Abstract
Visceral leishmaniasis is a disease caused by protozoa of the species Leishmania (Leishmania) infantum (syn = Leishmania chagasi) and Leishmania (Leishmania) donovani, which are transmitted by hematophagous insects of the genera Lutzomyia and Phlebotomus. The domestic dog (Canis familiaris) is considered the main urban reservoir of the parasite due to the high parasite load on its skin, serving as a source of infection for sandfly vectors and, consequently, perpetuating the disease in the urban environment. Some factors are considered important in the perpetuation and spread of canine visceral leishmaniasis (CVL) in urban areas, such as stray dogs, with their errant behavior, and houses that have backyards with trees, shade, and organic materials, creating an attractive environment for sandfly vectors. CVL is found in approximately 50 countries, with the number of infected dogs reaching millions. However, due to the difficulty of controlling and diagnosing the disease, the number of infected animals could be even greater. In the four continents endemic for CVL, there are reports of disease expansion in endemic countries such as Brazil, Italy, Morocco, and Tunisia, as well as in areas where CVL is not endemic, for example, Uruguay. Socio-environmental factors, such as migration, drought, deforestation, and global warming, have been pointed out as reasons for the expansion into areas where it had been absent. Thus, the objective of this review is to address (i) the distribution of CVL in endemic areas, (ii) the role of the dog in the visceral leishmaniasis epidemiology and the factors that influence dog infection and the spread of the disease, and (iii) the challenges faced in the control of CVL.
Keywords: Leishmania infantum, epidemiology, canine visceral leishmaniasis
1. Introduction
Leishmaniasis is a complex of diseases whose etiological agent is the digenetic protozoa (amastigote and promastigote forms) of the Leishmania genus. The transmission of the parasite occurs during the blood meal of female phlebotomine sandflies of the genus Phlebotomus in the Old World and Lutzomyia in the New World [1]. It is a disease considered neglected by the World Health Organization (WHO), with a wide geographic distribution and presence in 98 countries. Worldwide, it is estimated that there are about 12 million infected people, with approximately 700,000 to 1 million new cases reported each year [2,3,4,5]. Leishmaniasis has different clinical manifestations, which depend on the species of the parasite and the host’s immune response to the cutaneous, mucosal, and visceral forms [6].
Visceral leishmaniasis (VL) is the most severe form of the disease, with a high mortality rate in humans if left untreated [3]. It is estimated that 50,000 to 90,000 new cases of VL occur annually worldwide, with the majority occurring in Brazil and East African countries [5]. In the Americas, LV is present in more than 10 countries, such as Argentina, Bolivia, Brazil, Colombia, El Salvador, Guatemala, Honduras, Mexico, Paraguay, Uruguay, and Venezuela. In 2020, Brazil accounted for 97% (1933) of the total cases [7]. Leishmania (Leishmania) donovani is the species responsible for VL in Africa and Asia, while in the Mediterranean region and the Americas, the disease is caused mainly by Leishmania (Leishmania) infantum (syn = Leishmania chagasi) [6,8,9].
Many animals, such as armadillos, jackals, rodents, bats, cats, dogs, and mongooses, play an important role in the parasite’s life cycle, acting as hosts [3,10]. However, dogs are the main urban reservoirs of L. infantum in Brazil and several other countries [11,12]. In some regions, especially in South America, the seroprevalence can reach 33% on Margarita Island, Venezuela, and 36% in Jacobina, Brazil [13]. Canine visceral leishmaniasis (CVL) is present in approximately 50 countries; except for Antarctica and Oceania, all continents have endemic regions for the disease [14,15]. Dogs are often asymptomatic and, when symptomatic, can present a wide spectrum of clinical manifestations, such as anemia, emaciation, hepatosplenomegaly, renal changes, and onychogryphosis, which, if not treated, can lead to death [16]. Even when treated, the dogs remain infective to vectors, maintaining the presence of the disease in urban centers [17], and becoming a major public health problem.
Therefore, given the close relationship between the dog and the disease, as well as their strong interaction with humans, this review discusses the role of dogs in CVL epidemiology and the disease’s distribution in endemic areas, highlighting the main challenges in controlling CVL.
2. Distribution of Canine Visceral Leishmaniasis in the World
2.1. Africa
On the African continent, the most VL cases occur in northern countries, belonging to the Mediterranean basin and East Africa, where the main species are L. infantum and L. donovani, respectively [18].
In Algeria, a North African country, CVL is present throughout the country, with the prevalence of parasites varying depending on the region [19]. In the capital, Algiers, 37% of 1800 dogs had positive serology for CVL between 1990 and 1997 [20]. Also in Algiers, Adel et al. [21] showed in a study of 462 dogs conducted between 2004 and 2005 that stray dogs were those with the highest prevalence of leishmaniasis (11.7%), followed by animals from the national guard (9.7%), and those that lived on farms (5.9%), but there was no statistical difference among these groups. Between 2008 and 2009, there was an increase in the number of cases of CVL, from west to east, in the country’s coastal region, between the cities of Tlemcen and Jijel, which could be due to the increase in rainfall in the region, with some cities showing a statistical difference in the rise of CVL prevalence during this period [22]. Seropositive dogs were also found in Kabylia, a region located in the north, where the rate of seropositive animals was 36% [23], and in the Tiaret region, with 93/137 (67.88%) dogs testing positive for CVL through Leishmania nested PCR—LnPCR [24].
The first reported case of canine infection in Morocco was published in 1932 [25]. CVL caused by L. infantum is present in the north and central–south regions [26]. Recently, 29 dogs infected with L. infantum have been found in northwestern Morocco, in urban centers such as Rabat (21/29 = 72.4%), Benslimane (2/29 = 6.9%), Casablanca (2/29 = 6.9%), Kenitra (1/29 = 3.45%), Fes (1/29 = 3.45%), Rommani (1/29 = 3.45%), and Jorf al Melha (1/29 = 3.45%) [27].
In Tunisia, the infection of dogs by Leishmania was first described by Nicolle and Comte [28]. As in other countries, the prevalence of CVL varies according to the region. In the city of Medjez El Bab, Beja Governorate, in northern Tunisia, the canine infection rate in 1994 and 1995 was 18% and 22.3%, respectively, and the positivity rate was based on serological tests, including both the enzyme-linked immunosorbent assay (ELISA) and the immunofluorescence antibody test (IFAT). It is noteworthy that 90% of the animals were asymptomatic [29]. In Sfax, the central–south region, 250 animals were tested for CVL, with a seroprevalence rate of 6% [30]. In the Kairouan Governorate, in the central region of the country, in 2005, the prevalence was 21% in dogs from the rural area of El Alaa, in which 50% of the animals were asymptomatic [31]. Similarly, in a study by Zoghlami et al. [32], in 2013, in seven districts of the same governorate, the overall prevalence was 26.7%. Currently, a seroprevalence of 58.3% was obtained in a study conducted by Bouattour et al. [33] in eight locations, in different bioclimatic areas of the country. According to the authors, it is possible to conclude that there was an increase in CVL in all bioclimatic areas, in addition to confirming the spread of the disease to arid areas.
In Egypt, asymptomatic infected dogs were found in five provinces in the north of the country, with a seroprevalence rate of 21.3%, with the highest being found in the provinces of Giza and Cairo [34].
In East Africa, the main species related to VL is L. donovani, and its transmission is considered mainly anthroponotic [35]. However, some studies have highlighted that dogs can be an important reservoir of this species in this part of the continent [35,36].
The existence of dogs infected with Leishmania in Ethiopia has been reported in several studies (Table 1). While in Sudanese territory, Dereure et al. [36] were the first to advance the possibility of the dog acting as a reservoir host in Barbar El Fugarra, in the state of Gedaref, eastern Sudan, which is considered an endemic area for VL. At the same location, a study conducted by Dereure et al. [37], involving dogs with poor body condition, observed a seroprevalence for leishmaniasis of 72.5% (37/51), 74.3% (26/35), and 42.9% (15/35) in the years 1998, 1999, and 2000, respectively. In contrast, a study by Hassan et al. [38], conducted in in 2002 in 10 villages along the Rahad River, located in eastern Sudan, showed that 6.9% of the dogs were serologically positive for L. donovani. In the western part of the continent, cases of dogs infected with Leishmania, mostly by L. infantum, have been reported in Burkina Faso [39], Côte d’Ivoire [40], Nigeria [41], and Zambia in southern Africa [42] (Table 1).
2.2. The Americas
In the Americas, the main etiological agent of CVL is L. infantum, which was introduced during the period of the Portuguese and Spanish colonization through infected animals, such as dogs, which came from Europe to the New World with the colonizers [43].
In Argentina, a country where the number of human VL cases rose in 2019, presenting nine cases (0.35% of cases in the Americans) [44], infected dogs were found in the city of Posadas, the province of Misiones [45,46], where Cruz et al. [47] reported a 57.3% infection rate of dogs infected by L. infantum (63/110). In this study, the dogs were diagnosed by serological tests (such as the rK39-immunochromatographic test (rK39-ICT) and IFAT), in addition to molecular testing (nested PCR). One group came from two shelters, while the other consisted of dogs owned by individuals who visited a local veterinary clinic, including both symptomatic and asymptomatic animals. In Puerto Iguazú, located in the same province, 7.17% of 209 dogs evaluated were considered positive for L. infantum [48], using rK39-ICT, IFAT, and nested PCR techniques. More recently, infected dogs were identified by Fujisawa et al. [49] and Lamattina et al. [50], in which 28 of 160 dogs (17.5%) were found with positive serology. Similarly, in the Chaco region of Salta Province, about 1000 km away from the province of Misiones, the prevalence of CVL was 13% [51].
In Uruguay, an autochthonous outbreak that occurred in Arenitas Blancas, in the department of Salto, 22% of the animals studied tested positive for leishmaniasis [52].
Reports dated from 1982 demonstrate the presence of CVL in Bolivia, where L. infantum was isolated from five dogs in the Yungas area, which was confirmed by serology [53].
In Colombia, VL has two sources of transmission: in the northern departments of Sucre, Bolívar, and Córdoba, where the highest number of cases are registered; and the Middle Valley region of the Magdalena River, between the central and eastern parts of the Colombian Andes mountain range, in the departments of Cundinamarca, Tolima, and Huila [54]. Picón et al. [55] found a 12% seroprevalence in 2016–2017, when analyzing dogs in the cities of Guamo, Ortega, Flandes, Coyaima, and Melgar in the Tolima department, and Neiva in the Huila department. Interestingly, Arbeláez et al. [56] reported the first case of urban CVL in the city of Cali, in the Valle del Cauca department, southwestern Colombia, in a dog that had never lived in an endemic area and only roamed in areas where human or canine cases of the disease had not been reported. More recently, dogs infected with L. infantum were found within the urban perimeter of Sincelejo. The frequency of dogs with CVL is as high as the frequencies found 8 years ago in the rural areas of this municipality, which is in the Department of Sucre [57], located in the Colombian Caribbean, as well as in Pradera and Florida, in Valle del Cauca, southwest Colombia [58].
In a study conducted in Venezuela, the seroprevalence of dogs infected with L. infantum was found to be 5.2% (3/58) in the state of Lara. In the state of Yaracuy, no seropositive dogs for L. infantum’s antigen were detected. However, qPCR testing identified one positive case [59]. In a study conducted with randomly sampled dogs from February to March 2019 on El Tigre Island, located in Amapala, Honduras, the seroprevalence of L. infantum infection was 41% (44/107), according to the dual path platform (DPP) rapid test and ELISA. Additionally, the presence of parasite DNA was detected in 94% of the dogs that tested positive in serological tests [60].
According to PAHO, 97% of all human VL cases that occurred on the continent in 2019 were reported in Brazil [7], and the disease continues to spread [61,62]. Cases of canine infection have been reported in several state capitals, or in their metropolitan regions, such as Belo Horizonte [63,64], Boa Vista [65], Cuiabá [66,67], Florianópolis [68], Niterói [69], Porto Alegre [70], and Vitória [71]. The same was observed in inland cities, such as Piacatu [72], in the state of São Paulo; Divinópolis [73] and Governador Valadares [74,75] in Minas Gerais; Campina Grande [76] in Paraíba; and Santarém [77], in the state of Pará. The seroprevalence of CVL in Brazil varies from 4% to 75%, being related to geographic conditions, climate, and social aspects of the affected area [78].
It is known that the disease has reached areas where it had previously not been present. The expansion of CVL to non-endemic regions can be illustrated by reports, such as the occurrence in the French overseas department of French Guiana. In 2016, dogs with autochthonous infection by L. infantum were reported in Cayenne through molecular tests, with a prevalence of 3.1%. In addition, it was observed in dogs that performed military work, which had negative molecular and serological results prior to going to French Guiana, yet tested positive for infection when they returned to France after four months in the region, suggesting the possible existence of an autochthonous cycle of VL transmission in the department [79].
2.3. Asia
VL on the Asian continent is caused by L. infantum in the Middle East and Central Asia and L. donovani in the Indian subcontinent, a region that harbors the most cases in the world [80,81,82].
In Iran, VL is distributed in three foci, located in four provinces in the northwest and south and, recently, it has spread to other parts of the country [83,84]. The prevalence of CVL in endemic areas ranges from 14.2% to 17.4% [83], and several studies have shown the distribution of infected dogs throughout Iran (Table 1).
After more than 30 years without the occurrence of human and canine infection, cases of CVL in Israel were reported by Baneth et al. [85]. Nasereddin et al. [86] isolated strains of Leishmania, mainly L. infantum, in dogs in the northern and central regions of the country, as well as in the West Bank, where 215 domestic dogs from seven districts were examined, and the overall prevalence of leishmaniasis was 16.7% [87]. Samples from 189 dogs, obtained in the town of Alfei Menashe, in the Shomron region, were analyzed and 3.3% of the animals tested positive for infection by L. infantum [88].
CVL in Turkey was first reported in 1951 in Istanbul and Bursa [89] and, according to Toz et al. [90], it is a common disease throughout the country, being mainly caused by L. infantum. Recently, in a study conducted by Koenhemsi et al. [91] in Istanbul, 5/171 (2.92%) dogs of different breeds and ages tested positive for CVL. Infected dogs in Central Asia have been found in Kazakhstan, Turkmenistan, and Uzbekistan [92]. Nepal [93] and Pakistan [94] have also identified dogs infected with the parasite (Table 1).
VL is a major public health issue in China, being considered endemic or re-emerging in six provinces, including Xinjiang, Gansu, Sichuan, Shaanxi, Shanxi, and Inner Mongolia. Zoonotic VL is endemic, with dogs acting as the main reservoir of L. infantum [95,96]. Shang et al. [95], using real-time PCR, were able to detect Leishmania DNA in dogs from Wenchuan (23/98 = 23.5%), Heishui (20/71 = 28.2%), and Jiuzhaigou (35/145 = 24.1%) counties, all located in Sichuan province, southwest China, where the total positivity rate was 24.8%. In 2022, Sandy et al. [97] were the first to report the occurrence of CVL in Hong Kong. Dogs infected with Leishmania have also been found in the Philippines and Vietnam [98].
2.4. Europe
For many years, leishmaniasis has been the only tropical vector-borne disease endemic in southern Europe, where the majority of reported cases are due to zoonotic VL caused by L. infantum [99].
Among the Mediterranean Basin countries, Albania is one of the most affected countries by VL, and since the late 1980s, several L. infantum isolates have been obtained from both human and canine cases [100]. In Bosnia and Herzegovina, domestic, stray, and shelter dogs from different parts of the country have been tested for leishmaniasis. In total, 16.7% of the animals tested positive, indicating that a significant part of the canine population was infected [101].
The county of Split-Dalmatia, in Croatia, is considered an endemic area for CVL, with seroprevalence ranging from 0 to 42.8%. Dogs from the city of Split and from 12 villages in the region were tested, and 14.7% of the animals in Split were positive for L. infantum infection, with eight villages having seroprevalence ranging from 7.1% to 42.8%, depending on their location [102]. In another study conducted in 2008, the seroprevalence was 31% in Split-Dalmatia and 3.8% in Šibenik-Knin county [103].
Cyprus, an island country located in the eastern Mediterranean region, did not register any cases of CVL for a period of more than 20 years due to a decline in the number of sandflies and dogs. However, there has been an increase in the population of vectors and dogs, due to the end of malaria and echinococcosis control programs, which directly affected the vector and dog population on the island, resulting in the resurgence of CVL in several areas in the south [104,105]. Infected dogs were also found in the island’s northern region, with rates ranging from 1.9% to 13.2% [106].
CVL is endemic in the south of France, where several foci have been identified, such as Alpes-Maritimes, Cévennes, and Provence [107,108]. A seroprevalence of 29.6% was reported in Cévennes, according to study by Lachaud et al. [109]. Dogs infected with L. infantum were found in several French cities, including Toulon and Hyères, in the department of Var, in addition to Miramas, Istres, and Salon-de-Provence, in the department of Bouches-du-Rhône, and Solenzara, on the island of Corsica [110]. Currently, through data obtained by veterinarians, it is possible to verify that the incidence of dogs infected by L. infantum in a large part of French territory is between 0 and 5 cases/1000 dogs/year. However, the number of cases in parts of south-eastern France has reached 20 cases/1000 dogs/year. Sporadic cases have been reported in the north of the country, which is considered a non-endemic region for the disease [111].
