Abstract
According to modern concepts, the genus Hyssopus L. includes seven plant species (Hyssopus ambiguus (Trautv.) Iljin ex Prochorov. & Lebel; Hyssopus cuspidatus Boriss; Hyssopus latilabiatus C.Y.Wu & H.W. Li; Hyssopus macranthus Boriss.; Hyssopus officinalis L.; Hyssopus seravschanicus (Dubj.) Pazij; Hyssopus subulifolius (Rech.f.) Rech.f.). The plants are rich in various groups of biologically active substances with a wide spectrum of pharmacological action. This review presents a modern comprehensive overview of the botanical research, extraction methods, chemical composition and pharmacological activity of plants of the genus Hyssopus L. As a result of the review, it was established that the chemical composition of plant extracts of the genus Hyssopus L. depends on various factors (place of growth, weather conditions, chemotypes, extraction methods, etc.). For the further use of the plants, the extraction methods and low-molecular metabolites isolated from them (mono- and sesquiterpenoids, flavonoids, alkaloids, etc.) are discussed. The data from the review provide an assessment of the relevance.
Keywords: Hyssopus, distribution, compounds, essential oils, flavonoids, terpenoids, biological activity
1. Introduction
Currently, the genus Hyssopus L. (family Lamiaceae or Labiatae) has seven species [1,2], being one of the small genera. According to the Plants of the World Online (POWO) online taxonomic list [3,4], the genus Hyssopus is confirmed as including seven species distributed naturally in Europe, Asia, northern Africa, and also introduced in North America. These comprise Hyssopus ambiguus (Trautv.) Iljin ex Prochorov. & Lebel, Hyssopus cuspidatus Boriss., Hyssopus latilabiatus C.Y.Wu & H.W. Li, Hyssopus macranthus Boriss., Hyssopus officinalis L., Hyssopus seravschanicus (Dubj.) Pazij and Hyssopus subulifolius (Rech.f.) Rech.f. Moreover, according to World Flora Online (WFO) [4], H. ferganensis Boriss. and H. tianschanicus Boriss. are defined as a synonym for H. seravschanicus.
In the flora of Kazakhstan [5], the following four species are described: H. cuspidatus Boriss., H. ambiguus Iljin, H. macranthus Boriss. and H. tianschanicus Boriss. Later, M.S. Baitenov [6] listed approximately 15 species distributed in Eurasia from the Mediterranean Sea to Central Asia, confirming the presence of only four species in the territory of Kazakhstan.
Hyssopus has been known as a medicinal plant since the time of Hippocrates (circa 460–377 BC), who mentioned it in his writings. The most common representative of the genus is H. officinalis L. This species (leaves and flowers) is widely used in traditional medicine, cooking and perfumery. H. officinalis is widely found in Europe and North Africa. This plant is included in the official pharmacopoeias of France, Portugal, Romania, Sweden and Germany [7]; the herb is actively used in the food industry [8].
In the genus Hyssopus, the plants’ main function is providing essential oil for the cosmetic and perfume industry, especially for the production of oriental fragrances [9]. Hyssop essential oil exhibits antibacterial, antiviral and expectorant properties. This makes it an important ingredient in aromatherapy, pharmaceuticals, personal care products, food and beverages [9].
However, in our time, the culture of hyssop has been unnecessarily overlooked, with its industrial use significantly limited. The plants of the genus Hyssopus L. have potential for further research on the development of medicinal and cosmetic products with relatively high biological properties.
For a comprehensive review of the literature, we analyzed published data available through the following search engines: SciFinder®, Web of Science®, Scopus® and Google Scholar®. When searching for information, the following keywords were used: “Hyssopus”, “compounds”, “isolation”, “extraction” and “activity”. The available reviews either presented the phytochemical and pharmacological properties of only one species, H. officinalis L., or the reviews were devoted to one class of chemical compounds [8,9,10,11]; meanwhile, there were no reviews about other plants’ properties in the species Hyssopus L. In this regard, this paper aims to elucidate, using new data, the chemical composition of plants of the genus H. officinalis L., the pharmacological properties of isolated extracts, essential oils and individual compounds and their further use in the pharmaceutical, cosmetic and medical industries.
Plants of the genus Hyssop, like many other plants, contain various classes of biologically active compounds, such as flavonoids, essential oils, phenolic compounds and others. The purpose of our review is to collect and systematize the available data on the chemical composition of these plants and their potential pharmacological properties, since the Republic of Kazakhstan is actively developing the technology of new plant-based medicines, and possesses significant reserves of some species of Hyssop, such as H. cuspidatus Boriss., H. ambiguus Iljin, H. macranthus Boriss. and H. tianschanicus Boriss. In many countries, plants of the genus Hyssopus are traditionally used for medicinal, perfumery, cosmetic and nutritional purposes. Extensive research into the content of biologically active compounds in Hyssopus macranthus Boriss. and Hyssopus subulifolius (Rech.f.) has not yet been conducted, and in relation to H. angustifolius M. Bieb., H. tianschanicus Boriss., H. ambiguus Iljin, H. cretaceus Dub., H. seravschanicus (Dub.) Pazij and H. ferganensis Boriss, only individual classes have been studied to date. This article will help evaluate the scientific evidence for hyssop’s traditional use and determine its effectiveness and safety. In addition, further research into the chemical composition of hyssop plants may lead to the isolation of new biologically active compounds that can be used to create new medicines for clinical use.
2. Botany
The plants of this genus are characterized by the presence of elongated or oblong inflorescences, multi-flowered, spike-shaped and consisting of close or spread whorls, sitting in the axils of the leaves. The calyx is tubular-bell-shaped, with 15 veins, almost regular, with five almost equal teeth. The calyx is covered with glands, its surface is painted lilac-green and the inside is bare. The corolla is fused-petalled, two-lipped, hairy and with glands. The upper lip is almost flat, notched or bilobed; the lower one is three-lobed, with a larger middle blade. There are four stamens, one style, bifid at the apex and the nuts are oblong or oblong–ovate. The leaves range from linear to oblong. Life forms are represented by perennial herbaceous plants or subshrubs. The general distribution of representatives of the genus covers Europe, Asia and North Africa; additionally, introduced species have been noted in North America (Table 1).
Table 1.
The morphological properties of species of the genus Hyssopus growing in Kazakhstan.
| Indicators | H. cuspidatus | H. ambiguus | H. macranthus | H. seravschanicus | H. latilabiatus | H. officinalis | H. subulifolius |
|---|---|---|---|---|---|---|---|
| Life form | Subshrub | Subshrub | Subshrub | Subshrub | Subshrub | Subshrub | Subshrub |
| Leaves | Narrowly linear, with non-folding edges, with an awl-shaped tip at the apex | Glabrous, entire, narrowly linear, with edges turning downwards, with a vein protruding from the underside | Sessile, lily, pointed, narrowed at the base, twisting | Leaves linear, almost glabrous, with sparse short hairs, sharp, with curled edges | Leaves sessile, linear, glandular, base wedge-shaped, edges curving inward | Leaves are lanceolate, short-petiolate, short-hairy | Leaves are small, needle-shaped, short-petiolate, glabrous |
| Inflorescence | Multi-flowered, thin, tapering towards the apex | Multi-flowered, unbranched, dense | Thin, multi-flowered, tapering towards the apex | Long, narrow, consists of loose whorls | Short, apical, spicate, few flowers | Long, spicate, flowers sit in the axils of the upper leaves | Long, spicate, covered with small white hairs |
| Calyx | Regular, with 5 awl-shaped pointed teeth | 4–6 mm long, with five almost identical teeth, equal to one-third of the total length of the calyx | 4–6 mm long, covered with short hairs along the veins and along the edge of triangular, sharp teeth | 5–6 mm long, with triangular sharp teeth, two times shorter than the tube, painted blue | Tubular-bell-shaped, 5–6 mm long, purple, with five teeth, pubescent, glandular at the apex | 5–6 mm long, with five teeth, increased to one-third of the total length of calyx, with short hairs along veins, purple color | 6–8 mm long, purple, with five triangular teeth, pubescent with hairs along the veins |
| Corolla | Blue, up to 12 mm long, with a short tube, two-lipped, upper lip two-lobed, shorter than the lower, lower three-lobed, with a large middle lobe | Bluish–blue, 0.8–1 cm long, two-lipped, the upper lip is flat, bilobed, the lower lip is three-lobed with a large middle lobe | 10–15 mm long, blue–violet, short-pubescent on the outside, two-lipped, upper lip slightly notched, smaller than the lower, three-lobed. On the lower lip, the middle lobe is two times wider than the lateral ones | Blue–violet, about 1 cm long, with a narrow tube, about 5 mm, the upper lip is ovoid, equal to the lower, the middle large lobe is strongly prominent on the lower lip | Purple, 12–13 mm long, pubescent, glandular; the upper lip is straight, oblong; lower lip is broadened; middle lobe up to 1 mm, considerably wider than lateral lobes; lateral lobes are ovate | Purple, 10–15 mm long, two-lipped, upper lip with notch, shorter than lower lip. The lower lip with well-defined, downwardly bent middle lobe | White, two-lipped, 12–16 mm long, the upper lip with notch, short; the lower lip is three-lobed, large; middle lobe is round |
| Habitat | Grows in feather-fescue sepia, on rocky mountain slopes and on pebbles | Grows on crushed and rocky mountain slopes, on pebbles | Grows on saline flood meadows, rocky and gravelly slopes of hills and hills, on pebbles and coarse sandy soils | Grows on rocky and gravelly slopes and trails of mountains, on steppe areas | Grows on dry and rocky slopes, on stony screes | Grows in steppes, on dry hills, rocky slopes of hillsides | Grows on dry and stony soils, in dry forests and shrub thickets |
| Distribution in the Republic of Kazakhstan | Altai, Tarbagatai, Dzungarian Alatau | Irtysh, Eastern Small Hills, Karkaralinsky, Altai, Tarbagatai, Dzhungar Alatau | Irtysh, Western and Eastern small hills, Karkaraly, Zaisan, Bal-khash-Alakol, Altai, Tarbagatai | Kyrgyz Alatau, Western Tien Shan | Absent | Absent | Absent |
| General distribution * | Altai, Kazakhstan, Mongolia, China (Xinjiang) | Altai, Kazakhstan, Mongolia, West Siberia | Endemic of Kazakhstan | Afghanistan, Kirgizstan, Pakistan, Tadzhikistan, Uzbekistan | Endemic of China (Xinjiang) | South and central Europe, South Siberia, Mediterranean region, North Caucasus, Turkey, North Africa (Morocco) | Endemic of Afghanistan |
* Distribution of natural Hyssopus species in the world (according to POWO [3]). Based on international theories, the following four species of this genus grow in Kazakhstan: H. cuspidatus, H. ambiguus, H. macranthus and H. seravschanicus. All species in terms of life forms are subshrubs, with simple sessile leaves and multi-flowered inflorescences.
Thus, the main differences between the species are the structure of the leaf shape, the size of the inflorescences and the structure of the calyx and corolla of the flower. The habitat of all species is confined mainly to arid territories (mountain slopes, steppes), rocky or sandy soils. In the territory of Kazakhstan, three species have a wide range beyond its borders, covering Western Siberia, Mongolia, Central Asia and the Tien Shan. One species is endemic, whose range includes the northern, central and eastern territories [5]. Not all species are sufficiently studied botanically and chemically.
3. Methods for Isolating Extracts and Essential Oils from Plants of the Genus Hyssopus L.
Various methods and solvents are used to isolate polar and nonpolar secondary metabolites from plants of the genus Hyssopus. To isolate the components of essential oils, steam distillation with hydro-distillation is traditionally performed using either a Clevenger-type apparatus and the aerial parts of plants with a distillation time of 2–3 h [11] or a Dean–Stark apparatus with a distillation time of up to 4 h [12]. The volatile components from H. officinalis were isolated using the Soxhlet extraction method using pentane/diethyl ether and supercritical extraction with carbon dioxide [13]. The use of supercritical fluid extraction for Hyssopus at different conditions, including pressure, temperature, extraction times and modifier concentrations, using an orthogonal lattice design with matrix conditions influenced the extraction yield of major monoterpenoids [14]. In [15], the supercritical extraction of H. officinalis was carried out using carbon dioxide as an extractant. The effect of pressure (80, 100 and 150 bar) on the yield of the total extract was studied at a temperature of 313 K, a flow rate of 0.00323 kg/min and an average particle diameter of 0.49 mm, obtaining a strong correlation between the inverse values of the total extract yield and extraction time.
However, these methods also have disadvantages: they were developed only in laboratory conditions or in pilot plants, and require expensive and bulky equipment. To isolate the essential oil from H. officinalis, an effective and economically attractive technology, Détente Instantanée Contrôlée (DIC), was proposed. This is a thermomechanical process that involves exposing the raw material to saturated steam under high pressure for a brief duration, followed with a sharp drop in pressure inside the vacuum. In relation to other methods, DIC has several other benefits, including no solvents used, a higher extract quality making it environmentally friendly on an industrial scale, with a high speed, selectivity, automatic operation and performance under normal conditions. At the same time, the yield of H. officinalis extract turned out to be the highest when compared to the methods of hydro-distillation, ultrasonic extraction and the Soxhlet method [16]. For a relatively high yield of essential oil, the plant should be collected at the full-flowering stage.
Many methods have been used to extract and isolate plant phytochemicals from H. officinalis, such as homogenization, solvent extraction, maceration, grinding, ultrasonication and Soxhlet extraction.
