Abstract
Avocado tree wilt is a disease caused by Phytophthora cinnamomi Rands. Recently, this disease has been associated to Pythium amazonianum, another causal agent. Avocado tree wilt is being currently controlled with synthetic fungicides that kill beneficial microorganisms, polluting the environment and leading to resistance problems in plant pathogens. The current research work aims to provide alternative management using extracts from Proboscidea parviflora W. and Phaseolus lunatus L. to control the development of mycelia in P. amazonianum in vitro. Raw extracts were prepared at UAAAN Toxicology Laboratory, determining the inhibition percentages, inhibition concentrations and inhibition lethal times. Several concentrations of the plant extracts were evaluated using the poisoned medium methodology, showing that both extracts control and inhibit mycelial development, in particular P. lutatus, which inhibits mycelial growth at concentrations lower than 80 mg/L, being lower than P. parviflora extracts. These extracts are promising candidates for excellent control of Pythium amazonianum.
Keywords: plants, extracts, avocado, Phytophythium sp.
1. Introduction
Mexico is a global producer of avocado (Persea americana Mill.) and a consumer leader. There are 16,436,760 ha currently planted with avocado in the country, yielding an annual production of 10,686,454 tons. The states with higher levels of production are Michoacan, Jalisco, Estado de Mexico and Nayarit [1]. Among the most important diseases, avocado tree wilt caused by Phythopthora cinnamomi Rands is the main disease affecting this crop worldwide [2], besides having more than one thousand host plant species [3]. In 2016, Pythium amazonianum = Phytophytium sp. amazonianum [4,5,6] was reported for the first time as the causal agent of avocado wilt in Peribán Michoacán, Mexico. Molecular and morphological characterization describes Phytophytium mirpurense as a phylogenetically separate entity from Phythium and Phythopthora, since it is the only entity with internally proliferating papillate oogonia and cylindrical or lobed antheridia associated with the disease. In order to face the problems caused by this disease, avocado growers have been searching for management alternatives. However, synthetic fungicides provide poor control over this disease and the use of multiple applications leads to different problems like human toxicity [7,8,9], export rejects due to chemical residues, environmental damage [10] and impact on beneficial fauna [11]. Likewise, plant pathogens can develop resistance to active ingredients in synthetic fungicides, leading to efficiency problems and [12] the use of higher rates, or the development of new agricultural chemicals that are intended to replace those products to which fungi have shown resistance [13]. Due to the problems caused by the indiscriminate use of synthetic fungicides, it is necessary to develop natural alternatives to control fungal diseases. One of those strategies is the use of plant extracts, which are organic, biodegradable and harmless to human health and the environment [14]. However, the use of extracts is scarce due to the limited availability of products in the market, the difficulties in their formulation and the decrease in their efficacy in the field [15]. The use of plant extracts is an alternative for integrated crop management. Due to their low cost and their potential, plant extracts can be used to control and inhibit bacteria, as well as plant pathogenic fungi [16].
Furthermore, it is mentioned that Proboscidea parviflora W ethanolic and methanolic extracts have fungicidal effects that inhibit the growth of Aspergillus niger, Aspergillus flavus, Penicillium chrysogenum, Penicillium expansus, Fusarium poae and Fusarium moniliforme; although we do not know which metabolites are present in these species [17].
Proboscidea lousiana contains 47% triterpenes and 38% glucosiloxy-fatty acids [18]; phytoalexins have been found in Phaseolus vulgaris beans [19]. Phytoalexins have low molecular weight and are secondary metabolites of diverse chemical nature and antimicrobial activity, including terpenoids, alkaloids, glycosides and flavonoids, among others. These metabolites offer protection against pathogens and they can contribute directly to control of bacterial and plant pathogenic fungal growth. This research work seeks management alternatives, using highly effective inputs with lower environmental impact that can be part of Pythium amazonianum management practices. The aim was to prepare and assess the in vitro effect of Proboscidea parviflora W. and Phaseolus lunatus L. plant extracts on pathogens that affect avocado.