Between 2005 and 2010, samples from 5772 dogs from different parts of Greece were collected, with an average reported seroprevalence of 22.09%. It was shown to vary from 6.5% in western Macedonia to 50.2% on the island of Corfu, and of the 43 prefectures from which the canine samples were collected, 41 had seropositive dogs, showing that the disease is spreading [112]. According to studies conducted by Symeonidou et al. [113], it is possible to deduce that CVL remains a country-wide problem, since seropositive dogs were found throughout the Greek departments, and most prefectures had at least one seropositive animal, with seropositivity rates ranging from 0% to 53%. This variation in the prevalence of CVL cases in Greek territory is related to the varied geographical, climatic and socioeconomic conditions in the country, as well as the existence of dogs and vectors [113].
The main endemic areas for CVL in Italy include the central and southern regions and the islands of Sicily and Sardinia. In a study conducted on the island of Sardinia, involving dogs from a local kennel and those attending a veterinary hospital, the authors examined 1147 dogs, of which 15.4% (177/1147) were seropositive, similar to the seroprevalence of 17.7% previously reported for the mainland part of the country [114]. In recent decades, CVL has spread towards the north of the country, which had previously been considered a non-endemic area, with cases registered in Emilia-Romagna, Lombardia, Trentino-Alto Adige, Piedmont, and Vale D’Aosta [115,116,117,118,119]. Furthermore, the Republic of San Marino, an autonomous state located within Italian territory, presented a seroprevalence of 3.9% in 2012 [120], making the entire Italian Peninsula an endemic area for the infection [118].
In the Malta archipelago, 60 samples of dogs from different breeds were collected on the Islands of Malta and Gozo, and 20% tested positive for leishmaniasis through molecular testing [121].
In Portugal, according to Cardoso et al. [122], dogs testing positive for L. infantum are distributed throughout the mainland, namely the north, Central Region, Alentejo, Lisbon, and the Algarve. When analyzing 193 dogs living in kennels in the Algarve, Maia et al. [123], found a general CVL seroprevalence of 16.06%. Furthermore, between 2011 and 2014, 170 dogs were studied in the same region, and seroprevalence reached 18.2% [124]. Schallig et al. [125] showed that the number of seropositive dogs in Évora increased over a period of 20 years, with 9.4% in 1999 and 5.6% in 2010, as compared to 3.9% in 1990, the year of the initial study. In other study, conducted from May 2011 to February 2014, a total of 230 dogs from veterinary medical centers and animal shelters in southern Portugal were randomly separated in two groups. One analyzed group presented no clinical signs compatible with CVL, and the prevalence was 69% (107/155). In the other group, with clinical signs compatible with CVL, the prevalence was 42.7% (32/75) [126]. In contrast, between 2011 and 2012, 581 dogs from five shelters in Lisbon and Setúbal were analyzed, and 13.1% tested positive for L. infantum [127]. In a cross-sectional study by Almeida et al. [128], using samples from 1860 dogs from all parts of the country, the overall seroprevalence rate was 12.5%, with values ranging from 9.6% in the north to 17.2% in the Algarve.
CVL in Spain was considered restricted to the Mediterranean region. However, the disease has spread throughout most of the country, including the islands [129]. The CVL seroprevalence rate after a random analysis varies from 2% to 57.1% between regions [130], and these variations are related to environmental factors, geographic location, and vector dispersion [131]. Northern Spain is considered a non-endemic area, with low seroprevalence [130]. According to the results of a multicenter study by Diaz-Regañón et al. [132], the highest seroprevalence obtained in the north was found in the commune of Aragon (24.56%) and the lowest in Asturias (1.27%). Through a cross-sectional serological survey conducted between 2011 and 2016, Galvéz et al. [130], showed a high rate of seroprevalence in the southern Spanish provinces, such as Malaga (29.4%), Seville (25%), Murcia (23.7%), and the Balearic Islands (20%). In addition, for the first time, the seroprevalence of L. infantum on the Canary Islands was reported, with 2.45% of dogs also testing positive for leishmaniasis [129].
Cases of CVL have been reported in other European countries, such as Bulgaria [133], Georgia [134,135], Germany [136], Hungary [137], Kosovo [138], the Netherlands [136], North Macedonia [139], Romania [140,141,142], Slovenia [143], Switzerland [136], and the United Kingdom [144] (Table 1).
Table 1.
Percentage of CVL positive dogs and Leishmania species described in African, Asian, American, and European countries.
| Continent | Country | Location | Parasite Species | % of Dogs Positive for Leishmania (Diagnostic Test Used) | References |
|---|---|---|---|---|---|
| Africa | Ethiopia | Amhara region | L. donovani Complex * | 3.8% (RIFI and ELISA); 2.8% (PCR) | [145] |
| Kafta Humera district | L. donovani | 27.7% (DAT); 14.8% (KDRT) | [146] | ||
| Addis Zemen, Humera, and Sheraro |
L. donovani Complex * |
5.9% (PCR) | [147] | ||
| Benishangul-Gumuz region | NA | 13.9% (rK39 ICT); 5.6 (DAT) | [148] | ||
| Burkina-Faso | Bobo-Dioulasso | L. infantum | 5.88% (Serological test) | [39] | |
| Côte d’Ivoire | Abidjan and Yamoussoukro cities | L. infantum | 8.9% (PCR or IFAT) | [40] | |
| Nigeria | Oyo, Ogun, and Kwara | NA | 4.4% (ELISA) | [41] | |
| Zambia | Southern Province | L. infantum | NA (Serological tests and PCR) | [42] | |
| Asia | Iran | Meshkin-Shahr district | L. infantum | 15.8% (DAT) | [149] |
| Boyer Ahmad district | L. infantum | 10% (DAT) | [150] | ||
| Kouhsar district | L. infantum | 3.6% (DAT) | [151] | ||
| Khorasan Razavi province | L. infantum | 7.6% (IFAT) | [152] | ||
| Kerman province | L. infantum | 11.25% (DAT) | [153] | ||
| Kerman and Sistan-Baluchestan provinces | L. infantum | 15.4% (ELISA) | [154] | ||
| Tehran and Alborz provinces | L. infantum | 4.9% (DAT) | [155] | ||
| Meshkin-Shahr district | L. infantum | 23.4% (DAT) | [156] | ||
| Hamedan province | L. infantum | 3.95% (ELISA) | [157] | ||
| Jiroft district | L. infantum | 7.9% (DAT) | [158] | ||
| Ardabil, Alborz, and East-Azerbaijan provinces | L. infantum | 100% (DAT, rK39, and PCR) | [84] | ||
| Alborz province | L. infantum | 2.97% (DAT) | [159] | ||
| Golestan province | L. infantum | 18% (PCR | [160] | ||
| Nepal | Kathmandu |
L. donovani Complex * |
18.57% (PCR) | [93] | |
| Pakistan | Chilas, Abbotabad, Bagh, Poonch, and Muzafarabad districts |
L. donovani Complex * |
18% (DAT); 26.6% (ELISA) | [94] | |
| Philippines | NA | L. infantum | NA | [98] | |
| Vietnam | NA | L. infantum | NA | [98] | |
| America | Brazil | Belo Horizonte | L. infantum | 64.6% (IFAT) | [63] |
| L. infantum | 56.7% (PCR) | [64] | |||
| Boa Vista | L. infantum | 10.3% (RIFI) | [65] | ||
| Baixada Cuiabana | L. infantum | 16.58% (PCR) | [66] | ||
| Cuiabá | L. infantum | 1.14% (DPP and ELISA) | [67] | ||
| Florianópolis | L. infantum | 1.37% (ELISA and RIFI) | [68] | ||
| Niterói | NA | 15.5% (ELISA and IFAT) | [69] | ||
| Porto Alegre metropolitan area | NA | 4% (PCR) | [70] | ||
| Vitória | NA | 13% (ELISA); 6% (RIFI) | [71] | ||
| Piacatu | L. infantum | 16.08% (ELISA) | [72] | ||
| Divinópolis | NA | 4.63% (ELISA and IFAT) | [73] | ||
| Governador Valadares | NA | 30.2% (IFAT) | [74] | ||
| NA | 29% (ELISA and RIFI) | [75] | |||
| Campina Grande | NA | 8.4% (IFAT) and 4.3% (ELISA) | [76] | ||
| Santarém | L. infantum | 23.3% (ELISA and rK39) | [77] | ||
| Europe | Bulgaria | Petrich | L. infantum | NA (IFAT and PCR) | [133] |
| Georgia | Tbilisi and Kutaisi |
L. donovani Complex * |
20% (rK39) | [134] | |
| Kvareli and Sagarejo districts | 19.5% and 11.4% (rK39) | [135] | |||
| Germany | NA | NA | 11.8% (ELISA) | [136] | |
| Hungary | Tolna province | L. infantum | 30% (IFAT and PCR) | [137] | |
| Kosovo | Prizren, Gjakova, Rahovec, and Deçan | L. infantum | 18.49% (ELISA) | [138] | |
| The Netherlands | NA | NA | 32.4% (ELISA) | [136] | |
| North Macedonia | Skopje and Prilep | L. infantum | 2.5% (PCR) | [139] | |
| Romania | Vâlcea County | L. infantum | NA (FASTest®LEISH) | [140] | |
| Ramnicu Vâlcea | L. infantum | 8.75% (ELISA); 10% (PCR) | [161] | ||
| Galați | NA | 8.33% (ELISA) | [141] | ||
| Argeș County | L. infantum | 20.1% (PCR) | [142] | ||
| Slovenia | Kostelo | L. infantum | NA (IFAT) | [143] | |
| Switzerland | NA | NA | 12.2% (ELISA) | [136] | |
| United Kingdom | NA | L. infantum | NA (ELISA and PCR) | [144] |
IFAT: Indirect immunofluorescence reaction; ELISA: enzyme-linked immunosorbent assay; DAT: direct agglutination test; PCR: Polymerase Chain Reaction. NA: not applicable. * Classification that encompasses the species L. donovani, L. infantum (Old World), and L. chagasi (New World). Unfortunately, the majority of studies do not provide the sensitivity and specificity of the tests used for CVL diagnosis/detection. Exceptions include researches 66 [PCR—sensitivity (S): 94.7%, specificity (E): 100% (bone marrow); S: 91.8%, E: 100% (lymph node); S: 98%, E:100% (blood)] and 141 [ELISA—S: 95%, E: 96%].
3. Factors Influencing the Territorial Expansion and Transmission Maintenance of Visceral Leishmaniasis
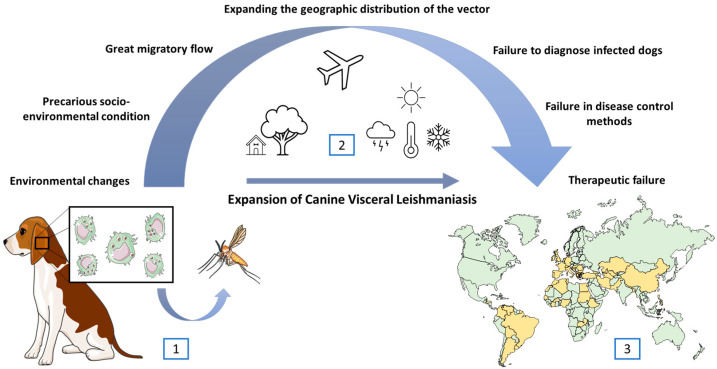
It is estimated that about 2.5 million dogs are infected on the European continent, also reaching millions in South America [162]. Furthermore, this number is believed to have increased mostly in the northern and eastern hemispheres [161]. Many factors contribute to the spreading of the disease, especially those related to environmental and demographic changes, such as long periods of drought, global warming, deforestation, urbanization, and migration [5,161,163,164] (Figure 1).
Figure 1.
The dog is considered the main reservoir of Leishmania infantum, as the presence of amastigotes of the parasite in its skin favors the infection of sandfly vectors and, consequently, the maintenance of the disease (1). Several environmental (global warming, deforestation), social (migration), and economic factors (poverty, lack of basic sanitation) are related to the permanence of CVL in endemic areas (2), as well as its expansion into regions where it had not previously existed. The countries represented in yellow on the map are those that have data in this review (3).
Environmental changes, such as global warming and deforestation, can affect the distribution of leishmaniasis in several ways. Among them are changes in vector biology, such as the effect of temperature on parasite development, vector competence [165,166], an increase in the vectors’ breeding season in a given area, and their presence in areas where they were once absent [166,167,168,169,170]. According to Santos et al. [171], the probabilities of the occurrence of the vector and cases of CVL and HVL in deforested areas were, respectively, 2.63, 2.07, and 3.18 times greater, as compared to non-deforested areas. Socioeconomic impacts are also an aggravating factor in spreading the disease. Over the years, several countries have suffered from a rise in the prevalence of neglected tropical diseases due to human migration from countries suffering from war and famine [172], as well as migration provoked by climatic factors, such as the one that occurred in Northeastern Brazil due to a prolonged drought, compelling many people to leave the countryside and settle in large cities, mainly on the outskirts [163]. Problems in accessing basic care in areas of conflict, such as during a civil war, as reported in Somalia and South Sudan [173,174], also present formidable challenges.
Living on the outskirts of an urban center offers a favorable environment for the occurrence of new outbreaks of CVL transmission, as it is often related to areas of recent habitation, close to open fields and wooded areas. Therefore, the disorganized occupation of this space, precarious living conditions, and the lack of basic infrastructure, in addition to frequent contact with domestic animals, especially infected dogs, create an ideal environment for the VL transmission cycle [164,175,176], which is often related to the houses that have gardens and backyards, where dogs are generally kept [177,178].
The presence of trees, birds, shade, and animal feces in these areas attracts sandflies, facilitating their contact with dogs [72,78,179]. According to the findings by Martín-Sánchez et al. [180] and Coura-Vital et al. [181], dogs that sleep outdoors are more likely to be infected, as compared to those that spend most of their time indoors. Studies have also shown the importance of stray dogs in the epidemiology of VL [81,151,153,158,179,182]. According to Babuadze et al. [134], the errant behavior of these dogs is a contributing factor to the spread of leishmaniasis into new areas.
It has been suggested that CVL normally precedes the appearance of cases in humans [183,184,185] and, according to some authors, there are no reports of infection in humans without the presence of infected dogs [73,175]. Therefore, the spatial overlap of human and canine VL cases in urban areas is an important condition in transmitting the disease to humans [75,119,186,187,188].
Some characteristics of dogs appear to favor Leishmania infection, such as breed, age, coat, and size. Studies have shown that Boxer, German Shepherd, Doberman, Foxhound, and Beagle breeds are among those most affected by Leishmania infection and disease progression [189,190,191,192,193]. Older animals had higher seroprevalence in some studies, and this may be related to the time of exposure to the vectors, as the older the animal is, the more contact with the vector it has [95,156,157,158]. Regarding the coat, the blood meal seems to be easier in dogs with short coats, which give the sandfly better access to the animal’s skin. In addition, it may be related to higher CO2 production, attracting vector insects [70,78,190,194]. This is also observed in larger dogs, as they have a larger contact area, making them more susceptible to bites [38,130,195]. In relation to sex, several authors found no difference between males and females regarding the risk of infection [152,153,159,181,196,197,198].
Thus, assorted factors are associated with the occurrence and spread of CVL, especially in urban and peri-urban areas. However, they must be more thoroughly studied to better understand the epidemiology of the disease.
4. The Dog’s Role in the Visceral Leishmaniasis Transmission Cycle
Dogs with visceral leishmaniasis (VL) play a key role in the transmission of protozoan parasites of the Leishmania donovani complex in urban areas. Deane [199] and Deane and Deane [200] contributed to the understanding of the epidemiology of L. infantum, documenting the participation of the dog as a domestic reservoir and the fox as a wild reservoir of the parasite. In this sense, there is a substantial overlap between locations where human cases have been reported and high canine seroprevalence, highlighting the close relationship between canine and human infections [184,201]. Most infected animals are asymptomatic (clinically healthy), but they can host the parasite in the skin [13,202]. In symptomatic dogs, the phlebotomine sandflies’ capacity for infection is greater when compared to asymptomatic dogs due to the high parasite load on the animals’ skin, facilitating infection. When evaluating the disease transmission potential in relation to the parasite load, Courtenay et al. [203] showed that, in sick and infectious animals, the spread of parasites to the skin was greater, especially in the ear skin, suggesting that this is the most infectious region for the sandflies. Thus, the dog represents an abundant source of the parasite to invertebrate hosts, being found in all foci of human disease, and is characterized as the main link in the VL transmission chain [204].
