Abstract
Metastasis remains the major driver of mortality in patients with cancer. The multistep metastatic process requires the concerted actions of several genes and involves tumor cell invasion, epithelial mesenchymal transition (EMT), shedding from primary tumor, intravasation, arrest, extravasation and colonization at a preferential site. Understanding this complex process would provide the basis for the development of molecularly targeted therapeutics aimed at the tumor cell or its interaction with the host microenvironment. The neuropeptide hormones endothelins (specially, ET-1) have been correlated with invasiveness and metastasis of several cancers and high ET-1 levels are associated with decreased disease-specific survival. The mechanism(s) by which ET-1 promotes metastasis are being gradually unraveled. Through preferential binding to cognate receptors (ETAR or ETBR), ET-1 triggers autocrine and paracrine signaling cascades in tumor, immune and stromal cells, at both primary and distant sites, supporting cancer progression and metastasis. In this review, we will summarize the role of the ET axis in metastasis of different cancers and potential targeting of ET receptors in the therapeutic setting.
Keywords: Endothelin, Metastasis, Inflammation
Introduction
ET-1, an endothelial cell-derived vasoconstrictor peptide, is an important member of the endothelin axis with myriad developmental, physiological and pathological functions (Kedzierski and Yanagisawa, 2001; Herrmann et al., 2009, 2007; Hagemann et al., 2007). The “endothelin axis” consists of three similar small peptides, ET-1, ET-2 and ET-3, two G-protein-coupled receptors, ETAR and ETBR, and two membrane-bound proteases, the ET-converting enzymes, ECE-1 and ECE-2 (Kedzierski and Yanagisawa, 2001), that activate the secreted pro forms of the peptide. ET-1 production is stimulated by a variety of cytokines and growth factors, hypoxia, and shear stress, while ETAR activation triggers signaling networks involved in cell proliferation, new vessel formation, invasion, inflammation and metastatic spread (Kedzierski and Yanagisawa, 2001; Bagnato et al., 2005; Giaid et al., 1990; Rosano et al., 2003, 2007a). ET-1 has been shown to activate the pro-inflammatory transcriptional factors AP-1 and NFκB in human monocytes and cancer cells and to stimulate the production of inflammatory cytokines IL-6, CCL2/MCP-1 and COX2, as well as matrix metalloproteinases (MMPs) activity, the key orchestrators of inflammation-mediated cancer cell invasiveness and metastasis (Kandalaft et al., 2009; Sutcliffe et al., 2009; Spinella et al., 2004a, 2004b). Moreover, elevated expression of ECE-1, ET-1 and its receptors have been detected in a variety of malignancies including prostate, ovarian, breast, melanoma, HNSCC, colorectal and bladder cancers (summarized in Bagnato et al. (2011)). In addition to their direct contribution to tumor growth and metastasis, members of the endothelin axis indirectly modulate tumor–host interactions in various milieus’ furthering tumor progression and metastasis. For example, ET-1 promotes autocrine/paracrine interactions between fibroblasts and cancer cells in prostate and HNSCC cells (Dawson et al., 2004) and modulates trafficking, differentiation, and activation of tumor-associated immune cells, possibly contributing to immune evasion and resistance to immunotherapy (Kandalaft et al., 2009; Grimshaw et al., 2002; Buckanovich et al., 2008; Said et al., 2011). ET-1 can induce expression of IL-6, CCL-2, as well as MMP and COX-2 activity, key orchestrators of inflammation-mediated cancer cell invasiveness and metastasis via AP-1 and NF-κB (Rosano et al., 2007a, 2001, 2007b; Sutcliffe et al., 2009; Grimshaw et al., 2002, 2004; Said et al., 2011; Browatzki et al., 2007; Spinella et al., 2007). Recently, we reported that tumor ET-1 triggers inflammation in the lung soon after the cancer cells lodged at this site and thus sets up a vicious cycle wherein inflammatory cells would enhance and facilitate the process of metastatic colonization (Said et al., 2011) (Fig. 1).
Fig. 1.