2. Materials and Methods
This research work was conducted at the Agricultural Parasitology Department of Universidad Autónoma Agraria Antonio Narro (UAAAN), Saltillo, Coah, Mexico.
2.1. Biological Material
The strain of Pythium amazonianum used in this experiment was isolated, purified and identified at UAAAN Toxicology Laboratory by [4]. The oomycete strains were white with aerial, cottony mycelium; microscopically the mycelium was hyaline, coenocytic, the oogonia with antheridia and spherical and globular sporangia.
2.2. Preparation of Assessed Extracts
Proboscidea parviflora W. was collected from Calera and Villa de Cos municipalities at Zacatecas, Mexico. The seed of Phaseolus lunatus L came from the growing region of San Miguel Totolapan municipality in Guerrero; these seeds were planted and cultivated under greenhouse conditions at UAAAN from October 2015 to January 2016. The extracts were prepared using different parts of the plant like roots, stems, leaves, flowers, green pods and seeds. The solvent was sterile distilled water, hexane and ethanol at 96%. A total of 200 g macerated plant material with 200 mL of sterile distilled water was mixed in a conventional blender (Oster). The samples were suspended in 800 mL of the solvent, obtaining a final volume of 1000 mL. All the preparations were stored in darkness at room temperature and they were stirred once a day for 30 days. After that time, the samples were filtered to separate the plant residues. The extracts were concentrated in a rotary evaporator (BUCHI R-200 Heathing Bath B-490, Marshall Scientific, Hampton, NH, USA) using the following temperatures: aqueous extracts at 90 °C, ethanolic extracts at 70 °C and hexanolic extracts at 60 °C. The samples of the aqueous macerated extracts were filtered. Raw extracts were kept in 30 mL amber-colored glass containers at 4 °C to preserve them.
2.3. Extracts’ ASSAY
Sixty-three extracts were evaluated using an in vitro bioassay technique (poisoned medium technique) with four replicates per dose. The raw extract was used to obtain the different concentrations. When PDA (BD Bioxon, Franklin Lakes, NJ, USA) reached a temperature between 35 °C and 40 °C, the extract quantities were added at the desired concentration. Explants with a diameter of 0.5 cm were introduced in sequence with the plant pathogens and they were incubated at 25 ± 2 °C in darkness (Lab-line Model 150, Barnstead, NH, USA) until the mycelia grew in the Petri dish that was used as a check test (PDA without extract), covering all the plate. Mycelial growth was measured every day with a digital vernier caliper and the readings were used to calculate the inhibition percentages, using the [20,21] formula:
| (1) |
2.4. Determination of Compounds in Extracts by Chromatography–Mass Spectrometry
The extracts with the greatest inhibitory effect were analyzed by chromatography and mass spectrometry in the Metabolomics and Mass Spectrometry Laboratory at LANGEBIO-CINVESTAV.
2.5. Analysis of the Results
The inhibition percentages obtained from the preliminary tests that were carried out to determine , and were analyzed using R statistical system, version 3.3.2 by Probit regression. On the other hand, the highest concentrations per extract (mg/L) obtained from the mycelial growth inhibiting percentages were studied by Probit regression using statistical system R, Version 3.3.2, in order to determine the mean lethal time.
3. Results
Sixty-three extracts from different parts of the plant (Table 1) were prepared. Eight of them showed an inhibitory effect over Pythium amazonianum at different concentrations.
Table 1.
Preparation methods of the different extracts of Proboscidea parviflora W. and Phaseolus lunatus L.