Michalsky et al. [205] showed that symptomatic animals had an average rate of sandfly infectivity of 83.3%, with 28.4% of the vectors being infected. In contrast, the rates of oligosymptomatic and asymptomatic animals were 16.7% and 33.3%, with the percentages of infected vectors at 5.1% and 5.4%, respectively. Verçosa et al. [206] also obtained similar results, proving the high parasite load in ear tissues. In this test, using cytological impressions from 22 different sites of the animal, it was observed that symptomatic dogs had amastigote forms of the parasite in at least one analyzed region. Conversely, no amastigote form was found in asymptomatic individuals. In addition, the study found that six-ninths of the symptomatic dogs were able to transmit the parasite to insect vectors. However, in contrast to the results obtained by Michalsky et al. [207], no asymptomatic animal was able to infect sandflies.
Although some studies consider that symptomatic animals are the main reservoirs, the importance of asymptomatic animals in the transmission of the parasite has been supported by studies such as those by Molina et al. [208], who even claimed that the clinical status of dogs did not influence the infectivity of P. perniciosus—three out of five asymptomatic dogs were able to transmit L. infantum to sandflies. In fact, the clinical status of the animals did not interfere with the infection of the vectors, according to Guarga et al. [207]. However, the percentage of females that fed slightly decreased as the clinical signs in the dogs increased, suggesting a possible predilection for healthy skin. Other studies, such as those by Solano-Gallego et al. [209], using bone marrow, conjunctiva, skin PCR, and ELISA, proved that asymptomatic animals could also be considered reservoirs of L. infantum. Corroborating these findings, Moshfe et al. [210] observed that 43.4% of the dogs without clinical signs had L. infantum DNA in peripheral blood, detected by PCR, while Coura-Vital et al. [211] showed for the first time that asymptomatic dogs were seronegative, yet PCR positive. According to the authors, these animals presented twice the risk of seroconversion, as compared to dogs with negative PCR results. More recently, in an endemic region of the state of Minas Gerais, Brazil, the parasite in 73% of asymptomatic dogs was found mainly on the skin [212].
Therefore, dogs carrying the parasite should be considered infectious for the vectors, regardless of any clinical signs [158,208]. In addition, these animals play an important role in the epidemiology of VL, since a large number of animals with skin parasitism is able to infect sandflies and thus perpetuate disease transmission in urban and peri-urban areas [188,213].
5. The Euthanasia Challenges for Controlling the Spread of Canine Visceral Leishmaniasis
In many endemic countries, the elimination of infected dogs has been recommended as a means of controlling VL [214]. In South America, euthanasia is officially recommended in Brazil, Colombia, and Venezuela. In addition, it is also recommended in Morocco and Tunisia, the Mediterranean region, and some Middle Eastern countries, such as Iran, Iraq, and Syria, as well as in Armenia, Azerbaijan, and Uzbekistan, in Central Asia [2,215]. Furthermore, in some countries, this practice is not officially recommended, although it is nonetheless carried out, for example, in Afghanistan, Albania, Saudi Arabia, Algeria, Kazakhstan, China, Georgia, Paraguay, and Tajikistan. On the European continent, euthanasia is generally performed on animals with a serious form of the disease, as a way to prevent prolonged suffering [2,215].
The practice of euthanasia is controversial among researchers, who still discuss its effectiveness in eliminating VL, not to mention it being strongly criticized from an ethical perspective, with resistance from dog owners and the professionals responsible for carrying out this work [216,217].
Furthermore, this practice faces other challenges. For instance, the limitation of serological tests should be highlighted, as shown by Grimaldi et al. [218], when evaluating the sensitivity of the rapid DPP test, which showed low sensitivity in asymptomatic dogs, with a rate of 47%, reaching 98% among the symptomatic animals. Serological tests, such as IFAT and ELISA, are also widespread but have cross-reaction problems and can generate false-positive and false-negative results [219]. This could lead to the improper elimination of dogs, while allowing infected animals to remain in the transmission cycle of the parasite [215,219].
The delay between diagnosis and the removal of animals is another issue and, according to Dantas-Torres et al. [215], newly infected dogs may remain undetected for months before a new investigation is conducted. Seronegative animals may be infected but have not yet undergone seroconversion, with these dogs roaming around freely and acting as a source of parasites for vector insects [220]. In fact, seronegative dogs displaying PCR positivity in the skin have previously been reported, but their epidemiological role remains unclear [184,211], and their potential infectivity is theoretically possible.
The replacement of euthanized dogs with other susceptible dogs also influences the effectiveness of euthanasia [215,220,221], as shown in studies by Moreira et al. [222] and by Nunes et al. [216] in endemic areas of Brazil.
Nevertheless, studies such as those by Ashford et al. [223], Costa et al. [224], Nunes et al. [225], Costa et al. [226], and Bermudi et al. [227], have shown that the elimination of infected dogs reduces the incidence of VL in humans and dogs. Conversely, some researchers, such as Dantas-Torres et al. [215], Dietze et al. [228], Paranhos-Silva et al. [229], and Vaz et al. [230], have shown the opposite.
In Brazil, thousands of dogs are eliminated every year and, even so, the prevalence of CVL remains high in several endemic foci. In fact, animal euthanasia is not capable of controlling zoonotic VL in the country [215]. This scenario corroborates a study by Costa et al. [231], which demonstrated that the elimination of dogs alone is not effective in areas of high transmission. However, dog screening and faster culling intervention (30 days after CVL diagnosis) during two years of VL transmission in the north of Minas Gerais State (Brazil) reduced the number of human cases by 75% and canine disease, cases as recently reported [232].
6. Discussion
The dog is considered a key element in the VL cycle caused by L. infantum, especially in the Americas and the Mediterranean basin.
The paucity of studies and surveys on the prevalence of CVL is noteworthy, as many regions have no data on it. As shown, CVL has been spreading into new territories due to various factors. One example is the migratory flow of dogs, which often travel to endemic areas with their human guardians or are bought in these areas, becoming a risk for the emergence and transmission of the disease in places that had not been considered endemic for VL [168,233].
According to Figueiredo et al. [234], some precautions can be taken to minimize the expansion, such as stricter control of the flow of these animals in endemic areas, mandatory serological screening of dogs that are traveling, and mandatory notification of positive cases. However, few countries have mandatory reporting for CVL [235].
Another factor to consider is the effectiveness of the detection methods. Could it be that the dogs in the cited studies were not positive or was it a reflection of the diagnostic method? Studies by Carvalho et al. [236] and Pessoa-e-Silva et al. [237] compared several serological and molecular methods used in the diagnosis of CVL and showed that the percentage of agreement between the tests varies from low to moderate. The efficiency of serological tests depends on several factors, especially the antigen used [218]. According to Travi et al. [214], the rK39 antigen was the most successful in the transition from the academic to the clinical environment, as it demonstrated high sensitivity and was able to detect an active infection. However, it does not have good specificity and sensitivity to identify asymptomatic infections, as compared to other serological methods (Table 2). Although molecular tests also present problems, they are better at detecting the parasite compared to serological tests [184,238]. Therefore, the number of infected dogs in some areas could be considered underestimated, since the accuracy of diagnostic methods is limited.
Table 2.
Sensitivity and specificity of serological tests used for CVL diagnosis.
| Test | Manufacturer | Sensitivity | Specificity | References |
|---|---|---|---|---|
| IFAT 1:40 | Bio-Manguinhos, Rio de Janeiro, Brazil | 96% | 18% *; 76% ** | [239] |
| IFAT 1:80 | 90% | 33% *; 93% ** | ||
| ELISA | Bio-Manguinhos, Rio de Janeiro, Brazil | 91% | 79% *; 98% ** | [239] |
| 84.20% | 95.6% | [240] | ||
| rK39 (Kalazar Detect) | InBios, Inc., Seattle, WA, USA | 88% | 74% *; 98% ** | [239] |
| 82.90% | 92.6% | [241] | ||
| 76.90% | 98.6% | [242] | ||
| 79.60% | 95.7% | [240] | ||
| FAST | Royal Tropical Institute, Amsterdam, The Netherlands | 93% | 68% *; 100% ** | [239] |
| DAT | Royal Tropical Institute, Amsterdam, The Netherlands | 96% | 33% *; 98% ** | [239] |
| TR DPP | Bio-Manguinhos, Rio de Janeiro, Brazil |
98% | 60% *; 98% ** | [239] |
| 21.74% | 92.59% | [243] | ||
| 89% | 70.2% | [244] | ||
| 85.70% | 79.6% | [241] | ||
| 93.70% | 95.9% | [242] | ||
| 97.90% | 93.6% | [240] |
IFAT: indirect immunofluorescence reaction; ELISA: enzyme-linked immunosorbent assay; DAT: direct agglutination test; FAST: fast agglutination screening test; TR DPP: dual path platform fast test. * Value referring to high transmission area and **, value referring to low transmission area, according to the authors.
Controlling the canine population is also extremely important in the epidemiology of CVL. Countries such as Argentina [245], Brazil [246], Colombia [247], Paraguay [248], Uruguay [249], among others in South America, recommend the euthanasia of L. infantum-infected dogs. However, as already shown, the practice of euthanasia is controversial for ethical and social reasons, in addition to being considered costly [250].
Thus, in addition to the control measures recommended by the Brazilian Ministry of Health, a vaccine for prophylactic purposes was commercially available until 2015 [251]. The sale of LeishTec®, the only vaccine available in Brazil, was suspended in 2023. The effectiveness of vaccines currently on the European market varies. CaniLeish® prevents clinical signs in 68.4% of cases and provides a protection level—defined as the percentage of vaccinated animals that remain asymptomatic (and not in terms of parasite protection)—of 92.7%. Similarly, LetiFend® has shown a 72% effectiveness in preventing clinical signs and helps reduce these signs in infected animals [252,253]. The levels of protection provided by these vaccines are not sufficient to prevent infection by L. infantum [17,251,254]. In fact, as shown in a study carried out by Cotrina et al. [253], dogs vaccinated with LetiFend® showed a 9.4% positivity rate for L. infantum, with no statistical difference compared to the control group (16.1%). For CaniLeish®, Oliva et al. [252], through a randomized double-blind controlled trial conducted in an endemic area for CVL, showed that vaccinated dogs presented 50.22% positivity for Leishmania, while the control group presented 66.66%. Furthermore, even when vaccinated, dogs can be a source of parasites for sandflies and, consequently, for other dogs and humans [255]. The treatment also has drawbacks, since the parasitological cure of infected animals is not achieved, and there is a high rate of recurrence and the possibility of resistance to the drugs [17,256]. Nonetheless, these alternatives have been used together with repellent collars, which have been widely accepted by dog owners in Europe [168] and are highly regarded for their effectiveness, according to studies by Gavgani et al. (Iran) [187], Sevá et al. (Brazil) [217], and Ribas et al. (Brazil) [257]. In a mathematical model, Sevá et al. [217] found that applying collars to 90% of dogs effectively eliminated the disease among humans and nearly wiped out the infection in the dog population as well. In 2021, the Brazilian Ministry of Health made the use of collars official as a way of controlling the disease in municipalities where transmission varies from high to very intense [258]. Furthermore, the sandflies antigens have been considered a promising strategy in blocking CVL transmission [17,251,254,256]. As such, there is a pressing need to find new methods and protocols to reduce limitations in the prevention, treatment, and control of CVL. Improvements in technology that facilitate the development of vaccines capable of disrupting the transmission of L. infantum from infected dogs could serve as a highly effective measure in preventing its spread. In fact, some studies have described how sandfly antigens interfere with the vector life cycle and help control de L. infantum infection after a blood meal. This is compatible with de hypothesis that effective control of CVL transmission can be achieved [17,251,254,256,259,260].
7. Conclusions
Based on the information presented in this review, it becomes evident that dogs play a pivotal role in the transmission cycle of visceral leishmaniasis caused by L. infantum. However, it is crucial to acknowledge that various other factors contribute significantly to this transmission dynamic. Environmental conditions, socioeconomic factors, climatic variations, as well as shortcomings in disease treatment and control efforts, all play significant roles in shaping the prevalence and spread of the disease. More comprehensive studies are needed to better understand these factors and find ways to overcome these failures.
Author Contributions
The following authors contributed to the search, analysis, and interpretation of data for the development of this article: D.F.V.-B., E.K.N.N., A.A.M.G., D.F.L., D.S.d.O., D.F.S.P., G.G.S., I.d.S.S.C., L.A.R., M.F.Z., R.M.d.S.M. and R.C.G. The following authors contributed to the revision, correction, and organization of this article: W.O.D., M.A.C.-F., A.S.G. and D.S.-L. The following authors coordinated the writing of this article: R.C.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data collected were reported in the text.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was financially supported through grants from CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil), CNPq (National Council for Scientific and Technological Development), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG); Departamento de Ciência e Tecnologia do Ministério da Saúde do Brasil; and Universidade Federal de Minas Gerais (UFMG), and Instituto Nacional de Ciência e Tecnologia em Doenças Tropicais (INCT-DT).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alves F., Bilbe G., Blesson S., Goyal V., Monnerat S., Mowbray C., Muthoni Ouattara G., Pécoul B., Rijal S., Rode J., et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 2018;31:e00048-18. doi: 10.1128/CMR.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alemayehu B., Alemayehu M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017;11:1. doi: 10.21767/1791-809X.1000519. [DOI] [Google Scholar]
- 4.Rossi M., Fasel N. How to Master the Host Immune System? Leishmania Parasites Have the Solutions! Int. Immunol. 2018;30:103–111. doi: 10.1093/intimm/dxx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Leishmaniasis. [(accessed on 25 March 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/Leishmaniasis.
- 6.McGwire B.S., Satoskar A.R. Leishmaniasis: Clinical Syndromes and Treatment. QJM. 2014;107:7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organização Panamericana da Saúde Leishmanioses: Informe Epidemiológico das Américas, No. 10 (Dezembro 2021) [(accessed on 20 February 2023)]. Available online: https://iris.paho.org/handle/10665.2/55386.