The authors of [17] proposed an innovative method for obtaining essential oil, which included the following stages: preliminary grinding, mixing with a reagent, infusion at temperature and the ratio of material and reagent, and hydro-distillation to obtain essential oil. The grinding of herbal essential oil raw materials was carried out to sizes of 5–15 mm, mixing with the reagent in a volume ratio from 1:5 to 1:8 and infusing the raw materials at a temperature of 22 to 24 °C for 3 to 5 h. Electro-activated water with a pH of 8.0 to 9.5 was used as a reagent, obtained via electrolysis of a 1–2% aqueous solution of NaCl, at a current of 0.5–0.6 A and a voltage of 36 V. The yield of hyssop essential oil’s medicinal content using the proposed technology ranged from 0.6 to 0.8%. The quality of the essential oil of H. officinalis was assessed using the ratio of the main components—pinocamphone and cis-pinocamphone—to the total content of essential oil components [17].
Ultrasonic extraction is one of the modern methods for obtaining compounds from plant organs. In [18], the authors obtained H. officinalis leaf extract using ultrasonic extraction with an ethanol/water/solvent ratio (50:50) and (80:20) from 10 to 20 min at 30 and 40 °C. The (80-40-20) solution identified the highest amount of antioxidant activity in the inhibition of DPPH radicals and beta-carotene–linoleic acid color analysis and determined the highest amount for phenolic compounds (193.3 ± 5.53 mg/g) and flavonoids (40.63 ± 2.36 mg/g).
A relatively high content of extractives was observed during the microwave extraction of H. officinalis (g/100 g of dry weight)—23.4 ± 0.36; with ultrasonic cavitation—20.6 ± 0.48; during extraction in the Soxhlet apparatus—15.1 ± 0.25 and during maceration—12.4 ± 0.14. However, the extraction methods also influenced the concentration of phenolic compounds in H. officinalis, with the highest to the lowest percentage of phenolic compounds as follows: microwave extraction > ultrasonic extraction > Soxhlet extraction > maceration [19].
Ahmadian et al. [20] demonstrated that ultrasound combined with cold atmospheric plasma as a pre-treatment improved the extraction of phenolic components from H. officinalis by approximately 22% compared to the use of ultrasound alone.
To obtain various extracts, polar (acetone, methanol, ethanol) and nonpolar solvents (hexane, petroleum ether) were used. The polar solvents were used more frequently and provided better results, both in terms of the concentration and biological potential. For a relatively high yield of extractives, the best solvent was aqueous alcohol at 70% [21].
To obtain extractives from the structure of the material, an extraction process using new physical methods can be used. One of the promising methods for intensifying extraction is the electrophysical method, where the material is treated with a pulsed electric field, which can be applied to substances that are polar dielectrics in physical nature. The authors of [22] presented the results of the intensification processes of polysaccharides from H. officinalis under the influence of electric current. The energy consumption for the H. officinalis extraction process was proved to be intensified with a pulsed electric current that was significantly lower than extraction via convection heating. The possibility of increasing the content of extractable polysaccharides by 48% after extraction was demonstrated. That is, this process makes it possible to reduce by three times the time required for obtaining water-soluble polysaccharides compared to traditional pharmacopoeia convection methods and, furthermore, to reduce energy costs by 20 times. The use of electric current can also lead to a reduction in the maximum processing temperature to 40 °C, which makes it possible to obtain aqueous alcoholic and alcoholic extracts, and to extract biologically active substances that are insoluble in water.
In summary, a literature review revealed a large number of extraction methods from the plants of the genus Hyssopus. Each of the traditional methods has its own advantages and disadvantages; the choice of one or another option depends on the purpose of using the processor. Therefore, the choice of the extraction system should be based on a careful analysis of the essential properties of the extract and its components.
4. Mono- and Sesquiterpenoids of Essential Oils from Plants of the Genus Hyssopus L.
The essential oils from plants of the genus Hyssopus are known for their medicinal and aromatic properties. These oils have antimicrobial, antiviral and expectorant properties, making them a valuable ingredient in aromatherapy, pharmaceuticals, personal care products, food and beverages. The most common H. officinalis essential oil is produced and distributed by various companies, including Now Foods, Katyani Exports, Ungerer & Company, Young Living, doTERRA, Edens Garden, Radha Beauty, Majestic Pure, Art Naturals, Healing Solutions, Native American Nutritionals and Rocky Mountain Oils.
The H. officinalis essential oil market has experienced significant growth in recent years, stimulated by the increased consumer attention toward the benefits of natural and organic products, the growing demand for alternative medicine and rising incomes. The market research shows that the H. officinalis essential oil market is poised for sustained growth, with opportunities for manufacturers, suppliers and distributors to capitalize on the rising demand. In connection with economic use, a more thorough study of the chemical composition of the H. officinalis essential oil and other species of this genus is necessary [23]. The herb H. officinalis is included as an official raw material in the pharmacopoeias of France, Portugal, Romania, Sweden and Germany.
An analysis of the available literature devoted to studying the composition of the H. officinalis essential oil showed that the information is fragmentary and often contradictory. Most frequently, summary data are provided on the quantitative content of the dominant components; in some cases, there is an analysis of the component composition of various morphological forms.
The component composition of the H. officinalis essential oil, which grows in various geographical areas, is reasonably well known. For example, studies of the chemical composition of the ethereal H. officinalis of various chemotypes (pinocamphonic, linalool, thymolic) are described. The data on the composition of the H. ambiguus (Trautv.) Iljin ex Prochorov. & Lebel, H. cuspidatus Boriss., H. officinalis L. and H. seavschanicus (Dubj.) Pazij essential oils are presented in Table 2.
Table 2.
Chemical composition of essential oils of different species in the genus Hyssopus L.
| Species | Location | Number of Identified Compounds | Main Compounds | Reference |
|---|---|---|---|---|
| H. officinalis, culture | Poland | From 27 to 36 | Cis-pinocamphone (40.07–45.45%) | [12] |
| H. ambiguus | Kazakhstan | 9 | 1,8-cineole (36.0–43.5%) | [24,25] |
| H. cuspidatus | Kazakhstan | 83 | Pinocarvone (27.06%), 1,8-cineole (10.76%), cis-pinocarveol (9.57%) | [26] |
| H. cuspidatus | China | 38 | Verbenone (23.84%), β-pinene (19.76%), pinocamphone (17.95%), 1,8-cineole (7.16%), myrtenol (7.06%) | [27] |
| H. cuspidatus | China | 36 | Germacrene D (18.67%), hexadecanoic acid (17.53%), germacrene B (15.61%), trans-caryophyllene (8.04%) | [28] |
| H. cuspidatus | China | 39 | Thymol (19.65%), pinocamphone (15.30%), γ-terpinene (14.63%), p-cymene (7.49%), β-pinene (6.57%) | [29] |
| H. officinalis | Iran | 14 | Camphor (23.61%), β-pinene (21.91%) | [30] |
| H. officinalis | Cultivated in Serbia | 18 | cis-pinocamphone (42.9%), pinocamphone (14.1%), germacrene-D-11-ol (5.7%), elemol (5.6%) | [31] |
| H. officinalis | Egypt | 26 | Cis-pinocamphone (34.00%), pinocamphone (21.27%), β-pinene (13.19%), β-phellandrene (13.10) | [32] |
| H. officinalis | Turkey | 34 | Cis-pinocamphone (57.27%), β-pinene (7.23%), terpinen-4-ol (7.13%), pinocarvone (6.49%) | [33] |
| H. officinalis subsp. angustifolius | Turkey | 51 | Pinocarvone (27.1%), β-pinene (19.0%), cis-pinocamphone (13.6%) | [34] |
| H. officinalis subsp. officinalis L. | Serbia | 59 | Cis-pinocamphone in f. albus (16.4%), in f. cyaneus (22.3%), in f. ruber (58.3%) | [35] |
| H. officinalis | East Lithuania | 63 | Pinocarvone (21.1–28.1%), cis-pinocamphone (11.5–15.9%), β-pinene (7.0–11.4%), germacrene D (3.7–5.5%), hedycaryol (4.1–4.8%) in four oils, cis-pinocamphone (16.8–33.6%) in two oils | [36] |
| H. officinalis | Russia | From 31 to 37 | White-flowered pinocamphone up to 44.99%, blue-flowered pinocamphone up to 20.85%, pink-flowered pinocamphone up to 45.23% | [37] |
| H. officinalis | Poland | 5 | Cis-pinocamphone (33.52%), pinocamphone (28.67%), β-pinene (8.12%), elemol (5.86%) | [38] |
| H. officinalis | Iran | 17 | pinocamphone (53.93%) | [39] |
| H. officinalis | Russia | 27 | Pinocamphone (63.55%) | [40] |
| H. officinalis f. cyaneus | Russia, cultivated | 68 | Pinocamphone (70%) | [41] |
| H. officinalis L. subsp. angustifolius | Iran | 25 and 22 | Purple landrace was cis-pinocamphone (55.14%), β-pinene (17.06%), pinocamphone (3.50%); White landrace of hyssop camphor (31.85%), cis-pinocamphone (30.11%), β-pinene (12.26%), pinocamphone (6.09%) |
[42] |
| H. officinalis ssp. officinalis | India | 21 | Pinocamphone (49.1%), β-pinene (18.4%), cis-pinocamphone (9.7%) | [43] |
| H. officinalis | Turkey | 24 | Pinocarvone (29.2%), trans-pinocamphone (27.2%), β-pinene (17.6%), cis-camphone (4.7%) | [44] |
| H. officinalis | Egypt | 33 | Cis-pinocamphone (26.85%), β-pinene (20.43%), pinocamphone (15.97%), α-elemol (7.96%) | [45] |
| H. officinalis | India | 33 | cis-pinocamphone (53.34%), β-pinene (9.91%), limonene (7.19%) | [46] |
| H. officinalis | Serbia | 74 | Pinocamphone (41.2%) | [47] |
| H. officinalis | Iran | 19 | Myrtenyl acetate (74.08%), camphor (6.76%), germacrene (3.39%) | [48] |
| H. officinalis | Poland | 52 | Cis-pinocamphone (22.53–28.74%), pinocamphone (11.41–17.99%), β-pinene (6.69–12.01%), elemol (5.02–7.57%), germacrene D (3.14–6.98%) | [49] |
| H. officinalis | Spain | 44 | 1,8-cineole (53%), β-pinene (16%) | [50] |
| H. officinalis | Poland | 74 | Cis-pinocamphone (20.05–43.02%), pinocamphone (1.68–19.62%) |
[51] |
| H. officinalis | Poland | 50 | White-flowered pinocamphone (51%), pink-flowered pinocamphone (28.8%), cis-pinocamphone (21.9%) | [52] |
| H. officinalis | Bulgaria | 46 | Cis-pinocamphone (48.98–50.77%), β-pinene (13.38–13.54%), pinocamphone (5.78–5.94%) |
[53] |
| H. officinalis | Iran | 36 | Cis-pinocamphone (38.47%), pinocamphone (13.32%), pinocarvone (5.34%) | [54] |
| H. officinalis | Cultivated in Bulgaria | 55 | Cis-pinocamphone (40.2%), pinocamphone (10.3%), β-pinene (14.2%) | [55] |
| H. officinalis | Egypt | - | White-flowered β-pinene (19.60%), pinocamphone (19.20%), camphor (16.3%) | [56] |
| H. officinalis | India | 47 | Cis-pinocamphone (38.1%), pinocarvone (20.3%), 1,8-cineole (12.2%) | [57] |
|
H. officinalis var. decumbens |
France | 16 | Linalool (49.6%), 1,8-cineole (13.3%), limonene (5.4%) | [58] |
| H. officinalis | Montenegro | 45 | Methyl eugenol (38.3%), limonene (37.4%), β-pinene (9.6%) | [59] |
| H. officinalis | Yugoslavia | Cis-pinocamphone (46.1%) | [60] | |
| H. officinalis | Spain | 21 | 1,8-cineole (52.89%), β-pinene (16.82%) | [61] |
| H. officinalis L. subsp. angustifolius (Bieb.) | Turkey | 34 | Pinocarvone (36.3%), pinocamphone (19.6%), β-pinene (10.6%), 1,8-cineole (7.2%), cis-pinocamphone (5.3%) | [62] |
| H. seravschanicus | Ukraine, in culture | 27 | Cis-pinocamphone (61.58%) | [63] |
| H. seravschanicus | Tajikistan | 87 | Cis-pinocamphone (57.0–88.9%), β-pinene (0.4–6.0%), 1,8-cineole (1.8–3.6%), camphor (0.5–4.0%), spathulenol (0.1–5.0%) | [64] |
| H. cretaceus | Russia | 45 | Cis-pinocamphone (60%), pinene (12.78%), myrtenyl acetate (7.17%) |
[65] |
| H. officinalis | Russia, Crym | 58 | Cis-pinocamphone (29.7–58.4%), pinocamphone (15.2–23.3%) | [66] |
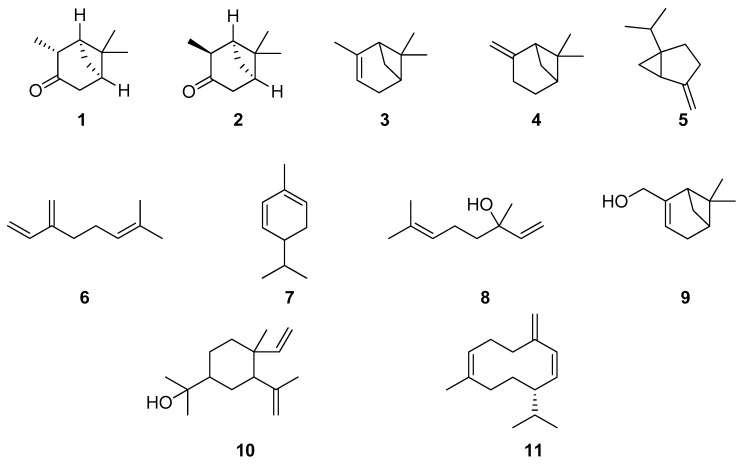
Many publications are devoted to the study of the species H. officinalis L. growing in European, Asian and African countries. The component composition and quantitative content of various constituents in essential oils may vary depending on soil, climatic and genetic factors [12,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]; however, the main ketones that are characteristic of this species are pinocamphone 1 and cis-pinocamphone 2 (their relative content varies ranging from 2.94 to 63.55%; these components are in dynamic equilibrium), α-pinene 3 and β-pinene 4, sabinene 5, myrcene 6, phellandrene 7, linalool 8, myrtenol 9, elemol 10 and germacrene-D 11 (Figure 1).