| Plant | Part of Plant | Solvent | Rest and Agitation Time | Temperature |
|---|---|---|---|---|
| Proboscidea parviflora W. | Root, Stem, Leave, Flower and Green pods. | Water | 30 days | 90 °C |
| Ethanol | 30 days | 70 °C | ||
| Hexane | 30 days | 60 °C | ||
| Aqueous filtered | 30 days | 0 °C | ||
| Ethanolic Filtered |
30 days | 0 °C | ||
| Hexanolico Filtered |
30 days | 0 °C | ||
| Aqueous macerated | 0 days | 0 °C | ||
| Phaseolus lunatus L. | Root, Stem, Leave and Seeds. | Water | 30 days | 90 °C |
| Ethanol | 30 days | 70 °C | ||
| Hexane | 30 days | 60 °C | ||
| Aqueous filtered | 30 days | 0 °C | ||
| Ethanolic Filtered |
30 days | 0 °C | ||
| Hexanolico Filtered |
30 days | 0 °C | ||
| Aqueous macerated | 0 days | 0 °C |
The preliminary test to determine the inhibitory concentrations (Ci) of the extracts included a biological window per extract (0.05, 0.1, 1, 10, 20, 40, 60, 80, 100, 400, 800, 1000, 2000, 6000 and 10,000 mg/L). Eight extracts showed inhibiting effects at the following rates: ethanolic of flower of P. parviflora (E.F.P.p), 2000, 2104.75 and 2265.76 mg/L; ethanolic of root of P. parviflora (E.R.P.p), 211.59, 691.6 and 1000 mg/L; aqueous filtered of green Pod of P. parviflora (F.A.V.v.P.p), 1, 2 and 4 mg/L; hexane of green pod of P. parviflora (H.V.v.P.p), 264.62, 725.67 and 2935 mg/L; aqueous filtered of leaves of P. lunatus (F.A.H.P.l), 0.05, 0.5 and 1 mg/L; aqueous filtered of seed of P. lunatus (F.A.S.P.l), 0.05, 0.1 and 1 mg/L; aqueous filtered of stem of P. lunatus (F.A.T.P.l), 10, 20 and 60 mg/L; and aqueous macerated of seed of P. lunatus (M.A.S.P.l), 0.1, 1 and 2 mg/L. Sixty-three extracts from different parts of the plant (Table 1) were prepared. Eight of them showed an inhibitory effect over Pythium amazonianum at different concentrations.
P. lunatus extracts F.A.H.P.l, F.A.S.P.l and F.A.T.P.l y M.A.S.P.l (Table 2) showed better inhibition rates. At concentrations of 0.05 mg/L (F.A.H.P.l y F.A.S.P.l), they inhibited 11.9 and 11.26% of P. amazonianum mycellian growth and, at 2 mg/L (M.A.S.P.l), they inhibited 100%, except in the case of F.A.T.P.l, where concentrations greater than 60 mg/L were required to inhibit 93.3% of the pathogen’s growth. None of these extracts exceeded 100 mg/L in concentration. P. parviflora extracts (F.A.V.v.P.p) had a behavior similar to the behavior of P. lunatus extracts, presenting an inhibiting effect at low concentrations (1, 2 and 4 mg/L), controlling mycelial growth at 27.4, 70.33 and 74.73%. E.R.P.p controlled 100% at 1000 mg/L, while E.F.P.p and H.V.v.P.p required concentrations over 1400 mg/mL to inhibit 100% of the pathogen’s mycelial growth. The inhibition percentage results differ from the data reported by [16]. Their research reported growth inhibition percentages (86.6%) of Fusarium poae under the ethanolic effects of P. parviflora prepared at 6% concentration. These results explain the low concentration requirements, without neglecting the fact that F. poae belongs to true deuteromycets fungi, with ketin cell walls (N-acetylglucosamine polymer), whereas the wall in oomycetes is made of β-1,3 y β-1,6 glycans [22].
Table 2.
Inhibitory effect (%) over Pythium amazonianum mycelial growth of Proboscidea parviflora W. and Phaseolus lunatus L. plant extracts after 120 h.
