Abstract
Prostaglandin E2 (PGE2), produced by macrophages, has important immune regulatory functions, suppressing a type 1 immune response and stimulating a type 2 immune response. Type 1 cytokines (interleukin-2 [IL-2], IL-12, and gamma interferon) increase in freshly isolated peripheral blood mononuclear cells (PBMCs) of animals with an early disease stage of bovine leukemia virus (BLV) infection, while IL-10 increases in animals with a late disease stage. Although IL-10 has an immunosuppressive role in the host immune system, IL-10 also inhibits BLV tax and pol mRNA levels in vitro. In contrast, IL-2 stimulates BLV tax and pol mRNA and p24 protein expression in cultured PBMCs. The inhibitory effect of IL-10 on BLV expression depends on soluble factors secreted by macrophages. Thus, we hypothesized that PGE2, a cyclooxygenase 2 (COX-2) product of macrophages, may regulate BLV expression. Here, we show that the level of COX-2 mRNA was decreased in PBMCs treated with IL-10, while IL-2 enhanced the level of COX-2 mRNA. Addition of PGE2 stimulated BLV tax and pol mRNA levels and reversed the IL-10 inhibition of BLV mRNA. In addition, the specific COX-2 inhibitor, NS-398, inhibited the amount of BLV mRNA detected. Addition of PGE2 increased BLV tax mRNA regardless of NS-398 addition. PGE2 inhibited antigen-specific PBMC stimulation, suggesting that stimulation of BLV tax and pol mRNA levels by PGE2 is independent of cell proliferation. These findings suggest that macrophage-derived COX-2 products, such as PGE2, regulate virus expression and disease progression in BLV infection.
Bovine leukemia virus (BLV), closely related to human T-cell leukemia virus type 1 (HTLV-1), is a type C retrovirus that infects bovine B cells and leads to enzootic bovine leukosis (16). The genomes of BLV and HTLV-1 are similarly arranged. In particular, the 3′ region that contains the tax, rex, R3, and G4 genes is unique to BLV and HTLV-1 (2). In addition, there are several features of pathogenesis that are shared by BLV and HTLV-1. For both viruses, many infected individuals develop antibodies, but clinical symptoms are relatively rare. Disease progression in BLV-infected animals is divided into three stages: serologically positive, persistent lymphocytosis negative (alymphocytotic [AL]); serologically positive, persistent lymphocytosis positive (persistently lymphocytotic [PL]); and tumor-bearing stages (usually lymphosarcoma). Most infected animals never display outward signs of disease and are referred to as asymptomatic or aleukemic. Fewer than 5% of infected animals develop malignant lymphosarcoma (11), while 30% of infected animals progress to persistent lymphocytosis, in which nonneoplastic B cells proliferate and leukocyte counts may exceed 10,000 cells/mm3 (17). Usually there is a long duration between these disease stages. The mechanism of disease progression from AL to PL or tumor-bearing stage is not clear.
Recent investigation has revealed that cytokine production plays a critical role in the progression of many different diseases (9, 32). In previous studies, we found cytokine polarization in BLV-infected animals at different disease stages (27, 29). Type 1 cytokines, such as interleukin-2 (IL-2), IL-12, and gamma interferon (IFN-γ), were expressed in high amounts in AL animals, while IL-10 was increased in PL and tumor-bearing animals. Other studies showed that antigen-specific lymphocyte proliferation (25) and cytotoxic γδ T-cell activity (P. S. Lundberg and G. A. Splitter, submitted for publication) were reduced in PL and tumor-bearing animals. Because IL-10 had an inhibitory effect on type 1 immune responses, increased IL-10 expression in late disease stages may result in suppressed cell-mediated immune responses. Nevertheless, we found that IL-10 inhibited BLV tax and pol mRNA levels, while IL-2 stimulated detection of BLV in peripheral blood mononuclear cells (PBMCs) in vitro (28). These data suggest that increasing levels of IL-10 during BLV infection may have a beneficial effect to suppress BLV expression in infected B cells. In addition, we found that macrophages have a critical role in BLV expression and secrete certain factor(s) to activate BLV tax and pol mRNA.
