Abstract
Inactivation of viral particles is the basis for several vaccines currently in use. Initial attempts to use simian immunodeficiency virus to model a killed human immunodeficiency virus type 1 (HIV-1) vaccine were unsuccessful, and limited subsequent effort has been directed toward a systematic study of the requirements for a protective killed HIV-1 vaccine. Recent insights into HIV-1 virion and glycoprotein structure and neutralization epitopes led us to revisit whether inactivated HIV-1 particles could serve as the basis for an HIV-1 vaccine. Our results indicate that relatively simple processes involving thermal and chemical inactivation can inactivate HIV-1 by at least 7 logs. For some HIV-1 strains, significant amounts of envelope glycoproteins are retained in high-molecular-weight fractions. Importantly, we demonstrate retention of each of three conformation-dependent neutralization epitopes. Moreover, reactivity of monoclonal antibodies directed toward these epitopes is increased following treatment, suggesting greater exposure of the epitopes. In contrast, treatment of free envelope under the same conditions leads only to decreased antibody recognition. These inactivated virions can also be presented by human dendritic cells to direct a cell-mediated immune response in vitro. These data indicate that a systematic study of HIV-1 inactivation, gp120 retention, and epitope reactivity with conformation-specific neutralizing antibodies can provide important insights for the development of an effective killed HIV-1 vaccine.
Although it has been over 15 years since human immunodeficiency virus type 1 (HIV-1) was first isolated, the virus remains an emerging pathogen worldwide (16). Researchers have developed potent chemotherapeutic strategies to treat HIV infection which have dramatically reduced the number of AIDS cases and progression to disease in the United States and Europe. Nonetheless, these regimens have not been uniformly successful. Therefore, it is clear that studies must be targeted at the identification and development of protective HIV vaccine immunogens. Cogent arguments exist for a variety of HIV-1 vaccine strategies, including one based on inactivated virions. This technology has worked successfully for a variety of viral, including retroviral, vaccines (17, 34, 43).
To date, the majority of efforts directed toward developing a preventative HIV-1 vaccine have focused on recombinant subunit vaccines, such as those consisting of envelope proteins, and the use of vector-based delivery systems (2). The only inactivated vaccine (REMUNE) to enter clinical trials for HIV-1 has been tested exclusively as a therapeutic vaccine (28). Live attenuated HIV-1 vaccines have also been considered based on protection of adult rhesus macaques seen using an attenuated strain of simian immunodeficiency virus (SIV) (8, 9, 18). The minimal success of subunit vaccines, coupled with the apparent ability of live attenuated SIV to protect against infection, indicates that protective immunity is possible but that multiple components or a complex virion structure may be required. Recently, cross-clade neutralizing antibodies have been found in mice following injection with formaldehyde-fixed viral envelope and cell membrane fusion partners (20). These results indicate that a cross-clade antibody response is possible when a conformationally correct antigen (Ag) is presented to the immune system.
Inactivated vaccines are theoretically advantageous since they represent a complex mixture of viral antigens closely resembling native virions. Ideally, inactivation would result in conservation of linear and conformational epitopes required for both humoral and cellular immune responses. Furthermore, inactivation protocols, in combination with chemical or biological procedures, could be designed to expose cryptic neutralization epitopes. In this manner, it might be possible to enhance the desired immunogenicity of the vaccine beyond that achieved by native virions.
Early efforts to model a killed HIV-1 vaccine using SIV in rhesus macaques were unsuccessful. Although protection against live challenge was conferred, it was the result of immune responses directed toward xenoantigens in the vaccine preparations rather than toward epitopes on SIV (3, 7). Recent work to model a killed HIV-1 vaccine using SIV has demonstrated that covalent modification of nucleocapsid zinc fingers by 2,2′-dithiodipyridine can preserve antigenic structures on the surface of SIVMne and HIVMN (4, 39). Moreover, SIV-specific antibodies were shown to be present following intravenous injection of 2,2′-dithiodipyridine-treated SIVMne into a juvenile pig-tailed macaque (14).
Despite the protection afforded by killed vaccines for other viral diseases, research devoted to developing a killed vaccine for HIV-1 has been minimal. This is primarily due to concerns regarding shedding of gp120 from virions, safety considerations surrounding vaccine preparation, and the failures of early SIV vaccine preparations. In the present work, we have systematically reexamined the concept of a killed HIV-1 vaccine. We have shown that virus can be inactivated by at least 7 logs and still associates with envelope through purification by ultrafiltration. Moreover, these virus preparations have maintained and/or enhanced binding capacity to broadly reactive, conformation-dependent neutralizing antibodies. Finally, we have determined that these preparations of HIV-1 can be used to elicit a prototypic Th1 recall in vitro response using cells from HIV-1-infected persons.
MATERIALS AND METHODS
Virus growth and cell culture.
The full-length infectious molecular clones (HIVSX and HIVNL4-3) and primary virus isolates have been described elsewhere (1, 13, 15, 30).
Virus stocks from plasmid DNA were made following electroporation of 25 μg of DNA into a donor pool of 5 × 106 phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) as described elsewhere (15). Virus stocks were propagated in PHA-stimulated PBMC.
Infectious viral titers were determined on allogeneic pools of PBMC. Half-log dilutions of viral stocks were applied to the cells for 16 h. Supernatants were changed at days 7 and 14 and harvested at day 21 to determine the 50% tissue culture infective dose for each virus, calculated by the method of Reed and Muench (36).
Reagents used in capture enzyme-linked immunosorbent assays (ELISAs).
Monoclonal antibody (MAb) 2G12 was a gift from H. Katinger, MAb IgG1b12 was a gift from D. Burton, MAb 17b was a gift from J. Robinson, MAb 447-52D was a gift from S. Zolla-Pazner, and soluble CD4 (sCD4) was obtained from the AIDS Reagent Repository. Plasmid CDM7-CD4Eγ1, coding for CD4-IgG (immunoglobulin G) (6), a gift from D. Camerini, was transfected (25 μg) into 293T cells (5 × 106) by standard methods (40). Supernatant was collected at 48 h, titrated, and used at a 1:10 dilution for all assays.
Thermal and chemical treatment of virus.