Figure 1.
Chemical structures of mono- and sesquiterpene molecules from plants of the genus Hyssop.
Blue-flowered plants reportedly contain more essential oil than pink- and white-flowered forms. In addition, plants with different flower colors have differences in the percentage of specific essential oil components [67]. Chromato-mass-spectral analysis of the content of volatile organic compounds in plants of the same variety, but differing in flower color, revealed features in the biosynthesis of secondary metabolites. The studies have shown that, in the white-flowered plants, the content of pinocamphone 1 was up to 44.99%, in the blue-flowered plants it was up to 20.85% and in the pink-flowered plants it was up to 45.23% [37].
In [68], details are provided of a study conducted to examine the component composition of the essential oil of forms of H. officinalis, manifested in white, blue and pink flowers. The study revealed that there are no significant differences in the hydrocarbon content between the white- and pink-flowered forms. However, the blue-flowered form had half the hydrocarbon content (4.4%). The white-flowered form had a high alcohol content (up to 8.69%), while the blue-flowered (up to 5.73%) and pink-flowered (up to 4.61%) forms had a lower alcohol content. In the blue- and pink-flowered forms, the content of aldehydes and ketones was the same (59.8% each); meanwhile, in the white-flowered form, it was slightly higher (up to 62.17%).
According to the studies, most species of H. officinalis synthesized components such as pinocamphone 1, cis-pinocamphone 2, β-pinene 4, sabinene 5, myrtenol 9 and elemol 10, as well as some others in small quantities. The second group of plants (20%) concentrated five main mono- and sesquiterpenoids, mainly consisting of pinocamphone 1 (up to 60%), β-pinene 4 (up to 6.2%), β-phellandrene 7 (up to 6.8%), spathulenol 12 (up to 3.5%) and myrtenol 9 (up to 6.3%), as well as (Z)-caryophyllene 13 (up to 3.5%). The third group of plants (10%), including H. officinalis, differed in their synthesis, mainly producing cis-pinocamphone 2 (up to 61.1%), β-pinene 4 (up to 10.5%), elemol 10 (up to 19%), β-eudesmol 14 (up to 7.6%) and a small amount of sesquiterpenes (up to 25%). The essential oil of all studied plants corresponded to the composition of H. officinalis, differing in the quantitative content of the main components. The content of individual hydrocarbons in the essential oil did not exceed 1.5%, and the largest amount of β-pinene 4 was 10.5%.
The main component of the H. ambiguus [24,25] and H. cuspidatus essential oils [26] is 1,8-cineole 15 (Figure 2). Samples of H. ambiguus essential oil were collected in the vicinity of the town of Karkaralinsk and the neighboring village. The varieties differ in qualitative and quantitative composition. Thus, in samples from the first point of growth, 3-carene 16, terpinen-4-ol 17 and germacrene D 11 were identified; meanwhile, in the raw materials from the second collection point, β-pinene 4 and β-myrcene 6 were identified. In Spain, 1,8-cineole 15 is a major component of the H. officinalis essential oil [61]. β-pinene 4, 1,8-cineole 15 and cis-pinocamphone 2 are the main compounds found in wild plants of the H. officinalis subspecies from Serbia [69], which also formed a major component in H. officinalis essential oil from Bulgaria [55].
Figure 2.
Chemical structures of isolated mono- and sesquiterpenes from plants of the genus Hyssop.
Moreover, 1,8-cineole 15 was also found in samples of H. cuspidatus essential oils growing in Altai and China [26,27]. The main components in these oils are pinocarvone 18 (27.06%), 1,8-cineole 15 (10.76%) and cis-pinocarveol 19 (9.57%). In Altai, the components were verbenone 20 (23.84%), β-pinene 4 (19.76%), pinocamphone 1 (17.95%), 1,8-cineole 15 (7.16%) and myrtenol 9 (7.06%). Thymol 21 was the main component of H. cuspidatus [29], growing in Taicheng, Xinjiang, China. In addition, thymol 21 (18.95%) was found in the H. officinalis essential oil of from Iran, and carvacrol 22 (7.73%) and β-bisabolol 23 (16.62%) were also found [70].
The monoterpenoid linalool 8 was found in significant quantities in the H. officinalis essential oil from France, amounting to almost 50%. In [71], 44 chemical constituents were detected in the H. officinalis essential oil cultivated in Italy using GC–MS analysis. The main chemical constituents detected were linalool 8 (47.7%) and methyl eugenol 24 (9.9%) [71].
In addition to the known compounds, six previously undescribed monoterpenoids 25–30 were isolated and identified from the n-BuOH fraction of H. cuspidatus [72]:
25—(1S,4S,5S)-4,6,6-trimethyl-1-(((2R,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)bicyclo[3.1.1]heptan-3-one.
26—(1R,4R,5R)-4,6,6-trimethyl-1-(((2R,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)bicyclo[3.1.1]heptan-3-one.
27—(1S,4R,5R)-4-hydroxy-4,6,6-trimethyl-1-(((2R,3S,4R,5R,6S)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)bicyclo[3.1.1]heptan-3-one.
28—(2R,3S,4R,5R,6S)-2-(((1S,3R,4R,5R)-3,4-dihydroxy-3,4,6,6-tetramethylbicyclo[3.1.1]heptan-1-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
29—(1R,5S)-6,6-dimethyl-4-oxobicyclo[3.1.1]hept-2-ene-2-carboxylic acid.
30—(2R,3R)-2-((E)-but-2-enoyloxy)-3,4-dimethylpentanoic acid.
Thus, a significant amount of information has been identified in the literature, studying the component composition of essential oils from plants of the genus Hyssopus. The genus Hyssopus is characterized by the representation of mono- and sesquiterpenoids of all biogenetic lines; in particular, the pinocamphone 1 line is most developed in H. officinalis, where it comprises more than half of the essential oil, reaching a maximum of 90%. The biogenetic lineage of cis-pinocamphone 2 is more common and was identified as the main component in almost a third of the species considered. In some species of Hyssopus, the essential oils contain large amounts of linalool 8, thymol 17 and 1,8-cineole 15, which are found in other species of the family Lamiaceae.
5. Steroids and Triterpenoids of Plants of the Genus Hyssopus L.
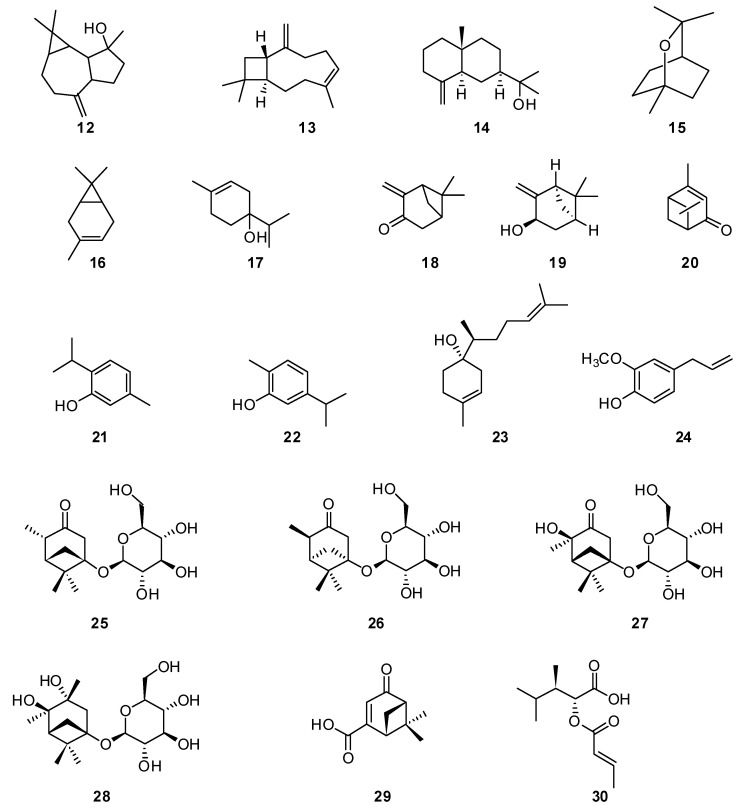
Many species of the family Lamiaceae accumulate significant amounts of triterpenoids, which are structurally and genetically similar to steroids. Of particular interest are the pentacyclic triterpene acids—ursolic 31 and oleanolic 32—which were found in the raw materials of some Hyssopus species (Figure 3) [73].
Figure 3.
Chemical structures of triterpenoid and steroid molecules from plants of the genus Hyssop.
The experiments showed that chloroform and 70% alcohol extracts obtained from the herb H. officinalis contain oleanolic acid 32 and ursolic acid 31. The best separation of triterpenoids occurred in the system petroleum ether–chloroform–acetic acid (10:4:0.4) [74].
Oleanolic acid 32, ursolic acid 31 and β-sitosterol 33 were isolated from the ethyl acetate fraction of the H. seravshanicus herb using CC and Sephadex LH-20 column chromatography in combination with semipreparative HPLC [75].
The authors of [76] studied the cell cultures of H. officinalis as a means of learning the extent of their ability to synthesize secondary metabolites. The TLC analysis of dichloromethane extracts of cultured cells revealed the presence of sterols and triterpenes [76].
Cell suspension cultures from H. officinalis hypocotyl-derived callus were found to produce two sterols named β-sitosterol 33 and stigmasterol 34; additionally, a number of known pentacyclic triterpenes with an oleanane and ursine skeleton were found. The triterpenes were recognized as oleanolic acid 32, ursolic acid 31, 2α, 3β-dihydroxyolean-12-en-28-oic acid 35, 2α, 3β-dihydroxyurs-12-en-28-oic acid 36, 2α, 3β, 24-trihydroxyolean-12-en-28-oic acid 37 and 2α,3β,24-trihydroxyurs-12-en-28-oic acid 38.
Daucosterol 39, ursolic acid 31 and 2α,3β,24-trihydroxy-12-en-28-ursolic acid 40 were obtained from the ethyl acetate fraction of H. cuspidatus aerial parts. The structures of these compounds were confirmed via analysis of mass and NMR data and compared with previously published data [77].
6. Phenolic Acids and Their Derivatives
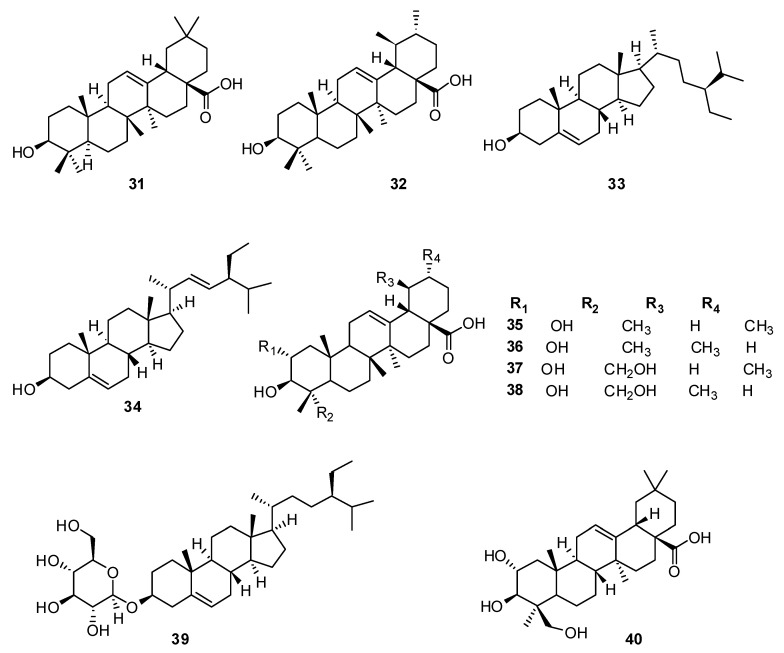
To date, about thirty phenolic acids and their derivatives have been isolated from the genus Hyssopus, including chlorogenic acid 41, protocatechuic acid 41, ferulic acid 43, lilac acid 44, hydroxybenzoic acid 45, caffeic acid 46, vanillic acid 47, p-coumaric acid 48, rosemary acid 49, gentisic acid 50 and phenylpropane 51 (Figure 4). These remain characteristic of the genus and are usually present in most Hyssopus species [78,79,80,81,82].
Figure 4.
Chemical structures of phenolic acids from plants of the genus Hyssop.
In terms of the dry raw materials, HPTLC analysis showed the presence of caffeic acid 46 (0.0064%) and ferulic acid 43 (0.034%) in the extract of H. officinalis [83].
HPLC DAD identified the following three phenolic acids: caffeic 46 (RT 8.65 min), ferulic 43 (RT 15.55 min) and rosemary 49 (RT 22.81 min) in a 70% ethanol extract of H. cuspidatus collected in 2018–2019. The contents of caffeic 46, ferulic 43, and rosmarinic acids 49 were 0.04–0.06%, 0.01–0.08 and 0.12–0.13% [84].
H. officinalis stem extract demonstrated the highest amount of total phenolic content at 374.60 ± 15.7 mg/g of gallic acid 52 [85].
