Abstract
Many approaches are currently being developed to deliver exogenous antigen into the major histocompatibility complex class I-restricted antigen pathway, leading to in vivo priming of CD8+ cytotoxic T cells. One attractive possibility consists of targeting the antigen to phagocytic or macropinocytic antigen-presenting cells. In this study, we demonstrate that strong CD8+ class I-restricted cytotoxic responses are induced upon intraperitoneal immunization of mice with different peptides, characterized as CD8+ T-cell epitopes, bound to 1-μm synthetic latex microspheres and injected in the absence of adjuvant. The cytotoxic response induced against a lymphocytic choriomeningitis virus (LCMV) peptide linked to these microspheres was compared to the cytotoxic T-lymphocyte (CTL) response obtained upon immunization with the nonreplicative porcine parvovirus-like particles (PPV:VLP) carrying the same peptide (PPV:VLP-LCMV) previously described (C. Sedlik, M. F. Saron, J. Sarraseca, I. Casal, and C. Leclerc, Proc. Natl. Acad. Sci. USA 94:7503–7508, 1997). We show that the induction of specific CTL activity by peptides bound to microspheres requires CD4+ T-cell help in contrast to the CTL response obtained with the peptide delivered by viral pseudoparticles. Furthermore, PPV:VLP are 100-fold more efficient than microspheres in generating a strong CTL response characterized by a high frequency of specific T cells of high avidity. Moreover, PPV:VLP-LCMV are able to protect mice against a lethal LCMV challenge whereas microspheres carrying the LCMV epitope fail to confer such protection. This study demonstrates the crucial involvement of the frequency and avidity of CTLs in conferring antiviral protective immunity and highlights the importance of considering these parameters when developing new vaccine strategies.
Different major histocompatibility complex (MHC)-restricted antigen presentation pathways operate for exogenous and endogenous antigens (17). Generally, exogenous antigens are internalized by professional antigen-presenting cells (APC) and degraded into acid pH endocytic compartments. The generated peptides are then loaded on MHC class II molecules and presented at the cell surface to CD4+ helper T lymphocytes. By contrast, endogenous antigens are degraded into the cytoplasm by the proteasome, and the resulting processed peptides are carried to the endoplasmic reticulum by a transporter associated with antigen processing followed by association with nascent MHC class I molecules in stable trimeric complexes with β2-microglobulin. These complexes are then transported to the cell surface by a conventional secretory pathway and presented to CD8+ cytotoxic T cells. Therefore, in most cases, administration of soluble protein antigens does not generate cytotoxic T-lymphocyte (CTL) responses. However, it is now well demonstrated that some particular exogenous antigens can be processed and presented to CD8+ T cells by APC following alternative class I-restricted antigen presentation pathways (22, 40, 54).
Thus, a great number of modified exogenous antigens were recently developed to induce MHC class I-restricted CTL activity, such as bacterial toxins (15, 43), noninfectious virus-like particles (VLP) (18, 27, 32, 45, 49), proteins associated with the lipidic structure or associated with adjuvants (immunostimulating complex, saponin) (19, 23, 34, 51), proteins complexed with heat shock proteins (28, 53), crude cell lysates, denatured aggregates (44), antigens encapsulated in biodegradable polymer microspheres (10, 36), and antigens coupled to synthetic beads (20, 24). Nevertheless the potential application of these approaches to human vaccination remains limited due to the toxicity of some components. A broad range of studies also reported the induction of CTLs with peptides associated with lipidic structures or bacteria or adjuvants. However, peptides did not prime specific CTL responses when injected in association with alum (35), the only adjuvant allowed for human use. Therefore, additional biocompatible delivery systems for human use are still needed to confer an efficient and safe immunogenicity to peptides.
The capacity of peptides linked to microspheres to induce CD8+ T-cell responses was never explored in vivo, whereas it was previously shown that ovalbumin linked to these carriers is able to activate specific CTLs through an alternative class I-restricted antigen presentation pathway (24, 25). In the present study, we first investigated the capacity of peptides containing a CD8+ T-cell epitope covalently coupled to 1-μm synthetic microspheres to induce CTL responses. Peptides corresponding to three different H-2d-restricted CD8+ T-cell epitopes bound to microspheres were able to elicit strong specific CTL responses in vivo in the absence of adjuvant but with the requirement for CD4+ T-cell help. We then compared the immunogenicity of the p118–132 peptide from lymphocytic choriomeningitis virus (LCMV) nucleoprotein (2, 55) either bound to synthetic microspheres or delivered by recombinant porcine parvovirus VLP (PPV:VLP) (49). We showed that both particulate vectors induced strong CD8+ T-cell responses. However, the PPV:VLP vector, which carries 100-fold less antigen, induced a high frequency of CTLs of high avidity compared with microspheres. Furthermore, only mice immunized with PPV:VLP carrying the LCMV peptide (PPV:VLP-LCMV) were protected from a lethal LCMV challenge. Our results conclusively show that the frequency of CTLs and their avidity for the antigen are important parameters for determining the ability to confer protective immunity.
MATERIALS AND METHODS
Mice and peptides.
Female BALB/c mice were purchased from Iffa Credo (L'Arbresle, France). H-2d-restricted CTL epitopes corresponding to the synthetic peptides RPQASGVYMGNLTAQ carrying the p118–132 sequence from the LCMV nucleoprotein (2, 55), GYKDGNEYI bearing the p91–99 sequence from the Listeria monocytogenes O listeriolysin (37), and RIQRGPGRAFVTIGK, bearing the p315–329 sequence from the V3 region of IIIB human immunodeficiency virus type 1 gp120 (51), were purchased from Neosystem (Strasbourg, France). Hen egg lysozyme (HEL) protein was purchased from Sigma (St. Louis, Mo.).