- 8.Maurìcio I.L., Stothard J.R., Miles M.A. The Strange Case of Leishmania chagasi. Parasitol. Today. 2000;16:188–189. doi: 10.1016/S0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 9.Lukeš J., Mauricio I.L., Schönian G., Dujardin J.-C., Soteriadou K., Dedet J.-P., Kuhls K., Tintaya K.W.Q., Jirků M., Chocholová E., et al. Evolutionary and Geographical History of the Leishmania donovani Complex with a Revision of Current Taxonomy. Proc. Natl. Acad. Sci. USA. 2007;104:9375–9380. doi: 10.1073/pnas.0703678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roque A.L.R., Jansen A.M. Wild and Synanthropic Reservoirs of Leishmania Species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014;3:251–262. doi: 10.1016/j.ijppaw.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roatt B.M., Aguiar-Soares R.D.D.O., Coura-Vital W., Ker H.G., Moreira N.D.D., Vitoriano-Souza J., Giunchetti R.C., Carneiro C.M., Reis A.B. Immunotherapy and Immunochemotherapy in Visceral Leishmaniasis: Promising Treatments for This Neglected Disease. Front. Immunol. 2014;5:272. doi: 10.3389/fimmu.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burza S., Croft S.L., Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 13.Moreno J., Alvar J. Canine Leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 14.Solano-Gallego L., Koutinas A., Miró G., Cardoso L., Pennisi M.G., Ferrer L., Bourdeau P., Oliva G., Baneth G. Directions for the Diagnosis, Clinical Staging, Treatment and Prevention of Canine Leishmaniosis. Vet. Parasitol. 2009;165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Dantas-Torres F., Solano-Gallego L., Baneth G., Ribeiro V.M., de Paiva-Cavalcanti M., Otranto D. Canine Leishmaniosis in the Old and New Worlds: Unveiled Similarities and Differences. Trends Parasitol. 2012;28:531–538. doi: 10.1016/j.pt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ulchar I., Celeska I., Stefanovska J., Jakimovska A. Hematological and Biochemical Parameters in Symptomatic and Asymptomatic Leishmania-Seropositive Dogs. Maced. Vet. Rev. 2015;38:175–182. doi: 10.14432/j.macvetrev.2015.06.045. [DOI] [Google Scholar]
- 17.Gonçalves A.A.M., Leite J.C., Resende L.A., Mariano R.M.D.S., Silveira P., Melo-Júnior O.A.D.O., Ribeiro H.S., de Oliveira D.S., Soares D.F., Santos T.A.P., et al. An Overview of Immunotherapeutic Approaches against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019;9:427. doi: 10.3389/fcimb.2019.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ready P. Epidemiology of Visceral Leishmaniasis. Clin. Epidemiol. 2014;6:147. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabbout N., Merzoug D., Chenchouni H. Ecological Status of Phlebotomine Sandflies (Diptera: Psychodidae) in Rural Communities of Northeastern Algeria. J. Arthropod Borne Dis. 2016;10:24–38. [PMC free article] [PubMed] [Google Scholar]
- 20.Harrat Z., Belkaid M. Les Leishmanioses Dans l’Algérois. Données Épidémiologiques; Proceedings of the 6ème Congrès International Francophone de Médecine Tropicale “Santé et Urbanisation en Afrique; [(accessed on 10 July 2023)]. Available online: https://pathexo.societe-mtsi.fr/documents/articles-bull/T96-3-DK42.pdf. [Google Scholar]
- 21.Adel A., Saegerman C., Speybroeck N., Praet N., Victor B., De Deken R., Soukehal A., Berkvens D. Canine Leishmaniasis in Algeria: True Prevalence and Diagnostic Test Characteristics in Groups of Dogs of Different Functional Type. Vet. Parasitol. 2010;172:204–213. doi: 10.1016/j.vetpar.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Adel A., Abatih E., Speybroeck N., Soukehal A., Bouguedour R., Boughalem K., Bouhbal A., Djerbal M., Saegerman C., Berkvens D. Estimation of Canine Leishmania Infection Prevalence in Six Cities of the Algerian Littoral Zone Using a Bayesian Approach. PLoS ONE. 2015;10:e0117313. doi: 10.1371/journal.pone.0117313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medkour H., Laidoudi Y., Lafri I., Davoust B., Mekroud A., Bitam I., Mediannikov O. Canine Vector-Borne Protozoa: Molecular and Serological Investigation for Leishmania spp., Trypanosoma spp., Babesia spp., and Hepatozoon spp. in Dogs from Northern Algeria. Vet. Parasitol. Reg. Stud. Rep. 2020;19:100353. doi: 10.1016/j.vprsr.2019.100353. [DOI] [PubMed] [Google Scholar]
- 24.Bia T., Sanchez C., Zait H., Kouidri M., Mabrouk S.K., Nieto J., Ammar S.S.M., Moreno J., Ahlem B.N. Diagnosis and Prevalence of Canine Leishmaniasis in the Atlas Shepherd Dog. Vet. Parasitol. Reg. Stud. Rep. 2022;36:100787. doi: 10.1016/j.vprsr.2022.100787. [DOI] [PubMed] [Google Scholar]
- 25.Jeaume G. Un Cas de Leishmaniose Naturelle Généralisée Chez Le Chien Au Maroc. Bulletiin Société Pathol. Exot. 1932;25:225–227. [Google Scholar]
- 26.Kahime K., Boussaa S., Nhammi H., Boumezzough A. Urbanization of Human Visceral Leishmaniasis in Morocco. Parasite Epidemiol. Control. 2017;2:1–6. doi: 10.1016/j.parepi.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idrissi H., Hakkour M., Duchateau L., Zanatta R., Kachani M., Azrib R., Daminet S., Kichou F., El Asatey S., Tazi N., et al. Canine Leishmaniasis in Morocco: A Descriptive Prospective Clinical Study. Vet. Med. Int. 2021;2021:6304127. doi: 10.1155/2021/6304127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolle C., Comte C. Origine Canine Du Kala-Azar. Bull. Soc. Pathol. Exot. 1908;1:299–301. [Google Scholar]
- 29.Diouani M.F., Ben Alaya Bouafif N., Bettaib J., Louzir H., Jedidi S., Ftaiti A., Zaatour A., Jomaa I., Dellagi K., Ben Ismail R., et al. Dogs L. infantum Infection from an Endemic Region of the North of Tunisia: A Prospective Study. Arch. Inst. Pasteur Tunis. 2008;85:55–61. [PubMed] [Google Scholar]
- 30.Chargui N., Haouas N., Gorcii M., Akrout Messaidi F., Zribi M., Babba H. Increase of Canine Leishmaniasis in a Previously Low-Endemicity Area in Tunisia. Parasite. 2007;14:247–251. doi: 10.1051/parasite/2007143247. [DOI] [PubMed] [Google Scholar]
- 31.Chargui N., Haouas N., Gorcii M., Lahmar S., Guesmi M., Ben Abdelhafidh A., Mezhoud H., Babba H. Use of PCR, IFAT and in Vitro Culture in the Detection of Leishmania infantum Infection in Dogs and Evaluation of the Prevalence of Canine Leishmaniasis in a Low Endemic Area in Tunisia. Parasite. 2009;16:65–69. doi: 10.1051/parasite/2009161065. [DOI] [PubMed] [Google Scholar]
- 32.Zoghlami Z., Chouihi E., Barhoumi W., Dachraoui K., Massoudi N., Helel K.B., Habboul Z., Hadhri M.H., Limam S., Mhadhbi M., et al. Interaction between Canine and Human Visceral Leishmaniases in a Holoendemic Focus of Central Tunisia. Acta Trop. 2014;139:32–38. doi: 10.1016/j.actatropica.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Bouattour A., Amri A., Belkhiria J.A., Rhim A., Fezaa O., Gantier J.-C., M’ghirbi Y. Canine Leishmaniosis in Tunisia: Growing Prevalence, Larger Zones of Infection. PLoS Negl. Trop. Dis. 2021;15:e0009990. doi: 10.1371/journal.pntd.0009990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selim A., Shoulah S., Abdelhady A., Alouffi A., Alraey Y., Al-Salem W. Seroprevalence and Risk Factors Associated with Canine Leishmaniasis in Egypt. Vet. Sci. 2021;8:236. doi: 10.3390/vetsci8100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leta S., Dao T.H.T., Mesele F., Alemayehu G. Visceral Leishmaniasis in Ethiopia: An Evolving Disease. PLoS Negl. Trop. Dis. 2014;8:e3131. doi: 10.1371/journal.pntd.0003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dereure J., Boni M., Pratlong F., el Hadi Osman M., Bucheton B., el-Safi S., Feugier E., Musa M.K., Davoust B., Dessein A., et al. Visceral Leishmaniasis in Sudan: First Identifications of Leishmania from Dogs. Trans. R. Soc. Trop. Med. Hyg. 2000;94:154–155. doi: 10.1016/s0035-9203(00)90253-0. [DOI] [PubMed] [Google Scholar]
- 37.Dereure J., El-Safi S.H., Bucheton B., Boni M., Kheir M.M., Davoust B., Pratlong F., Feugier E., Lambert M., Dessein A., et al. Visceral Leishmaniasis in Eastern Sudan: Parasite Identification in Humans and Dogs; Host-Parasite Relationships. Microbes Infect. 2003;5:1103–1108. doi: 10.1016/j.micinf.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Hassan M.M., Osman O.F., El-Raba’a F.M., Schallig H.D., Elnaiem D.-E.A. Role of the Domestic Dog as a Reservoir Host of Leishmania donovani in Eastern Sudan. Parasit. Vectors. 2009;2:26. doi: 10.1186/1756-3305-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangare I., Djibougou A., Koudraogo B., Drabo F., Diabate A., Laure Banu A., Fournet F., Price H., Tinga Guig R., Kounbobr D.R. First Detection of Leishmania infantum in Domestic Dogs from Burkina Faso (West Africa) Res. J. Parasitol. 2016;12:27–32. doi: 10.3923/jp.2017.27.32. [DOI] [Google Scholar]
- 40.Medkour H., Laidoudi Y., Athias E., Bouam A., Dizoé S., Davoust B., Mediannikov O. Molecular and Serological Detection of Animal and Human Vector-Borne Pathogens in the Blood of Dogs from Côte d’Ivoire. Comp. Immunol. Microbiol. Infect. Dis. 2020;69:101412. doi: 10.1016/j.cimid.2019.101412. [DOI] [PubMed] [Google Scholar]
- 41.Adediran O.A., Kolapo T.U., Uwalaka E.C. Seroprevalence of Canine Leishmaniasis in Kwara, Oyo and Ogun States of Nigeria. J. Parasit. Dis. 2016;40:510–514. doi: 10.1007/s12639-014-0535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squarre D., Chambaro H.M., Hayashida K., Moonga L.C., Qiu Y., Goto Y., Oparaocha E., Mumba C., Muleya W., Bwalya P., et al. Autochthonous Leishmania infantum in Dogs, Zambia, 2021. Emerg. Infect. Dis. 2022;28:888–890. doi: 10.3201/eid2804.212378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhls K., Alam M.Z., Cupolillo E., Ferreira G.E.M., Mauricio I.L., Oddone R., Feliciangeli M.D., Wirth T., Miles M.A., Schönian G. Comparative Microsatellite Typing of New World Leishmania infantum Reveals Low Heterogeneity among Populations and Its Recent Old World Origin. PLoS Negl. Trop. Dis. 2011;5:e1155. doi: 10.1371/journal.pntd.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Organização Pan-Americada da Saúde Leishmanioses: Informe Epidemiológico das Américas, dezembro 2020. [(accessed on 12 October 2022)]. Available online: https://iris.paho.org/handle/10665.2/53091.
- 45.Salomon O., Sinagra A., Nevot M., Barberian G., Paulin P., Estevez J., Riarte A., Estevez J. First Visceral Leishmaniasis Focus in Argentina. Mem. Inst. Oswaldo Cruz. 2008;103:109–111. doi: 10.1590/S0074-02762008000100018. [DOI] [PubMed] [Google Scholar]
- 46.Acardi S.A., Liotta D.J., Santini M.S., Romagosa C.M., Salomón O.D. Detection of Leishmania infantum in Naturally Infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis Familiaris in Misiones, Argentina: The First Report of a PCR-RFLP and Sequencing-Based Confirmation Assay. Mem. Inst. Oswaldo Cruz. 2010;105:796–799. doi: 10.1590/S0074-02762010000600011. [DOI] [PubMed] [Google Scholar]
- 47.Cruz I., Acosta L., Gutiérrez M.N., Nieto J., Cañavate C., Deschutter J., Bornay-Llinares F.J. A Canine Leishmaniasis Pilot Survey in an Emerging Focus of Visceral Leishmaniasis: Posadas (Misiones, Argentina) BMC Infect. Dis. 2010;10:342. doi: 10.1186/1471-2334-10-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acosta L., Díaz R., Torres P., Silva G., Ramos M., Fattore G., Deschutter E.J., Bornay-Llinares F.J. Identification of Leishmania infantum in puerto iguazú, misiones, argentina. Rev. Inst. Med. Trop. Sao Paulo. 2015;57:175–176. doi: 10.1590/S0036-46652015000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujisawa K., Silcott-Niles C., Simonson P., Lamattina D., Humeres C.A., Bhattacharyya T., Mertens P., Thunissen C., O’Rourke V., Pańczuk M., et al. Emergent Canine Visceral Leishmaniasis in Argentina: Comparative Diagnostics and Relevance to Proliferation of Human Disease. PLoS Negl. Trop. Dis. 2021;15:e0009552. doi: 10.1371/journal.pntd.0009552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamattina D., Berrozpe P.E., Casas N., Moya S.L., Giuliani M.G., Costa S.A., Arrabal J.P., Martínez M.F., Rivero M.R., Salas M., et al. Twice upon a Time: The Progression of Canine Visceral Leishmaniasis in an Argentinean City. PLoS ONE. 2019;14:e0219395. doi: 10.1371/journal.pone.0219395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barroso P.A., Marco J.D., Locatelli F.M., Cardozo R.M., Hoyos C.L., Mora M.C., García Bustos M.F., López-Quiroga I., Mimori T., Gentile A.G., et al. Visceral Leishmaniasis Caused by Leishmania infantum in Salta, Argentina: Possible Reservoirs and Vectors. Am. J. Trop. Med. Hyg. 2015;93:334–339. doi: 10.4269/ajtmh.14-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satragno D., Faral-Tello P., Canneva B., Verger L., Lozano A., Vitale E., Greif G., Soto C., Robello C., Basmadjián Y. Autochthonous Outbreak and Expansion of Canine Visceral Leishmaniasis, Uruguay. Emerg. Infect. Dis. 2017;23:536–538. doi: 10.3201/eid2303.160377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Pont F., Mollinedo S., Mouchet J., Desjeux P. Leishmaniose en Bolivie. IV. Le Chien Dans les Cycles des Leishmanioses en Bolivie. Mem. Inst. Oswaldo Cruz. 1989;84:417–421. doi: 10.1590/S0074-02761989000300019. [DOI] [PubMed] [Google Scholar]
- 54.Zambrano-Hernandez P.C., Ayala Sotelo M.S., Fuya Oviedo O.P., Montenegro Puentes C.A., Aya Vanegas N.M., Rodriguez Toro J.G., Becerra Osorio S.L., Aguilera Jaramillo G., Lozano Polanco C.A., Rojas Garcia M.C., et al. Brote Urbano de Leishmaniasis Visceral En Neiva (Huila), 2012. Rev. Salud Pública. 2015;17:514–527. doi: 10.15446/rsap.v17n4.44663. [DOI] [PubMed] [Google Scholar]
- 55.Picón Y., Almario G., Rodríguez V., Garcia N.V. Seroprevalence, Clinical, and Pathological Characteristics of Canine Leishmaniasis in a Central Region of Colombia. J. Vet. Res. 2020;64:85–94. doi: 10.2478/jvetres-2020-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arbeláez N., Moreno J., Murillo J., Montoya A., Robledo S.M., Vélez A., Vélez I.D. First Report of an Urban Case of Canine Visceral Leishmaniasis in the Municipality of Cali, Colombia. Am. J. Trop. Med. Hyg. 2020;102:289–293. doi: 10.4269/ajtmh.19-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rueda-Concha K.L., Payares-Mercado A., Guerra-Castillo J., Melendrez J., Arroyo-Munive Y., Martínez-Abad L., Cochero S., Bejarano E.E., Paternina L.E. Circulación de Leishmania infantum y Trypanosoma Cruzi En Perros Domésticos de Áreas Urbanas de Sincelejo, Región Caribe de Colombia. Biomédica. 2022;42:633–649. doi: 10.7705/biomedica.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pérez-Ramírez R.D., Lugo-Vargas R., Petano-Duque J.M., Cruz-Méndez J.S., Rondón-Barragán I.S. First Study on Microscopic and Molecular Detection of Acanthocheilonema Reconditum and Leishmania infantum Coinfection in Dogs in Southwest Colombia. Vet. World. 2023;16:94–103. doi: 10.14202/vetworld.2023.94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivas A.K., Alcover M.M., Martínez-Orellana P., Montserrat-Sangrà S., Nachum-Biala Y., Fisa R., Riera C., Baneth G., Solano-Gallego L. Serological and Molecular Survey of Leishmania Infection in Dogs from Venezuela. Vet. Parasitol. Reg. Stud. Rep. 2020;21:100420. doi: 10.1016/j.vprsr.2020.100420. [DOI] [PubMed] [Google Scholar]
