Abstract
Handwriting kinematics (HWKs) were assessed in the randomized controlled ALPINE study of 2 long-acting injectable antipsychotics started during an acute exacerbation of schizophrenia. This exploratory analysis examined the relationship between baseline HWKs and response to acute antipsychotic treatment. Adults with acute schizophrenia were assigned to aripiprazole lauroxil or paliperidone palmitate (groups combined for this analysis). Treatment response was defined as ≥20% reduction from baseline in Positive and Negative Syndrome Scale (PANSS) total score at week 4. Two HWK measures, peak velocity (decreases with greater dysfunction) and percentage of nonballistic movements (%NBM; increases with greater dysfunction), were captured in 4 handwriting tasks (complex loops, maximum speed circles, overlay circles, and left-right loops). Peak velocity and %NBM at baseline were compared between responders and nonresponders. The analysis included 143 patients (mean baseline PANSS total score, 94.5). PANSS responders (n = 67 [46.9%]) had a lower mean peak velocity (i.e., slower pen movements) on all HWK tasks at baseline compared with nonresponders (n = 76): complex loops, 8.8 versus 12.1 cm/s; maximum speed circles, 18.0 versus 23.7 cm/s; overlay circles, 12.6 versus 17.2 cm/s; and left-right loops, 11.2 versus 14.6 cm/s. PANSS responders had a greater %NBM on 3 tasks compared with nonresponders: complex loops, 57.1% versus 47.4%; overlay circles, 30.6% versus 24.3%; and left-right loops, 58.7% versus 47.0%. In this exploratory analysis, PANSS responders to aripiprazole lauroxil or paliperidone palmitate treatment at week 4 had lower baseline HWK movement velocities and greater baseline %NBM versus nonresponders, suggesting that baseline HWKs might predict response to these antipsychotic drugs.
Keywords: exploratory analysis, biomarkers, predictors of response, motor function, dopamine dysfunction
Introduction
Antipsychotic medication is an essential component of an evidence-based treatment plan for patients with schizophrenia.1 However, many patients with acute schizophrenia do not have an adequate response to a short-term trial of antipsychotic therapy.2 One of the challenges in advancing the pharmacologic treatment of schizophrenia is the lack of established biomarkers of neurotransmitter system dysfunction that can be used in exploring mechanisms of how and when patients respond to antipsychotic treatment.2,3 Measures predictive of antipsychotic treatment response have been observed, including higher central D2 dopamine receptor occupancy during the course of therapy4,5 and early (week 2) response in clinical trials,6 but few baseline predictors of response to antipsychotic drugs have been identified.7 Some studies have reported that baseline or antipsychotic-induced parkinsonism may be associated with a favorable clinical response to treatment8,9; however, in other studies, parkinsonism has been associated with poor treatment response in first-episode psychosis10,11 and long-term treatment.12 Differences in how parkinsonism was assessed (clinical vs. instrumental) and differences in patient characteristics across the studies could account for this discrepancy. Thus, there is an unmet need for quantitative predictors of acute response to antipsychotic medications as tools for understanding therapeutic response in schizophrenia.
Neuroimaging and clinical studies suggest that handwriting kinematics, such as handwriting size, speed, or fluency, are mediated by a striatal dopaminergic mechanism.13–16 Dopamine D2 receptor occupancy during antipsychotic treatment is a significant predictor of treatment response,4 and correlations between change in handwriting kinematics and dopamine D2 receptor occupancy (using positron emission tomography) have been observed.17,18 This work is validated by studies demonstrating that handwriting kinematic measures are sensitive to antipsychotic medication effects in patients with schizophrenia.19,20 Specifically, motor impairment can be detected in medication-exposed versus unmedicated patients with schizophrenia using kinematic measures,19 and greater impairment in certain handwriting kinematic variables is observed with increasing antipsychotic dose.19,20 Changes in handwriting kinematic measures also have been associated with independent measures of motor slowing in patients with schizophrenia treated with antipsychotic medications.21 Therefore, handwriting kinematics might be a useful biomarker of striatal dopamine integrity and provide insight into the therapeutic response to drugs that act on dopamine D2 receptors.
