Abstract
Disruption of Epstein-Barr virus (EBV) latency is mediated by ZEBRA, the protein product of the immediate-early EBV gene, BZLF1. In vitro, phorbol 12-myristate 13-acetate (PMA), a potent activator of protein kinase C (PKC), induces reactivation of EBV. However, the physiological stimuli responsible for the disruption of viral latency are not well characterized. Transforming growth factor beta 1 (TGF-β1) has also been shown to trigger the reactivation of EBV in Burkitt lymphoma cell lines; however, the effect of TGF-β1 on ZEBRA expression has not been reported. To further understand this phenomenon, we have investigated the effect of TGF-β1 on ZEBRA expression. Our results indicate that the treatment of different EBV-positive Burkitt's lymphoma cell lines with TGF-β1 induces a time-dependent activation of BZLF1 transcription with a corresponding increase in the production of the protein ZEBRA. TGF-β1 has been shown to exert its effects through a wide range of intracellular routes; in the present study, we have explored these pathways. Transient expression of Smad proteins on their own had no effect on ZEBRA expression. A specific inhibitor of p38 mitogen-activated protein kinase (MAPK), SB203580, did not affect TGF-β1-induced ZEBRA expression, whereas treatment with the MAPK/ERK kinase inhibitors, PD98059 and U0126, dramatically decreased this induction. This suggests that TGF-β1 effect on BZLF1 expression requires the MAPK pathway. However, in Raji and B95-8 cells additional routes can be used, as (i) the inhibition of ZEBRA induction by PD98059 or U0126 was incomplete, whereas these inhibitors completely abolished PMA-induced ZEBRA expression, (ii) TGF-β1 induction of ZEBRA expression occurs in PKC-depleted cells, (iii) in Raji and in B95-8 cells, the effect of TGF-β1 and PMA are additive. Transient transfection of the EBV-negative B-cell line DG75 with a BZLF1 promoter-fusion construct (Zp-CAT) showed that under conditions where the BZLF1 promoter is activated by PMA treatment, TGF-β1 had no significant effect on the expression of the chloramphenicol acetyltransferase gene. Furthermore, TGF-β1 induction of BZLF1 transcripts is dependent on de novo protein synthesis, which suggests that TGF-β1 induces BZLF1 expression by an indirect mechanism.
Epstein-Barr virus (EBV), the causative agent of infectious mononucleosis, is associated with a growing number of malignant diseases, which include nasopharyngeal carcinoma, African Burkitt lymphoma (BL), Hodgkin's disease, non-Hodgkin's lymphoma in immunocompromised individuals (48), and peripheral T-cell lymphoma (54). In vitro, EBV infection of human B lymphocytes results in the immortalization of these cells with the virus maintained in a latent state, expressing a minimum of six nuclear (EBNA 1, 2, 3A, 3B, 3C, and EBNA LP) and three latent membrane (LMP1, LMP2A, and LMP2B) proteins.
EBV activation from latency is initiated by the expression of the BZLF1 gene product ZEBRA, also known as EB1 and Zta (11, 12). ZEBRA shares partial amino acid homology to a region in the product of the cellular proto-oncogene, c-fos. ZEBRA transactivates various EBV promoters through binding to AP-1-like sites and cyclic AMP (cAMP)-responsive element consensus sequences (9, 21, 40, 58). The BZLF1 transcripts are derived from either one of two promoters, Zp and Rp, as a 1-kb monocistronic or a 3-kb bicistronic mRNA, respectively, (41). The more proximal promoter, Zp, contains elements responsive to phorbol esters and anti-immunoglobulin G (anti-IgG) (7, 15, 22).
It has been reported that EBV can be reactivated in immunocompromised hosts, e.g., organ-transplanted and AIDS patients (6, 62). In such hosts, reactivation leads to increased susceptibility to development of EBV-positive non-Hodgkin's-type B-cell lymphoma (27–29). In addition, the reactivation of EBV in nasopharyngeal carcinoma has also been reported (42). However, the factors responsible for the reactivation of the virus in vivo are not known. To further our understanding of EBV reactivation, it is essential to identify the physiological stimuli and to determine the mechanism(s) leading to this phenomenon. In vitro, reactivation of the lytic cycle in latently infected B cells can be achieved by treatment with various agents, such as phorbol 12-myristate 13-acetate (PMA), Ca2+ ionophore, anti-IgG, human herpesvirus 6 infection, and transforming growth factor beta 1 (TGF-β1) (5, 10, 17, 20, 56, 64).
TGF-β1 regulates a wide range of physiological and pathological cellular processes, including differentiation, immune response, inflammation, extracellular matrix synthesis, angiogenesis, and wound healing in humans (39). Different studies have reported that TGF-β1 regulates the expression of various genes. However, the signal-transducing mechanism of the cytokine is not completely understood. TGF-β1 has been shown to exert its effects through a wide range of intracellular routes. Recent studies from several laboratories reported that Smads are intermediate effector proteins that transduce the TGF-β1 signal from the plasma membrane to the nucleus (14, 30, 43). TGF-β1 can induce gene expression via c-Jun N-terminal kinase (JNK) activation (3, 31, 60) or p38 mitogen-activated protein kinase (MAPK) (1, 26). The role of MAPK/ERK in the TGF-β1 signaling pathway has also been described (4, 61). Protein kinase C (PKC) has also been shown to be involved in PMA- and anti-IgG-induced EBV reactivation (13, 15) and could also play an important role in the signal transduction by TGF-β1 (25, 50, 53).
This study was undertaken to elaborate on the role of TGF-β1 in EBV reactivation. As ZEBRA expression is a key step in the switch from latency to lytic cycle, this study focused on the expression of this transactivator.
MATERIALS AND METHODS
Cell culture and reagents.
The EBV-positive B-cell lines Daudi, P3HR1, Raji, B95-8, and Mutu I and the EBV-negative BL cell line DG75 were maintained in RPMI 1640 supplemented with 100 UI of penicillin/ml, 100 μg of streptomycin/ml, and 10% heat-inactivated fetal calf serum (GIBCO BRL).
Purified recombinant TGF-β1 was purchased from R&D Systems (Minneapolis, Minn.), PMA, anisomycin, and cycloheximide were from Sigma Chemical Co. (St. Louis, Mo.), 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H7), bisindolylmaleimide (GF-109203X) [GFX], HA-1004, PD98059, and SB203580 were from Alexis (San Diego, Calif.), and U0126 was from Promega (Madison, Wis.).
Western blot analysis.