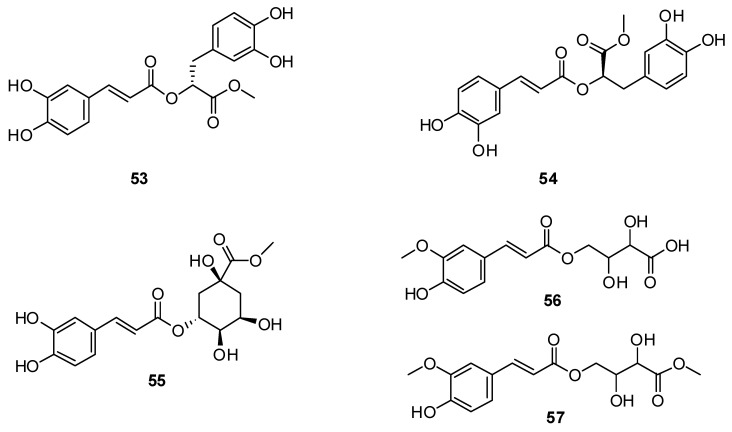
From 17 kg of H. cuspidatus, 17 compounds were obtained, from which rosmarinic acid 49 and methyl rosmarinate 53, 4-O-caffeoylquinic acid methyl ester 54, 3-O-caffeoylquinic acid methyl ester 55 and caffeic acid 46 were isolated for the first time [77]. In addition, two new phenolic acids (Figure 5), (E)-2,3-dihydroxy-4-((3-(4-hydroxy-3-methoxyphenyl)acryloyl)oxy)butanoic acid 56 and (E)-methyl 2,3-dihydroxy-4-((3-(4-hydroxy-3-methoxyphenyl)acryloyl)oxy)butanoate 57, were identified for the first time from H. cuspidatus, along with eleven known polyphenolic compounds [86].
Figure 5.
Chemical structures of derivatives of phenolic compounds from plants of the genus Hyssop.
Hydroxycinnamic acids were revealed as composing the majority of the extract isolated from H. officinalis, among which rosmarinic acid dominates 49. The content of vitamins was also determined in this extract including levels of ascorbic acid (9.50 mg/100 g) and carotenoids (0.66 mg/100 g) [67].
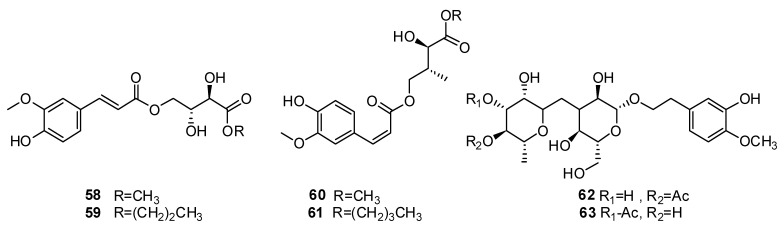
H. cuspidatus is a famous spice in Central Asia. In addition to the essential oil, non-volatile new compounds have been isolated from this plant species. The authors of [87] identified 64 compounds using LC-MS/MS, with phenolic compounds being the dominant components. The systematic separation and purification of H. cuspidatus ethanol extract resulted in the isolation of 34 compounds. The following 6 compounds (Figure 6) were identified as new compounds: hyssopusine A 58, hyssopusine B 59, hyssopusine C 60, hyssopusine D 61, 4′ ′-acetyldarendoside A 62 and 3′ ′-acetyldarendoside A—63, and 18 compounds were isolated from H. cuspidatus extract for the first time.
Figure 6.
Chemical structures of new compounds from H. cuspidatus.
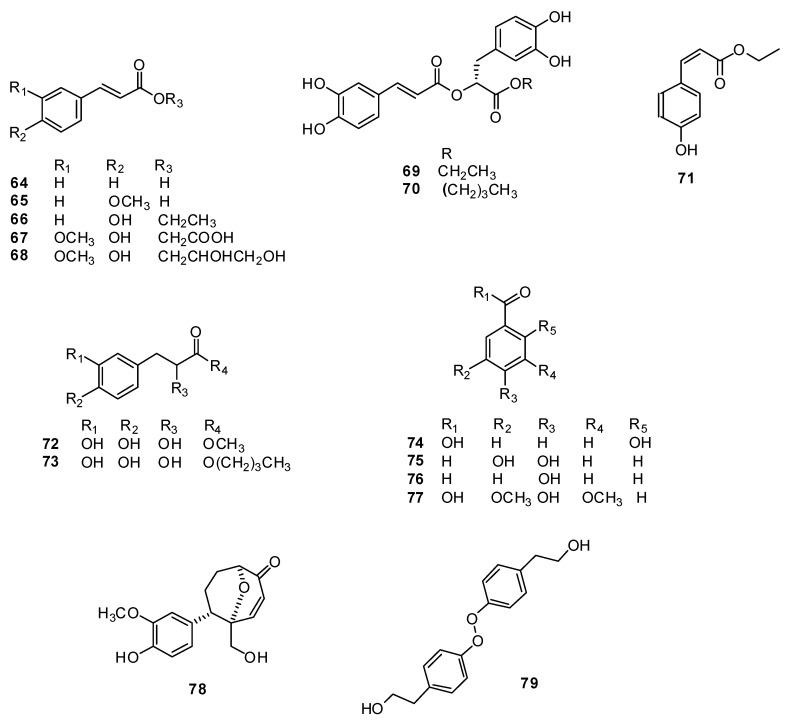
Among them were the following: €-cinnamic acid 64, 4-methoxycinnamic acid 65, ethyl p-coumarate 66, ferulic acid 43, carboxymethyl isoferulate 67, 1-O-feruloylglycerol 68, rosmarinic acid 49, methyl rosmarinate 53, ethyl rosmarinate 69, butyl rosmarinate 70, cis-p-coumaric acid ethyl ester 71, 5R-5-hydroxy methyl-2 (5H)-furanone 72, n-butyl-3,4-dihydroxy-phenyllactate 73, p-hydroxybenzoic acid 45, salicylic acid 74, protocatechuic aldehyde 75, p-hydroxybenzaldehyde 76, syringic acid 77, (+)-ligusticumtone 78 and p-hydroxy phenethyl alcohol 79 (Figure 7).
Figure 7.
Chemical structures of molecules of polyphenolic compounds from plants of the genus Hyssop.
7. Compounds of a Flavonoid and Flavone Glycoside Nature
At present, about thirty flavonoids (flavones, flavanones, flavonols and flavanols) and their derivatives have been identified, isolated from various species of Hyssopus.
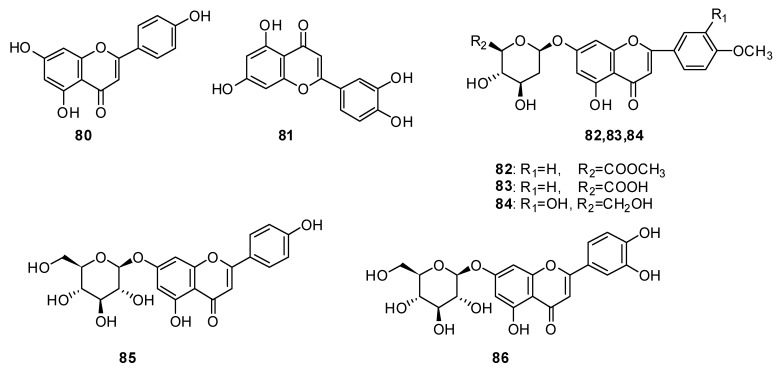
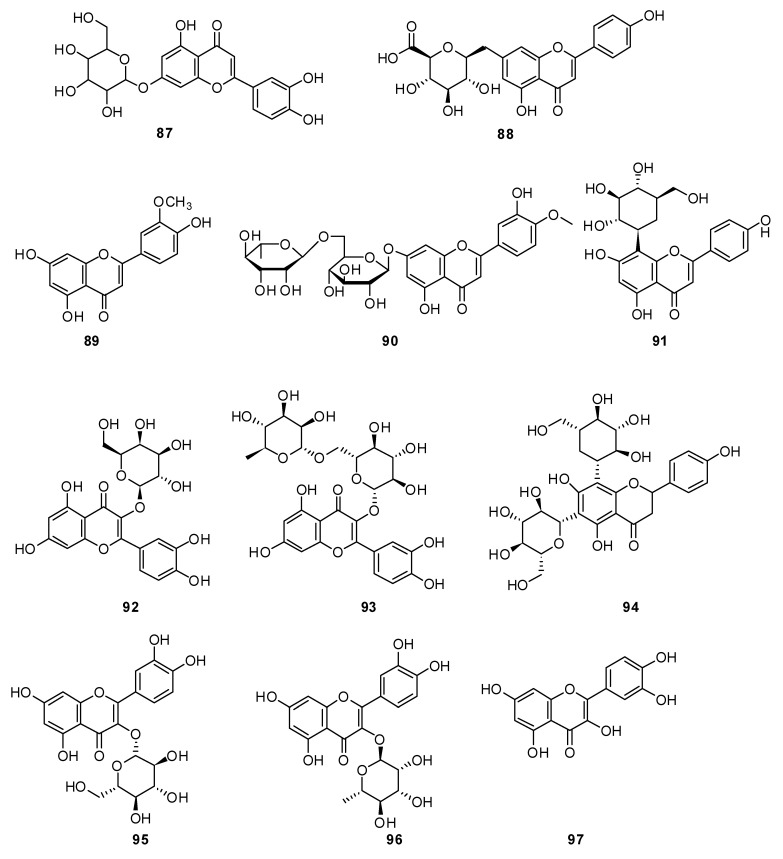
Apigenin 80, luteolin 81, acacetin-7-O-β−methyl glucuronide 82, acacetin-7-O-β−glucuronide 83, diosmetin 7-O-β−D-glucoside 84, apigenin 7-O-β−glucoside 85, luteolin-7-O-β-D-glucoside 86 and luteolin-7-O-β−D-galactoside 87 were first isolated from the genus H. officinalis (Figure 8) [88].
Figure 8.
Chemical structures of isolated flavonoids and flavonoid glycosides from plants of the genus Hyssop. Part 1.
Apigenin 7-O-β-D-glucuronide 88 was determined to be the main flavonoid of H. officinalis from Iran [48]. The following six flavonoids were isolated individually from the herb H. officinalis: chrysoeriol 89, diosmin 90, vitexin 91, hyperoside 92, rutin 93 and vicenin 94 (Figure 9) [89].
Figure 9.
Chemical structures of isolated flavonoids and flavonoid glycosides from plants of the genus Hyssop. Part 2.
Three flavonoid glycosides were detected in H. officinalis using HPLC-MS—isoquercitrin 95, rutin 93 and quercitrin 96, as well as two flavonoid aglycones—quercetin 97 and luteolin 81. Isoquercitrin 95 was the flavonoid discovered in the highest amount (32.78 ± 0.23 µg/g) [90].
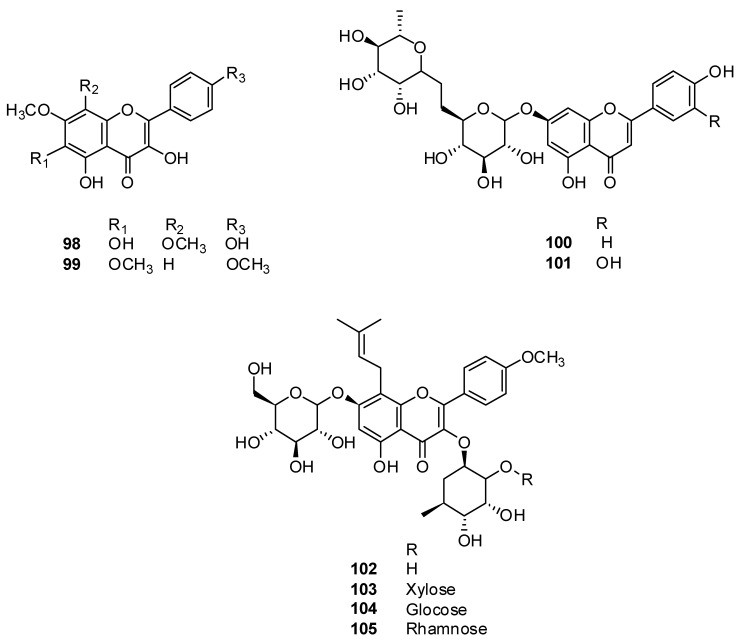
In China, scientists isolated for the first time eight flavonoids (Figure 10) from the H. cuspidatus herb: 5,6,4′-trihydroxyl-7,8-dimethoxyflavone 98, salvigenin 99, apigenin-7-rutinoside 100, luteolin-7-O-α-L-rhamnosyl (1 → 6)-β-D-glucoside 101, icariin 102, 4-methoxy-5-hydroxy-8–3,3-dimethylallyl flavone-3-O-β-D-xylopyranosyl (1 → 2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside 103, 4′-methoxy-5-hydroxy-8–3,3-dimethylallyl flavone-3-O-β-D-glucopyranosyl (1 → 2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside 104 and 4′-methoxy-5-hydroxy-8–3,3-dimethylallylflavone-3-O-β-L-rhamnopyranosyl (1 → 2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside 105 [87].
Figure 10.
Chemical structures of isolated flavonoids and flavnoid glycosides from H. cuspidatus.
