Abstract
BACKGROUND & AIMS:
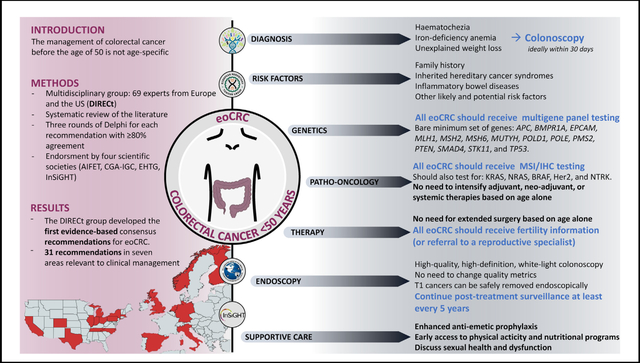
Patients with early-onset colorectal cancer (eoCRC) are managed according to guidelines that are not age-specific. A multidisciplinary international group (DIRECt), composed of 69 experts, was convened to develop the first evidence-based consensus recommendations for eoCRC.
METHODS:
After reviewing the published literature, a Delphi methodology was used to draft and respond to clinically relevant questions. Each statement underwent 3 rounds of voting and reached a consensus level of agreement of ≥80%.
RESULTS:
The DIRECt group produced 31 statements in 7 areas of interest: diagnosis, risk factors, genetics, pathology-oncology, endoscopy, therapy, and supportive care. There was strong consensus that all individuals younger than 50 should undergo CRC risk stratification and prompt symptom assessment. All newly diagnosed eoCRC patients should receive germline genetic testing, ideally before surgery. On the basis of current evidence, endoscopic, surgical, and oncologic treatment of eoCRC should not differ from later-onset CRC, except for individuals with pathogenic or likely pathogenic germline variants. The evidence on chemotherapy is not sufficient to recommend changes to established therapeutic protocols. Fertility preservation and sexual health are important to address in eoCRC survivors. The DIRECt group highlighted areas with knowledge gaps that should be prioritized in future research efforts, including age at first screening for the general population, use of fecal immunochemical tests, chemotherapy, endoscopic therapy, and post-treatment surveillance for eoCRC patients.
CONCLUSIONS:
The DIRECt group produced the first consensus recommendations on eoCRC. All statements should be considered together with the accompanying comments and literature reviews. We highlighted areas where research should be prioritized. These guidelines represent a useful tool for clinicians caring for patients with eoCRC.
Keywords: Recommendation, Clinical, Young, 50 Years, Colorectal Cancer
Graphical Abstract
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e7. Upon completion of this module, successful learners will be able to evaluate high-risk symptoms of early-onset colorectal cancer, explain the utility of tumor testing and germline testing in this setting, integrate the oncological management with the fertility management, and cite the surveillance protocol after cure from colorectal cancer <50 years.
Colorectal cancer (CRC) diagnosed before the age of 50 is referred to as early-onset CRC (eoCRC). Numerous studies have reported that the epidemiology of eoCRC has changed over the past decades1; since the 1990s, there has been an increase in incidence rates of eoCRC across the globe in both high- and low-income countries.2–4 The rate of increase in eoCRC incidence is accelerating, such that it is projected to become a significant public health threat.2,3
In contrast, during the last decades, CRC incidence and mortality rates have decreased in individuals older than 50 living in high-income countries5 because of effective screening programs6,7 and healthier lifestyle habits (decreased smoking, increased aspirin use).8,9 Moreover, advances in surgery, radiotherapy, and systemic therapy have reduced morbidity and increased survival.10–13 Currently, there is significant interest in determining the most appropriate strategies for diagnosis, treatment, and follow-up of eoCRC. There are several knowledge gaps regarding the appropriate management of eoCRC patients, including whether they should receive different surgical, adjuvant, neoadjuvant, and supportive treatments. In the past decade, sufficient evidence has been gathered to warrant the first international evidence-based consensus guidelines. The primary aims of this document are to collect and summarize all available evidence on eoCRC and to provide high-quality risk assessment and disease management guidance for healthcare professionals who care for eoCRC patients.
These recommendations from the DIRECt group (Delphi Initiative Recommendations on eoCRC) received the endorsement of 4 scientific societies: the Associazione Italiana Familiarità Ereditarietà Tumori (AIFET), the Collaborative Group of the Americas on Inherited Gastrointestinal Cancers (CGA-IGC), the European Hereditary Tumor Group (EHTG), and the International Society for Gastrointestinal Hereditary Tumours (InSiGHT).
Methods
The first 2 consensus votes were held online because of the severe acute respiratory syndrome–associated coronavirus (SARS-CoV2) pandemic. The third voting round was held during the DIRECt22 congress in Milan (September 2022). All votes were registered anonymously. The DIRECt consensus was led by a non-voting chairman (GMC) and included a multidisciplinary, international scientific panel of 69 professionals/experts divided into 7 working groups (Table 1, Supplementary Figure 2). Expertise was defined according to publications and clinical expertise. The scientific panel defined, developed, and reviewed the recommendations. Each recommendation was graded according to the Oxford Center for Evidence Based Medicine levels of evidence (LE) (Supplementary Tables 1 and 2).14 Unlike other ranking schemes that focus on therapeutic interventions and harms, the Oxford system has the additional benefit to appraise evidence on epidemiology, risk factors, accuracy of diagnostic tests, and rare and common harms. Therefore, the Oxford system was preferred because of its distinguishing ability to cover multiple questions. Briefly, in the Oxford system each article receives a LE; systematic reviews receive the highest LE (LE 1A), whereas randomized controlled studies and cohort studies are ranked on the basis of the design (retrospective vs prospective), the length of follow-up, the percentage of follow-up, and the width of confidence intervals (CIs) (LE 1B–2B). Individual case-control studies, ecological studies, outcome studies, nonconsecutive cohort studies, and audit studies provide lower LE (LE 2C–4). The lowest LE is represented by expert opinion, bench-research, and “first principle” research (LE 5). After the evaluation of each article, the recommendations are graded (GR) on the basis of the consistency of findings from all studies. If all studies find similar results, the recommendations receive a higher grade. All recommendations were based on a critical appraisal of the available evidence, as summarized in Supplementary Appendices 2–7. The appendices explain the results, interpretation, and LE of all the articles that support each statement. The timeline and methods of the DIRECt recommendations are detailed in Supplementary Figure 1 and Supplementary Appendix 1, respectively.
Table 1.
Distribution of Experts
| Chairs of the Working Panels | Balaguer F, Hampel H, Kupfer SS, Repici A, Sartore-Bianchi A, Seppälä TT, Valentini V |
| Consensus non-voting chairman | Cavestro GM |
| Scientific Board | Boland CR, Brand RE, Caccialanza R, Cascinu S, Dekker E, Daca-Alvarez M, Deni F, Dominguez-Valentin M, Houwen BBSL, Kastrinos F, Mannucci A, Meldolesi E, Möslein G, Murphy CC, Nass K, Ng K, Oliani C, Papaleo E, Patel SG, Puzzono M, Remo A, Ripamonti CI, Syngal S, Turi S, Urso ED, Valle L, Zuppardo RA, Stoffel EM |
| Consensus participants | Buffart TE, Burke CA, Cannizzaro R, Cercek A, Crosbie EJ, Danese S, Eng C, Goel A, Guillem JG, Kahi C, Kalady MF, Kühn F, Laghi L, Latchford A, Liska D, Lu KH, Lynch P, Malesci A, Mauri G, Moller P, Monahan KJ, Ricciardiello L, Siena S, Singh SK, Stadler ZK, Stanich PP, Vanni VS, Vilar E, Yurgelun MB |
| Representative of the non-governmental organizations for patients | Davis A, Vitaloni M |
The agreement/disagreement level was scored on a 6-point scale, with the option of providing anonymous feedback during the first 2 virtual consensus and the third discussion rounds (Supplementary Table 3 and Supplementary Figure 3). The level of agreement was expressed as a percentage of each point of the scale. At the end of at least 3 rounds of voting, statements receiving ≥80% agreement were accepted.
The format recommendations comprised the question, statement, LE, strength of recommendation, and final percentage of agreement. All statements are accompanied by qualifying comments, which were written and reviewed by each working group and the entire scientific panel. Statements and their accompanying comments are meant to be read together as a whole.
Results
The DIRECt consensus produced 31 recommendations for patients diagnosed with eoCRC ≥18 years old based on 145 articles (summarized in Supplementary Appendices 2–7). When appropriate, issues related to colon or rectal cancers specifically are highlighted; in cases where statements applied to both colon and rectal cancer, the term colorectal cancer (CRC) was used. All statements are summarized in Tables 2–4 (Table 2: diagnosis, risk factors, and genetics; Table 3: pathology, oncology; Table 4: endoscopic diagnosis and treatment, therapy, and supportive care). Areas of controversy are described throughout the main text and summarized in Table 5.
Table 2.
Statements Pertaining to the Diagnosis (D), Risk Factors (R), and Genetics (G) of Early-Onset Colorectal Cancer
| Question and statement | Level of evidence, grade of recommendation, agreement level, and clarity |
|---|---|
|
| |
| Diagnosis of early onset colorectal cancer (D) | |
| D.1: What is the age cutoff to define eoCRC? EoCRC is defined as CRC diagnosed younger than age 50. |
LE 2A; GR B Agreement: 91.7% (A+ 50.0%|A 41.7%|A− 8.3%) |
| D.2: Which symptoms and clinical signs prompt evaluation for eoCRC? Symptoms and signs that should prompt evaluation for eoCRC include (but are not limited to) any of the following: hematochezia, unexplained iron deficiency anemia, or unexplained weight loss. |
LE 2B; GR B Agreement: 90.7% (A+ 53.5%| A 37.2%|A− 7.0%|D− 2.3%) |
| D.3: Which test(s) should be used to evaluate eoCRC signs and symptoms? A diagnostic colonoscopy is recommended for evaluation of alarming symptoms and signs of eoCRC. |
LE 2B; GR B Agreement: 85.4% (A+ 56.1%|A 29.3%|A− 7.3%|D− 4.9% |D 2.4%) |
| D.4: When should colonoscopy be performed for alarming symptoms? A colonoscopy should be expedited, ideally within 30 days after referral to a healthcare professional. |
LE 2B; GR C Agreement: 86.5% (A+ 35.1%|A 51.4%|A− 10.8%|D+ 2.7%) |
| Risk factors of early-onset colorectal cancer (R) | |
| R.1: Does family history of CRC influence eoCRC detection? A family cancer history can inform risk assessment for syndromic and non-syndromic CRC. Therefore, a thorough family history should be routinely collected for all individuals. In addition, in non-syndromic cases, CRC family history can facilitate the identification of high-risk individuals who may benefit from starting screening at an earlier age. |
LE 1A; GR A Agreement: 89.2% (A+ 27.0%|A 62.2%|A− 8.1%|D 2.7%) |
| R.2: What other risk factors increase the risk of eoCRC? Some studies have identified male sex, race and ethnicity, obesity, diabetes, alcohol consumption, and hyperlipidemia as potential risk factors for eoCRC. However, at this time the evidence is insufficient to recommend earlier CRC screening based on these factors. |
LE 1B; GR A Agreement: 92.5% (A+ 27.5%|A 65.0%|A− 7.5%) |
| Genetics of early-onset colorectal cancer (G) | |
| G.1: Which eoCRC patients should receive germline genetic testing and when? A. All eoCRC patients should be offered multi-gene panel germline genetic testing and genetic counseling for those with a positive germline finding. B. Genetic testing should be performed before treatment to maximize clinical utility, when feasible, but should not substantially delay treatment. |
LE 1B; GR A Agreement: 100% (A+ 66.7%|A 33.3%) LE 2A; GR B Agreement: 91.9% (A+ 32.4%|A 59.5%|A− 8.1%) |
| G.2: What genes should be included in germline multi-gene panel tests for eoCRC patients? Germline genetic testing for CRC patients diagnosed younger than age 50 should include at a minimum: |
LE 1B; GR B Agreement: 97.1% (A+ 38.2%|A 58.8%|A− 2.9%) |
| • APC, BMPR1A, EPCAM, MLH1, MSH2, MSH6, MUTYH, POLD1, POLE, PMS2, PTEN, SMAD4, STK11, and TP53. | |
| Where available and not cost-prohibitive testing should also include: | |
| • The following genes that are reasonably prevalent in CRC and change clinical management: BRCA1, BRCA2, ATM, CHEK2, PALB2, and possibly, but less prevalent, BRIP1, BARD1, CDKN2A, CDH1, RAD51C, and RAD51D. | |
| • The following genes that have been associated with CRC or polyposis: AXIN2, GREM1, MLH3, MSH3, MBD4, NTHL1, RNF43, and RPS20. | |
| G.3: Are polygenic risk scores useful for identifying patients at risk for eoCRC? Although emerging data suggest polygenic risk scores (PRS) may provide information that could improve CRC risk stratification, their performance has not been formally validated, and they are not yet ready for clinical use. |
LE 2B; GR B Agreement: 100% (A+ 44.8%|A 55.2%) |
Table 4.