We hypothesized that one of the soluble factor(s) may be prostaglandin E2 (PGE2) that is produced by cyclooxygenase 2 (COX-2). PGE2 is the only prostaglandin produced by macrophages in response to inflammatory cytokines, such as IL-1 and tumor necrosis factor alpha (TNF-α), that is known to inhibit cell-mediated immune response and stimulate type 2 cytokine production (12, 14, 18, 26, 36). There are two COX enzymes, referred to as COX-1 and COX-2, that produce prostaglandins from arachidonic acid (10). While COX-1 is a constitutive enzyme that is expressed at its highest concentrations in the kidney, stomach, platelets, and vascular endothelium, COX-2 is an inducible enzyme whose expression is regulated by growth factors, tumor promoters, or cytokines. COX-2 is expressed in a few specialized tissues and cells, including macrophages and follicular dendritic cells. The functions of COX-2 and prostaglandins are very important in regulating normal physiological processes (8, 19), as well as the immune response. Here, we demonstrate that IL-10 decreased detection of COX-2 mRNA by PBMCs, while conversely, IL-2 increased COX-2 mRNA. Although PGE2 reduced antigen-specific PBMC proliferation, PGE2 increased detection of BLV and reversed the IL-10 inhibition of BLV tax and pol mRNA levels. In addition, BLV acts as an autocrine stimulator to increase the levels of BLV tax and pol mRNA and COX-2 mRNA.
COX-2 mRNA from PBMCs was inhibited by IL-10 and enhanced by IL-2.
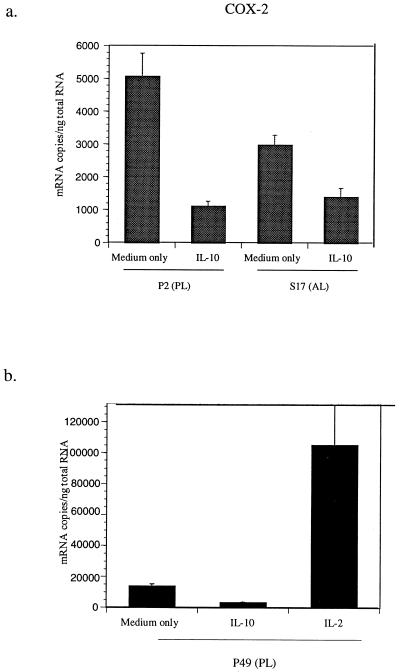
In a previous study, we found that human recombinant IL-10 (hrIL-10) inhibited BLV tax and pol mRNA, while IL-2 enhanced the detection of BLV tax and pol mRNA and BLV p24 protein in PBMCs in vitro (27). Also, IL-10-mediated regulation of BLV expression was macrophage dependent. Because COX-2, an important enzyme for prostaglandin synthesis, is mainly expressed by macrophages (18, 26, 36), we hypothesized that COX-2 and its products may affect BLV expression regulated by IL-2 and IL-10. Quantitative competitive PCR (QC-PCR) was performed to determine COX-2 mRNA levels in PBMCs cultured with or without hrIL-10. Transcripts of COX-2 mRNA were quantified by a competitive reverse transcriptase PCR (RT-PCR) assay using standard curve methodology. Validation of this assay and synthesis of native and competitor standards has been published previously (37, 38). A standard curve was created by RT-PCR using a constant amount of competitor RNA (2 aM) together with twofold serial dilutions of native RNA (15 to 0.11 aM). Unknown mRNA samples were diluted as needed, reverse transcribed and amplified with the same amount of competitor RNA, and compared to the standard curve. Reverse transcription was carried out in 1× RT buffer (Promega), 0.2 mM deoxynucleoside triphosphates, 100 μM random hexamer, and 40 U of Moloney murine leukemia virus RT for 1.5 h at 37°C, followed by 95°C for 10 min in a final volume of 20 μl. Four microliters of RT reaction mixture were then PCR amplified (30 s, 95°C; 30 s, 57°C; 30 s, 72°C) for 30 cycles, followed by 72°C for 5 min in 20 μl of 1× PCR buffer (Promega, Madison, Wis.), 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 0.4 μM upstream and downstream primers, and 0.5 U of Taq DNA polymerase. PCR products were separated on a 5% polyacrylamide gel electrophoresis (PAGE) gel and stained with ethidium bromide. Bands observed in gels were quantified using Collagea software (Fotodyne, Heartland, Wis.). Densitometric values for the standard curve were plotted as follows: log([native RNA]/[competitor RNA]) versus log [native RNA (aM)]. Addition of hrIL-10 reduced the detection of COX-2 mRNA in PBMCs from both AL and PL animals (Fig. 1a). These results paralleled the effects of IL-10 inhibition of BLV tax and pol mRNA levels (27). In contrast, IL-2 dramatically increased detection of COX-2 mRNA (Fig. 1b). These results suggest that cytokines, such as IL-2 and IL-10, can have opposing effects on the regulation of COX-2 mRNA.