Coupling of peptides or protein to microspheres.
Each single peptide antigen or HEL protein was covalently linked to the surface of a 1-μm-diameter latex particle (Polysciences, Warrington, Pa.) using glutaraldehyde (Sigma) as previously described in detail (48). The amount of peptide bound to beads was measured by the difference in absorbance between the solution before and after the linkage.
Preparation of chimeric VLP expressing the LCMV p118–132 peptide.
The construction, characterization, and purification of recombinant chimeric or empty PPV:VLP were previously described in detail (49). Briefly, the PPV VP2 gene was expressed either with the p118–132 peptide sequence (PPV:VLP-LCMV) or without this sequence (PPV:VLP) in a baculovirus vector system. After infection of Sf 9 insect cells, the recombinant VLP were purified by salt precipitation with 20% ammonium sulfate followed by dialysis. Characterization of PPV:VLP and PPV:VLP-LCMV obtained by CsCl sedimentation analysis and electron microscopy revealed properties identical to those of native PPV virions. The molecular weight (MW) of the LCMV peptide represented 3% of the MW of the PPV:VLP-LCMV.
Mouse immunization.
For both delivery systems, mice were immunized twice by the intraperitoneal (i.p.) route at a 21-day interval in the absence of adjuvant. Spleens were surgically removed 7 days after the last injection.
In vitro cytotoxicity assay.
After immunization of mice with peptide bound to beads or with PPV:VLP-LCMV, spleen cells were in vitro stimulated with 1 μM priming peptide in the presence of syngeneic irradiated naive spleen cells during 5 days. The cytotoxic activity of these effector cells was tested on 51Cr-labeled P815 (H-2d), EL4 (H-2b), RDM4 (H-2k), or 1T22 (H-2q) target cells pulsed with a 50 μM concentration of the respective peptide or on 51Cr-labeled LCMV-infected J774 target cells. The released radioactivity in the supernatant was measured. The percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was generated by adding 1 N HCl to P815 target cells or 1% Triton X-100 to J774 cells, and spontaneous release was obtained with target cells incubated without effector cells.
LDA.
The LCMV-specific effector CTL frequencies present in culture after in vitro stimulation were determined by limiting dilution assays (LDA) as previously described (21). Briefly, between 20 and 40,000 cells from 5-day in vitro stimulation cultures were assayed for cytotoxicity on 104 51Cr-labeled P815 target cells pulsed with 50 μM p118–132 peptide. Each dilution was tested in 24 replicate wells, and supernatants were counted for radioactivity after 5 h of incubation. A well was considered positive if the amount of 51Cr released exceeded by 3 standard deviations the mean of amounts from control wells containing target cells alone. Effector cell frequencies were calculated as previously described (52).
The number of LCMV-specific CTL precursor cells present in immunized mice was determined as follows. Microcultures were performed under LDA conditions with 50 to 100,000 splenocytes from immunized mice in 24 replicate wells. Each microculture contained 104 syngeneic irradiated naive spleen cells and 1 μg of p118–132 peptide/ml. Three days latter, interleukin 2 (IL-2) was added in each microculture at 10 U/ml. On day 10, the microculture in each well was split and assayed for cytotoxicity on 104 p118–132 peptide-pulsed and unpulsed 51Cr-labeled P815 target cells. Frequencies were determined as mentioned above.
In vitro inhibition of CTL activity.
Spleen cells (107/ml) stimulated for 5 days, resulting in effector cells, were preincubated with 10 μg of anti-CD4 (GK1.5) (8) or anti-CD8 (H35.17.2) (38) monoclonal antibodies (MAb; purified from ascitic fluid preparations)/ml for 1 h at 4°C. Cells were washed and incubated with goat anti-rat immunoglobulins coupled to magnetic microbeads (Dynal, Compiègne, France) for 30 min at 4°C. Subsequently, retained CD4+ or CD8+ cells were removed by three passages on a magnetic field. The depleted populations were controlled by FACScan and contained less than 5% CD4+ or CD8+ cells, respectively. The resulting populations were used as effector cells for the cytotoxicity assay and were added to peptide-pulsed target cells. Phosphate-buffered saline (PBS)-treated stimulated spleen cells were also tested as a control.
In vivo inhibition of CTL induction.
Mice were i.p. injected with 300 μg of anti-CD4 or anti-CD8 MAb on days −1, 0, and +1 and once a week throughout the immunization period as described for Fig. 4. The spleen cells were then removed, and, prior to in vitro stimulation with peptide, the resulting populations were controlled by FACScan and contained less than 1% CD4+ or CD8+ cells, respectively.
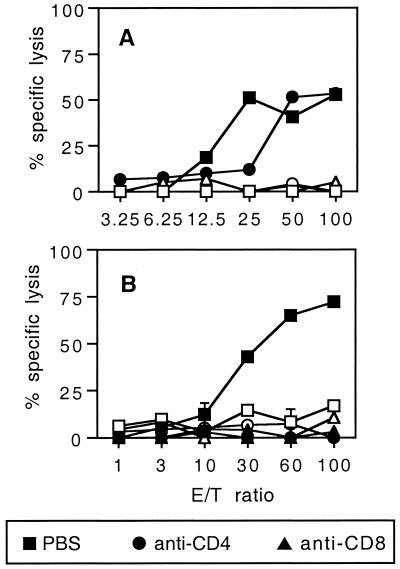
FIG. 4.