Handwriting kinematics were included as an exploratory outcome in the ALPINE (Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness) study, a randomized, controlled trial of long-acting injectable (LAI) antipsychotic treatment started during an acute exacerbation of schizophrenia.22 ALPINE evaluated the efficacy and safety of a 2-month formulation of aripiprazole lauroxil (AL; ARISTADA23), with paliperidone palmitate (PP; INVEGA SUSTENNA24) as an active control. The LAI formulations have the methodologic advantage of eliminating undetected medication gaps associated with oral antipsychotic formulations.25 Significant improvement in the primary efficacy outcome, change in Positive and Negative Syndrome Scale (PANSS)26 total score from baseline to week 4, was observed in each treatment group.22 The ALPINE study provided an opportunity to evaluate as a proof of concept whether blinded baseline handwriting kinematics can serve as a behavioral or biologic predictor of future therapeutic response to acute antipsychotic treatment.22 The objective of this exploratory analysis was to assess whether handwriting kinematic parameter values measured at randomization to 1 of 2 first-line LAI atypical antipsychotics for the treatment of acute schizophrenia are associated with severity of clinical symptoms at 4 weeks in the subgroup of ALPINE patients who completed a baseline handwriting kinematic assessment.
Methods
The ALPINE study (ClinicalTrials.gov identifier: NCT03345979) was designed and carried out in accordance with the principles of Good Clinical Practice that have their origin in the Declaration of Helsinki and its amendments27 and in accordance with local regulations and International Council for Harmonisation guidelines.28 The study protocol and amendment were approved by the independent ethics committee/institutional review board (IRB) for each study site, and the IRB registered with the Office for Human Research Protections and the U.S. Food and Drug Administration for the ALPINE study was the Copernicus Group IRB (IRB00001313). All patients received information about the nature, purpose, and possible risks and benefits of the study and provided written informed consent before any study-specific procedures were conducted.
Patients
The ALPINE study enrolled acutely ill adults (age 18–65 years) with schizophrenia, diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria,29 requiring hospitalization. Enrollment criteria included a PANSS total score range between 80 and 120, with individual item scores of ≥4 on at least 2 of the following items: P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), or P6 (suspiciousness/persecution). Key exclusion criteria included a primary diagnosis other than schizophrenia, current risk of suicide, or recent LAI antipsychotic treatment. Patients with a history of treatment resistance and of poor or inadequate clinical response to aripiprazole, risperidone, or paliperidone were also excluded. Additional enrollment criteria are reported in the primary ALPINE publication.22 The current exploratory analysis included all ALPINE patients who completed a handwriting kinematic assessment at baseline and remained on trial for at least 25 days.
Study Design
The ALPINE study included a screening period of up to 7 days and 25 weeks of double-blind treatment. Patients were hospitalized during the screening period and first 2 weeks of treatment (with a possible additional week based on clinical need; supplementary figure 1). Prior antipsychotic medications were discontinued during a washout period of 2–5 days before patients were randomly assigned to start either AL or PP LAI treatment on study day 1. Patients without prior exposure to AL, PP, or both received test doses during the first 2 days of inpatient screening to establish tolerability. After study day 1 (randomization), no other oral antipsychotic was allowed. Patients assigned to AL were administered AL 1064 mg every 8 weeks, started with a 1-day initiation regimen (AL NanoCrystal Dispersion [ARISTADA INITIO30] plus a single 30 mg dose of oral aripiprazole; supplementary figure 1). Patients assigned to PP were administered PP 156 mg every 4 weeks, started with a single injection of PP 234 mg.
The primary efficacy outcome measure in ALPINE was change in PANSS total score from baseline (prior to the first dose of study drug) to week 4 within each treatment group, and ALPINE results have been published.22 ALPINE study assessments included for this exploratory analysis were PANSS, the Clinical Global Impression–Severity (CGI-S) scale,31 the Abnormal Involuntary Movement Scale,32 the Barnes Akathisia Rating Scale,33 the Simpson-Angus Scale,34 and a handwriting kinematic assessment. Each assessment was administered at baseline and at on-therapy study visits; the timing of assessments included in the current analysis is shown in supplementary figure 1.
Handwriting kinematics were assessed at screening (screening day 1 or 2; defined as baseline for the handwriting kinematic analysis) and at several visits over the treatment period. For this exploratory analysis, only the baseline handwriting kinematic assessment was evaluated as a potential predictor.
The primary clinical outcome of interest for the current analysis was the relationship between baseline handwriting kinematics and subsequent treatment response as assessed by change from baseline to the week 4 assessment. A dichotomized end point was used in the exploratory analysis to minimize variability associated with change scores. Two definitions of treatment response were used: (1) a 20% or greater reduction from baseline in PANSS total score and (2) a 2-point or greater improvement from baseline in CGI-S score.