Cells were harvested, washed briefly with phosphate-buffered saline, resuspended in a buffer composed of 100 mM Tris-Cl (pH 7.6), 50 mM NaCl, 2 mM EDTA, 0.5% NP-40, phenylmethylsulfonyl fluoride (100 μg/ml), and 1 μg each of leupeptin, pepstatin and aprotinin per ml, and sonicated; protein concentrations were determined by the Bradford assay. Equal amounts of protein in loading buffer, heated for 5 min at 100°C and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels were transferred by electroblotting to a nitrocellulose membrane (Schleicher & Schuell, Ecquevilly, France). The membrane was stained with Ponceau S sodium salt (Sigma) to verify that the same amount of protein was deposited in each lane. Anti-ZEBRA monoclonal antibodies (MAbs) Z125 and Z130 (E. Drouet, Faculté de Pharmacie, Grenoble, France), were used as primary antibodies, and horseradish peroxidase-conjugated IgG (Interchim, Montluçon, France) was used as the secondary antibody; blots were developed by enhanced chemiluminescence (Interchim).
Immunohistology.
Frozen cytospun slides were air dried, fixed in acetone for 10 min, and air dried. Endogenous peroxidases were quenched with H2O2 (0.3% in methanol) for 15 min. Slides were immersed in washing buffer (WB; BioGenex, San Ramon, Calif.) and placed in coverplates (Shandon) filled with Power Block universal blocking reagent (1/10 dilution; BioGenex) for 10 min. Primary MAb Z130 was applied to the slides for 1 h. The slides were washed twice with WB and incubated for 30 min at room temperature with a biotinylated rabbit anti-mouse secondary antibody (1/200 dilution; Dako, Copenhagen, Denmark). After two 3-min washes with WB, slides were incubated for 30 min with streptavidin peroxidase (1/20 dilution; Vector Laboratories). Following two 3-min washes, the chromogen 3-amino-9-ethylcarbazole (Sigma) was added for 5 min. After washing in WB, slides were counterstained with Mayer's hematoxylin and mounted in aqueous medium (gel mount Microm).
RNA isolation and Northern blot analysis.
Total RNA was isolated on a Qiagen (Courtaboeuf, France) column according to the manufacturer's instructions. Poly(A)+ RNA was isolated using oligo(dT)-cellulose as instructed by the supplier (Pharmacia, Courtaboeuf, France). Poly(A)+ RNA was separated by electrophoresis through a 1% agarose-formaldehyde gel. The RNA was transferred onto a nylon membrane (Hybond N; Amersham, Courtaboeuf, France), and blots were prehybridized at 42°C for 3 h in 50% formamide–5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–5× Denhardt's solution–0.5% SDS–100 μg of denatured salmon sperm DNA/ml. The membranes were probed successively with random-primed [32P]dCTP-labeled BZLF1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs (106 cpm/ml) as recommended by the manufacturer (Promega, Charbonnieres, France). Following overnight incubation at 42°C, membranes were washed twice for 15 min with 2× SSPE–0.1% SDS at room temperature, twice with 2× SSPE–0.1% SDS at 65°C, and once with 0.5× SSPE–0.1% SDS at 65°C (10 min).
Plasmids, transfection, and CAT assays.
Plasmid −221Zp-CAT, generously provided by A. Sergeant (ENS, Lyon, France), contains BZLF1 promoter bp −221 to +12, relative to the transcription initiation site, cloned upstream of the bacterial chloramphenicol acetyltransferase (CAT) reporter gene. The Ia1 germ line reporter construct, pAI-D-CAT, was kindly provided by P. Sideras, Umeå University, Umeå Sweden (37). The latter plasmid was used as a positive control of TGF-β1 response. Plasmids containing Smad2, Smad3, Smad4, and Smad7 coding sequences driven by the cytomegalovirus promoter were kindly provided by Peter ten Dijke, Ludwig Institute for Cancer Research, Uppsala, Sweden.
Plasmid DNA (10 μg) purified on two CsCl2 density gradients was mixed with 107 cells in 500 μl of RPMI 1640. The cells were exposed to a single pulse at 230 V and 960 μF (Bio-Rad, Richmond, Calif.). The transfected cells were resuspended in 10 ml of complete culture medium, and TGF-β1 or PMA was added immediately following transfection. Cells were harvested 48 h later, washed with phosphate-buffered saline, suspended in 100 μl of 25 mM Tris-HCl (pH 7.5), and frozen in liquid nitrogen. Cells were disrupted by three freeze-thaw cycles in liquid nitrogen and 37°C. Cell debris was removed by centrifugation at 12,000 rpm for 10 min, and protein concentration was determined by the Bradford assay. For CAT assays, equal amounts of protein were incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A as described previously (23) or in the presence of N-butyryl coenzyme A (52).
RESULTS
TGF-β1 induces ZEBRA expression in EBV-infected BL cells.
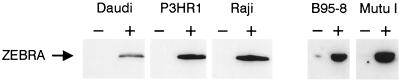
To determine whether TGF-β1 treatment could induce ZEBRA expression, EBV-positive B-cell lines B95-8, Raji, and Mutu I were cultured overnight in absence or in presence of TGF-β1 (1 or 5 ng/ml), and ZEBRA expression was determined by immunoblotting. TGF-β1 induced ZEBRA expression in the cell lines tested (Raji, P3HR1, Daudi, B95-8, and Mutu I) (Fig. 1). Similar results were obtained whether cells were cultured in the presence or absence of serum, and no expression of ZEBRA was observed in control cells treated with the vehicle alone (data not shown).
FIG. 1.
TGF-β1-induced ZEBRA expression in EBV-infected BL cells. Cells were incubated in absence or in presence of TGF-β1 (5 ng/ml) for 18 h. Cells were lysed, and equal amounts of protein were separated by SDS-PAGE and Western blotted with anti-ZEBRA antibodies as described in Materials and Methods. The arrow indicates the position of ZEBRA.
Kinetics of BZLF1 mRNA and ZEBRA expression in TGF-β1-stimulated Raji cells.
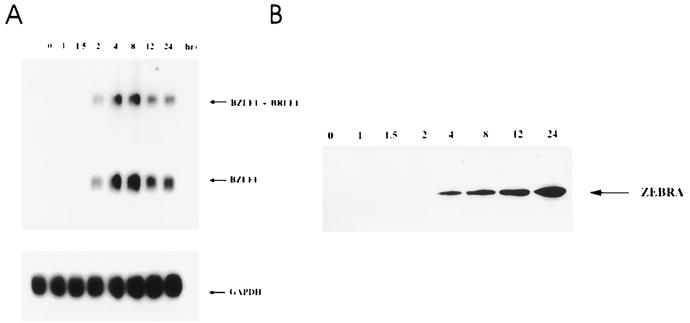
Since TGF-β1 induced the expression of ZEBRA protein, we then looked at the appearance of the 1- and 3.0-kb mRNAs of the BZLF1 gene. Raji cells were exposed to TGF-β1 for various periods of time and assayed for BZLF1 RNA expression by Northern blotting or for ZEBRA expression by Western blotting.