In vivo induction of CD8+ effector CTLs by LCMV beads requires a CD4+ helper T-cell activity. (A) BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads or HEL beads. Ten days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2. These effector cells were then treated with PBS or anti-CD4 or anti-CD8 MAb as described in Materials and Methods. (B) Mice were treated on days −1, 0, 1, 7, 14, and 20 with anti-CD4 or anti-CD8 MAb or with PBS and were injected i.p. on days 0 and 21 with 109 LCMV beads. Spleens were removed 7 days after the last immunization and cells were stimulated in vitro as described for Fig. 2. Cytotoxic activity on 51Cr-labeled p118–132-pulsed P815 target cells (solid symbols) and on cells incubated with medium alone (open symbols) was measured. Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
Virus protection experiment.
LCMV (strain Arm/53b; 101.7 PFU) was inoculated intracerebrally (30 μl) to perform protection experiments. Death and survival were recorded during 21 days after the viral challenge. Clearance of the virus was checked by an LCMV antigen capture enzyme-linked immunosorbent assay using mouse kidneys as previously described (4).
Single IFN-γ-producing cell enzyme-linked immunospot (ELISPOT) assay.
Ninety-six-well multiscreen filtration plates (Millipore, Molsheim, France) were coated with 4 μg of rat anti-mouse gamma interferon (IFN-γ) antibody (clone R4-6A2; Pharmingen, San Diego, Calif.)/ml overnight at room temperature. Plates were then washed and blocked with RPMI 1640 supplemented with 10% fetal calf serum for 1 h. Serial twofold dilutions of spleen cells from immunized mice were added into the wells along with 5 × 105 γ-irradiated (3,000 rad) syngeneic feeder cells and 10 U of recombinant murine IL-2 (Pharmingen)/ml. Cells were incubated for 36 h either with or without p118–132 peptide at 1 μg/ml. Assays were arrested by extensive washes followed by incubation with 4 μg of biotinylated rat anti-mouse IFN-γ antibody (clone XMG 1.2; Pharmingen)/ml. Plates were developed by incubation with streptavidin-alkaline phosphatase (Pharmingen) and 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (Sigma) as the substrate. The frequency of IFN-γ-producing cells was determined by counting the number of spot-forming cells (SFC) in each well, and the results were expressed as the number of SFC per spleen.
RESULTS
Induction of CTL responses by peptides bound to synthetic microspheres.
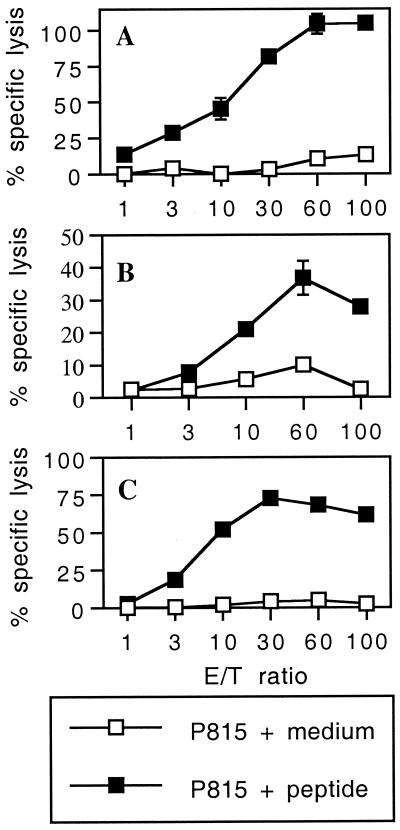
To study the capacity of 1-μm synthetic microspheres to induce cytotoxic responses to CD8+ epitopes, BALB/c mice were i.p. immunized with three synthetic peptides corresponding to H-2d-restricted CD8+ T-cell epitopes (p118–132 from LCMV nucleoprotein, p91–99 from Listeria listeriolysin, and p315–329 from human immunodeficiency virus type 1 gp120) covalently bound to microparticles and injected without adjuvant. In these three models, immunization of mice with peptides linked to beads was able to stimulate strong specific cytotoxic activities against the respective peptide (Fig. 1).
FIG. 1.