- 60.Segura G.B.R., Ochoa W.H.S., da Matta V.L.R., Martínez M., Tercero C.R., Gonzalez R.R., Pacheco C.M.S., Flores G.V.A., Silveira F.T., Henriquez M.M.R., et al. Can Domestic Dogs Be Considered a Good Reservoir of Leishmania (L.) infantum chagasi in an Endemic Area of Nonulcerated Cutaneous Leishmaniasis in Southern Honduras? Rev. Inst. Med. Trop. Sao Paulo. 2023;65:e24. doi: 10.1590/s1678-9946202365024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maia-Elkhoury A.N.S., Alves W.A., de Sousa-Gomes M.L., de Sena J.M., Luna E.A. Visceral Leishmaniasis in Brazil: Trends and Challenges. Cad. Saude Publica. 2008;24:2941–2947. doi: 10.1590/S0102-311X2008001200024. [DOI] [PubMed] [Google Scholar]
- 62.Harhay M.O., Olliaro P.L., Costa D.L., Costa C.H.N. Urban Parasitology: Visceral Leishmaniasis in Brazil. Trends Parasitol. 2011;27:403–409. doi: 10.1016/j.pt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Silva E.S., Gontijo C.M., Pacheco R.S., Fiuza V.O., Brazil R.P. Visceral Leishmaniasis in the Metropolitan Region of Belo Horizonte, State of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2001;96:285–291. doi: 10.1590/S0074-02762001000300002. [DOI] [PubMed] [Google Scholar]
- 64.Alves Souza N., Souza Leite R., de Oliveira Silva S., Groenner Penna M., Figueiredo Felicori Vilela L., Melo M.N., de Andrade A.S.R. Detection of Mixed Leishmania Infections in Dogs from an Endemic Area in Southeastern Brazil. Acta Trop. 2019;193:12–17. doi: 10.1016/j.actatropica.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Guerra J.A.O., Barros M.L.B., Fé N.F., Guerra M.V.F., Castellon E., Paes M.G., Sherlock Í.A. Leishmaniose Visceral Entre Índios No Estado de Roraima, Brasil: Aspectos Clínicoepidemiológicos de Casos Observados No Período de 1989 a 1993. Rev. Soc. Bras. Med. Trop. 2004;37:305–311. doi: 10.1590/S0037-86822004000400004. [DOI] [PubMed] [Google Scholar]
- 66.Dias A.F.L.R., Almeida A.B.P.F., Rodrigues J.Y., Nakazato L., Fujimori M., Sousa V.R.F. Cytological and Molecular Detection of Leishmania spp. in Different Biological Tissues of Dogs in Areas Endemic for Visceral Leishmaniasis. Arq. Bras. Med. Vet. Zootec. 2019;71:2103–2106. doi: 10.1590/1678-4162-10775. [DOI] [Google Scholar]
- 67.Menegatti J.A., Oliveira Júnior G.J., Silva L.C.F., Oliveira A., Bica D.L.C., Santos P.V.B.A., Cunha Filho L.F.C., Lunardi M. Fauna Flebotomínica e Soroprevalência Para Leishmaniose Visceral Canina em Área Urbana Na Região Centro-Oeste do Brasil. Arq. Bras. Med. Vet. Zootec. 2020;72:1197–1205. doi: 10.1590/1678-4162-11549. [DOI] [Google Scholar]
- 68.Steindel M., Menin Á., Evangelista T., Stoco P.H., Marlow M.A., Fleith R.C., Pilati C., Grisard E.C. Outbreak of Autochthonous Canine Visceral Leishmaniasis in Santa Catarina, Brazil. Pesqui. Veterinária Bras. 2013;33:490–496. doi: 10.1590/S0100-736X2013000400013. [DOI] [Google Scholar]
- 69.De Oliveira A.C., Figueiredo F.B., Silva V.L., Santos F.N., de Souza M.B., Madeira M.D.F., Abrantes T.R., Périssé A.R.S. Canine visceral leishmaniasis case investigation in the jacare region of niteroi, rio de janeiro, brazil. Rev. Inst. Med. Trop. Sao Paulo. 2015;57:325–332. doi: 10.1590/S0036-46652015000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riboldi E., Carvalho F., Romão P.R.T., Barcellos R.B., Bello G.L., Ramos R.R., de Oliveira R.T., Júnior J.P.A., Rossetti M.L., Dallegrave E. Molecular Method Confirms Canine Leishmania Infection Detected by Serological Methods in Non-Endemic Area of Brazil. Korean J. Parasitol. 2018;56:11–19. doi: 10.3347/kjp.2018.56.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tonini M.A.L., Lemos E.M., Reis A.B., Vital W.C., Dias E.S., Dietze R. First Description of Autochthonous Canine Visceral Leishmaniasis in the Metropolitan Region of Vitória, State of Espírito Santo, Brazil. Rev. Soc. Bras. Med. Trop. 2012;45:754–756. doi: 10.1590/S0037-86822012000600019. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues T.F., Benitez A.D.N., Sevá A.D.P., Okamura L.H., Galvão A.B., Gomes J.F., Bresciani K.D.S., Cardoso T.C. Spatial and Seroepidemiology of Canine Visceral Leishmaniasis in an Endemic Southeast Brazilian Area. Rev. Soc. Bras. Med. Trop. 2020;53:e20190525. doi: 10.1590/0037-8682-0525-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teixeira-Neto R.G., da Silva E.S., Nascimento R.A., Belo V.S., de Oliveira C.D.L., Pinheiro L.C., Gontijo C.M.F. Canine Visceral Leishmaniasis in an Urban Setting of Southeastern Brazil: An Ecological Study Involving Spatial Analysis. Parasit. Vectors. 2014;7:485. doi: 10.1186/s13071-014-0485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barata R.A., Peixoto J.C., Tanure A., Gomes M.E., Apolinário E.C., Bodevan E.C., de Araújo H.S., Dias E.S., Pinheiro A.D.C. Epidemiology of Visceral Leishmaniasis in a Reemerging Focus of Intense Transmission in Minas Gerais State, Brazil. Biomed. Res. Int. 2013;2013:1–6. doi: 10.1155/2013/405083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinheiro A.D.C., da Costa A.S.V., de Oliveira R.S., Reis M.L.C. Epidemiological Aspects and Spatial Distribution of Visceral Leishmaniasis in Governador Valadares, Brazil, between 2008 and 2012. Rev. Soc. Bras. Med. Trop. 2020;53:e20190216. doi: 10.1590/0037-8682-0216-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brito F.G., Langoni H., da Silva R.C., Rotondano T.E.D.F., de Melo M.A., da Paz G.S. Canine Visceral Leishmaniasis in the Northeast Region of Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016;22:15. doi: 10.1186/s40409-016-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valadas S., Minervino A.H.H., Lima V.M.F., Soares R.M., Ortolani E.L., Gennari S.M. Occurrence of Antibodies Anti-Neospora Caninum, Anti-Toxoplasma Gondii, and Anti-Leishmania chagasi in Serum of Dogs from Pará State, Amazon, Brazil. Parasitol. Res. 2010;107:453–457. doi: 10.1007/s00436-010-1890-2. [DOI] [PubMed] [Google Scholar]
- 78.Costa D.N.C.C., Blangiardo M., Rodas L.A.C., Nunes C.M., Hiramoto R.M., Tolezano J.E., Bonfietti L.X., Bermudi P.M.M., Cipriano R.S., Cardoso G.C.D., et al. Canine Visceral Leishmaniasis in Araçatuba, State of São Paulo, Brazil, and Its Relationship with Characteristics of Dogs and Their Owners: A Cross-Sectional and Spatial Analysis Using a Geostatistical Approach. BMC Vet. Res. 2018;14:229. doi: 10.1186/s12917-018-1550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medkour H., Davoust B., Dulieu F., Maurizi L., Lamour T., Marié J.-L., Mediannikov O. Potential Animal Reservoirs (Dogs and Bats) of Human Visceral Leishmaniasis Due to Leishmania infantum in French Guiana. PLoS Negl. Trop. Dis. 2019;13:e0007456. doi: 10.1371/journal.pntd.0007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alam M.Z., Kovalenko D.A., Kuhls K., Nasyrova R.M., Ponomareva V.I., Fatullaeva A.A., Razakov S.A., Schnur L.F., Schönian G. Identification of the Agent Causing Visceral Leishmaniasis in Uzbeki and Tajiki Foci by Analysing Parasite DNA Extracted from Patients’ Giemsa-Stained Tissue Preparations. Parasitology. 2009;136:981–986. doi: 10.1017/S0031182009006465. [DOI] [PubMed] [Google Scholar]
- 81.Akter S., Alam M.Z., Nakao R., Yasin G., Kato H., Katakura K. Molecular and Serological Evidence of Leishmania Infection in Stray Dogs from Visceral Leishmaniasis–Endemic Areas of Bangladesh. Am. J. Trop. Med. Hyg. 2016;95:795–799. doi: 10.4269/ajtmh.16-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karunaweera N.D., Ferreira M.U. Leishmaniasis: Current Challenges and Prospects for Elimination with Special Focus on the South Asian Region. Parasitology. 2018;145:425–429. doi: 10.1017/S0031182018000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharifi I., Aflatoonian M.R., Daei Parizi M.H., Hosseininasab A., Mostafavi M., Bamorovat M., Aghaei Afshar A., Mohebali M., Keshavarz H., Daneshvar H., et al. Visceral Leishmaniasis in Southeastern Iran: A Narrative Review. Iran. J. Parasitol. 2017;12:1–11. [PMC free article] [PubMed] [Google Scholar]
- 84.Dalimi A., Mohammadiha A., Mohebali M., Mirzaei A., Mahmoudi M. Molecular Identification and Intra-Species Variations among Leishmania infantum Isolated from Human and Canine Visceral Leishmaniasis in Iran. Iran. J. Parasitol. 2018;13:567–576. [PMC free article] [PubMed] [Google Scholar]
- 85.Baneth G., Dank G., Keren-Kornblatt E., Sekeles E., Adini I., Eisenberger C.L., Schnur L.F., King R., Jaffe C.L. Emergence of Visceral Leishmaniasis in Central Israel. Am. J. Trop. Med. Hyg. 1998;59:722–725. doi: 10.4269/ajtmh.1998.59.722. [DOI] [PubMed] [Google Scholar]
- 86.Nasereddin A., Baneth G., Schönian G., Kanaan M., Jaffe C.L. Molecular Fingerprinting of Leishmania infantum Strains Following an Outbreak of Visceral Leishmaniasis in Central Israel. J. Clin. Microbiol. 2005;43:6054–6059. doi: 10.1128/JCM.43.12.6054-6059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamarsheh O., Nasereddin A., Damaj S., Sawalha S., Al-Jawabreh H., Azmi K., Amro A., Ereqat S., Abdeen Z., Al-Jawabreh A. Serological and Molecular Survey of Leishmania Parasites in Apparently Healthy Dogs in the West Bank, Palestine. Parasit. Vectors. 2012;5:183. doi: 10.1186/1756-3305-5-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baneth G., Nachum-Biala Y., Adamsky O., Gunther I. Leishmania Tropica and Leishmania infantum Infection in Dogs and Cats in Central Israel. Parasit. Vectors. 2022;15:147. doi: 10.1186/s13071-022-05272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ozbel Y., Oskam L., Ozensoy S., Turgay N., Alkan M.Z., Jaffe C.L., Ozcel M.A. A Survey on Canine Leishmaniasis in Western Turkey by Parasite, DNA and Antibody Detection Assays. Acta Trop. 2000;74:1–6. doi: 10.1016/S0001-706X(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 90.Toz S.O., Culha G., Zeyrek F.Y., Ertabaklar H., Alkan M.Z., Vardarlı A.T., Gunduz C., Ozbel Y. A Real-Time ITS1-PCR Based Method in the Diagnosis and Species Identification of Leishmania Parasite from Human and Dog Clinical Samples in Turkey. PLoS Negl. Trop. Dis. 2013;7:e2205. doi: 10.1371/journal.pntd.0002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koenhemsi L., Fabrizio V., Mariella P., Antonella M., Or E. Seroprevalence of Leishmaniosis Among Healthy Dogs in Istanbul. Isr. J. Vet. Med. 2020;75:31–34. [Google Scholar]
- 92.Strelkova M.V., Ponirovsky E.N., Morozov E.N., Zhirenkina E.N., Razakov S.A., Kovalenko D.A., Schnur L.F., Schönian G. A Narrative Review of Visceral Leishmaniasis in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, the Crimean Peninsula and Southern Russia. Parasit. Vectors. 2015;8:330. doi: 10.1186/s13071-015-0925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Díaz-Regañón D., Agulla B., Piya B., Fernández-Ruiz N., Villaescusa A., García-Sancho M., Rodríguez-Franco F., Sainz Á. Stray Dogs in Nepal Have High Prevalence of Vector-Borne Pathogens: A Molecular Survey. Parasit. Vectors. 2020;13:174. doi: 10.1186/s13071-020-04057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rab M.A., Frame I.A., Evans D.A. The Role of Dogs in the Epidemiology of Human Visceral Leishmaniasis in Northern Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1995;89:612–615. doi: 10.1016/0035-9203(95)90409-3. [DOI] [PubMed] [Google Scholar]
- 95.Shang L., Peng W., Jin H., Xu D., Zhong N., Wang W., Wu Y., Liu Q. The Prevalence of Canine Leishmania infantum Infection in Sichuan Province, Southwestern China Detected by Real Time PCR. Parasit. Vectors. 2011;4:173. doi: 10.1186/1756-3305-4-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J.-Y., Ha Y., Gao C.-H., Wang Y., Yang Y.-T., Chen H.-T. The Prevalence of Canine Leishmania infantum Infection in Western China Detected by PCR and Serological Tests. Parasit. Vectors. 2011;4:69. doi: 10.1186/1756-3305-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandy J., Matthews A., Nachum-Biala Y., Baneth G. First Report of Autochthonous Canine Leishmaniasis in Hong Kong. Microorganisms. 2022;10:1873. doi: 10.3390/microorganisms10091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colella V., Nguyen V.L., Tan D.Y., Lu N., Fang F., Zhijuan Y., Wang J., Liu X., Chen X., Dong J., et al. Zoonotic Vectorborne Pathogens and Ectoparasites of Dogs and Cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020;26:1221–1233. doi: 10.3201/eid2606.191832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dujardin J.-C., Campino L., Cañavate C., Dedet J.-P., Gradoni L., Soteriadou K., Mazeris A., Ozbel Y., Boelaert M. Spread of Vector-Borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Velo E., Bongiorno G., Kadriaj P., Myrseli T., Crilly J., Lika A., Mersini K., Di Muccio T., Bino S., Gramiccia M., et al. The Current Status of Phlebotomine Sand Flies in Albania and Incrimination of Phlebotomus neglectus (Diptera, Psychodidae) as the Main Vector of Leishmania infantum. PLoS ONE. 2017;12:e0179118. doi: 10.1371/journal.pone.0179118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colella V., Hodžić A., Iatta R., Baneth G., Alić A., Otranto D. Zoonotic Leishmaniasis, Bosnia and Herzegovina. Emerg. Infect. Dis. 2019;25:385–386. doi: 10.3201/eid2502.181481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Živičnjak T., Martinković F., Marinculić A., Mrljak V., Kučer N., Matijatko V., Mihaljević Ž., Barić-Rafaj R. A Seroepidemiologic Survey of Canine Visceral Leishmaniosis among Apparently Healthy Dogs in Croatia. Vet. Parasitol. 2005;131:35–43. doi: 10.1016/j.vetpar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 103.Živičnjak T., Martinković F., Khoury C., Bongiorno G., Bosnić S., Lukačević D., Maroli M. Serological and Entomological Studies of Canine Leishmaniosis in Croatia. Vet. Arh. 2011;81:99–110. [Google Scholar]
- 104.Deplazes P., Grimm F., Papaprodromou M., Cavaliero T., Gramiccia M., Christofi G., Christofi N., Economides P., Eckert J. Canine Leishmaniosis in Cyprus Due to Leishmania infantum MON 1. Acta Trop. 1998;71:169–178. doi: 10.1016/S0001-706X(98)00064-3. [DOI] [PubMed] [Google Scholar]
- 105.Mazeris A., Soteriadou K., Dedet J.P., Haralambous C., Tsatsaris A., Moschandreas J., Messaritakis I., Christodoulou V., Papadopoulos B., Ivović V., et al. Leishmaniases and the Cyprus Paradox. Am. J. Trop. Med. Hyg. 2010;82:441–448. doi: 10.4269/ajtmh.2010.09-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruh E., Taylan Ozkan A. Leishmaniasis in Northern Cyprus. Eur. J. Ther. 2019;25:1–5. doi: 10.5152/EurJTher.2019.18077. [DOI] [Google Scholar]
- 107.Chamaillé L., Tran A., Meunier A., Bourdoiseau G., Ready P., Dedet J.-P. Environmental Risk Mapping of Canine Leishmaniasis in France. Parasit. Vectors. 2010;3:31. doi: 10.1186/1756-3305-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pomares C., Marty P., Bañuls A.L., Lemichez E., Pratlong F., Faucher B., Jeddi F., Moore S., Michel G., Aluru S., et al. Genetic Diversity and Population Structure of Leishmania infantum from Southeastern France: Evaluation Using Multi-Locus Microsatellite Typing. PLoS Negl. Trop. Dis. 2016;10:e0004303. doi: 10.1371/journal.pntd.0004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lachaud L., Chabbert E., Dubessay P., Dereure J., Lamothe J., Dedet J.-P., Bastien P. Value of Two PCR Methods for the Diagnosis of Canine Visceral Leishmaniasis and the Detection of Asymptomatic Carriers. Parasitology. 2002;125:197–207. doi: 10.1017/S0031182002002081. [DOI] [PubMed] [Google Scholar]