Handwriting Kinematics Assessment
Handwriting kinematics were assessed using a noninking pen and a Wacom (Portland, OR) Intuos UD 9 × 12 in. (22.5 × 30 cm) digitizing tablet (sampling rate, 100 samples/s; root mean square accuracy, 0.01 cm), which captured and recorded pen movements, attached to a notebook computer running MovAlyzeR (version 6.1; NeuroScript, Tempe, AZ) software (figure 1A). Seven handwriting tasks were administered, 5 using the dominant hand (normal writing size, left-right loops [“llllllll”]; normal complex loops [“lleellee”]; normal overlay circles drawn at comfortable speed; normal overlay circles drawn at maximum speed; larger stroke size, overlay circles drawn at comfortable speed) and 2 using the nondominant hand (normal overlay circles and large overlay circles drawn at comfortable speed).
Fig. 1.
Handwriting kinematics assessment. (A) Workflow. (B) Analysis of nonballistic movements.aaAdapted from Caligiuri, et al, 2019.21
Prior to the start of data collection, the patient was shown a visual copy of the task, given instructions, and provided with a practice period to ensure familiarity with the use of the inkless stylus on the tablet. Patients were instructed to begin writing loops or circles continuously until told by the examiner to stop and lift the pen. Tasks were administered in random order with a block of 5 trials for each condition. Each trial lasted 10 s with a 2-s interval between trials. When the 5 trials were completed, the program paused while the administrator provided a new set of instructions for the next task.
Four handwriting tasks like those previously found to be sensitive to dopamine or dopamine dysfunction in patients with Parkinson’s disease or schizophrenia15,19,20,35,36 were included in this analysis: simple left-right loops, complex loops, overlay circles, and maximum speed overlay circle drawing, each using the dominant hand (sample traces shown in figure 1A). For each task, 2 kinematic measures were considered for this exploratory study: peak velocity and percentage of nonballistic handwriting movements (%NBM). Lower peak velocity scores—indicating slower pen movements—correspond with greater dysfunction. Ballistic movements are rapid movements that are not adjusted during their course (as in cursive writing); conversely, nonballistic movements are generally slower, with adjustments in trajectory based on peripheral feedback21 (figure 1B). Thus, a higher %NBM corresponds with greater dysfunction.
Statistical Analysis
Patients who had a baseline handwriting kinematic assessment and a week 4 efficacy assessment (handwriting kinematics analysis population) were included in this exploratory analysis, and data from AL and PP treatment arms were pooled. Baseline characteristics were summarized for the handwriting kinematics analysis population and for PANSS responders versus nonresponders. Peak velocity and %NBM at baseline were calculated for each task. Mean (SE) peak velocity and %NBM at baseline were summarized for treatment responders and nonresponders at week 4 based on PANSS response and separately based on CGI-S response. For each task, effect size of peak velocity and of %NBM for treatment response based on PANSS and CGI-S definitions separately were estimated based on Cohen’s d. Predictive effects of other baseline characteristics on PANSS responder status were assessed based on Cohen’s d (continuous characteristics) or odds ratio (95% CIs; dichotomous characteristics). To address potential selection bias, t-tests were used to compare baseline handwriting kinematic scores from patients who completed the week 4 assessment with those from patients without a week 4 assessment.
Because the baseline assessment was conducted soon after admission (supplementary figure 1), while some patients were, not receiving antipsychotic medication and others were treated with multiple first- and second-generation antipsychotics, it is possible that variability in antipsychotic burden influenced handwriting kinematics. Therefore, we conducted an additional analysis that redefined baseline to assess this possibility. Because the washout period in the main study was to be completed no later than day 5 post-randomization, and all patients had been initiated on a stable dose of an LAI antipsychotic by day 15, we redefined the baseline handwriting kinematic assessment as that conducted on day 15, when the variability related to D2-antagonizing medications was effectively eliminated. Mean (SE) baseline handwriting kinematics scores for PANSS responders and nonresponders at week 4 were determined with baseline defined as study day 15, and differences in mean scores for responders and nonresponders were calculated for screening and study day 15 baseline measures.
Results
Patients
A total of 200 patients were randomized in the ALPINE study. Of those, 167 patients had baseline handwriting kinematic assessments and 143 also had a week 4 efficacy assessment (PANSS and CGI-S) and were included in this exploratory analysis. Patients included in the handwriting analysis had a mean (SD) age of 42.7 (10.18) years, and 76% were men. Mean (SD) PANSS total score at baseline was 94.5 (8.28); mean (SD) baseline CGI-S score was 4.88 (0.63). At week 4, PANSS response (≥20% improvement from baseline) was observed in 67/143 (47%) patients; CGI-S response (≥2-point improvement from baseline) was observed in 41/143 (29%) patients. Baseline demographic and clinical characteristics are presented for PANSS responders and nonresponders in table 1.