FIG. 1.
IL-10 inhibits detection of COX-2 mRNA (a and b), while IL-2 stimulates detection of COX-2 mRNA (b). PBMCs from PL animals and AL animals were cultured for 3 days with or without IL-10 and IL-2, and QC-PCR was performed. PCR products were separated on a 5% PAGE gel and stained with ethidium bromide. Quantification of gels was accomplished using Collagea software. Densitometric values for the standard curve were plotted as follows: log10([native RNA]/[competitor RNA]) versus log10 [native RNA (aM)]. The amounts of COX-2 mRNA were calculated based on the standard curve. The data is representative of three different PL animals tested (P2 [a] and P49 [b]) and three AL animals tested (S17 [a]). Standard errors of means are shown from at least three experiments on cells from the same animal.
PGE2, a COX-2 product, enhances detection of BLV tax and pol mRNA and reverses IL-10 inhibition of BLV tax and pol mRNA.
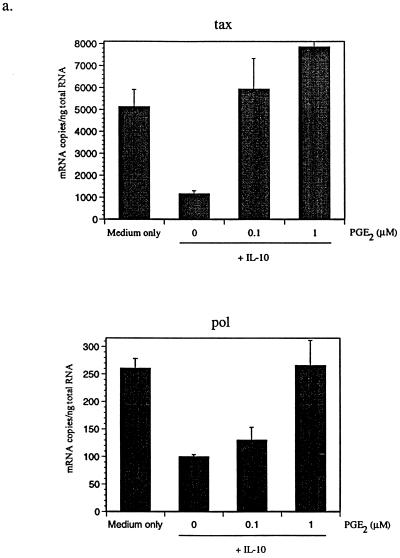
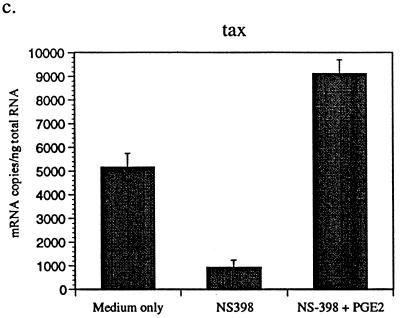
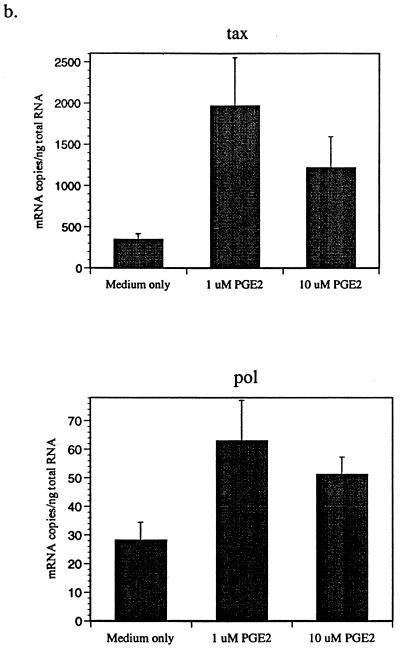
PGE2 is the only COX-2 product expressed by macrophages (14). To determine whether PGE2 affects BLV expression, BLV-infected PBMCs were cultured with or without PGE2 in the presence of hrIL-10. Subsequently, the tax and pol mRNA produced by PBMCs from BLV-infected animals was measured by QC-PCR (27). For standardization, PCR was performed using this serially twofold diluted standard plasmid, with concentrations ranging from 8,192 to 16 fg/μl for tax and from 2,048 to 4 fg/μl for pol and with a fixed amount of mimic (10 fg/μl). Synthesized cDNA from each sample and fixed amounts of mimic were added to the same tube and amplified simultaneously with tubes for a standard reaction. Gel photographs were scanned, and the amplified DNA bands were analyzed by densitometry using the NIH Image program, version 1.61, with standard curves constructed with Cricket graph. The amount of cytokine produced was determined by comparing the density ratios of sample and standard reaction mixtures. As expected, we observed that different animals have different viral loads. Usually, PL animals have more viral load than AL animals. While IL-10 reduced detection of BLV tax and pol mRNA, PGE2 reversed this IL-10 inhibitory effect (Fig. 2a). Addition of PGE2 alone also enhanced BLV tax and pol mRNA levels (Fig. 2b). These results suggest that macrophage-derived PGE2 may stimulate BLV expression from infected B cells and that IL-10 may inhibit BLV expression by reducing PGE2 production. At