RNA and proteins were extracted starting at 1 h and ending at 24 h poststimulation. The cDNA probe used for Northern analysis recognizes both the monocistronic BZLF1 (1.0-kb) and the bicistronic BZLF1-BRLF1 (3-kb) mRNAs transcribed from the Zp and Rp promoters, respectively. The results (Fig. 2A) indicate that as early as 90 min poststimulation, both the 1- and 3.0-kb RNAs were expressed. Induction was maximal at 8 h, declined by 12 h, and remained stable thereafter. Western blot analysis showed that ZEBRA expression was detected at 4 h postinduction and continued to increase throughout the entire 24 h of culture (Fig. 2B).
FIG. 2.
Kinetics of TGF-β1-induced expression of BZLF1 RNA and ZEBRA in Raji cells. (A) Raji cells were treated with TGF-β1 (5 ng/ml) for the indicated time periods. Poly(A)+ RNA was isolated and analyzed by Northern blot analysis. The blots were probed with 32P-labeled BZLF1 cDNA. Equal loading was assessed by rehybridization with 32P-labeled GAPDH cDNA. (B) Raji cells treated with TGF-β1 (5 ng/ml) for the indicated time periods were lysed, and equal amounts of protein separated by SDS-PAGE were Western blotted with anti-ZEBRA antibodies as described in Materials and Methods. The position of ZEBRA is indicated by an arrow.
ZEBRA expression is not affected by overexpression of Smad proteins.
Smad proteins are known to trigger TGF-β1 signaling. It has been shown that cotransfection of Smad3 and Smad4 expression vectors mimics the effect of the cytokine in TGF-β1-responsive cells and that Smad7 expression inhibits this effect (14, 59).
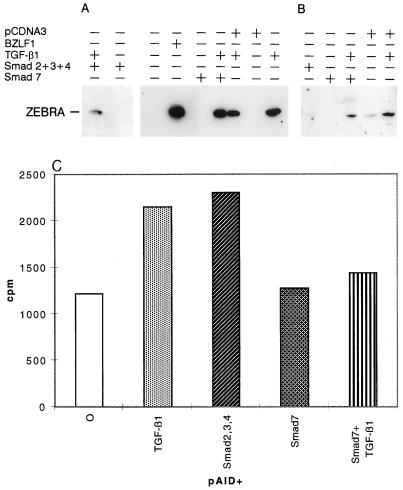
We cotransfected Mutu I or B95-8 cells with expression vectors for Smad2, Smad3, and Smad4. The Ia1 germ line reporter construct pAI-D-CAT was included as positive control for the TGF-β1 effect. When placed under the control of the pAI-D promoter, in Mutu I cells, the coexpression of Smad2, Smad3, and Smad4 activated CAT gene expression to the same extent as TGF-β1 activation (Fig. 3C). However, coexpression of Smad2, -3, and -4 had no effect on ZEBRA expression (Fig. 3A and B), whereas TGF-β1 induces its expression in the same cells. Similarly, expression of Smad7 in Mutu I reduces pAI-D-CAT expression brought about by TGF-β1 induction (Fig. 3), while no effect was observed on TGF-β1 induction of ZEBRA if Smad7 was expressed (Fig. 3A and B). These findings suggest that Smad proteins by themselves are not sufficient for TGF-β1 signaling effects on ZEBRA induction.
FIG. 3.
Effect of overexpression of Smad proteins on ZEBRA expression. Mutu I cells (A) or B95-8 cells (B) were transfected by electroporation with plasmids encoding Smad proteins, control plasmid pCDNA3, or a plasmid containing the BZLF1 coding sequence under the control of the cytomegalovirus promoter. Five hours after transfection, the cells were treated with or without TGF-β1 (2 ng/ml). After 24 h, cells were harvested and lysed, and equal amounts of protein separated by SDS-PAGE were Western blotted with anti-ZEBRA antibodies as described in Materials and Methods. The position of ZEBRA is indicated by the arrow. (C) Mutu I cells were transfected by electroporation with pAI-D-CAT in the absence or presence of plasmids encoding Smad proteins. Five hours after transfection, the cells were treated with or without TGF-β1 (2 ng/ml). After 24 h, cells were harvested and CAT activity was assayed in 50 μg of cell extract by enzymatic butyrylation of radiolabeled chloramphenicol.
Effect of PKC and PKA inhibitors on ZEBRA-induced expression by TGF-β1.
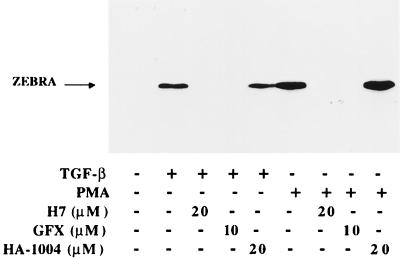
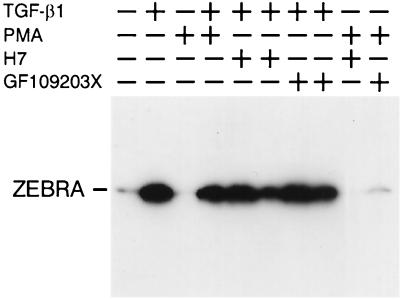
Since previous reports suggested that PKC may play an important role in TGF-β1 signal transduction (25, 50, 53), we examined the effect of specific inhibitors of PKC and PKA on TGF-β1 BZLF1 induction. Raji cells were pretreated for 1 h with the protein kinase inhibitors before stimulation by TGF-β1 or PMA, and induced ZEBRA expression was analyzed by Western blotting. As shown in Fig. 4, GFX and H7, potent inhibitors of PKC which interact with the catalytic subunit of the enzyme, inhibit both PMA- and TGF-β1-induced ZEBRA expression. These findings suggest that in Raji cells, PKC not only mediates the PMA effect but also may be involved in mediating the effect of TGF-β1 on BZLF1 gene expression. Conversely, in Mutu I cells, in which ZEBRA is not induced by PMA treatment, GFX or H7 has no effect on TGF-β1-mediated ZEBRA induction (Fig. 5), suggesting that diacylglycerol (DAG)-inducible PKC isoforms are not activated in these cells.
FIG. 4.
Effect of protein kinase inhibitors on TGF-β1- or PMA-induced ZEBRA expression in Raji cells. Raji cells were pretreated for 1 h, with or without the inhibitors listed, prior to stimulation with TGF-β1 (5 ng/ml) or PMA (20 ng/ml). The cells were lysed, and equal amounts of protein separated by SDS-PAGE were analyzed by Western blotting with anti-ZEBRA MAbs. The position of ZEBRA is indicated by the arrow.