8. Other Connections
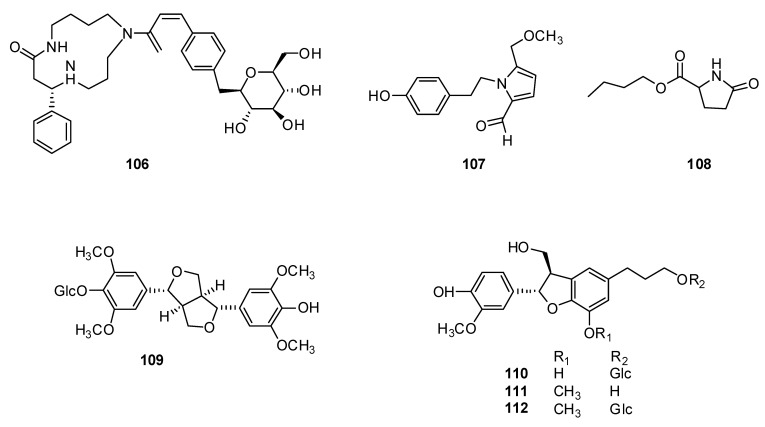
In addition, Chinese scientists isolated a new macrocyclic spermidine alkaloid, hyssopusizine 106, from an H. cuspidatus 95% ethanol extract [91] with 16 known compounds; this included for the first time the nitrogen-containing compounds (Figure 11) pyrrolezanthine-6-methylether 107 and n-butyl pyroglutamate 108 from the plants of the genus Hyssopus.
Figure 11.
Chemical structures of isolated nitrogen-containing and polyphenolic compounds from H. cuspidatus.
In addition, the lignan-type compounds syringaresinol 4′-O-β-D-glucopyranoside 109, dihydrodehydrodiconiferyl alcohol 9′-O-β-D-glucopyranoside 110, dihydrodehydrodiconiferyl alcohol 111 and (7R, 8S)-4,3′,9-trihydroxyl-3-methoxyl-7,8-dihydro-benzofuran-1′-propyl-neolignan 9′-O-β-D-glucopyranoside 112 were isolated from this compound [91].
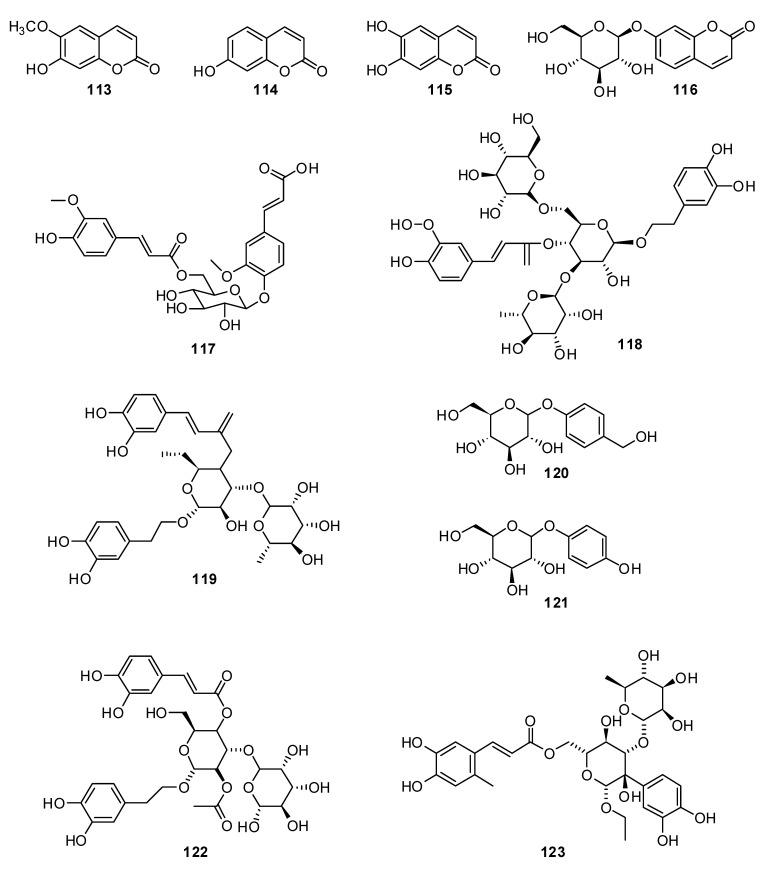
When conducting a qualitative analysis of the various groups of natural compounds in the H. officinalis herb, it is to be expected that phenolic compounds (coumarins, flavonoids, phenolcarboxylic acids, tannins, mainly condensed groups), polysaccharides, nitrogen-containing compounds (amino acids, nitrogenous bases), organic acids (citric, oxalic acid, tartaric acid, ascorbic acid), triterpene compounds and carotenoids will be encountered [89]. When quantitatively determined, it was found that the content of the sum of the nitrogenous bases in the studied H. officinalis herb ranged from 0.50% to 0.57% (including choline—from 0.08% to 0.10%; ascorbic acid—from 0.13% to 0.38%; the amount of free organic acids—from 5.07% to 13.87%; triterpene compounds—from 0.04% to 0.08%; tannins—from 18.32% to 19.24%; carotenoids—5.70 mg/g and essential oil—from 0.60% to 1.98%). The amino acids of the H. officinalis herb were represented by the following 11 compounds: aspartic acid, threonine, serine, glycine, alanine, valine, leucine, tyrosine, lysine, phenylalanine and histidine. The dominant free amino acids were threonine and serine (7.63 mg/g). The raw materials of H. officinalis were established to contain inorganic substances such as iron, potassium, sodium, calcium, magnesium, aluminum, silicon, copper, zinc, silver, strontium, phosphorus, manganese and titanium. The predominant macroelements were potassium (1%), calcium (1%) and magnesium (1%), and the microelements were silicon (0.3%) and aluminum (0.1%). Two coumarins (Figure 12), scopoletin 113 and umbelliferon 114, were also isolated from a 70% ethanol extract.
Figure 12.
Chemical structures of isolated polyphenolic compounds from H. cuspidatus.
The coumarins esculetin 114 and umbelliferon hexoside 116 were isolated and identified in H. cuspidatus [91].
The new glycoside hyssopuside 117 was isolated together with other phenolic glycosides 118–123 from H. cuspidatus [83].
9. Biological Activities
The species within the genus Hyssopus have primarily been evaluated for their potential anti-inflammatory, antioxidant, antibacterial, antifungal, and anti-asthmatic effects [47,52,53,64].
At present, H. officinalis is widely used in the culinary, food and medical industries. The green shoots of hyssop, cut before flowering, are used for medicinal purposes. In folk medicine, hyssop is used in the form of an infusion as an expectorant for chronic bronchitis, for asthma, as well as in chronic gastritis, as a wound-healing agent and an anti-sweating agent. A decoction of hyssop is used to wash the eyes and mouth during inflammatory processes and is used as a means of improving digestion [79].
An infusion of hyssop is recommended for older people as a general health drink and is also used for compresses for rheumatism, bruises, conjunctivitis and as a weak diuretic and carminative.
Using hyssop greens in the diet promotes digestion, increases appetite, tones the body and acts as a general tonic. Hyssop raw materials are used for bronchitis, catarrh of the upper respiratory tract, bronchial asthma, angina pectoris, neuroses, joint diseases, chronic colitis, flatulence, diabetes, as an anthelmintic and also as an antiseptic. Infusions and decoctions are used externally to wash the eyes, for stomatitis, diseases of the nasopharynx, for compresses for hemorrhages, bruises and as a wound-healing agent [83].
A range of the main pharmacological effects of different Hyssopus species are presented in Table 3.
Table 3.
Pharmacological activity of extracts, essential oils and individual compounds isolated from plants of the species Hyssopus L.
| Pharmacological Activity | Test Sample | Place of Growth | References |
|---|---|---|---|
| Antioxidant activity | H. officinalis essential oil; IC50 = 24.0 ± 0.2 µg/mL | Serbia | [47] |
| H. officinalis methanolic extract; IC50 = 0.50 µg/mL | India | [83] | |
| H. cuspidatus 70% ethanol extract AOA; IC50 of 0.0245 mg/mL | China | [84] | |
| H. cuspidatus compounds 109–111 ABTS; IC50 27.2–45.5 μM | China | [91] | |
| H. cuspidatus compounds 48, 52, 69 showed high AOA | China | [87] | |
| H. officinalis methanolic extracts DPPH; IC50 = 56.04–199.89 µg/mL, FRAP = 0.667–0.959 mmol Fe2+/g | Serbia | [92] | |
| N-butanol extract; IC50 = 25 mg/mL | Iran | [90] | |
| H. officinalis extract had a moderate lipid peroxidation and antioxidant activity | Iran | [93] | |
| Antimicrobial activity |
H. officinalis essential oil MIC: B. cereus, 14.20 µL/mL; E. coli, 227.25 µL/mL; E. faecalis, 454.50 µL/mL; P. aeruginosa, 454.50 µL/mL; S. enteritidis, 227.25 µL/mL; S. aureus, 227.25 µL/mL; S. epidermidis, 227.25 µL/mL; P. hauseri, 227.25 µL/mL H. officinalis essential oil MBC: B. cereus, 28.40 µL/mL, 227.25 µL/mL; E. faecalis, 454.50 µL/mL; P. aeruginosa, 454.50 µL/mL; S. enteritidis, 227.25 µL/mL; S. aureus, 227.25 µL/mL; S. epidermidis, 227.25 µL/mL; P. hauseri, 454.50 µL/mL |
Serbia | [51] |
|
H. officinalis essential oil growth of inhibition zones in the case of typical strains: S. aureus, 17.00 ± 0.20 mm; E. coli, 14.00 ± 0.56 mm; E. faecalis, 8.33 ± 0.33 mm; S. pyogenes, 11.00 ± 0.57 mm; C. albicans, 11.50 ± 0.20 mm H. officinalis essential oil growth of inhibition zones in the case of clinical strains: S. aureus, 20.00 ± 0.10 mm; E. coli, 10.66 ± 0.88 mm; S. pyogenes, 11.33 ± 0.33 mm; C. albicans, 12.00 ± 0.80 mm |
Czech Republic | [94] | |
| H. officinalis L. subsp. aristatus (Godr.) Nyman essential oil MIC against S. aureus and E. coli, 400 µg/mL | Montenegro and Serbia | [95] | |
| H. officinalis ethanolic extract biofilm formation against E. coli (95%). K. pneumoniae biofilm had a resistant biofilm structure between all tested bacteria (16.41%) | Iran | [96] | |
| H. officinalis ethanolic extract MIC: B. cereus, 1.562 µg/µL; S. marcescens, 6.25 µg/µL; P. aeruginosa, 3.125 µg/µL | Iran | [93] | |
| H. officinalis hydrolate had activity against natural test objects and recombinant bacteria E. coli (p Xen-lux) | Russia | [7] | |
| H. seravschanicus essential oil MIC: B. cereus and S. aureus, 312 µg/mL; P. aeruginosa, E. coli, C. albicans and A. niger, 625 µg/mL | Tajikistan | [64] | |
|
H. officinalis methanolic extract MIC: B. cereus and S. aureus, 25 mg/mL; P. aeruginosa and E. coli, 50 mg/mL H. officinalis methanolic extract MBC: B. cereus and S. aureus, 50 mg/mL; P. aeruginosa and E. coli, 100 mg/mL |
Iran | [97] | |
|
H. officinalis L. white-flowered essential oil MIC and MBC: S. aureus (10 mg/mL, 20 mg/mL), S. epidermidis (5 mg/mL, 10 mg/mL), B. subtilis (5 mg/mL, 5 mg/mL), M. luteus (2.5 mg/mL, 5 mg/mL), E. coli (5 mg/mL, 10 mg/mL), K. pneumoniae (5 mg/mL, 10 mg/mL), P. aeruginosa (5 mg/mL, 10 mg/mL) H. officinalis L. pink-flowered essential oil MIC and MBC: S. aureus (5 mg/mL, 10 mg/mL), S. epidermidis (2.5 mg/mL, 5 mg/mL), B. subtilis (0.625 mg/mL, 2.5 mg/mL), M. luteus (2.5 mg/mL, 5 mg/mL), E. coli (5 mg/mL, 5 mg/mL), K. pneumoniae (5 mg/mL, 10 mg/mL), P. aeruginosa (5 mg/mL, 10 mg/mL) |
Poland | [52] | |
| Antifungal activity |
Compounds 105, 109–110 from H. cuspidatus exhibited inhibitory effects against the proliferation of C. albicans with inhibitory zone diameters from 7.5 to 12.0 mm | China | [91] |
| H. officinalis essential oil showed activity against S. pyogenes, S. aureus, C. albicans and E. coli with inhibition zone diameters of 19.0 ± 0.1 mm, 18.0 ± 1.7 mm, 20.3 ± 1.8 mm and 15.0 ± 1.0 mm | Turkey | [33] | |
| H. officinalis L. var decumbens (Jordan & Fourr.) Briq. from France (Banon) and H. officinalis L. from Italy (Piedmont) essential oils were active against C. albicans, C. krusei and C. tropicis | France and Italy | [98] | |
|
H. officinalis L. white-flowered essential oil MIC and MBC: C. albicans (0.625 mg/mL, 2.5 mg/mL), C. parapsilosis (1.25 mg/mL, 5 mg/mL) H. officinalis L. pink-flowered essential oil MIC and MBC: C. albicans (0.625 mg/mL, 2.5 mg/mL), C. parapsilosis (0.625 mg/mL, 1.25 mg/mL) |
Poland | [52] | |
| H. officinalis essential oil demonstrated inhibition of ATPase enzyme and increased the membrane permeability in Candida species. This effect was caused due to synergistic effects of chemical constituents from essential oils like β-pinene, α-pinene, trans-pinocamphone and cis-pinocamphone | Bulgaria | [53] | |
| Anti-inflammatory activity |
Phenolic glycoside 116 isolated from H. cuspidatus could reduce NO production and inhibit TNF-α, IL-6 and IL-1β | China | [92] |
| H. cuspidatus essential oil had an anti-inflammatory effect of 0.4 mL/kg, which exceeds aspirin. H. cuspidatus essential oil was noted in inhibiting the production of TNF-α, IL-1β, IL-6 and PGE2 and significantly reduced the MDA and NO levels | China | [99] | |
| H. officinalis extract at doses of 25, 50 and 75 mg/kg/bw (13.33 ± 3.1, 20 ± 3.1, 19.83 ± 2.8) demonstrated high anti-inflammatory effects against Xylene-induced ear edema | Iran | [100] | |
| Anti-asthmatic activity |
Treatment with H. cuspidatus extract reduced the amount of sputum and decreased the infiltration of inflammatory cells around the bronchi in mice. The extract had a significant ameliorative effect on ovalbumin-induced asthma | China | [101] |
| H. cuspidatus ethanolic extract and the rosmarinic acid isolated from it had anti-asthmatic activity | China | [102] | |
| H. officinalis L. extract affected interleukin-4, -6 and -17 and interferon-γ levels in asthmatic mice and inhibited the invasion of EOS | China | [103] | |
| Antitumor activity |
H. officinalis L. essential oil using the MTT test showed antitumor activity against the tumor cell lines SW480, MDA-MB 231, HeLa and MRC-5 | Serbia | [92] |
| ZnO nanoparticles using H. officinalis extract disrupted spermatogenesis, the sperm maturation process and sperm motility. The IC50 for the PC3 cell line treated with ZnO nanoparticles for 24 and 48 h was recorded at 8.07 and 5 μg/mL, and induced apoptosis was 26.6% ± 0.05, 44% ± 0.12 and 80% ± 0.07 for the PC3 cells | Iran | [104] | |
| H. officinalis ethanolic extract concentration of 500 mg/mL showed 82% cytotoxic effect for breast cancer cells | India | [19] | |
| Antidiabetic activity | Aerial parts of H. officinalis L. were screened for determination of antidiabetic activity using an alpha-amylase inhibition assay, namely, a starch iodine assay model, and found an IC50 = 0.8366 mg/mL | India | [105] |
| Antiviral activity |
H. officinalis L. methanolic extract demonstrated antiviral effects against HSV at an oral dose of 125 mg/kg in mice | Iran | [106] |
| H. officinalis methanolic extract demonstrated significant anti-HIV activity due to the high content of caffeic acid | USA | [107] | |
| The polysaccharide MAR-10 isolated from the methanol extract of H. officinalis leaves inhibited human immunodeficiency virus type 1 replication in HUT78 T cells and peripheral blood mononuclear cells in a concentration-dependent manner | China | [108] | |
| Antispasmodic activity |