| Extracts | Concentration mg/L | Inhibition % | Standard Deviation |
|---|---|---|---|
| E.F.P.p | 2000 | 2.14 | 1.60 |
| 2104.75 | 62.09 | 11.27 | |
| 2265.76 | 100 | 0 | |
| E.R.P.p | 211.59 | 17 | 7.38 |
| 691.6 | 52.2 | 14.61 | |
| 1000 | 100 | 0 | |
| F.A.V.v.P.p | 1 | 27.4 | 9.70 |
| 2 | 70.33 | 3.53 | |
| 4 | 74.73 | 2.11 | |
| H.V.v.P.p | 264.62 | 14.56 | 8.09 |
| 725.67 | 57.93 | 15.27 | |
| 2935 | 100 | 0 | |
| F.A.H.P.l | 0.05 | 11.9 | 2.91 |
| 0.5 | 63.86 | 0.86 | |
| 1 | 78.33 | 2.35 | |
| F.A.S.P.l | 0.05 | 11.26 | 2.21 |
| 0.1 | 25.36 | 6.08 | |
| 1 | 75.03 | 0.96 | |
| F.A.T.P.l | 10 | 80.7 | 5.00 |
| 20 | 83.43 | 2.52 | |
| 60 | 93.3 | 0.38 | |
| M.A.S.P.l | 0.1 | 13.7 | 4.66 |
| 1 | 47.03 | 9.43 | |
| 2 | 100 | 0 |
E.F.P.p: ethanolic of flower of P. parviflora; E.R.P.p: ethanolic of root of P. parviflora; F.A.V.y.P.p: aqueous filtered of green pod of P. parviflora; H.V.v.P.p: hexane of green pod of P. parviflora; F.A.H.P.1: aqueous filtered of leaves of P. lunatus; F.A.S.P.1: aqueous filtered of seed of P. lunatus; F.A.T.P.1: aqueous filtered of stem of P. lunatus; M.A.S.P.1: aqueous macerated of seed of P. lunatus.
P. parviflora extracts (E.F.P.p, E.R.P.p and H.V.v.P.p) showed a lethal time of 50% inhibition over the growth of P. amazonianum within the first 19.57 h, reaching 95% inhibition after 116.10 h. F.A.V.v.P.p inhibited 50% after 95.37 h and 95% after 258.86 h. P. lunatus extracts (F.A.T.P.l, F.A.H.P.l and F.A.S.P.l) showed a similar effect to F.A.V.v.P.p but required a longer time to inhibit 50% of the mycelial growth, opposite to M.A.S.P.l, which, just like E.F.P.p, E.R.P.p and H.V.v.P.p, required less time to control 50% of the mycelial growth. We observed that higher rates of the plant extracts inhibited more than 50% of the mycelial growth during the first 24 h, except M.A.S.P.l, which has the same effect at low concentrations (Table 3).
Table 3.
Mean lethal time (TL50) of Pythium amazonianum inhibition under the influence of Proboscidea parviflora W. and Phaseolus lunatus L. extracts.
| Extracts | Df | Tl50 | Ltl | Utl | Tl05 | Tl95 | Intercept | Slope | p Value |
|---|---|---|---|---|---|---|---|---|---|
| E.F.P.p | 19 | 19.57 | 17.87 | 21.20 | 3.30 | 116.10 | −2.74 | 2.12 | 2.02 × 10−14 |
| E.R.P.p | 19 | 19.57 | 17.87 | 21.20 | 3.30 | 116.10 | −2.74 | 2.12 | 2.029 × 10−14 |
| F.A.V.v.P.p | 19 | 95.37 | 91.50 | 99.76 | 35.13 | 258.86 | −7.50 | 3.79 | 7.31 × 10−84 |
| H.V.v.P.p | 19 | 19.57 | 17.87 | 21.20 | 3.30 | 116.10 | −2.74 | 2.12 | 2.02 × 10−14 |
| F.A.H.P.l | 19 | 62.94 | 59.70 | 66.31 | 11.57 | 342.22 | −4.02 | 2.23 | 7.66 × 10−90 |
| F.A.S.P.l | 19 | 72.63 | 69.89 | 75.53 | 15.79 | 333.88 | −4.62 | 2.48 | 2.535 × 10−15 |
| F.A.T.P.l | 19 | 28.29 | 26.57 | 29.93 | 3.88 | 205.97 | −2.76 | 1.90 | 8.50 × 10−19 |
| M.A.S.P.l | 19 | 19.57 | 17.87 | 21.20 | 3.30 | 116.10 | −2.74 | 2.12 | 2.02 × 10−14 |
Df: degrees of freedom, TL: lethal time, LTL: lower time limit and UTL: upper time limit. E.F.P.p: ethanolic of flower of P. parviflora; E.R.P.p: ethanolic of root of P. parviflora; F.A.V.y.P.p: aqueous filtered of green pod of P. parviflora; H.V.v.P.p: hexane of green pod of P. parviflora; F.A.H.P.1: aqueous filtered of leaves of P. lunatus; F.A.S.P.1: aqueous filtered of seed of P. lunatus; F.A.T.P.1: aqueous filtered of stem of P. lunatus; M.A.S.P.1: aqueous macerated of seed of P. lunatus.