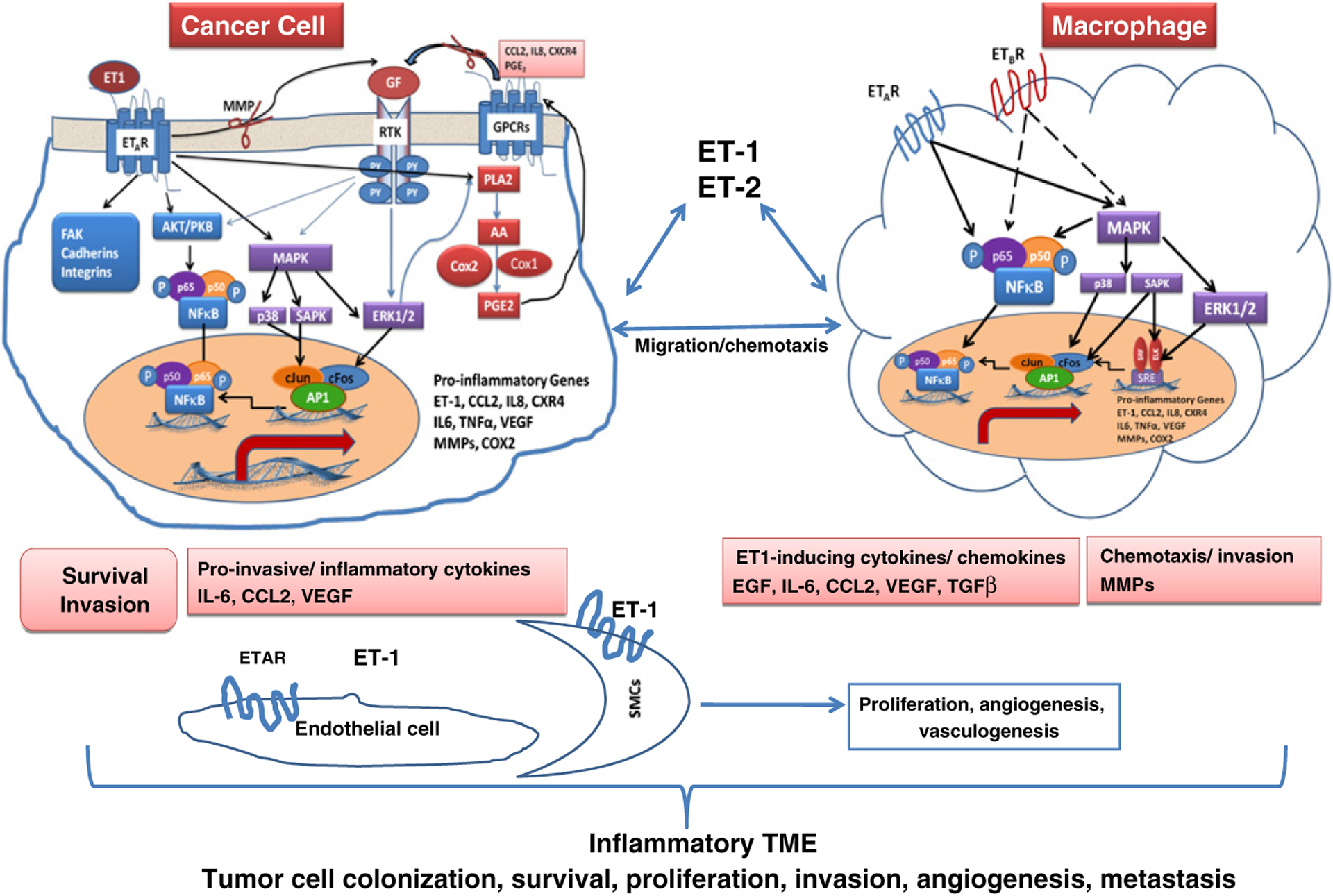
- In tumor cells: Binding of ET-1 to the ETAR triggers multiple signal-transduction pathways, leading to cell survival and invasion. ET-1 increases its own secretion as well as the secretion of cytokines and growth factors IL-6, CCL2 (MCP-1), and VEGF as well as inflammatory mediators COX2 and prostaglandins-E2 (PGE-2).
- In macrophages: ET-1 induces macrophage chemotaxis through ETAR and ETBR. Binding of ET-1 to both receptors in macrophages triggers signaling pathways converging in NFκB and AP-1 activation with subsequent induction of IL-6, CCL2, VEGF, EGF, TGFβ, MMPs and COX2, subsequently, stimulating tumor cell invasiveness, recruitment and colonization in preferential metastatic sites.
- In endothelial cells and vascular smooth muscle cells: ET-1 stimulates endothelial and VSMCs proliferation, angiogenesis and vasculogenesis through interaction with ETAR receptor.
Ovarian cancer
Ovarian cancer arises from the surface of the ovary. Hence cancer cells shed from the primary tumor can disseminate as floating single cells or spheroids in the peritoneal fluid and lead to implants on the mesothelial lining of the peritoneal cavity or deeply penetrate the omentum. In the ovarian cancer microenvironment, high ET-1 levels were detected in the peritoneal fluid in patients with malignant ascites (Bagnato et al., 2005). In vivo analysis of the ET-1 axis demonstrated a higher expression of ET-1 and ETAR in primary and metastatic tumors than in normal ovarian tissues. Interestingly, ET-1-producing cells also expressed functional ETAR, but not ETBR, indicating that in ovarian tumor cells, ET-1 likely acts as an autocrine factor selectively through the ETAR (Bagnato et al., 2005). In human ovarian tumors, ET-1 axis overexpression is associated with ascites formation, malignant progression, advanced tumor stage, and enhanced tumor angiogenesis (Bagnato et al., 1999, 2005). Here the ET-1/ETAR axis drives multiple signaling networks involving epidermal growth factor receptors, integrins, Wnt, and MMPs (Bagnato and Rosano, 2007) that regulate cell survival, invasiveness, angiogenesis, vascular permeability and EMT (Rosano et al., 2010). ET-1 axis has been shown to be regulated by and induce activation of hypoxia-inducible factor 1α (HIF-1α) and EMT transcription factors as β-catenin/TCF, SNAIL and SLUG, suggesting that the ET-1 axis contributes to the complex cooperation between the intracellular signaling pathways and extracellular signals triggering EMT (Bagnato et al., 2005; Spinella et al., 2004a, 2004b, 2007; Rosano et al., 2001, 2007b, 2010; Bagnato and Rosano, 2007). Genome-wide expression profiling of ovarian carcinoma identified ETAR as a key gene related to chemoresistance (Rosano et al., 2010). Interestingly, paclitaxel and platinum resistant ovarian cancer cells showed phenotypic changes consistent with EMT, providing strong evidence linking ETAR signaling pathways to chemoresistance, EMT and stemness of ovarian cancer cells (Rosano et al., 2010).