Table 1.
Baseline Demographic and Clinical Characteristics for Responders vs Nonresponders Based on Change in PANSS Total Score (≥20% Change from Baseline at Week 4)
| Baseline Characteristic | PANSS Response at Week 4 (N = 143a) | Effect Sizeb for Responders vs Nonresponders | |
|---|---|---|---|
| Responders (n = 67) |
Nonresponders (n = 76) |
||
| Age, y, mean (SD) | 42.3 (9.61) | 43.0 (10.72) | 0.07 |
| Sex, male, n (%) | 48 (71.64) | 60 (78.95) | 1.48 (0.69, 3.19) |
| PANSS total score, mean (SD) | 94.7 (7.86) | 94.3 (8.68) | 0.05 |
| PANSS Positive score, mean (SD) | 26.3 (3.08) | 25.0 (3.35) | 0.40 |
| PANSS Negative score, mean (SD) | 22.4 (3.42) | 23.3 (3.89) | 0.24 |
| PANSS General Psychopathology score, mean (SD) | 46.0 (5.56) | 46.0 (4.94) | 0.01 |
| CGI-S, mean (SD) | 4.9 (0.66) | 4.8 (0.61) | 0.13 |
| SAS score, mean (SD) | 0.2 (0.57) | 0.2 (0.97) | 0.03 |
| BARS total score, mean (SD) | 0.04 (0.21) | 0 | 0.32 |
| AIMS total score, mean (SD) | 0.01 (0.12) | 0.36 (1.40) | 0.33 |
| AIMS total score > 0, n (%) | 1 (1.49) | 9 (11.84) | 8.87 (1.09, 71.95) |
| Patients not on antipsychotic medications within 30 days prior to first dose of study drug, n (%) | 21 (31.34) | 20 (26.32) | 1.28 (0.62, 2.64) |
| Patients on anticholinergic medications within 30 days prior to first dose of study drug, n (%) | 3 (4.48) | 6 (7.89) | 1.83 (0.44, 7.62) |
aMissing handwriting data at baseline (n = 33) were mainly due to issues with handwriting test procedures.
bEffect size, a measure of the difference in the baseline characteristic for responders vs nonresponders, was calculated as Cohen’s d for continuous characteristics and odds ratios (95% CIs) for dichotomous characteristics.
AIMS, Abnormal Involuntary Movement Scale; BARS, Barnes Akathisia Rating Scale; CGI-S, Clinical Global Impressions-Severity scale; PANSS, Positive and Negative Syndrome Scale; SAS, Simpson-Angus Scale.
Handwriting Kinematics Predictor Analysis
Peak velocity
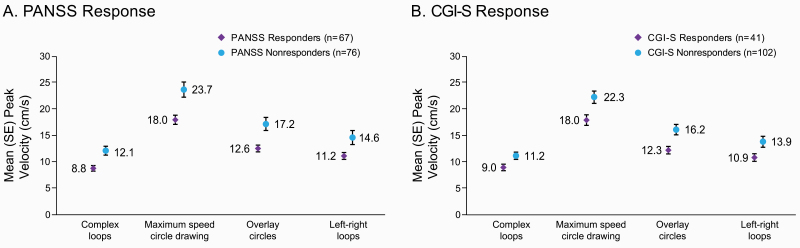
PANSS responders (n = 67) had a lower baseline mean peak velocity on all handwriting kinematic tasks compared with nonresponders (n = 76), indicating slower pen movements during completion of handwriting tasks at baseline. Mean (SE) peak velocity for each of the 4 handwriting tasks is shown for PANSS responders and nonresponders in figure 2A. Effect size (Cohen’s d) of the 4 tasks on PANSS response ranged from 0.39 to 0.56 (table 2). CGI-S responders (n = 41) also had lower peak velocities (slower pen movements) on each task at baseline compared with nonresponders (n = 102; figure 2B). Effect size on CGI-S response ranged from 0.33 to 0.45 (table 2).
Fig. 2.
Mean ± SE peak velocity in handwriting kinematic tasks at baseline, Week 4 respondersa vs nonresponders.b (A) PANSS Response. (B) CGI-S Response.aPANSS response defined as ≥20% reduction from baseline in PANSS total score; CGI-S response defined as ≥2-point improvement from baseline in CGI-S score.
bFor PANSS nonresponders, n = 75 for complex loops and left-right loops tasks. For CGI-S nonresponders, n = 101 for complex loops and left-right loops tasks.