the higher concentration of PGE2, BLV tax and pol mRNA levels were slightly diminished, indicating that the stimulatory effect of PGE2 was maximal at 1 μM. To confirm the effects of PGE2 to increase BLV mRNA levels, a selective COX-2 inhibitor, NS-398 (21), was added to PBMC cultures. As expected, the level of BLV tax mRNA was dramatically suppressed by the COX-2 inhibitor (Fig. 2c). In contrast, addition of PGE2 increased BLV tax mRNA detection regardless of NS-398 addition (Fig. 2c). These data suggest that a selective COX-2 inhibitor may reduce the level of BLV mRNA in infected B cells and that PGE2 addition may bypass the NS-398 inhibition of COX-2 activity.
FIG. 2.
PGE2 increases detection of BLV tax and pol mRNA. BLV-infected AL and PL PBMCs were cultured for 3 days with IL-10 (10 ng/ml) and increasing concentrations of PGE2 (0.1 and 1 μM) (a). BLV-infected PBMCs were also incubated with PGE2 (1 and 10 μM) alone (b) and/or NS-398 (20 μM) (c). QC-PCR was performed as described in the text. The bands were analyzed using densitometry in the NIH Image program, version 1.61, and representative data are from one of three (a and b) or two (c) different AL and PL animals. Standard errors of means are shown from at least three experiments on cells from the same animal.
Purified BLV increases the level of COX-2 mRNA and has an autocrine effect that increases the levels of BLV tax and pol mRNA.
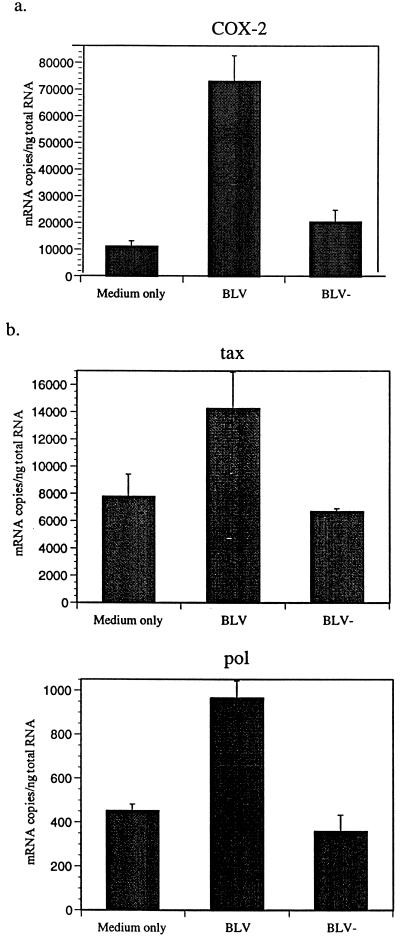
Numerous factors can stimulate COX-2 and PGE2 expression by macrophages. To investigate how BLV infection might affect COX-2 and BLV expression, BLV proteins were purified from the supernatant of the BL3* cell line using metrizamide density gradient centrifugation. Purified BLV antigens were confirmed by sodium dodecyl sulfate-PAGE and immunoblotting with anti-gp51 and -p24 antibodies (data not shown). When purified BLV was added to PBMC cultures, COX-2 mRNA detection rapidly increased (Fig. 3a). Also, detection of BLV tax and pol mRNA increased by addition of purified BLV (Fig. 3b), suggesting that BLV antigens regulate BLV expression by an autocrine mechanism. To remove the possibility that other factors isolated during BLV antigen purification could affect COX-2 and BLV expression, a BLV-negative reagent was prepared from the BL3 cell line, which does not produce any BLV antigens. BLV gp51 and p24 were not detected by immunoblotting in the purified BLV-negative material. The BL3 purified material could not enhance COX-2 mRNA or BLV tax and pol mRNA (Fig. 3).