In ovarian cancer, ETBR has been recently shown to control T-cell homing to tumors and failure of immunotherapy as vaccine failure was associated with poor accumulation of T cells at the tumor site, in spite of detectable systemic antitumor immune response. ETBR blockade with specific antagonist BQ-788 greatly enhanced the efficacy of vaccines in both preventing recurrence and therapy directed at established tumors. BQ-788 did not increase systemic immune response to the vaccine in vivo, but rather greatly enhanced T-cell infiltration in tumors following vaccine (Kandalaft et al., 2009). Activation of a paracrine ET-1/ETBR axis was also found between tumor cells and endothelial cells, whereby tumor cells overexpress and release ET-1 whereas the latter express ETBR. This axis suppresses T-cell homing (even in the presence of tumor inflammation), and can be disrupted by ETBR blockade, which in vivo markedly enhances tumor immune therapy (Kandalaft et al., 2009). ETBR blockade is likely to have also direct antiangiogenic effects through suppression of endothelial nitric oxide. Interestingly, ETBR upregulation predicts poor outcome in ovarian cancer (Buckanovich et al., 2008).
Bladder cancer
Gene expression data from several independent studies of human bladder cancer have revealed that ET1 and ETAR were overexpressed in muscle invasive disease and that the degree of expression is associated with reduced patient survival (Said et al., 2011). This was confirmed at the protein level by ET-1 immunohistochemistry in human bladder cancer tissue microarrays (Said et al., 2011). Interestingly, ET-1 expression was positively correlated with the proinvasive and proinflammatory mediators IL-6, CCL2, COX2 as well as MMP2 and MMP9. However, an apparent paradox that has arisen in the field regarding the role of ETAR in cancer is the finding that pharmacologic blockade of this receptor has minimal effect on primary tumor growth (Titus et al., 2005; Wulfing et al., 2005a, 2005b) yet affects experimental metastasis (Said et al., 2011; Titus et al., 2005) and that clinical trials with ETAR inhibitors in patients with advanced cancers appear to have minimal effect (Bagnato et al., 2011; Nelson et al., 2008; Ohlmann et al., 2011; Clezardin, 2011; Nelson et al., 2011).
To decipher the role of ET-1/ETAR axis in bladder cancer metastasis we used a comprehensive model whereby the endothelin axis, via ETAR, was shown to drive bladder cancer lung colonization by regulating key factors in the microenvironment of disseminated tumor cells (Said et al., 2011). Using combined genetic and pharmacologic approaches in human bladder cancer cell lines in vitro, we reported the role of ET-1 in autocrine regulation of proliferation in bladder cancer cell lines and that ET-1 stimulates migration, invasion, and proteolytic activity of such cells through ETAR. In vivo, using human and murine models of experimental and spontaneous lung metastasis, we showed that tumor ET-1 is a paracrine mediator of early metastatic colonization of the lung through triggering an early inflammatory response in the lung characterized by macrophage influx, and production of pro-inflammatory mediators MCP-1/CCL2, IL-6, and COX-2. Interestingly, the IL-6, MCP-1/CCL2, COX-2, and MMPs induced by ET-1 are also known to breach the lung vasculature and enable extravasation of cancer cells on dissemination of these cells to the lungs (Qian et al., 2009; Gupta and Massague, 2006; Gupta et al., 2007; Mantovani, 2009). This ET-1 or ETAR mediated early inflammatory response led to subsequent development of macroscopic lung metastases. Interestingly, pharmacologic inhibition of ETAR by ZD4054 prior to injection of tumor cells reduced the early inflammatory response and subsequent development of lung metastases. In contrast, when administration of ZD4054 was initiated after establishment of the early inflammatory response, the reduction in lung inflammation and clinical lung metastases was less dramatic. We also showed that the cellular effectors of this ET-1/ETAR axis are likely macrophages since metastasis was suppressed by macrophage depletion by liposomal clodronate nanoparticles. Given this data we speculate that in clinical trials where pharmacologic inhibition of ETAR did not affect primary tumor growth or established high-volume metastatic disease, these tumors were no longer dependent on macrophages for their maintenance or growth.