Statements Pertaining to Endoscopic Detection and Treatment (E), Therapy (T), and Supportive Care (C) of Early-Onset Colorectal Cancer
| Question and statement | Level of evidence, grade of recommendation, agreement level, and clarity |
|---|---|
|
| |
| Endoscopic detection, diagnosis, and treatment of early-onset colorectal cancer (E) | |
| E.1: Should additional endoscopic technologies be routinely used to improve the diagnostic capabilities for eoCRC? We suggest high-quality, high-definition white-light endoscopy as the standard modality for colonoscopy. There is currently insufficient evidence for the routine use of adjuncts such as dye or virtual chromoendoscopy, add-on devices, and artificial intelligence systems. |
LE 5; GR D Agreement: 96.4% (A+ 42.9%|A 53.6% |D 3.6%) |
| E.2: Are standard quality metrics for colonoscopy appropriate? Standard quality metrics for diagnostic and surveillance colonoscopy in eoCRC have not been established for adenoma detection rate. However, other established standard key performance indicators should be applied. |
LE 2A; GR B Agreement: 93.1% (A+ 27.6 %|A 65.5%|A− 3.4% |D− 3.4%) |
| E.3: What diagnostic workup is necessary before surgery for eoCRC? Complete evaluation of the colon should be performed before surgical treatment, with colonoscopy preferred to computed tomography-colonography. If complete colonoscopy is not technically feasible, a complete colonoscopy should be done within 3–6 months postoperatively. |
LE 2A; GR B Agreement: 96.7% (A+ 23.3%|A 73.3%|D− 3.3%) |
| E.4: Should T1 CRC receive endoscopic therapy in rectal or colonic eoCRC? There is insufficient evidence to recommend T1 CRC be managed differently in eoCRC. |
LE 2B; GR B Agreement: 97.1% (A+ 35.3%|A 61.8%IA− 2.9%) |
| E.5: What endoscopic follow-up is recommended after treatment? A. Patients with non-syndromic eoCRC should receive standard surveillance after the CRC curative resection (at 1 and 3 years) and should continue colonoscopies at a minimum of every 5 years. B. Patients diagnosed with hereditary CRC syndromes should receive variant- and phenotype-specific surveillance intervals. |
LE2B; GRC Agreement: 89.7% (A+ 31.0%|A 58.6%|A− 10.3%) LE 2A; GR B Agreement: 96.8% (A+ 16.1%|A 80.6% |D 3.2%) |
| Treatment of early-onset colorectal cancer (T) | |
| T.1: Should the surgical approach differ for eoCRC? A. Standard segmental resections should be offered to eoCRC. Extended surgery to reduce metachronous cancer risk should only be considered for individuals with a demonstrated risk-enhancing predisposition. B. In the presence of a demonstrated risk-enhancing predisposition, an extended colorectal resection should be recommended by incorporating the variant-specific guidance, patient characteristics, and patient preference. C. For individuals with eoCRC with high risk of gynecologic cancers (due to specific syndromic likely pathogenic/pathogenic variants), combined surgery with colorectal resection and prophylactic hysterectomy with or without bilateral oophorectomy may be considered (if childbearing has been completed). |
LE 2B; GR C Agreement: 96.8% (A+ 22.6%|A 74.2%| D 3.2%) LE 2A; GR B Agreement: 87.0% (A+ 21.7%|A 65.2%|A− 8.7%|D 4.3%) LE 2B; GR C Agreement: 93.8% (A+ 18.8%|A 75.0%|A− 6.3%) Clarity: 92.6% |
| T.2: Which information should patients receive about the risk of infertility related to treatment of eoCRC? Clinicians should provide eoCRC patients with referral to a reproductive medicine specialist before treatment and/or infertility information to discuss: (1) The impact of cancer diagnosis and treatments on reproductive function and on potential risks for infertility. (2) Fertility preservation options, ovarian transposition, and issues related to cryo- preservation storage after fertility preservation. (3) Pregnancy-related and menopause-related issues after gonadotoxic treatment or underlying condition and other childbearing and parenting options. |
LE 2B; GR B Agreement: 81.6% (A+ 13.2%|A 68.4%|A− 13.2%|D− 2.6%|D 2.6%) |
| T.3: Which criteria make patients candidates for fertility preservation? The following criteria should be considered: the estimated risk of gonadotoxicity, the characteristics of the proposed treatment, the patient’s characteristics, and the disease stage and severity. |
LE 3A; GR C Agreement: 85.7% (A+ 17.1%|A 68.6%|A− 8.6%|D+ 2.9%) |
| Supportive care of early-onset colorectal cancer (C) | |
| C.1: Are there peculiarities in the management of cancer-related symptoms in eoCRC (ie, pain, fatigue, nausea, vomiting, constipation, diarrhea, cachexia)? A. For symptom management, patients with eoCRC should be managed as recommended in the ASCO and ESMO guidelines for the general population with CRC. B. Patients with eoCRC may be more prone to chemotherapy-induced nausea and vomiting (CINV) compared with patients with later-onset CRC, particularly female patients with low body mass index. Therefore, enhanced prophylaxis may be considered. C. Patients with eoCRC can benefit from early personalized physical activity and nutritional support programs. Such programs could favor the maintenance and recovery of muscle mass. D. Patients with eoCRC benefit from discussions about sexual health and dysfunction resulting from cancer or its treatment. Psychosocial and/or psychosexual counseling should be offered to improve sexual response, body image, intimacy and relationship issues, and overall sexual functioning and satisfaction. |
LE 1B; GR A Agreement: 96.2% (A+ 30.8%|A 65.4%|D 3.8%) LE 3B; GR C Agreement: 100% (A+ 34.6%|A 65.4%) LE 3B; GR B Agreement: 88.0% (A+ 24.0%|A 64.0%|A− 12.0%) LE 4; GR D Agreement: 91.3% (A+ 34.8%|A 56.5%|A− 8.7%) |
| C.2: How should supportive care programs be organized for eoCRC patients? For eoCRC patients, a multidisciplinary team including psychosocial support and fertility preservation experts should be made available because of the specific psychosocial and informational needs (symptom management, fears, and behavior modifications). |
LE 4; GR C Agreement: 91.3% (A+ 26.1%|A 65.2%|A− 8.7%) |
Table 3.
Statements Pertaining to the Pathology and Oncological Treatment (O) of Early-Onset Colorectal Cancer
| Question and statement | Level of evidence, grade of recommendation, agreement level, and clarity |
|---|---|
|
| |
| Pathology and oncological treatment of early-onset colorectal cancer (O) | |
| O.1: Is it necessary to test tumors for mismatch repair deficiency with immunohistochemistry or microsatellite instability analysis? All CRCs should undergo evaluation for mismatch repair (MMR) phenotype (with either immunohistochemistry staining for MMR proteins or microsatellite instability testing) preferably in the pretreatment setting on biopsies when feasible. |
LE 1B; GR A Agreement: 100% (A+ 92.6%|A 7.4%) |
| O.2: Which molecular markers are necessary for targeted treatments in eoCRC? Molecular profiling should not be different in eoCRC compared with CRC in older patients, and it should include testing for DNA mismatch repair phenotype/MSI, KRAS, NRAS, BRAF, Her2, and NTRK. |
LE 2B; GR C Agreement: 96.3% (A+ 88.9%|A 7.4%|D− 3.7%) |
| O.3: What is the adjuvant postoperative treatment in eoCRCs? There is no evidence that adjuvant therapy in resected colorectal cancer (stage II at high risk and stage III) should differ between eoCRC patients and patients older than 50 years. |
LE 1B; GR B Agreement: 97.1% (A+ 37.1%|A 60.0%|D− 2.9%) |
| O.4: What is the role of neoadjuvant and systemic treatment in rectal and colon eoCRC? A. There is no evidence that neoadjuvant therapy in locally advanced rectal cancer should differ between eoRC patients and patients older than 50 years. B. There is no evidence that systemic therapy should differ between eoCRC patients and patients older than 50 years. |
LE 1B; GR B Agreement: 93.3% (A+ 40.0%|A 53.3%|A− 6.7%) LE 1B; GR B Agreement: 93.5% (A+ 35.5%|A 58.1%|A− 6.5%) |
Table 5.
Areas of Uncertainty on eoCRC and Proposed Research Agenda
| Areas of controversy | Issues raised |
|---|---|
|
| |
| Topic: diagnosis of eoCRC | |
| Fecal immunochemical test | • FIT vs colonoscopy for alarming signs and symptoms: (1) no cost-effectiveness analysis, (2) higher risk of false negatives with FIT, (3) FIT use may prolong diagnostic delays, (4) FIT may be useful for patients with vague symptoms (ie, not alarming) • Positive FIT follow-up: (1) unknown referral rate to colonoscopy after positive FIT, (2) non-zero risk of non-compliance to follow-up colonoscopy • Screening FIT: (1) unknown diagnostic rate, (2) unknown survival benefit, (3) unknown cost- benefit ratio in many countries • Unclear whether a positive FIT is a sign of eoCRC: lack of data |
| Sigmoidoscopy versus colonoscopy | • Advantages of sigmoidoscopy: (1) eoCRC often left-sided, (2) sigmoidoscopy marginally faster than colonoscopy, (3) no need for a complete bowel preparation • Advantages of colonoscopy: (1) similar overall costs, (2) similar need for hospital access, (3) lower risk of false negatives |
| Time to colonoscopy | • Diagnostic delay: (1) 30 days are ideal but difficult to achieve under some circumstances (difficult access to care, incomplete insurance coverage), (2) highlight the need for a timely diagnosis Alarming signs/symptoms + positive FIT: proceed to colonoscopy with the highest priority |
| Topic: Risk factors of eoCRC | |
| Family history | • Accuracy of family histories: (1) a 2-generation family history is often difficult to obtain under routine circumstances, (2) dedicated hospitals may have more time for such tasks, (3) risk assessment tools may provide a framework for history taking |
| Risk factors | • Many risk factors identified, but insufficient evidence to recommend earlier access to screening. Further studies necessary on the additional risk factors to include in CRC screening programs (besides age). |
| Topic: genetics of eoCRC | |
| Ranking the genes by importance | • Costs of germline testing: (1) not all healthcare systems may afford large gene panels, (2) prioritize the most important genes if needed, (3) no cost-benefit analysis on additional genes, (4) further studies necessary before recommending large panels in low resources settings |
| Risk assessment tools | • Utility: (1) all with eoCRC should receive germline testing |
| Polygenic risk scores | • Utility: (1) potentially estimate lifetime risk of CRC, (2) further evidence and validation studies in diverse populations are needed before clinical use |
| Topic: Oncological treatment of eoCRC | |
| Adjuvant therapy | • Aggressive adjuvant therapy: (1) eoCRC often receive more aggressive regimens, (2) increased toxicity, but no evidence of a survival benefit, (3) further randomized clinical trials should include endpoints to evaluate benefits to patients with eoCRC |
| Neoadjuvant therapy, adding oxaliplatin | • Oxaliplatin addition: (1) post hoc analysis of one large phase II trial suggested that adding oxaliplatin to standard chemoradiotherapy in eoRC improved disease-free survival and overall survival compared with older individuals, (2) no prospectively analyzed randomized clinical trial data, (3) future randomized clinical trials should include endpoints pertaining to eoRC patients specifically. |
| Rectum, neoadjuvant therapy | • Total neoadjuvant therapy: (1) more and more centers are adopting this strategy as standard management of individuals with rectal cancer, regardless of age, (2) not enough evidence to hypothesize that this should differ for rectal eoCRC, (3) further clinical trials should include endpoints pertaining to eoCRC patients specifically. |
| Immune checkpoint inhibitors therapy | • Use: (1) not enough evidence to hypothesize a different use for younger patients, (2) higher prevalence of LS among eoCRC, therefore higher likelihood of MSI-H CRC, (3) further clinical trials should include endpoints pertaining to eoCRC patients specifically. |
| IHC/MMR assessment | • Biopsies vs surgical specimens: (1) ideally, IHC/MMR assessment before treatment, (2) biopsies provide results comparable with staining on surgical specimens, (3) biopsies do not carry a risk of false-negative IHC/MMR results |
| Targeted therapies | • Use: (1) not enough evidence to hypothesize a different use for younger patients, (2) further clinical trials should include endpoints pertaining to eoCRC patients specifically |
| Topic: Endoscopy of eoCRC | |
| Clearing colonoscopy | • Ideally, the diagnostic colonoscopy should clear the colon of all synchronous lesions, particularly when multiple polyps are present. It should be emphasized that younger patients do not require an extended surgical resection by default. The scientific panel suggests a clearing colonoscopy to further discourage the use of an extended surgical resection. |
| Post-treatment follow-up | • Surveillance protocol: (1) insufficient evidence to support an intensified surveillance protocol, (2) insufficient evidence to discharge patients with eoCRC from follow-up, (3) suggestion to continue post-treatment surveillance and not to discharge the patient, (4) significant knowledge gap, (5) further studies necessary on the risk of metachronous CRC and the time of surveillance discharge • Hereditary CRC, family history of CRC, or inflammatory bowel diseases: should receive posttreatment surveillance according to their specific guidelines. |
| Secondary prevention of CRC | • Aspirin use: (1) insufficient evidence on the secondary prevention of eoCRC, (2) optimal dosage for cancer prevention unclear after CRC • Other medications: (1) insufficient evidence |
| Topic: Treatment of eoCRC | |
| Standard vs extensive surgical resections | • Extended surgical resections: (1) no evidence to support more extensive resections, unless a distinctly higher risk of CRC is demonstrated, (2) the scientific panel currently discourages further analysis on extensive colorectal surgeries based on early age alone • Factors besides age: (1) can be considered, including (but not limited to) a polyposis phenotype, a colitis-associated CRC, and a genetically higher risk of CRC. Such characteristics do not pertain to these guidelines. |
| Synchronous gynecologic surgery | • Indications: (1) eoCRC is not an indication for hysterectomy with or without oophorectomy, (2) however, other indications may justify hysterectomy with or without oophorectomy at the time of CRC surgery, (3) consider age of the patient, risk for gynecologic cancers, and reproductive desires when offering a gynecologic prophylactic surgery • Ovarian transposition: (1) patients requiring radiotherapy may benefit from ovarian transposition at the time of colorectal surgery, (2) further evidence is necessary |
| Fertility preservation | • Information provider: (1) any healthcare professional, if adequately trained, (2) multidisciplinary teams for eoCRC patients may benefit from having a gynecologist • Ovarian damage: (1) CRC-directed chemotherapeutic agents can be gonadotoxic, (2) female patients with eoCRC should receive information on their ovarian health • Menopause: little to no evidence on the menopausal issues on patients with eoCRC receiving treatment. Research necessary • Male reproductive health: scarce evidence on male factors. Further research necessary on the reproductive needs, issues, and desires |
| Supportive care of eoCRC | |
| Nausea and vomiting | • (1) Higher risk of chemotherapy-induced nausea and vomiting, (2) enough evidence to contemplate the use of enhanced antiemetic prophylaxis with new-generation antiemetic, (3) further evidence may be needed. |
| Nutritional support and physical therapy | • (1) Higher risk of significant weight loss than patients with CRC at an older age, (2) little to no evidence on the use of nutritional support and physical support programs in patients with eoCRC. |
Section I – Diagnosis (D)
D.1: Comment.
