Abstract
A human lymphoid cell line (F172-D8) excreting a human immunodeficiency virus type 1 (HIV-1) anti-gp41 monoclonal antibody was used to construct a plasmid containing the cDNA of the single-chain variable fragment (scFvD8) corresponding to this antibody. A stable human osteosarcoma cell line was obtained which expressed the scFvD8 protein in the cytoplasm. Whereas a cell line transfected with a control construct (pCI-neo) was readily and productively infected with laboratory (Ba-L) or primary HIV-1 isolates, the scFvD8 cell line did not support productive infection. Binding of the virus, internalization, and reverse transcription were not altered by scFvD8 expression, but gp160 expression was dramatically reduced. These data suggest that cytoplasmic expression of this artificial single-chain antibody can interfere with gp160 expression, thereby reducing the production of mature viral envelope proteins.
The gene therapeutic approach involving intracellular immunization (for reviews, see references 20 and 29) has been used extensively to inhibit human immunodeficiency virus type 1 (HIV-1) replication by using single-chain variable-fragment (scFv) or Fab antibody fragments directed against regulatory viral proteins such as Rev (9, 10, 11, 12, 13, 14, 32) or Tat (22, 23), enzymatic viral proteins like integrase (1, 15, 19, 27) or reverse transcriptase (26, 30), and structural viral proteins like p17 (31) or various epitopes of the envelope proteins (3, 4, 5, 21, 33). It can be a useful strategy, as it does not involve the production of complete antibodies in the extracellular environment. Antibody fragments lacking the Fc fragment are generally produced and retained inside the cell, avoiding the phenomenon of antibody-dependent enhancement or Fc receptor-mediated antibody-dependent enhancement in HIV-1 infection. Such a strategy applied to gp41 may be useful for abolishing the normal maturation of the transmembrane protein and consequently disturbing the formation of the virion envelope inside the cell before budding of the viral particle. As well as gp120, gp41 is crucial for HIV entry into cells but is subject to lower genetic variability (24, 28) and therefore constitutes a target of choice for intracellular immunization.
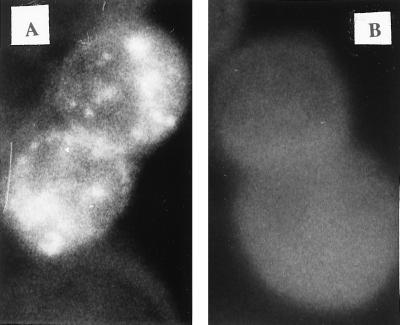
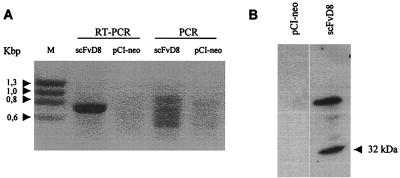
The single-chain antibody scFvD8 was derived from the parental F172-D8 cell line immortalized with Epstein-Barr virus and excreting an anti-gp41 monoclonal antibody directed against an extracellular conserved epitope (residues 609 to 620) of HIV-1 transmembrane gp41. scFvD8 cDNA was first cloned into prokaryotic vector pCANTAB5E in frame with the E-tag sequence and was expressed in Escherichia coli in accordance with the manufacturer's recommendations (Recombinant Phage Antibody System Mouse scFv Module; Pharmacia Biotech, Orsay, France) with the use of human primers derived from D8 cDNA sequences already published (6, 7). scFvD8 construction from one prokaryotic clone, positive in a gp160 MN/LAI (gift from Pasteur Mérieux Connaught, Lyon, France) enzyme-linked immunosorbent assay (ELISA), was then subcloned with the E-tag sequence into pCI-neo under control of the cytomegalovirus promoter and a Kozak sequence to ensure a high level of expression (16, 17). After verification of the sequence of the insert on both strands, as well as the ability of the construct to express the full scFvD8 fragment in an in vitro transcription-coupled translation assay (data not shown), the