FIG. 5.
Effect of PMA and protein kinase inhibitors on TGF-β1-induced ZEBRA expression in Mutu I cells. Mutu I cells were pretreated for 1 h with or without the inhibitors listed, in the absence or presence of TGF-β1 (2 ng/ml) or PMA (20 ng/ml). The cells were lysed, and equal amounts of protein separated by SDS-PAGE were analyzed by Western blotting with anti-ZEBRA MAbs. The position of ZEBRA is indicated by the arrow.
The addition of HA-1004, an inhibitor of cAMP-dependent protein kinase, to Raji cell cultures had no effect on the TGF-β1-mediated ZEBRA expression. PKA does not appear to be involved in this effect of TGF-β1.
TGF-β1- and PMA-induced ZEBRA expression is additive.
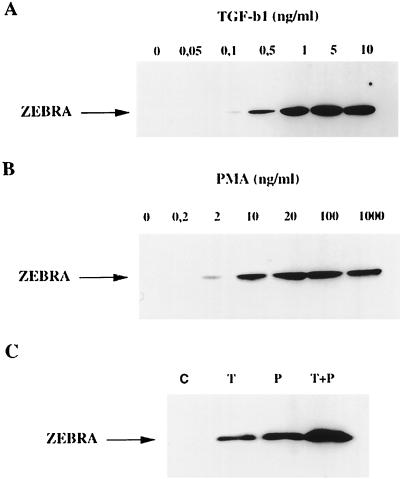
A dose-dependent TGF-β1 (0 to 10 ng/ml) induction of ZEBRA expression in Raji cells showed the protein induced with a dose as low as 0.5 ng/ml, with a maximum effect observed at 5 ng/ml (Fig. 6A). The tumor-promoting phorbol ester PMA was previously shown to induce BZLF1 expression (13, 38); we therefore compared the stimulating effect of this agent to that of TGF-β1 in Raji cells. As could be expected, PMA induced ZEBRA expression in a dose-dependent manner, with maximum induction attained at 20 ng/ml (Fig. 6B). Furthermore, the stimulation observed with maximal concentrations of TGF-β1 (10 ng/ml) and PMA (100 ng/ml) was additive (Fig. 6C). This additive effect of PMA and TGF-β1 on ZEBRA expression was also seen in B95-8 cells (data not shown).
FIG. 6.
Additive effect of TGF-β1 and PMA on ZEBRA expression. (A and B) Raji cells were treated with the indicated concentrations of TGF-β1 or PMA for 18 h. (C) Raji cells were treated with either vehicle (C), TGF-β1 (10 ng/ml; T), PMA (100 ng/ml; P), or TGF-β1 (10 ng/ml) plus PMA (100 ng/ml) (T+P) for 18 h. Cells were lysed, and equal amounts of protein were separated by SDS-PAGE and analyzed by Western blotting with anti-ZEBRA antibodies as described in Materials and Methods. The position of ZEBRA protein is indicated by the arrow.
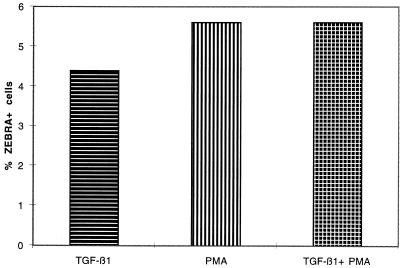
We compared the fraction of cells producing ZEBRA in response to the various inducing agents. Figure 7 shows that treatment of Raji cells with TGF-β1, PMA, or both TGF-β1 and PMA induces ZEBRA expression in 74 of 1,645 (4.45%), 88 of 1,566 (5.6%), and 74 of 1,322 (5.6%) cells, respectively. A chi-square test showed no statistical difference (P = 0.27). This result shows that the additive effect of PMA and TGF-β1 on ZEBRA expression was not due to a higher number of cells responding to the combined treatment.
FIG. 7.
Percentage of Raji cells producing ZEBRA. Raji cells were treated for 18 h with or without TGF-β1 (5 ng/ml), PMA (20 ng/ml) or TGF-β1 (5 ng/ml) plus PMA (20 ng/ml). Detection of ZEBRA expression was performed by immunochemistry as described in Materials and Methods. ZEBRA-positive and -negative cells were counted on low-magnification photographs. More than 1,300 total cells were counted for each treatment, and a chi-square test was performed for statistical analysis.
These findings provide evidence that activation of ZEBRA expression by TGF-β1 is mediated by a pathway distinct from that used for the stimulation by PMA.
TGF-β1 induction of ZEBRA expression requires a non-PMA-inducible protein kinase.
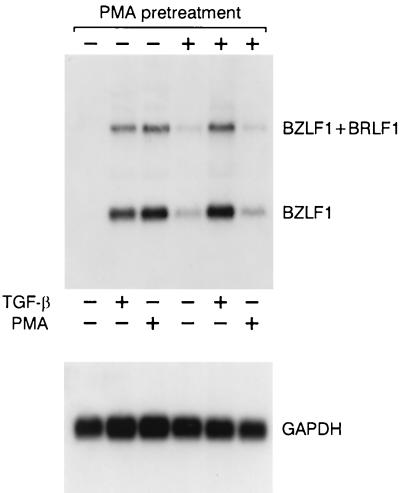
Different isoforms of PKC (α, β, and δ) are down-regulated by chronically treating cell culture with PMA. This procedure depletes these isoforms and desensitizes the enzymes to subsequent activation by PMA (34, 55, 57). We exposed Raji cells to PMA (300 ng/ml) or vehicle (dimethyl sulfoxide [DMSO]) for 48 h, and mRNA expression was evaluated following the addition of either TGF-β1 or PMA. As shown in Fig. 8, PMA pretreatment caused a marked decrease in the induction of BZLF1 expression by PMA compared to that seen in controls pretreated with the vehicle alone. In contrast, TGF-β1 promoted a strong response which was even higher than that seen in cells which were not pretreated with PMA.
FIG. 8.
Effect of PMA-sensitive PKC down-regulation on TGF-β1- or PMA-induced BZLF1 expression. Raji cells were pretreated with PMA (300 ng/ml) or control vehicle (DMSO) for 48 h prior to stimulation with TGF-β1 (10 ng/ml) or PMA (20 ng/ml) for 4 h. The cells were then harvested, mRNA was isolated, and the Northern blot was probed with the 32P-labeled BamHI Z fragment cDNA. Equal loading was assessed by rehybridization with 32P-labeled GAPDH cDNA.