Thermal treatment of HIV-1 was performed by loading samples into thin-wall 0.5-ml microtubes and heating them for the indicated times at various temperatures in a heat block filled with water. Formaldehyde (10% ultrapure; Polysciences) was freshly diluted in phosphate-buffered saline (PBS) and added to the virus as indicated. After incubation, an equal volume of 0.2% bovine serum albumin (BSA) in PBS was added to quench residual aldehyde. The buffer was removed by diafiltration in a 100-kDa-cutoff ultrafiltration device (Millipore) by centrifugation at 10,000 rpm. The filtrate (approximately 95% of the volume) was removed by aspiration, and PBS was added to the upper cell to reconstitute the sample to the original volume. After mixing by inversion, the device was centrifuged at 10,000 rpm. This process was repeated a total of four times, resulting in a 160,000-fold dilution of the low-molecular-weight molecules including residual aldehydes.
gp120 capture ELISA.
Capture of gp120 was performed as described previously (26). In brief, 80-μl aliquots of clarified culture supernatant were incubated with 20 μl of human anti-gp120 MAb or with CD4-IgG (2 to 10 μg/ml) in a U-bottom microtiter plate. Where appropriate, the sample was preincubated with sCD4 (2 ng/well) in PBS with 0.2% BSA for 30 min at 37°C. Samples and antibodies were allowed to react in the liquid phase for 45 min at 37°C. Triton X-100 was added to a final concentration of 1% for 15 min at 37°C. This concentration of detergent will not disrupt the immune complex (41). At the end of the incubation period, the contents were transferred to an ELISA plate precoated with sheep anti-gp120 (International Enzyme). The gp120-antibody complex was captured onto the plate at 37°C for 60 min. After washing, the plate was incubated with goat anti-human IgG (horseradish peroxidase conjugated; Accurate Chemical) for 45 min at 37°C. Following a final wash, 200 μl of tetramethylbenzidine substrate was added to each well for 20 min. The reaction was terminated by addition of 4 N H2SO4 (final concentration of 0.8 N), and the optical density (OD) was read at 450 nm (Molecular Dynamics). A standard serial dilution of concentrated HIVSX was used as a standard to normalize gp120 binding in all assays.
Fractionation of virion-bound and soluble gp120.
Five hundred microliters of clarified culture supernatant was added to the top reservoir of a NanoSep (Pall Filtration) device with a mean molecular size cutoff of 300 kDa. The device was spun at 8,000 rpm for 6 min or until the retentate was reduced to 20 μl. PBS with 0.2% BSA was added to the top reservoir, and the volume was reconstituted to the original level. The filtrate fraction containing molecules of <300 kDa was collected from the bottom receptacle.
Fractionation of virus on a Percoll gradient.
After ultrafiltration, HIVSX was either held at 4°C or heated to 62°C for 10 min. Next, 200 μl of each preparation was layered onto 1.8 ml of undiluted Percoll (Pharmacia), and the samples were centrifuged at 56,000 × g for 60 min at 4°C. Fractions (100 μl) were removed from the top of the gradient. The samples which were previously held at 4°C were then heated to 62°C for 10 min to normalize OD readings for the ELISA. HIV p24 was measured by capture ELISA (Coulter), and gp120 ELISA was as described above, using CD4-IgG as the capture antibody.
ELISPOT for IFN-γ-secreting cells.
Human dendritic cells (DC) were prepared by the method of Fan et al. (12). In brief, 2 × 105 CD14-depleted PBMC were cultured overnight in 96-well round-bottom plates in serum-free medium (AimV; Gibco/BRL). Nonadherent cells were removed, and the adherent cells were incubated in AimV containing 1,000 U of recombinant human interleukin-4 (IL-4; R&D Systems), 1,000 U of recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Immune Sciences), and 10 U of recombinant gamma interferon (IFN-γ; R&D Systems) per ml. On day 4, antigen (Ag) was added to duplicate wells for 16 to 18 h. The cells were extensively washed to remove nonprocessed Ag and cytokines, and 2 × 105 autologous PBMC were added to the Ag-pulsed DC for 6 h. PBMC incubated for 6 h with PHA (2.5 μg/ml) were included as a positive control in some experiments. Next, the PBMC were transferred to a 96-well polyvinylidene difluoride (PVDF)-backed plate (Millipore) coated with anti-IFN-γ MAb 1-D1K (Mabtech) and incubated at 37°C for 48 h. The enzyme-linked spot (ELISPOT) assay was performed after removal of all residual cells as instructed by the manufacturer (Mabtech). Spot-forming cells were detected after a 5-min incubation with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (Pierce). Spots were counted under ×40 magnification with a stereomicroscope. In accordance with convention, responses were considered to be positive if the well contained >5 spots, and this number was at least twice that of the negative control (medium). Frequencies of Ag-specific cells were calculated after subtraction of spots contained in the medium control well.
RESULTS
Thermal treatment of HIVSX results in increased binding to CD4.
Thermal inactivation is commonly used as a means of efficiently inactivating retroviruses (32, 33, 35, 37, 44, 46). McDougal et al. (23) demonstrated that HIV-1 was inactivated at 60°C at rate of 1 log each 24 s in the liquid state. Therefore, we conducted experiments to determine whether heat treatment could be used to inactivate HIV-1 while still maintaining the antigenic properties of the virus. To this end, we determined whether thermal treatment of HIV-1 affected CD4 binding to gp120.
These studies used the R5-tropic virus HIVSX (30), which contains the HIVJRFL envelope in an HIVNL4-3 backbone. Thermal treatment of HIVSX with an infectivity titer of 106.25 resulted in a 10-fold decrease in infectivity after 1 min, a 5.75-log decrease in infectivity after 3 min, and at least a 6.25-log decrease in infectivity after 10 min at 62°C (Table 1). This translates to a log decline in infectivity every 1.6 min. Thermal treatment of a second stock of HIVSX for 30 min at 62°C resulted in at least a 7-log decline in infectivity when tested on PBMC (data not shown). Treatment of HIVNL4-3 for the same time period also resulted in at least a 7-log decline in infectivity (data not shown).
TABLE 1.
