Acoustic tweezers enable contactless, dynamic, precise, and multifunctional manipulation of bioparticles in Petri dishes.
Abstract
Acoustic tweezers are a promising technology for the biocompatible, precise manipulation of delicate bioparticles ranging from nanometer-sized exosomes to millimeter-sized zebrafish larva. However, their widespread usage is hindered by their low compatibility with the workflows in biological laboratories. Here, we present multifunctional acoustic tweezers that can manipulate bioparticles in a disposable Petri dish. Various functionalities including cell patterning, tissue engineering, concentrating particles, translating cells, stimulating cells, and cell lysis are demonstrated. Moreover, leaky surface acoustic wave–based holography is achieved by encoding required phases in electrode profiles of interdigitated transducers. This overcomes the frequency and resolution limits of previous holographic techniques to control three-dimensional acoustic beams in microscale. This study presents a favorable technique for noncontact and label-free manipulation of bioparticles in commonly used Petri dishes. It can be readily adopted by the biological and medical communities for cell studies, tissue generation, and regenerative medicine.
INTRODUCTION
Acoustic tweezers use modulated acoustic waves to form virtual tweezers that enable precise manipulation of a wide range of particles scaling in sizes from nanometers to millimeters (1–7). Recently, acoustic tweezers have garnered increased interest from the biomedical research community, as they can perform noncontact, label-free, and precise manipulation of bioparticles (1, 8–11). With these merits, acoustic tweezers have been used in a wide range of biomedical applications, including patterning and printing cells (12–15), separating and sorting cells (16–19), controlling cell-cell interactions (20, 21), single-cell analysis (22–24), concentrating bioparticles (25–31), acousto-mechanical phenotyping (32, 33), constructing tissues (34–38), generating and translating droplets (39–41), rotating multicellular organisms (42, 43), and isolating extracellular vesicles (44, 45).
Despite recent advances in acoustic tweezer–based solutions for applications in biomedical research, most of the previously developed acoustic tweezers have limited compatibility with the workflow in biomedical laboratories and lack reusability (1, 12–21). These earlier devices usually require customized microfluidic channels/chambers that require costly and time-consuming steps for fabrication and sterilization (46–49). These customized structures are often not accessible for many biological and clinical laboratories for downstream cell cultivation or optical characterization methods. In addition, they usually require experienced engineers to design and operate them. Hence, for acoustic tweezers to become a common instrument in biological and clinical laboratories, they need to become accessible, easy to use, and compatible with existing workflows.
With the goal of bridging the gap between acoustic innovations and the biological/clinical benchtop, we present acoustic tweezers with three configurations that enable unique, noncontact manipulation of bioparticles in Petri dishes, the most common cell culture plate found in biomedical laboratories. By leveraging tunable standing acoustic waves, acoustic streaming vortices, and holographic interdigital transducers (IDTs), the resulting acoustic tweezers have achieved multiple functionalities, including multiconfiguration cell patterning, construction of large arrays of cell clusters for cell-cell interaction studies, construction of three-dimensional (3D) tissues with micrometer-scaled cellular architectures, construction of large cell spheroids, concentration of bioparticles, translation of cells, transient stimulation of cells, and lysis of cells in a small region, all in Petri dishes. Moreover, our multifunctional acoustic tweezers in Petri dishes are reusable and easy to use while maintaining sterile conditions. We believe the acoustic tweezers presented here, with their numerous bioparticle manipulation functionalities, will be of interest to the biological and biomedical communities.
To enable multifunctional acoustic tweezers in Petri dishes, the primary challenge is how to effectively generate and dynamically modulate various acoustic wavefields in Petri dishes by using acoustic transducers placed outside the Petri dishes. Here, we present three different configurations (Fig. 1) of acoustic tweezers to address this challenge for realizing different bioparticle manipulation functionalities. The first configuration (Fig. 1A) takes advantage of a piezoelectric transducer array placed around a Petri dish; the transducer array generates standing acoustic waves that are strong enough for patterning bioparticles in the Petri dish. By tuning the frequency, phase, and amplitude of the excitation for each transducer in the array, the generated acoustic fields can be modulated to form different interference patterns. Thus, bioparticles in the resulting acoustic fields are redistributed to form different patterns. On the basis of these features, our device paves the way for multiconfiguration cell patterning, construction of large arrays of cell clusters in Petri dishes for cell-cell interaction studies, as well as creation of 3D tissues with different micrometer-scaled cellular architectures, such as cell networks, cages, and bundles. Using this device configuration, nanometer-scaled F-actins inside cells were aligned by acoustic waves. In addition, this configuration allows dynamic acoustic field–based translation of micrometer-scale single cells directly in a Petri dish.
Fig. 1. Illustrations of the acoustic tweezer devices for manipulating bioparticles in Petri dishes.
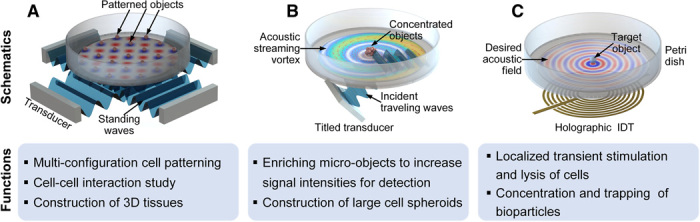
(A) A Petri dish is placed on top of the tweezer device, which is composed of an array of piezoelectric transducers that can generate standing acoustic waves in a fluid layer in the Petri dish. Manipulation is achieved by modulating the excitation frequency, phase, and amplitude of each transducer. This device allows multiconfiguration cell patterning, cell-cell interaction studies, and construction of 3D tissues. (B) This device configuration has a tilted piezoelectric transducer, which generates oblique incident traveling acoustic waves. Because the incident acoustic waves are asymmetric with respect to the center of the Petri dish, a large acoustic steaming vortex is generated in a fluid layer in the Petri dish. This drives bioparticles in the fluid layer to the vortex center, which enables functions such as the concentration of bioparticles for signal enhancement and the construction of large cell spheroids. (C) This device configuration has a holographic IDT placed under the Petri dish to generate high-frequency acoustic waves. By customizing the electrode profile of the holographic IDT, different desired acoustic fields/beams such as 3D focused or vortex beams can be constructed to manipulate cells in the Petri dish. By switching between different holographic IDT designs, multiple functions can be achieved including localized transient stimulation and lysis of cells, as well as concentration and trapping of bioparticles.
In the second configuration (Fig. 1B), a tilted piezoelectric transducer (or a narrow-beam IDT) that is placed under a Petri dish is used to generate an acoustic streaming vortex in a fluid layer (or even only a sessile droplet) in the Petri dish. Bioparticles in the fluid layer (or the sessile droplet) can be concentrated at the vortex center. This second configuration enables the rapid concentration of bioparticles in Petri dishes to enhance signal intensities for sensing and detection as well as easily enables the construction of large cell spheroids in Petri dishes. Compared to previous acoustic wave-based, cellular concentration/assembly devices that require complex microfluidic channels/chambers (25–28) or direct contact with the devices’ substrates (28–30), our tweezing device can perform concentration in disposable Petri dishes without direct contact to solid surfaces by using acoustic streaming vortices. This greatly reduces cross-contamination from multiple uses.
The third configuration (Fig. 1C) uses holographic IDTs that are developed by encoding the required phases for acoustic holography to the electrode pattern of the IDTs. This enables the generation of desired high-frequency, 3D acoustic fields for manipulating cells in Petri dishes. By customizing the electrode patterns, two specific types of holographic IDTs have been designed. One design has a 3D focused acoustic beam with highly focused acoustic energy at the beam center and the other design has a 3D acoustic vortex beam with a local Gor’kov potential minimum at the vortex center. HeLa cells are experimentally stimulated in a Petri dish using a 3D focused acoustic beam with a fluorescent marker of calcium signaling. Furthermore, the rapid lysis of cells in a selected small region of the Petri dish is also demonstrated. The 3D vortex beam, on the other hand, offers a method for concentration and trapping of bioparticles trapped at the vortex center. Compared to previous IDTs (2, 50, 51), the holographic IDTs encoded with phase information in the electrode patterns greatly extend the IDTs’ capabilities for customizing surface acoustic waves (SAWs). Compared to previous acoustic holography techniques, which were primarily used for low-frequency acoustic waves (52–54), the holographic IDTs presented here can be used to construct custom high-resolution acoustic fields in a high-frequency range above 10 MHz, which is well suited for cell manipulation.