recombinant plasmid was used to transfect (ExGen 500; Euromedex, Souffelweyersheim, France) a human osteosarcoma (HOS) cell line expressing the CD4 receptor and CCR-5 coreceptor of HIV-1 (8, 18). In parallel, HOS cells were transfected under the same conditions with the vector pCI-neo lacking an insert. Expression and location of the scFvD8 protein within transiently transfected cells were determined by immunofluorescence on trypsinized and fixed cells coated with the anti-E-tag antibody (Pharmacia Biotech) and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (DAKO, Trappes, France) as the secondary antibody for staining. As shown in Fig. 1A, the cytoplasm of scFvD8 cells was strongly stained. After dilution cloning of the scFvD8-transfected cells in neomycin (500 μg/ml; Life Technologies, Cergy Pontoise, France), one stable cellular clone (scFvD8 cell line) was selected which was strongly positive when tested for the presence of scFvD8 mRNA by reverse transcription (RT-PCR; Titan One Tube RT-PCR System; Roche Diagnostics, Meylan, France) with the primers used to amplify the scFv construct (Fig. 2A). PCR was negative in the absence of RT, as was RT-PCR on a pCI-neo cell line. The scFvD8 protein was found in the lysate of the scFvD8 cell line by Western blotting using the anti-E-tag antibody and a secondary anti-mouse immunoglobulin G conjugated to horseradish peroxidase (Biosys, Compiègne, France) for detection with chemiluminescent reagents (Covalight; Valbiotech, Paris, France) (Fig. 2B). A higher-molecular-weight band was also visible which could correspond to the expression of a dimeric form of the scFvD8 protein. The antibody fragment was not detected in scFvD8 cell culture supernatants (data not shown).
FIG. 1.
Indirect immunofluorescent staining for scFvD8 protein expression in transiently transfected HOS cells. Panels: A, scFvD8-transfected cells; B, pCI-neo-transfected cells. Magnification, ×4,000.
FIG. 2.
(A) RT-PCR analysis of scFv mRNA in transfected cells. Lanes: M, HaeIII-digested φX174 replicative-form DNA (molecular size marker); RT-PCR, RT-PCR carried out on total RNA extracted from scFvD8 or pCI-neo cells; PCR, amplification of the same total RNA samples without the initial RT step. The expected size of the correctly amplified scFvD8 fragment is 765 bp. (B) Expression of scFvD8 protein in lysates of stably transfected HOS cells. The Western blot assay used an anti-E-tag antibody.
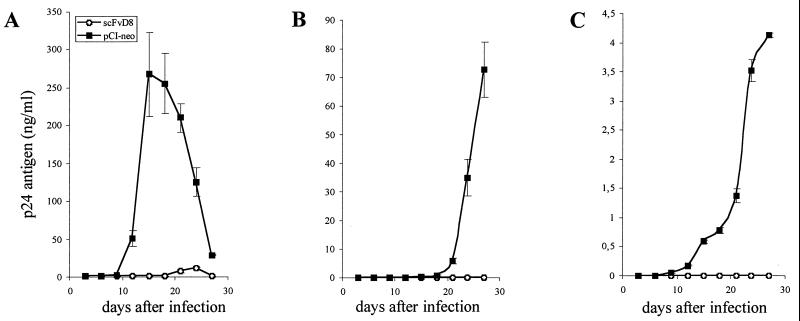
In order to see whether the expression of scFvD8 renders the HOS cell line resistant to HIV-1 replication, we infected the scFvD8 or pCI-neo cell line with HIV-1 Ba-L at a multiplicity of infection (MOI) of 0.0001 titrated on HOS cells. As shown in Fig. 3A, HIV-1 Ba-L infection was strongly inhibited in HOS cells expressing scFvD8, as measured (and previously described [2]) by the level of HIV-1 p24 in culture supernatants. Infection of the pCI-neo cell line was detectable by ELISA 12 days after infection and was maximal on day 15 (268 ± 55 ng of p24 per ml). Detectable infection of the scFvD8 cell line was delayed, with the maximal concentration of p24 reaching only 12 ± 1 ng/ml on day 24. Infection of scFvD8 cells with two different primary HIV-1 isolates, 91US656 (MOI of 0.01) and 92US060 (MOI of 0.003), supplied by the National Institutes of Health was also strongly inhibited, as shown in Fig. 3B and C. The infection was less efficient than that with laboratory strain Ba-L; however, the initial viral load was higher. Primary isolates