FIG. 3.
BLV proteins stimulate COX-2 mRNA (a) and BLV tax and pol mRNA (b). BLV proteins were purified from BL3* supernatant, and BLV-negative reagent was prepared from BL3 supernatant using metrizamide density gradient centrifugation. BLV-infected PBMCs were cultured for 3 days with similar volumes of purified BLV (10 μg/ml) and BLV-negative (BLV−) materials. COX-2 and BLV tax and pol mRNA were quantified as described above. Shown are representative data (PL animals) of experiments with three different AL and PL animals. Standard errors of means are shown from at least three experiments on cells from the same animal.
PGE2 suppresses antigen-specific PBMC proliferation.
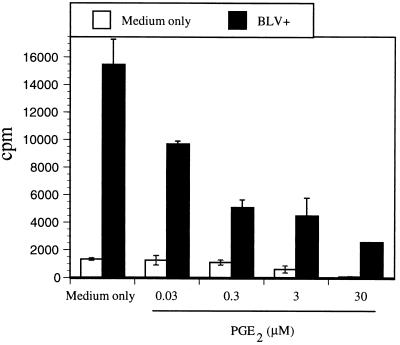
To determine if PBMC proliferation and BLV expression were correlated, different concentrations of PGE2 were added to the PBMC cultures with or without BL3* supernatant as a viral antigen source (23). Following incubation of PBMCs for 3 to 5 days, cells were harvested for further experiments. In cell proliferation assays, [3H]thymidine was added 8 to 12 h before harvest and the radioactivity of the harvested cells was measured by a β-counter (MATRIX 9600; Packard, Meriden, Conn.). Medium alone was used as a control in proliferation assay. Addition of BL3* supernatant increased PBMC proliferation more than 10-fold. However, antigen-specific PBMC proliferation was dramatically suppressed with increasing concentrations of PGE2 (Fig. 4). Spontaneous cell proliferation in the absence of antigens was also slightly reduced by addition of PGE2 (Fig. 4). Thus, PGE2 enhanced BLV detection but suppressed antigen-specific PBMC proliferation. The concentration of cells differed between transcription and proliferation assays, as fewer cells were optimal in the U-bottom wells for the proliferation assay.
FIG. 4.
PGE2 inhibits BLV-specific PBMC proliferation. BLV-infected PBMCs from AL and PL animals were cultured with BL3* supernatant (BLV+) or medium alone (BLV−) for 5 days with different concentrations of PGE2 (0.03 to 30 μM). Proliferation was assessed by [3H]thymidine incorporation using a β-scintillation counter. The data are representative of experiments with four different AL and PL animals. Standard errors of means are shown.
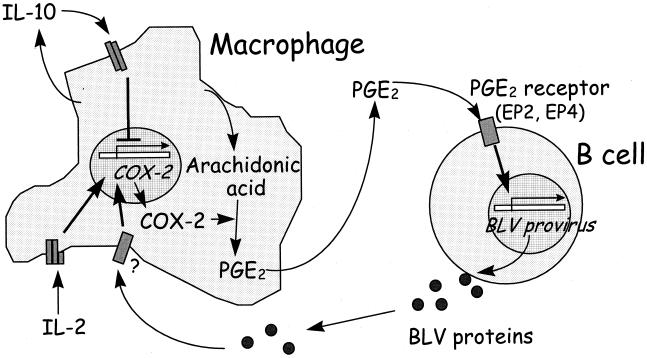
The results presented here demonstrate that IL-10 inhibited detection of BLV tax and pol mRNA and reduced COX-2 transcription from macrophages, while PGE2 activated BLV tax and pol mRNA. These data indicate that regulation of BLV is closely related to signals induced by PGE2. Both AL and PL animals, but not noninfected animals, produced similar findings when tested with IL-2, IL-10, and PGE2, supporting a common mechanism of pathogenesis in infected animals. Also, in spite of the relatively low IL-10 levels, AL animals produced more IL-10 than uninfected animals, and AL and PL animals responded similarly to IL-10 and PGE2. These findings suggest that macrophages produce PGE2 and have a central role in regulating BLV expression in infected B cells (Fig. 5).