RESULTS
Multiconfiguration cell patterning and translation of cells in Petri dishes
Arranging cells and other bioparticles into desired patterns play an important role in numerous biological and biomedical studies, such as cell-cell interaction studies (20, 21), tissue engineering (34–38), and regenerative medicine (55, 56). The acoustic tweezer configuration presented in Fig. 1A is designed for custom cell patterning directly in Petri dishes. In this configuration, a piezoelectric transducer array is placed in a thin ultrasonic couplant layer and a Petri dish sits on the couplant layer. Incident acoustic waves are generated in the thin couplant layer by using the transducer array. The generated acoustic waves are converted to antisymmetric Lamb waves in the Petri dish bottom and then leak into the culture medium in the dish to form standing acoustic wave patterns. In our configuration, the acoustic wave interaction with curved sidewall can be minimized, reducing wave pattern distortion. In addition, the cell solution in the Petri dish does not need to cover the entire dish bottom because acoustic waves leak into the solution that is in contact with the dish bottom. The numerical analysis suggests that the transmission coefficient is around 0.8 at 3.4 MHz (fig. S1). The standing acoustic waves introduce Gor’kov potential wells, which are usually located at pressure nodes for cells with acoustic impedances larger than that of the culture medium (13). These potential wells function as virtual, noncontact tweezers that can attract cells to the potential minima for patterning and translating cells.
The standing wave patterns generated by the acoustic tweezer device depend on the excitation frequency, phase, and applied voltage to each piezoelectric transducer of the device. Therefore, by tuning these excitation parameters, different standing wave patterns can be generated for multiple cell positioning. Analytical simulations are used to predict the resulting cell patterns, and experiments with U937 cells are performed to verify these analytical predictions and to demonstrate this multiconfiguration cell patterning capability. When parallel piezoelectric transducers are excited at 7.47 MHz, the analytical potential field solution shows a parallel line–like pattern with a spacing of 99 μm along the x direction (Fig. 2A). Cells in this potential field will be gradually moved toward the local potential minima at the parallel lines. As expected, cells form a parallel line–like pattern (Fig. 2D) that matches well with the distribution of potential minima (Fig. 2A). Besides parallel line–like patterns, two orthogonal transducer pairs excited at different frequencies can be used to construct grid-like patterns (20). For example, when the two transducer pairs generate acoustic waves at 3.45 and 3.42 MHz, respectively, 25 cell clusters are assembled with both green (stained with CellTracker Green CMFDA dye) and orange (stained with CellTracker Orange CMRA dye) fluorescent cells in each cluster. These cell clusters are distributed in a grid-like pattern (Fig. 2E) that matches with the predicted distribution of potential wells (Fig. 2B). These experimental results verify that the designed acoustic tweezers can achieve multiconfiguration cell patterning in a Petri dish by tuning the excitation amplitudes and frequencies of the transducer arrays. This cell patterning function can potentially be used to generate a large array of cell clusters with two or more types of cells in each cluster for high-throughput, cell-cell interaction studies.
Fig. 2. Analytical and experimental results for multiconfiguration cell patterning and single-cell translation.
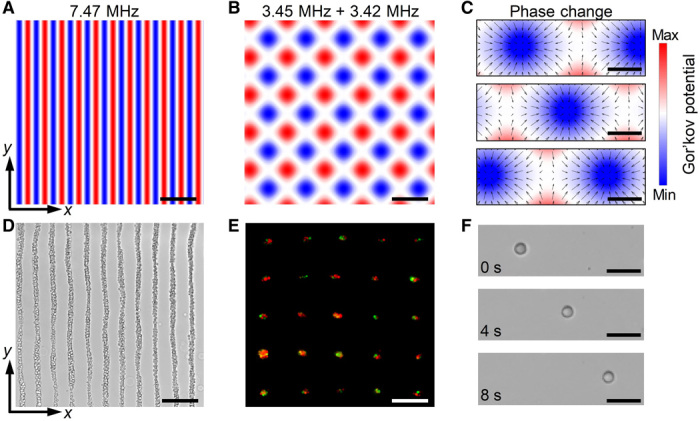
(A to C) Analytical solutions for generated Gor’kov potential fields with different excitation parameters. (A) When a pair of parallel transducers is excited at 7.47 MHz, the potential wells are distributed in parallel line–like patterns with spacings of 99 μm along the x direction. (B) When two orthogonal transducer pairs are excited at different frequencies of 3.45 and 3.42 MHz, respectively, the potential wells are distributed in a grid-like pattern. (C) The positions of potential wells can be gradually changed by tuning the phase difference between the excitation frequencies for two parallel pairs of transducers. (D to F) Experimental results (microscope images) that confirm the analytical solutions in (A) to (C). (D) U937 cells are distributed in a parallel line–like pattern. (E) Cell clusters with both green (stained with CellTracker Green CMFDA dye) and orange (stained with CellTracker Orange CMRA dye) fluorescent cells are assembled and distributed in a grid-like pattern. (F) Time-elapsed sequence of microscope images shows that a HeLa cell in a Petri dish is translated horizontally by a Gor’kov potential well. The distributions of cells in (D) to (F) agree well with the calculated distributions of potential wells in (A) to (C). Scale bars, 200 μm (A, B, D, and E) and 40 μm (C and F).
Besides cell patterning, the dynamic translation of single cells in a Petri dish is demonstrated. This is achieved by dynamically tuning the positions of Gor’kov potential wells through the modulation of the excitation phases for a pair of parallel transducers, as illustrated by the analytical potential fields in Fig. 2C. A time sequence of microscope images (Fig. 2F) verifies that a HeLa cell in a Petri dish is translated horizontally by moving the Gor’kov potential well. Movie S1 further shows that the HeLa cell can be linearly translated back and forth by tuning the position of the Gor’kov potential well generated in the Petri dish.
Engineering 3D tissues in Petri dishes
Tissue engineering offers great opportunities for regenerative medicine and disease modeling (55, 56). Recent biomimetic studies have demonstrated that the cell-cell spatial relationships in tissues are critical for the growth, differentiation, and maturation of artificial tissues as well as maintaining the tissue function (55, 56). However, current tissue engineering approaches such as 3D templating and direct cell printing techniques have limitations in the construction of microscale cellular structures that are necessary for reproducing native cellular organization (55–57). The limitations of these approaches include a limited selection of biomaterials, fabrication time, and costly specialized equipment (12–15, 55, 56). In addition, 3D templating requires physical molds or scaffolds to guide cells (57). A potential solution to overcome these limitations is to use acoustic tweezers to directly pattern many cells in a disposable Petri dish and constructing 3D tissues with these patterned cells. Using this method, large 3D tissues containing cellular structures with resolutions down to a few micrometers can be rapidly constructed in a Petri dish.