are often less infectious in vitro than adapted laboratory strains at the same dose. A p24 maximum of 73 ± 9.5 ng/ml was measured in the supernatant of the pCI-neo cell line 27 days after infection with 91US656, and 4 ± 0.04 ng/ml was measured after infection with 92US060. The inhibition of infection in the scFvD8 cell line was complete, as no p24 was detectable in the supernatants after 30 days of infection with the two primary B isolates. However, the parental antibody F172-D8 itself is not neutralizing in vitro. A recent study has reported a similar phenomenon for an scFv directed against gp41 which was not neutralizing in vitro but was able to neutralize different strains of HIV-1 when expressed intracellularly (33). These results confirm that the mechanisms of intracellular and extracellular neutralization are distinct.
FIG. 3.
Inhibition of HIV-1 infection of the scFvD8 cell line as measured by p24 Gag protein levels in supernatants. (A) Infection with laboratory strain Ba-L at an MOI of 0.0001. (B) Infection with primary isolate 91US656 (MOI, 0.01). (C) Infection with primary isolate 92US060 (MOI, 0.003). The values represent the means and standard deviations of triplicate determinations.
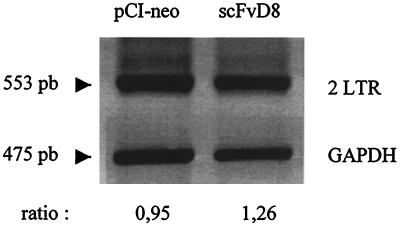
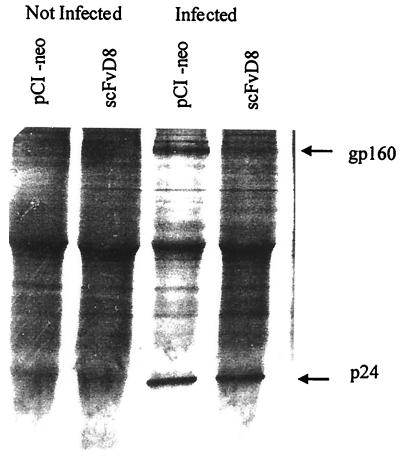
To determine if the very early steps of infection were altered by intracellular scFvD8 expression, amplification of the double long terminal repeat (LTR) circular form of viral DNA was performed as previously described (2) on pCI-neo- or scFvD8-transfected cell lines 24 h after infection with a 10-fold higher dose of HIV-1 strain Ba-L (MOI, 0.001) than those neutralized by scFvD8 in order to clearly follow these very early steps of viral maturation. Levels of the HIV-1 double LTR circular form were quite similar in cell lines expressing scFvD8 or transfected with pCI-neo alone, as shown in Fig. 4. These results suggest that the preceding steps of viral binding, penetration of the cell by the core, and reverse transcription were not altered. An immunoprecipitation assay was performed in order to determine whether production of the gp160 protein, to which the parental F172-D8 antibody binds strongly, was affected by the expression of scFvD8 in the cytoplasm of the infected cell line. The scFvD8 and pCI-neo cell lines were infected with an MOI of 0.0001 of HIV-1 strain Ba-L, and after 15 days in culture (the peak of infection in the pCI-neo cell line), they were starved in cysteine-free medium for 2 h and then radiolabeled for 24 h with 25 μCi of [35S]cysteine per million cells. The cell lysates were immunoprecipitated as described previously (2) with strongly anti-HIV-1 gp160-positive and anti-HIV-1 p24-positive serum (gift from Christine Cartier) diluted 100 times. As shown in Fig. 5, the two cell lines (pCI-neo and scFvD8) produced equivalent amount of p24 intracellularly but gp160 was not visible in the scFvD8 cell line after infection. Other viral proteins were not clearly immunoprecipitated with that serum. A Western blot assay using the F172-D8 antibody also showed strong inhibition of gp160 and gp41 expression in scFvD8 cell line lysate compared with that of the control cell line, as when they were infected with a 10-fold higher dose of the strain Ba-L virus (data not shown). Contrary to previous studies in which HIV-1 anti-envelope scFv antibodies were targeted to the endoplasmic reticulum of transfected cells (5, 21, 33) and interfered with gp41 or gp120 expression, scFvD8 produced in the cytoplasm of HOS cell interfered with gp160 precursor expression. Gag protein p24 seemed to be retained in the cytoplasm of scFvD8-transfected cells and not secreted in the supernatant, as no p24 was detected by ELISA 15 days after infection, but equal amounts of p24 were immunoprecipitated in the scFvD8 and pCI-neo cell lines.