CGI-S, Clinical Global Impression-Severity scale; PANSS, Positive and Negative Syndrome Scale.
Table 2.
Mean (SE) Baseline Handwriting Kinematics Scores for Responders vs Nonresponders at Week 4
| Responders | Nonresponders | Effect Size (Cohen’s d) |
|
|---|---|---|---|
| Peak velocity, mean (SE), cm/s | |||
| PANSS response,a n | 67 | 76b | |
| Complex loops | 8.8 (0.53) | 12.1 (0.84) | 0.55 |
| Maximum speed circle drawing | 18.0 (0.87) | 23.7 (1.44) | 0.56 |
| Overlay circles | 12.6 (0.64) | 17.2 (1.24) | 0.54 |
| Left-right loops | 11.2 (0.65) | 14.6 (1.33) | 0.39 |
| CGI-S response,c n | 41 | 102d | |
| Complex loops | 9.0 (0.62) | 11.2 (0.69) | 0.36 |
| Maximum speed circle drawing | 18.0 (1.00) | 22.3 (1.17) | 0.41 |
| Overlay circles | 12.3 (0.71) | 16.2 (0.99) | 0.45 |
| Left-right loops | 10.9 (0.72) | 13.9 (1.04) | 0.33 |
| %NBM, mean (SE) | |||
| PANSS response,a n | 67 | 76b | |
| Complex loops | 57.1 (2.82) | 47.4 (3.28) | 0.38 |
| Maximum speed circle drawing | 9.2 (1.52) | 9.1 (1.53) | 0.01 |
| Overlay circles | 30.6 (3.01) | 24.3 (3.12) | 0.24 |
| Left-right loops | 58.7 (3.50) | 47.0 (3.59) | 0.40 |
| CGI-S response,c n | 41 | 102d | |
| Complex loops | 59.6 (3.67) | 48.9 (2.69) | 0.41 |
| Maximum speed circle drawing | 9.2 (2.12) | 9.2 (1.25) | <0.01 |
| Overlay circles | 32.9 (3.85) | 25.0 (2.63) | 0.31 |
| Left-right loops | 63.4 (4.50) | 48.1 (2.99) | 0.52 |
aPANSS response defined as ≥20% reduction from baseline in PANSS total score.
bFor PANSS nonresponders, n = 75 for complex loops and left-right loops tasks.
cCGI-S response defined as ≥2-point improvement from baseline in CGI-S score.
dFor CGI-S nonresponders, n = 101 for complex loops and left-right loops tasks.
%NBM, percentage of nonballistic movements; CGI-S, Clinical Global Impression-Severity Scale; PANSS, Positive and Negative Syndrome Scale.
Percent Age of Nonballistic Movements
PANSS responders had a greater %NBM at baseline compared with nonresponders on the complex loops, overlay circles, and left-right loops tasks (figure 3A), with effect sizes ranging from 0.24 to 0.40 (table 2). For the maximum speed circle drawing task, %NBM was similar for PANSS responders and nonresponders (figure 3A; table 2). The same pattern was observed for the CGI-S definition of response: CGI-S responders had a greater %NBM compared with nonresponders on the complex loops, overlay circles, and left-right loops tasks at baseline, with effect sizes ranging from 0.31 to 0.52, whereas %NBM was similar for responders versus nonresponders on the maximum speed circle drawing task (figure 3B; table 2).
Fig. 3.
Mean ± SE percentage of nonballistic movements in handwriting kinematic tasks at baseline, week 4 respondersa vs nonresponders.b A. PANSS response. B. CGI-S response.aPANSS response defined as ≥20% reduction from baseline in PANSS total score; CGI-S response defined as ≥2-point improvement from baseline in CGI-S score.
bFor PANSS nonresponders, n = 75 for complex loops and left-right loops tasks. For CGI-S nonresponders, n = 101 for complex loops and left-right loops tasks.
%NBM, percentage of nonballistic movement; CGI-S, Clinical Global Impression-Severity scale; PANSS, Positive and Negative Syndrome Scale.
Week 4 clinical efficacy assessments were unavailable for 24 of the 167 patients with baseline handwriting kinematic data. No significant differences were observed between completers and noncompleters for the week 4 assessment of peak velocity or %NBM in any of the handwriting tasks (supplementary table 1).