- 110.Aoun O., Mary C., Roqueplo C., Marié J.-L., Terrier O., Levieuge A., Davoust B. Canine Leishmaniasis in South-East of France: Screening of Leishmania infantum Antibodies (Western Blotting, ELISA) and Parasitaemia Levels by PCR Quantification. Vet. Parasitol. 2009;166:27–31. doi: 10.1016/j.vetpar.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Le Rutte E.A., van der Wilt L.S., Bulstra C.A., Nieboer D., Kontoroupis P., de Vlas S.J., Richardus J.H. Incidence and Geographical Distribution of Canine Leishmaniosis in 2016–2017 in Spain and France. Vet. Parasitol. Reg. Stud. Rep. 2021;25:100613. doi: 10.1016/j.vprsr.2021.100613. [DOI] [PubMed] [Google Scholar]
- 112.Ntais P., Sifaki-Pistola D., Christodoulou V., Messaritakis I., Pratlong F., Poupalos G., Antoniou M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013;89:906–915. doi: 10.4269/ajtmh.13-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Symeonidou I., Angelou A., Theodoridis A., Sioutas G., Papadopoulos E. Canine Leishmaniosis in Greece: An Updated Countrywide Serological Study and Associated Risk Factors. Pathogens. 2021;10:1129. doi: 10.3390/pathogens10091129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tamponi C., Scarpa F., Carta S., Knoll S., Sanna D., Gai C., Pipia A.P., Dessì G., Casu M., Varcasia A., et al. Seroprevalence and Risk Factors Associated with Leishmania infantum in Dogs in Sardinia (Italy), an Endemic Island for Leishmaniasis. Parasitol. Res. 2021;120:289–300. doi: 10.1007/s00436-020-06973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferroglio E., Maroli M., Gastaldo S., Mignone W., Rossi L. Canine Leishmaniasis, Italy. Emerg. Infect. Dis. 2005;11:1618–1620. doi: 10.3201/eid1110.040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baldelli R., Piva S., Salvatore D., Parigi M., Melloni O., Tamba M., Bellini R., Poglayen G. Canine Leishmaniasis Surveillance in a Northern Italy Kennel. Vet. Parasitol. 2011;179:57–61. doi: 10.1016/j.vetpar.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 117.Santi A., Renzi M., Baldelli R., Calzolari M., Caminiti A., Dell’Anna S., Galletti G., Lombardini A., Paternoster G., Tamba M. A Surveillance Program on Canine Leishmaniasis in the Public Kennels of Emilia-Romagna Region, Northern Italy. Vector-Borne Zoonotic Dis. 2014;14:206–211. doi: 10.1089/vbz.2013.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendoza-Roldan J., Benelli G., Panarese R., Iatta R., Furlanello T., Beugnet F., Zatelli A., Otranto D. Leishmania infantum and Dirofilaria Immitis Infections in Italy, 2009–2019: Changing Distribution Patterns. Parasit Vectors. 2020;13:193. doi: 10.1186/s13071-020-04063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moirano G., Zanet S., Giorgi E., Battisti E., Falzoi S., Acquaotta F., Fratianni S., Richiardi L., Ferroglio E., Maule M. Integrating Environmental, Entomological, Animal, and Human Data to Model the Leishmania infantum Transmission Risk in a Newly Endemic Area in Northern Italy. One Health. 2020;10:100159. doi: 10.1016/j.onehlt.2020.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salvatore D., Di Francesco A., Parigi M., Poglayen G., Battistini M., Baldelli R. Programma di Sorveglianza della Leishmaniosi Canina in un Canile della Repubblica di San Marino. Vet. Ital. 2013;49:341–346. doi: 10.12834/VetIt.1302.01. [DOI] [PubMed] [Google Scholar]
- 121.Headington C.E., Barbara C.H., Lambson B.E., Hart D.T., Barker D.C. Diagnosis of Leishmaniasis in Maltese Dogs with the Aid of the Polymerase Chain Reaction. Trans. R. Soc. Trop. Med. Hyg. 2002;96:S195–S197. doi: 10.1016/S0035-9203(02)90076-3. [DOI] [PubMed] [Google Scholar]
- 122.Cardoso L., Mendão C., Madeira de Carvalho L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi Sensu Lato, Anaplasma spp. and Leishmania infantum in Apparently Healthy and CVBD-Suspect Dogs in Portugal—A National Serological Study. Parasit. Vectors. 2012;5:62. doi: 10.1186/1756-3305-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maia C., Dionisio L., Afonso M.O., Neto L., Cristovao J.M., Campino L. Leishmania Infection and Host-Blood Feeding Preferences of Phlebotomine Sandflies and Canine Leishmaniasis in an Endemic European Area, the Algarve Region in Portugal. Mem. Inst. Oswaldo Cruz. 2013;108:481–487. doi: 10.1590/S0074-0276108042013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maia C., Coimbra M., Ramos C., Cristóvão J., Cardoso L., Campino L. Serological Investigation of Leishmania infantum, Dirofilaria Immitis and Angiostrongylus Vasorum in Dogs from Southern Portugal. Parasit. Vectors. 2015;8:152. doi: 10.1186/s13071-015-0771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schallig H.D., Cardoso L., Semião-Santos S.J. Seroepidemiology of Canine Leishmaniosis in Évora (Southern Portugal): 20-Year Trends. Parasit. Vectors. 2013;6:100. doi: 10.1186/1756-3305-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maia C., Altet L., Serrano L., Cristóvão J.M., Tabar M.D., Francino O., Cardoso L., Campino L., Roura X. Molecular Detection of Leishmania infantum, Filariae and Wolbachia Spp. in Dogs from Southern Portugal. Parasit. Vectors. 2016;9:170. doi: 10.1186/s13071-016-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maia C., Alwassouf S., Cristóvão J.M., Ayhan N., Pereira A., Charrel R.N., Campino L. Serological Association between Leishmania infantum and Sand Fly Fever Sicilian (but Not Toscana) Virus in Sheltered Dogs from Southern Portugal. Parasit. Vectors. 2017;10:92. doi: 10.1186/s13071-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Almeida M., Maia C., Cristóvão J.M., Morgado C., Barbosa I., Ibars R.F., Campino L., Gonçalves L., Cortes S. Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms. 2022;10:2262. doi: 10.3390/microorganisms10112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Montoya-Alonso J.A., Morchón R., Costa-Rodríguez N., Matos J.I., Falcón-Cordón Y., Carretón E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.564429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gálvez R., Montoya A., Cruz I., Fernández C., Martín O., Checa R., Chicharro C., Migueláñez S., Marino V., Miró G. Latest Trends in Leishmania infantum Infection in Dogs in Spain, Part I: Mapped Seroprevalence and Sand Fly Distributions. Parasit. Vectors. 2020;13:204. doi: 10.1186/s13071-020-04081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Montoya A., Gálvez R., Checa R., Sarquis J., Plaza A., Barrera J.P., Marino V., Miró G. Latest Trends in L. infantum Infection in Dogs in Spain, Part II: Current Clinical Management and Control According to a National Survey of Veterinary Practitioners. Parasit. Vectors. 2020;13:205. doi: 10.1186/s13071-020-04080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Díaz-Regañón D., Roura X., Suárez M.L., León M., Sainz Á. Serological Evaluation of Selected Vector-Borne Pathogens in Owned Dogs from Northern Spain Based on a Multicenter Study Using a Commercial Test. Parasit. Vectors. 2020;13:301. doi: 10.1186/s13071-020-04172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsatchev I., Kyriazis I.D., Boutsini S., Karagouni E., Dotsika E. First Report of Canine Visceral Leishmaniasis in Bulgaria. Turk. J. Vet. Anim. Sci. 2010;34:465–469. doi: 10.3906/vet-0905-16. [DOI] [Google Scholar]
- 134.Babuadze G., Alvar J., Argaw D., de Koning H.P., Iosava M., Kekelidze M., Tsertsvadze N., Tsereteli D., Chakhunashvili G., Mamatsashvili T., et al. Epidemiology of Visceral Leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014;8:e2725. doi: 10.1371/journal.pntd.0002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Babuadze G., Farlow J., de Koning H.P., Carrillo E., Chakhunashvili G., Murskvaladze M., Kekelidze M., Karseladze I., Kokaia N., Kalandadze I., et al. Seroepidemiology and Molecular Diversity of Leishmania donovani Complex in Georgia. Parasit. Vectors. 2016;9:279. doi: 10.1186/s13071-016-1558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miró G., Wright I., Michael H., Burton W., Hegarty E., Rodón J., Buch J., Pantchev N., von Samson-Himmelstjerna G. Seropositivity of Main Vector-Borne Pathogens in Dogs across Europe. Parasit. Vectors. 2022;15:189. doi: 10.1186/s13071-022-05316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tánczos B., Balogh N., Király L., Biksi I., Szeredi L., Gyurkovsky M., Scalone A., Fiorentino E., Gramiccia M., Farkas R. First Record of Autochthonous Canine Leishmaniasis in Hungary. Vector-Borne Zoonotic Dis. 2012;12:588–594. doi: 10.1089/vbz.2011.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xhekaj B., Alishani M., Rexhepi A., Jakupi X., Sherifi K. Serological Survey of Canine Leishmaniasis in Southwestern Region of Kosovo. Vet. Ital. 2020;56:47–50. doi: 10.12834/VetIt.1345.7407.5. [DOI] [PubMed] [Google Scholar]
- 139.Tasić-Otašević S., Savić S., Jurhar-Pavlova M., Stefanovska J., Stalević M., Ignjatović A., Ranđelović M., Gajić B., Cvetkovikj A., Gabrielli S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals. 2022;12:911. doi: 10.3390/ani12070911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mircean V., Dumitrache M.O., Mircean M., Bolfa P., Györke A., Mihalca A.D. Autochthonous Canine Leishmaniasis in Romania: Neglected or (Re)Emerging? Parasit. Vectors. 2014;7:135. doi: 10.1186/1756-3305-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cîmpan A.A., Diakou A., Papadopoulos E., Miron L.D. Serological study of exposure to Leishmania in dogs living in shelters, in south-east romania. Rev. Rom. Med. Vet. 2019;29:54–58. [Google Scholar]
- 142.Cazan C.D., Ionică A.M., Matei I.A., D’Amico G., Muñoz C., Berriatua E., Dumitrache M.O. Detection of Leishmania infantum DNA and Antibodies against Anaplasma spp., Borrelia burgdorferi s.l. and Ehrlichia canis in a Dog Kennel in South-Central Romania. Acta Vet. Scand. 2020;62:42. doi: 10.1186/s13028-020-00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kotnik T. Dog Leishmaniasis in Slovenia: A Probable Creation of the First Enzootic Focus—A Case Report. Vet. Arh. 2020;90:317–322. doi: 10.24099/vet.arhiv.0380. [DOI] [Google Scholar]
- 144.McKenna M., Attipa C., Tasker S., Augusto M. Leishmaniosis in a Dog with No Travel History outside of the UK. Vet. Rec. 2019;184:441. doi: 10.1136/vr.105157. [DOI] [PubMed] [Google Scholar]
- 145.Bashaye S., Nombela N., Argaw D., Mulugeta A., Herrero M., Nieto J., Chicharro C., Cañavate C., Aparicio P., Vélez I.D., et al. Risk Factors for Visceral Leishmaniasis in a New Epidemic Site in Amhara Region, Ethiopia. Am. J. Trop. Med. Hyg. 2009;81:34–39. doi: 10.4269/ajtmh.2009.81.34. [DOI] [PubMed] [Google Scholar]
- 146.Kalayou S., Tadelle H., Bsrat A., Abebe N., Haileselassie M., Schallig H.D.F.H. Serological Evidence of Leishmania donovani Infection in Apparently Healthy Dogs Using Direct Agglutination Test (DAT) and Rk39 Dipstick Tests in Kafta Humera, North-West Ethiopia. Transbound. Emerg. Dis. 2011;58:255–262. doi: 10.1111/j.1865-1682.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 147.Rohousova I., Talmi-Frank D., Kostalova T., Polanska N., Lestinova T., Kassahun A., Yasur-Landau D., Maia C., King R., Votypka J., et al. Exposure to Leishmania spp. and Sand Flies in Domestic Animals in Northwestern Ethiopia. Parasit. Vectors. 2015;8:360. doi: 10.1186/s13071-015-0976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bejano S., Shumie G., Kumar A., Asemahagn E., Damte D., Woldie S., Mulugeta A., Manaye N., Genetu A., Gadisa E., et al. Prevalence of Asymptomatic Visceral Leishmaniasis in Human and Dog, Benishangul Gumuz Regional State, Western Ethiopia. Parasit. Vectors. 2021;14:39. doi: 10.1186/s13071-020-04542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharifdini M., Mohebali M., Keshavarz H., Hosseininejad M., Hajjaran H., Akhoundi B., Foroushani A.R., Zarei Z., Charehdar S. Neospora Caninum and Leishmania infantum Co-Infection in Domestic Dogs (Canis Familiaris) in Meshkin-Shahr District, Northwestern Iran. Iran. J. Arthropod Borne Dis. 2011;1:60–68. [PMC free article] [PubMed] [Google Scholar]
- 150.Moshfe A., Mohebali M., Afshoun E., Mousavizadeh A., Zarei Z., Abidi H., Akhoundi B., Barati V., Joukar S. Canine Visceral Leishmaniasis in Boyer Ahmad District, Kohgiluyeh & Boyer Ahmad Province, Southwest of Iran. Iran. J. Parasitol. 2012;7:75–81. [PMC free article] [PubMed] [Google Scholar]
- 151.Haddadzade H., Fattahi R., Mohebali M., Akhoundi B., Ebrahimzade E. Seroepidemiologcal Investigation of Visceral Leishmaniasis in Stray and Owned Dogs in Alborz Province, Central Iran Using Direct Agglutination Test. Iran. J. Parasitol. 2013;8:152–157. [PMC free article] [PubMed] [Google Scholar]
- 152.Sabzevari S., Razmi G.R., Naghibi A., Khoshnegah J. A Serological Study of Leishmania infantum in Dogs of Khorasan Razavi Province, Iran. J. Parasit. Dis. 2013;37:189–191. doi: 10.1007/s12639-012-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bamorovat M., Sharifi I., Mohammadi M.A., Fasihi Harandi M., Mohebali M., Malekpour Afshar R., Babaei Z., Ziaali N., Aflatoonian M.R. Canine Visceral Leishmaniasis in Kerman, Southeast of Iran: A Seroepidemiological, Histopathological and Molecular Study. Iran. J. Parasitol. 2014;9:342–349. [PMC free article] [PubMed] [Google Scholar]
- 154.Mahshid M., Baharak A., Iraj S., Sina K., Javad K., Mehdi B. Seroprevalence of Canine Visceral Leishmaniasis in Southeast of Iran. J. Parasit. Dis. 2014;38:218–222. doi: 10.1007/s12639-012-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Malmasi A., Janitabar S., Mohebali M., Akhoundi B., Maazi N., Aramoon M., Khorrami N., Seifi H.A. Seroepidemiologic Survey of Canine Visceral Leishmaniasis in Tehran and Alborz Provinces of Iran. J. Arthropod Borne Dis. 2014;8:132–138. [PMC free article] [PubMed] [Google Scholar]
- 156.Barati M., Mohebali M., Alimohammadian M.H., Khamesipour A., Akhoundi B., Zarei Z. Canine Visceral Leishmaniasis: Seroprevalence Survey of Asymptomatic Dogs in an Endemic Area of Northwestern Iran. J. Parasit. Dis. 2015;39:221–224. doi: 10.1007/s12639-013-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gharekhani J., Heidari H., Hajian-Bidar H., Abbasi-Doulatshahi E., Edalati-Shokat H. Prevalence of Anti-Leishmania infantum Antibodies in Dogs from West of Iran. J. Parasit. Dis. 2016;40:964–967. doi: 10.1007/s12639-014-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Afshar M.J.A., Sharifi I., Bamorovat M., Mohebali M., Bahreini M.S., Naderi A. Canine Visceral Leishmaniasis; A Seroepidemiological Survey in Jiroft District, Southern Kerman Province, Southeastern Iran in 2015. Iran. J. Parasitol. 2018;13:67–71. [PMC free article] [PubMed] [Google Scholar]
- 159.Heidari A., Mohebali M., Vahed M., Kabir K., Zarei Z., Akhoundi B., Elikaee S., Barati H., Sezavar M., Keshavarz H., et al. Molecular and Seroepidemiological Survey of Visceral Leishmaniasis in Owned Dogs (Canis Familiaris) in New Foci of Rural Areas of Alborz Province, Central Part of Iran: A Cross-Sectional Study in 2017. J. Arthropod Borne Dis. 2020;14:38–46. doi: 10.18502/jad.v14i1.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fakhar M., Derakhshani-nia M., Gohardehi S., Karamian M., Hezarjaribi H.Z., Mohebali M., Akhoundi B., Sharbatkhori M. Domestic Dogs Carriers of Leishmania infantum, Leishmania tropica and Crithidia fasciculata as Potential Reservoirs for Human Visceral Leishmaniasis in Northeastern Iran. Vet. Med. Sci. 2022;8:2329–2336. doi: 10.1002/vms3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dumitrache M.O., Nachum-Biala Y., Gilad M., Mircean V., Cazan C.D., Mihalca A.D., Baneth G. The Quest for Canine Leishmaniasis in Romania: The Presence of an Autochthonous Focus with Subclinical Infections in an Area Where Disease Occurred. Parasit. Vectors. 2016;9:297. doi: 10.1186/s13071-016-1583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marcondes M., Rossi C.N. Leishmaniose Visceral No Brasil. Braz. J. Vet. Res. Anim. Sci. 2013;50:341–352. doi: 10.11606/issn.2318-3659.v50i5p341-352. [DOI] [Google Scholar]