Historically, CRC screening has started at age 50 for average-risk individuals in the United States. As a result, CRC diagnoses in patients aged <50 have been referred to as early- or young-onset in the literature. Some U.S. societies have recently recommended lowering the average-risk population screening age to 45 years.15–20 For the purpose of continuity and consistency in research, we recommend using the term early-onset CRC (eoCRC) and defining this as CRC diagnosed younger than 50 years of age. However, with changes in age for population-based screening, it will be critical to assess whether there are differences in risk factors, diagnosis, and/or outcomes for those age 45 and older compared with those younger than age 45. Several terms have been used to describe CRC in the youngest age groups.21–23 The Scientific Panel suggested “very early onset” for CRC diagnoses before 35 years based on definitions used in previous studies.23–26 Age 18 is conventionally used to distinguish adult- from pediatric/adolescent-onset cancers.
D.2: Comment.
The most common symptoms and signs of eoCRC are hematochezia (ie, rectal bleeding) (46%), iron deficiency anemia (13.0%), and weight loss (10.0%).27–32 Hematochezia and iron deficiency anemia (ferritin <15 ng/dL) confer a hazard ratio of 10.66 and 10.81 for eoCRC, respectively, with higher risk for men compared with women and for ages 40–49 compared with age <30.33 More rectal cancers were noted among those with hematochezia compared with iron deficiency anemia (38% vs 20%, respectively).33 It should be noted that the American Gastroenterological Association Practice Guidelines recommend gastrointestinal (GI) evaluation for men and postmenopausal women with iron deficiency anemia; in premenopausal women with iron deficiency anemia, GI evaluation received a conditional recommendation with several caveats related to patient preferences.27 In a case-control study of eoCRC, of which 40% were rectal cancers, weight loss of ≥5 kg (>11 pounds) within 5 years was associated with higher odds of eoCRC (odds ratio [OR], 2.23).29
Other common symptoms at CRC diagnosis include abdominal pain, abdominal distention, change in bowel habits, and fatigue.28,34–38 However, because abdominal pain and changes in bowel habits are common and non-specific and there is conflicting evidence as to how often abdominal pain and changes in bowel habits are associated with eoCRC,30,39 endoscopic evaluation is currently not recommended for all young adults without other alarming symptoms or CRC risk factors. The decision to proceed with further diagnostic testing in an individual who presents with abdominal pain, bowel habit changes, or both should be individualized.
The systematic review found 10 studies on anemia, hematochezia, and unexplained weight loss in the literature, 5 of which had LE 2b, with similar results across studies. There were 5 studies on abdominal pain and changes to bowel habits, 2 of which had LE 2b but with significant differences across studies. Two studies compared the differences in symptomatic presentation between eoCRC and late-onset CRC (loCRC), one of which had LE 2b.
D.3: Comment.
Colonoscopy is recommended for the diagnostic evaluation of individuals with hematochezia, unexplained iron deficiency anemia, or unexplained weight loss. Colonoscopy should be complete to the cecum and of high quality. The use of colonoscopy for evaluation of other symptoms (including a change in bowel habits or abdominal pain) is discussed in section D.2.
The use of alternative diagnostic modalities, including fecal immunochemical tests (FIT), for symptomatic individuals remains controversial. An expanded statement that included FIT reached 67% agreement only (A+, 30.0%; A, 37.5%, A−, 20.0%; D−, 7.5%; D, 5.0%) and was therefore eliminated (“A diagnostic colonoscopy is recommended for evaluation of alarming symptoms and signs of eoCRC (and in case of FIT positivity)”). Recent studies have found that FIT performs well in both symptomatic and asymptomatic patients younger than age 50.40–42 However, the reasons for such disagreement include that a positive FIT result would still require a colonoscopy, which may lead to delays in diagnosis. Delays in obtaining a colonoscopy are associated with an increased risk of advanced-stage disease.43,44 Therefore, FIT is not recommended for symptomatic patients. Triaging patients with low-risk symptoms with FIT may be an option (ie, change in bowel habits or abdominal pain). However, for high-risk symptoms (hematochezia, unexplained iron deficiency anemia, or unexplained weight loss) diagnostic colonoscopy remains the modality of choice.32
The systematic review found 3 studies on the use of FIT and colonoscopy for asymptomatic individuals, 2 of whom had LE 1b. However, all studies had a selection bias, and there were inconsistent results across them. There were 2 studies on the use of FIT in symptomatic individuals, both with LE 1b and with similar findings. There was 1 study on the use of colonoscopy in symptomatic individuals, with LE 2b.
D.4: Comment.
EoCRC patients are often diagnosed at later stages (stage III/IV). Some studies reported that diagnostic delays contribute to advanced disease at presentation.44–46 However, recent data suggest that the increased incidence of advanced-stage disease in eoCRC may not be fully explained by delays in workup.47,48 According to one study, stage III/IV eoCRCs tend to present with alarming symptoms that prompt expedited endoscopic evaluation compared with stage I/II eoCRC.47 The following recommendations should therefore be followed49: assessment of CRC risk, timely workup of symptoms, and referral for colonoscopy. Optimally, colonoscopy should be performed within 30 days of presentation with alarming symptoms.49
The systematic review found 4 studies on the diagnostic delay of eoCRC; only one had LE 2b, and the others with lower LE, but all showed consistent results. Three studies evaluated the hypothesis that a longer diagnostic delay was associated with a more advanced disease stage at diagnosis; all 3 studies had LE 3b and provided unconclusive and conflicting results.
A full summary of relevant evidence for D.2,28–39,47,50–53 D.3,32,40–42,54,55 and D.433,36,38,45–47,51 is available in Supplementary Appendix 2.
Section II: Risk Factors (R)
R.1: Comment.
Family history of cancer should include all cancer diagnoses to identify hereditary syndromes (implicated in 13% of eoCRC),56,57 as well as to quantify risk for non-syndromic familial CRC. About 28% of patients with eoCRC have a family history of CRC,58,59 which is not significantly different compared with the loCRC population. Individuals with a family history of CRC should undergo more intensive surveillance than the general population, starting at an earlier age. However, definitions of who should undergo more intensive surveillance vary widely by country. There is a consensus that having at least 2 first-degree relatives with CRC and/or at least 1 first-degree relative diagnosed with CRC before the age of 50–60 years are associated with a significant increase in risk for CRC. In these situations, screening colonoscopy starting at 40 years (or 10 years before the age at diagnosis of the youngest affected relative) is usually recommended. A recent study showed that up to 16% of eoCRC could be prevented56 if colonoscopy was performed at the age recommended by guidelines based on family history.17,18,58–60
Validated risk assessment tools can facilitate family history taking and identification of patients who would benefit from germline genetic testing, such as the Colon Cancer Risk Assessment Tool and the PREMM5.61,62 The PREMM5 tool can be used to determine the likelihood of a pathogenic variant (PV)/likely pathogenic variant (LPV) in a Lynch syndrome (LS) gene. However, it is recommended that all patients with eoCRC should undergo multigene germline panel testing, regardless of the results of risk assessment tools (see G.1).
The systematic review found 6 studies evaluating the prevalence of a family history of CRC among individuals with eoCRC, 3 of whom had LE 2b, and they all concluded that there was a strong predisposition for having a family history of CRC among younger patients. Five studies evaluated the clinical outcomes of taking family histories, with 2 studies having LE 1a, and they all concluded that a family history of CRC increases the risk of eoCRC.
R.2: Comment.
Most patients diagnosed with eoCRC have no obvious risk factors. A minority of eoCRC patients have a predisposing condition such as hereditary CRC syndromes (13% of cases), longstanding inflammatory bowel diseases (<1% of cases), or a family history of CRC (28%)29,63,64; however, the majority of individuals affected with eoCRC would have been considered at average risk for colorectal neoplasia.
In the United States, black individuals have a higher CRC incidence and mortality compared with other racial and ethnic groups.15,65 However, the recent increase in eoCRC is largely driven by an increase in rectal cancer among white males.66–70 Some studies have proposed other risk factors for eoCRC, including male sex, hyperlipidemia, obesity (especially during adolescence), metabolic syndrome, alcohol consumption, type II diabetes, and high intake of simple sugars.36,71–75 There is controversial evidence on cigarette smoking, hypertension, chronic kidney disease, dietary patterns, sedentary behavior, and in utero, pediatric, and occupational exposures.36,72–74,76–87 Although many of these proposed risk factors have been combined to produce CRC risk scores,88,89 no CRC risk score has received formal validation for clinical use.
The systematic review found 6 studies evaluating the risk of eoCRC among different ethnicities, with 2 studies having LE 1b. They all concluded that black individuals have a higher risk of eoCRC, but the incidence and prevalence of eoCRC have remained the same in recent years, whereas it has increased among white individuals. Eight studies with LE 1a, 1b, or 2b evaluated the known CRC risk factors (male sex, hyperlipidemia, obesity, metabolic syndrome, alcohol consumption, and type II diabetes), and they generally agreed that these represent risk factors for eoCRC as well. Seventeen more studies evaluated other risk factors, with controversial and inconsistent findings.
A full summary of relevant evidence for R.121,30,31,39,42,47,51,53,54,56,59,74,76,78,90–102 and R.29,30,31, 36,65–67,70–89,91,103,104 is available in Supplementary Appendix 3.
Section III: Genetics (G)
G.1: Comment.
The advent of next-generation sequencing (NGS) has allowed multigene panel testing to be performed on various cohorts of cancer patients including those with eoCRC. The prevalence of germline LPV and PV in cancer susceptibility genes is 13.0% (range, 9.0%–26.4%) among patients with eoCRC (excluding MUTYH heterozygotes), but it is even higher among patients younger than 35 (23.0%).105 This is comparable with the 18%–24%106 prevalence of germline LPV/PVs among ovarian cancer patients for whom genetic testing is recommended.
The management of hereditary CRC syndromes should be incorporated into surgical planning. Genetic testing before surgery may permit optimization of the surgical plan,107–109 including a discussion of extent of colonic resection and indications for gynecologic surgery (section T.1).
Thirteen studies analyzed the prevalence of PV/LPVs in cancer susceptibility genes in individuals with eoCRC. There were 7 studies with LE 2b and 6 studies with LE 1b. The prevalence of LS was variable from 0% to 18.3%. The prevalence of other, non-LS, hereditary predisposition PV/LPV ranged from 2.3% to 26.4%.