Assessment of the Impact of Baseline Medication Variability
Results of the predictor analysis were similar with baseline redefined as study day 15, although differences between week 4 PANSS responders and nonresponders were observed in %NBM on fewer tasks. Using the study day 15 baseline, responders differed from nonresponders on peak velocity in all tasks and %NBM for complex loops (supplementary table 2).
Assessment of Other Baseline Characteristics
Effect size estimates for the strengths of the relationships between PANSS responder status and other baseline measures are given in table 1. Among continuous demographic and clinical characteristics evaluated as baseline predictors, Cohen’s d values were highest for PANSS Positive (0.40) and PANSS Negative (0.24) scores.
Discussion
This exploratory analysis characterized the relationship between baseline handwriting kinematic measures and subsequent therapeutic response to 2 atypical LAI antipsychotics in patients with acute exacerbation of schizophrenia enrolled in the ALPINE study. Patients who achieved a treatment response after 4 weeks of AL or PP therapy (based on either the PANSS or CGI-S definition) had lower peak movement velocities at baseline on each handwriting task examined compared with those who did not achieve response based on either PANSS or CGI-S criteria. PANSS or CGI-S responders also had greater %NBM at baseline compared with nonresponders on the complex loop, overlay circles, and left-right loops tasks, but not for maximum speed circle drawing. The observed association between baseline handwriting kinematic measures and response to AL or PP treatment 4 weeks later suggests that these handwriting kinematic measures may serve as a biomarker for understanding response to antipsychotic treatment.
Lower peak velocities and greater %NBM are associated with neuromotor slowing in patients taking antipsychotic medications.21 Thus, the pattern of results from this exploratory analysis indicate that patients who achieved at least 20% improvement from baseline in PANSS total score (or ≥2-point improvement in CGI-S score) by study week 4 may have exhibited impairments at baseline compared with those who did not achieve that treatment response, potentially including subclinical bradykinesia. It is important to note that these handwriting kinematic measures were previously demonstrated to be sensitive to subclinical neuromotor slowing not detected by traditional clinical observation.21 Results from several lines of research indicate that abnormal handwriting kinematics such as these are mediated by a striatal dopaminergic mechanism: decreased dopamine transporter density13 and increased D2 receptor occupancy by risperidone14 in the striatum are both correlated with handwriting movement impairment, and treatment with dopaminergic medications is associated with reductions in handwriting velocity and fluency in patients with Parkinson’s disease or schizophrenia.15,19,20 Therefore, our results may suggest that the patients who have an increased likelihood of achieving symptom improvement after 4 weeks of LAI antipsychotic treatment (as indicated by reduced peak velocity and increased %NBM) are those with reduced striatal dopaminergic tone at baseline.
Evidence from studies of handwriting in Parkinson’s disease15,35 and transcranial sonography in healthy individuals37 suggests that abnormal handwriting kinematics as reported here may reflect reduced striatal dopaminergic tone or hypodopaminergia. If lower peak velocities and greater %NBM measured prior to treatment do reflect striatal dopamine hypofunction, our findings would indicate that patients with a better response to AL or PP treatment may be those with reduced striatal dopaminergic tone prior to initiating pharmacotherapy. However, further work would be needed to explore this hypothesis, taking into account the differing proposed mechanisms of action of the active moieties of AL and PP.38 Importantly, handwriting kinematics may be a useful biomarker of striatal dopamine integrity and as such could provide insight into the therapeutic response to antidopaminergic drugs. Advances in the development of this tool may allow its wider use examining links between striatal dopaminergic function in schizophrenia and clinical response to antipsychotic treatment. Future innovation in the technology for collecting and analyzing handwriting kinematics might one day allow for its use in the clinic as a tool for monitoring dopaminergic function over the course of antipsychotic treatment to potentially inform treatment decisions.
This analysis had several limitations. Handwriting kinematic scores from ALPINE cannot be compared with published controls owing to methodologic differences. Because the study population was limited to those who met inclusion and exclusion criteria and completed the 4-week treatment period, these results may not generalize to all patients with schizophrenia, or to those treated with other antipsychotics. In this analysis, treatment response cannot necessarily be specifically attributed to LAI antipsychotic treatment because of the lack of a placebo control arm in the ALPINE study design. Furthermore, AL and PP treatment groups were pooled for this exploratory analysis as the analysis was not powered for between-group comparisons. Additionally, it is possible, if not likely, that the lower handwriting kinematic scores of patients who subsequently responded to LAI antipsychotic treatment reflect reduced striatal dopaminergic tone at baseline resulting from prior or recent antipsychotic treatment. To the extent that some patients entered the trial while not receiving antipsychotics while others were being treated with combinations of first- and second-generation antipsychotics, it is difficult to attribute the abnormal handwriting kinematic scores to antipsychotic burden. As shown in supplementary table 2, handwriting kinematic scores were similarly predictive of treatment response whether using the screening or study day 15 assessment as baseline. According to the study design, the washout period was to be completed no later than day 5 post-randomization, at which time all patients were initiated on a stable dose of an LAI antipsychotic. By day 15, variability related to prior D2-antagonizing medications was effectively eliminated.