Breast cancer
Elevated expression of ETAR in primary breast carcinoma is associated with reduced disease-free survival time (Wulfing et al., 2003). ETAR-positive carcinomas frequently show staining for ETAR in fibroblasts suggesting the existence of a paracrine axis since breast cancer cells express ET-1 (Wulfing et al., 2003; Kojima and Nihei, 1995). High ET-1/ETAR-positive tumors were associated with clinicopathological markers of aggressiveness and poor prognosis such as tumor size, poor differentiation, high grade, Her-2/neu overexpression, lymphovascular invasion, inflammation as well as with incidence of local recurrence or distant metastasis (Wulfing et al., 2003). The above findings suggested that ET-1 and ETAR may be useful prognostic biomarkers in breast carcinomas and may help to identify patients who may benefit from adjuvant therapy (Wulfing et al., 2003). Mechanistic studies revealed that endothelins (1 and 2) induce breast cancer cell invasiveness through HIF1α, inflammatory cytokines/chemokines, and MMPs (Grimshaw et al., 2004; Grimshaw, 2005; Yamashita et al., 1995; Wilson et al., 2006; Hagemann et al., 2005). ET-1 secretion in breast cancer cells is induced by EGF via EGFR and HER2 and involves MAPK-dependent signaling. In turn, an ET-1/ETAR-dependent regulation of EGFR protein expression and phosphorylation (at Tyr845) was observed and conferred an additional anti-proliferative and anti-invasive effect to ETAR blocker atrasentan, in trastuzumab treated cells (Fischgrabe et al., 2010). ETAR antagonism also has additive antitumor activity in breast cancer cells treated with aromatase inhibitors in vitro and in vivo (Smollich et al., 2010). ETAR blocker, ZD4045 was equipotent to aromatase inhibitors and in combination, exerted an additive effect on in vitro cells and in vivo in tumor xenografts (Smollich et al., 2010).
Recently, ET-1 was found to mediate the invasive properties of triple-negative breast cancer (TNBC) (Ha et al., 2011) as a downstream effector of lactoferrin (Lf) which efficiently downregulates levels of ER-alpha, PR, and HER-2 in a proteasome-dependent manner (Ha et al., 2011) with subsequent loss of responsiveness of breast cancer cells to ER- or HER-2-targeted therapies. Lactoferrin-induced increased invasion of breast cancer cells was mediated via transcriptional stimulation of ET-1 and was effectively blocked by antagonists of the ET-1 receptor. Co-overexpression of lactoferrin and ET-1 in tumors as well as elevated circulating levels was observed in serum from TNBC as compared with samples from ER-, PR-, and HER-2-positive breast tumors. Thus targeting Lf–ET-1 axis in TNBC represents a new promising approach (Ha et al., 2011).
Prostate cancer
ET-1 plasma concentrations are elevated in men with metastatic and hormone refractory prostate cancer compared to those with locally confined disease or healthy control (Nelson et al., 1995). Immunohistochemistry revealed that primary prostate cancers and prostate cancer bone metastases were usually positive for ET-1 expression (Nelson et al., 1995, 1996). Prostate cancers express higher ET-1 and ETAR but less ETBR than normal prostate tissue (Nelson et al., 1996). The in vitro mitogenic effect of exogenous ET-1 on prostate cancer cell lines is modest, but it enhances the effects of a variety of growth factors such as basic fibroblast growth factor b (bFGF), insulin-like growth factor (IGF), and platelet-derived growth factor (PDGF) (Nelson et al., 2003), and in conjunction with VEGF, plays a major role in tumor angiogenesis. ET-1, through ETAR, transactivates EGFR (Konety and Nelson, 2001), a finding that was verified in a recent study by Wang et al. (2011). The ET-1/ETAR-EGFR pathway was shown to activate the PI3K/AKT pathway, which is known to play an important role in prostate cancer progression (Nelson et al., 2003). ET-1 also activates focal adhesion kinase (FAK) and elevates intracellular calcium in several prostate cancer cell lines (Dawson et al., 2004, 2006; Nelson et al., 2003). ET-1 expression is also associated with the transition from androgen-dependent to androgen-independent disease (Nelson et al., 1995, 1996). Increased levels of ET-1 are also produced by upregulation of ECE-1 in tumor associated stromal cells (Dawson et al., 2004, 2006).
Colorectal cancer
Preoperative plasma big ET-1 concentration is a predictor of overall survival in patients with colorectal cancer suggesting its utility in selecting high-risk, lymph node-negative patients for adjuvant therapy (Elahi and Everson, 2004; Arun et al., 2002, 2004; Hoosein et al., 2007; Lloyd et al., 2011). In another study, however, elevated serum ET-1 levels in patients with colorectal cancer did not seem to be of prognostic value for survival (Peeters et al., 2000). Immunohistochemistry and immunoelectron microscopy demonstrated increased ET-1 expression in colorectal cancer including endothelial and stromal cells within and surrounding primary and liver metastases (Shankar et al., 1998; Simpson et al., 2000). Patients with colorectal cancer with and without liver metastases had elevated plasma levels of ET-1 (Elahi and Everson, 2004; Shankar et al., 1998). Portal vein chemotherapy with ETAR antagonist BQ123 in a rodent model (Elahi and Everson, 2004; Asham et al., 2001) produced a significant reduction in micrometastasis specifically when given at the time of, but not after, tumor cell inoculation suggesting the role of ET-1/ETAR in promoting tumor implantation and the initial establishment of micrometastases (Asham et al., 2001). The frequent overexpression of the ET-1 gene in human colon cancers was reported to be a direct consequence of genetic alterations of β-catenin signaling in these tumors as inactivating mutations in the APC gene or activating mutations in β-catenin lead to the formation of β-catenin/TCF4 complex on the ET-1 promoter, which in turn activates transcription of the gene. ET-1 would contribute to β-catenin’s oncogenic program by providing antiapoptotic and growth-promoting functions (Kim et al., 2004).