- 163.Desjeux P. The Increase in Risk Factors for Leishmaniasis Worldwide. Trans. R. Soc. Trop. Med. Hyg. 2001;95:239–243. doi: 10.1016/S0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 164.Barbosa I.R., Carlota F.C., Andrade-Neto V.F. de Seroepidemiological Survey of Canine Leishmania Infections from Peripheral Areas in Natal, Northeast Brazil. Open Microbiol. J. 2015;9:43–47. doi: 10.2174/1874285801509010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ready P.D. Leishmaniasis Emergence and Climate Change. Rev. Sci. Tech. 2008;27:399–412. doi: 10.20506/rst.27.2.1803. [DOI] [PubMed] [Google Scholar]
- 166.El Omari H., Chahlaoui A., Talbi F.Z., El Mouhdi K., El Ouali Lalami A. Impact of Climatic Factors on the Seasonal Fluctuation of Leishmaniasis Vectors in Central Morocco (Meknes Prefecture) Can. J. Infect. Dis. Med. Microbiol. 2020;2020:6098149. doi: 10.1155/2020/6098149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gramiccia M., Gradoni L. The Current Status of Zoonotic Leishmaniases and Approaches to Disease Control. Int. J. Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 168.Ready P.D. Leishmaniasis Emergence in Europe. Euro Surveill. 2010;15:19505. doi: 10.2807/ese.15.10.19505-en. [DOI] [PubMed] [Google Scholar]
- 169.Koch L.K., Kochmann J., Klimpel S., Cunze S. Modeling the Climatic Suitability of Leishmaniasis Vector Species in Europe. Sci. Rep. 2017;7:13325. doi: 10.1038/s41598-017-13822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abdullah A.Y.M., Dewan A., Shogib M.R.I., Rahman M.M., Hossain M.F. Environmental Factors Associated with the Distribution of Visceral Leishmaniasis in Endemic Areas of Bangladesh: Modeling the Ecological Niche. Trop. Med. Health. 2017;45:13. doi: 10.1186/s41182-017-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dos Santos C.V.B., Sevá A.D.P., Werneck G.L., Struchiner C.J. Does Deforestation Drive Visceral Leishmaniasis Transmission? A Causal Analysis. Proc. R. Soc. B Biol. Sci. 2021;288:20211537. doi: 10.1098/rspb.2021.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.El-Sayed A., Kamel M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020;27:22336–22352. doi: 10.1007/s11356-020-08896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Al-Salem W., Herricks J.R., Hotez P.J. A Review of Visceral Leishmaniasis during the Conflict in South Sudan and the Consequences for East African Countries. Parasit. Vectors. 2016;9:460. doi: 10.1186/s13071-016-1743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sunyoto T., Potet J., Boelaert M. Visceral Leishmaniasis in Somalia: A Review of Epidemiology and Access to Care. PLoS Negl. Trop. Dis. 2017;11:e0005231. doi: 10.1371/journal.pntd.0005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Carvalho A.G., Luz J.G.G., Rodrigues L.D., Dias J.V.L., Fontes C.J.F. High Seroprevalence and Peripheral Spatial Distribution of Visceral Leishmaniasis among Domestic Dogs in an Emerging Urban Focus in Central Brazil: A Cross-Sectional Study. Pathog. Glob. Health. 2018;112:29–36. doi: 10.1080/20477724.2018.1438229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carvalho A.G., Luz J.G.G., Rodrigues L.D., Dias J.V.L., Fontes C.J.F. Factors Associated with Leishmania spp. Infection in Domestic Dogs from an Emerging Area of High Endemicity for Visceral Leishmaniasis in Central-Western Brazil. Res. Vet. Sci. 2019;125:205–211. doi: 10.1016/j.rvsc.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 177.Luz J.G.G., Carvalho A.G., Naves D.B., Dias J.V.L., Fontes C.J.F. Are Backyard Characteristics Relevant Factors for the Occurrence of Human Visceral Leishmaniasis in Central-Western Brazil? Trans. R. Soc. Trop. Med. Hyg. 2020;114:276–283. doi: 10.1093/trstmh/trz110. [DOI] [PubMed] [Google Scholar]
- 178.Teixeira A.I.P., Silva D.M., de Freitas L.R.S., Romero G.A.S. A Cross-Sectional Approach Including Dog Owner Characteristics as Predictors of Visceral Leishmaniasis Infection in Dogs. Mem. Inst. Oswaldo Cruz. 2020;115:e190349. doi: 10.1590/0074-02760190349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cortes S., Afonso M.O., Alves-Pires C., Campino L. Stray Dogs and Leishmaniasis in Urban Areas, Portugal. Emerg. Infect. Dis. 2007;13:1431–1432. doi: 10.3201/eid1309.070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martín-Sánchez J., Morales-Yuste M., Acedo-Sánchez C., Barón S., Díaz V., Morillas-Márquez F. Canine Leishmaniasis in Southeastern Spain. Emerg. Infect. Dis. 2009;15:795–798. doi: 10.3201/eid1505.080969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coura-Vital W., Marques M.J., Veloso V.M., Roatt B.M., Aguiar-Soares R.D.D.O., Reis L.E.S., Braga S.L., Morais M.H.F., Reis A.B., Carneiro M. Prevalence and Factors Associated with Leishmania infantum Infection of Dogs from an Urban Area of Brazil as Identified by Molecular Methods. PLoS Negl. Trop. Dis. 2011;5:e1291. doi: 10.1371/journal.pntd.0001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Melo S.N., Teixeira-Neto R.G., Werneck G.L., Struchiner C.J., Ribeiro R.A.N., Sousa L.R., de Melo M.O.G., Carvalho C.G., Jr., Penaforte K.M., Manhani M.N., et al. Prevalence of Visceral Leishmaniasis in A Population of Free-Roaming Dogs as Determined by Multiple Sampling Efforts: A Longitudinal Study Analyzing the Effectiveness of Euthanasia. Prev. Vet. Med. 2018;161:19–24. doi: 10.1016/j.prevetmed.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 183.Werneck G.L., Costa C.H.N., Walker A.M., David J.R., Wand M., Maguire J.H. Multilevel Modelling of the Incidence of Visceral Leishmaniasis in Teresina, Brazil. Epidemiol. Infect. 2007;135:195–201. doi: 10.1017/S0950268806006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Araújo V.E.M., Pinheiro L.C., Almeida M.C.D.M., Menezes F.C.D., Morais M.H.F., Reis I.A., Assunção R.M., Carneiro M. Relative Risk of Visceral Leishmaniasis in Brazil: A Spatial Analysis in Urban Area. PLoS Negl. Trop. Dis. 2013;7:e2540. doi: 10.1371/journal.pntd.0002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leite B.M.M., Solcà M.D.S., Santos L.C.S., Coelho L.B., Amorim L.D.A.F., Donato L.E., Passos S.M.D.S., Almeida A.O.D., Veras P.S.T., Fraga D.B.M. The Mass Use of Deltamethrin Collars to Control and Prevent Canine Visceral Leishmaniasis: A Field Effectiveness Study in a Highly Endemic Area. PLoS Negl. Trop. Dis. 2018;12:e0006496. doi: 10.1371/journal.pntd.0006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oliveira C.D.L., Assunção R.M., Reis I.A., Proietti F.A. Spatial Distribution of Human and Canine Visceral Leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994–1997. Cad. Saude Publica. 2001;17:1231–1239. doi: 10.1590/S0102-311X2001000500023. [DOI] [PubMed] [Google Scholar]
- 187.Gavgani A.S.M., Mohite H., Edrissian G.H., Mohebali M., Davies C.R. Domestic Dog Ownership in Iran Is a Risk Factor for Human Infection with Leishmania infantum. Am. J. Trop. Med. Hyg. 2002;67:511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- 188.Rodrigues A.C.M., Melo A.C.F.L., Júnior A.D.S., Franco S.O., Rondon F.C.M., Bevilaqua C.M.L. Epidemiologia Da Leishmaniose Visceral No Município de Fortaleza, Ceará. Pesqui. Veterinária Bras. 2017;37:1119–1124. doi: 10.1590/s0100-736x2017001000013. [DOI] [Google Scholar]
- 189.Gaskin A.A., Schantz P., Jackson J., Birkenheuer A., Tomlinson L., Gramiccia M., Levy M., Steurer F., Kollmar E., Hegarty B.C., et al. Visceral Leishmaniasis in a New York Foxhound Kennel. J. Vet. Intern. Med. 2002;16:34. doi: 10.1892/0891-6640(2002)016<0034:VLIANY>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 190.França-Silva J.C., da Costa R.T., Siqueira A.M., Machado-Coelho G.L.L., da Costa C.A., Mayrink W., Vieira E.P., Costa J.S., Genaro O., Nascimento E. Epidemiology of Canine Visceral Leishmaniosis in the Endemic Area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet. Parasitol. 2003;111:161–173. doi: 10.1016/S0304-4017(02)00351-5. [DOI] [PubMed] [Google Scholar]
- 191.Giorgobiani E., Chitadze N., Chanturya G., Grdzelidze M., Jochim R.C., Machablishvili A., Tushishvili T., Zedginidze Y., Manjgaladze M.K., Iashvili N., et al. Epidemiologic Aspects of an Emerging Focus of Visceral Leishmaniasis in Tbilisi, Georgia. PLoS Negl. Trop. Dis. 2011;5:e1415. doi: 10.1371/journal.pntd.0001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Palatnik-de-Sousa C.B., Day M.J. One Health: The Global Challenge of Epidemic and Endemic Leishmaniasis. Parasit. Vectors. 2011;4:197. doi: 10.1186/1756-3305-4-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toepp A.J., Monteiro G.R.G., Coutinho J.F.V., Lima A.L., Larson M., Wilson G., Grinnage-Pulley T., Bennett C., Mahachi K., Anderson B., et al. Comorbid Infections Induce Progression of Visceral Leishmaniasis. Parasit. Vectors. 2019;12:54. doi: 10.1186/s13071-019-3312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Belo V.S., Werneck G.L., Barbosa D.S., Simões T.C., Nascimento B.W.L., da Silva E.S., Struchiner C.J. Factors Associated with Visceral Leishmaniasis in the Americas: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2013;7:e2182. doi: 10.1371/journal.pntd.0002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thomaz Soccol V., Pasquali A.K.S., Pozzolo E.M., Leandro A.D.S., Chiyo L., Baggio R.A., Michaliszyn M.S., Silva C., Cubas P.H., Peterlle R., et al. More than the Eyes Can See: The Worrying Scenario of Canine Leishmaniasis in the Brazilian Side of the Triple Border. PLoS ONE. 2017;12:e0189182. doi: 10.1371/journal.pone.0189182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cabrera M.A.A., Paula A.A., Camacho L.A.B., Marzochi M.C.A., Xavier S.C., da Silva A.V.M., Jansen A.M. Canine Visceral Leishmaniasis in Barra de Guaratiba, Rio de Janeiro, Brazil: Assessment of Risk Factors. Rev. Inst. Med. Trop. Sao Paulo. 2003;45:79–83. doi: 10.1590/S0036-46652003000200005. [DOI] [PubMed] [Google Scholar]
- 197.Almeida A.d.B.P.F.d., Mendonça A.J., Sousa V.R.F. Prevalência e Epidemiologia Da Leishmaniose Visceral Em Cães e Humanos, Na Cidade de Cuiabá, Mato Grosso, Brasil. Ciência Rural. 2010;40:1610–1615. doi: 10.1590/S0103-84782010005000102. [DOI] [Google Scholar]
- 198.dos Santos J.M.L., Dantas-Torres F., Mattos M.R.F., Lino F.R.L., Andrade L.S.S., de Souza R.C.A., Brito M.E.F.D., de Brito M.E.F., Brandão-Filho S.P., Simões-Mattos L. Prevalência de Anticorpos Antileishmania spp. Em Cães de Garanhuns, Agreste de Pernambuco. Rev. Soc. Bras. Med. Trop. 2010;43:41–45. doi: 10.1590/S0037-86822010000100010. [DOI] [PubMed] [Google Scholar]
- 199.Deane L.M. Ph.D. Thesis. Universidade de São Paulo; São Paulo, Brazil: 1956. Leishmaniose Visceral No Brasil. Estudos Sobre Reservatórios e Transmissores Realizados No Estado do Ceará. [Google Scholar]
- 200.Deane L.M., Deane M.P. Visceral Leishmaniosis in Brazil. Geographical Distribution and Transmission. Rev. Inst. Med. Trop. 1962;4:198–212. [PubMed] [Google Scholar]
- 201.Fraga D.B.M., Solcà M.S., Silva V.M.G., Borja L.S., Nascimento E.G., Oliveira G.G.S., Pontes-de-Carvalho L.C., Veras P.S.T., dos-Santos W.L.C. Temporal Distribution of Positive Results of Tests for Detecting Leishmania Infection in Stray Dogs of an Endemic Area of Visceral Leishmaniasis in the Brazilian Tropics: A 13 Years Survey and Association with Human Disease. Vet. Parasitol. 2012;190:591–594. doi: 10.1016/j.vetpar.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 202.Miró G., Gálvez R., Fraile C., Descalzo M.A., Molina R. Infectivity to Phlebotomus perniciosus of Dogs Naturally Parasitized with Leishmania infantum after Different Treatments. Parasit. Vectors. 2011;4:52. doi: 10.1186/1756-3305-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Courtenay O., Carson C., Calvo-Bado L., Garcez L.M., Quinnell R.J. Heterogeneities in Leishmania infantum Infection: Using Skin Parasite Burdens to Identify Highly Infectious Dogs. PLoS Negl. Trop. Dis. 2014;8:e2583. doi: 10.1371/journal.pntd.0002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maia C., Nunes M., Cristóvão J., Campino L. Experimental Canine Leishmaniasis: Clinical, Parasitological and Serological Follow-Up. Acta Trop. 2010;116:193–199. doi: 10.1016/j.actatropica.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 205.Michalsky É.M., Rocha M.F., da Rocha Lima A.C.V.M., França-Silva J.C., Pires M.Q., Oliveira F.S., Pacheco R.S., dos Santos S.L., Barata R.A., Romanha Á.J., et al. Infectivity of Seropositive Dogs, Showing Different Clinical Forms of Leishmaniasis, to Lutzomyia Longipalpis Phlebotomine Sand Flies. Vet. Parasitol. 2007;147:67–76. doi: 10.1016/j.vetpar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 206.Verçosa B., Lemos C., Mendonça I., Silva S., de Carvalho S., Goto H., Costa F. Transmission Potential, Skin Inflammatory Response, and Parasitism of Symptomatic and Asymptomatic Dogs with Visceral Leishmaniasis. BMC Vet. Res. 2008;4:45. doi: 10.1186/1746-6148-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guarga J.L., Lucientes J., Peribáñez M.A., Molina R., Gracia M.J., Castillo J.A. Experimental Infection of Phlebotomusperniciosus and Determination of the Natural Infection Rates of Leishmania infantum in Dogs. Acta Trop. 2000;77:203–207. doi: 10.1016/S0001-706X(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 208.Molina R., Amela C., Nieto J., San-Andrés M., González F., Castillo J.A., Lucientes J., Alvar J. Infectivity of Dogs Naturally Infected with Leishmania infantum to Colonized Phlebotomus perniciosus. Trans. R. Soc. Trop. Med. Hyg. 1994;88:491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 209.Solano-Gallego L., Morell P., Arboix M., Alberola J., Ferrer L. Prevalence of Leishmania infantum Infection in Dogs Living in an Area of Canine Leishmaniasis Endemicity Using PCR on Several Tissues and Serology. J. Clin. Microbiol. 2001;39:560–563. doi: 10.1128/JCM.39.2.560-563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moshfe A., Mohebali M., Edrissian G., Zarei Z., Akhoundi B., Kazemi B., Jamshidi S., Mahmoodi M. Canine Visceral Leishmaniasis: Asymptomatic Infected Dogs as a Source of L. infantum Infection. Acta Trop. 2009;112:101–105. doi: 10.1016/j.actatropica.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 211.Coura-Vital W., Reis A.B., Fausto M.A., Leal G.G.D.A., Marques M.J., Veloso V.M., Carneiro M. Risk Factors for Seroconversion by Leishmania infantum in a Cohort of Dogs from an Endemic Area of Brazil. PLoS ONE. 2013;8:e71833. doi: 10.1371/journal.pone.0071833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lopes J.V., Michalsky É.M., Lara Silva F.D.O., Lima A.C.V.M.R., de Avelar D.M., da Costa A.A.J., França-Silva J.C., Regina-Silva S., Fortes-Dias C.L., Dias E.S. Seroprevalence and Molecular Characterization of Leishmania in Dogs from an Endemic Area of Zoonotic Visceral Leishmaniasis in Brazil. Int. J. Vet. Sci. Med. 2017;5:70–74. doi: 10.1016/j.ijvsm.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miguel D.C., Guarnier D.C. Canine and Human Leishmaniasis: Disease Progression to Brazilian Urbanized Areas. Int. J. Trop. Dis. 2019;2:23. doi: 10.23937/2643-461X/1710023. [DOI] [Google Scholar]