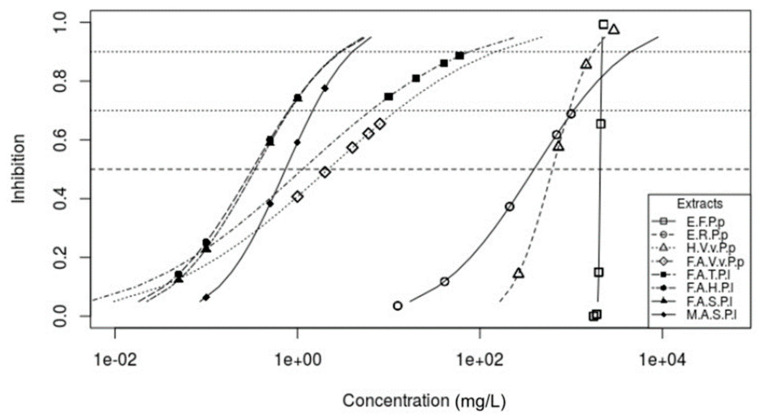
At , and P. parviflora extracts showed the following behavior: E.F.P.p showed an inhibitory effect within the concentrations proposed by Probit assay (Table 4), coinciding with the inhibition percentages (Table 1). According to Probit regression curve displacement (Figure 1), there is no need to exceed 2200 mg/L to inhibit 90% of the pathogen’s mycelial growth. H.V.v.P.p behaved similarly, without exceeding those concentrations. However, according to Probit analysis, E.R.P.p and F.A.V.v.P.p (Table 4) required slightly higher rates to achieve similar inhibiting effects. On the other hand, P. lunatus extracts (F.A.H.P.l, F.A.S.P.l, F.A.T.P.l and M.A.S.P.l) are similar to P. parviflora extracts (E.R.P.p and F.A.V.v.P.p), where Probit analysis (Table 4) suggested using slightly higher concentrations without exceeding 100 mg/L. Regarding our inhibition concentration estimations, we agree with the proposal of [23], who mentioned that, using a by-product of the agricultural industry (wine vinasse) at concentrations of 50 and 70 mg/L, it is possible to inhibit 100% of Pythium aphanidermatum and Phytophthora parasitica growth. Likewise, [24] studied a methanolic extract of Origanum mejorana L. at 8000 mg/L and a synthetic product (Metalaxyl at 750 mg/L) to inhibit Phytophthora infestans at 100%.
Table 4.
Inhibitory concentrations (, and ) of Proboscidea parviflora W. and Phaseolus lunatus L. extracts over Pythium amazonianum mycelial growth.
