Tevethia, personal communication). To take full advantage of new technology to better understand the role of CD8+ T cells in immunity of humans and experimental animals requires that more CD8-recognized epitopes be defined. We and others are participating in this objective.
Acknowledgments
This work was supported by grants AI 14981 and AI 46462.
REFERENCES
- 1.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaney J E, Jr, Nobusawa E, Brehm M A, Bonneau R H, Mylin L M, Fu T M, Kawaoka Y, Tevethia S S. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callan M F C, Steven N, Krausa P, Moss P A H, Wilson J D K, Bell J I, Rickson A B, McMichael A J. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 5.Murali-Krishna K, Altman J D, Suresh M, Sourdive D, Zajac A, Miller J, Slansky J, Ahmed R. Counting antigen specific CD8+ T cells: a re-evaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 6.Rouse B T, Larsen H S, Wagner H. Frequency of cytotoxic T lymphocyte precursors to herpes simplex virus type 1 as determined by limiting dilution analysis. Infect Immun. 1983;39:785–792. doi: 10.1128/iai.39.2.785-792.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1620. [PubMed] [Google Scholar]
- 8.Wallace M E, Keating R, Heath W R, Carbone F R. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson J D K, Ogg G S, Allen R L, Goulder P J R, Kelleher A, Sewell A K, O'Callaghan C A, Rowland-Jones S L, Callan M F, McMichael A J. Oligoclonal expansion of CD8+ T cells in chronic HIV infection are antigen specific. J Exp Med. 1998;188:785–790. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]