G.2: Comment.
Among eoCRC patients, 2%–16% have LS, and up to 14% have PV/LPVs in other cancer susceptibility genes.110–122 LS is the most common genetic diagnosis among eoCRC patients, and LS genes include the DNA-mismatch repair genes MLH1, MSH2, MSH6, and PMS2, as well as EPCAM 3′ deletions. Colorectal polyposis syndromes account for 2%–3% of eoCRC and include familial adenomatous polyposis (associated with PV/LPV in APC), MUTYH-associated polyposis (associated with biallelic PV/LPV in MUTYH), juvenile polyposis (associated with PV/LPV in SMAD4, BMPR1A), and PeutzJeghers syndrome (associated with PV/LPV in STK11). Some of the newer genes associated with polyposis or CRC (GREM1, POLE, POLD1, AXIN2, MSH3, MLH3, MBD4, RNF43, and RPS20) were not included in most prior studies because they were discovered relatively recently. Studies have also identified PV/LPVs in other highly actionable high-penetrance genes that have not previously been associated with CRC (TP53, BRCA1, BRCA2, and PALB2) at a prevalence higher than that of some of the known polyposis genes.122–124 Notably, there is emerging evidence that ATM may be a CRC susceptibility gene.115 At this time, RNF43, RPS20, and MBD4 do not have actionable recommendations for clinical management. However, they are included in this statement because of their potential association with serrated polyposis syndrome or CRC.
As of the time of writing these guidelines, our recommendations about which genes to include in the multigene panel testing for eoCRC patients are based on their known association with CRC or polyposis, the prevalence of PV/LPVs in each gene among eoCRC patients, and the clinical actionability of genetic findings (Supplementary Tables 4–6). Germline genetic testing for eoCRC patients should include at a minimum the following: APC, BMPR1A, EPCAM, MLH1, MSH2, MSH6, MUTYH, POLD1, POLE, PMS2, PTEN, SMAD4, STK11, and TP53.Where available and not cost-prohibitive, testing should also include the following genes, which are reasonably prevalent in CRC and change clinical management: BRCA1, BRCA2, ATM, CHEK2, PALB2, and possibly, but less prevalent, BRIP1, BARD1, CDKN2A, CDH1, RAD51C, and RAD51D, and the following genes, which have been associated with CRC or polyposis: AXIN2, GREM1, MLH3, MSH3, MBD4, NTHL1, RNF43, and RPS20.
G.3: Comment.
Single nucleotide polymorphisms (SNPs) have been identified through genome-wide association studies as associated with increases or decreases in risk for CRC. A variety of SNPs have been shown to modestly increase the relative risk of CRC (range, 1.46–2.82), and these have been combined (with and without other lifestyle factors) to create polygenic risk scores (PRS). However, the clinical utility of the various PRS remains thus far unproven. Important limitations of PRS include that most were developed using data from predominantly white individuals of European ancestry and have not been validated in diverse populations. One genome-wide association study took data from 12,197 individuals younger than 50 and 95,865 individuals older than 50. It categorized the resulting 95 SNPs into a PRS that could correlate more strongly with eoCRC than with loCRC.
In a subanalysis, the same study conducted a PRS classification to identify individuals who would benefit the most from anticipatory screening at age 45. We encourage further study of the performance of PRS and its validity in non-white non-European individuals.
A full summary of relevant evidence for G.1,110–122 G.2,110–122,125,126 and G.3127–142 is available in Supplementary Appendix 4 and Supplementary Tables 4–6.
Section IV: Pathology and Oncological Treatment (O)
O.1: Comment.
All patients with CRCs, regardless of age at diagnosis, must be tested for DNA mismatch repair deficiency (MMR-d) by immunohistochemistry (IHC) staining for MMR proteins MLH1, MSH2, MSH6, and PMS2 or microsatellite instability (MSI) by polymerase chain reaction or NGS. MMR-d or MSI-high (MSI-H) tumors are associated with LS, a decreased response to 5-fluorouracil–based chemotherapy, an enhanced response to immunotherapy, and in general have an improved prognosis compared with MMR-proficient tumors (MMR-p).143,144
Pretreatment MMR-d testing is particularly critical in 2 scenarios. (1) In metastatic CRC, patients with MMRd metastatic CRC should be treated with immunotherapy as a part of first-line systemic treatment, regardless of age at diagnosis. (2) In non-metastatic CRC, the presence of MMR-d may implicate a diagnosis of LS, for which an extended colectomy may be recommended.145–148 The longer the life expectancy, determined by the patient’s age and disease stage, the greater the benefit of more extensive colonic resection for reducing the risk of metachronous tumors.145–147
The rate of MMR-d CRC is higher among eoCRC than among loCRC.21,64,149 The MMR/MSI status can be assessed on diagnostic colon tumor biopsies or surgical specimens. Although pathologists may prefer to test the surgical specimen to analyze the normal matched mucosa, MMR testing on pretreatment biopsies is usually preferable.144 IHC/MSI tumor testing results are often available before the results of germline panel testing. In cases of metastatic, non-resectable tumors, MMR testing can be performed on biopsies. Moreover, in locally advanced rectal cancer, MMR testing is best investigated on biopsies collected before neoadjuvant therapy, because the tumor may regress during chemoradiotherapy.144
All patients with eoCRC should undergo germline genetic testing and receive genetic counseling, regardless of the results of MMR-d/MSI testing, to identify other high-penetrance PVs beyond LS.124,150
The systematic review found 3 cohort studies on the prevalence of IHC/MSI in eoCRC, only one with LE 3b but all with similar findings. Six studies compared the IHC/MSI characteristics of eoCRC against loCRC, 2 with LE 1b and 3 with LE 2b. They all showed similar findings.
O.2: Comment.
MMR-d/MSI, KRAS, NRAS, BRAF, and Her2 should be tested in all patients with metastatic CRC, regardless of age at diagnosis, to guide therapy selection. Initial studies noted that eoCRC tumors exhibit fewer somatic mutations in APC and TP53 and exhibit consensus molecular subtype 1 more often than loCRC.64,151–154 However, when eoCRC was compared with loCRC with complete clinical annotation and the genomics of sporadic eoCRC were analyzed by tumor sidedness, there were no significant differences in the mutational landscape.105 Moreover, MSI-H eoCRC, particularly MLH1-deficient, non-LS, BRAF-wild-type, MLH1-methylation negative tumors, should be tested for NTRK.50,151,155 Finally, anti-EGFR inhibitors are a reasonable component of first-line treatment for metastatic left-sided (including but not limited to rectal cancer) and RAS/RAF-wild-type eoCRC.
The systematic review found 3 studies with LE 2b–3b supporting the hypothesis that eoCRC has biological markers different than loCRC. However, 2 LE 1b studies showed that there was no statistically significant difference in the biological markers between eoCRC and loCRC.
O.3: Comment.
Various reports have suggested that eoCRC may display a more aggressive behavior than CRC of older individuals.31,156–163 This has been explained by delayed diagnosis resulting in more advanced tumor stage,164 more aggressive molecular and pathologic subtypes, and/or lower pathologic complete response rates to neoadjuvant chemoradiotherapy.163 However, recent lines of evidence challenge this observation.104,152,153,165,166
Because of their young age and robust performance status, patients with eoCRC often receive more aggressive multimodality treatment.162,167,168 However, more aggressive treatment strategies have not translated into a statistically significant survival benefit.50,105,143,155,169,170
The systematic review found 7 studies that supported that eoCRC is more aggressive than loCRC, with 1 LE 1b study and 3 LE 3b studies. However, 5 studies reported that the survival rates and the prognosis of eoCRC and loCRC do not differ, with 1 LE 1b study and 3 LE 2b studies. Seven studies did not support the use of more aggressive systemic therapies for eoCRC, with 2 studies having LE 1b. Only 2 studies suggested a benefit from more aggressive systemic therapy, but both had a low LE.
O.4: Comment.
There is scarce evidence regarding the impact of age on the efficacy of neoadjuvant chemoradiotherapy and the outcomes of locally advanced rectal cancer. Many publications describe a more aggressive attitude of clinicians and surgeons treating stage III and IV eoCRC, as well as on the part of eoCRC patients, but this aggressiveness has often not conferred a significant survival benefit.165,171 One post hoc analysis suggested that adding oxaliplatin to standard 5-fluorouracil–based chemoradiotherapy for locally advanced rectal cancer may improve disease-free survival and overall survival in patients younger than 60.172 However, numerous phase III trials have shown no benefit (and increased toxicity) from adding oxaliplatin to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients of any age, and adding oxaliplatin is thus not part of standard neoadjuvant therapy. In the absence of randomized clinical trial data prospectively comparing eoRC versus loRC, standard 5-fluorouracil–based chemoradiotherapy in eoRC patients does not contain oxaliplatin. Finally, there are data from randomized controlled trials that total neoadjuvant therapy with sequential radiotherapy and combination chemotherapy may improve complete pathologic response rate, disease-free survival, and overall survival compared with standard chemoradiotherapy, but no data specifically on eoRC.173–176
Age of onset is not a criterion to change the use of immune checkpoint inhibitors in MSI-H CRC. Likewise, early age of onset is not a criterion to drive treatment, and the current consensus is that eoCRC and loCRC patients should receive similar systemic treatments.177–180
Two cohort studies reported the outcomes of neoadjuvant use on eoRC, and 3 retrospective studies (2 LE 3b, 1 LE 2b) suggested that eoRC has lower response rates to neoadjuvant therapy compared with loRC. However, a case-control study (LE 2b) concluded that there was no significant difference in survival between loCRC and eoCRC.
A full summary of relevant evidence for O.1,21,37,64,105,143,151,152,162,167 O.2,64,151–154 O.3,31,50,104, 105,143,152,153,155–162,165–170 162–164,167,171,172,181,182 and O.4 is available in Supplementary Appendix 5.
Section V: Endoscopy (E)
E.1: Comment.
The overall miss rates for colonoscopic detection of adenomas and advanced adenomas are 26% and 9%, respectively.183–185 To maximize the detection of adenomas and CRC, good bowel preparation and high-quality endoscopic techniques are necessary.183–185 High-definition white-light endoscopy, dye- or virtual chromoendoscopy, and certain add-on devices can increase the adenoma detection rate (ADR) of colonoscopy exams.185–187 The degree to which artificial intelligence systems can improve detection of colorectal neoplasia is currently being evaluated. To date, no study has compared these endoscopic techniques for detection of eoCRC or its precursors.
E.2: Comment.
Standard endoscopic quality metrics for polyp detection should be applied for colonoscopy exams performed on young patients. A high ADR is associated with decreases in incidence of post-colonoscopy CRC and CRC-related mortality.188,189 Current guidelines propose a minimum ADR of 25% overall,183 but there is limited evidence regarding the expected ADR in young patients. Although ADRs in younger age groups are likely lower than the ADR observed in the 50- to 75-year age group (28.4% vs 35.6%, P < .001),190 the absolute difference remains small. Nevertheless, if the number of average risk 45- to 49-year-olds undergoing colonoscopy increases compared with older populations, this could further lower the ADR. We encourage more research to determine a minimum ADR. Other colonoscopy quality metrics (cecal intubation rate, bowel preparation, and post-polypectomy recommendations) should be applied equally regardless of patient age.
The systematic review yielded 2 studies on the prevalence of adenoma, advanced adenoma, and CRC among individuals younger than the age of 50, with 1 LE 1a study. Three studies tested the hypothesis that a lower ADR should be used for individuals younger than 50; 2 LE 1b studies supported a change in the ADR, but 1 LE 2b study did not.
E.3: Comment.
The prevalence of synchronous CRCs and adenomas reaches 10% and 60%, respectively,191 and many of these (43% and 80% of cases, respectively) are located in a different area within the colon.191,192 Therefore, all patients should undergo a complete colonoscopy exam before surgery if feasible.193 Alternatively, colonoscopy should be performed intraoperatively or 3–6 months after recovery from surgery to exclude synchronous lesions.
EoCRCs present at a more advanced stage.182,194 Staging studies for CRC should not differ on the basis of patient age (computed tomography of chest, abdomen, and pelvis, complete blood count, blood chemistries, and carcinoembryonic antigen).195 Pelvic magnetic resonance imaging or lower endoscopic ultrasound is necessary for rectal cancer staging.196
Patients undergoing curative resection for colon cancer should undergo a follow-up colonoscopy 1 year after the resection (or 1 year after the performance of the colonoscopy that was performed to clear the colon of synchronous disease). Patients undergoing curative resection for rectal cancer could undergo rectal ultrasound or flexible sigmoidoscopy every 3–6 months during the first 2 years after resection.197–202
E.4: Comment.