In vivo induction of CTL responses by synthetic peptides bound to 1-μm microspheres. BALB/c mice were immunized i.p. on days 0 and 21 with 109 beads bound to various synthetic peptides: LCMV p118–132 (A), Listeria p91–99 (B), or HIV p315–329 (C). Eight or 11 days after the last injection, spleen cells from immune mice were stimulated in vitro with priming peptide p118–132 (A), p91–99 (B), or p315–329 (C) in the presence of irradiated syngeneic spleen cells. The cytotoxic activity of these effector cells on 51Cr-labeled P815 target cells pulsed with the respective peptide or incubated with medium alone was measured. Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
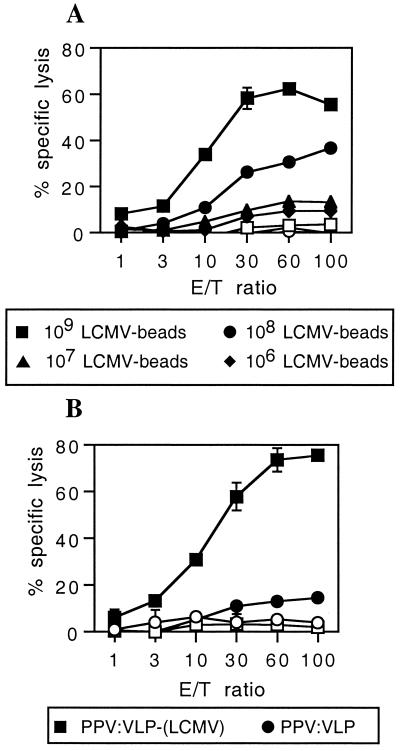
We previously demonstrated that recombinant PPV:VLP carrying the p118–132 LCMV peptide were able to stimulate in vivo strong CD8+ CTL responses (49). To further investigate the efficiency of synthetic microspheres to provide cytotoxic immunogenicity to peptides, we compared the immunogenicity of the LCMV p118–132 peptide carried by 1-μm synthetic microparticles with its immunogenicity when carried by 0.025-μm PPV:VLP. Figure 2 shows that strong cytotoxic responses were obtained after i.p. immunization of mice with both LCMV-carrying beads (LCMV beads) (A) and PPV:VLP-LCMV (B) in the absence of adjuvant. The cytotoxic response induced by LCMV beads was specific for the LCMV peptide. Indeed, no cytotoxic response against LCMV peptide-coated target cells was obtained after immunization with lysozyme (HEL)-carrying beads (Fig. 2A). It is noteworthy that the cytotoxic activity of spleen cells from mice immunized with 10 μg of PPV:VLP-LCMV was comparable to the cytotoxic activity induced by immunization of mice with 109 LCMV beads (Fig. 2). These results indicate that PPV:VLP-LCMV are more immunogenic than LCMV beads, since 10 μg of PPV:VLP-LCMV corresponds to 0.3 μg of the LCMV peptide whereas the most efficient dose of LCMV beads for inducing CTL activity was 109 LCMV beads, a dose which corresponds to 20 μg of p118–132 peptide. Nevertheless, 108 LCMV beads (2 μg of p118–132 peptide) administered to mice were still able to stimulate a CTL response. However, the injection of 107 LCMV beads, corresponding to the amount of the LCMV peptide delivered by 10 μg of PPV:VLP-LCMV, did not induce a CTL response.
FIG. 2.
Comparison of the CTL responses induced by the LCMV synthetic peptide delivered by two different types of particulate vectors. BALB/c mice were immunized i.p. on days 0 and 21 (A) with various doses of LCMV beads or with 109 control HEL beads (B) and with 10 μg of PPV:VLP-LCMV or control PPV:VLP. Seven days after the last immunization, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of syngeneic spleen cells and cytotoxic activity was measured on 51Cr-labeled P815 target cells pulsed with the p118–132 peptide (solid symbols) or incubated with medium alone (open symbols). Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
It should be mentioned that both delivery systems induce long-lasting cytotoxic responses since LCMV-specific CTL activity generated by LCMV beads persisted at least 5 months after the last injection (data not shown) and at least 9 months after the last injection of PPV:VLP-LCMV (49).
Characterization of effector cells induced by peptide bound to microparticles.
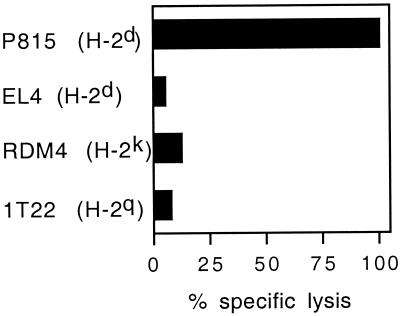
First, to characterize the CTLs induced by peptides covalently linked to synthetic microspheres, the cytotoxic activity of effector cells induced by injection of mice with the H-2d-restricted LCMV peptides bound to beads against target cells expressing different MHC haplotypes was assessed. Lytic activity was only observed on peptide-pulsed H-2d P815 target cells, indicating that the 1-μm particulate form of peptides induced in vivo MHC class I-restricted cytotoxic cells (Fig. 3). Second, effector cells were depleted of CD4+ or CD8+ T cells before incubation with target cells. As shown on Fig. 4A, PBS-treated effector cells exhibited an efficient CTL activity against LCMV peptide-coated target cells. In contrast, CD8+-depleted effector cells were not able to kill peptide-pulsed P815 target cells, whereas the lytic activity of effector cells depleted of CD4+ T cells was not modified.
FIG. 3.
CTL responses induced by LCMV beads are mediated by MHC class I-restricted cytotoxic cells. BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads. Seven days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2 and cytotoxic activity was measured on 51Cr-labeled p118–132-pulsed target cells from various H-2 haplotypes at a 60:1 effector/target ratio. Lysis obtained with the target cells incubated with medium alone was less than 10% and is not shown. Data represent the means of percent specific lysis from duplicate samples.
We then analyzed the in vivo requirement for CD4+ lymphocytes to elicit a CTL response consequent to LCMV bead immunization. For this purpose, mice were treated with anti-CD4 or anti-CD8 MAb before and after immunization with the LCMV beads. As illustrated on Fig. 4B, in vivo CD8+ T-cell depletion totally inhibited the induction of a CTL response. Moreover, depletion of CD4+ cells led also clearly to abrogation of CTL induction. These data showed that CD4+ T cells are strictly required for CTL activation by LCMV microparticles.
Comparison between the capacities of LCMV peptides delivered by synthetic microspheres and PPV:VLP to protect mice against a lethal LCMV challenge.
We then investigated the capacities of CTLs induced by LCMV beads and PPV:VLP-LCMV to lyse virus-infected target cells and to protect mice against a lethal viral challenge. Both delivery systems administered i.p. to mice elicited cytotoxic T cells able to lyse virus-infected J774 target cells (Table 1), demonstrating that the CTLs induced by these immunogens were able to recognize the naturally processed peptide on LCMV-infected cells. Surprisingly, while PPV:VLP-LCMV induced full protection of mice against an LCMV challenge (Table 1) associated with viral clearance (data not shown), all mice immunized with LCMV beads died after the viral challenge, indicating that the LCMV-specific CTL response induced by these immunogens was not protective.