H. officinalis L. essential oil inhibited the acetylcholine- and BaCl2-induced contractions, with an IC50 of 37 μg/mL and 60 μg/mL, respectively | China | [109] |
| Anti-leishmaniasis activity | H. officinalis extract ointment showed significant effectiveness against cutaneous leishmaniasis due to the release of nitric acid and tumor necrosis factor from the macrophages | Iran | [110] |
| Anticonvulsant activity | The water hyssop extracts, having a concentration of 100 mg/kg, showed anticonvulsant action and caused a significant increase in iNOS gene expression in the hippocampus | Iran | [111] |
| Insecticidal activity | H. cuspidatus essential oil possessed fumigant toxicity against S. zeamais adults, with an LD50 = 24.44 μg/adult and LC50 = 16.72 mg/L | China | [29] |
| Mosquito larvicidal activity | H. officinalis essential oil in an acute toxicity study against Culex mosquitos revealed an LC50 of more than 90 μL/L | France | [71] |
| Myorelaxation activity | The inhalation of hyssop essential oil increased the immobile position and may have caused a sedative effect in mice | Iran | [112] |
10. Conclusions
The chemical composition of the plant H. officinalis, which is one of the most popular species, distributed mainly from the Eastern Mediterranean to Central Asia, has been sufficiently studied. The plant has traditionally been used for medicinal purposes. The raw materials contain essential oils, flavonoids and polyphenolic acids. The flower tips contain ursolic acid and the glucoside diosmin. The main components of the essential oil are bicyclic monoterpenes (L-pinocamphene, cis-pinocamphone, pinocarvone, β-pinene), depending on the chemotype of the plant. The main components of H. officinalis include apigenin, quercetin, diosmin, luteolin and their glycosides, chlorogenic, protocatechuic, ferulic, lilac, p-hydroxybenzoic, caffeic and other acids. H. officinalis has a moderate antioxidant effect and antimicrobial activity against Gram-positive and Gram-negative bacteria; pronounced antifungal, insecticidal and antiviral properties were also identified in vitro. The studies on animal models have indicated muscle relaxant and antiplatelet properties. However, human studies, investigation of adverse reactions and clinical trials are lacking and further study is needed.
For the H. ambiguous plant growing in Central Kazakhstan, the chemical composition of the essential oil was ascertained. The main component is 1,8-cineol; therefore, its antimicrobial properties were studied and, based on the data obtained, methods were developed for obtaining essential oil compositions with a pleasant odor for the further development of a high-quality inhalation form. In addition, the creation of relatively inexpensive therapeutic, treatment and prophylactic agents that can effectively combat the infectious diseases of the upper respiratory tract was investigated. The plant was not studied for the content of other biologically active compounds.
H. cuspidatus is a famous Chinese plant and is frequently used in traditional Uyghur folk medicine to treat cough, asthma, bronchitis and rheumatism. The raw material contains essential oil, which is currently the most studied functional natural component; additionally, polyphenols, flavonoids, triterpenes and steroids are studied as the other principal components. Currently, a number of new compounds have been isolated from this plant species. Modern pharmacological research has shown that H. cuspidatus can reduce or improve airway inflammation, lower blood sugar, eliminate phlegm and relieve cough, and also has many biological properties such as antibacterial, antioxidant and antitumor.
H. seravschanicus is distributed in the mountain forests, valleys and gorges of Central Asia. The plant has been used in medicinal practice since ancient times; however, in modern folk and scientific medicine, it is only occasionally used. The plant is cultivated in some European countries. The chemical composition of the plant has not been sufficiently studied, but it has been identified that it contains essential oil, flavonoids, glycosides and steroids. In Tajikistan, the plant is being used to develop antimicrobial medicinal forms based on the essential oil.
The scientific research has also confirmed its antispasmodic properties and has shown that hyssop essential oil has both an antiseptic and sedative effect.
The long-term studies from scientific centers in a number of countries, including China, Russia, Iran, Bulgaria, Turkey, Bulgaria, Tajikistan, Kazakhstan, etc., have focused on the chemical composition and pharmacological activity of the Hyssopus species and have shown that the chemical composition of the plants varies significantly, based on abiotic and biotic factors, as well as extraction methods. The variability in the chemical composition (both qualitative and quantitative) of a plant extract or essential oil can lead to significant differences in its pharmacological activity. Currently, not all Hyssopus species have been studied for their chemical composition and biological activity.
At the same time, there is no information about existing or proposed non-clinical and clinical developments and side effects, which therefore requires additional research.
The extracts, essential oils and individual compounds isolated from Hyssopus are attracting increasing attention as a valuable source for drug development and complementary health products. At the same time, it is necessary to take into account the isolation procedures, different chemotypes, time and place of collection and different biological activities for the development of new drugs based on the Hyssopus species; it is clear that plants of the genus require more careful and in-depth study.
Thus, the biologically active compounds of plants of the genus Hyssopus are a promising source for the development and introduction into medicine of new innovative highly effective herbal medicines with a wide spectrum of action.
Acknowledgments
The authors of the article express gratitude to the university management for the opportunity to carry out this research.
Author Contributions
Conceptualization, G.A. and M.I.; writing—original draft preparation, Y.L. (Yana Levaya), Y.L. (Yekaterina Lakomkina) and M.S.; writing—review and editing, G.A. and M.I.; visualization, G.A. and Y.L. (Yana Levaya); supervision, G.A. and M.I.; project administration, M.I.; funding acquisition, M.I. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan; grant number AP19677164 “Development of new cosmeceutical agents of antioxidant action based on domestic plant raw materials”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Govaerts R. World Checklist of Selected Plant Families. In: Bánki O., Roskov Y., Vandepitte L., DeWalt R.E., Remsen D., Schalk P., Orrell T., Keping M., Miller J., et al., editors. Catalogue of Life Checklist. Science; Almaty, Kazakhstan: 2017. [DOI] [Google Scholar]
- 2.Harley R.M., Atkins S., Budantsev A.L., Cantino P.D., Conn B.J., Grayer R., Harley M.M., de Kok R.P.J., Krestovskaja T., Morales R., et al. Flowering Plants. Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae) In: Kubitzki K., Kadereit J.W., editors. The Families and Genera of Vascular Plants. Volume 7. Springer; Berlin/Heidelberg, Germany: 2004. pp. 167–275. [Google Scholar]
- 3.Kew Plants of the World Online. [(accessed on 15 March 2024)]. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30022219-2#distributions.
- 4.WFO Hyssopus L. [(accessed on 16 May 2024)]. Available online: http://www.worldfloraonline.org/taxon/wfo-4000018903.
- 5.Pavlov N.V. Flora of Kazakhstan. Volume 7. Science; Almaty, Kazakhstan: 1964. pp. 440–442. [Google Scholar]
- 6.Baytenov M.S. Flora of Kazakhstan. Gylym; Almaty, Kazakhstan: 2001. Generic complex of flora; p. 183. [Google Scholar]
- 7.Burtseva Y.V., Kuldyrkaeva E.V., Mekhonoshina I.S., Timasheva L.A., Pekhova O.A., Katsev A.M. Study of the chemical composition and biological action of Hyssopus officinalis L. Hydrolate. Med. Pharm. J. Pulse. 2023;25:25–34. doi: 10.33380/2305-2066-2023-12-4-1526. [DOI] [Google Scholar]
- 8.Jankovský M., Landa T. Genus Hyssopus L.—Recent knowledge. Hort. Sci. 2002;29:119–123. doi: 10.17221/4474-HORTSCI. [DOI] [Google Scholar]
- 9.Tahir M., Khushtar M., Fahad M., Rahman M.A. Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.) J. Appl. Pharm. Sci. 2018;8:132–140. doi: 10.7324/JAPS.2018.8721. [DOI] [Google Scholar]
- 10.Fathiazad F., Hamedeyazdan S. A review on Hyssopus officinalis L.: Composition and biological activities. Afr. J. Pharm. Pharmacol. 2011;5:959–1966. doi: 10.5897/AJPP11.527. [DOI] [Google Scholar]
- 11.Sharifi-Rad J., Quispe C., Kumar M., Akram M., Amin M., Iqbal M., Koirala N., Sytar O., Kregiel D., Nicola S., et al. Hyssopus Essential Oil: An Update of Its Phytochemistry, Biological Activities, and Safety Profile. Oxidative Med. Cell. Longev. 2022;2022:8442734. doi: 10.1155/2022/8442734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesołowska A., Jadczak D., Grzeszczuk M. Essential oil composition of hyssop (Hyssopus officinalis L.) cultivated in north-western Poland. Herba Pol. 2010;56:57–65. [Google Scholar]
- 13.Kerrola K., Galambosi B., Kallio H. Volatile components and odor intensity of four phenotypes of hyssop (Hyssopus officinalis L.) J. Agric. Food Chem. 1994;42:776–781. doi: 10.1021/jf00039a035. [DOI] [Google Scholar]
- 14.Kazazi H. Supercriticial fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chem. 2007;105:805–811. doi: 10.1016/j.foodchem.2007.01.059. [DOI] [Google Scholar]
- 15.Mićić V., Begić S., Dugić P., Petrović Z. Application of mathematical model of naik for determination of total extract yield of hyssop obtained by supercritical extraction with carbon dioxide. Contemp. Mater. 2017;6:68–72. doi: 10.7251/COMEN1701067M. [DOI] [Google Scholar]
- 16.Rashidi S., Eikani M.H., Ardjmand M. Extraction of Hyssopus officinalis L. essential oil using instant controlled pressure drop process. J. Chromatogr. A. 2018;1579:9–19. doi: 10.1016/j.chroma.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Kanevskaya A.A., Tarasov V.E. Current State, Problems and Prospects for the Development of Agricultural Science. IT “ARIAL; Simferopol, Russia: 2023. Peculiarities of processing and study of the chemical composition of medicinal hyssop Hyssopus officinalis L. pp. 67–68. [Google Scholar]
- 18.Aziminezhad H., Esmaeilzadehkenari R., Raftani Amiri Z. Investigating the effect of extraction of bath ultrasound in different conditions on antioxidant properties of Hyssop (Hyssopus officinalis) extract. FSCT. 2020;17:159–168. doi: 10.29252/fsct.17.01.14. [DOI] [Google Scholar]
- 19.Nile S.H., Nile A.S., Keum Y.S. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. Biotech. 2017;7:76. doi: 10.1007/s13205-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadian S., Kenari R.E., Amiri Z.R., Sohbatzadeh F., Khodaparast M.H.H. Effect of ultrasound-assisted cold plasma pretreatment on cell wall polysaccharides distribution and extraction of phenolic compounds from hyssop (Hyssopus officinalis L.) Int. J. Biol. Macromol. 2023;233:123557. doi: 10.1016/j.ijbiomac.2023.123557. [DOI] [PubMed] [Google Scholar]
- 21.Benea A., Ciobanu C., Ciobanu N., Pompus I., Cojocaru-Toma M. Polyphenolic content and antioxidant activity of Hyssopus officinalis L. from the Republic of Moldova. Mold. Med. J. 2022;65:41–46. doi: 10.52418/moldovan-med-j.65-2.22.06. [DOI] [Google Scholar]
- 22.Malyushevskaya A., Koszelnik P., Yushchishina A., Mitryasova O., Gruca-Rokosz R. Green Approach to Intensify the Extraction Processes of Substances from Plant Materials. J. Ecol. Eng. 2022;23:197–204. doi: 10.12911/22998993/150060. [DOI] [Google Scholar]
- 23.LinkedIn. [(accessed on 10 August 2023)]. Available online: https://www.linkedin.com/pulse/hyssop-oil-market-size-analyzing-trends-forecasting?trk=pulse-article_more-articles_related-content-card.