Head and neck cancer
Activation of the endothelin axis is a feature of head and neck squamous cell carcinoma (HNSCC) (Hinsley et al., 2012). High pretreatment levels of plasma big ET-1 levels were generally associated with posttreatment distant failure in patients with advanced-stage nasopharyngeal carcinoma, (NPC) (Hearnden et al., 2009; Wen et al., 2011). ETAR was overexpressed in 74% of NPC and its expression was found to be a robust independent determinant of survival and an independent predictor of distant metastasis (Hinsley et al., 2012; Hearnden et al., 2009). Awano et al. (2006) demonstrated that ET-1 is able to act in an autocrine manner to stimulate the proliferation of HNSCC cells via both, ETAR and ETBR. In this study, ET-1 was able to stimulate the migration of HNSCC cells, an effect dramatically amplified by the presence of oral fibroblasts. This paracrine stimulation of HNSCC motility results from the ET-1-stimulated proteolytic release of bioactive ligands from fibroblasts, mediated in part by ADAM17, transactivating EGFR on HNSCC cells, triggering an increase in COX-2 expression (Hinsley et al., 2012; Wen et al., 2011; Awano et al., 2006). Interestingly, ETAR antagonist ABT-627 can inhibit the growth and metastasis of NPC cells and increase sensitivity to chemotherapy (Mai et al., 2006). The EDNRA/H323H polymorphism was found to be an independent prognostic marker for overall survival in patients with locally advanced NPC. Patients with EDNRA/H323H had poorer 5-year overall survival compared to patients with wild-type genotype. The functional significance of this polymorphism has yet to be elucidated (Wen et al., 2011).
Melanoma
ET-1, ET-3 and ETBR are implicated in melanocyte transformation and melanoma progression and ETBR is the major endothelin receptor expressed by normal and transformed melanocytes (Spinella et al., 2007). Gene expression profiling of human melanoma biopsies and cell lines indicated positive correlation of ETBR with aggressive phenotype (Imokawa et al., 1992). ET-1 was shown to be secreted by keratinocytes in response to UV, stimulating proliferation, chemotaxis, and pigment production in melanocytes, and promoting melanocyte survival and inhibiting the UV-induced apoptosis through the phosphatidylinositol 3-kinase (PI3K)-Akt pathway (Hachiya et al., 2004; Kadekaro et al., 2005). UV-induced ET-1 via ETBR down-regulates E-cadherin expression concomitant with increased expression of N-cadherin, MMP-2, MMP-9, and αvβ3 and α2β1 integrins and inhibits intercellular communication by inducing phosphorylation of gap junctional protein connexin 43 (Spinella et al., 2007; Jamal and Schneider, 2002). In addition, ET-1/ETBR upregulates melanoma cell adhesion molecule (MCAM), a marker of melanoma aggressiveness and metastasis, in primary and metastatic melanoma cell lines (Mangahas et al., 2004). In melanoma cell lines, ET increases HIF-1α with subsequent upregulation of vascular endothelial growth factor (VEGF), COX-1/COX-2 expression and activity and PGE2 production in normoxic and hypoxic conditions (Spinella et al., 2007). COX-1/COX-2 inhibitors blocked ET-induced PGE2 and VEGF secretion, MMP activation and cell invasion, indicating that both enzymes function as downstream mediators of ET-1 induced invasive properties. In melanoma xenografts, ETBR blockade suppressed HIF-1α accumulation, tumor growth, neovascularization, VEGF expression and MMP-2 (Spinella et al., 2007). Moreover, ET-1 and −3 induced secretion of prometastatic CXC chemokines (Mangahas et al., 2005) further implicating the proinflammatory properties of ET-1 in melanoma invasiveness and metastasis.
The endothelin axis in bone metastasis
In addition to the effects of ET-1 on tumor growth and invasiveness, the paracrine effects of tumor-produced ET-1 on bone cells may create a fertile growth environment for tumor cells in bone. In the bone microenvironment, tumor-derived ET-1 stimulates mitogenesis in osteoblasts, which express both ETAR and ETBR, and it decreases osteoclast activity and motility (Nelson et al., 2003), and therefore is involved in the formation of osteoblastic lesions that are frequently observed in patients with metastatic prostate cancer and, to a lesser extent, in metastatic breast cancer (Nelson et al., 2003). In preclinical models, osteoblastic bone metastases elicited by human and murine breast cancer cell lines were inhibited by ETAR antagonist atrasentan (Guise et al., 2003; Yin et al., 2003) and the mixed (ETAR and ETBR) inhibitor bosentan (Dreau et al., 2006). The effect of the ETA receptor-selective antagonist ABT-627 to block ET-1-stimulated osteoblast proliferation and new bone formation was specific because it did not block FGF-2-stimulated new bone formation (Guise et al., 2003). In prostate cancer, ET-1 production was down-regulated by androgens and up-regulated by the bone-associated factors transforming growth factor β (TGFβ), EGF, IL-1-β, IL-1-α, and TNF-α (Le Brun et al., 1999). Co-cultures of prostate cancer and bone have demonstrated that ET-1 production is increased by prostate cancer cells in contact with bone (Chiao et al., 2000). These effects are mediated via ETAR (Nelson et al., 1995, 1996).