Compared with surgery, endoscopic resection of colonic T1 CRC may offer a similar 5-year cancer-free survival.203 However, the long-term risk of recurrence after endoscopic resection of T1 eoCRC is unknown. Therefore, the endoscopic resection modalities and pre-procedural workup should be no different than for other T1 CRCs.186,187
High-definition endoscopy and chromoendoscopy (dye- or virtual) are recommended.185–187 The risk of submucosal invasion depends on size, vascular invasion, glandular pattern, Paris classification, and type of laterally spreading tumor lesions. Rectal lesions should be staged with lower endoscopic ultrasound or pelvic magnetic resonance imaging before initiation of treatment.196,204 Superficially invasive T1 CRCs (Kudo Vi, Sano IIIa, LST-NG, and rectal LST-GM) should be removed endoscopically en bloc with endoscopic submucosal dissection or endoscopic mucosal resection. Deeply invasive CRCs (Kudo Vn, Sano IIIb) are not amenable to endoscopic resection. Radical surgery with lymphadenectomy is recommended if histopathology shows lymphovascular invasion, submucosal invasion >1000 μm, high-grade budding, positive/non-evaluable vertical margins, or poor differentiation (G3).186,205 There are limited data on full-thickness resections in eoCRC, but we encourage further investigations.
Five studies analyzed the use of endoscopic treatment for T1 CRC among patients younger than 50. Two LE 1a studies, 1 LE 2b study, and 1 LE 3b study all supported the use of endoscopic treatment of T1 eoCRC. Only 1 LE 2b study suggested that T1 eoCRC has a higher propensity for lymph node metastasis and cautioned against endoscopic treatment.
E.5: Comment.
Endoscopic surveillance after curative resection of CRC can prevent local recurrences and meta-chronous CRC.198 The detection of interval high-risk neoplastic lesions should prompt shortening of the endoscopy intervals (size, number, and histologic features), as should a genetic diagnosis that requires more intensive colonoscopic surveillance. The European Society of Gastrointestinal Endoscopy (ESGE), National Comprehensive Cancer Network (NCCN), and U.S. Multi-Society Task Force (USMSTF) guidelines endorse similar follow-up endoscopic surveillance intervals after CRC resection (at 1, 3, and 5 years).147,180,185,198 Patients with eoCRC may have a higher risk for metachronous CRC after surgery compared with patients with loCRC,52 and the risk for metachronous neoplasia may extend further in time.101,206 Therefore, eoCRC survivors may not be safely dismissed from post-treatment surveillance. Post-CRC colonoscopic surveillance is recommended at similar intervals for eoCRC and loCRC in the absence of interval advanced colorectal neoplasia and/or diagnosis of a genetic condition requiring more intensive surveillance. There is not enough evidence on the effectiveness of aspirin for secondary prevention after eoCRC treatment; the decision to give aspirin should be individualized, and no recommendation could be endorsed at the time of writing this guideline. We acknowledge a significant knowledge gap in this area.
Three studies found that the risk of metachronous CRC is higher in eoCRC patients compared with loCRC, including 1 LE1a and 2 LE2b. Four more studies did not conclude that the risk of metachronous CRC was higher, although with inconsistent observational times and inconsistent follow-ups.
A full summary of relevant evidence for E.2,39,53,91,190,207 E.3,156,203,208–211 andE.451,91,102,182,206,210,212 is available in Supplementary Appendix 6.
Session VI: Treatment (T)
T.1: Comment.
More extensive surgical resection cannot be recommended for eoCRC patients who do not have a distinct risk-enhancing predisposition (particularly a hereditary CRC syndrome or ulcerative colitis).102,209,213 Although subtotal or total colectomy offers a benefit in reducing risk for metachronous CRC in patients with LS and familial adenomatous polyposis, more extensive surgery did not offer a survival benefit in LS.107,108,214–216
For patients with LS, the cumulative incidence of gynecologic cancers (endometrial or ovarian) before 50 years of age is high (the risk of endometrial cancer by age 50 in female carriers of MSH2 and MSH6 PV/LPVs exceeds that of CRC, and it is roughly the same among MLH1 female carriers).217 One-third of LS patients are diagnosed with CRC before risk-reducing gynecologic surgery.210 Simultaneous hysterectomy with or without bilateral salpingo-oophorectomy at the time of CRC resection may be an option for female LS carriers age >35 years who have completed childbearing. A decision regarding risk-reducing gynecologic surgery should be individualized, taking into account the woman’s age and childbearing status, and must follow a detailed discussion regarding risks and benefits.218–220 The negative consequences of a surgical menopause preclude bilateral oophorectomy at the time of risk-reducing gynecologic surgery in very young women (<40 years). For women aged 40–50 years undergoing risk-reducing gynecologic surgery, estrogen replacement therapy may be a consideration to prevent the negative sequelae of bilateral salpingo-oophorectomy.
The systematic review gathered 3 studies on the use of more extended surgical resections for patients with eoCRC, and they all concluded that the use of extended surgical resections should be discouraged.
T.2: Comment.
Loss of fertility is a known side effect of cancer treatment. Unfortunately, eoCRC survivors often do not receive comprehensive fertility information.221–223 All patients of reproductive age should receive information on the risk of infertility and the option of fertility preservation before initiation of potentially gonadotoxic treatment.224 Options for fertility preservation include ovarian transposition before initiation of radiotherapy, sperm banking, and cryopreservation of oocytes, embryos, and ovarian tissue.
Three studies investigated the access to fertility preservation among patients with eoCRC, and they all concluded that patients often receive inadequate counseling and are offered limited access to fertility services. Most patients in these 3 studies were female, which further highlights the lack of fertility care particularly among eoCRC male patients.
T.3: Comment.
One of the major issues to consider when choosing a treatment plan includes the risk of gonadal failure and/or uterine damage with the proposed treatment program.225 The risk of treatment-induced gonadotoxicity depends on the use of alkylating agents,226 the patient’s age,226,227 and the patient’s ovarian reserve.228 There is no homogeneous definition for premature ovarian failure, but ovarian reserve tests may be useful to assess this (ie, blood levels of antiMüllerian hormone and the antral follicle count).
Moreover, one also needs to factor in the overall prognosis of the patient, the potential risks of delaying treatment, the impact of pregnancy on the risk of recurrence, and the risk of hormonal manipulation on CRC.219,229 Studies about fertility preservation generally enroll individuals with eoCRC.222 However, no interventional study has directly compared the clinical outcomes of different fertility preservation techniques in individuals with eoCRC specifically.
Two small studies (LE 4) evaluated the effects of oxaliplatin on fertility markers. One cohort study analyzed the fertility rates of patients with eoCRC, but with an intrinsic selection bias (all patients had LS).
A full summary of relevant evidence for T.1,50,104,213 T.2,221–223 and T.3226,227,230 is available in Supplementary Appendix 7.
Session VII: Supportive Care (C)
C.1: Comment.
The assessment and treatment of pain are important for every patient with cancer, and a comprehensive set of guidelines was recently published by the European Society of Medical Oncology (ESMO).231 Abdominal pain is common during CRC, particularly within the context of advanced disease. A continuous assessment of pain (characteristics, duration, and intensity) should be an integral part of cancer care using standardized scales. In the absence of vomiting and dysphagia, oral analgesics are preferred. In the case of severe pain, strong opiates may be required for symptom control.231 Adjuvant drugs, antidepressants, invasive techniques, psychological therapy, and palliative antitumor treatment can also be considered.
The assessment and treatment of fatigue are important and addressed for every patient with cancer, and the recently published ESMO guidelines provide general recommendations on the management of this common cancer-related symptom.232
Patients with eoCRC often suffer from chemotherapy-induced nausea and vomiting (CINV), particularly women with low body mass index.233 Therefore, enhanced prophylactic use of antiemetic drugs can be considered in this population234,235; however, there are no data available on the effectiveness of tailored antiemetic regimens specific for eoCRC.
The assessment and treatment of constipation, diarrhea, and cachexia should follow the recently published ESMO and American Society of Clinical Oncology guidelines.219,236–239 Similar to patients with loCRC, eoCRC patients may be responsive to early physical activity programs and nutritional support to maintain and recover muscle mass and counteract cachexia.240
Finally, eoCRC patients may be more reluctant than older patients to discuss concerns about side effects with their healthcare providers and may be especially hesitant to address issues such as sexual dysfunction.241 Therefore, clinicians and members of the healthcare team should proactively discuss sexual health and potential dysfunction resulting from cancer or its treatment because these issues are particularly relevant for eoCRC survivors.242
Three studies assessed the prevalence of comorbidities among eoCRC patients, with 1 LE 1b study. There was 1 LE 1b study supporting the use of physical therapy during and after cancer treatment.
C.2: Comment.
Pain should be managed by a multidisciplinary team and should include psychosocial support.231 Inadequate pain control contributes to poor quality of life and negative emotional status. Young adults and adolescents with cancer can present with needs that are different from those of their adult and pediatric counterparts.243,244 They may experience similar side effects, but these symptoms may have a greater impact on daily activities including work and childcare.245 Furthermore, younger patients also have unique psychosocial and informational needs, including those that concern educational/work pursuits and goals.243 There may also be more difficulties in the management of symptoms, fears, and behavior modifications.246
There were 2 LE 4 studies concerning the organization of supportive care programs among individuals with eoCRC, including the management of sleeping, sexual, intimacy, nutritional, and social care.
A full summary of relevant evidence for C.138,165,233,240 and C.2245,247 is available in Supplementary Appendix 8.
Discussion
The DIRECt group provides the first comprehensive, evidence-based, practical consensus recommendations for the best management of patients with eoCRC. There are some important differences in the management of eoCRC compared with loCRC (Table 6). We strongly recommend that the diagnosis of CRC be carefully considered for individuals younger than 50 who present with alarming symptoms. Risk assessment for CRC includes the presence of a CRC family history and the personal history of individual risk factors and comorbidities. We strongly recommend that all patients with newly diagnosed eoCRC undergo both germline multigene panel testing and IHC/MSI tumor testing, ideally before surgery. There is insufficient evidence to recommend changes to the endoscopic, surgical, and oncologic treatment based on age alone. However, therapeutic decisions should be individualized on the basis of additional factors (ie, higher risk of metachronous CRC, results from germline and somatic testing, fertility desires, concomitant indications for gynecologic cancer, and higher risk of CINV). We recommend that all newly diagnosed eoCRC patients receive counseling on fertility preservation before treatment starts, as well as psychosocial support.
Table 6.
Guideline Differences Between Early-Onset Colorectal Cancer (eoCRC) and CRC Diagnosed After the Age of 50 Years
| CRC before age 50 | CRC after age 50 | |
|---|---|---|
|
| ||
| Genetics | Germline multigene panel testing always recommended | Germline multigene panel testing recommended under specific circumstances (tumor and clinical features suggestive of hereditary cancer syndromes) |
| Family history | Family history: mandatory | Family history: recommended |
| Surgery | No difference yet | |
| Chemotherapy | No difference yet | |
| Targeted therapy | No difference yet | |
| Fertility | All patients should receive information on fertility preservation options | Most patients are not candidates for fertility preservation |
| Sexuality | No difference yet | |
| Nausea and vomiting | Consider enhanced antiemetic prophylaxis | Regular antiemetic prophylaxis |
| Endoscopic management | No difference yet | |
| Post-treatment follow-up | Should not be discharged from follow-up | Follow country-specific guidelines for post-treatment surveillance |
All statements received an agreement rate of at least 80%. The systematic analysis and appraisal of the literature showed that the LE in this disease is low in some specific areas.
The lack of sufficiently high-quality data on some topics demands further investigation (Table 5). During the in-person DIRECt22 meeting in Milan, we identified significant knowledge gaps that should be prioritized in future research agendas, including outcomes of screening in young populations at average and increased risk for CRC (especially in European Union countries, where data remain limited); identification of risk factors for eoCRC; examining outcomes of specific neoadjuvant, adjuvant, and systemic therapy in eoCRC; long-term outcomes after surgery vs endoscopic resections; and appropriate follow-up schedules and surveillance intervals after curative resection. However, there was global consensus regarding the importance of individual risk assessment for determining the optimal age to initiate CRC screening. One topic of discussion was whether the systemic treatment of eoCRC should differ compared with loCRC; at this time data are limited because of the absence of randomized controlled trials specific to eoCRC; therefore, no change to the treatment of CRC should be made on the basis of age alone.
These recommendations resulted from a critical appraisal of the best available evidence and expert evaluation of the most recently published data on eoCRC. A consensus process contributed to their elaboration and validated the conclusions drawn from the literature. The DIRECt guidelines are the first for eoCRC, and they represent a useful tool for diagnosis, management, and prevention of eoCRC in clinical settings.
Supplementary Material
Acknowledgments
The authors thank Fondazione Internazionale Menarini for the support in the organization of the first international congress on eoCRC (DIRECt22), held in Milan in September 2022. The authors thank M. Vitaloni and A. Davis, patient representatives on behalf of the “Digestive Cancers Europe” and “Fight Colorectal Cancer” patient associations, respectively. The authors acknowledge the valuable help from Dr G. Dell’Anna and Dr R. Ponz De Leon Pisani in carrying out the systematic search on PubMed, Embase, and Scopus.