FIG. 5.
Possible linkage between BLV expression by B cells and COX-2 and PGE2 produced by macrophages. Arrows represent positive regulation, and a blunt line represents negative regulation.
The E series of prostaglandins are widely known as immunosuppressive products produced by macrophages, follicular dendritic cells, and fibroblasts (10, 17). These prostaglandins, especially PGE2, can downregulate many aspects of B- and T-cell functions. PGE2 production is triggered by inflammatory cytokines, such as IL-1 and TNF-α, that are produced in viral and bacterial infections (4, 20). Increasing PGE2 negatively regulates type 1 cytokines, such as IL-2, IFN-γ, and TNF-α, by increasing production of type 2 cytokines, such as IL-10 (13). Thus, PGE2 may have a central role in regulating production of type 1 and type 2 cytokines. PGE2 activates a humoral immune response, stimulating B-cell differentiation and immunoglobulin class switching (26). In this paper, we show that PGE2 stimulated detection of BLV tax and pol mRNA and inhibited PBMC proliferation. These findings suggest that enhancement of BLV expression by PGE2 may not depend on cell proliferation. Ironically, B cells are the only PBMCs that are significantly infected with BLV (22), while macrophages are the only source of PGE2 in PBMCs (14). However, B cells have a number of PGE2 receptors that regulate B-cell activation (7). Signal transduction by PGE2 receptors mediates increased cyclic AMP (cAMP) production (3). BLV long terminal repeats (LTRs) contain a cAMP-response element (CRE) that facilitates BLV gene transcription (1, 39). Tax stabilizes CRE-binding protein (CREB) to bind CRE in LTRs (5). Thus, CREB and Tax may activate BLV expression of infected B cells. In addition, protein kinase C (PKC) increases BLV expression with increased Ca2+ influx (15). Therefore, increased PGE2 production by macrophages may stimulate BLV tax and pol mRNA expression through cAMP-dependent PKA and/or PKC signal transduction pathway. BLV LTRs also contain NF-κB binding sites that facilitate BLV transcription (6). Recently, antiinflammatory agents, such as aspirin and salicylate, reportedly inhibit the activity of IκB kinase-β, which facilitates the degradation of IκB and activates NF-κB (40). Antiinflammatory agents that inhibit prostaglandin synthesis may suppress BLV expression via NF-κB inhibition.
We demonstrate that BLV functions as a stimulant of COX-2 expression. Although PGE2 enhances IL-10 expression (13) to inhibit COX-2 and BLV expression, a synergistic effect of BLV expression, opportunistic infections (35), pregnancy (19), and/or stress (8) could induce disease progression in BLV infection. COX-2 and PGE2 also inhibit programmed cell death and facilitate tumor formation (33, 34), and thus these activities might promote lymphosarcoma and leukemia with other carcinogenic factors, such as Bcl-2 and BLV Tax protein, in BLV infection. Thus, in other retrovirus infections, the inhibitory function of IL-10 on human immunodeficiency virus expression has been reported (24, 30, 31). Most studies have utilized macrophage cell lines and primary macrophages, while the studies with T-cell lines or primary T cells failed to demonstrate the IL-10 inhibition of human immunodeficiency virus expression. Therefore, macrophages may have a direct role in regulating retrovirus expression responding to IL-10 (32). Our preliminary data showed that bovine herpes virus type 1 and Brucella abortus, two common opportunistic infections in cattle, activated COX-2 mRNA expression (D. Pyeon and G. A. Splitter, unpublished data). We anticipate that further research regarding PGE2 and opportunistic infections will reveal additional clues to solve the complicated mechanisms of disease progression in retrovirus infections.
To examine whether inhibitors of PGE2 or COX-2 would be efficacious for treatment of BLV infection, in vivo studies are necessary. Infected animals could be treated with a PGE2 inhibitor, such as indomethacin, and viral load and BLV expression could be measured. Treatment with a PGE2 inhibitor may reduce BLV load in BLV-infected animals and would support a role for PGE2 to stimulate BLV replication and disease progression in vivo. This study provides an additional strategy to treat retrovirus infection combined with currently available antiretroviral treatment.