Thermal and chemical treatment of HIV-1 reduces infectivitya
| Treatment time (min) | Treatment condition
|
|
|---|---|---|
| Thermal | Thermal + formaldehyde | |
| 0 | 106.25 | 106.25 |
| 1 | 105.25 | 102 |
| 3 | 100.5 | <10 |
| 10 | <10 | <10 |
Endpoint dilution studies were performed as described in Materials and Methods. Pools of three donors were used for allogeneic infections. Quadruplicate wells were established, and p24 was tested at day 21. Clarified culture supernatants from HIVSX-infected cells grown in medium containing 10% fetal calf serum were collected. Serum proteins were removed by ultracentrifugation at 40,000 × g, followed by ultrafiltration through a 300-kDa-cutoff membrane. Infectivity titers were calculated by the method of Reed and Muench. Treatment of HIV-1 was performed as described in Materials and Methods.
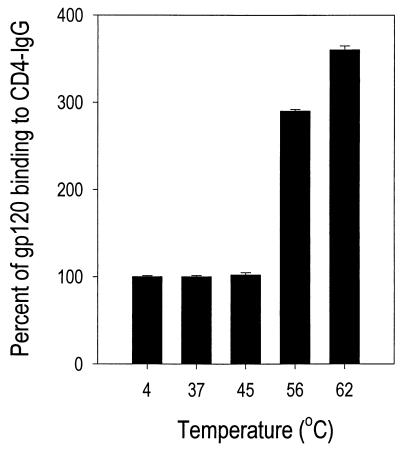
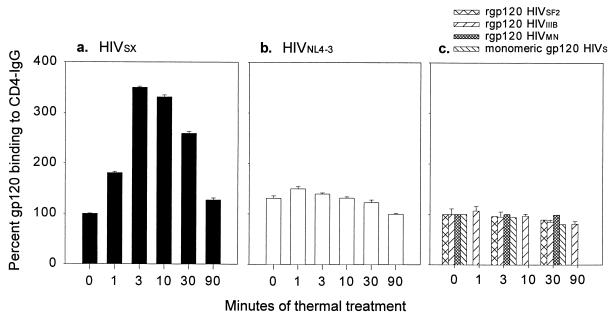
To determine whether retention of antigenic properties was temperature dependent, we treated HIVSX for 30 min at various temperatures. As shown in Fig. 1, binding of heat-treated HIVSX gp120 to CD4-IgG increased as a function of treatment temperature. Interestingly, binding of gp120 to CD4-IgG was approximately fourfold higher to a virus preparation that was heated at 62°C than to one which was held at 4°C. In contrast to gp120, thermal treatment reduced the recognition of p24 in commercial p24 antigen capture assays 10-fold (data not shown). This finding is likely the result of destruction of the epitope recognized by the MAb used in the commercial ELISA, as comparable levels of p24 were detected in heat-treated and nontreated virion preparations by Western blotting (data not shown). Since heat treatment at 62°C resulted in at least a 7-log reduction in infectivity and in enhanced recognition of gp120 by CD4, we further examined the effects of treatment on gp120 binding over time while holding temperature constant. The increase in binding of HIVSX to CD4 reached a peak at 3 min (350% of baseline) after heating and then declined to baseline (127%) after 90 min (Fig. 2a). Enhanced binding of gp120 to CD4 was not seen with laboratory-adapted X4-tropic virus HIVNL4-3, which differs from HIVSX only by envelope sequences (Fig. 2b). Of note, enhanced binding was also not observed with each of three recombinant gp120 (rgp120) molecules or monomeric HIVSX separated from virion-associated gp120 by ultrafiltration (Fig. 2c). These data indicate that there are differences in the structures of monomeric gp120 and gp120 forms associated with virions.
FIG. 1.
Thermal treatment of HIV-1 increases binding to CD4. HIVSX was prepared in serum-free medium following infection of a three-donor pool of PBMC. Clarified culture supernatants containing 160 ng of HIV p24 per ml were aliquoted for single use, and aliquots were heated at the indicated temperatures. Thermal treatment of HIV-1 was performed by loading samples into thin-wall tubes and heating them for 3 min at the indicated temperatures in a heat block filled with water. The sample was immediately cooled to 4°C. Samples from each temperature point were assessed by gp120 capture ELISA using rCD4-IgG as the first antibody. Data are expressed as percent binding to rCD4-IgG relative to HIVSX held at 4°C (set at 100%; OD was 0.590 for gp120 in the untreated sample). Mean ± standard error are shown for three independent experiments.
FIG. 2.
CD4 binding to heat-treated HIV-1 changes as a function of time. HIVSX and HIVNL4-3 were prepared in serum-free medium following infection of a three-donor pool of PBMC. The rgp120 was purchased from Intracell. Clarified culture supernatants containing 200 ng of HIV p24 per ml were aliquoted for single use. For these experiments, 16 ng of p24 per well of HIVSX and HIVNL4-3 and 20 ng of rgp120 per well were used. Thermal treatment of HIV-1 was performed by loading samples into thin-wall tubes and heating them for the indicated times at 62°C in a heat block filled with water. At the end of the incubation period, the sample was immediately cooled to 4°C and held on ice until all samples were acquired. Samples were assessed by gp120 capture ELISA using rCD4-IgG as the first antibody. Data are expressed as percent binding to CD4-IgG following treatment at 62°C relative to untreated sample for each preparation as described in Fig. 1. Mean ± standard error are shown for three independent experiments. (a) HIVSX (OD was 1.3 for gp120 in untreated sample); (b) HIVNL4-3 (OD was 1.1 for gp120 in untreated sample); (c) rgp120 (ODs were 2.2 for gp120 in untreated sample HIVSF2, 2.22 for gp120 in untreated rgp120 HIVIIIB, 2.7 for gp120 in untreated rgp120 HIVMN, and 0.27 for monomeric HIVSX gp120 in untreated sample). Mock supernatants from uninfected PBMC were negative for gp120 binding at all time points (data not shown).
Low-molecular-weight gp120 is not shed following thermal treatment.
Shedding of HIV-1 envelope has been described for some strains of HIV-1 (24, 27, 31) and has been cited (reviewed in reference 5) as one of the major impediments to developing an inactivated HIV-1 vaccine. Therefore, we examined whether thermal treatment of HIVSX resulted in shedding of gp120 from virions. For these studies, we fractionated envelope by ultrafiltration through a 300-kDa molecular mass exclusion membrane (45). This procedure allowed us to distinguish virion-associated (retentate) gp120 from unbound or free (filtrate) gp120 (Table 2). The amount of gp120 in each fraction was measured by capture ELISA as described above.