We have experimentally demonstrated the creation of 3D fibroblast tissues directly in a Petri dish based on the multiconfiguration cell patterning capabilities of our acoustic tweezers. NIH 3T3 fibroblasts in fibrin gels are used for this demonstration. Using fibrin as a 3D microenvironment offers the advantage of tuning the viscoelastic properties of gels to mimic native tissues by adjusting the prepolymer concentration of fibrinogen (37). The cross-linking time is tuned by changing the concentration of thrombin. In our experiment, fibroblasts are first suspended in a 0.5-ml culture medium containing thrombin (10 U/ml). They are then mixed with a 0.2-ml serum/antibiotic-free medium containing fibrinogen (20 mg/ml) and added to a Petri dish placed at the center of our acoustic device (Fig. 1A). Applying standing acoustic waves, the fibroblasts are translated to pressure nodes and trapped there, thus forming cell patterns. Although this culture medium mixed with fibrinogen and thrombin has a high viscosity, the fibroblasts can still be rapidly (<5 s) patterned by our acoustic tweezers before cross-linking. The standing acoustic waves are turned off after 1 min, when the cross-linking gel can hold patterned fibroblasts at their positions. In the next 10 min, the fibrin solution gradually forms a stable 3D gel containing acoustically patterned fibroblasts. This entire Petri dish, with the fixed 3D gel, can now be removed from tweezer device for subsequent cell culture and analysis. By using sterile, portable, and disposable Petri dishes, our acoustic wave–based method can be readily adapted to downstream assay requirements. Compared to recently presented ultrasound-assisted biofabrication methods that immerse acoustic transducers in cell inks (58, 59), our platform can be used for cell patterning and hydrogel fabrication in Petri dishes without placing transducers in the dish, thus reducing the chance of cross-contamination between fabrication of different cellular constructs.
The architectures of postculturing fibroblast structures can be affected by the initial fibroblast patterns constructed by acoustic waves, the cell growth, migration and interaction, and the material properties and compositions of hydrogels. In this study, we constructed different fibroblast patterns using acoustics and investigated the effects of initial cell patterns on postculturing cellular architectures. After culturing different patterns of fibroblasts for 30 hours, we observe that fibroblasts interlink with each other and form a 3D network with multiple microscale cellular structures (Fig. 3A), cages with different sizes (Fig. 3B), and unidirectional bundles with different diameters (Fig. 3C). Our experimental results show that when the Gor’kov potential wells are in grid-like distributions (e.g., Fig. 2B), fibroblasts cultured in a hydrogel for 30 hours tend to form a network-like structure (Fig. 3A). If a large number of fibroblasts are gathered in a cluster by a potential well, our results show that fibroblasts in a cluster have a chance of forming a cage-like structure (Fig. 3B). The formation of a cage-like structure might be related to the cell migration, growth, and/or interaction. When Gor’kov potential wells are distributed as parallel line–like patterns, fibroblasts tend to weave with each other, forming unidirectional bundles (Fig. 3C). The diameters of unidirectional bundles can be controlled by adjusting the seeding densities of the fibroblasts. A confocal microscopy image of a unidirectional bundle (Fig. 3D) is taken to characterize the F-actins (proteins in fibroblasts), which play an import role for cellular force transmission and mechanotransduction (60). It is known that regions with highly aligned F-actins usually have high talin tension, whereas regions with less well-aligned F-actins have low talin tension (60). Our confocal image in Fig. 3D shows that nearly all the F-actins (green) in a fibroblast bundle are well aligned along the axial directional of the bundle. This observation implies that the F-actin alignment at the nanometer scale introduces mechanical anisotropy to the fibroblast bundle. This configuration will likely result in a higher tensile Young’s modulus along the axial direction than the moduli in other directions. Our acoustic tweezers provide an easy-to-use, low-contamination, and reusable platform for the fabrication of hydrogels containing patterned cellular structures. Our platform can also be used to investigate the effects of initial cell patterns on the postculturing cellular architectures and protein distributions.
Fig. 3. Creation of 3D tissues with patterned fibroblasts in fibrin gels using acoustic tweezers.
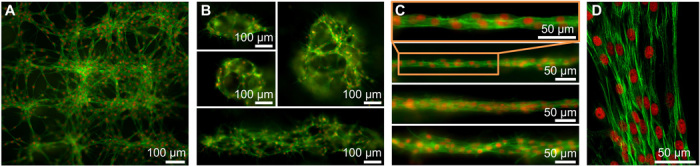
Fluorescence microscopy images show tissues constructed with multiple microscale cellular structures, such as (A) a network, (B) cages with different sizes, and (C) unidirectional bundles with different diameters. The tissues are acoustically patterned with fibroblasts and then cultured for 30 hours. (D) A confocal microscopy image of a bundle of fibroblasts shows that F-actins are aligned along the axial direction of the bundle. For fluorescence and confocal microscopy, Alexa Fluor 488 Phalloidin and 7-aminoactinomycin D are used to selectively stain F-actins (green) and DNA (red), respectively.
Concentrating microparticles in Petri dishes
Concentrating bioparticles is of great interest to many biochemical and biomedical applications (25–31). For example, detecting low concentrations of bacteria, cells, or other bioparticles with biomarkers usually requires a concentration step to enhance the signal intensity and signal-to-noise ratio and improve the limit of detection for diagnosis (26–31). Also, the efficient concentration of cells can be used for producing cell agglomerates that are essential for drug testing and tissue engineering (25, 31). Here, we developed an acoustic-based device that generates an acoustic streaming vortex (19) for concentrating bioparticles in a thin liquid layer (or a sessile droplet) in a Petri dish. Figure 4A shows the mechanism for concentrating bioparticles in a thin liquid layer in a Petri dish. A 45° tilted piezoelectric transducer is placed under the Petri dish to generate incident traveling acoustic waves at the transducer’s thickness-mode resonance frequency. The traveling waves are transmitted into the bottom of the Petri dish via a couplant layer and then leak into the thin liquid layer in the Petri dish. The incident waves are laterally offset from the center of the Petri dish to break the symmetry, as illustrated in the schematic (Fig. 4A). The combined effect of asymmetric traveling waves and the circular boundary of the Petri dish induces a large acoustic steaming vortex, which concentrates bioparticles at the vortex center in the liquid layer. A proof-of-concept experiment is performed to concentrate polystyrene particles in a thin water layer spread in a Petri dish (movie S2). The time-elapsed images (Fig. 4, B to D) show that 5-μm polystyrene particles are concentrated at the vortex center within 10 s of switching on the piezoelectric transducer at the resonance frequency of 3 MHz.
Fig. 4. Concentrating micro-objects in a Petri dish via acoustic streaming vortices.
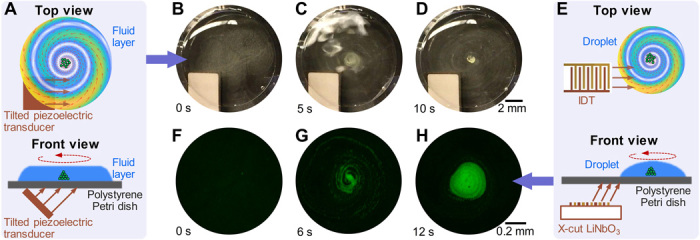
(A) Schematics of concentrating micro-objects in a Petri dish using an acoustic streaming vortex generated by a tilted piezoelectric transducer. Traveling acoustic waves generated by the piezoelectric transducer first transmit to the bottom of the Petri dish through a couplant layer and then leak into the fluid layer in the Petri dish. Traveling waves in the fluid layer further induce an acoustic streaming vortex that drives micro-objects to the center of the Petri dish for enrichment. (B to D) Photos taken at 0, 5, and 10 s after the piezoelectric transducer is switched on at the transducer’s resonance frequency of 3.0 MHz. The time-sequential images show that 5-μm polystyrene particles are gradually concentrated at the center of the Petri dish. (E) Schematics of concentrating micro-objects in a sessile droplet at the center of a Petri dish using an acoustic streaming vortex generated by an IDT. High-frequency narrow-beam traveling acoustic waves generated by the IDT can transmit to the droplet and further induce an acoustic streaming vortex that drives micro-objects in the droplet to the vortex center. The oblique incident angle is induced by the difference between the wavelengths of SAWs and bulk acoustic waves. (F to H) Fluorescence microscopy images taken at 0, 6, and 12 s after the IDT is switched on at the IDT’s resonance frequency of 10.3 MHz. The time-sequential images show that 5-μm polystyrene particles (green) are gradually concentrated at the center of the acoustic streaming vortex. Meanwhile, the fluorescence intensity gradually increases and becomes much higher at 12 s than the intensity at the beginning. Photo credit: Zhenhua Tian, Duke University.