Taken together, these results strongly suggest that PMA activates BZLF1 expression through DAG-sensitive PKC, whereas GFX-sensitive protein kinase(s) (other than DAG-sensitive PKCs, or other isoforms of PKC) mediate the TGF-β1 effect.
Effect of MAPK inhibitors on TGF-β1 induction of ZEBRA expression.
The role of MAPK kinase (4, 61) as well as that of p38 MAPK in the TGF-β1 signaling pathway has been described elsewhere (1, 26). Treatment of Mutu I, Raji, and B95-8 cells with SB203580 (120 nM) had no effect on the rate of ZEBRA production through TGF-β1 induction in any of these cell lines (data not shown). This result demonstrate that the p38 MAPK is not required in TGF-β1 signaling pathway for ZEBRA induction.
Transfection of a dominant negative form of JNK coding sequences (24) in Mutu I, Raji, and B95-8 cells had no effect on TGF-β1-mediated ZEBRA induction, indicating that JNK is not involved in TGF-β1 signaling for ZEBRA expression. The dominant negative form of JNK is efficiently expressed in Mutu I, Raji, and B95-8 cells, as measured by its activity on transfected TRE-CAT plasmid (not shown).
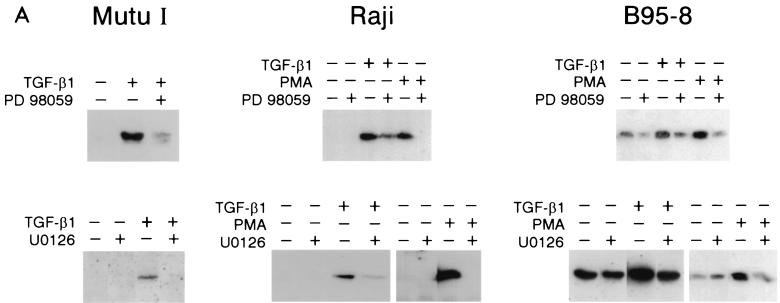
We investigated the effect MAPK/ERK kinase inhibitors on the induction of ZEBRA expression by TGF-β1 or PMA using PD098059, which prevents the MEK1,2 activation by Raf and U0126, which inhibits both active and inactive MEK1,2 (18). Mutu I, Raji, and B95-8 cells were pretreated for 2 h with 100 μM PD98059, and TGF-β1 (2 or 5 ng/ml) or PMA (20 ng/ml) was then added for 18 h. The results are presented in Fig. 9A. TGF-β1-induced production of ZEBRA in Mutu I, Raji, and B95-8 cells is reduced by pretreatment with PD98059. In B95-8 cells, this inhibition affects only the induced and not the basal ZEBRA production. Experiments performed with U0126 gave similar results. These results show that MAPK/ERK kinase pathway is required for TGF-β1 induction of ZEBRA expression.
FIG. 9.
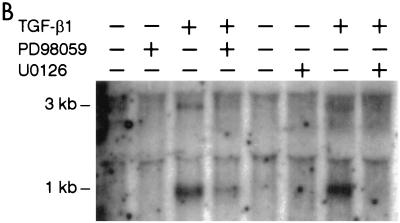
Effect of protein kinase inhibitors on TGF-β1- or PMA-induced BZLF1 expression. (A) Mutu I, Raji, and B95-8 cells were pretreated for 2 h with PD98059 (100 μM), U0126 (50 μM), or vehicle (DMSO) before the addition of TGF-β1 (2 ng/ml) or PMA (20 ng/ml). Fifteen hours later, cells were harvested and resuspended in Laemmli sample buffer. Equal amounts of protein were separated by SDS-PAGE and analyzed by Western blotting with anti-ZEBRA antibodies as described in Materials and Methods. (B) Mutu I cells pretreated for 2 h with PD98059 (100 μM), U0126 (50 μM), or vehicle before the addition of TGF-β1 (2 ng/ml). Four hours later, cells were harvested, and total RNA was extracted and analyzed on Northern blots probed with 32P-labeled BZLF1 cDNA.
The partial effects on ZEBRA expression observed with PD98050 and U0126 in Raji and B95-8 cells suggest that these cells use an additional route(s) to mediate the TGF-β1 effect on ZEBRA expression.
Conversely, PD98050 and U0126 completely inhibited PMA-induced ZEBRA expression in Raji and B95-8 cells. Thus, the signal-transducing pathway of PMA appears to implicate solely that of MAPK/ERK kinase. In Mutu I cells, while PD98059 exhibits partial TGF-β1-mediated ZEBRA induction, U0126 completely inhibits this induction. Since U0126 inhibits the MAPK/ERK signaling pathway at the level of both Raf and MEK activation, an additional pathway leading to MEK activation could occur through TGF-β1 signaling. Nevertheless, as inhibition of TGF-β1 induction by U0126 is complete in Mutu I cells, it would appear that no pathway other than the one leading to MAPK/ERK kinase is used.
These results were confirmed by Northern blot analysis. Figure 9B shows that the induction of the 1- and 3-kb BZLF1 transcripts by TGF-β1 is partially inhibited by PD98059 in Mutu I cells, while U0126 completely inhibits this induction. These results show that both the induction and the inhibition of BZLF1 expression occur at the transcriptional level.
The Zp BZLF1 promoter does not respond to TGF-β1 in the EBV-negative cell line DG75.
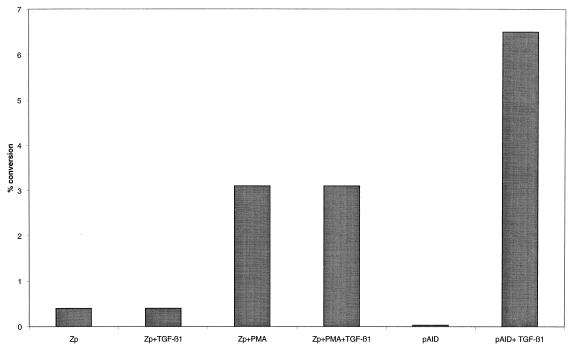
TGF-β1 stimulation results in the induction of BZLF1 mRNA and protein expression. BZLF1 gene induction is initiated by activation of its promoter by lytic cycle inducers. The EBV-negative BL cell line DG75 was transfected with a construct containing BZLF1 promoter nucleotides −221 to +12 linked to the bacterial CAT reporter −221Zp-CAT. The cells were then induced with TGF-β1 (10 ng/ml) and/or PMA (20 ng/ml). The Ia1 germ line reporter construct pAI-D-CAT was included as the positive control. An increase in CAT activity was observed following −221Zp-CAT stimulation with PMA, whereas no significant activation was detected in response to TGF-β1 (Fig. 10). Since TGF-β1 activated the pAI-D promoter, the lack of a response for Zp-CAT could not be attributed to failure of TGF-β1 signal transduction in DG75 cells.
