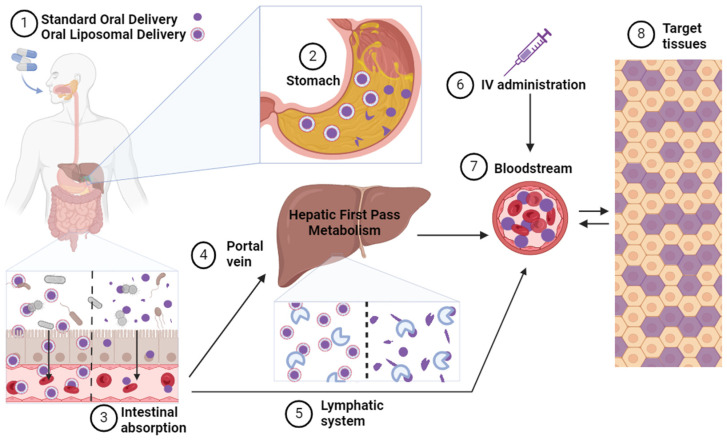
Figure 3.
NMN Administration Routes and Metabolic Processing. (1) Following ingestion of either a standard or liposomal formulation of NMN, (2) the capsule undergoes breakdown in the stomach, with liposomal formulations offering enhanced protection from degradation. (3) The standard NMN formulation undergoes extensive metabolism in the intestine by gut bacteria and intestinal enzymes before absorption into the enterocyte, while liposomes fuse with the intestinal lining, facilitating the absorption of encapsulated NMN. From the enterocyte, (4) standard NMN is absorbed into the portal vein and transported to the liver, where it undergoes first-pass metabolism and further enzymatic breakdown. (5) absorbed liposomes exit the enterocytes via the lymphatic system and enter the bloodstream, bypassing first-pass hepatic metabolism. (6) Intravenous administration of NMN directly introduces NMN into the bloodstream without undergoing processing in the intestine or liver. (7) Ultimately, NMN molecules enter the bloodstream for potential uptake into target tissues, albeit the amount reaching these tissues varies depending on the route of administration and the metabolic processing experienced en route. Figure created in BioRender.