Figure 4E illustrates the mechanism for concentrating bioparticles in a sessile microliter droplet in a Petri dish. To generate an acoustic streaming vortex in a microliter droplet, incident acoustic waves with a narrow beam (with a width nearly the same as or smaller than the droplet diameter) are preferred. A small-width IDT on an X-cut LiNbO3 substrate is used for generating narrow-beam acoustic waves, which are transmitted into the Petri dish and then leak into the droplet. The LiNbO3 substrate is parallel to the Petri dish bottom. Because the traveling SAWs in the substrate have a larger wavelength than that of the bulk acoustic waves in the coupling medium, incident waves that travel toward the Petri dish bottom have an oblique angle. On the other hand, the IDT is laterally offset from the center of the droplet to break the symmetry and this induces an acoustic streaming vortex for concentrating bioparticles in the droplet. A proof-of-concept experiment is performed to concentrate 5-μm polystyrene particles in a sessile droplet on a Petri dish (movie S2). The time sequence of fluorescence images (Fig. 4, F to H) shows that most polystyrene particles are gradually transported to the vortex center along a spiral path and finally form a large cluster. As expected, the fluorescence intensity gradually increases as the particles concentrate, which means that this acoustic method can potentially be used for enhancing the signal intensity for detecting low-concentration bioparticles.
High-frequency, holographic acoustic tweezers in Petri dishes
Holographic techniques enable the construction of versatile optical or acoustic fields through interference patterns (52–54). Recently, holographic acoustic tweezers have been designed to manipulate objects ranging from the micrometer to centimeter scale (52–54). On one hand, printed passive holograms functioning like acoustic lenses have been designed to shape the transmission field of acoustic waves (52, 53) so that particles in the resulting acoustic field can be arranged to form complex patterns. On the other hand, active ultrasonic phased arrays have been developed to dynamically generate the desired acoustic fields (54). By modulating the input phase and/or amplitude of each transducer in the array, the generated acoustic fields can be dynamically changed for particle manipulation. Even with these advances, previous holographic acoustic tweezers are still limited by their manipulation resolution because they work at relatively low frequencies with large wavelengths. For example, at 2 MHz, a passive hologram will have a wavelength of 740 μm (52) and active arrays at 40 kHz have a wavelength of 8.5 mm (54). Scaling down those holographic techniques for acoustic waves with smaller wavelengths at higher frequencies, making them suitable for cell manipulation, is very challenging. Therefore, holographic acoustic tweezers that are easy to implement and capable of operating from 10s to 100s of MHz, with resulting wavelengths comparable to the sizes of cells, are highly desirable for biomedical applications.
Here, we use IDTs to realize high-frequency, holographic acoustic tweezers for cell manipulation. As shown in Fig. 1C, the acoustic tweezer device has a holographic IDT placed under a Petri dish for generating the desired high-frequency acoustic field in the fluid layer in the Petri dish. The phase information for the desired holographic acoustic field is stored in the electrode design of the IDT. Several other factors also need to be considered for designing the electrode profile such as the direction-dependent phase velocities of SAWs in LiNbO3 wafers, the conversion from SAWs to pressure waves, and the electric-mechanical coupling for generating SAWs. Therefore, before designing holographic IDTs, the propagation of SAWs in X-cut LiNbO3 substrates is investigated (section S1). Our numerical results unveil two intriguing properties: (i) omnidirectional center-symmetric SAWs generate anti–center-symmetric electric potential fields (fig. S2) and (ii) an IDT with symmetrically arranged fingers generates antisymmetric SAWs (fig. S3). These properties have not been reported previously and are usually omitted in the design of IDT-based acoustic tweezers. If these properties are not considered, the designed IDT-based acoustic tweezers could introduce adverse effects. For example, two IDTs (mirroring each other) in an X-cut substrate generate a node at the center point between them (fig. S4). This is contrary to the result when using a common Y128-cut LiNbO3 substrate, where a pair of symmetric IDTs will generate an antinode at the center.
The procedures for designing holographic IDTs are presented in section S2. A schematic in the x-o-z plane for illustrating the design method and geometrical relations of holographic IDTs is given in fig. S5. To generate a desired acoustic field in a target plane using holographic IDTs, waves in the target plane are backpropagated to the excitation plane, i.e., the top surface of the LiNbO3 substrate. Then, the phase profile in the excitation plane is extracted from the backpropagated waves and is used to determine the geometry of the IDTs. Using this design method, two types of holographic IDTs (Fig. 5, A and D) have been designed to generate 3D focused and vortex beams, respectively. Finite element simulations are performed using the piezoelectric and acoustic modules in the COMSOL Multiphysics software (section S2) to visualize the 3D holographic interactions of the acoustic beams.
Fig. 5. Holographic IDTs for customized 3D high-frequency acoustic beams.
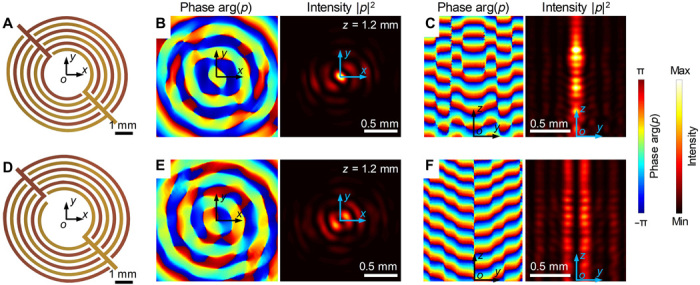
(A and D) Schematics of two different holographic IDTs for generating 3D focused beams and acoustic vortices. With respect to the IDT design in (A), numerical simulation results of a generated focused acoustic beam at 10 MHz in (B) z = 1.2 mm and (C) y-o-z planes. With respect to the IDT design in (D), numerical simulation results of the generated acoustic vortex beam at 10 MHz are in (E) z = 1.2 mm and (F) y-o-z planes. In the simulation results (B), (C), (E), and (F), the left and right images represent the phase arg(p) and intensity |p|2 of acoustic waves. The field results are obtained through finite element modeling simulations. Details of the numerical simulation setup and 3D results can be found in section S2.
For the designed holographic IDT in Fig. 5A, the numerical simulation results at 10 MHz in z = 1.2 mm (Fig. 5B) and y-o-z planes (Fig. 5C) show a 3D focused acoustic beam whose energy is highly concentrated at the beam center. A 3D plot of the numerically modeled acoustic pressure field is given in Fig. 6A, and the 3D acoustic intensity field is given in fig. S6. These 3D fields show that acoustic waves are highly concentrated in the confined narrow beam. Movie S3 dynamically shows the phase-dependent variation of the beam’s pressure field. For the designed holographic IDT in Fig. 5D, the numerical simulation results at 10 MHz in z = 1.2 mm (Fig. 5E) and y-o-z planes (Fig. 5F) show an acoustic vortex beam (61) with a topological charge of −1. At the beam center, there is a local intensity minimum. 3D views of the acoustic pressure and intensity fields for the generated vortex beam are given in fig. S7. Movie S4 dynamically shows the phase-dependent variation of the vortex beam. At the acoustic beam center, there is a local intensity minimum and a microscale Gor’kov potential well is formed. Compared to a recent study of generating a 4.4-MHz acoustic vortex beam with a flat piezoelectric transducer that is based on specific curved electrodes inspired by the Archimedes-Fermat spiral (5), our study distinguishes itself in the following regards: (i) Our design method considers the anisotropic material properties of piezoelectric substrates, (ii) our design method considers the relation between dynamic electric potential fields and acoustic fields, and (iii) our design method can be generally applied to develop different holographic IDTs. By encoding the optimized phase information into an IDT’s electrode shape based on the design method presented in section S2, one can develop holographic IDTs for generating other types of acoustic beams. Previously, we have demonstrated the generation of bottle-shaped and nonparaxial beams at low frequencies (<200 kHz) through metamaterial-based phase engineering (62–64). In the future, we will encode similar phase information to electrode profiles; we expect that the holographic IDTs can generate various acoustic beams at high frequencies. Moreover, we will optimize the electrode profiles of IDTs through iterative approaches (52, 65); we expect that the optimized holographic IDTs can construct prescribed, arbitrarily shaped acoustic fields at high frequencies.