Conclusion
The ET axis is deeply implicated in the malignant process and tumor progression in several tumor types. In addition components of this axis have potential as prognostic and predictive biomarkers. The prometastatic effect of ET/ETRs in most cancers involves paracrine effects regulating tumor-stromal interactions and involving pro-inflammatory cytokines/chemokines, COX2 and the matrix metalloproteinase. Indeed the identification of endothelin and biomarkers associated with it (Said et al., 2011) in primary tumors begets the design of clinical trials with endothelin axis inhibitors either after radical surgery or in combination with standard of care chemo or radiotherapy to hamper metastatic seeding. Interestingly, despite significant evidence for a role in cancer, prostate cancer has been the only human tumor where significant clinical studies have been undertaken to explore the benefits of interrupting the ET axis.
Acknowledgments
This study was supported by NIH grant CA143971 to D. Theodorescu.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.lfs.2012.03.040.
References
- Arun C, London NJ, Hemingway DM. Prognostic significance of elevated endothelin-1 levels in patients with colorectal cancer. Int J Biol Markers 2004;19:32–7. [DOI] [PubMed] [Google Scholar]
- Arun C, Swift B, Porter KE, West KP, London NJ, Hemingway DM. The role of big endothelin-1 in colorectal cancer. Int J Biol Markers 2002;17:268–74. [DOI] [PubMed] [Google Scholar]
- Asham E, Shankar A, Loizidou M, Fredericks S, Miller K, Boulos PB, et al. Increased endothelin-1 in colorectal cancer and reduction of tumour growth by ET(A) receptor antagonism. Br J Cancer 2001;85:1759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano S, Dawson LA, Hunter AR, Turner AJ, Usmani BA. Endothelin system in oral squamous carcinoma cells: specific siRNA targeting of ECE-1 blocks cell proliferation. Int J Cancer 2006;118:1645–52. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol 2011;163:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Rosano L. Epithelial-mesenchymal transition in ovarian cancer progression: a crucial role for the endothelin axis. Cells Tissues Organs 2007;185:85–94. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res 1999;59:720–7. [PubMed] [Google Scholar]
- Bagnato A, Spinella F, Rosano L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer 2005;12:761–72. [DOI] [PubMed] [Google Scholar]
- Browatzki M, Pfeiffer CA, Schmidt J, Kranzhofer R. Endothelin-1 induces functionally active CD40 protein via nuclear factor-kappaB in human vascular smooth muscle cells. Eur J Med Res 2007;12:129–33. [PubMed] [Google Scholar]
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008;14:28–36. [DOI] [PubMed] [Google Scholar]
- Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR, et al. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer 2000;83:360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clezardin P Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res 2011;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Maitland NJ, Berry P, Turner AJ, Usmani BA. Expression and localization of endothelin-converting enzyme-1 in human prostate cancer. Exp Biol Med (Maywood) 2006;231:1106–10. [PubMed] [Google Scholar]
- Dawson LA, Maitland NJ, Turner AJ, Usmani BA. Stromal-epithelial interactions influence prostate cancer cell invasion by altering the balance of metallopeptidase expression. Br J Cancer 2004;90:1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreau D, Karaa A, Culberson C, Wyan H, McKillop IH, Clemens MG. Bosentan inhibits tumor vascularization and bone metastasis in an immunocompetent skin-fold chamber model of breast carcinoma cell metastasis. Clin Exp Metastasis 2006;23:41–53. [DOI] [PubMed] [Google Scholar]
- Elahi MM, Everson NW. Prognosis of colorectal cancer patients with elevated endothelin-1 concentrations. Asian J Surg 2004;27:4–9. [DOI] [PubMed] [Google Scholar]
- Fischgrabe J, Gotte M, Michels K, Kiesel L, Wulfing P. Targeting endothelin A receptor enhances anti-proliferative and anti-invasive effects of the HER2 antibody trastuzumab in HER2-overexpressing breast cancer cells. Int J Cancer 2010;127:696–706. [DOI] [PubMed] [Google Scholar]
- Giaid A, Hamid QA, Springall DR, Yanagisawa M, Shinmi O, Sawamura T, et al. Detection of endothelin immunoreactivity and mRNA in pulmonary tumours. J Pathol 1990;162:15–22. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ. Endothelins in breast tumour cell invasion. Cancer Lett 2005;222:129–38. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C, Balkwill FR. A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Res 2004;64:2461–8. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol 2002;32:2393–400. [DOI] [PubMed] [Google Scholar]
- Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer 2003;97:779–84. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006;127:679–95. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 2007;446:765–70. [DOI] [PubMed] [Google Scholar]