TABLE 2.
Retention of HIV envelope following heat treatmenta
| Virus | % of virion-bound gp120
|
|
|---|---|---|
| 4°C | 62°C | |
| HIVSX | 83 | 88 |
| HIVNL4-3 | 50 | 53 |
| LTS | ||
| 1 | 96 | 69 |
| 3 | 80 | 66 |
| Rapid progressors | ||
| 1 | 99 | 87 |
| 2 | 100 | 81 |
| Seroconverter 13 | 75 | 71 |
| rgp120 (HIVIIIB) | 4 | 4 |
Representative data from two independent experiments are expressed as percent gp120 binding to CD4-IgG after treatment as indicated. Samples were heated at 62°C for 30 min. Virus preparations (26 ng of p24 in 200 μl) were fractionated by centrifugal ultrafiltration through a 300,000-Da cutoff device (Pall-Filtron NanoSep 300). Retentates were reconstituted in PBS to the original volume. The gp120 in the filtrate (F) and retentate (R) fractions were determined by gp120 capture ELISA. The percentage of virion-bound gp120 was calculated as R/(R + F) × 100. rgp120 (Intracell) was derived from HIVIIIB and produced in baculovirus. The assay was performed in triplicate.
Consistent with published reports, the amount of low-molecular-weight gp120 associated with virion preparations was strain dependent (24, 27, 31). Our data also indicated that similar amounts of HIVSX gp120 remained associated with the high-molecular-weight fraction regardless of incubation temperature (4°C versus 62°C). As expected, HIVNL4-3 retained less virion-associated gp120 than HIVSX at 4°C but did not show any further loss of gp120 following heating to 62°C. Recombinant gp120 was not retained in the ultrafiltration device at either temperature (Table 2). These results confirm and extend previous observations indicating that virion shedding of gp120 is strain dependent (27, 31). More importantly, they indicate that antigenic gp120 is likely to be retained in a high-molecular-weight form following thermal treatment.
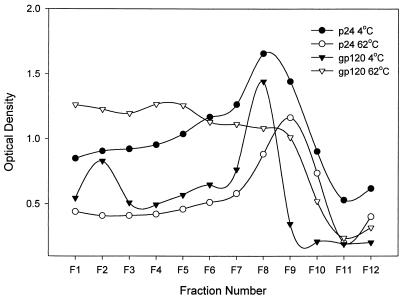
We addressed the nature of the gp120 after heat treatment of the virions by velocity gradient sedimentation of heated and unheated virus on Percoll gradients. Fractions were analyzed by ELISA for gp120 and for p24 (Fig. 3). These data indicate that a substantial amount of envelope does not associate with intact virion cores after heat treatment. The sedimentation suggests a structure greater than monomeric envelope, perhaps associated with membrane or virion fragments. This finding is consistent with our previous data demonstrating copurification of gp120 with p24 by ultrafiltration.
FIG. 3.
Fractionation of virus on a Percoll gradient suggests that a substantial amount of envelope does not cosediment with p24 after heat treatment. After ultrafiltration, HIVSX was either held at 4°C or heated to 62°C for 10 min. Next, 200 μl of virus was layered onto 1.8 ml of undiluted Percoll (Pharmacia), and the samples were centrifuged at 56,000 × g for 60 min at 4°C. Fractions (100 μl) were removed from the top of the gradient. The samples which were previously held at 4°C were then heated to 62°C for 10 min to normalize OD readings for the ELISA. HIV p24 was measured by capture ELISA (Coulter); gp120 ELISA was as described in the text using CD4-IgG as the capture antibody.
Thermal treatment of HIV-1 results in enhanced binding of antibodies to conformation-dependent epitopes.
The above results demonstrate that the gp120 domain recognized by CD4 is conserved and enhanced for CD4-IgG recognition following thermal treatment. A number of other epitopes that are dependent on maintenance of a proper conformation of gp120 have been described. Since some MAbs directed toward these epitopes can neutralize HIV-1 infectivity, we tested the ability of these antibodies to bind to heat-treated HIV-1 preparations. Three conserved antibody binding domains (19, 25, 47) were tested: (i) the CD4 binding domain (CD4 BS), (ii) the cryptic epitope induced by CD4 binding which overlaps the chemokine receptor binding site (CD4i), and (iii) the heavily glycosylated region of gp120 (2G12 specific). The V3 tip motif which is conserved in 75% of HIV-1 isolates was also tested. All of these antibodies recognize conformation-dependent epitopes, and all are neutralizing with the exception of the two CD4 BS antibodies 205-46-9 and 205-43-1. Our data indicated that binding to each of these epitopes was maintained. Furthermore, with the exception of 2G12, binding by these antibodies to HIVSX was increased following heat treatment (Table 3 and Fig. 4a). We extended these findings to a panel of five primary isolates derived from HIV-infected long-term survivors (LTS) and from one seroconverter. Similar to the results with HIVSX, we found enhanced binding to CD4-IgG (Table 4). To eliminate the possibility that heat treatment simply results in more efficient capture of envelope during ELISA, we used virus to directly coat the microtiter plate in the absence of capture antibody. In this instance, thermal treatment of the microtiter plate followed by incubation with CD4-IgG also resulted in enhanced binding (325%) to CD4-IgG (data not shown).
TABLE 3.