Fig. 6. Experimental results for localized cell disruption and transient cell stimulation.
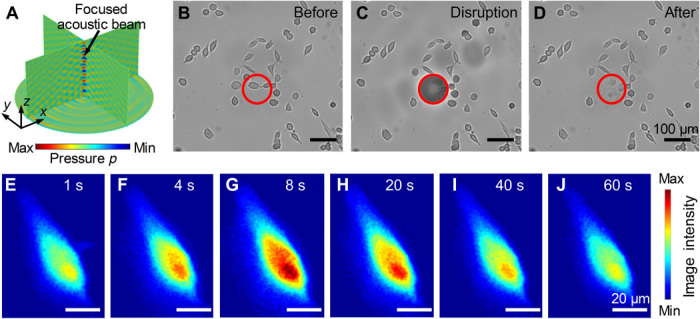
(A) Numerical simulation results for the 3D pressure field in a Petri dish as generated by a holographic IDT. The resulting focused acoustic beam is at the center of the figure. (B to D) Time-elapsed microscope images showing that HeLa cells within the 100-μm-diameter red circle are disrupted by a focused acoustic beam. (E to J) Time-elapsed imaging showing induced transient stimulation with focused acoustic pulses in 0.1 ms, resulting in an increase in the intracellular Ca2+ concentration in a HeLa cell. After 60 s, the Ca2+ concentration returns to a normal level. The calcium indicator Fluo-4 AM is used for calcium imaging. The image intensity indicates the relative level of Ca2+ concentration.
A 3D focused acoustic beam (Fig. 6A) generated by our holographic IDT has a high acoustic energy concentrated in a microscale region. This makes it suitable for localized transient disruption and stimulation of cells. An experiment was performed using the focused acoustic beam in a periodic burst mode to selectively disrupt HeLa cells in a target region of a Petri dish (movie S5). The time-sequential microscopy images (Fig. 6, B to D) show that only the HeLa cells within the marked red circle with a diameter of 100 μm are quickly disrupted. This demonstrates that the focused acoustic beam generated by our holographic IDT can achieve selective cell disruption/lysis in a microscale target region. Another experiment was performed using a short-duration (0.1 ms) pulse of focused acoustic waves for transient stimulation of a HeLa cell in a Petri dish (movie S6). Imaging of the calcium concentration (Fig. 6, E to J) using Fluo-4 acetoxymethyl (AM) as an indicator shows an instant increase in the intracellular Ca2+ concentration. Hence, the acoustic stimulation has induced the initiation of calcium signaling (24). The experimental results in Fig. 6 verify that our holographic IDT can generate a focused acoustic beam with high energy for cell disruption and stimulation in a prescribed region. Compared to the commonly used sonication-based cell disruption methods (66, 67), our holographic IDT can target cells in a microscale region. Besides these two functionalities demonstrated in Fig. 6, we believe that our holographic IDT can potentially be used for the lysis of a cell at a prescribed position for extracting the DNA in that cell, delivery of DNA into a cell, transient stimulation of signal transduction in cells, and other acoustic stimulation–based cell biology studies.
DISCUSSION
This study presents acoustic tweezers with three configurations for noncontact manipulation of bioparticles in Petri dishes. The acoustic tweezers can effectively generate standing acoustic waves, acoustic streaming vortices, and 3D focused/vortex beams directly in Petri dishes. We have experimentally demonstrated multiple functionalities, including multiconfiguration cell patterning, construction of 3D tissues, concentration of microparticles, translation of cells, transient stimulation of cells, and lysis of cells within a micrometer-scale target region.
The acoustic tweezer setup, as shown in Fig. 1A, is an array of piezoelectric transducers placed around a Petri dish. The array generates standing acoustic waves in the fluid layer in the Petri dish. By tuning the frequency, phase, and amplitude of the excitation signal for each transducer in the array, the generated standing wave patterns can be modulated for multiconfiguration cell patterning. For example, parallel line–like and grid-like cell patterns are demonstrated, as well as tuning the distance between cell clusters. We experimentally constructed a large array of cell clusters that can potentially be used for high-throughput, cell-cell interaction studies. The experimental cell patterns agree with our simulation acoustic fields. In addition to cell patterning, the bioengineering of tissues with acoustically patterned microscale cellular structures has also been experimentally demonstrated. Using these acoustic tweezers, 3D tissues with different micrometer-scaled cellular architectures, including cell networks, different size cages, and different diameter bundles, were constructed in Petri dishes. Moreover, these acoustic tweezers can be used to introduce mechanical anisotropy to 3D cellular structures, resulting in customized mechanical properties such as the Young’s modulus and Poisson’s ratio. Furthermore, we believe that we have demonstrated, for the first time, that the nanometer-scaled F-actins inside cells can be aligned after acoustic patterning. Although this study only uses fibroblasts for demonstrating assembly of 3D cellular structures, these acoustic tweezers should be equally applicable to tissue engineering with other types of cells, such as neural, cardiac, and vascular cells.
Another tweezer design offers an efficient way of concentrating bioparticles in microliter liquid volumes. Acoustic tweezers based on the configuration in Fig. 1B placed a tilted piezoelectric transducer (or a narrow-beam IDT) under a Petri dish. It generates an acoustic streaming vortex in a fluid layer (or a sessile droplet) in the Petri dish so that bioparticles in the fluid layer (or the sessile droplet) are driven to the vortex center by acoustic streaming for enrichment. We have experimentally demonstrated the concentration of 5-μm polystyrene particles in a fluid layer and a sessile droplet. Such concentration methods potentially can be used for enhancing signal intensities (e.g., for fluorescent detection) and for construction of large cell spheroids.
The holographic IDTs have been designed by encoding the required phase information into the IDT’s electrode design. Acoustic tweezers with the configuration shown in Fig. 1C take advantage of a holographic IDT placed under a Petri dish for generating high-frequency 3D acoustic fields in the Petri dish. By carefully engineering the electrodes, different holographic IDTs can be designed for generating custom acoustic fields/beams. As a proof of concept, two types of holographic IDTs (Fig. 5, A and D) have been designed. Our simulation results successfully show one type for generating a 3D focused beam with acoustic energy highly focused at the beam center and another type for generating a 3D vortex beam with a local potential minimum at the beam center. A 3D focused beam was demonstrated for localized lysis of HeLa cells in a small target region of a Petri dish (Fig. 6, B to D) and for stimulating a HeLa cell cultured in a Petri dish (Fig. 6, E to J). The 3D vortex beam has the potential to trap a cell at the center of the vortex. In the future, we will use iterative approaches (52, 65) to further optimize the electrode profile of the holographic IDTs so that more complex high-frequency acoustic beams (such as bottle-shaped and nonparaxial beams) can be generated. Compared to traditional IDTs (2, 50, 51), the holographic IDTs, which have the phase information encoded in their electrode designs, should greatly extend the capabilities of IDTs by generating more complex SAWs and bulk waves. Compared to previous holographic acoustic techniques, which mainly use low-frequency bulk acoustic waves (52–54), these acoustic tweezers based on holographic IDTs can project high-resolution acoustic fields in the high-frequency range above 10 MHz for cell manipulation and stimulation. Moreover, these holographic IDTs can easily be fabricated using standard lithography, thin-film metal evaporation, and lift-off techniques (13, 16). These holographic IDTs offer a way to engineer high-frequency acoustic waves and may advance multiple acoustic techniques, such as acoustic tweezers, high-resolution acoustic imaging, and on-chip acoustic sensors.