TABLE 1.
Comparison of the antiviral activities and protective effects of the CTL responses induced by the LCMV peptide delivered by two types of particulate vectors
| Vector for in vivo immunizationa | % Specific lysisb of J774 cells
|
% Viral protectionc | |
|---|---|---|---|
| Noninfected | Infected | ||
| LCMV beads | 0 | 33 | 0 |
| HEL beads | 0 | 0 | 0 |
| PPV:VLP-LCMV | 0 | 22.1 | 100 |
| PPV:VLP | 0.6 | 7 | 0 |
Mice were immunized i.p. on days 0 and 21 with 109 antigen beads or with 10 μg of PPV:VLP-LCMV or control PPV:VLP.
Cytotoxic activity of the effector cells was measured at a 50:1 effector/target ratio on 51Cr-labeled J774 target cells either noninfected or infected with LCMV after in vitro stimulation. Data represent the means from quadruplicate samples.
Seven days after the last injection, 10 mice per group were challenged intracerebrally with 101.7 PFU of LCMV. Data represent the percentages of surviving mice after a 21-day period and are the cumulative results of two experiments.
Comparison of the frequencies of LCMV-specific T cells induced by LCMV beads and PPV:VLP-LCMV.
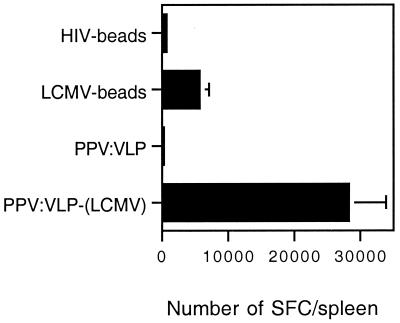
The discrepancy between the capacity of PPV:VLP and beads to induce a protective antiviral immunity could be related to the frequency of effector T cells activated by these two delivery systems. Thus, in order to quantify the specific T-cell responses induced by the PPV:VLP and microsphere delivery systems, we determined the number of IFN-γ-producing cells in response to in vitro-specific LCMV peptide stimulation following in vivo immunization. Spleen cells removed from immunized mice were cultured in vitro for 36 h with or without the p118–132 LCMV peptide, and a single IFN-γ-producing cell ELISPOT assay was performed. As shown in Fig. 5, the size of the LCMV-specific T-cell population was remarkably higher in mice immunized with PPV:VLP-LCMV than in mice immunized with LCMV beads. One of 3,359 spleen cells from PPV:VLP-LCMV-immunized mice produced IFN-γ in response to specific peptide stimulation versus 1 of 20,546 cells for mice injected with LCMV beads. The low number of IFN-γ-producing spleen cells observed following immunization with control beads or PPV:VLP (Fig. 5) or in the absence of in vitro peptide stimulation of spleen cells (data not shown) indicates the specificity of the response. The frequencies of lytic LCMV-specific CTL precursors induced by PPV:VLP or beads were also determined by LDA (Table 2). This frequency was 1 of 80,600 for mice immunized with PPV:VLP-LCMV and was too low to be detectable for mice immunized with LCMV beads. As already observed following LCMV infection, the frequencies obtained by ELISPOT were around 20-fold higher than the precursor frequencies determined by LDA (33). Together, these results demonstrate that PPV:VLP have a very high capacity to induce a specific T-cell response compared to synthetic microspheres, since they stimulate 6 times more specific T cells than microspheres while carrying 100 times less LCMV peptide.
FIG. 5.
Immunization with PPV:VLP-LCMV induces a high frequency of LCMV-specific T cells compared with immunization with LCMV beads. BALB/c mice were immunized i.p. on days 0 and 21 with 109 HIV or LCMV beads or 10 μg of PPV:VLP or PPV:VLP-LCMV. Seven days after the last injection, the frequency of LCMV-specific T cells in the spleen was measured by single-cell IFN-γ ELISPOT assay in the presence of p118–132 as described in Materials and Methods. Data are expressed as the number of SFC per spleen and represent the means obtained with six mice in two independent experiments. No SFC were obtained in the absence of p118–132.
TABLE 2.
Comparison of the frequencies of ex vivo LCMV-specific precursor CTLs and effector CTLs after in vitro stimulation of spleens from mice immunized by two types of particulate vectors
| Vector for in vivo immunizationa | Frequency (no. of mice tested) of:
|
|
|---|---|---|
| Ex vivo CTL precursorsb | Effector CTLs after in vitro culturec | |
| Control | Undd (6) | Und (10) |
| LCMV beads | Und (3) | 1/3,076 ± 1/1330 (6) |
| PPV:VLP-LCMV | 1/80,600 ± 1/17,000 (3) | 1/3,196 ± 1/1,761 (6) |
Mice were immunized i.p. on days 0 and 21 with 109 antigen beads or with 10 μg of PPV:VLP-LCMV. Control mice were immunized under the same conditions with HEL beads or control PPV:VLP.
LCMV-specific precursor CTL frequencies were determined 7 days after the last injection by LDA as described in Materials and Methods. Results are expressed as the mean ± standard deviation of the frequencies obtained with the different mice tested.
LCMV-specific effector CTL frequencies were determined after 5 days of in vitro stimulation by LDA as described in Materials and Methods. Results are expressed as the means ± standard deviations of the frequencies obtained with the different mice tested.