Percent enhancement in recognition by conformation-dependent antibodies following heat treatmenta
| Virus | % gp120 binding to:
|
|||||
|---|---|---|---|---|---|---|
| CD4 BS
|
2G12 specific (2G12) | V3 specific (447-52D) | ||||
| CD4-IgG | IgG1b12 | 205-46-9 | 205-43-1 | |||
| HIVSX | 243 | 193 | 255 | 295 | 94 | 128 |
| HIVNL4-3 | 83 | 100 | ND | ND | 97 | 112 |
Aliquots of HIVSX or HIVNL4-3 (130 ng of p24/ml) were harvested in serum-free medium and held at 4°C or heated to 62°C for 30 min. Binding using the indicated reagents was performed for 45 min at 37°C in 200 μl. The retention of epitopes was assessed by HIV gp120 capture ELISA. Control reactivity without Ag was <0.1 for each antibody. Antibody concentrations were determined by titration. IgG1b12 was used at 2,000 ng/ml; ODs at 4°C were 0.54 ± 0.03 for HIVSX and 0.31 ± 0.08 for HIVNL4-3. 205-46-9 was used at 2,000 ng/ml; OD at 4°C was 0.700 ± 0.06 for HIVSX. 205-43-1 was used at 2,000 ng/ml; OD at 4°C was 0.900 ± 0.02 for HIVSX. 2G12 was used at 400 ng/ml; ODs at 4°C were 1.92 ± 0.05 for HIVSX and 1.43 ± 0.04 for HIVNL4-3. 447-52D was used at 2,000 ng/ml; ODs at 4°C were 1.31 ± 0.06 for HIVSX and 0.34 ± 0.06 for HIVNL4-3. CD4-IgG was synthesized in 293T cells and used at a 1:10 dilution; ODs at 4°C were 1.04 ± 0.05 for HIVSX and 1.02 ± 0.04 for HIVNL4-3. The assay was performed in triplicate. Data are representative of three independent experiments. ND, not done.
FIG. 4.
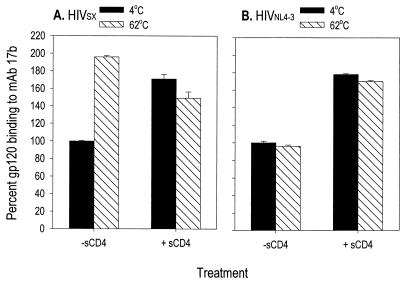
Thermal treatment of HIV-1 results in exposure of the cryptic CD4-induced epitope. HIVSX and HIVNL4-3 were prepared in serum-free medium following infection of a three-donor pool of PBMC. Clarified culture supernatants containing 200 ng of HIV p24 per ml were aliquoted for single use. HIVSX and HIVNL4-3 were each used at 16 ng/well. Samples treated with sCD4 to induce exposure of the 17b epitope were preincubated with 2 ng of sCD4 per well in PBS with 0.2% BSA for 30 min at 37°C. MAb 17b was incubated with HIVSX at a concentration of 2 μg/ml and with HIVNL4-3 at a concentration of 10 μg/ml. Thermal treatment of HIV-1 was performed by loading samples into tubes and heating them for 30 min at 62°C in a heat block filled with water. Binding to 17b was assessed by capture ELISA. Data are expressed as percent binding to 17b. Mean ± standard error are shown for three independent experiments. (A) HIVSX (OD was 1.4 for gp120 in untreated sample); (b) HIVNL4-3 (OD was 1.01 for gp120 in untreated sample).
TABLE 4.
Percent enhancement in recognition by CD4-IgG of primary viral isolates following heat treatmenta
| Virus | % Enhancement (mean ± SE) |
|---|---|
| LTS | |
| 1 | 440 ± 155 |
| 3 | 147 ± 19 |
| 4 | 237 ± 84 |
| 5 | 122 ± 18 |
| 8 | 233 ± 57 |
| Seroconverter 13 | 369 ± 176 |
Data are expressed as percent gp120 binding to CD4-IgG at 62°C relative to binding at 4°C. Aliquots of viral isolates were held at 4°C or heated to 62°C for 30 min. Binding using the indicated reagents was performed for 45 min at 37°C in 200 μl. The retention of epitopes was assessed by HIV gp120 capture ELISA. Control reactivity without Ag was <0.1. CD4-IgG was synthesized in 293T cells and used at a 1:10 dilution. The data are the means from three independent experiments (each experiment was run in triplicate).
Of particular note, binding to gp120 by MAb 17b, which recognizes the CD4i domain (42), was reproducibly increased (198% of control) after heat treatment of HIVSX as shown in Fig. 4A. This increase in binding of gp120 to 17b was comparable to that observed after binding of unheated material to sCD4 (165% of control). In contrast, binding of HIVNL4-3 gp120 to 17b did not increase following heat treatment in the absence of sCD4 (Fig. 4b). These data demonstrate that inactivation regimens can be devised which not only will kill the virion but also may generate potentially better vaccine candidates through enhancing exposure of otherwise cryptic epitopes.
Chemical treatment of HIV-1 can alter the kinetics of enhancement of envelope structures.
We have shown that heat treatment can preserve antigenic structures and can inactivate HIV-1 by at least 7 logs. Nonetheless, additional inactivating agents are necessary for ultimate use in a killed HIV vaccine. To this end, we tested the impact of treatment with formaldehyde on antigenic epitopes of the HIV-1 envelope.
Treatment of HIVSX with a relatively low concentration of formaldehyde (0.02%) for 1 h at 37°C followed by thermal treatment for 1 min reduced infectivity 4.25 logs and reduced infectivity at least 6.25 logs by 3 min (Table 1). Thermal treatment alone for 1 min resulted in a 1-log decrease in infectivity. These findings indicate that treatment of virion stocks with 0.02% formaldehyde can rapidly decrease viral infectivity titers.
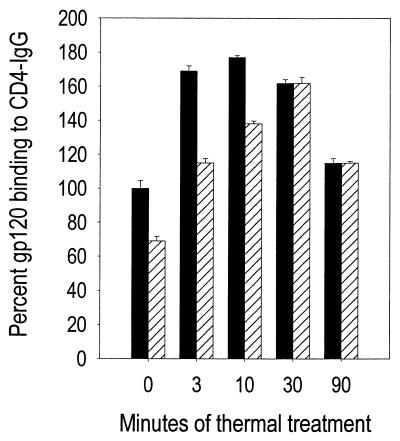
In the absence of thermal treatment, 0.02% formaldehyde treatment for 1 h at 37°C reduced binding of gp120 to CD4-IgG (from 100% to 66%) (Fig. 5). On the other hand, incubation with formaldehyde followed by thermal treatment resulted in binding of CD4 to gp120 that continued to rise from 3 to 30 min (111% of control to 162% of control). This is in contrast to the kinetics of thermal treatment alone, where binding reaches a maximum between 3 and 10 min following treatment. Experiments conducted using formaldehyde at higher concentrations demonstrated that as little as 0.1% formaldehyde at 62°C for 2 h reduced binding to neutralization epitopes by 90% (data not shown). These data indicate that a second inactivating agent can be included without disruption of antigenic structures.
FIG. 5.