- Ha NH, Nair VS, Reddy DN, Mudvari P, Ohshiro K, Ghanta KS, et al. Lactoferrin-Endothelin-1 Axis Contributes to the Development and Invasiveness of Triple-Negative Breast Cancer Phenotypes. Cancer Res 2011;71:7259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol 2004;165:2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell 2007;12:300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Binder C, Binder L, Pukrop T, Trumper L, Grimshaw MJ. Expression of endothelins and their receptors promotes an invasive phenotype of breast tumor cells but is insufficient to induce invasion in benign cells. DNA Cell Biol 2005;24:766–76. [DOI] [PubMed] [Google Scholar]
- Hearnden V, Lomas H, Macneil S, Thornhill M, Murdoch C, Lewis A, et al. Diffusion studies of nanometer polymersomes across tissue engineered human oral mucosa. Pharm Res 2009;26:1718–28. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Bogemann M, Bierer S, Eltze E, Toma MI, Kopke T, et al. The role of the endothelin axis and microvessel density in bladder cancer - correlation with tumor angiogenesis and clinical prognosis. Oncol Rep 2007;18:133–8. [PubMed] [Google Scholar]
- Herrmann E, Tiemann A, Eltze E, Bolenz C, Bremer C, Persigehl T, et al. Endothelin-A-receptor antagonism with atrasentan exhibits limited activity on the KU-19-19 bladder cancer cell line in a mouse model. J Cancer Res Clin Oncol 2009;135: 1455–62. [DOI] [PubMed] [Google Scholar]
- Hinsley EE, Hunt S, Hunter KD, Whawell SA, Lambert DW. Endothelin-1 stimulates motility of head and neck squamous carcinoma cells by promoting stromal-epithelial interactions. Int J Cancer 2012;130:40–7. [DOI] [PubMed] [Google Scholar]
- Hoosein MM, Dashwood MR, Dawas K, Ali HM, Grant K, Savage F, et al. Altered endothelin receptor subtypes in colorectal cancer. Eur J Gastroenterol Hepatol 2007;19:775–82. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem 1992;267:24675–80. [PubMed] [Google Scholar]
- Jamal S, Schneider RJ. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest 2002;110:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 2005;65:4292–9. [DOI] [PubMed] [Google Scholar]
- Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B Receptor, a New Target in Cancer Immune Therapy. Clin Cancer Res 2009;15:4521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. ENDOTHELIN SYSTEM: The Double-Edged Sword in Health and Disease. Annu Rev Pharmacol Toxicol 2001;41:851–76. [DOI] [PubMed] [Google Scholar]
- Kim TH, Xiong H, Zhang Z, Ren B. [beta]-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene 2004;24:597–604. [DOI] [PubMed] [Google Scholar]
- Kojima K, Nihei Z. Expression of endothelin-1 immunoreactivity in breast cancer. Surg Oncol 1995;4:309–15. [DOI] [PubMed] [Google Scholar]
- Konety BR, Nelson JB. Nonandrogenic mediators of prostatic growth. Hematol Oncol Clin North Am 2001;15:459–76. [DOI] [PubMed] [Google Scholar]
- Le Brun G, Aubin P, Soliman H, Ropiquet F, Villette JM, Berthon P, et al. Upregulation of endothelin 1 and its precursor by IL-1beta, TNF-alpha, and TGF-beta in the PC3 human prostate cancer cell line. Cytokine 1999;11:157–62. [DOI] [PubMed] [Google Scholar]
- Lloyd GM, Neal CP, Arun C, London NJ, Hemingway DM. The prognostic value of circulating big endothelin-1 in patients undergoing potentially curative resection for colorectal cancer. Colorectal Dis 2011;13:290–5. [DOI] [PubMed] [Google Scholar]
- Mai H-Q, Zeng Z-Y, Feng K-T, Ye Y-L, Zhang C-Q, Liang W-J, et al. Therapeutic targeting of the endothelin a receptor in human nasopharyngeal carcinoma. Cancer Sci 2006;97:1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangahas CR, dela Cruz GV, Friedman-Jimenez G, Jamal S. Endothelin-1 induces CXCL1 and CXCL8 secretion in human melanoma cells. J Invest Dermatol 2005;125:307–11. [DOI] [PubMed] [Google Scholar]
- Mangahas CR, dela Cruz GV, Schneider RJ, Jamal S. Endothelin-1 upregulates MCAM in melanocytes. J Invest Dermatol 2004;123:1135–9. [DOI] [PubMed] [Google Scholar]
- Mantovani A Cancer: Inflaming metastasis. Nature 2009;457:36–7. [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer 2003;3:110–6. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res 1996;56:663–8. [PubMed] [Google Scholar]
- Nelson JB, Fizazi K, Miller K, Higano CS, Moul JW, Morris T, et al. Phase III study of the efficacy and safety of zibotentan (ZD4054) in patients with bone metastatic castration-resistant prostate cancer (CRPC). J Clin Oncol 2011;29. abstr 117. [Google Scholar]
- Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med 1995;1:944–9. [DOI] [PubMed] [Google Scholar]
- Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 2008;113:2478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann C-H, Merseburger A, Suttmann H, Schilling D, Trojan L, Kempkensteffen C, et al. Novel options for the treatment of castration-resistant prostate cancer. World J Urol 2011:1–9. [DOI] [PubMed] [Google Scholar]