Acknowledgments
This work was supported by National Cancer Institute grant R01 CA59127, BARD 95-34339-2556, and the College of Agricultural and Life Sciences.
REFERENCES
- 1.Adam E, Kerkhofs P, Mammerickx M, Kettmann R, Burny A, Droogmans L, Willems L. Involvement of the cyclic AMP-responsive element binding protein in bovine leukemia virus expression in vivo. J Virol. 1994;68:5845–5853. doi: 10.1128/jvi.68.9.5845-5853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandersen S, Carpenter S, Christensen J, Storgaard T, Viuff B, Wannemuehler Y, Belousov J, Roth J A. Identification of alternatively spliced mRNAs encoding potential new regulatory proteins in cattle infected with bovine leukemia virus. J Virol. 1993;67:39–52. doi: 10.1128/jvi.67.1.39-52.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An S, Yang J, So S W, Zeng L, Goetzl E J. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- 4.Bachwich P R, Chensue S W, Larrick J W, Kunkel S L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986;136:94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- 5.Boros I M, Tie F, Giam C Z. Interaction of bovine leukemia virus transactivator Tax with bZip protein. Virology. 1995;214:207–214. doi: 10.1006/viro.1995.9939. [DOI] [PubMed] [Google Scholar]
- 6.Brooks P A, Nyborg J K, Cockerell G L. Identification of an NF-kappa B binding site in the bovine leukemia virus promoter. J Virol. 1995;69:6005–6009. doi: 10.1128/jvi.69.10.6005-6009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D M, Phipps R P. Characterization and regulation of prostaglandin E2 receptors on normal and malignant murine B lymphocytes. Cell Immunol. 1995;161:79–87. doi: 10.1006/cimm.1995.1011. [DOI] [PubMed] [Google Scholar]
- 8.Chancellor-Freeland C, Zhu G F, Kage R, Beller D I, Leeman S E, Black P H. Substance P and stress-induced changes in macrophages. Ann N Y Acad Sci. 1995;771:472–484. doi: 10.1111/j.1749-6632.1995.tb44703.x. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Shearer G M. A TH1--TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt D, Smith W L. Yes, but do they still get headaches? Cell. 1995;83:345–348. doi: 10.1016/0092-8674(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 11.Esteban E N, Thorn R M, Ferrer J F. Characterization of the blood lymphocyte population in cattle infected with the bovine leukemia virus. Cancer Res. 1985;45:3225–3230. [PubMed] [Google Scholar]
- 12.Fedyk E R, Brown D M, Phipps R P. PGE2 regulation of B lymphocytes and T helper 1 and T helper 2 cells: induction of inflammatory versus allergic responses. Adv Exp Med Biol. 1997;407:237–242. doi: 10.1007/978-1-4899-1813-0_35. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Sharma S, Mao J T, Dubinett S M. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol. 1996;157:5512–5520. [PubMed] [Google Scholar]
- 14.Kennedy M S, Stobo J D, Goldyne M E. In vitro synthesis of prostaglandins and related lipids by populations of human peripheral blood mononuclear cells. Prostaglandins. 1980;20:135–145. doi: 10.1016/0090-6980(80)90013-1. [DOI] [PubMed] [Google Scholar]
- 15.Kerkhofs P, Adam E, Droogmans L, Portetelle D, Mammerickx M, Burny A, Kettmann R, Willems L. Cellular pathways involved in the ex vivo expression of bovine leukemia virus. J Virol. 1996;70:2170–2177. doi: 10.1128/jvi.70.4.2170-2177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kettmann R, Portetelle D, Mammerickx M, Cleuter Y, Dekegel D, Galoux M, Ghysdael J, Burny A, Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci USA. 1976;73:1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama H, Nakanishi H, Kajikawa O, Yoshikawa H, Tsubaki S, Yoshikawa T, Saito H. T and B lymphocytes in persistently lymphocytotic and leukemic cattle. Jpn J Vet Sci. 1983;45:471–475. doi: 10.1292/jvms1939.45.471. [DOI] [PubMed] [Google Scholar]