Chemical treatment of HIV-1 in conjunction with thermal treatment maintains enhanced antigenicity of envelope structures. HIVSX was prepared as described for Fig. 1. A stock solution of formaldehyde was freshly diluted in PBS and added to the virus, resulting in a final concentration of 0.02%. After 1 h at 37°C, an equal volume of 0.2% BSA in PBS was added to quench residual aldehyde. The buffer was removed by diafiltration through a 100-kDa-cutoff ultrafiltration device (Millipore) by centrifugation at 10,000 rpm. The filtrate (approximately 95% of the volume) was removed by aspiration, and PBS was added to the top cell to reconstitute the sample to the original volume. After inversion, the device was centrifuged at 10,000 rpm. This process was repeated a total of four times, resulting in a 160,000-fold dilution of the low-molecular-weight molecules, including residual aldehyde. Thermal treatment of HIV-1 was performed as previously described. At the end of the incubation period, the sample was immediately cooled to 4°C. Samples from each temperature point were assessed by gp120 capture ELISA using recombinant CD4-IgG as the first antibody. Data are expressed as percent binding to CD4-IgG relative to untreated (time zero) HIVSX held at 4°C. ■, thermal treatment; ▨, formaldehyde plus thermal treatment.
Heat-inactivated HIV-1 can stimulate Ag-specific memory cells to produce IFN-γ.
Traditionally killed vaccines elicit humoral, not cellular, immune responses (5). There is, however, evidence for protective cell-mediated immune responses following immunization with other killed viral, including retroviral, vaccines (22, 34, 43). Thus, we tested the ability of our inactivated vaccine preparation to elicit an Ag-specific recall response using an ELISPOT assay for IFN-γ production (21).
These studies were performed using DC from HIV-infected and uninfected persons to process and present Ags. Autologous PBMC were tested for the ability to produce IFN-γ in response to our inactivated vaccine preparation, a potent recall Ag (tetanus toxoid), a mitogen (PHA), and a superantigen (Staphylococcus enterotoxin A [SEA]). Tetanus toxoid and SEA were included as controls to demonstrate that these donors were capable of producing IFN-γ in response to antigenic stimulation. All HIV-infected persons studied here were infected for at least 7 years and are considered to be LTS. We chose to study HIV-1 LTS since defective recall responses would be likely in other individuals (38). As shown in Table 5, Ag-specific cells were detected in response to tetanus toxoid and SEA in all persons tested. HIV-infected but not HIV-seronegative donors demonstrated the capacity to make IFN-γ in response to thermally treated HIVSX. None of the donors tested made IFN-γ after exposure to mock-infected cell culture supernatants. These data imply that T cells from seropositive individuals can be induced to produce IFN-γ in an Ag-specific fashion upon exposure to inactivated vaccine preparations.
TABLE 5.
Detection of antigen-specific cells by ELISPOTa
| Donor | Titer
|
|||||
|---|---|---|---|---|---|---|
| Medium | PHA | SEA | Tetanus toxoid | Heat-inactivated HIVSX | Mock infected | |
| HIV seropositive | ||||||
| 60016b | <1/200,000 | 1/1,471 | NDe | 1/28,571 | 1/33,333 | ND |
| 60030c | <1/200,000 | ND | 1/2,595 | 1/23,529 | 1/26,667 | <1/200,000 |
| 60031d | <1/200,000 | ND | 1/1,235 | 1/10,526 | 1/12,903 | <1/200,000 |
| HIV seronegative | ||||||
| 60001 | <1/200,000 | ND | 1/2,702 | 1/31,746 | <1/200,000 | <1/200,000 |
| 60002 | <1/200,000 | ND | 1/2,381 | 1/12,500 | <1/200,000 | <1/200,000 |
Duplicate wells of DC were cultured in serum-free medium supplemented with 1,000 U each of IL-4 and GM-CSF per ml and 10 U of IFN-γ per ml for 4 days. On day 4, DC were pulsed with tetanus toxoid (2 μg/ml), SEA (1 μg/ml), heat-inactivated (for 30 min at 62°C) HIVSX (40 ng/ml), or supernatants derived from mock-infected cultures from the same donors in whom HIVSX was grown (volume equal to that for heat-inactivated HIV) for 16 h. After 16 h, the Ags and cytokines were removed and 2 × 105 autologous PBMC were added to the DC for 6 h. The PBMC were then transferred to 96-well PVDF-backed plates (Millipore) coated with anti-IFN-γ MAb 1-D1K (Mabtech) and were incubated at 37°C for 48 h. The ELISPOT assay was performed as instructed by the manufacturer (Mabtech). All HIV-infected donors were on antiretroviral therapy for at least 1 year.
Viral load, <400 copies/ml; CD4+ T cells, 540/mm3.
Viral load, <400 copies/ml; and CD4+ T cells, 800/mm3.
Viral load, <40 copies/ml; CD4+ T cells, 921/mm3.
ND, not determined.
DISCUSSION
We show here that thermal treatment of HIVSX for 10 min at 62°C can result in at least a 6.25-log reduction of infectivity. Moreover, we could retain the association of envelope with virion particles following ultrafiltration of thermal treated virus through a 300-kDa molecular mass cutoff device. Thermally treated HIVSX retained binding of gp120 to antibodies that define major neutralization epitopes. In fact, with the exception of the epitope defined by MAb 2G12, heat-treated HIVSX demonstrated increased binding of gp120 to the MAbs that recognize major neutralization epitopes of HIV-1 envelope. The addition of a second inactivating agent (formaldehyde) resulted in more rapid kinetics of inactivation while maintaining the property of thermally induced enhancement of envelope binding to CD4. Finally, heat-treated HIVSX was able to induce a cell-mediated recall response in vitro as measured in cells from an HIV-1 infected LST.
The perceived difficulty of inactivating HIV-1 without losing or destroying viral envelope has been cited as a major stumbling block to the development of an inactivated vaccine (reviewed in reference 5). Retroviral envelopes can be easily shed, particularly following concentration by ultracentrifugation or sucrose gradient banding, common methods used by most investigators for concentration of HIV-1 (11). We used ultrafiltration of HIV-1 and were able to maintain gp120 as a high-molecular-weight structure in association with virion particles. We do not know the biochemical nature of the gp120 structure. Analysis following fractionation of virus on a Percoll gradient suggests that a substantial amount of envelope does not cosediment with p24. It may be in the form of oligomeric envelope or lipid membrane fragments. Further biochemical analyses will be required to definitively determine the structure of the inactivated virus preparations.