Although previous acoustic tweezers have been demonstrated for the manipulation of cells, most of them require customized microfluidic channels/chambers, which usually require time-consuming and costly steps for fabrication and sterilization and hence are not frequently used in biological and biomedical laboratories (1, 12–21). This study develops acoustic tweezer devices that can directly manipulate bioparticles in the most common laboratory cell culture plate, the Petri dish. This leverages all the advantages, such as low cost, disposability, sterility, and existing assay workflow optimized around handling Petri dishes. Moreover, on the basis of standing acoustic waves, acoustic streaming vortices, and high-frequency acoustic holography, our devices can readily be used in different assays for biomedical research, including cell-cell interaction studies, construction of 3D tissues, construction of large cell spheroids, concentration of bioparticles, translation of cells, transient stimulation of cells, and lysis of cells in a small target region. Future work will focus on the experimental characterization of generated acoustic fields by our devices as well as rapidly adapting our acoustic tweezer devices to demonstrate more of the aforementioned capabilities and hopefully move the technology toward becoming the widespread standard method for noncontact manipulation of cells.
MATERIALS AND METHODS
Design, fabrication, and operation of acoustic tweezer devices
A schematic of the acoustic device is presented in Fig. 1A. The acoustic device is composed of an array of piezoelectric transducers arranged in a customized holder fabricated using 3D printing (J750, Stratasys). In this proof-of-concept study, the array has two pairs of piezoelectric transducers that are orthogonal to each other. Arrays with more transducer pairs can also be designed to generate more complex patterns for cell manipulation. The piezoelectric transducers (material SM111, Steiner & Martins Inc., USA) have a thickness of 2.1 mm. Their first-, second-, third-, and fourth-order thickness-mode resonant frequencies are around 1.0, 3.4, 5.4, and 7.5 MHz, respectively. At these resonant frequencies, the piezoelectric transducers can efficiently generate strong pressure waves in water with wavelengths around 1480, 435, 274, and 197 μm, respectively. The excitation signal for each piezoelectric transducer is generated with a function generator (AFG3102C, Tektronix) and amplified with a power amplifier (25A250A, Amplifier Research). Through the modulation of the phase, amplitude, and frequency of each input signal, the generated standing pressure waves in the Petri dish can be controlled to form different wave patterns for cell manipulation.
A schematic of the acoustic device for concentrating bioparticles is presented in Fig. 1B. This device uses the SM111 piezoelectric transducer with a thickness of 0.7 mm and a first-order thickness-mode resonant frequency of 3 MHz. The piezoelectric transducer is tilted at 45° to generate oblique incident traveling acoustic waves. The excitation signal for the piezoelectric transducer is generated with a function generator (AFG3102C, Tektronix) and then amplified with a power amplifier (25A250A, Amplifier Research). The generated traveling waves are transmitted into the fluid medium in the Petri dish through the dish’s bottom. There is a couplant layer between the Petri dish and the transducer. The incident waves are laterally offset from the center of the Petri dish to break the symmetry. The combined effect of asymmetric traveling waves and the circular boundary of the Petri dish induces a large acoustic steaming vortex in the fluid layer in the Petri dish. The generated acoustic streaming vortex concentrates bioparticles in the fluid layer at the vortex center.
A schematic of the acoustic device for transient cell stimulation is given in Fig. 1C. A holographic IDT is placed under the Petri dish to generate SAWs in an X-cut LiNbO3 substrate. Through the solid-fluid interaction, SAWs excite pressure waves in the ultrasound couplant layer between the Petri dish and the LiNbO3 substrate. The excited pressure waves then transmit into the fluid medium in the Petri dish for cell manipulation. The holographic IDT under the Petri dish can be changed to generate various acoustic fields in the Petri dish for different applications. In this proof-of-concept study, we have designed two types of holographic IDTs, which can generate a 3D focused beam (Fig. 5A) or vortex beam (Fig. 5D) at 10 MHz, respectively. Holographic IDTs working at other frequencies can be designed by following the procedures given in section S2. To fabricate holographic IDTs, a double layer of chrome and gold (Cr/Au, 50 Å/800 Å) was deposited on a photoresist-patterned X-cut LiNbO3 wafer (thickness 500 μm, double-side polished) followed by a lift-off technique (13, 16). The input voltage signals for the fabricated IDTs were generated with a function generator (AFG3102C, Tektronix) and amplified with a power amplifier (25A250A, Amplifier Research).
In each acoustofluidic experiment, a polystyrene Petri dish with cells or other microparticles was placed above the acoustic transducers using a 3D printed holder. The fluid-type ultrasound couplant or deionized water was added between the Petri dish and acoustic transducers to more efficiently transmit acoustic waves generated by the transducers into the fluid medium in the Petri dish. After each experiment, the Petri dish with cells or other micro-objects was removed for cell culture or downstreaming analyses. Hence, the acoustic devices can be reused with different Petri dishes for subsequent assays. Because all of the test samples are isolated in the Petri dish, this method also avoids cross-contamination between different cell cultures.
Microparticle and cell sample preparation
Polystyrene beads (5 μm; Bangs Laboratories Inc.) were suspended in deionized water to an approximate concentration of 3 × 106 ml−1. The HeLa cells and NIH 3T3 fibroblasts were both purchased from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Life Technologies) containing 10% fetal bovine serum (Gibco, Life Technologies) and 1% penicillin-streptomycin (Mediatech). The U937 cells were purchased from ATCC and cultured in RPMI 1640 media (Gibco, Life Technologies) containing 10% fetal bovine serum (Gibco, Life Technologies) and 1% penicillin-streptomycin (Mediatech). All the cells were maintained in a cell culture incubator (Nu-4750, NuAire) with a temperature of 37°C and a CO2 level of 5%. For multiconfiguration cell patterning experiments, the HeLa (or U937) cells were harvested and resuspended in fresh DMEM (or RPMI 1640 media) to an approximate concentration of 1.5 × 106 ml−1.
Construction of 3D fibrin gels containing acoustically patterned fibroblasts
Fibroblasts were suspended in 0.5 ml of fresh culture medium with thrombin (10 U/ml) (T4648, Sigma-Aldrich). Then, the medium with fibroblasts and thrombin was mixed with a 0.2-ml serum/antibiotic-free medium containing fibrinogen (20 mg/ml) (F8630, Sigma-Aldrich). This mixture was added to a Petri dish placed at the center of our acoustic device (Fig. 1A). After the piezoelectric transducers were turned on, fibroblasts were moved to pressure nodes and trapped there, gradually forming 3D patterns in 5 s. The piezoelectric transducers were turned off after 1 min, when the cross-linking gel can fix the patterned fibroblasts at their positions. Over the next 10 min, the fibrin solution cross-links slowly and finally forms a stable 3D gel with the acoustically patterned fibroblasts. This gel is kept in the Petri dish and is moved to an incubator for cell culture. After 30 hours, fibroblasts interlink with each other and form 3D tissues with different microscale cellular structures such as a network, cages with different sizes, and unidirectional bundles with different diameters.
Cell staining
In the fluorescent image (Fig. 2E), U937 cells were stained with either the green fluorescent dye (CellTracker Green CMFDA, Invitrogen) or an orange fluorescent dye (CellTracker Orange CMRA, Invitrogen). In the fluorescent (Fig. 3, A to C) and confocal microscopy images (Fig. 3D), the F-actins and DNA of fibroblasts in 3D fibrin gels were stained with Alexa Fluor 488 Phalloidin (A12379, Life Technologies) and 7-aminoactinomycin D (A1310, Life Technologies), respectively. Before staining, fixation and permeabilization of fibroblasts were performed using a kit (554714, BD Cytofix/Cytoperm) containing both a fixation/permeabilization solution and a wash buffer. After staining, the fibrin gels were washed with phosphate buffered saline (Gibco, Life Technologies).
In the cell stimulation assay, to visualize and characterize the change of intracellular calcium concentration, the cell-permeant fluorescent calcium indicator Fluo-4 AM ester (F14201, Thermo Fisher Scientific) was used. The cells were incubated with 1 μM Fluo-4 AM for 60 min at 37°C followed by a washing step with fresh culture medium.