- 24.Lakomkina E.V., Akhmetova S.B., Atazhanova G.A., Ishmuratova M.Y. Component composition and antimicrobial activity of samples of Hyssopus ambiguus (Trautv.) Iljin essential oil collected in the territory of the Karaganda region. Pharm. Kazakhstan. 2023;5:374–379. doi: 10.53511/PHARMKAZ.2023.46.10.049. [DOI] [Google Scholar]
- 25.Lakomkina E.V., Atazhanova G.A., Akhmetova S.B., Zilfikarov I.N. Development of composition and technology for obtaining antimicrobial composition based on mono- and sesquiterpenoids. Pharm. Pharmacol. 2023;11:114–126. doi: 10.19163/2307-9266-2023-11-2-114-126. [DOI] [Google Scholar]
- 26.Duzbayeva N., Ibrayeva M., Kabdysalym K., Mukazhanova Z., Adhikari A. Component composition and biological activity of essential oil of Hyssopus cuspidatus plants. Rep. Natl. Acad. Sci. Repub. Kazakhstan. 2023;4:169–178. doi: 10.32014/2023.2518-1483.251. [DOI] [Google Scholar]
- 27.Zhou X., Hai-Yan G., Tun-Hai X., Tian S. Physicochemical evaluation and essential oil composition analysis of Hyssopus cuspidatus Boriss from Xinjiang, China. Pharmacogn. Mag. 2010;6:278–281. doi: 10.4103/0973-1296.71790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ablizl P., Cong Y., Musa M., Zhu Y., Kasimu R. Chemical composition of the essential oil of Hyssopus cuspidatus from Xinjiang, China. Chem. Nat. Compd. 2009;45:445. doi: 10.1007/s10600-009-9309-y. [DOI] [Google Scholar]
- 29.Li H.T., Zhao N.N., Yang K., Liu Z.L., Wang Q. Chemical composition and toxicities of the essential oil derived from Hyssopus cuspidatus flowering aerial parts against Sitophilus zeamais and Heterodera avenae. J. Med. Plants Res. 2013;7:343–348. [Google Scholar]
- 30.Moulodi F., Khezerlou A., Zolfaghari H., Mohamadzadeh A., Alimoradi F. Chemical Composition and Antioxidant and Antimicrobial Properties of the Essential Oil of Hyssopus officinalis L. J. Kermanshah Univ. Med. Sci. 2018;22:e85256. doi: 10.5812/jkums.85256. [DOI] [Google Scholar]
- 31.Mitić V., Đorđević S. Essential oil composition of Hyssopus officinalis L. Cultivated in Serbia. Facta Univ. Ser. Phys. Chem. Technol. 2000;2:105–108. [Google Scholar]
- 32.Eldeghedy H.I., El-Gendy A.E.-N.G., Nassrallah A.A., Aboul-Enein A.M., Omer E.A. Essential oil composition and biological activities of Hyssopus officinalis and Perilla frutescens. Int. J. Health Sci. 2022;6:9963–9982. doi: 10.53730/ijhs.v6nS6.12566. [DOI] [Google Scholar]
- 33.Kizil S., Haşimi N., Tolan V., Kilinç E., Karataş H. Chemical Composition, Antimicrobial and Antioxidant Activities of Hyssop (Hyssopus officinalis L.) Essential Oil. Not. Bot. Horti Agrobot. Cluj-Napoca. 2010;38:99–103. doi: 10.15835/nbha3834788. [DOI] [Google Scholar]
- 34.Kürkçüoğlu M., Eser S.A., Başer K.H.C. Composition of the essential oil of the Hyssopus officinalis L. subsp. angustifolius (Bieb.) Arcangeli. Nat. Volatiles Essent. Oils. 2016;3:15–19. [Google Scholar]
- 35.Aćimović M., Stanković J., Cvetković J.M., Kiprovski B., Marjanović-Jeromela A., Rat M., Malenčić D. Analiza hemijskog sastava etarskihul jarazličitih genotipov aizopaiz IFVCNS kolekcijelekovitogbilja. Ann. Agron. 2019;43:38–45. [Google Scholar]
- 36.Bernotienė G., Butkienė R. Essential oils of Hyssopus officinalis L. cultivated in East Lithuania. Chemija. 2010;21:135–138. [Google Scholar]
- 37.Radjabov G.K., Musaev A.M., Islamova F.I., Aliev A.M. Analysis of the accumulation of volatile organic compounds in Hyssopus officinalis L. plants introduced in mountain conditions. Sustain. Dev. Mt. Territ. 2023;15:174–181. doi: 10.21177/1998-4502-2023-15-1-174-181. [DOI] [Google Scholar]
- 38.Zawiślak G. Morphological characters of Hyssopus officinalis L. and chemical composition of its essential oil. Mod. Phytomorphol. 2013;4:93–95. doi: 10.5281/zenodo.161195. [DOI] [Google Scholar]
- 39.Saebi A., Minaei S., Mahdavian A.R., Ebadi M.T. Quantity and Quality of Hyssop (Hyssopus officinalis L.) Affected by Precision Harvesting. Int. J. Hortic. Sci. Technol. 2021;8:291–304. doi: 10.22059/ijhst.2020.298266.346. [DOI] [PubMed] [Google Scholar]
- 40.Velikorodov A.V., Kovalev V.B., Kurbanova F.K., Shchepetova E.V. Chemical composition of Hyssopus officinalis L. essential oil cultivated in the Astrakhan region. Chem. Plant Raw Mater. 2015;3:71–76. [Google Scholar]
- 41.Plugatar Y.V., Bulavin I.V., Ivanova N.N., Miroshnichenko N.N., Saplev N.M., Shevchuk O.M., Feskov S.A., Naumenko T.S. Study of the Component Composition of Essential Oil, Morphology, Anatomy and Ploidy Level of Hyssopus officinalis f. cyaneus Alef. Horticulturae. 2023;9:480. doi: 10.3390/horticulturae9040480. [DOI] [Google Scholar]
- 42.Pirbalouti A.G., Bajalan I., Malekpoor F. Chemical compositions and antioxidant activity of essential oils from inflorescences of two landraces of hyssop [Hyssopus officinalis L. subsp. angustifolius (Bieb.)] cultivated in Southwestern, Iran. J. Essent. Oil Bear. Plants. 2019;22:1074–1081. doi: 10.1080/0972060X.2019.1641431. [DOI] [Google Scholar]
- 43.Garg S. Composition of essential oil from an annual crop of Hyssopus officinalis grown in Indian plains. Flavour Fragr. J. 1999;14:170–172. doi: 10.1002/(SICI)1099-1026(199905/06)14:3<170::AID-FFJ808>3.0.CO;2-Q. [DOI] [Google Scholar]
- 44.Figueredo G., Özcan M.M., Chalchat J.C., Bagci Y., Chalard P. Chemical Composition of Essential Oil of Hyssopus officinalis L. and Origanum acutiden. Jeobp. 2012;15:300–306. doi: 10.1080/0972060X.2012.10644051. [DOI] [Google Scholar]
- 45.Said-Al Ahl H.A.H., Abbas Z.H., Sabra A.S., Tkachenko K.G. Essential Oil Composition of Hyssopus officinalis L. Cultivated in Egypt. Int. J. Plant Sci. Ecol. 2015;1:49–53. [Google Scholar]
- 46.Khan R., Shawl A.S., Tantry M.A. Determination and seasonal variation of chemical constituents of essential oil of Hyssopus officinalis growing in Kashmir valley as incorporated species of western Himalaya. Chem. Nat. Compd. 2012;48:502–505. doi: 10.1007/s10600-012-0290-5. [DOI] [Google Scholar]
- 47.Aćimović M., Pezo L., Zeremski T., Lončar B., Marjanović Jeromela A., Stanković Jeremic J., Cvetković M., Sikora V., Ignjatov M. Weather Conditions Influence on Hyssop Essential Oil Quality. Processes. 2021;9:1152. doi: 10.3390/pr9071152. [DOI] [Google Scholar]
- 48.Fathiazad F., Mazandarani M., Hamedeyazdan S. Phytochemical analysis and antioxidant activity of Hyssopus officinalis L. from Iran. Adv. Pharm. Bull. 2011;1:63–67. doi: 10.5681/apb.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zawislak G. Essential oil composition of Hyssopus officinalis L. grown in Poland. J. Essent. Oil-Bear. Plant. 2016;19:699–705. doi: 10.1080/0972060X.2014.935034. [DOI] [Google Scholar]
- 50.Ortiz de Elguea-Culebras G., Sánchez-Vioque R., Berruga M.I., Herraiz-Peñalver D., González-Coloma A., Andrés M.F., Santana-Méridas O. Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. super, and Santolina chamaecyparissus. Chem. Biodivers. 2018;15:e1700313. doi: 10.1002/cbdv.201700313. [DOI] [PubMed] [Google Scholar]
- 51.Aćimović M., Todosijević M., Varga A., Kiprovski B., Tešević V., Čabarkapa I., Sikora V. Bioactivity of essential oils from cultivated winter savory, sage and hyssop. Lekovitesirovine. 2019;39:11–17. doi: 10.5937/leksir1939011A. [DOI] [Google Scholar]
- 52.Baj T., Korona-Głowniak I., Kowalski R., Malm A. Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers. Open Chem. 2018;16:317–323. doi: 10.1515/chem-2018-0032. [DOI] [Google Scholar]
- 53.Hristova Y., Wanner J., Jirovetz L., Stappen I., Iliev I., Gochev V. Chemical composition and antifungal activity of essential oil of Hyssopus officinalis L. from Bulgaria against clinical isolates of Candida species. Biotechnol. Biotechnol. Equip. 2015;29:592–601. doi: 10.1080/13102818.2015.1020341. [DOI] [Google Scholar]
- 54.Moghtader M. Comparative evaluation of the essential oil composition from the leaves and flowers of Hyssopus officinalis L. J. Hortic. For. 2014;6:1–5. doi: 10.5897/JHF2013.0318. [DOI] [Google Scholar]
- 55.Tsankova E.T., Konaktchiev A.N., Genova E.M. Chemical composition of the essential oils of two Hyssopus officinalis taxa. J. Essent. Oil Res. 1993;5:609–611. doi: 10.1080/10412905.1993.9698292. [DOI] [Google Scholar]
- 56.Salma A.S., El Sawi A.A. Chemical and physiological studies on Anise hyssop [Agastache foeniculumpursh] and hyssop [Hyssopus officinalis L.] plants grown in Egypt as new spices. Bull. Natl. Res. Cent. 2002;27:25–35. [Google Scholar]
- 57.Shah N.C. Chemical constituents of Hyssopus officinalis L.: ‘ZufahYabis’ A Unani drug from UP Himalaya, India. Indian Perfum. 1991;35:49–52. [Google Scholar]
- 58.Salvatore G., D’Andrea A., Nicoletti M. A pinocamphone poor oil of Hyssopus officinalis L. var. decumbens from France (Barton) J. Essent. Oil Res. 1998;10:563–567. doi: 10.1080/10412905.1998.9700972. [DOI] [Google Scholar]
- 59.Gorunovic M.S., Bogavac P.M., Chalchat J.C., Chabard J.L. Essential oil of Hyssopus officinalis L., Lamiaceae of Montenegro origin. J. Essent. Oil Res. 1995;7:39–43. doi: 10.1080/10412905.1995.9698459. [DOI] [Google Scholar]
- 60.Cvijovic M., Djukic D., Mandic L., Acamovic-Djokovic G., Pesakovic M. Composition and antimicrobial activity of essential oils of some medicinal and spice plants. Chem. Nat. Compd. 2010;46:481–483. doi: 10.1007/s10600-010-9652-z. [DOI] [Google Scholar]
- 61.Vallejo M.C.G., Herraiz J.G., Pérez-Alonso M.J., Velasco-Negueruela A. Volatile oil of Hyssopus officinalis L. from Spain. J. Essent. Oil Res. 1995;7:567–568. doi: 10.1080/10412905.1995.9698590. [DOI] [Google Scholar]
- 62.Özer H., Şahin F., Kılıç H., Güllüce M. Essential oil composition of Hyssopus officinalis L. subsp. angustifolius (Bieb.) Arcangeli from Turkey. Flavour Fragr. J. 2005;20:42–44. doi: 10.1002/ffj.1421. [DOI] [Google Scholar]
- 63.Kovtun-Vodyanytska S.M., Levchuk I.V., Golubets O.V., Rakhmetov D.B. Biochemical composition of essential oil of Hyssopus seravschanicus (Dubj.) Pazij. (Lamiaceae) introduced into the conditions of Ukraine (Forest-Steppe Zone) Stud. Biol. 2023;17:61–68. doi: 10.30970/sbi.1701.702. [DOI] [Google Scholar]
- 64.Sharopov F.S., Kukaniev M.A., Thompson R.M., Satyal P., Setzer W.N. Composition and antimicrobial activity of the essential oil of Hyssopus seravschanicus growing wild in Tajikistan. Der Pharma Chem. 2012;4:961–966. [Google Scholar]
- 65.Shevchuk O.M., Korotkov O.I., Malaeva E.V., Feskov S.A. Component composition of Hyssopus cretaceous Dubj essential oil. and Hyssopus officinalis L. Ind. Bot. 2019;19:49–54. [Google Scholar]
- 66.Pekhova O.A., Timasheva L.A., Danilova I.L., Belova I.V. Dynamics of accumulation of biologically active substances in Hyssopus officinalis L. plants grown in the pediguntary zone of the Crimea. Tauride Bull. Agrar. Sci. 2021;4:138–148. [Google Scholar]
- 67.Grebennikova O., Paliy A., Khlypenko L., Rabotyagov V. Biologically active substances of Hyssopus officinalis L. Orbital Mag. 2017;1:21–28. [Google Scholar]
- 68.Rabotyagov V.D., Shibko A.N. Research of the Component Composition of Hyssopus officinalis L. Essential Oil. Collect. Sci. Work. GNBS. 2014;139:94–106. [Google Scholar]