Und, undetectable.
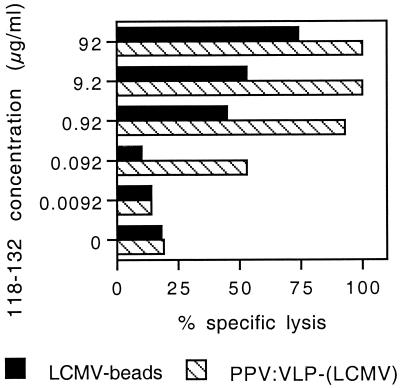
We then analyzed the CTL avidity for the p118–132 peptide by testing the capacity of effector cells to lyse P815 target cells pulsed with a large range of peptide concentrations (92 to 0.0092 μg/ml). As shown in Fig. 6, immunization with LCMV beads induced CTLs that exhibited a significant lytic activity on P815 target cells coated with a minimal concentration of 0.92 μg/ml of the p118–132 peptide. In contrast, CTLs induced in response to PPV:VLP-LCMV were still able to kill P815 target cells incubated with 0.092 μg/ml of the peptide. These results were not due to a lower frequency of specific CTLs induced in mice immunized with microspheres, since after 5 days of in vitro stimulation with the p118–132 peptide, the numbers of LCMV-specific effector T cells were almost the same for both groups (1 of 3,076 for spleen cells from mice immunized with LCMV beads versus 1 of 3,196 for spleen cells from mice immunized with PPV:VLP-LCMV (Table 2). So, the observed differences in the CTL avidity experiment were clearly due to the difference in the avidity of the CTL populations induced following immunization with the two vectors and not to a difference in effector cell numbers. These results therefore confirm the differences in the quality of CTL responses induced by the two delivery systems.
FIG. 6.
Comparison of the peptide affinities of the CTL response induced by the LCMV peptide delivered by two different particulate vectors. BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads or with 10 μg of PPV:VLP-LCMV. Ten days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2. Cytotoxic activity of these effector cells on 51Cr-labeled P815 target cells pulsed with various concentrations of the p118–132 peptide or incubated with medium alone was measured. Data represent the means of percent specific lysis from duplicate samples obtained at a 100:1 effector/target ratio.
DISCUSSION
In the present study, we demonstrated for the first time that synthetic peptides corresponding to CD8+ CTL epitopes covalently bound to 1-μm synthetic microspheres can stimulate in vivo MHC class I-restricted CD8+ CTL responses in the absence of adjuvant. We then compared the induction of specific CTL responses against the CD8+ T-cell p118–132 epitope from the LCMV nucleoprotein carried by these microparticules with that when the epitope was delivered by 0.025-μm viral PPV:VLP. While the optimal cytotoxic activities induced by both delivery systems, as tested by a chromium release cytotoxicity assay, seem to be similar, important differences were detected. Indeed, the in vivo induction of equivalent CTL activities required 100 times less LCMV peptide when the peptide was delivered by PPV:VLP than when it was carried by microspheres. Under these conditions, PPV:VLP induced six times more LCMV-specific T cells than the microspheres. These results show clearly that PPV:VLP are highly immunogenic compared to microspheres.
A major difference between PPV:VLP and microspheres as particulate carriers is their sizes, which might be crucial for determining the antigen presentation pathway involved in each antigen delivery system. Indeed, to initiate a CTL response, MHC-bound peptides have to be presented to naive T cells by professional APC expressing high levels of MHC class I molecules and adhesion and costimulator molecules. Due to their size, proteins bound to synthetic microspheres are processed and presented by macrophages after internalization by phagocytosis (20, 24), and the antigen is transferred from phagosomes into cytosol via a common pathway with endogenous proteins for MHC class I presentation (25). It has been shown that this mechanism is of primary importance for the initiation of CTL responses to viruses that infect only nonhematopoietic cells (50). Most likely, peptides bound to microspheres could follow the same pathway. In contrast, PPV:VLP could be captured by phagocytosis but also by a broad range of other nonphagocytic uptake mechanisms such as fluid phase pinocytosis or receptor-mediated endocytosis via Fc receptors, mannose receptors, and/or scavenger receptors (1, 12, 31, 42). It is noteworthy that receptor-mediated uptake of antigens by APC can result in a 100-fold-more-efficient presentation to T cells (11). Thus, the high efficiency of class I-restricted presentation by APC observed for PPV:VLP might suggest that the uptake and the processing of these particles followed specific mechanisms compared to the uptake and processing of LCMV beads.
Importantly, we demonstrated that CD4+ T-helper cells are strictly required to promote induction of CTL by LCMV beads in contrast to what we previously demonstrated for PPV:VLP-LCMV (49). It has been already shown that induction of a CTL response by synthetic peptides injected in incomplete Freund's adjuvant required T-cell help (14, 16), and the p118–132 LCMV peptide used in this study contains both class I- and class II-restricted epitopes (14). Furthermore, we have previously shown that proteins or peptides covalently linked to synthetic microspheres are able to induce CD4+ T-cell responses in the absence of adjuvant (47). All these observations are in agreement with the CD4+ T-cell dependence of CTL induction by LCMV beads, previously described for low doses of protein linked to microspheres (41).