- 18.Kurland J I, Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978;147:952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lala P K. Similarities between immunoregulation in pregnancy and in malignancy: the role of prostaglandin E2. Am J Reprod Immunol. 1989;20:147–152. doi: 10.1111/j.1600-0897.1989.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 20.Lehmmann V, Benninghoff B, Droge W. Tumor necrosis factor-induced activation of peritoneal macrophages is regulated by prostaglandin E2 and cAMP. J Immunol. 1988;141:587–591. [PubMed] [Google Scholar]
- 21.Masferrer J L, Zweifer B S, Manning P T, Hauser S D, Leahy K M, Smith W G, Isakson P C, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirsky M L, Olmstead C A, Da Y, Lewin H A. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J Virol. 1996;70:2178–2183. doi: 10.1128/jvi.70.4.2178-2183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirsky M L, Da Y, Lewin H A. Detection of bovine leukemia virus proviral DNA in individual cells. Genome Res. 1993;2:333–340. doi: 10.1101/gr.2.4.333. [DOI] [PubMed] [Google Scholar]
- 24.Naif H M, Chang J, Ho-Shon M, Li S, Cunningham A L. Inhibition of human immunodeficiency virus replication in differentiating monocytes by interleukin 10 occurs in parallel with inhibition of cellular RNA expression. AIDS Res Hum Retrovir. 1996;12:1237–1245. doi: 10.1089/aid.1996.12.1237. [DOI] [PubMed] [Google Scholar]
- 25.Orlik O, Splitter G A. Progression to persistent lymphocytosis and tumor development in bovine leukemia virus (BLV)-infected cattle correlates with impaired proliferation of CD4+ T cells in response to gag- and env-encoded BLV proteins. J Virol. 1996;70:7584–7593. doi: 10.1128/jvi.70.11.7584-7593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phipps R P, Stein S H, Roper R L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 27.Pyeon D, Splitter G A. Interleukin-12 p40 mRNA expression in bovine leukemia virus-infected animals: increase in alymphocytosis but decrease in persistent lymphocytosis. J Virol. 1998;72:6917–6921. doi: 10.1128/jvi.72.8.6917-6921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyeon D, Splitter G A. Regulation of interleukin-2 and -10 on bovine leukemia virus tax and pol mRNA levels. J Virol. 1999;73:8427–8434. doi: 10.1128/jvi.73.10.8427-8434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyeon D, O'Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saville M W, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–3599. [PubMed] [Google Scholar]
- 31.Schuitemaker H. IL-4 and IL-10 as potent inhibitors of HIV1 replication in macrophages in vitro: a role for cytokines in the in vivo virus host range? Res Immunol. 1994;145:588–592. doi: 10.1016/s0923-2494(05)80038-0. [DOI] [PubMed] [Google Scholar]
- 32.Shearer G M, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 33.Sheng H, Shao J, Morrow J D, Beauchamp R D, DuBois R N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 34.Sheng H, Shao J, Kirkland S C, Isakson P, Coffey R J, Morrow J, Beauchamp R D, DuBois R N. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Investig. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver R M, Edwin S S, Trautman M S, Simmons D L, Branch D W, Dudley D J, Mitchell M D. Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Investig. 1995;95:725–731. doi: 10.1172/JCI117719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin-10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai S-J, Wiltbank M C. Quantification of mRNA using competitive RT-PCR with standard-curve methodology. BioTechniques. 1996;21:862–866. doi: 10.2144/96215st04. [DOI] [PubMed] [Google Scholar]
- 38.Tsai S-J, Wiltbank M C. Prostaglandin F2alpha regulates distinct physiological changes in early and mid-cycle bovine corpora lutea. Biol Reprod. 1998;58:346–352. doi: 10.1095/biolreprod58.2.346. [DOI] [PubMed] [Google Scholar]
- 39.Willems L, Kettmann R, Chen G, Portetelle D, Burny A, Derse D. A cyclic AMP-responsive DNA-binding protein (CREB2) is a cellular transactivator of the bovine leukemia virus long terminal repeat. J Virol. 1992;66:766–772. doi: 10.1128/jvi.66.2.766-772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin M J, Yamamoto Y, Gaynor R B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]