FIG. 4.
Amplification of the HIV-1 double LTR circular DNA form in scFvD8 or pCI-neo cells 24 h after infection with HIV-1 strain Ba-L (MOI, 0.001). Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was done in parallel. Nonsaturated signals were analyzed with Molecular Analysis software. The ratios of the nonsaturated signals of the double LTR to the GAPDH PCR bands are given.
FIG. 5.
Immunoprecipitation of pCI-neo or scFvD8 cell lysates with anti-HIV-1 gp160-positive and anti-HIV-1 p24-positive serum. Cells were not infected or infected with strain Ba-L at an MOI of 0.0001 for 15 days.
The epitope recognized by parental antibody D8 is located between the two heptad repeat regions in the loop of the transmembrane glycoprotein of HIV-1, and it contains one cysteine of the disulfide bridge and a glycosylation site. In mature gp41, this region is not easily accessible to antibodies and may play a critical role in determining the structural conformation of the protein, especially in the folding of the two heptad repeat regions. Furthermore, it is a very conserved motif in the different known strains of HIV-1 (25).
Consequently, in order to block the early steps of envelope glycoprotein maturation in different strains of HIV-1, scFvD8 seems to be the antibody fragment of choice for intracellular immunization, especially against primarily isolates.
Acknowledgments
I. Legastelois was a fellow of SIDACTION. This work was supported by grants from INSERM, SIDACTION, and ANRS.
We thank M. Mortier for technical assistance in the obtention of the F172/D8 cell line and A. Kay, R. Sodoyer, and R. El Habib for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Barsov E V, Huber W E, Marcotrigiano J, Clark P K, Clark A D, Arnold E, Hughes S H. Inhibition of human immunodeficiency virus type 1 integrase by the Fab fragment of a specific monoclonal antibody suggests that different multimerization states are required for different enzymatic functions. J Virol. 1996;70:4484–4494. doi: 10.1128/jvi.70.7.4484-4494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartier C, Sivard P, Tranchat C, Decimo D, Desgranges C, Boyer V. Identification of three major phosphorylation sites within HIV-1 capsid: role of phosphorylation during the early steps of infection. J Biol Chem. 1999;274:19434–19440. doi: 10.1074/jbc.274.27.19434. [DOI] [PubMed] [Google Scholar]
- 3.Chen J D, Yang Q, Yang A G, Marasco W A, Chen S Y. Intra- and extracellular immunization against HIV-1 infection with lymphocytes transduced with an AAV vector expressing a human anti-gp120 antibody. Hum Gene Ther. 1996;7:1515–1525. doi: 10.1089/hum.1996.7.13-1515. [DOI] [PubMed] [Google Scholar]
- 4.Chen S Y, Bagley J, Marasco W A. Intracellular antibodies as a new class of therapeutic molecules for gene therapy. Hum Gene Ther. 1994;5:595–601. doi: 10.1089/hum.1994.5.5-595. [DOI] [PubMed] [Google Scholar]
- 5.Chen S Y, Khouri Y, Bagley J, Marasco W A. Combined intra- and extracellular immunization against human immunodeficiency virus type 1 infection with a human anti-gp120 antibody. Proc Natl Acad Sci USA. 1994;91:5932–5936. doi: 10.1073/pnas.91.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David D, Zouali M. Variable region light chain genes encoding human antibodies to HIV-1. Mol Immunol. 1995;32:77–88. doi: 10.1016/0161-5890(94)00109-e. [DOI] [PubMed] [Google Scholar]