- 214.Travi B.L., Cordeiro-da-Silva A., Dantas-Torres F., Miró G. Canine Visceral Leishmaniasis: Diagnosis and Management of the Reservoir Living among Us. PLoS Negl. Trop. Dis. 2018;12:e0006082. doi: 10.1371/journal.pntd.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dantas-Torres F., Miró G., Bowman D.D., Gradoni L., Otranto D. Culling Dogs for Zoonotic Visceral Leishmaniasis Control: The Wind of Change. Trends Parasitol. 2019;35:97–101. doi: 10.1016/j.pt.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 216.Nunes C.M., de Lima V.M.F., de Paula H.B., Perri S.H.V., de Andrade A.M., Dias F.E.F., Burattini M.N. Dog Culling and Replacement in an Area Endemic for Visceral Leishmaniasis in Brazil. Vet. Parasitol. 2008;153:19–23. doi: 10.1016/j.vetpar.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 217.Sevá A.P., Ovallos F.G., Amaku M., Carrillo E., Moreno J., Galati E.A.B., Lopes E.G., Soares R.M., Ferreira F. Canine-Based Strategies for Prevention and Control of Visceral Leishmaniasis in Brazil. PLoS ONE. 2016;11:e0160058. doi: 10.1371/journal.pone.0160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Grimaldi G., Teva A., Ferreira A.L., dos Santos C.B., Pinto I.D.S., de-Azevedo C.T., Falqueto A. Evaluation of a Novel Chromatographic Immunoassay Based on Dual-Path Platform Technology (DPP® CVL Rapid Test) for the Serodiagnosis of Canine Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012;106:54–59. doi: 10.1016/j.trstmh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 219.Coura-Vital W., Ker H.G., Roatt B.M., Aguiar-Soares R.D.O., Leal G.G.D.A., Moreira N.D.D., Oliveira L.A.M., de Menezes Machado E.M., Morais M.H.F., Corrêa-Oliveira R., et al. Evaluation of Change in Canine Diagnosis Protocol Adopted by the Visceral Leishmaniasis Control Program in Brazil and a New Proposal for Diagnosis. PLoS ONE. 2014;9:e91009. doi: 10.1371/journal.pone.0091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.de Sousa-Paula L.C., da Silva L.G., Sales K.G.D.S., Dantas-Torres F. Failure of the Dog Culling Strategy in Controlling Human Visceral Leishmaniasis in Brazil: A Screening Coverage Issue? PLoS Negl. Trop. Dis. 2019;13:e0007553. doi: 10.1371/journal.pntd.0007553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Courtenay O., Quinnell R.J., Garcez L.M., Shaw J.J., Dye C. Infectiousness in a Cohort of Brazilian Dogs: Why Culling Fails to Control Visceral Leishmaniasis in Areas of High Transmission. J. Infect. Dis. 2002;186:1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- 222.Moreira E.D., Mendes de Souza V.M., Sreenivasan M., Nascimento E.G., Pontes de Carvalho L. Assessment of an Optimized Dog-Culling Program in the Dynamics of Canine Leishmania Transmission. Vet. Parasitol. 2004;122:245–252. doi: 10.1016/j.vetpar.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 223.Ashford D.A., David J.R., Freire M., David R., Sherlock I., Eulálio M.D.C., Sampaio D.P., Badaro R. Studies on Control of Visceral Leishmaniasis: Impact of Dog Control on Canine and Human Visceral Leishmaniasis in Jacobina, Bahia, Brazil. Am. J. Trop. Med. Hyg. 1998;59:53–57. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 224.Costa C.H.N., Tapety C.M.M., Werneck G.L. Controle Da Leishmaniose Visceral Em Meio Urbano: Estudo de Intervenção Randomizado Fatorial. Rev. Soc. Bras. Med. Trop. 2007;40:415–419. doi: 10.1590/S0037-86822007000400009. [DOI] [PubMed] [Google Scholar]
- 225.Nunes C.M., Pires M.M., da Silva K.M., Assis F.D., Filho J.G., Perri S.H.V. Relationship between Dog Culling and Incidence of Human Visceral Leishmaniasis in an Endemic Area. Vet. Parasitol. 2010;170:131–133. doi: 10.1016/j.vetpar.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 226.Costa D.N.C.C., Bermudi P.M.M.B., Rodas L.A.C., Nunes C.M., Hiramoto R.M., Tolezano J.E., Cipriano R.S., Cardoso G.C.D., Codeço C.T., Chiaravalloti-Neto F. Human Visceral Leishmaniasis and Relationship with Vector and Canine Control Measures. Rev. Saude Publica. 2018;52:92. doi: 10.11606/S1518-8787.2018052000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bermudi P.M.M., Costa D.N.C.C., Nunes C.M., Tolezano J.E., Hiramoto R.M., Rodas L.A.C., Cipriano R.S., Blangiardo M., Chiaravalloti-Neto F. Canine Serological Survey and Dog Culling and Its Relationship with Human Visceral Leishmaniasis in an Endemic Urban Area. BMC Infect. Dis. 2020;20:401. doi: 10.1186/s12879-020-05125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dietze R., Barros G.B., Teixeira L., Harris J., Michelson K., Falqueto A., Corey R. Effect of Eliminating Seropositive Canines on the Transmission of Visceral Leishmaniasis in Brazil. Clin. Infect. Dis. 1997;25:1240–1242. doi: 10.1086/516096. [DOI] [PubMed] [Google Scholar]
- 229.Paranhos-Silva M., Nascimento E.G., Melro M.C.B.F., Oliveira G.G.S., dos Santos W.L.C., Pontes-de-Carvalho L.C., Oliveira-dos-Santos A.J. Cohort Study on Canine Emigration and Leishmania Infection in an Endemic Area for American Visceral Leishmaniasis. Implications for the Disease Control. Acta Trop. 1998;69:75–83. doi: 10.1016/S0001-706X(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 230.Vaz T.P., Gama-Melo M.O., Quaresma P.F., Gontijo C.M.F., Santos G., Barbosa F.S., Fontes G. Evaluation of the Euthanasia of Seropositive Dogs for Canine Visceral Leishmaniasis as the Only Method of Controling the Disease in the Enzootic Area in the Midwestern Minas Gerais. Pesqui. Veterinária Bras. 2020;40:107–112. doi: 10.1590/1678-5150-pvb-6165. [DOI] [Google Scholar]
- 231.Costa D.N.C.C., Codeço C.T., Silva M.A., Werneck G.L. Culling Dogs in Scenarios of Imperfect Control: Realistic Impact on the Prevalence of Canine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2013;7:e2355. doi: 10.1371/journal.pntd.0002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.França-Silva J.C., Giunchetti R.C., Mariano R.M.D.S., Machado-Coelho G.L.L., Teixeira L.D.A.S., Barata R.A., Michalsky É.M., Rocha M.F., Fortes-Dias C.L., Dias E.S. The Program for the Control of Visceral Leishmaniasis in Brazil: The Effect of the Systematic Euthanasia of Seropositive Dogs as a Single Control Action in Porteirinha, a Brazilian City with an Intense Transmission of Visceral Leishmaniasis. Pathogens. 2023;12:1060. doi: 10.3390/pathogens12081060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Latif A.A., Nkabinde B., Peba B., Matthee O., Pienaar R., Josemans A., Marumo D., Labuschagne K., Abdelatief N.A., Krüger A., et al. Risk of Establishment of Canine Leishmaniasis Infection through the Import of Dogs into South Africa. Onderstepoort J. Vet. Res. 2019;86:e1–e11. doi: 10.4102/ojvr.v86i1.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Figueiredo F.B., de Lima F.E.F., Jr., Tomio J.E., Indá F.D.M.C., Corrêa G.L.B., Madeira M.D.F. Leishmaniose Visceral Canina: Dois Casos Autóctones No Município de Florianópolis, Estado de Santa Catarina. Acta Sci. Vet. 2012;40:1026. [Google Scholar]
- 235.Vilas V.J.D.R., Maia-Elkhoury A.N.S., Yadon Z.E., Cosivi O., Sanchez-Vazquez M.J. Visceral Leishmaniasis: A One Health Approach. Vet. Rec. 2014;175:42–44. doi: 10.1136/vr.g4378. [DOI] [PubMed] [Google Scholar]
- 236.De Carvalho F.L.N., de Oliveira Riboldi E., Bello G.L., Ramos R.R., Barcellos R.B., Gehlen M., Halon M.L., Romão P.R.T., Dallegrave E., Rossetti M.L.R. Canine Visceral Leishmaniasis Diagnosis: A Comparative Performance of Serological and Molecular Tests in Symptomatic and Asymptomatic Dogs. Epidemiol. Infect. 2018;146:571–576. doi: 10.1017/S0950268818000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pessoa-e-Silva R., Vaitkevicius-Antão V., de Andrade T.A.S., de Oliveira Silva A.C., de Oliveira G.A., Trajano-Silva L.A.M., Nakasone E.K.N., de Paiva-Cavalcanti M. The Diagnosis of Canine Visceral Leishmaniasis in Brazil: Confronting Old Problems. Exp. Parasitol. 2019;199:9–16. doi: 10.1016/j.exppara.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 238.Lopes E.G., Sevá A.P., Ferreira F., Nunes C.M., Keid L.B., Hiramoto R.M., Ferreira H.L., Oliveira T.M.F.S., Bigotto M.F.D., Galvis-Ovallos F., et al. Serological and Molecular Diagnostic Tests for Canine Visceral Leishmaniasis in Brazilian Endemic Area: One out of Five Seronegative Dogs Are Infected. Epidemiol. Infect. 2017;145:2436–2444. doi: 10.1017/S0950268817001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.de Mendonça I.L., Batista J.F., Schallig H., Cruz M.D.S.P.E., Alonso D.P., Ribolla P.E.M., Costa D.L., Costa C.H.N. The Performance of Serological Tests for Leishmania infantum Infection Screening in Dogs Depends on the Prevalence of the Disease. Rev. Inst. Med. Trop. Sao Paulo. 2017;59:e39. doi: 10.1590/s1678-9946201759039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ribeiro V.M., Miranda J.B., Marcelino A.P., de Andrade H.M., Reis I.A., Cardoso M.S., Gontijo C.M.F., Paz G.F. Performance of Different Serological Tests in the Diagnosis of Natural Infection by Leishmania infantum in Dogs. Vet. Parasitol. 2019;274:108920. doi: 10.1016/j.vetpar.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 241.Herrera G., Castillo A., Ayala M.S., Flórez C., Cantillo-Barraza O., Ramirez J.D. Evaluation of Four Rapid Diagnostic Tests for Canine and Human Visceral Leishmaniasis in Colombia. BMC Infect. Dis. 2019;19:747. doi: 10.1186/s12879-019-4353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Salomón O.D., Pérez A.A., Riarte A.R., Casas N., Fragueiro-Frías V., Negri V., Santini M.S., Liotta D.J. Performance of Rapid Tests for Canine Visceral Leishmaniasis Diagnosis in Argentina. Medicina. 2020;80:103–110. [PubMed] [Google Scholar]
- 243.Teixeira A.I.P., Silva D.M., Vital T., Nitz N., de Carvalho B.C., Hecht M., Oliveira D., Oliveira E., Rabello A., Romero G.A.S. Improving the Reference Standard for the Diagnosis of Canine Visceral Leishmaniasis: A Challenge for Current and Future Tests. Mem. Inst. Oswaldo Cruz. 2019;114:e180452. doi: 10.1590/0074-02760180452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Figueiredo F.B., de Vasconcelos T.C.B., Madeira M.D.F., Menezes R.C., Maia-Elkhoury A.N.S., Marcelino A.P., Werneck G.L. Validation of the Dual-Path Platform Chromatographic Immunoassay (DPP® CVL Rapid Test) for the Serodiagnosis of Canine Visceral Leishmaniasis. Mem. Inst. Oswaldo Cruz. 2018;113:e180260. doi: 10.1590/0074-02760180260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Programa Nacional de Leishmaniasis IV Reunión Nacional. [(accessed on 23 April 2024)]. Available online: https://www.argentina.gob.ar/sites/default/files/2021/07/pnl2009web_1_0.pdf.
- 246.Ministério da Saúde Manual de Vigilância e Controle da Leishmaniose Visceral. [(accessed on 6 February 2022)]; Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral_1edicao.pdf.
- 247.Subdirección de Vigilancia y Control en Salud Pública . Instituto Nacional de Salud Guía Protocolo para la Vigilancia en Salud Pública de Leishmaisniasis. Subdirección de Vigilancia y Control en Salud Pública; Madrid, Spain: [Google Scholar]
- 248.Ministerio de Salud Pública y Bienestar . Social Reporte de Leishmaniasis Visceral en Humanos y Caninos, Paraguay, Período 2016 al 2018. Ministerio de Salud Pública y Bienestar; Asunción, Paraguay: [Google Scholar]
- 249.Ministerio de Salud Guía de Diagnóstico . Tratamiento y Control de la Leishmaniasis Visceral en Uruguay. Ministerio de Salud Guía de Diagnóstico; Bogotá, Colombia: 2016. [Google Scholar]
- 250.Costa D.N.C.C., Codeço C.T., Bermudi P.M.M., Rodas L.A.C., Nunes C.M., Hiramoto R.M., Tolezano J.E., Chiaravalloti Neto F. Controle Da Leishmaniose Visceral Canina Por Eutanásia: Estimativa de Efeito Baseado Em Inquérito e Modelagem Matemática. Cad. Saude Publica. 2020;36:e00221418. doi: 10.1590/0102-311x00221418. [DOI] [PubMed] [Google Scholar]
- 251.Leite J.C., Gonçalves A.A.M., de Oliveira D.S., Resende L.A., Boas D.F.V., Ribeiro H.S., Pereira D.F.S., da Silva A.V., Mariano R.M.D.S., Reis P.C.C., et al. Transmission-Blocking Vaccines for Canine Visceral Leishmaniasis: New Progress and Yet New Challenges. Vaccines. 2023;11:1565. doi: 10.3390/vaccines11101565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Oliva G., Nieto J., Foglia Manzillo V., Cappiello S., Fiorentino E., Di Muccio T., Scalone A., Moreno J., Chicharro C., Carrillo E., et al. A Randomised, Double-Blind, Controlled Efficacy Trial of the LiESP/QA-21 Vaccine in Naïve Dogs Exposed to Two Leishmania infantum Transmission Seasons. PLoS Negl. Trop. Dis. 2014;8:e3213. doi: 10.1371/journal.pntd.0003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fernández Cotrina J., Iniesta V., Monroy I., Baz V., Hugnet C., Marañon F., Fabra M., Gómez-Nieto L.C., Alonso C. A Large-Scale Field Randomized Trial Demonstrates Safety and Efficacy of the Vaccine LetiFend® against Canine Leishmaniosis. Vaccine. 2018;36:1972–1982. doi: 10.1016/j.vaccine.2018.02.111. [DOI] [PubMed] [Google Scholar]
- 254.Giunchetti R.C., Silveira P., Resende L.A., Leite J.C., de Oliveira Melo-Júnior O.A., Rodrigues- Alves M.L., Costa L.M., Lair D.F., Chaves V.R., dos Santos Soares I., et al. Canine Visceral Leishmaniasis Biomarkers and Their Employment in Vaccines. Vet. Parasitol. 2019;271:87–97. doi: 10.1016/j.vetpar.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 255.Velez R., Gállego M. Commercially Approved Vaccines for Canine Leishmaniosis: A Review of Available Data on Their Safety and Efficacy. Trop. Med. Int. Health. 2020;25:540–557. doi: 10.1111/tmi.13382. [DOI] [PubMed] [Google Scholar]
- 256.De Lana M., Giunchetti R.C. Dogs as a Model for Chemotherapy of Chagas Disease and Leishmaniasis. Curr. Pharm. Des. 2021;27:1741–1756. doi: 10.2174/1381612826666201228142703. [DOI] [PubMed] [Google Scholar]
- 257.Ribas L.M., Zaher V.L., Shimozako H.J., Massad E. Estimating the Optimal Control of Zoonotic Visceral Leishmaniasis by the Use of a Mathematical Model. Sci. World J. 2013;2013:810380. doi: 10.1155/2013/810380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ministério da Saúde, Brasil. Secretaria de Vigilância em Saúde . Nota Técnica Nº 5/2021-CGZV/DEIDT/SVS/MS. Ministério da Saúde; Brasília, Brasil: 2021. [(accessed on 14 October 2022)]. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leishmaniose-visceral/arquivos/sei_ms-nota-tecnica-n-5_leishpdf.pdf. [Google Scholar]
- 259.Oliveira D.S., Zaldívar M.F., Gonçalves A.A.M., Resende L.A., Mariano R.M.D.S., Pereira D.F.S., Conrado I.D.S.S., Costa M.A.F., Lair D.F., Vilas-Boas D.F., et al. New Approaches to the Prevention of Visceral Leishmaniasis: A Review of Recent Patents of Potential Candidates for a Chimeric Protein Vaccine. Vaccines. 2024;12:271. doi: 10.3390/vaccines12030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Graciano R.C.D., Ribeiro J.A.T., Macêdo A.K.S., de S Lavareda J.P., de Oliveira P.R., Netto J.B., Nogueira L.M., Machado J.M., Camposda-Paz M., Giunchetti R.C., et al. Recent Patents Applications in Red Biotechnology: A Mini-Review. Recent. Pat. Biotechnol. 2019;13:170–186. doi: 10.2174/1872208313666190114150511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected were reported in the text.