For the cell viability assays, calcein AM (C3100MP, Life Technologies) and SYTOX orange (S11368, Life Technologies) stains were used to identify live and dead cells, respectively. Cell viability was obtained by counting the numbers of live and dead cells. If the stained cells showed any measurable orange fluorescence, they were counted as dead cells.
Image acquisition and analysis
Optical and fluorescence microscopy images/videos were acquired using an inverted microscope (TE2000-U, Nikon) with a charge-coupled device digital camera (CoolSNAP HQ2, Photometrics). A MATLAB script was written to analyze the fluorescence microscopy images and to extract the image intensities. Confocal microscopy images were acquired using a high-sensitivity, point-scanning, confocal imaging system (LSM-880, Zeiss). Millimeter-scaled photos were taken using a digital camera (EOS Rebel T3i, Canon).
Numerical and analytical simulations
Numerical simulations of acoustic waves in a solid-fluid-solid system and SAWs in an X-cut LiNbO3 substrate are performed using the commercial finite element modeling and analysis software COMSOL Multiphysics. The simulation details can be found in sections S1 and S2. Analytical simulations are performed using a recently developed model for predicting acoustic wavefields and Gor’kov potential fields generated by a circular array of plane-wave transducers (14).
Acknowledgments
Funding: We acknowledge support from the NIH (R01GM132603, R01GM135486, UG3TR002978, R33CA223908, R01GM127714, and R01HD086325), United States Army Medical Research Acquisition Activity (W81XWH-18-1-0242), and NSF (ECCS-1807601). Author contributions: Z.T., Z.W., and P.Z. conceived the idea. Z.T., Z.W., and Y.W. performed acoustic experiments and data analyses. S.Y. and H.B. fabricated the IDTs and customized plastic holders. Y.L. and Z.Y. cultured and prepared cells. Z.W. performed cell staining and confocal microscopy imaging. P.Z. prepared solutions for constructing 3D fibrin gels. Z.T. and Y.G. performed numerical and analytical simulations. All the authors contributed to the paper writing. Z.T., H.B., J.M., and T.D.N. contributed to the paper revision. T.J.H. provided overall guidance and supervised the study. Competing interests: T.J.H. has cofounded a start-up company, Ascent Bio-Nano Technologies Inc., to commercialize technologies involving acoustofluidics and acoustic tweezers. All other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/37/eabb0494/DC1
REFERENCES AND NOTES
- 1.Ozcelik A., Rufo J., Guo F., Gu Y., Li P., Lata J., Huang T. J., Acoustic tweezers for the life sciences. Nat. Methods 15, 1021–1028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friend J., Yeo L. Y., Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 83, 647–704 (2011). [Google Scholar]
- 3.Oberti S., Neild A., Dual J., Manipulation of micrometer sized particles within a micromachined fluidic device to form two-dimensional patterns using ultrasound. J. Acoust. Soc. Am. 121, 778–785 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Dual J., Schwarz T., Acoustofluidics 3: Continuum mechanics for ultrasonic particle manipulation. Lab Chip 12, 244–252 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Baudoin M., Gerbedoen J.-C., Riaud A., Matar O. B., Smagin N., Thomas J.-L., Folding a focalized acoustical vortex on a flat holographic transducer: Miniaturized selective acoustical tweezers. Sci. Adv. 5, eaav1967 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riaud A., Baudoin M., Matar O. B., Becerra L., Thomas J.-L., Selective manipulation of microscopic particles with precursor swirling rayleigh waves. Phys. Rev. Appl. 7, 024007 (2017). [Google Scholar]
- 7.Marzo A., Caleap M., Drinkwater B. W., Acoustic virtual vortices with tunable orbital angular momentum for trapping of mie particles. Phys. Rev. Lett. 120, 044301 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Wilson R., Reboud J., Bourquin Y., Neale S. L., Zhang Y., Cooper J. M., Phononic crystal structures for acoustically driven microfluidic manipulations. Lab Chip 11, 323–328 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Reboud J., Bourquin Y., Wilson R., Pall G. S., Jiwaji M., Pitt A. R., Graham A., Waters A. P., Cooper J. M., Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc. Natl. Acad. Sci. U.S.A. 109, 15162–15167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiklund M., Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab Chip 12, 2018–2028 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Hultström J., Manneberg O., Dopf K., Hertz H. M., Brismar H., Wiklund M., Proliferation and viability of adherent cells manipulated by standing-wave ultrasound in a microfluidic chip. Ultrasound Med. Biol. 33, 145–151 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Collins D. J., Morahan B., Garcia-Bustos J., Doerig C., Plebanski M., Neild A., Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves. Nat. Commun. 6, 8686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo F., Mao Z. M., Chen Y. C., Xie Z. W., Lata J. P., Li P., Ren L. Q., Liu J. Y., Yang J., Dao M., Suresh S., Huang T. J., Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Natl. Acad. Sci. U.S.A. 113, 1522–1527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Z., Yang S., Huang P.-H., Wang Z., Zhang P., Gu Y., Bachman H., Chen C., Wu M., Xie Y., Huang T. J., Wave number-spiral acoustic tweezers for dynamic and reconfigurable manipulation of particles and cells. Sci. Adv. 5, eaau6062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernassau A. L., Glynne-Jones P., Gesellchen F., Riehle M., Hill M., Cumming D. R. S., Controlling acoustic streaming in an ultrasonic heptagonal tweezers with application to cell manipulation. Ultrasonics 54, 268–274 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Ren L., Yang S., Zhang P., Qu Z., Mao Z., Huang P.-H., Chen Y., Wu M., Wang L., Li P., Huang T. J., Standing surface acoustic wave (SSAW)-based fluorescence-activated cell sorter. Small 14, e1801996 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M. V., Tovar A. R., Lee A. P., Lateral cavity acoustic transducer as an on-chip cell/particle microfluidic switch. Lab Chip 12, 139–145 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Antfolk M., Magnusson C., Augustsson P., Lilja H., Laurell T., Acoustofluidic, label-free separation and simultaneous concentration of rare tumor cells from white blood cells. Anal. Chem. 87, 9322–9328 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Collins D. J., Khoo B. L., Ma Z. C., Winkler A., Weser R., Schmidt H., Han J., Ai Y., Selective particle and cell capture in a continuous flow using micro-vortex acoustic streaming. Lab Chip 17, 1769–1777 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Guo F., Li P., French J. B., Mao Z. M., Zhao H., Li S. X., Nama N., Fick J. R., Benkovic S. J., Huang T. J., Controlling cell-cell interactions using surface acoustic waves. Proc. Natl. Acad. Sci. U.S.A. 112, 43–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S., Guo F., Chen Y., Ding X., Li P., Wang L., Cameron C. E., Huang T. J., Standing surface acoustic wave based cell coculture. Anal. Chem. 86, 9853–9859 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim H. G., Shung K. K., Quantification of inter-erythrocyte forces with ultra-high frequency (410 MHz) single beam acoustic tweezer. Ann. Biomed. Eng. 45, 2174–2183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M. G., Park J., Lim H. G., Yoon S., Lee C., Chang J. H., Shung K. K., Label-free analysis of the characteristics of a single cell trapped by acoustic tweezers. Sci. Rep. 7, 14092 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Z., Sun Y., Chen D., Tay D., Chen W., Deng C. X., Fu J., Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Sci. Rep. 3, 2176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K., Wu M., Guo F., Li P., Chan C. Y., Mao Z. M., Li S., Ren L., Zhang R., Huang T. J., Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab Chip 16, 2636–2643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordin M., Laurell T., Two-hundredfold volume concentration of dilute cell and particle suspensions using chip integrated multistage acoustophoresis. Lab Chip 12, 4610–4616 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Garg N., Westerhof T. M., Liu V., Liu R., Nelson E. L., Lee A. P., Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsyst. Nanoeng. 4, 17085 (2018). [Google Scholar]