| EXTRACT | Ci | Concentration mg/L | Lower Fiducial Limits 95% | Upper Fiducial Limits 95% |
|---|---|---|---|---|
| E.F.P.p | 2075.19 | 2034.05 | 2117.33 | |
| 2114.29 | 2076.48 | 2172.06 | ||
| 2172.05 | 2126.83 | 2266.83 | ||
| E.R.P.p | 391.65 | 231.32 | 766.67 | |
| 1062 | 575.68 | 3074 | ||
| 4483 | 1819 | 26939 | ||
| F.A.V.v.P.p | 2.16 | 0.38 | 3.89 | |
| 12.14 | 5.89 | 909.51 | ||
| 146.32 | 26.82 | 2716 | ||
| H.V.v.P.p | 622.12 | 516.39 | 734.22 | |
| 948.19 | 803.43 | 1142 | ||
| 1742 | 1411 | 2330 | ||
| F.A.H.P.l | 0.32 | 0.28 | 0.36 | |
| 0.79 | 0.68 | 0.95 | ||
| 2.98 | 2.32 | 4.04 | ||
| F.A.S.P.l | 0.34 | 0.30 | 0.38 | |
| 0.82 | 0.70 | 0.97 | ||
| 2.89 | 2.27 | 3.84 | ||
| F.A.T.P.l | 1.13 | 0.21 | 2.56 | |
| 6.33 | 2.93 | 9.46 | ||
| 75.75 | 52.53 | 150.75 | ||
| M.A.S.P.l | 0.73 | 0.50 | 1.10 | |
| 1.47 | 0.99 | 2.81 | ||
| 3.97 | 2.23 | 12.78 |
Ci (50, 70, 90)—inhibitory concentration in mg/L; E.F.P.p: ethanolic of flower of P. parviflora; E.R.P.p: ethanolic of root of P. parviflora; F.A.V.y.P.p: aqueous filtered of green pod of P. parviflora; H.V.v.P.p: hexane of green pod of P. parviflora; F.A.H.P.1: aqueous filtered of leaves of P. lunatus; F.A.S.P.1: aqueous filtered of seed of P. lunatus; F.A.T.P.1: aqueous filtered of stem of P. lunatus; M.A.S.P.1: aqueous macerated of seed of P. lunatus.
Figure 1.
Probit regression depicting the inhibition of Pythium amazonianum mycelial growth under the effects of Proboscidea parviflora W. and Phaseolus lunatus L. Inhibition vs. Concentration.
The extracts with the highest inhibitory effect were F.A.S.P.l and H.V.v.v.P.p, which were analyzed by a positive and negative mode scanning system using chromatography–mass spectrometry (Table 5 and Table 6).
Table 5.
Determination of compounds present in the aqueous filtered extract of P. lunatus L. (F.A.S.P.l) seed in positive mode and negative.
| POSITIVE MODE | NEGATIVE MODE | |||||
|---|---|---|---|---|---|---|
| COMPOUND | CHEMICAL FORMULA | RETENTION TIME (min) |
COMPOUND | CHEMICAL FORMULA | RETENTION TIME (min) |
|
| A | Cycasin | C8H16N2O7 | 1.21 | 1-[(5-Amino-5-carboxypentyl)amino]-1-deoxyfructose | C12H24N2O7 | 1.21 |
| B | Asparaginyl-Glycine | C6H11N3O4 | 16.36 | Glutamyl-Asparagine | C9H14N3O6- | 0.83 |
| C | Avocadene 2-acetate | C19H36O4 | 20.73 | 1,3-Octadiene | C8H14 | 0.87 |
| D | Gabapentin | C9H17NO2 | 23.98 | Estriol-3-glucuronide | C24H32O9 | 1.93 |
| E | Pentadecanoylglycine | C17H33NO3 | 30.34 | Nebularine | C10H12N4O4 | 2.00 |
| F | Phosphoglycolic acid | C2H5O6P | 0.75 | Imidazolelactic acid | C6H8N2O3 | 3.09 |
| G | Biphenyl | C12H10 | 26.86 | |||
| H | (S)-Isosclerone | C10H10O3 | 0.87 | |||
Table 6.
Chromatography and mass spectrometry positive and negative mode of the aqueous filtered extract of P. lunatus L. (F.A.S.P.l) seed.