FIG. 10.
Response to TGF-β1 and PMA of a transiently transfected BZLF1 promoter-driven CAT reporter plasmid in DG75 cells. The BZLF1 promoter reporter construct −221Zp-CAT and the pAI-D-CAT construct were transfected into DG75 cells by electroporation. Immediately following transfection, cells were treated with TGF-β1 (10 ng/ml) and/or PMA (20 ng/ml). After 48 h, cells were harvested and the level of CAT activity was determined by quantification of acetylated chloramphenicol species with a PhosphorImager (Molecular Dynamics).
The presence of a positive TGF-β1 response element(s) outside the −221-bp region, or negative regulatory element(s) in the −221-bp region, was assayed with constructs containing extensions of the Zp promoter 5′ region to −500 bp and various deletions in the −221-bp Zp, respectively. These insertions or deletions had no effect on TGF-β1 inductivity of Zp (data not shown). A similar negative result was obtained with the promoter of the 3-kb transcript.
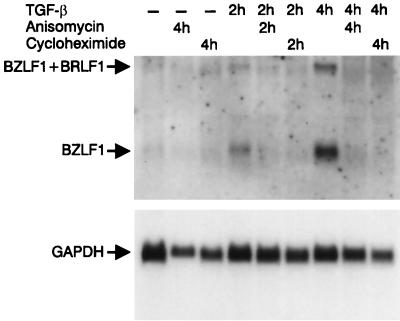
To determine if TGF-β1 induction of BZLF1 transcripts is dependent on de novo protein synthesis, Mutu I cells were treated with TGF-β1 (2 ng/ml) in the presence or absence of either one or both of two inhibitors of protein synthesis (anisomycin or cycloheximide). Cells were harvested 2 or 4 h after addition of TGF-β1, and RNA prepared from these cells was analyzed for the presence of BZLF1 transcripts by Northern blotting. As seen in Fig. 11, each inhibitor (or both together [data not shown]) completely abolished the appearance of both the 1- and 3-kb BZLF1 transcripts, which suggests that induction of the lytic cycle is dependent on de novo protein synthesis.
FIG. 11.
Effect of protein synthesis inhibitors on TGF-β1-induced BZLF1 expression. Mutu I cells were treated (or not) with TGF-β1 (1 ng/ml) for indicated time periods, with or without 10 μM anisomycin or 40 μM cycloheximide. Total RNA was isolated and probed with 32P-labeled BZLF1 cDNA on Northern blots. Equal loading was assessed by rehybridization with [32P]GAPDH cDNA.
DISCUSSION
It was reported that TGF-β1 induces EA expression in EBV latently infected B cells (10, 16); however, the molecular mechanisms involved in the effect of TGF-β1 are unknown. The present study was aimed at defining the molecular mechanisms by which EBV is reactivated following exposure to TGF-β1. Since the expression of ZEBRA is an early event which precedes EA production, we focused our study on the activation of ZEBRA by this cytokine. Our results indicate that exposure of different EBV genome-positive B cells to TGF-β1 results in the expression of the immediate-early EBV protein, ZEBRA. This supports earlier observations that low levels of TGF-β1 can induce the lytic cycle in latently infected B cells (16).
Northern blot analysis showed that stimulation by TGF-β1 in Raji cells involves the simultaneous expression of both the 1- and 3.0-kb RNAs, suggesting that both promoters could be activated by changes in the activity of cellular factors associated with TGF-β1 treatment. Although the BZLF1 transcripts are produced in a short period of time following TGF-β1 induction, de novo protein synthesis is required to produce this effect.
Depending on the cell type, TGF-β1 signal transduction has implicated virtually every second messenger pathway including cAMP, inositol phosphate hydrolysis, calcium influx, DAG, immediate-early genes c-jun and c-fos, p21ras, p38, JNK, and PKC (25, 35, 36, 44–46). More recently, a pathway involving Smad proteins has been documented (43). However, overexpression of Smad proteins is not able to mimic the TGF-β1 effect on ZEBRA expression, suggesting that on their own, Smads are not sufficient in mediating TGF-β1 induction of ZEBRA.
The DAG-inducible PKC could be a step in this TGF-β1 signaling pathway, as the PKC inhibitors H7 and GFX are able to inhibit TGF-β1-mediated ZEBRA induction. Activation of PKC leads to the MAPK/ERK pathway (13, 19). Furthermore, several lines of evidence suggest that the MAPK/ERK pathway is involved in TGF-β1 induction of ZEBRA in B95-8 and in Raji cells since incubation with PD98059 or U0126, potent inhibitors of this pathway, inhibit the TGF-β1 ZEBRA induction. It remains to be determined if ras activation of raf is involved in this pathway. It has been shown that the MAPK/ERK pathway is involved in anti-Ig activation of ZEBRA expression in Akata cells (51). We now provide evidence for the use of this pathway in triggering the TGF-β1 induction of ZEBRA. Nevertheless, in B95-8 and in Raji cells an additional route (or routes) could also be used since (i) the inhibition by PD98059 or U0126 was incomplete (inhibitors which completely abolished PMA-induced ZEBRA expression in the same cell lines), (ii) TGF-β1 induction of ZEBRA expression occurred in PKC-depleted cells, and (iii) the effects of TGF-β1 and PMA are additive in Raji and in B95-8 cells. This model is represented in Fig. 12A. The proposed additional pathway does not involve the p38 MAPK or JNK because TGF-β1 ZEBRA induction is not affected by incubation with the p38 MAPK inhibitor SB203580 or by transient expression of a dominant negative JNK mutant.
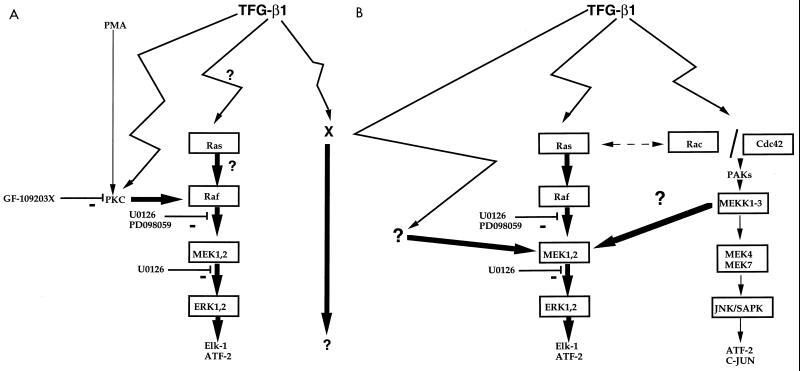
FIG. 12.