As a proof-of-concept analysis, the goal of the present study was to identify a new avenue for future biomarker or response predictors of pharmacotherapy in schizophrenia. Secondary analyses of clinical response were not explored. For example, decomposing the PANSS total score into individual subscales would allow a more granular examination of handwriting kinematics as a predictor of response to positive symptoms, the primary target of antipsychotics, versus other symptoms. Treating clinical response as a dimensional variable might reveal stronger associations between handwriting kinematics and outcome. However, dichotomizing patients into responders and nonresponders for this exploratory analysis was consistent with design of the parent ALPINE trial. Future studies will be needed to further characterize the relationship between handwriting kinematics and clinical improvement with LAI antipsychotic treatment, including an analysis of changes in handwriting kinematics over time, testing relationships between clinical and motor changes, and an examination of the relationship between baseline handwriting motor scores and time to treatment response. Finally, although the current results support handwriting kinematics as a biomarker of striatal dopamine dysfunction, additional work also would be needed to ascertain possible links between handwriting kinematics as used in this study and antipsychotic-induced adverse effects such as extrapyramidal symptoms. Because sample sizes for patients with baseline severity ratings greater than 0 on traditional rating scales, such as the AIMS, SAS, or BARS, were small, we were unable to test whether baseline handwriting kinematics were associated with clinically observed extrapyramidal symptoms in the current analysis.
The current findings have both clinical and mechanistic implications. Clinically, replication of these results could enable early and targeted medication management for patients transitioning from inpatient to outpatient status. Specifically, motor assessment such as ours could predict therapeutic response to LAI antipsychotics, and possibly noninjectable antipsychotics, upon discharge. Our results also provide insight into the relationships between response to antipsychotic treatment and central regulation of neuromotor dysfunction. Interestingly, elucidating the role of neuromotor dysfunction in psychotic illness has emerged as a proposed modification of the National Institute of Mental Health research domain criteria framework.39–41
Conclusions
In the ALPINE study, baseline measures of handwriting kinematics assessed before antipsychotic initiation were associated with treatment response measured at 4 weeks. The pattern of baseline handwriting kinematics, with lower peak velocity and higher %NBM in responders versus nonresponders, suggests that patients who achieved either PANSS or CGI-S response after 4 weeks of AL or PP treatment had greater baseline striatal dopamine dysfunction relative to those with a poorer treatment response. The current results indicate that handwriting kinematics warrant further exploration as a biomarker for therapeutic response to antidopaminergic treatments for schizophrenia.
Supplementary Material
Acknowledgments
The authors thank Yangchun Du, PhD, and Isaac Zhao, ScM, of Alkermes, Inc., for statistical support.
Funding
This work was supported by Alkermes, Inc. (Waltham, MA, USA). Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, and Jane Phillips, PhD, of Peloton Advantage, LLC (Parsippany, NJ, USA), an OPEN Health company, and funded by Alkermes, Inc. The authors are entirely responsible for the scientific content of this article.
Disclosures
Michael P Caligiuri served as a paid consultant on the ALPINE study; he discloses no stock options/ownership in Alkermes, Inc. Peter J Weiden, Anna Legedza, and Amy Claxton are former employees of Alkermes, Inc., and may own stock options in the company. Sergey Yagoda is an employee of Alkermes, Inc., and may own stock options in the company.