- 28.Park J., Destgeer G., Kim H., Cho Y., Sung H. J., In-droplet microparticle washing and enrichment using surface acoustic wave-driven acoustic radiation force. Lab Chip 18, 2936–2945 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Li H., Friend J. R., Yeo L. Y., Surface acoustic wave concentration of particle and bioparticle suspensions. Biomed. Microdevices 9, 647–656 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Destgeer G., Cho H., Ha B. H., Jung J. H., Park J., Sung H. J., Acoustofluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 16, 660–667 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Kurashina Y., Takemura K., Friend J., Cell agglomeration in the wells of a 24-well plate using acoustic streaming. Lab Chip 17, 876–886 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Augustsson P., Karlsen J. T., Su H.-W., Bruus H., Voldman J., Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping. Nat. Commun. 7, 11556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Liu Z. Z., Shin D. M., Chen Z. G., Cho Y., Kim Y.-J., Han A., A continuous-flow acoustofluidic cytometer for single-cell mechanotyping. Lab Chip 19, 387–393 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Lata J. P., Guo F., Guo J. S., Huang P.-H., Yang J., Huang T. J., Surface acoustic waves grant superior spatial control of cells embedded in hydrogel fibers. Adv. Mater. 28, 8632–8638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naseer S. M., Manbachi A., Samandari M., Walch P., Gao Y., Zhang Y. S., Davoudi F., Wang W., Abrinia K., Cooper J. M., Khademhosseini A., Shin S. R., Surface acoustic waves induced micropatterning of cells in gelatin methacryloyl (GelMA) hydrogels. Biofabrication 9, 015020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang B., Shin J., Park H.-J., Rhyou C., Kang D., Lee S.-J., Yoon Y.-s., Cho S.-W., Lee H., High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat. Commun. 9, 5402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouyer C., Chen P., Güven S., Demirtaş T. T., Nieland T. J. F., Padilla F., Demirci U., A bio-acoustic levitational (BAL) assembly method for engineering of multilayered, 3D brain-like constructs, Using human embryonic stem cell derived neuro-progenitors. Adv. Mater. 28, 161–167 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Armstrong J. P. K., Puetzer J. L., Serio A., Guex A. G., Kapnisi M., Breant A., Zong Y., Assal V., Skaalure S. C., King O., Murty T., Meinert C., Franklin A. C., Bassindale P. G., Nichols M. K., Terracciano C. M., Hutmacher D. W., Drinkwater B. W., Klein T. J., Perriman A. W., Stevens M. M., Engineering anisotropic muscle tissue using acoustic cell patterning. Adv. Mater. 30, e1802649 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demirci U., Picoliter droplets for spinless photoresist deposition. Rev. Sci. Instrum. 76, 065103 (2005). [Google Scholar]
- 40.Wixforth A., Acoustically driven planar microfluidics. Superlattice Microst. 33, 389–396 (2003). [Google Scholar]
- 41.Zhang S. P., Lata J., Chen C., Mai J., Guo F., Tian Z., Ren L., Mao Z., Huang P.-H., Li P., Yang S., Huang T. J., Digital acoustofluidics enables contactless and programmable liquid handling. Nat. Commun. 9, 2928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed D., Ozcelik A., Bojanala N., Nama N., Upadhyay A., Chen Y. C., Hanna-Rose W., Huang T. J., Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 7, 11085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J., Yang S., Chen C., Hartman J. H., Huang P.-H., Wang L., Tian Z., Zhang P., Faulkenberry D., Meyer J. N., Huang T. J., Surface acoustic waves enable rotational manipulation of Caenorhabditis elegans. Lab Chip 19, 984–992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu M., Ouyang Y., Wang Z., Zhang R., Huang P.-H., Chen C., Li H., Li P., Quinn D., Dao M., Suresh S., Sadovsky Y., Huang T. J., Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. U.S.A. 114, 10584–10589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezeli M., Gidlöf O., Evander M., Bryl-Górecka P., Sathanoori R., Gilje P., Pawłowski K., Horvatovich P., Erlinge D., Marko-Varga G., Laurell T., Comparative proteomic analysis of extracellular vesicles isolated by acoustic trapping or differential centrifugation. Anal. Chem. 88, 8577–8586 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Li P., Huang T. J., Applications of acoustofluidics in bioanalytical chemistry. Anal. Chem. 91, 757–767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M., Ozcelik A., Rufo J., Wang Z., Fang R., Jun Huang T., Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 5, 32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nama N., Huang P.-H., Huang T. J., Costanzo F., Investigation of acoustic streaming patterns around oscillating sharp edges. Lab Chip 14, 2824–2836 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed D., Chan C. Y., Lin S.-C. S., Muddana H. S., Nama N., Benkovic S. J., Jun Huang T., Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles. Lab Chip 13, 328–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y. Q., Luo J. K., Nguyen N. T., Walton A. J., Flewitt A. J., Zu X. T., Li Y., McHale G., Matthews A., Iborra E., Du H., Milne W. I., Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 89, 31–91 (2017). [Google Scholar]
- 51.Meng L., Cai F., Li F., Zhou W., Niu L., Zheng H., Acoustic tweezers. J. Phys. D Appl. Phys. 52, 273001 (2019). [Google Scholar]
- 52.Melde K., Mark A. G., Qiu T., Fischer P., Holograms for acoustics. Nature 537, 518–522 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Melde K., Choi E., Wu Z. G., Palagi S., Qiu T., Fischer P., Acoustic fabrication via the assembly and fusion of particles. Adv. Mater. 30, 1704507 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Marzo A., Drinkwater B. W., Holographic acoustic tweezers. Proc. Natl. Acad. Sci. U.S.A. 116, 84–89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khademhosseini A., Langer R., A decade of progress in tissue engineering. Nat. Protoc. 11, 1775–1781 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Bajaj P., Schweller R. M., Khademhosseini A., West J. L., Bashir R., 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 16, 247–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y. S., Yue K., Aleman J., Mollazadeh-Moghaddam K., Bakht S. M., Yang J. Z., Jia W. T., Dell’Erba V., Assawes P., Shin S. R., Dokmeci M. R., Oklu R., Khademhosseini A., 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 45, 148–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chansoria P., Narayanan L. K., Schuchard K., Shirwaiker R., Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication 11, 035015 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Chansoria P., Shirwaiker R., Characterizing the process physics of ultrasound-assisted bioprinting. Sci. Rep. 9, 13889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A., Anderson K. L., Swift M. F., Hanein D., Volkmann N., Schwartz M. A., Local tension on talin in focal adhesions correlates with F-actin alignment at the nanometer scale. Biophys. J. 115, 1569–1579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demore C. E. M., Yang Z., Volovick A., Cochran S., MacDonald M. P., Spalding G. C., Mechanical evidence of the orbital angular momentum to energy ratio of vortex beams. Phys. Rev. Lett. 108, 194301 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Tian Z., Yu L., Elastic phased diffraction gratings for manipulation of ultrasonic guided waves in solids. Phys. Rev. Appl. 11, 024052 (2019). [Google Scholar]
- 63.Tian Z., Shen C., Li J., Reit E., Gu Y., Fu H., Cummer S. A., Huang T. J., Programmable acoustic metasurfaces. Adv. Funct. Mater. 29, 1808489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian Z., Shen C., Li J., Reit E., Bachman H., Socolar J. E. S., Cummer S. A., Jun Huang T., Dispersion tuning and route reconfiguration of acoustic waves in valley topological phononic crystals. Nat. Commun. 11, 762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mellin S. D., Nordin G. P., Limits of scalar diffraction theory and an iterative angular spectrum algorithm for finite aperture diffractive optical element design. Opt. Express 8, 705–722 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Fykse E. M., Olsen J. S., Skogan G., Application of sonication to release DNA from Bacillus cereus for quantitative detection by real-time PCR. J. Microbiol. Methods 55, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- 67.J. M. Walker, The Protein Protocols Handbook (Humana Press, 2009). [Google Scholar]
- 68.Campbell J. J., Jones W. R., Propagation of surface waves at the boundary between a piezoelectric crystal and a fluid medium. IEEE Trans. Sonics Ultrasonics 17, 71–76 (1970). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/37/eabb0494/DC1