| POSITIVE MODE | NEGATIVE MODE | |||||
|---|---|---|---|---|---|---|
| COMPOUND | CHEMICAL FORMULA | RETENTION TIME (min) |
COMPOUND | CHEMICAL FORMULA | RETENTION TIME (min) |
|
| A | Dimethindene | C20H24N2 | 11.30 | Cyclocurcumin | C21H20O6 | 11.09 |
| B | 1-Hydroxyacorenone | C15H22O3 | 4.96 | (S)-Reticuline | C19H23NO4 | 1.30 |
| C | Geranylacetone | C13H22O | 11.24 | Austdiol | C12H12O5 | 10.98 |
| D | Dehydroisochalciporone | C16H19NO | 11.61 | 2-Isopropyl-1,4-hexadiene | C9H16 | 0.87 |
| E | 2-Methylphenyl 2-methylpropanoate | C11H14O2 | 11.76 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid | C21H34NO3+ | 16.86 |
| F | Valyl-Hydroxyproline | C10H18N2O4 | 11.80 | N-Methylschinifoline | C16H17NO2 | 20.70 |
| G | Unknown | C12H16N2O4 | 11.80 | |||
| H | Unknown | C10H17O5- | 12.14 | |||
4. Conclusions
Only eight extracts showed significant inhibitory effect. P. lunatus showed out-standing control of the plant pathogen at concentrations lower than 80 mg/L, while higher concentrations of P. parviflora were required. In regards to mean lethal time, P. parviflora extracts required less time to produce inhibitory effects, while P. lunatus extracts required longer for producing antifungal response. These extracts are promising candidates for excellent control of Pythium amazonianum; however, it is suggested to continue evaluating these extracts for the control of various phytopathogenic fungi and oomycetes.
Author Contributions
All authors have contributed equally to the conduct of the research and the writing of this research paper. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
Author Antonio Orozco Plancarte is involved as paid employees in CULTA S. A. de C. V. Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.SIAP Sistema de Información Agroalimentaria y Pesquera. Situación Actual de Aguacate en México. [(accessed on 6 March 2024)];2023 Available online: http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/AvanceNacionalCultivo.do.
- 2.Telíz O.D., Mora A.A. El Aguacate y su Manejo Integrado Segunda Edición. Editorial Mundi Prensa; Bogotá, Colombia: 2007. pp. 192–202. [Google Scholar]
- 3.Hardman R.A. Pathogen profile Phytophthora cinnamomi. Mol. Plant Pathol. 2005;6:589–604. doi: 10.1111/J.1364-3703.2005.00308.X. [DOI] [PubMed] [Google Scholar]
- 4.Hernández A., Ochoa Y.M., Cerna E., Delgado J.C., Beltrán M., Flores U., Tapia L.M. Pythium sp. amazonianum como agente causal de la tristeza del aguacate en Peribán, Michoacán. Memoria de congreso. Rev. Mex. De Fitopatol. 2016;34:112. [Google Scholar]
- 5.Spies C.F.J., Mazzola M., Botha W.J., Rijstd M., Mostert L., Mcleod A. Oogonial biometry and phylogenetic analyses of the Pythium vexans species group from woody agricultural hosts in South Africa reveal distinct groups within this taxon. Fungal Biol. 2010;115:157–168. doi: 10.1016/j.funbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Robideau G.P., De Cock A.W.A.M., Coffey M.D., Voglmayr H., Brouwer H., Bala K., Chitty D.W., De Saulniers N., Eggertson Q.A., Gachon C.M.M., et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011;11:1002–1011. doi: 10.1111/j.1755-0998.2011.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Cock A.W.A.M., Lodhi A.M., Rintoul T.L., Bala K., Robideau G.P., Gloria Z., Coffey M.D., Shahzad S., Lévesque C.A. Phytopythium: Molecular phylogeny and systematics. Persoonia. 2015;34:25–39. doi: 10.3767/003158515X685382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whalen M.M., Wilson S., Gleghorn C., Loganathan B.G. Brief exposure to triphenyltin produces irreversible inhibition of the cytotoxic function of human natural killer cells. Environ. Res. 2003;92:213–220. doi: 10.1016/S0013-9351(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 9.Paulitz T., Bélanger R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001;39:103–133. doi: 10.1146/annurev.phyto.39.1.103. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez M.R., Jacobo J.L. Impacto ambiental del uso de plaguicidas en huertos de manzano del noroeste de Chihuahua, México. Rev. Mex. De Fitopatol. 2002;20:168–173. [Google Scholar]