In contrast, PPV:VLP-LCMV seem to behave like short optimal peptides, which do not require CD4+ T-cell help to generate in vivo CTL responses (9, 13), although the VLP are also able to activate CD4+ T lymphocytes (29). The CD4+ T-cell independence of the CTL responses induced by the PPV:VLP could be due to the high efficiency of the LCMV epitope processing and presentation from PPV:VLP, leading to a high density of antigen-MHC class I complexes at the surface of the APC. Alternatively, this difference could be due to a direct effect of PPV:VLP on the maturation of APC, such as dendritic cells. Indeed, recent studies have shown that T-helper cells deliver a signal to dendritic cells after the recognition of antigens on the cells. This signal leads to dendritic cell maturation and then to a direct CTL stimulation by these dendritic cells (5, 39, 46). Nevertheless, the CD4+ help step can be bypassed by modulation of surface molecule CD40 or by viral infection of dendritic cells (39). As a consequence, the independence of CD4+ T cells for CTL induction may result from direct maturation of dendritic cells following interaction with the antigen as mentioned by Lanzavecchia (26). Some molecules, such as lipopolysaccharide (7) and double-stranded RNA (6), and influenza virus (6) have the capacity to maturate dendritic cells and to induce the migration of these cells to the T-cell areas. In the same way, PPV:VLP, which are very efficient inducers of CTL responses, may induce the maturation of dendritic cells to a state where they can directly deliver cosignals that stimulate specific CD8+ T cells without CD4+ T-cell help. In contrast to PPV:VLP, for inert delivery systems such as microspheres, CD4+ T-helper-mediated CD40 triggering would be absolutely required to induce CTL responses.
During maturation, dendritic cells upregulate MHC molecules, increasing epitope-MHC class I complex density expressed on APC. This might explain why a 100-fold smaller amount of peptide carried by PPV:VLP-LCMV than by LCMV beads was required to activate a CTL response. Mature dendritic cells upregulate costimulatory molecules and produce cytokines such as IL-12 which are required for efficient CTL activation. It was recently shown that CD8α+ dendritic cells lead to Th1 differentiation by producing IL-12 while CD8α− dendritic cells induce a Th2 response (30). Thus, it can be suggested that PPV:VLP activate specifically CD8α+ dendritic cells since PPV:VLP administered without adjuvant induce a Th1 response (29), while proteins covalently bound to microspheres are not able to polarize the Th response (47). Altogether, these observations suggest that the high immunogenicity of PPV:VLP could be a consequence of their capacity to stimulate APC maturation, thus increasing the ability to stimulate T cells via expression of costimulatory molecules and/or cytokine production.
Most importantly, we demonstrated that the CTL response induced by LCMV bead immunization was not efficient in protecting mice against a lethal viral challenge, although these CTLs killed peptide-pulsed or virus-infected target cells. Thus, the in vivo ability of CTLs to clear virus cannot be predicted from their capacity to kill infected cells. This discrepancy could be linked to the high number of specific T cells observed in PPV:VLP-LCMV-immunized mice. Moreover, Alexander-Miller et al. (3) have shown by CTL transfer experiments in SCID mice that the high avidity of CTL generated in vitro is a crucial parameter for the clearance of viral infection. In our model, we demonstrated that the VLP carrying the LCMV peptide are able to generate in vivo specific CTLs exhibiting a high avidity for the antigen compared to LCMV beads. Altogether, these findings showed that the high immunogenicity of PPV:VLP, linked to the frequency and the avidity of CTL responses, is essential to confer antiviral protective immunity. All these observations indicate that these recombinant parvovirus particles could provide an efficient and safe strategy to develop effective vaccination and immunotherapy. Nevertheless, the mechanisms of the activation of APC leading to the efficient presentation of PPV:VLP should be investigated to determine the key events of its high immunogenicity.
ACKNOWLEDGMENTS
This work was supported by grants from Agence National de Recherche sur le SIDA (ANRS) and Association pour la recherche sur le Cancer (ARC) to C.L.
We thank J. Sarraseca for technical assistance in the preparation of PPV:VLP.
Footnotes
Collaborative project between the Institut Pasteur and Ingenasa (EEC Biotechnology project BI04-CT96-024).
REFERENCES
- 1.Abraham R, Singh N, Mukhopadhyay A, Basu S K, Bal V, Rath S. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J Immunol. 1995;154:1–8. [PubMed] [Google Scholar]
- 2.Aichele P, Hengartner H, Zinkernagel R M, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmeyer R, Girard M, van der Werf S, Mimic V, Seigneur L, Saron M F. Attenuated mengo virus: a new vector for live recombinant vaccines. J Virol. 1995;69:3193–3196. doi: 10.1093/benz/9780199773787.article.b00034516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 6.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, de Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loker M R, Pierres M, Fitch F W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1-5: similarity of L3T4 to human Leu3/T4 molecule. J Immunol. 1984;131:2445–2451. [PubMed] [Google Scholar]
- 9.Dyall R, Vasovic L V, Molano A, Nikolic-Zugic J. CD4-independent in vivo priming of murine CTL by optimal MHC class I-restricted peptides derived from intracellular pathogens. Int Immunol. 1995;7:1205–1212. doi: 10.1093/intimm/7.8.1205. [DOI] [PubMed] [Google Scholar]
- 10.Eldridge J H, Staas J K, Meulbroek J A, McGhee J R, Tice T R, Gilley R M. Biodegradable microspheres as a vaccine delivery system. Mol Immunol. 1991;28:287–294. doi: 10.1016/0161-5890(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 11.Engering A J, Cella M, Fluitsma D, Brockhaus M, Hoefsmit E C, Lanzavecchia A, Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 12.Ezekowitz R A, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fayolle C, Abdel-Motal U M, Berg L, Deriaud E, Jondal M, Leclerc C. Induction of CTL by optimal-length peptides does not require CD4+ T cell help. Immunology. 1996;89:41–45. doi: 10.1046/j.1365-2567.1996.d01-704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991;147:4069–4073. [PubMed] [Google Scholar]