Proposed scheme for a second messenger pathway triggered by TGF-β1 leading to the induction of ZEBRA. (A) In Raji and B95-8 cells, TGF-β1 causes activation of PKC and/or initiation of the Raf-MEK-ERK MAPK cascade. TGF-β1 also used an activity determined by the method described by Gorman et al. (23) as an additional signaling pathway to mediate the induction of ZEBRA expression. (B) In Mutu I cells, the Raf-MEK-ERK MAPK cascade is used as signal transducer. Moreover a cross talk between pathways will lead to a Raf-independent MEK1,2 activation. Thick arrows represent proposed routes. PD98059 acts by inhibiting the activation of MEK1,2 by Raf kinase, U0126 acts both on inactive and active forms of MEK1,2. GF-109203X is a potent inhibitor of PKC.
In Mutu I cells, U0126 completely abolished TGF-β1-induced ZEBRA expression, showing that only the MAPK/ERK pathway is used. As PD98059 only partially inhibits the TGF-β1 effect, a pathway leading to activation of MEK1,2 could be used. This activation might be due to MEKK1,3, as shown by Yujiri et al. (63). The model is represented in Fig. 12B.
The MAPK pathway is also activated by the EBV latent membrane protein LMP1 (19), which plays a critical role in the regulation of cell growth and differentiation. This might suggest that the lytic cycle requires a some step of differentiation.
We observed that the Zp BZLF1 promoter does not respond to TGF-β1 in transfected cells and that TGF-β1 induction of BZLF1 transcripts is dependent on de novo protein synthesis. These data are in agreement with the two-step induction model proposed by Flemington and Speck (21), in which induction of the EBV lytic cycle requires an initial activation signal of sufficient magnitude to allow expression of enough ZEBRA to autoactivate Zp.
TGF-β1, a potent immunosuppressive cytokine which suppresses T-cell responses and deactivates macrophage effector functions, is produced by a wide variety of cells (39). In addition, B-lymphoma cells and Hodgkin's Reed-Sternberg cells have been shown to produce TGF-β1 (33, 47). Therefore, in vivo, TGF-β1-mediated EBV reactivation may occur through a paracrine or an autocrine mode. Furthermore, EBV binding (2) and ZEBRA (8) have been shown to induce TGF-β1 expression. Thus, a vicious cycle may be initiated, whereby replication of EBV and production of TGF-β1 amplify one another. In immunodeficient individuals, this may lead to an increase in the number of EBV-infected cells and thus favor the development of EBV-associated diseases. Moreover, high levels of the cytokine may perpetuate the immunosuppressive circuits.
ACKNOWLEDGMENTS
We thank A. Alberga for critically reading the manuscript and Azzedine Atfi for helpful discussion. We thank E. Drouet for the anti-ZEBRA monoclonal antibodies, A. Sergeant for the Zp-CAT plasmid, P. Sideras for pAI-D-CAT, Peter ten Dijke for plasmids containing sequences encoding Smad2, Smad3, Smad4, and Smad7 proteins, Roger Davis for the plasmid with the sequence of the dominant negative form of JNK, and E. Connault for technical assistance.
This project was supported by ARC (9474) and by the Ligue Nationale contre le Cancer (SF-98).
REFERENCES
- 1.Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A, Menezes J. Binding of the Epstein-Barr virus to human platelets causes the release of transforming growth factor-b. J Immunol. 1997;159:3984–3988. [PubMed] [Google Scholar]
- 3.Atfi A, Buisine M, Mazars A, Guespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-beta through stress-activated protein kinase/c-jun N-terminal kinase(SAPK/JNK) signaling pathway. J Biol Chem. 1999;272:24731–24734. doi: 10.1074/jbc.272.40.24731. [DOI] [PubMed] [Google Scholar]
- 4.Axmann A, Seidel D, Reimann T, Hempel U, Wenzel K W. Transforming growth factor-beta1-induced activation of the Raf-MEK-MAPK signaling pathway in rat lung fibroblasts via a PKC dependent mechanism. Biochem Biophys Res Commun. 1998;249:456–460. doi: 10.1006/bbrc.1998.9188. [DOI] [PubMed] [Google Scholar]
- 5.Bauer G, Hofler P, zur Hausen H. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducers. Virology. 1982;121:184–194. doi: 10.1016/0042-6822(82)90128-3. [DOI] [PubMed] [Google Scholar]
- 6.Birx D L, Redfield R R, Tosato G. Defective regulation of Epstein-Barr virus infection in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related disorders. N Engl J Med. 1986;314:874–879. doi: 10.1056/NEJM198604033141403. [DOI] [PubMed] [Google Scholar]
- 7.Borras A M, Strominger J L, Speck S L. Characterization of the ZI domains in the Epstein-Barr virus BZLF1 gene promoter: role in phorbol ester induction. J Virol. 1996;70:3894–3901. doi: 10.1128/jvi.70.6.3894-3901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayrol C, Flemington E K. Identification of cellular targets genes of the Epstein-Barr virus transactivator Zta: activation of transforming growth factor βigh3 (TGF-βigh3) and TGF-β1. J Virol. 1995;69:4206–4212. doi: 10.1128/jvi.69.7.4206-4212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y N, Dong D L Y, Hayward G S, Hayward D. The Epstein-Barr virus Zta transactivator: a member of the bZip family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990;64:3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chasserot-Golaz S, Shuster C, Dietrich J B, Beck G, Lawrence D A. Antagonistic action of RU38486 on the activity of transforming growth factor-b in fibroblasts and lymphoma cells. J Steroid Biochem. 1988;30:381–385. doi: 10.1016/0022-4731(88)90127-6. [DOI] [PubMed] [Google Scholar]
- 11.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Dallie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:2343–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogenous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies A H, Grand R J A, Evans F J, Rickinson A B. Induction of Epstein-Barr virus lytic cycle by tumor-promoting and non-tumor-promoting phorbol esters requires active protein kinase C. J Virol. 1991;65:6838–6844. doi: 10.1128/jvi.65.12.6838-6844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier J M. Direct binding of Smad3 and Smad4 to critical TGF-β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabata M, Speck S H, Mulder C, Sairenji T. Regulation of the BZLF1 promoter of Epstein-Barr virus by second messengers in anti-immunoglobulin-treated B cells. Virology. 1994;198:446–454. doi: 10.1006/viro.1994.1056. [DOI] [PubMed] [Google Scholar]
- 16.Di Renzo L, Altiok A, Klein G, Klein E. Endogenous TGF-β contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma cell lines. Int J Cancer. 1994;57:914–919. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 17.Fagionni A, Zompetta C, Grimaldi S, Barile G, Frati L, Lazdins J. Calcium modulation activates Epstein-Barr virus genome in latently infected cells. Science. 1986;232:1554–1556. doi: 10.1126/science.3012779. [DOI] [PubMed] [Google Scholar]