- 7.David D, Goossens D, Desgranges C, Thèze J, Zouali M. Molecular characterization of human monoclonal antibodies specific for several HIV proteins: analysis of the VH3 family expression. Immunol Lett. 1995;47:107–112. doi: 10.1016/0165-2478(95)00078-j. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marmon S, Sutton R E, Hill C M, David C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Duan L, Zhu M, Bagasra O, Pomerantz R J. Intracellular immunization against HIV-1 infection of human T lymphocytes: utility of anti-Rev single chain variable fragments. Hum Gene Ther. 1995;6:1561–1573. doi: 10.1089/hum.1995.6.12-1561. [DOI] [PubMed] [Google Scholar]
- 10.Duan L, Zhang H, Oakes J W, Bagasra O, Pomerantz R J. Molecular and virological effects of intracellular anti-Rev single-chain variable fragments on the expression of various human immunodeficiency virus-1 strains. Hum Gene Ther. 1994;5:1315–1324. doi: 10.1089/hum.1994.5.11-1315. [DOI] [PubMed] [Google Scholar]
- 11.Duan L K, Bagasra O, Laughlin M A, Oakes J W, Pomerantz R J. Potent inhibition of human immunodeficiency virus type 1 replication by intracellular anti-Rev single chain antibody. Proc Natl Acad Sci USA. 1994;91:5075–5079. doi: 10.1073/pnas.91.11.5075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Ho W Z, Lai J P, Bouhamdan M, Duan L, Pomerantz R J, Starr S E. Inhibition of HIV type 1 replication in chronically infected monocytes and lymphocytes by retrovirus-mediated gene transfer of anti-rev single-chain variable fragments. AIDS Res Hum Retroviruses. 1998;14:1573–1580. doi: 10.1089/aid.1998.14.1573. [DOI] [PubMed] [Google Scholar]
- 13.Inouye R T, Du B, Boldt-Houle D, Ferrante A, Park I O, Hamer S M, Duan L, Groopman J E, Pomerantz R J, Terwilliger F. Potent inhibition of human immunodeficiency virus type 1 in primary T cells and alveolar macrophages by combination anti-Rev strategy delivered in adeno-associated virus vector. J Virol. 1997;71:4071–4078. doi: 10.1128/jvi.71.5.4071-4078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junker U, Kalfoglou C S, Moon J J, Beck M K, Kaneshima H, Böhnlein E. Inhibition of human immunodeficiency virus type 1 replication in myelomonocytic cells derived from retroviral vector-transduced peripheral blood progenitor cells. Hum Gene Ther. 1998;9:333–340. doi: 10.1089/hum.1998.9.3-333. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura Y, Ishikawa T, Okui N, Kobayashi N, Kanda T, Shimada T, Miyake K, Yoshiike K. Inhibition of replication of HIV-1 at both early and late stages of the viral life cycle by single-chain antibody against integrase. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;20:105–114. doi: 10.1097/00042560-199902010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 17.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Landau N L, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy-Mintz P, Duan L, Zhang H, Hu B, Dornadula G, Zhu M, Kulkosky J, Bizub-Bender D, Skalka A M, Pomerantz R J. Intracellular expression of single-chain variable fragments to inhibit early stages of the viral life cycle by targeting human immunodeficiency virus type 1 integrase. J Virol. 1996;70:8821–8832. doi: 10.1128/jvi.70.12.8821-8832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20.Marasco W A. Intrabodies: turning the humoral immune system outside in for intracellular immunization. Gene Ther. 1997;4:11–15. doi: 10.1038/sj.gt.3300346. [DOI] [PubMed] [Google Scholar]