- 69.Džamić A.M., Soković M.D., Novaković M., Jadranin M., Ristić M.S., Tešević V., Marin P.D. Composition, antifungal and antioxidant properties of Hyssopus officinalis L. subsp. pilifer (Pant.) Murb. essential oil and deodorized extracts. Ind. Crops Prod. 2013;51:401–407. doi: 10.1016/j.indcrop.2013.09.038. [DOI] [Google Scholar]
- 70.Dehghanzadeh N., Ketabchi S., Alizadeh A. Essential oil composition and antibacterial activity of Hyssopus officinalis L. grown in Iran. Asian J. Exp. Biol. Sci. 2012;3:767–771. [Google Scholar]
- 71.Benelli G., Pavela R., Canale A., Cianfaglione K., Ciaschetti G., Conti F., Maggi F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017;66:166–171. doi: 10.1016/j.parint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Aihaiti K., Li J., Xu N.N., Tang D., Aisa H.A. Monoterpenoid derivatives from Hyssopus cuspidatus Boriss. and their bioactivities. Fitoterapia. 2023;165:105432. doi: 10.1016/j.fitote.2023.105432. [DOI] [PubMed] [Google Scholar]
- 73.Zotov E.P. Triterpenoids and steroids of Hissopus seravshnicus and Hissopus ferganensis. Chem. Pharmac. Mag. 1975;2:259–260. doi: 10.1007/BF00570696. [DOI] [Google Scholar]
- 74.Nikitina A.S., Popova O.I. Research of triterpene compounds of medicinal Hyssop cultivated in the conditions of the Stavropol region. Fundam. Res. 2011;11:430–432. [Google Scholar]
- 75.Shomirzoeva O., Li J., Numonov S., Mamat N., Shataer D., Lu X., Aisa H.A. Chemical components of Hyssopus seravshanicus: Antioxidant activity, activations of melanogenesis and tyrosinase, and quantitative determination by UPLC-DAD. Nat. Prod. Res. 2017;33:866–870. doi: 10.1080/14786419.2017.1408105. [DOI] [PubMed] [Google Scholar]
- 76.Skrzypek Z., Wysokińska H. Sterols and Triterpenes in Cell Culture of Hyssopus officinalis L. C J. Biosci. 2003;58:308–312. doi: 10.1515/znc-2003-5-602. [DOI] [PubMed] [Google Scholar]
- 77.Shomirzoeva O., Li J., Numonov S., Atolikshoeva S., Aisa H.A. Chemical components of Hyssopus cuspidatus Boriss.: Isolation and identification, characterization by HPLC-DAD-ESI-HRMS/MS, antioxidant activity and antimicrobial activity. Nat. Prod. Res. 2020;34:534–540. doi: 10.1080/14786419.2018.1488710. [DOI] [PubMed] [Google Scholar]
- 78.Murakami Y., Omoto T., Asai I., Shimomura K., Yoshihira K., Ishimaru K. Rosmarinic acid and related phenolics in transformed root cultures of Hyssopus officinalis. Plant Cell Tissue Organ Cult. 1998;53:75–78. doi: 10.1023/A:1006007707722. [DOI] [Google Scholar]
- 79.Varga E., Hajdu Z., Veres K., Mathe I., Nemeth E., Pluhar Z., Bernath J. Investigation of variation of the production of biological and chemical compounds of Hyssopus officinalis L. Acta. Pharm. Hung. 1998;68:183–188. [PubMed] [Google Scholar]
- 80.Kochan E., Wysokinska H., Chmiel A., Grabias B. Rosmarinic acid and other phenolic acids in hairy roots of Hyssopus officinalis. Biosciences. 1999;54:11–16. doi: 10.1515/znc-1999-1-204. [DOI] [Google Scholar]
- 81.Zgorka G., Głowniak K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001;26:79–81. doi: 10.1016/S0731-7085(01)00354-5. [DOI] [PubMed] [Google Scholar]
- 82.Benedec D., Oniga I., Tipercius B., Popescu H. Preliminary research of some polyphenolic compounds from Hyssopus officinalis L. (Lamiaceae) Farmacia. 2002;50:54–57. [Google Scholar]
- 83.Srivastava A., Awasthi K., Kumar B., Misra A., Srivastava S. Pharmacognostic and Pharmacological Evaluation of Hyssopus officinalis L. (Lamiaceae) Collected from Kashmir Himalayas, India. Pharmacogn. J. 2018;10:690–693. doi: 10.5530/pj.2018.4.114. [DOI] [Google Scholar]
- 84.Zhao L., Ji Z., Li K., Wang B., Zeng Y., Tian S. HPLC-DAD analysis of Hyssopus Cuspidatus Boriss extract and mensuration of its antioxygenation property. BMC Complement. Med. Ther. 2020;20:228. doi: 10.1186/s12906-020-03016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alinezhad H., Azimi R., Zare M., Ebrahimzadeh M.A., Eslami S., Nabavi S.F., Nabavi S.M. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int. J. Food Prop. 2013;16:1169–1178. doi: 10.1080/10942912.2011.578319. [DOI] [Google Scholar]
- 86.Liu X., Wang G., Lv C., Song Z., Bao Y., Wang S., Huang Y. Two newphenolic acids and one newphenolic glycoside from Hyssopus cuspidatus Boriss and their anti-inflammatory activities. Phytochem. Lett. 2021;46:15–20. doi: 10.1016/j.phytol.2021.09.007. [DOI] [Google Scholar]
- 87.Aihaiti K., Li J., Yaermaimaiti S., Liu L., Xin X., Aisa H.A. Non-volatile compounds of Hyssopus cuspidatus Boriss and their antioxidant and antimicrobial activities. Food Chem. 2022;374:131638. doi: 10.1016/j.foodchem.2021.131638. [DOI] [PubMed] [Google Scholar]
- 88.Abduwaki M., Eshbakova K.A., Dong J.-C., Aisa H.A. Flavonoids from flowers of Hyssopus cuspidatus. Chem. Nat. Compd. 2014;50 doi: 10.1007/s10600-014-1116-4. [DOI] [Google Scholar]
- 89.Sen T.V. Ph.D. Thesis. Kursk State Medical University of the Federal Agency for Health and Social Development; Kursk, Russia: 2006. Pharmacognostic Study of Medicinal Hyssop. Author’s Abstract. [Google Scholar]
- 90.Vlase L., Benedec D., Hanganu D., Damian G., Csillag I., Sevastre B., Mot A.C., Silaghi-Dumitrescu R., Tilea I. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules. 2014;19:5490–5507. doi: 10.3390/molecules19055490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aihaiti K., Li J., Yaermaimaiti S., Yin K., Aisa H.A. A new macrocyclic spermidine alkaloid from the aerial part of Hyssopus cuspidatus Boriss. Nat. Prod. Res. 2022;37:2113–2119. doi: 10.1080/14786419.2022.2027935. [DOI] [PubMed] [Google Scholar]
- 92.Mićović T. Antioxidant, antigenotoxic and cytotoxic activity of essential oils and methanol extracts of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae) subsp. Aristatus (godr.) nyman (Lamiaceae) Plants. 2021;10:711. doi: 10.3390/plants10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rastkari N., Samadi N., Ahmadkhaniha R., Rozita A., Leila A. Chemical Composition And Biological Activities of Hyssopus Officinalis Cultivatedin Iran. NPAIJ. 2007;3:87–91. [Google Scholar]
- 94.Salamon I., Kryvtsova M., Bucko D., Tarawneh A.H. Chemical characterization and antimicrobial activity of some essential oils after their industrial large-scale distillation. J. Microbiol. Biotechnol. Food Sci. 2019;8:965–969. doi: 10.15414/jmbfs.2018.8.3.965-969. [DOI] [Google Scholar]
- 95.Micovi T., Ušjak D., Milenkovic M., Samardžić S., Maksimovic Z. Antimicrobial activity of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae) essential oils from Montenegro and Serbia. Lekovitesirovine. 2023;43:e173. doi: 10.61652/leksir2343e173M. [DOI] [Google Scholar]
- 96.Hassanshahian M., Saadatfar A., Masoumi F. Antimicrobial Properties of Hyssopus officinalis Extract Against Antibiotic-Resistant Bacteria in Planktonic and Biofilm Form. Biol. J. Microorg. 2019;7:91–101. [Google Scholar]
- 97.Sayyahi J., Mobaiyen H., Jafari B., Jafari-Sales A. Antibacterial effects of methanolic extracts of Reum ribes L. and Hyssopus officinalis on some standard pathogenic bacteria. Jorjani Biomed. J. 2019;7:34–44. doi: 10.29252/jorjanibiomedj.7.3.34. [DOI] [Google Scholar]
- 98.Mazzanti G., Battinelli L., Salvatore G. Antimicrobial properties of the linalol-rich essential oil of Hyssopus officinalis L. var decumbens (Lamiaceae) Flavour. Fragr. J. 1998;13:289–294. doi: 10.1002/(SICI)1099-1026(1998090)13:5<289::AID-FFJ750>3.0.CO;2-A. [DOI] [Google Scholar]
- 99.Zhang H., Hou Y., Ehbal T., Jiang M., Li F. A comparative analysis of the anti-inflammatory effects of Hyssopus cuspidatus Boriss. essential oil and aspirin on chronic inflammation models in mice. Int. J. Clin. Exp. Med. 2019;12:8261–8270. [Google Scholar]
- 100.Salehi A., Setorki M. Analgesic and anti-inflammatory effects of ethanolic extract of Hyssopus officinalis in mice. J. Gorgan Univ. Med. Sci. 2018;20:42–47. [Google Scholar]
- 101.Ling-Fei K., Xiao-Juan R., Pan Y., Tuo Q., Xiao-Hui Z., Yu-Tong K., Bo C., Wen-Ling S., Tian-Le G., Cai T. The influence of Hyssopus cuspidatus Boriss extract on lipid mediators metabolism network in asthmatic mice. Front. Pharmacol. 2023;14:1066643. doi: 10.3389/fphar.2023.1066643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qin T., Rong X., Zhang X., Kong L., Kang Y., Liu X., Hu M., Liang H., Tie C. Lipid Mediators Metabolic Chaos of Asthmatic Mice Reversed by Rosmarinic Acid. Molecules. 2023;28:3827. doi: 10.3390/molecules28093827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma X., Ma X., Ma Z., Wang Z., Sun Z., Yu W., Li F., Ding J. Effect of Hyssopus officinalis L. on inhibiting airway inflammation and immune regulation in a chronic asthmatic mouse model. Exp. Ther. Med. 2014;8:1371–1374. doi: 10.3892/etm.2014.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahimi Kalateh Shah Mohammad G., Karimi E., Oskoueian E., Homayouni-Tabrizi M. Anticancer properties of green-synthesised zinc oxide nanoparticles using Hyssopus officinalis extract on prostate carcinoma cells and its effects on testicular damage and spermatogenesis in Balb/C mice. Andrologia. 2020;52:e13450. doi: 10.1111/and.13450. [DOI] [PubMed] [Google Scholar]
- 105.Liu X., Su J., Wang G., Zheng L., Wang G., Sun Y., Bao Y., Wang S., Huang Y. Discovery of Phenolic Glycoside from Hyssopus cuspidatus Attenuates LPS-Induced Inflammatory Responses by Inhibition of iNOS and COX-2 Expression through Suppression of NF-B Activation. Int. J. Mol. Sci. 2021;22:12128. doi: 10.3390/ijms222212128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Behbahani M. Anti-viral activity of the methanolic leaf extract of an Iranian medicinal plant “Hyssopus officinalis” against herpes simplex virus. JMPR. 2009;3:1118–1125. [Google Scholar]
- 107.Kreis W., Kaplan M.H., Freeman J., Sun D.K., Sarin P.S. Inhibition of HIV replication by Hyssop officinalis extracts. Antivir. Res. 1990;14:323–337. doi: 10.1016/0166-3542(90)90051-8. [DOI] [PubMed] [Google Scholar]
- 108.Gollapudi S., Sharma H.A., Aggarwal S., Byers L.D., Ensley H.E., Gupta S. Isolation of a previously unidentified polysaccharide (MAR-10) from Hyssop officinalis that exhibits strong activity against human immunodeficiency virus type 1. Biochem. Biophys. Res. Commun. 1995;210:145–151. doi: 10.1006/bbrc.1995.1639. [DOI] [PubMed] [Google Scholar]
- 109.Mazzanti G., Lu M., Salvatore G. Spasmolytic action of the essential oil from Hyssopus officinalis L. var. decumbens and its major components. Phytother. Res. 1998;12:S92–S94. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S92::AID-PTR261>3.0.CO;2-9. [DOI] [Google Scholar]
- 110.Kermanjani A., Jalalizadegan B., Tabatabaie F. Comparison of Hyssopus officinalis, Tussilago farfara, Carum copticum Extracts versus Systemic Glucantime in the Treatment of Cutaneous leishmaniasis in Balb/c Mice. Adv. Stud. Biol. 2015;7:49–54. doi: 10.12988/asb.2015.41054. [DOI] [Google Scholar]
- 111.Gholami M., Jafari F., Baradaran Z., Amri J., Azhdari-Zarmehri H., Sadegh M. Effects of aqueous extract of Hyssopus officinalis on seizures induced by pentylenetetrazole and hippocampus mRNA level of iNOS in rats. Avicenna J. Phytomed. 2020;10:213. [PMC free article] [PubMed] [Google Scholar]
- 112.Lim W.C. Stimulative and sedative effects of essential oils upon inhalation in mice. Arch. Pharmacal. Res. 2005;28:770–774. doi: 10.1007/BF02977341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.