Author Contributions
MPC, PJW, AL, SY, and AC made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work and the drafting or the critical revision of the paper for intellectual content. All authors approved the final version of the paper and its publication. Finally, all authors agreed to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. Washington, DC: American Psychiatric Association; 2019. [Google Scholar]
- 2.Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8:303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brink WJ, Palic S, Köhler I, de Lange ECM. Access to the CNS: biomarker strategies for dopaminergic treatments. Pharm Res. 2018;35:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A. positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–559. [DOI] [PubMed] [Google Scholar]
- 5.Nordstrom AL, Farde L, Wiesel FA, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227–235. [DOI] [PubMed] [Google Scholar]
- 6.Stern S, Linker S, Vadodaria KC, Marchetto MC, Gage FH. Prediction of response to drug therapy in psychiatric disorders. Open Biol. 2018;8:180031. Available at: https://royalsocietypublishing.org/doi/10.1098/rsob.180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16:505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75:65–75. [DOI] [PubMed] [Google Scholar]
- 9.Venkatasubramanian G, Rao NP, Arasappa R, Kalmady SV, Gangadhar BN. A longitudinal study of relation between side-effects and clinical improvement in schizophrenia: is there a neuro-metabolic threshold for second generation antipsychotics? Clin Psychopharmacol Neurosci. 2013;11:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A, Chakos M, Koreen A, et al. Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry. 1995;152:1724–1729. [DOI] [PubMed] [Google Scholar]
- 11.Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52:139–145. [DOI] [PubMed] [Google Scholar]
- 12.Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544–549. [DOI] [PubMed] [Google Scholar]
- 13.Hermann W, Eggers B, Barthel H, et al. Correlation between automated writing movements and striatal dopaminergic innervation in patients with Wilson’s disease. J Neurol. 2002;249:1082–1087. [DOI] [PubMed] [Google Scholar]
- 14.Regenthal R, Kunstler U, Hesse S, Sabri O, Preiss R. D2 dopamine receptor occupancy, risperidone plasma level and extrapyramidal motor symptoms in previously drug-free schizophrenic patients. Int J Clin Pharmacol Ther. 2005;43:370–378. [DOI] [PubMed] [Google Scholar]
- 15.Tucha O, Mecklinger L, Thome J, et al. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson’s disease. J Neural Transm. 2006;113:609–623. [DOI] [PubMed] [Google Scholar]
- 16.Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naïve adolescents at high risk for psychosis. J Vis Exp 2013;81:e50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuenstler U, Juhnhold U, Knapp WH, Gertz HJ. Positive correlation between reduction of handwriting area and D2 dopamine receptor occupancy during treatment with neuroleptic drugs. Psychiatry Res. 1999;90:31–39. [DOI] [PubMed] [Google Scholar]
- 18.Künstler U, Hohdorf K, Regenthal R, Seese A, Gertz HJ. Diminution of hand writing area and D2-dopamine receptor blockade. Results from treatment with typical and atypical neuroleptics. Nervenarzt 2000;71:373–379. [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr J. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Hum Mov Sci. 2009;28:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caligiuri MP, Teulings HL, Dean CE, Niculescu AB 3rd, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caligiuri MP, Teulings HL, Dean CE, Lohr JB. The nature of bradykinesia in schizophrenia treated with antipsychotics. Psychiatry Res. 2019;273:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiden PJ, Claxton A, Kunovac J, et al. Efficacy and safety of a 2-month formulation of aripiprazole lauroxil with 1-day initiation in patients hospitalized for acute schizophrenia transitioned to outpatient care: phase 3, randomized, double-blind, active control ALPINE study. J Clin Psychiatry. 2020;81:19m13207. [DOI] [PubMed] [Google Scholar]
- 23.Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2021. [Google Scholar]
- 24.Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2021. [Google Scholar]
- 25.Brissos S, Veguilla MR, Taylor D, Balanza-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. 2013. Available at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed January 12, 2022.
- 28.European Medicines Agency. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). E6 R1. Guideline for Good Clinical Practice. 2002. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002874.pdf. Accessed January 12, 2022.
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 30.Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2021. [Google Scholar]
- 31.Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:217–222. [Google Scholar]
- 32.Guy W. Abnormal involuntary movement scale (AIMS). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:534–537. [Google Scholar]
- 33.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 34.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 35.Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Hum Mov Sci. 2006;25:510–522. [DOI] [PubMed] [Google Scholar]
- 36.Thomas M, Lenka A, Kumar Pal P. Handwriting analysis in parkinson’s disease: current status and future directions. Move Disord Clin Pract. 2017;4:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange KW, Mecklinger L, Walitza S, et al. Brain dopamine and kinematics of graphomotor functions. Hum Mov Sci. 2006;25:492–509. [DOI] [PubMed] [Google Scholar]
- 38.Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walther S, Bernard JA, Mittal VA, Shankman SA. The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med. 2019;49:212–216. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Mental Health. Strategic Plan for Research. 2021. https://www.nimh.nih.gov/sites/default/files/documents/about/strategic-planning-reports/NIMH-Strategic-Plan-for-Research-2021-Update.pdf. Accessed January 5, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.