Abbreviations used in this paper:
- ADR
adenoma detection rate
- CI
confidence interval
- CINV
chemotherapy-induced nausea and vomiting
- CRC
colorectal cancer
- DIRECt
Delphi Initiative Recommendations on EoCRC
- eoCRC
early-onset colorectal cancer
- ESGE
European Society of Gastrointestinal Endoscopy
- ESMO
European Society of Medical Oncology
- FIT
fecal immunochemical testing
- GI
gastrointestinal
- IHC
immunohistochemistry
- LE
level of evidence
- loCRC
late-onset colorectal cancer
- LPV
likely pathogenic variants
- LS
Lynch syndrome
- MMR
mismatch repair
- MMR-d
mismatch repair deficiency
- MMR-p
mismatch repair proficiency
- MSI
microsatellite instability
- MSI-H
microsatellite instability high
- NCCN
National Comprehensive Cancer Network
- NGS
next-generation sequencing
- OR
odds ratio
- PICO
population, intervention, comparison, and outcome
- PRS
polygenic risk scores
- PV
pathogenic variants
- RR
relative risk
- SNP
single nucleotide polymorphism
- USMSTF
U.S. Multi-Society Task Force
- USPSTF
U.S. Preventive Service Task Force
Footnotes
Conflicts of interest
Refer to the online form to see conflicts of interest.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.12.006.
References
- 1.Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Heal 2019;4:e137–e147. [DOI] [PubMed] [Google Scholar]
- 2.Vuik FER, Nieuwenburg SAV, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–2185. [DOI] [PubMed] [Google Scholar]
- 4.Patel SG, Karlitz JJ, Yen T, et al. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol 2022;7:262–274. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335–349.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breekveldt ECH, Lansdorp-Vogelaar I, Toes-Zoutendijk E, et al. Colorectal cancer incidence, mortality, tumour characteristics, and treatment before and after introduction of the faecal immunochemical testing-based screening programme in the Netherlands: a population-based study. Lancet Gastroenterol Hepatol 2022;7:60–68. [DOI] [PubMed] [Google Scholar]
- 7.Chiu HM, Jen GHH, Wang YW, et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut 2021;70:2321–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flor LS, Reitsma MB, Gupta V, et al. The effects of tobacco control policies on global smoking prevalence. Nat Med 2021;27:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boakye E, Uddin SMI, Obisesan OH, et al. Aspirin for cardiovascular disease prevention among adults in the United States: trends, prevalence, and participant characteristics associated with use. Am J Prev Cardiol 2021;8:100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 11.Modest DP, Karthaus M, Fruehauf S, et al. Panitumumab plus fluorouracil and folinic acid versus fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer: the randomized PANAMA trial (AIO KRK 0212). J Clin Oncol 2022;40:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iveson TJ, Sobrero AF, Yoshino T, et al. Duration of adjuvant doublet chemotherapy (3 or 6 months) in patients with high-risk stage II colorectal cancer. J Clin Oncol 2021;39:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.André T, Vernerey D, Mineur L, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, International Duration Evaluation of Adjuvant (IDEA) France, phase III trial. J Clin Oncol 2018;36:1469–1477. [DOI] [PubMed] [Google Scholar]
- 14.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM evidence levels of evidence (introductory document). Oxford Cent Evidence-Based Med, 2011. [Google Scholar]
- 15.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 17.Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol 2021; 116:458–479. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:1016–1030. [DOI] [PubMed] [Google Scholar]
- 19.Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol 2021;116:458–479. [DOI] [PubMed] [Google Scholar]
- 20.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022;162:285–299. [DOI] [PubMed] [Google Scholar]
- 21.Khan SA, Morris M, Idrees K, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg 2016;51:1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djursby M, Madsen MB, Frederiksen JH, et al. New pathogenic germline variants in very early onset and familial colorectal cancer patients. Front Genet 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasperson KW, Vu TM, Schwab AL, et al. Evaluating Lynch syndrome in very early onset colorectal cancer probands without apparent polyposis. Fam Cancer 2010;9:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durno C, Aronson M, Bapat B, et al. Family history and molecular features of children, adolescents, and young adults with colorectal carcinoma. Gut 2005;54:1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terdiman JP, Levin TR, Allen BA, et al. Hereditary nonpolyposis colorectal cancer in young colorectal cancer patients: high-risk clinic versus population-based registry. Gastroenterology 2002;122:940–947. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Farrington SM, Petersen GM, et al. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med 1995;1:348–352. [DOI] [PubMed] [Google Scholar]
- 27.Ko CW, Siddique SM, Patel A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology 2020;159:1085–1094. [DOI] [PubMed] [Google Scholar]
- 28.Vajravelu RK, Mehta SJ, Lewis JD, et al. Understanding characteristics of who undergoes testing is crucial for the development of diagnostic strategies to identify individuals at risk for early-age onset colorectal cancer. Gastroenterology 2021;160:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low EE, Demb J, Liu L, et al. Risk factors for early-onset colorectal cancer. Gastroenterology 2020;159:492–501.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed AR, Thakkar P, Horne ZD, et al. Old vs new: risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol 2019;11:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frostberg E, Rahr HB. Clinical characteristics and a rising incidence of early-onset colorectal cancer in a nationwide cohort of 521 patients aged 18–40 years. Cancer Epidemiol 2020;66. [DOI] [PubMed] [Google Scholar]
- 32.Krigel A, Zhou M, Terry MB, et al. Symptoms and demographic factors associated with early-onset colorectal neoplasia among individuals undergoing diagnostic colonoscopy. Eur J Gastroenterol Hepatol 2020;32:821–826. [DOI] [PubMed] [Google Scholar]
- 33.Demb J, Liu L, Murphy CC, et al. Young-onset colorectal cancer risk among individuals with iron-deficiency anaemia and haematochezia. Gut 2021;70:1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dozois EJ, Boardman LA, Suwanthanma W, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore) 2008;87:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glover M, Mansoor E, Panhwar M, et al. Epidemiology of colorectal cancer in average risk adults 20–39 years of age: a population-based national study. Dig Dis Sci 2019;64:3602–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leo M Di, Zuppardo RA, Puzzono M, et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs late-onset colorectal cancer. Eur J Gastroenterol Hepatol 2021;33:1153–1160. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan A, Antoniou E, Morkos M, et al. Is colorectal cancer associated with altered bowel habits in young patients? ANZ J Surg 2021;91:943–946. [DOI] [PubMed] [Google Scholar]
- 38.Zhu C, Ji M, Dai W, et al. Clinicopathological characteristics of Chinese colorectal cancer patients under 30 years of age: implication in diagnosis and therapy. Curr Cancer Drug Targets 2015;15:27–34. [DOI] [PubMed] [Google Scholar]
- 39.Butterly LF, Siegel RL, Fedewa SA, et al. Colonoscopy outcomes in average-risk screening equivalent young adults: data from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2021;116:171–179. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza N, Delisle TG, Chen M, et al. Faecal immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2WW pathway: a diagnostic accuracy study. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Souza N, Monahan KJ, Benton S, et al. Finding the needle in the haystack: the diagnostic accuracy of the faecal immunochemical test for colorectal cancer in younger symptomatic patients. Colorectal Dis 2021:codi.15786. [DOI] [PubMed] [Google Scholar]
- 42.Jung Y, Park CH, Kim NH, et al. Colorectal cancer screening with the fecal immunochemical test in persons aged 30 to 49 years: focusing on the age for commencing screening. Gastrointest Endosc 2017;86:892–899. [DOI] [PubMed] [Google Scholar]
- 43.Corley D, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.San Miguel Y, Demb J, Martinez M, et al. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology 2021;160:1997–2005.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandhu GS, Anders R, Blatchford P, et al. High incidence of prolonged rectal bleeding and advanced stage cancer in earlyonset colorectal cancer patients. Color Cancer 2020;9:CRC31. [Google Scholar]
- 46.Scott RB, Rangel LE, Osler TM, et al. Rectal cancer in patients under the age of 50 years: the delayed diagnosis. Am J Surg 2016;211:1014–1018. [DOI] [PubMed] [Google Scholar]
- 47.Chen FW, Sundaram V, Chew TA, et al. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol 2017;15:728–737.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavestro GM, Mannucci A, Zuppardo RA, et al. Early onset sporadic colorectal cancer: worrisome trends and oncogenic features. Dig Liver Dis 2018;50:521–532. [DOI] [PubMed] [Google Scholar]
- 49.Burnett-Hartman AN, Lee J, Demb J, et al. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer. Gastroenterology 2021;160:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol 2007;14:2759–2765. [DOI] [PubMed] [Google Scholar]
- 51.Kim TJ, Kim ER, Hong SN, et al. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional-based retrospective study. Med (United States) 2016;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandhu GS, Anders R, Blatchford P, et al. High incidence ofprolonged rectal bleeding and advanced stage cancer in earlyonset colorectal cancer patients. Available at: 10.2217/crc-2020-00122020;9:CRC31. Accessed October 13, 2021. [DOI] [Google Scholar]
- 53.Bilal M, Singh S, Le TT, et al. Select group of patients might benefit from early colonoscopic screening for colorectal cancer. Surg Endosc 2020;34:4463–4471. [DOI] [PubMed] [Google Scholar]
- 54.Yeh JH, Lin CW, Wang WL, et al. Positive fecal immunochemical test strongly predicts adenomas in younger adults with fatty liver and metabolic syndrome. Clin Transl Gastroenterol 2021;12:e00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim NH, Park JH, Park DI, et al. The fecal immunochemical test has high accuracy for detecting advanced colorectal neoplasia before age 50. Dig Liver Dis 2017;49:557–561. [DOI] [PubMed] [Google Scholar]
- 56.Stanich PP, Pelstring KR, Hampel H, et al. A high percentage of early-age onset colorectal cancer is potentially preventable. Gastroenterology 2021;160:1850–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daca Alvarez M, Quintana I, Terradas M, et al. The inherited and familial component of early-onset colorectal cancer. Cells 2021;10:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leerdam ME van, Roos VH, Hooft JE van, et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Georg Thieme Verlag, 2019:1082–1093. [DOI] [PubMed] [Google Scholar]
- 59.Wong MCS, Chan CH, Lin J, et al. Lower relative contribution of positive family history to colorectal cancer risk with increasing age: a systematic review and meta-analysis of 9.28 million individuals. Am J Gastroenterol 2018;113:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemminki K, Li X. Familial colorectal adenocarcinoma from the Swedish Family-Cancer Database. Int J Cancer 2001;94:743–748. [DOI] [PubMed] [Google Scholar]
- 61.Kastrinos F, Allen JI, Stockwell DH, et al. Development and validation of a colon cancer risk assessment tool for patients undergoing colonoscopy. Am J Gastroenterol 2009;104:1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kastrinos F, Uno H, Ukaegbu C, et al. Development & validation of the PREMM5 model for comprehensive risk assessment of lynch syndrome. J Clin Oncol 2017;35:2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 2020;158:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019;125:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamath SD, Torrejon N, Wei W, et al. Racial disparities negatively impact outcomes in early-onset colorectal cancer independent of socioeconomic status. Cancer Med 2021;10:7542–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy CC, Wallace K, Sandler RS, et al. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology 2019;156:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman R, Schmaltz C, Jackson C, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med 2015;4:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia S, Pruitt SL, Singal AG, et al. Colorectal cancer incidence among Hispanics and non-Hispanic whites in the United States. Cancer Causes Control 2018;29:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muller C, Ihionkhan E, Stoffel EM, et al. Disparities in early-onset colorectal cancer. Cells 2021;10:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montminy EM, Zhou M, Maniscalco L, et al. Trends in the incidence of early-onset colorectal adenocarcinoma among black and white US residents aged 40 to 49 years, 2000–2017. JAMA Netw open 2021;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breau G, Ellis U. Risk factors associated with young-onset colorectal adenomas and cancer: a systematic review and meta-analysis of observational research. Cancer Control 2020;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joh H-K, Lee DH, Hur J, et al. Simple sugar and sugar-sweetened beverage intake during adolescence and risk of colorectal cancer precursors. Gastroenterology 2021;161:128–142.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Sullivan DE, Sutherland RL, Town S, et al. Risk factors for early-onset colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022;20:1229–1240.e5. [DOI] [PubMed] [Google Scholar]
- 74.Lei X, Song S, Li X, et al. Excessive body fat at a young age increases the risk of colorectal cancer: a systematic review and meta-analysis. Nutr Cancer, 2020. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Boakye D, Chen X, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology 2022;162:1088–1097.e3. [DOI] [PubMed] [Google Scholar]
- 76.Khan NA, Hussain M, Rahman AU, et al. Dietary practices, addictive behavior and bowel habits and risk of early onset colorectal cancer: a case control study. Asian Pacific J Cancer Prev 2015;16:7967–7973. [DOI] [PubMed] [Google Scholar]
- 77.Kim JY, Jung YS, Park JH, et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol 2016;22:3611–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20–39 years old). Clin Gastroenterol Hepatol 2019;17:115–122. [DOI] [PubMed] [Google Scholar]