- Peeters CF, Thomas CM, Sweep FC, Span PN, Wobbes T, Ruers TM. Elevated serum endothelin-1 levels in patients with colorectal cancer; relevance for prognosis. Int J Biol Markers 2000;15:288–93. [DOI] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 2009;4:e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano L, Di Castro V, Spinella F, Nicotra MR, Natali PG, Bagnato A. ZD4054, a specific antagonist of the endothelin A receptor, inhibits tumor growth and enhances paclitaxel activity in human ovarian carcinoma in vitro and in vivo. Mol Cancer Ther 2007a;6:2003–11. [DOI] [PubMed] [Google Scholar]
- Rosano L, Di Castro V, Spinella F, Tortora G, Nicotra MR, Natali PG, et al. Combined Targeting of Endothelin A Receptor and Epidermal Growth Factor Receptor in Ovarian Cancer Shows Enhanced Antitumor Activity. Cancer Res 2007b;67:6351–9. [DOI] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Bagnato A. The importance of endothelin axis in initiation, progression, and therapy of ovarian cancer. Am J Physiol Regul Integr Comp Physiol 2010;299:R395–04. [DOI] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, et al. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res 2003;63:2447–53. [PubMed] [Google Scholar]
- Rosano L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, et al. Endothelin-1 Induces Tumor Proteinase Activation and Invasiveness of Ovarian Carcinoma Cells. Cancer Res 2001;61:8340–6. [PubMed] [Google Scholar]
- Said N, Smith S, Sanchez-Carbayo M, Theodorescu D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest 2011;121:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Loizidou M, Aliev G, Fredericks S, Holt D, Boulos PB, et al. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg 1998;85:502–6. [DOI] [PubMed] [Google Scholar]
- Simpson RA, Dickinson T, Porter KE, London NJ, Hemingway DM. Raised levels of plasma big endothelin 1 in patients with colorectal cancer. Br J Surg 2000;87:1409–13. [DOI] [PubMed] [Google Scholar]
- Smollich M, Gotte M, Fischgrabe J, Macedo LF, Brodie A, Chen S, et al. ETAR antagonist ZD4054 exhibits additive effects with aromatase inhibitors and fulvestrant in breast cancer therapy, and improves in vivo efficacy of anastrozole. Breast Cancer Res Treat 2010;123:345–57. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, et al. Endothelin-1 and Endothelin-3 Promote Invasive Behavior via Hypoxia-Inducible Factor-1 {alpha} in Human Melanoma Cells. Cancer Res 2007;67:1725–34. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1-induced prostaglandin E2-EP2, EP4 signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J Biol Chem 2004a;279:46700–5. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Inhibition of cyclooxygenase-1 and −2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin Cancer Res 2004b;10:4670–9. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AM, Clarke DL, Bradbury DA, Corbett LM, Patel JA, Knox AJ. Transcriptional regulation of monocyte chemotactic protein-1 release by endothelin-1 in human airway smooth muscle cells involves NF-kappaB and AP-1. Br J Pharmacol 2009;157:436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus B, Frierson HF Jr, Conaway M, Ching K, Guise T, Chirgwin J, et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res 2005;65:7320–7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Li Q, Wang H, Liu G, Jing Q, et al. Identifying novel prostate cancer associated pathways based on integrative microarray data analysis. Comput Biol Chem 2011;35:151–8. [DOI] [PubMed] [Google Scholar]
- Wen Y-F, Qi B, Liu H, Mo H-Y, Chen Q-Y, Li J, et al. Polymorphisms in the Endothelin-1 and Endothelin A Receptor Genes and Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma. Clin Cancer Res 2011;17:2451–8. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Burchell J, Grimshaw MJ. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res 2006;66:11802–7. [DOI] [PubMed] [Google Scholar]
- Wulfing P, et al. Expression of endothelin-1, endothelin-A, and endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res 2003;9(11):4125–31. [PubMed] [Google Scholar]
- Wulfing C, Eltze E, Piechota H, Abol-Enein H, Wulfing P, Bode ME, et al. Expression of endothelin-1 and endothelin-A and -B receptors in invasive bladder cancer. Oncol Rep 2005a;13:223–8. [PubMed] [Google Scholar]
- Wulfing C, Eltze E, Yamini J, Wulfing P, Bierer S, Bocker W, et al. Expression of the endothelin axis in bladder cancer: relationship to clinicopathologic parameters and long-term survival. Eur Urol 2005b;47:593–600. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Ogawa M, Sakai K. Prognostic significance of three novel biologic factors in a clinical trial of adjuvant therapy for node-negative breast cancer. Surgery 1995;117(6):601–8. [DOI] [PubMed] [Google Scholar]
- Yin JJ, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A 2003;100(19):10954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