We also demonstrated retention of several major neutralization epitopes on viral envelope following treatment with heat. This is in contrast to data described by Rossio et al. (39), where heating HIV-1 at 56°C for 2 h resulted in loss of binding to gp120 MAb IgG1b12, as measured by p24 assay of virions after precipitation with IgG1b12. The differences between their results and ours may be due to their use of p24 as an assay method, different viral strains, and different conditions of treatment. Moreover, we found that binding of gp120 to some of these epitopes was significantly enhanced following thermal treatment. These induced sites include epitopes recognized by potent neutralizing antibodies, including that recognized by MAb 17b, which has been postulated to be partially occluded or cryptic in native virions. These data are particularly interesting in light of a report from LaCasse et al. examining immunogenicity of formalin-fixed fusion partners consisting of a cell line expressing HIV gp120 and a cell line expressing CD4 and either R5 or X4; these authors demonstrated induction of broadly reactive neutralizing antibodies following injection of transgenic mice with the fusion partners (20). They interpreted the data as providing evidence for the induction of neutralizing antibodies against antigenic structures transiently produced during infection. Although these authors suggest that the neutralizing antibodies raised in transgenic mice recognize gp41 domains important for fusion, it is possible that epitopes on gp120 are also involved. Because we observed enhancement of binding to known epitopes, it is possible that thermal treatment also results in the exposure of other antigenic sites.
The mechanism by which heat increases binding to conformation-dependent neutralization epitopes is not known. The simplest hypothesis is that thermal treatment increases the number of epitopes available to bind to antibody. In addition, cross-linking of reactive side chains (10) by reagents including formaldehyde can stabilize protein structures. Therefore, the combined use of these two inactivating reagents could lead to exposure of relevant, and perhaps novel, epitopes in a stable protein structure, consistent with the increased binding that we observed with antibodies recognizing the V3, CD4 binding, and CD4-induced epitopes of gp120.
As with other retroviral diseases, it is likely that a successful HIV vaccine will need to induce both a humoral and a cell-mediated immune response (reviewed in reference 5). In addition to demonstrating increased recognition of relevant envelope epitopes by broadly reactive neutralizing antibodies, heat-inactivated preparations also elicited memory cell-mediated immune responses in vitro. Although it is not clear which cell subset produced IFN-γ in response to our vaccine preparation, it is likely that the cytokine was secreted by CD4 cells since the DC were given an exogenous Ag for processing. If these preparations can be modified to include an adjuvant which might target the vaccine Ag to the endogenous pathway for processing, then induction of humoral and cellular immune responses would theoretically be possible.
Early attempts to develop an inactivated SIV vaccine failed after it was ascertained that most, if not all, immune reactivity was directed at xenoantigens on the surface of the virions (3). Because those experimental approaches and conditions of inactivation differed from the ones described here (3, 7, 29), they do not allow for direct comparison. However, our own unpublished data indicate that maintenance of HIV-1 gp120 epitopes is highly sensitive to formaldehyde. Either increasing the incubation time from 60 to 120 min (0.02% formaldehyde at 37°C) or changing the formaldehyde concentration from 0.02 to 0.1% (60 min at 37°C) resulted in a decrease in recognition of antigenic epitopes by 90%. Although treatment under the conditions of Fig. 5 (0.02% formaldehyde for 60 min at 37°C) did reduce binding to CD4 from 100 to 68%, the combination of formaldehyde treatment with heat was able to maintain the property of enhancement seen with heat alone after 30 min of thermal treatment. The combined use of two distinct inactivating agents (heat and formaldehyde) supports the notion that a killed HIV vaccine which retains envelope specific antigenicity is possible.
Together with other data from the field, the data presented here suggest that it may be possible to develop a killed HIV vaccine which could elicit protective humoral and cellular immune responses. Future studies to determine immunogenicity in animal models will be necessary to correlate in vitro responses with the generation of protective in vivo responses.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank B. P. Dorman, J. P. Moore, J. Binley, and M. Fung for helpful discussions, and we thank C. R. Rinaldo, Jr., for protocols related to DC culture. We thank P. Marhabi for technical assistance; J. P. Morgan (UCLA Center for AIDS Research HIV Virology Laboratory) for performing the p24 assays; D. Burton, H. Katinger, J. Robinson, and S. Zolla-Pazner for kindly providing reagents; and B. Poon and E. Withers-Ward for critical reading of the manuscript. We also sincerely thank the volunteers who donated blood for this study.
This work was supported by Universitywide AIDS Research Program grant F98-LA-134 (K. Grovit-Ferbas), NIH-R21-AI42687, and the Center for AIDS Research of the University of California at Los Angeles (National Institutes of Health grant AI/MH28697). Virus specimens were also provided by H. W. Sheppard of the San Francisco Men's Health Study (NIH-NOI-AI-82515).