- 21.Marasco W A, Haseltine W A, Chen S Y. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc Natl Acad Sci USA. 1993;90:7889–7893. doi: 10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhashilkar A M, Biswas D K, Vecchio J L, Pardee A B, Marasco W A. Inhibition of human immunodeficiency virus type 1 replication in vitro by a novel combination of anti-Tat single-chain intrabodies and NF-κB antagonists. J Virol. 1997;71:6486–6494. doi: 10.1128/jvi.71.9.6486-6494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mhashilkar A M, Bagley J, Chen S Y, Szilvay A M, Helland D G, Marasco W A. Inhibition of HIV-1 infection by anti-Tat single chain intrabodies. EMBO J. 1995;14:1542–1551. doi: 10.1002/j.1460-2075.1995.tb07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modow S, Hahn B H, Shaw G M, Gallo R C, Wong-Staal F, Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1997;61:570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers G, Wain-Hobson S, Henderson L E, Korber B, Jeang K T, Pavlakis G N. Human retroviruses and AIDS. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1997. [Google Scholar]
- 26.Gargano, Biocca S, Bradbury A, Cattaneo A. Human recombinant antibody fragments neutralizing human immunodeficiency virus type 1 reverse transcriptase provide an experimental basis for the structural classification of the DNA polymerase family. J Virol. 1996;70:7706–7712. doi: 10.1128/jvi.70.11.7706-7712.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen B M, Haugan I R, Berg K, Olsen L, Brown P O, Helland D E. Monoclonal antibodies against human immunodeficiency virus type 1 integrase: epitope mapping and differential effects on integrase activities in vitro. J Virol. 1996;70:1580–1587. doi: 10.1128/jvi.70.3.1580-1587.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peer Y V, Janssens W, Heyndrickx L, Fransen K, Groen G, Rupert De Wachter Phylogenetic analysis of the env gene of HIV-1 isolates taking into account individual nucleotide substitution rates. AIDS. 1996;10:1485–1494. doi: 10.1097/00002030-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rondon I J, Marasco W A. Intracellular antibodies (intrabodies) for gene therapy of infectious disease. Annu Rev Microbiol. 1997;51:257–283. doi: 10.1146/annurev.micro.51.1.257. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen F, Duan L, Zhu M, Bagasra O, Pomerantz R J. Targeting human immunodeficiency virus type 1 reverse transcriptase by intracellular expression of single-chain variable fragments to inhibit early stages of viral life cycle. J Virol. 1996;70:3392–3400. doi: 10.1128/jvi.70.6.3392-3400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tewari D, Goldstein S L, Notkins A L, Zhou P. cDNA encoding a single-chain antibody to HIV p17 with cytoplasmic or nuclear retention signals inhibits HIV-1 replication. J Immunol. 1998;161:2642–2647. [PubMed] [Google Scholar]
- 32.Wu Y, Duan L, Zhu M, Hu B, Kubota S, Bagasra O, Pomerantz R J. Binding of intracellular anti-Rev single chain variable fragments to different epitopes of human immunodeficiency virus type 1 Rev: variations in viral inhibition. J Virol. 1996;70:3290–3297. doi: 10.1128/jvi.70.5.3290-3297.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins A L. Cells transfected with a non-neutralizing antibody gene are resistant to HIV infection: targeting the endoplasmic reticulum and trans-Golgi network. J Immunol. 1998;160:1489–1496. [PubMed] [Google Scholar]