- 79.Murphy CC, Cirillo PM, Krigbaum NY, et al. In utero exposure to 17a-hydroxyprogesterone caproate and risk of cancer in offspring. Am J Obstet Gynecol 2022;226:132.e1–132.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng X, Hur J, Nguyen LH, et al. Comprehensive assessment of diet quality and risk of precursors of early-onset colorectal cancer. J Natl Cancer Inst 2021;113:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puzzono M, Mannucci A, Grannò S, et al. The role of diet and lifestyle in early-onset colorectal cancer: a systematic review. Cancers 2021;13:5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gausma V, Liang PS, O’Connell K, et al. Evaluation of early-life factors and early-onset colorectal cancer among men and women in the UK Biobank. Gastroenterology 2022;162:981–983.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Puzzono M, Mannucci A, Leo M Di, et al. Diet and lifestyle habits in early-onset colorectal cancer. a pilot case-control study. Dig Dis 2022;40:710–718. [DOI] [PubMed] [Google Scholar]
- 84.McDowell R, Perrott S, Murchie P, et al. Oral antibiotic use and early-onset colorectal cancer: findings from a case-control study using a national clinical database. Br J Cancer 2022;126:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen LH, Liu P-H, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr 2018;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hur J, Otegbeye E, Joh HK, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021;70:2330–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim H, Lipsyc-Sharf M, Zong X, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 2021;161:1208–1217.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu J, Li Y, Yu J, et al. A risk scoring system to predict the individual incidence of early-onset colorectal cancer. BMC Cancer 2022;22:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Archambaut AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst 2022;114:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kolb J, Hu J, DeSanto K, et al. Early-age onset colorectal neoplasia in average-risk individuals undergoing screening colonoscopy: a systematic review and meta-analysis. Gastroenterology 2021;161:1145–1155.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enweren, Cho MY, Demb J, et al. Systematic review of prevalence, risk factors, and risk for metachronous advanced neoplasia in patients with young-onset colorectal adenoma. Clin Gastroenterol Hepatol 2021;19:680–689.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 2019;157:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen C, Stock C, Hoffmeister M, et al. Optimal age for screening colonoscopy: a modeling study. Gastrointest Endosc 2019;89:1017–1025.e12. [DOI] [PubMed] [Google Scholar]
- 94.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services Task Force. JAMA 2016;315:2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trivedi PD, Mohapatra A, Morris MK, et al. Prevalence and predictors of young-onset colorectal neoplasia: insights from a nationally representative colonoscopy registry. Gastroenterology 2022;162:1136–1146.e5. [DOI] [PubMed] [Google Scholar]
- 96.Reif de Paula T, Haas EM, Keller DS. Colorectal cancer in the 45-to-50 age group in the United States: a National Cancer Database (NCDB) analysis. Surg Endosc 2022;36:6629–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen J, Wu Y, Mo M, et al. Risk factors associated with early-onset colorectal neoplasm in Chinese youth: a prospective population-based study. Front Oncol 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002;346:1781–1785. [DOI] [PubMed] [Google Scholar]
- 99.Yip B, Holub J, Lieberman D. Increasing prevalence of polyps > 9 mm in young adults aged 40 to 59 years undergoing colonoscopy from 2002 to 2014. Gastroenterology 2021;160:1863–1865.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yen T, Scolaro J, Montminy E, et al. Spectrum of advanced colorectal neoplasia and anticipated yield of average-risk screening in veterans under age 50. Clin Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 101.Levin TR, Jensen CD, Chawla NM, et al. Early screening of African Americans (45–50 years old) in a fecal immunochemical test–based colorectal cancer screening program. Gastroenterology 2020;159:1695–1704.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen FW, Yang L, Cusumano VT, et al. Early-onset colorectal cancer is associated with a lower risk of metachronous advanced neoplasia than traditional-onset colorectal cancer. Dig Dis Sci 2022;67:1045–1053. [DOI] [PubMed] [Google Scholar]
- 103.Yeo H, Betel D, Abelson JS, et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer 2017;16:293–299.e6. [DOI] [PubMed] [Google Scholar]
- 104.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 2016;122:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cercek A, Chatila WK, Yaeger R, et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J Natl Cancer Inst 2021;113:1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2016;2:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malik SS, Lythgoe MP, McPhail M, et al. Metachronous colorectal cancer following segmental or extended colectomy in Lynch syndrome: a systematic review and meta-analysis. Fam Cancer 2018;17:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duraes L, et al. Role of genetic testing in surgical decision making for patients with HNPCC. Fam Cancer 2017;16:S101–S102. [Google Scholar]
- 110.LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 2020; 22:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang T, Wang F, Wang Y, et al. Germline mutational profile of Chinese patients under 70 years old with colorectal cancer. Cancer Commun 2020;40:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhunussova G, Afonin G, Abdikerim S, et al. Mutation spectrum of cancer-associated genes in patients with early onset of colorectal cancer. Front Oncol 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.You YN, Borras E, Chang K, et al. Detection of pathogenic germline variants among patients with advanced colorectal cancer undergoing tumor genomic profiling for precision medicine. Dis Colon Rectum 2019;62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mork ME, Rodriguez A, Bannon SA, et al. Outcomes of diseasespecific next-generation sequencing gene panel testing in adolescents and young adults with colorectal cancer. Cancer Genet 2019;235–236:77–83. [DOI] [PubMed] [Google Scholar]
- 115.AlDubayan SH, Giannakis M, Moore ND, et al. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet 2018; 102:401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology 2018;154:897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017;3:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeRycke MS, Gunawardena S, Balcom JR, et al. Targeted sequencing of 36 known or putative colorectal cancer susceptibility genes. Mol Genet Genomic Med 2017;5:553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chubb D, Broderick P, Dobbins SE, et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat Commun 2016;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mork ME, You YN, Ying J, et al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J Clin Oncol 2015;33:3544–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Toh MR, Chiang JB, Chong ST, et al. Germline pathogenic variants in homologous recombination and DNA repair genes in an Asian cohort of young-onset colorectal cancer. JNCI Cancer Spectr 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol 2017;35:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang PY, Chang SC, Wang MC, et al. Pathogenic germline mutations of DNA repair pathway components in early-onset sporadic colorectal polyp and cancer patients. Cancers (Basel) 2020;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jansen AML, Ghosh P, Dakal TC, et al. Novel candidates in early-onset familial colorectal cancer. Fam Cancer 2020;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uson PLS, Riegert-Johnson D, Boardman L, et al. Germline cancer susceptibility gene testing in unselected patients with colorectal adenocarcinoma: a multicenter prospective study. Clin Gastroenterol Hepatol 2022;20:e508–e528. [DOI] [PubMed] [Google Scholar]
- 126.Fernández-Rozadilla C, Álvarez-Barona M, Quintana I, et al. Exome sequencing of early-onset patients supports genetic heterogeneity in colorectal cancer. Sci Rep 2021;11:11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Middeldorp A, Jagmohan-Changur S, Eijk R Van, et al. Enrichment of low penetrance susceptibility loci in a Dutch familial colorectal cancer cohort. Cancer Epidemiol Biomarkers Prev 2009;18:3062–3067. [DOI] [PubMed] [Google Scholar]
- 128.Song N, Shin A, Park JW, et al. Common risk variants for colorectal cancer: an evaluation of associations with age at cancer onset. Sci Rep 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giráldez MD, López-Dóriga A, Bujanda L, et al. Susceptibility genetic variants associated with early-onset colorectal cancer. Carcinogenesis 2012;33:613–619. [DOI] [PubMed] [Google Scholar]
- 130.Holst S Von, Picelli S, Edler D, et al. Association studies on 11 published colorectal cancer risk loci. Br J Cancer 2010;103:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He J, Wilkens LR, Strain DO, et al. Generalizability and epidemiologic characterization of eleven colorectal cancer GWAS hits in multiple populations. Cancer Epidemiol Biomarkers Prev 2011;20:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet 2019;51:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Archambault AN, Su YR, Jeon J, et al. Cumulative burden of colorectal cancer–associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology 2020;158:1274–1286.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Northcutt MJ, Shi Z, Zijlstra M, et al. Polygenic risk score is a predictor of adenomatous polyps at screening colonoscopy. BMC Gastroenterol 2021;21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas M, Sakoda LC, Hoffmeister M, et al. Genome-wide modeling of polygenic risk score in colorectal cancer risk. Am J Hum Genet 2020;107:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X, Timofeeva M, Spiliopoulou A, et al. Prediction of colorectal cancer risk based on profiling with common genetic variants. Int J Cancer 2020;147:3431–3437. [DOI] [PubMed] [Google Scholar]
- 137.Jia G, Lu Y, Wen W, et al. Evaluating the utility of polygenic risk scores in identifying high-risk individuals for eight common cancers. JNCI Cancer Spectr 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo F, Weigl K, Carr PR, et al. Use of polygenic risk scores to select screening intervals after negative findings from colonoscopy. Clin Gastroenterol Hepatol 2020;18:2742–2751.e7. [DOI] [PubMed] [Google Scholar]
- 139.Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology 2020;159:129–138.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saunders CL, Kilian B, Thompson DJ, et al. External validation of risk prediction models incorporating common genetic variants for incident colorectal cancer using UK Biobank. Cancer Prev Res 2020;13:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fahed AC, Wang M, Homburger JR, et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun 2020;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmit SL, Edlund CK, Schumacher FR, et al. Novel common genetic susceptibility loci for colorectal cancer. J Natl Cancer Inst 2019;111:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jin Z, Dixon JG, Fiskum JM, et al. Clinicopathological and molecular characteristics of early-onset stage III colon adenocarcinoma: an analysis of the ACCENT database. J Natl Cancer Inst 2021;113:1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Remo A, Fassan M, Lanza G. Immunohistochemical evaluation of mismatch repair proteins in colorectal carcinoma: the AIFEG/GIPAD proposal. Pathologica 2016;108:104–109. [PubMed] [Google Scholar]
- 145.Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020;69:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seppälä TT, Latchford A, Negoi I, et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg 2021;108:484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2021;19:329–359. [DOI] [PubMed] [Google Scholar]
- 148.Møller P, Seppälä T, Bernstein I, et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 2017;66:1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path-MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mannucci A, Sloane Furniss C, Ukaegbu CI, et al. Comparison of colorectal and endometrial microsatellite instability tumor analysis and Premm 5 risk assessment for predicting pathogenic germline variants on multigene panel testing. J Clin Oncol 2020; 38:4086–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg 2003;90:205–214. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y, Chen Z, Huang J, et al. Clinicopathological and molecular characteristics of early-onset vs late-onset colorectal cancer according to tumor location. Int J Clin Oncol 2022. [DOI] [PubMed] [Google Scholar]
- 153.Narayan RR, Aveson VG, Chou JF, et al. Association of genomic profiles and survival in early onset and screening-age colorectal cancer patients with liver metastases resected over 15 years. J Surg Oncol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jácome AA, Vreeland TJ, Johnson B, et al. The prognostic impact of RAS on overall survival following liver resection in early versus late-onset colorectal cancer patients. Mol Diagnostics Br J Cancer 2021;124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg 2017;152:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang C-T, Guo Z-X, Wang P, et al. Higher LNM rate and poorer prognosis of early-onset compared to late-onset T1 stage colorectal cancer: a large-population based study. Am J Cancer Res 2021;11:3176–3188. [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, Lu H, Yang H, et al. Impact of age on risk of lymph node positivity in patients with colon cancer. J Cancer 2019; 10:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lieu CH, Renfro LA, Gramont A De, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD clinical trials program. J Clin Oncol 2014;32:2975–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Foppa C, Tamburello S, Maroli A, et al. Early age of onset is an independent predictor for worse disease-free survival in sporadic rectal cancer patients: a comparative analysis of 980 consecutive patients. Eur J Surg Oncol 2022;48:857–863. [DOI] [PubMed] [Google Scholar]
- 160.McClelland PH, Liu T, Ozuner G. Early-onset colorectal cancer in patients under 50 years of age: demographics, disease characteristics, and survival. Clin Colorectal Cancer 2021. [DOI] [PubMed] [Google Scholar]