- 15.Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J Immunol. 1996;156:4697–4706. [PubMed] [Google Scholar]
- 16.Gao X M, Zheng B, Liew F Y, Brett S, Tite J. Priming of influenza virus-specific cytotoxic T lymphocytes in vivo by short synthetic peptides. J Immunol. 1991;147:3268–3273. [PubMed] [Google Scholar]
- 17.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths J C, Harris S J, Layton G T, Berrie E L, French T J, Burns N R, Adams S E, Kingsman A J. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral responses by a Gag:V3 fusion. J Virol. 1993;67:3191–3198. doi: 10.1128/jvi.67.6.3191-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding C V, Collins D S, Slot J W, Geuze H J, Unanue E R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 20.Harding C V, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 21.Hoffenbach A, Langlade-Demoyen P, Dadaglio G, Vilmer E, Michel F, Mayaud C, Autran B, Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–462. [PubMed] [Google Scholar]
- 22.Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 23.Ke Y, Li Y, Kapp J A. Ovalbumin injected with complete Freund's adjuvant stimulates cytolytic responses. Eur J Immunol. 1995;25:549–553. doi: 10.1002/eji.1830250237. [DOI] [PubMed] [Google Scholar]
- 24.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock K L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacsovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 26.Lanzavecchia A. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 27.Layton G T, Harris S J, Gearing A J H, Hill-Perkins M, Cole J S, Griffiths J C, Burns N R, Kingsman A J, Adams S E. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J Immunol. 1993;151:1097–1107. [PubMed] [Google Scholar]
- 28.Li Z, Srivastava P K. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo-Man R, Rueda P, Sedlik C, Deriaud E, Casal I, Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur J Immunol. 1998;28:1401–1407. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajack B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dentritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manca F, Fenoglio D, Li Pira G, Kunkl A, Celada F. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med. 1991;173:37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel M-L, Mancini M, Schlienger K, Tiollais P. Recombinant hepatitis B surface antigen as a carrier of human immunodeficiency virus epitopes. Res Virol. 1993;144:263–267. doi: 10.1016/s0923-2516(06)80038-5. [DOI] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Zhou F, Reddy R, Huang L, Rouse B T. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992;175:609–612. doi: 10.1084/jem.175.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nixon D F, Hioe C, Chen P D, Bian Z, Kuebler P, Li M L, Qiu H, Li X M, Singh M, Richardson J, McGee P, Zamb T, Koff W, Wang C Y, O'Hagan D. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine. 1996;14:1523–1530. doi: 10.1016/s0264-410x(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 36.O'Hagan D T, Rahman D, Mcgee J P, Jeffery H, Davies M C, Williams P, Davis S S, Challacombe S J. Biodegradable microparticles as controlled release antigen delivery systems. Immunology. 1991;73:239–242. [PMC free article] [PubMed] [Google Scholar]
- 37.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierres M, Goridis C, Goldstein P. Inhibition of murine T-cell mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94,000 and 180,000 molecular weight. Eur J Immunol. 1982;12:60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- 39.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 40.Rock K L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 41.Rock K L, Clark K. Analysis of the role of MHC class II presentation in the stimulation of cytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHC class I presentation pathway. J Immunol. 1996;156:3721–3726. [PubMed] [Google Scholar]
- 42.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saron M F, Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc Natl Acad Sci USA. 1997;94:3314–3319. doi: 10.1073/pnas.94.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirmbeck R, Böhm W, Melber K, Reimann J. Processing of exogenous heat-aggregated (denatured) and particulate (native) hepatitis B surface antigen for class I-restricted epitope presentation. J Immunol. 1995;155:4676–4684. [PubMed] [Google Scholar]
- 45.Schirmbeck R, Melber K, Kuhröber A, Janowicz Z A, Reimann J. Immunization with soluble hepatitis B virus surface protein elicits murine H-2 class I-restricted CD8+ cytotoxic T lymphocyte responses in vivo. J Immunol. 1994;152:1110–1119. [PubMed] [Google Scholar]
- 46.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 47.Sedlik C, Deriaud E, Leclerc C. Lack of Th1 or Th2 polarization of CD4+ T cell response induced by particulate antigen targeted to phagocytic cells. Int Immunol. 1997;9:91–103. doi: 10.1093/intimm/9.1.91. [DOI] [PubMed] [Google Scholar]
- 48.Sedlik C, Rojas M, Leclerc C. Activation of B cells by 1 μm particulate lysozyme or peptides: a Th-dependent pathway requiring CD40-CD40 ligand interaction. Int Immunol. 1998;10:1111–1119. doi: 10.1093/intimm/10.8.1111. [DOI] [PubMed] [Google Scholar]
- 49.Sedlik C, Saron M, Sarraseca J, Casal I, Leclerc C. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc Natl Acad Sci USA. 1997;94:7503–7508. doi: 10.1073/pnas.94.14.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi H, Germain R N, Moss B, Berzofsky J A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 class II-restricted help for itself. J Exp Med. 1990;171:571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 53.Udono H, Srivastava P K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Motal U M A, Berg L, Jondal M. In vivo priming of cytotoxic T lymphocyte responses in relation to in vitro up-regulation of major histocompatibility complex class I molecules by short synthetic peptides. Eur J Immunol. 1992;22:3085–3090. doi: 10.1002/eji.1830221209. [DOI] [PubMed] [Google Scholar]