REFERENCES
- 1.Adachi A, Gendelman E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and non-human cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AIDS Vaccine Evaluation Group. Protocol outlines. AVEG information handbook. [Online.] http://camelot.emmes.com/avctn/index.htm. 1999. [Google Scholar]
- 3.Arthur L O, Bess J W, Jr, Sowder II R C, Benveniste R E, Mann D L, Chermann J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 4.Arthur L O, Bess J W J, Chertova E N, Rossio J L, Esser M T, Benveniste R E, Henderson L E, Lifson J D. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S311–S319. [PubMed] [Google Scholar]
- 5.Burton D R, Moore J P. Why do we not have an HIV vaccine and how can we make one? Nat Med. 1998;4:495–498. doi: 10.1038/nm0598supp-495. [DOI] [PubMed] [Google Scholar]
- 6.Camerini D, Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the principal virus binding site. Cell. 1990;60:747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- 7.Cranage M P, Polyanskaya N, McBride B, Cook N, Ashworth L A, Dennis M, Baskerville A, Greenaway P J, Corcoran T, Kitchin P. Studies on the specificity of the vaccine effect elicited by inactivated simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1993;9:13–22. doi: 10.1089/aid.1993.9.13. [DOI] [PubMed] [Google Scholar]
- 8.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1942. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res Hum Retroviruses. 1992;8:411–421. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- 10.Duque H, Marshall R L, Israel B A, Letchworth G J. Effects of formalin inactivation on bovine herpes virus-1 glycoproteins and antibody response elicited by formalin-inactivated vaccines in rabbits. Vaccine. 1989;7:513–520. doi: 10.1016/0264-410x(89)90275-2. [DOI] [PubMed] [Google Scholar]
- 11.Einfeld D, Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci USA. 1988;85:8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Z, Huang X L, Zheng L, Wilson C, Borowski L, Liebmann J, Gupta P, Margolick J, Rinaldo C. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J Immunol. 1997;159:4973–4982. [PubMed] [Google Scholar]
- 13.Ferbas J, Kaplan A H, Hausner M A, Hultin L E, Matud J L, Liu Z, Panicali D L, Nerng-Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick R J, Benveniste R E, Gagliardi T D, Wiltrout T A, Busch L K, Bosche W J, Coren L V, Lifson J D, Bradley P J, Henderson L E, Arthur L O. Nucleocapsid protein zinc-finger mutants of simian immunodeficiency virus strain mne produce virions that are replication defective in vitro and in vivo. Virology. 1999;253:259–270. doi: 10.1006/viro.1998.9513. [DOI] [PubMed] [Google Scholar]
- 15.Grovit-Ferbas K, Ferbas J, Gudeman V, Sadeghi S, Goetz M B, Giorgi J V, Chen I S, O'Brien W A. Potential contributions of viral envelope and host genetic factors in a human immunodeficiency virus type 1-infected long-term survivor. J Virol. 1998;72:8650–8658. doi: 10.1128/jvi.72.11.8650-8658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer S M, Katzenstein D A, Hughes M D, Gundacker H, Schooley R T, Haubrich R H, Henry W K, Lederman M M, Phair J P, Niu M, Hirsch M S, Merigan T C. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 17.Issel C J, Horohov D W, Lea D F, Adams W V J, Hagius S D, McManus J M, Allison A C, Montelaro R C. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992;66:3398–3408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 21.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Zhou X, Orvell C, Lederer E, Ljunggren H G, Jondal M. Heat-inactivated Sendai virus can enter multiple MHC class I processing pathways and generate cytotoxic T lymphocyte responses in vivo. J Immunol. 1995;154:3147–3155. [PubMed] [Google Scholar]
- 23.McDougal J S, Martin L S, Cort S P, Mozen M, Heldebrant C M, Evatt B L. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J Clin Investig. 1985;76:875–877. doi: 10.1172/JCI112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKeating J A, McKnight A, Moore J P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore J P, Binley J. HIV. Envelope's letters boxed into shape. Nature. 1998;393:630–631. doi: 10.1038/31359. [DOI] [PubMed] [Google Scholar]
- 26.Moore J P, Jarrett R F. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses. 1988;4:369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- 27.Moore J P, McKeating J A, Weiss R A, Sattentau Q J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 28.Moss R B, Giermakowska W K, Savary J R, Theofan G, Daigle A E, Richieri S P, Jensen F C, Carlo D J. A primer on HIV type 1-specific immune function and REMUNE. AIDS Res Hum Retroviruses. 1998;14(Suppl. 2):S167–S175. [PubMed] [Google Scholar]
- 29.Murphey-Corb M, Martin L N, Davison-Fairburn B, Montelaro R C, Miller M, West M, Ohkawa S, Baskin G B, Zhang Z-Y, Putney S D, Allison A C, Eppstein D A. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989;246:1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien W A, Koyanagi Y, Namazie A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien W A, Mao S-H, Cao Y, Moore J P. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J Virol. 1994;68:5264–5269. doi: 10.1128/jvi.68.8.5264-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piszkiewicz D, Thomas W, Lieu M, Cabradilla C D, Andrews J, Kim J, Bourret E, McDougal J S, Cort S P. Heat inactivation of human immunodeficiency virus in lyophilized anti-inhibitor coagulant complex (Autoplex) Thromb Res. 1986;44:701–707. doi: 10.1016/0049-3848(86)90171-4. [DOI] [PubMed] [Google Scholar]
- 33.Piszkiewicz D, Thomas W, Lieu M Y, Tse D, Sarno L. Virus inactivation by heat treatment of lyophilized coagulation factor concentrates. Curr Stud Hematol Blood Transfus. 1989;56:44–54. doi: 10.1159/000416556. [DOI] [PubMed] [Google Scholar]
- 34.Pu R, Tellier M C, Yamamoto J K. Mechanism(s) of FIV vaccine protection. Leukemia. 1997;11(Suppl. 3):98–101. [PubMed] [Google Scholar]
- 35.Quinnan G V J, Wells M A, Wittek A E, Phelan M A, Mayner R E, Feinstone S, Purcell R H, Epstein J S. Inactivation of human T-cell lymphotropic virus, type III by heat, chemicals, and irradiation. Transfusion. 1986;26:481–483. doi: 10.1046/j.1537-2995.1986.26587020131.x. [DOI] [PubMed] [Google Scholar]
- 36.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 37.Resnick L, Veren K, Salahuddin S Z, Tondreau S, Markham P D. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986;255:1887–1891. [PubMed] [Google Scholar]
- 38.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 39.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W J, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, Bess J W J, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, Arthur L O, Henderson L E, Lifson J D. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 16.30–16.40. [Google Scholar]
- 41.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teller M C, Soos J, Pu R, Pollock D, Yamamoto J K. Development of FIV-specific cytolytic T-lymphocyte responses in cats upon immunisation with FIV vaccines. Vet Microbiol. 1997;57:1–11. doi: 10.1016/s0378-1135(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 44.Tersmette M, de Goede R E, Over J, De Jonge E, Radema H, Lucas C J, Huisman H G, Miedema F. Thermal inactivation of human immunodeficiency virus in lyophilised blood products evaluated by ID50 titrations. Vox Sang. 1986;51:239–243. doi: 10.1111/j.1423-0410.1986.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 45.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 46.Winkelman L, Feldman P A, Evans D R. Severe heat treatment of lyophilised coagulation factors. Curr Stud Hematol Blood Transfus. 1989;56:55–69. doi: 10.1159/000416557. [DOI] [PubMed] [Google Scholar]
- 47.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]