- 161.Chen JN, Zhang QW, Pan YB, et al. Young-onset early colorectal cancer had similar relative survival to but better overall survival than conventional early colorectal cancer: a large population-based study. Front Oncol 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Georgiou A, Khakoo S, Edwards P, et al. Outcomes of patients with early onset colorectal cancer treated in a UK specialist cancer center. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y, Yan L, Wu Y, et al. Worse treatment response to neoadjuvant chemoradiotherapy in young patients with locally advanced rectal cancer. BMC Cancer 2020;20:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Y, Wang Y, Liu X, et al. Worse prognosis in young patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy: a comparative study. Medicine (Baltimore) 2020;99:e21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blanke CD, Bot BM, Thomas DM, et al. Impact of young age on treatment efficacy and safety in advanced colorectal cancer: a pooled analysis of patients from nine first-line phase III chemotherapy trials. J Clin Oncol 2011;29:2781–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang L, Hirano Y, Heng G, et al. Better cancer-specific survival in younger patients with stage III colorectal cancer: a propensity score matching study from Japan. Anticancer Res 2020;40:4365–4372. [DOI] [PubMed] [Google Scholar]
- 167.Zaborowski AM, Murphy B, Creavin B, et al. Clinicopathological features and oncological outcomes of patients with youngonset rectal cancer. Br J Surg 2020;107:606–612. [DOI] [PubMed] [Google Scholar]
- 168.Xie X, Yin J, Zhou Z, et al. Young age increases the risk for lymph node metastasis in patients with early colon cancer. BMC Cancer 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fontana E, Meyers J, Sobrero A, et al. Early-onset colorectal adenocarcinoma in the IDEA database: treatment adherence, toxicities, and outcomes with 3 and 6 months of adjuvant fluoropyrimidine and oxaliplatin. J Clin Oncol 2021;39:4009–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 2015;150:402–409. [DOI] [PubMed] [Google Scholar]
- 171.Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in young-onset metastatic colorectal cancer: findings from Cancer and Leukemia Group B (Alliance)/SWOG 80405. J Natl Cancer Inst 2022;114:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hofheinz RD, Arnold D, Fokas E, et al. Impact of age on the efficacy of oxaliplatin in the preoperative chemoradiotherapy and adjuvant chemotherapy of rectal cancer: a post hoc analysis of the CAO/ARO/AIO-04 phase III trial. Ann Oncol 2018;29:1793–1799. [DOI] [PubMed] [Google Scholar]
- 173.Bahadoer RR, Dijkstra EA, Etten B van, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 174.Fokas E, Schlenska-Lange A, Polat B, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol 2022;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 2022;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–715. [DOI] [PubMed] [Google Scholar]
- 177.Cutsem E Van, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 178.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018 clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2018;16:874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44–70. [DOI] [PubMed] [Google Scholar]
- 180.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 6.2020: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 2020;18:807–815. [Google Scholar]
- 181.Calderillo G, Herrera M, Lopez H, et al. Impact of age on efficacy of neoadjuvant chemoradiotherapy (CRT) in locally advanced rectal cancer (LARC). Ann Oncol 2016;27:ii38. [Google Scholar]
- 182.You YN, Dozois EJ, Boardman LA, et al. Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Ann Surg Oncol 2011;18:2469–2476. [DOI] [PubMed] [Google Scholar]
- 183.Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy 2017;49:378–397. [DOI] [PubMed] [Google Scholar]
- 184.Dekker E, Houwen BBSL, Puig I, et al. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2020; 52:899–923. [DOI] [PubMed] [Google Scholar]
- 185.Bisschops R, East JE, Hassan C, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guide-line—update 2019. Endoscopy 2019;51:1155–1179. [DOI] [PubMed] [Google Scholar]
- 186.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015;47:829–854. [DOI] [PubMed] [Google Scholar]
- 187.Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2017;49:270–297. [DOI] [PubMed] [Google Scholar]
- 188.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795–1803. [DOI] [PubMed] [Google Scholar]
- 190.Shaukat A, Rex DK, Shyne M, et al. Adenoma detection rates for 45–49 year old screening population. Gastroenterology 2022;162:957–959.e1. [DOI] [PubMed] [Google Scholar]
- 191.Krause C, Kruis W. Synchronous pathologic findings in patients with colorectal cancer and preoperative incomplete colonoscopy. Int J Colorectal Dis 2019;34:1407–1412. [DOI] [PubMed] [Google Scholar]
- 192.Isler JT, Brown PC, Lewis GF, et al. The role of preoperative colonoscopy in colorectal cancer. Dis Colon Rectum 1987;30:435–439. [DOI] [PubMed] [Google Scholar]
- 193.Ito S, Hotta K, Imai K, et al. Ultrathin colonoscopy can improve complete preoperative colonoscopy for stenotic colorectal cancer: prospective observational study. Dig Endosc 2021;33:621–628. [DOI] [PubMed] [Google Scholar]
- 194.Healy M, Bednarski BK, Eng C, et al. Higher propensity for nodal metastases among young-onset rectal cancers. Dis Colon Rectum 2019;62:E322–E322. [Google Scholar]
- 195.Alawadi Z, Phatak UR, Hu CY, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 2017;123:1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Taylor FGM, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY Study. J Clin Oncol 2014;32:34–43. [DOI] [PubMed] [Google Scholar]
- 197.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2006;130:1865–1871. [DOI] [PubMed] [Google Scholar]
- 198.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2016;150:758–768.e11. [DOI] [PubMed] [Google Scholar]
- 199.Pita-Fernández S, Alhayek-Aí M, González-Martín C, et al. Intensive follow-up strategies improve outcomes in non-metastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015;26:644–656. [DOI] [PubMed] [Google Scholar]
- 200.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013;100:75–82. [DOI] [PubMed] [Google Scholar]
- 201.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006;56:160–167. [DOI] [PubMed] [Google Scholar]
- 202.Anon AGA. institute guidelines for colonoscopy surveillance after cancer resection: clinical decision tool. Gastroenterology 2014;146:1413–1414. [DOI] [PubMed] [Google Scholar]
- 203.Cao B, Min L, Zhu S, et al. Long-term oncological outcomes of local excision versus radical resection for early colorectal cancer in young patients without preoperative chemoradiotherapy: a population-based propensity matching study. Cancer Med 2018;7:2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc 2011;74:347–354. [DOI] [PubMed] [Google Scholar]
- 205.Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30:1299–1311. [DOI] [PubMed] [Google Scholar]
- 206.Kim SB, Lee HJ, Park SJ, et al. Comparison of colonoscopy surveillance outcomes between young and older colorectal cancer patients. J Cancer Prev 2017;22:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol 2019;25:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oh EH, Kim N, Hwang SW, et al. Comparison of long-term recurrence-free survival between primary surgery and endoscopic resection followed by secondary surgery in T1 colorectal cancer. Gastrointest Endosc 2021;94:394–404. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Q, Sun L, Tang C, et al. Inverse association of age with risk of lymph node metastasis in superficial colorectal cancer: a large population-based study. Oncologist 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dang H, Dekkers N, Cessie S le, et al. Risk and time pattern of recurrences after local endoscopic resection of T1 colorectal cancer: a meta-analysis. Clin Gastroenterol Hepatol 2022;20:e298–e314. [DOI] [PubMed] [Google Scholar]
- 211.Yeh JH, Tseng CH, Huang RY, et al. Long-term outcomes of primary endoscopic resection vs surgery for T1 colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:2813–2823.e5. [DOI] [PubMed] [Google Scholar]
- 212.Anderson JC, Robinson CM, Butterly LF. Young adults and metachronous neoplasia: risks for future advanced adenomas and large serrated polyps compared with older adults. Gastrointest Endosc 2020;91:669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Klos CL, Montenegro G, Jamal N, et al. Segmental versus extended resection for sporadic colorectal cancer in young patients. J Surg Oncol 2014;110:328–332. [DOI] [PubMed] [Google Scholar]
- 214.Renkonen-Sinisalo L, Seppälä TT, Järvinen HJ, et al. Subtotal colectomy for colon cancer reduces the need for subsequent surgery in Lynch syndrome. Dis Colon Rectum 2017;60:792–799. [DOI] [PubMed] [Google Scholar]
- 215.Heneghan HM, Martin ST, Winter DC. Segmental vs extended colectomy in the management of hereditary nonpolyposis colorectal cancer: a systematic review and meta-analysis. Color Dis 2015;17:382–389. [DOI] [PubMed] [Google Scholar]
- 216.Anele CC, Adegbola SO, Askari A, et al. Risk of metachronous colorectal cancer following colectomy in Lynch syndrome: a systematic review and meta-analysis. Color Dis 2017;19:528–536. [DOI] [PubMed] [Google Scholar]
- 217.Dominguez-Valentin M, Sampson JR, Seppälä TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med 2020;22:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Seppälä TT, Dominguez-Valentin M, Crosbie EJ, et al. Uptake of hysterectomy and bilateral salpingo-oophorectomy in carriers of pathogenic mismatch repair variants: a Prospective Lynch Syndrome Database report. Eur J Cancer 2021;148:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Crosbie EJ, Ryan NAJ, Arends MJ, et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med 2019;21:2390–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schmeler KM, Lynch HT, Chen L, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261–269. [DOI] [PubMed] [Google Scholar]
- 221.Stal J, Yi S, Cohen-Cutler S, et al. Fertility preservation discussions between young adult rectal cancer survivors and their providers: sex-specific prevalence and correlates. Oncologist 2022;27:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Selter J, Huang Y, Grossman Becht LC, et al. Use of fertility preservation services in female reproductive-aged cancer patients. Am J Obstet Gynecol 2019;221:328.e1–328.e16. [DOI] [PubMed] [Google Scholar]
- 223.Rogers JE, Woodard TL, Dasari A, et al. Fertility discussions in young adult stage III colorectal cancer population: a single-center institution experience. Support Care Cancer 2021;29:7351–7354. [DOI] [PubMed] [Google Scholar]
- 224.Holowatyj AN, Eng C, Lewis MA. Incorporating reproductive health in the clinical management of early-onset colorectal cancer. JCO Oncol Pract 2022;18:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Futur Oncol 2016;12:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cercek A, Siegel CL, Capanu M, et al. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer 2013;12:163–167. [DOI] [PubMed] [Google Scholar]
- 227.Stupart D, Win AK, Winship IM, et al. Fertility after young-onset colorectal cancer: a study of subjects with Lynch syndrome. Colorectal Dis 2015;17:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Overbeek A, Berg MH van den, Leeuwen FE van, et al. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: a systematic review. Cancer Treat Rev 2017;53:10–24. [DOI] [PubMed] [Google Scholar]
- 229.Anon. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril 2018;110:380–386. [DOI] [PubMed] [Google Scholar]
- 230.Levi M, Shalgi R, Brenner B, et al. The impact of oxaliplatin on the gonads: from bedside to the bench. Mol Hum Reprod 2015;21:885–893. [DOI] [PubMed] [Google Scholar]
- 231.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol 2018;29:iv166–iv191. [DOI] [PubMed] [Google Scholar]
- 232.Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol 2020;31. [DOI] [PubMed] [Google Scholar]
- 233.Fontana E, Meyers JP, Sobrero AF, et al. Early-onset colorectal adenocarcinoma in the IDEA database: treatment adherence, toxicities, and outcomes with 3 and 6 months of adjuvant fluoropyrimidine and oxaliplatin. J Clin Oncol 2021;39:4009–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol 2020;38:2782–2797. [DOI] [PubMed] [Google Scholar]
- 235.Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–v133. [DOI] [PubMed] [Google Scholar]
- 236.Larkin PJ, Cherny NI, Carpia D La, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO clinical practice guidelines. Ann Oncol 2018;29:iv111–iv125. [DOI] [PubMed] [Google Scholar]
- 237.Bossi P, Antonuzzo A, Cherny NI, et al. Diarrhoea in adult cancer patients: ESMO clinical practice guidelines. Ann Oncol 2018;29:iv126–iv142. [DOI] [PubMed] [Google Scholar]
- 238.Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 239.Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines. ESMO Open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Munsie C, Ebert J, Joske D, et al. The benefit of physical activity in adolescent and young adult cancer patients during and after treatment: a systematic review. J Adolesc Young Adult Oncol 2019;8:512–524. [DOI] [PubMed] [Google Scholar]
- 241.Carter J, Lacchetti C, Andersen BL, et al. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology clinical practice guideline adaptation of Cancer Care Ontario guideline. J Clin Oncol 2018;36:492–511. [DOI] [PubMed] [Google Scholar]
- 242.Averyt JC, Nishimoto PW. Addressing sexual dysfunction in colorectal cancer survivorship care. J Gastrointest Oncol 2014;5:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rogers JE, Johnson B. The reality of early-onset colorectal cancer: highlighting the needs in a unique but emerging population. Dig Med Res 2021;4:63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sansom-Daly UM, Wakefield CE, Patterson P, et al. End-of-life communication needs for adolescents and young adults with cancer: recommendations for research and practice. J Adolesc Young Adult Oncol 2020;9:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rogers JE, Woodard TL, Gonzalez GMN, et al. Colorectal cancer during pregnancy or postpartum: case series and literature review. Obstet Med 2022;15:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Barr RD, Ferrari A, Ries L, et al. Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr 2016;170:495–501. [DOI] [PubMed] [Google Scholar]
- 247.Perl G, Nordheimer S, Lando S, et al. Young patients and gastrointestinal (GI) tract malignancies: are we addressing the unmet needs? BMC Cancer 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