- 11.Anderson B.S., Hunt J.W., Phillips B.M., Nicely P.A., Vlaming V., Connor V., Richard N. Integrated assessment of the impacts of agricultural drainwater in the Salinas River (California, USA) Environ. Pollut. 2003;3:525–532. doi: 10.1016/s0269-7491(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 12.Chapagain B.P., Wiesman Z., Lahkim L.T. In vitro study of the antifungal activity of saponin-rich extracts against prevalent phytopathogenic fungi. Ind. Crops Prod. 2007;26:109–115. [Google Scholar]
- 13.Bajwa R., Kjalidy A., Cheema T.S. Antifungal Activity of Allelopathic Plant Extracts III: Growth Response of Some Pathogenic Fungi to Aqueous Extract of Parthenium hysterophorus. Plant Pathol. J. 2003;2:145–156. doi: 10.3923/ppj.2003.145.156. [DOI] [Google Scholar]
- 14.Bravo L.L., Bermúdez T.K., Montes B.R. Inhibición de Fusarium moniliforme mediante polvos vegetales y algunos de sus componentes químicos. Manejo Integr. De Plagas. 2000;57:29–34. [Google Scholar]
- 15.Castillo-Reyes F., Castillo-Quiroz D., Muñoz-Flores H.J., Sánchez A.R. Uso de bio-pesticidas de origen vegetal en el manejo de enfermedades de cultivos en Mexico. Mitigación Del Daño Ambient. Agroaliment. Y For. De México. 2018;4:107–121. [Google Scholar]
- 16.Maselli P.A.G., Rosales L.G., Guevara Y. Uso de extractos vegetales sobre Xanthomonas phaseoli, causante de la quemazón en Phaseolus vulgaris L. Rev. Digit. CENIAP HOY. 2006;12:1690–4117. [Google Scholar]
- 17.Tequida M., Cortez M., Rosas E.C., López S., Corrales C. Efecto de extractos alcohólicos de plantas silvestres sobre la inhibición de crecimiento de Aspergillus flavus, Aspergillus niger, Penicillium chrysogenum, Penicillium expansum, Fusarium moniliforme y Fusarium poae. Rev. Iberoam. De Micol. 2002;19:84–88. [PubMed] [Google Scholar]
- 18.Asai T., Sakai T., Ohyama K., Fujimoto Y. n-Octyl α-L-Rhamnopyranosyl-(1→2) –β-D-glucopyranoside Derivaties from the Glandular Trichome Exudate of Geranium carolinianum. Chem. Pharm. Bull. 2011;59:747–752. doi: 10.1248/cpb.59.747. [DOI] [PubMed] [Google Scholar]
- 19.Torres L.D. Bachelor’s Thesis. Pontificia Universidad Javeriana Facultad de Ciencias Microbiologia Industrial Bogotá; Bogotá, Colombia: 2010. Detección de fitoalexinas en plantas de fríjol (Phaseolus vulgaris) en respuesta a inoculación con aislamientos de actinomicetos. [Google Scholar]
- 20.Ochoa Y.M., Cerna E., Landeros J., Hernández S., Delgado J.C. Evaluación in vitro de la actividad antifúngica de cuatro extractos vegetales metanólicos para el control de tres especies de Fusarium spp. Phyton. 2012;81:69–73. [Google Scholar]
- 21.William E., Niklaus J.G. Introducción a los oomicetes. Plant Health Instructor. 2010;12 doi: 10.1094/PHI-I-2012-0220-01. [DOI] [Google Scholar]
- 22.Rodríguez A., Morales D., Ramírez M.A. Efecto de extractos vegetales sobre el crecimiento in vitro de hongos fitopatógenos. Instituto Nacional de Ciencias Agrícolas La Habana, Cuba. Cultiv. Trop. 2000;21:79–82. [Google Scholar]
- 23.Milagrosa V.N., Vicente N., Diánez F., De Cara M., Tello J.C. Vinazas y hongos del suelo. Agroecología. 2007;2:39–45. [Google Scholar]
- 24.Gamboa R., Hernández F.D., Guerrero E., Sánchez A., Lira R.H. Inhibición del crecimiento micelial de Rhizoctonia solani Kühn y Phytophthora infestans Mont. (De Bary) con extractos vegetales metanólicos de hojasén (Flourensia cernua DC.), mejorana (Origanum majorana L.) y trompetilla [Bouvardia ternifolia (Ca.) Schlecht.] Rev. Mex. De Fitopatol. 2003;21:13–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.