- 18.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stredley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magloda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;17:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 19.Fenton M, Sinclair A J. Divergent requirements for the MAPK(ERK) signal transduction pathway during initial virus infection of quiescent primary B cells and disruption of Epstein-Barr virus latency by phorbol esters. J Virol. 1999;73:8913–8916. doi: 10.1128/jvi.73.10.8913-8916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flamand L, Stefanescu I, Ablashi D V, Menezes J. Activation of the Epstein-Barr virus replicative cycle by human herpesvirus 6. J Virol. 1993;67:6768–6777. doi: 10.1128/jvi.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flemington E K, Speck S H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flemington E K, Speck S H. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 25.Halstead J, Kemp K, Ignotz R A. Evidence for the involvement of phosphatidylcholine-phospholipase C and protein kinase C in transforming growth factor-beta signaling. J Biol Chem. 1995;270:13600–13603. doi: 10.1074/jbc.270.23.13600. [DOI] [PubMed] [Google Scholar]
- 26.Hannigan M, Zhan L, Ai Y, Huang C K. The role of p38 MAP kinase in TGF-beta-1-induced signal transduction in human neutrophils. Biochem Biophys Res Commun. 1998;246:55–58. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- 27.Hanto D W, Najarian J S. Advances in the diagnosis and treatment of EBV-associated lymphoproliferative diseases in immunocompromised hosts. J Surg Oncol. 1985;30:215–220. doi: 10.1002/jso.2930300406. [DOI] [PubMed] [Google Scholar]
- 28.Hanto D W, Fizzera G, Gajl-Peczalska J, Simmons R L. Epstein-Barr virus, immunodeficiency, and B-cell lymphoproliferation. Transplantation. 1985;39:461–672. doi: 10.1097/00007890-198505000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hanto D W, Gajl-Peczalska J, Fizzera G, Arthur D C, Balfour H N, McClain K, Simmons R L, Najarian J S. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative disease occuring after renal transplantation. Ann Surg. 1983;198:356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldin C H, Miyazono K, ten Dijke P. TGF-β signaling from the cell membrane to nucleus through Smad proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 31.Hocevar B, Brown T, Howe P. TGF beta induces fibronectin synthesis through c-jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe P H, Bascom C C, Cunningham M R, Leof E B. Regulation of transforming growth factor-b1 action by multiple transducing pathways: evidence for both G protein-dependent and -independent signaling. Cancer Res. 1989;49:6024–6031. [PubMed] [Google Scholar]
- 33.Hsu S M, Lin J, Xie S S, Hsu P L, Rich S. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin's Reed-Sternberger and by reactive T lymphocytes in Hodgkin's disease. Hum Pathol. 1993;24:249–255. doi: 10.1016/0046-8177(93)90034-e. [DOI] [PubMed] [Google Scholar]
- 34.Isakov N, McMahon P, Altman A. Selective post-transcriptional down-regulation of protein kinase C isoenzymes in leukemic T cells chronically treated with phorbol ester. J Biol Chem. 1990;265:2091–2097. [PubMed] [Google Scholar]
- 35.Kataoka R, Sherlock J, Lanier S M. Signaling events initiated by transforming growth factor-β1 that require Gia1. J Biol Chem. 1993;268:19851–19857. [PubMed] [Google Scholar]
- 36.Kim S J, Angel P, Lafyatis R, Hattori K, Kim K Y, Sporn M B, Karin M, Roberts A B. Autoinduction of transforming growth factor β1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lars N, Sideras P. The human Ia 1 and Ia 2 germline proter elements: proximal positive and distal negative may regulate the tissue specific expression of Ca 1 and Ca 2 germline transcripts. Int Immunol. 1993;5:271–282. doi: 10.1093/intimm/5.3.271. [DOI] [PubMed] [Google Scholar]
- 38.Laux G, Freese U K, Fisher R, Polack A, Kofler E, Bornkamm G W. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology. 1988;162:503–507. doi: 10.1016/0042-6822(88)90496-5. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence D A. Transforming growth factor-β: a general review. Eur Cytokine Netw. 1996;7:363–374. [PubMed] [Google Scholar]
- 40.Lieberman P M, Hardwick J M, Sample J, Hayward G S, Hayward D. The Zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990;64:1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manet E, Gruffat H, Trescol-Biemont M C, Moreno I, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNA's generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martel-Renoir D, Grunewald V, Touitou R, Schwaab G, Joab I. Qualitative analysis of the expression of lytic genes in nasopharygeal carcinoma biopsies. J Gen Virol. 1995;76:1401–1408. doi: 10.1099/0022-1317-76-6-1401. [DOI] [PubMed] [Google Scholar]
- 43.Massagué TGF-β signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 44.Mulder K M, Morris S L. Activation of p 21ras by transforming growth factor β in epithelial cells. J Biol Chem. 1992;267:5029–5031. [PubMed] [Google Scholar]
- 45.Mulder K M, Humphrey L E, Choi H G, Childress-Fields K E, Brattain M G. Evidence for c-myc in the signaling pathway for TGF-beta in well-differentiated human colon carcinoma cells. J Cell Physiol. 1990;145:501–507. doi: 10.1002/jcp.1041450316. [DOI] [PubMed] [Google Scholar]
- 46.Muldoon L L, Rodland K D, Magun B E. Transforming growth factor beta and epidermal growth factor alter calcium influx and phosphatidylinositol turnover in rat-1 fibroblasts. J Biol Chem. 1988;263:18834–18841. [PubMed] [Google Scholar]
- 47.Newcom S R, Kadin M E, Ansari A A, Diehl V. L-428 nodular sclerosing Hodgkin's cell secretes a unique transforming growth factor-beta active at physiologic pH. J Clin Investig. 1988;82:1915–1921. doi: 10.1172/JCI113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley M M, Chanock R M, Monath T P, Melnick J L, Roizman B, Strauss S E, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 49.Roberts M L, Cooper N R. Activation of a Ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology. 1998;240:93–99. doi: 10.1006/viro.1997.8901. [DOI] [PubMed] [Google Scholar]
- 50.Saltis J, Bobik A. Regulation by protein kinase C of transforming growth factor-beta 1 action on the proliferation of vascular smooth muscle from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1996;23:573–575. doi: 10.1111/j.1440-1681.1996.tb02783.x. [DOI] [PubMed] [Google Scholar]
- 51.Satoh T, Hoshikawa Y, Satoh Y, Kurata T, Sairenji T. The interaction of mitogen-activated protein kinases to Epstein-Barr virus activation in Akata cells. Virus Genes. 1998;18:57–64. doi: 10.1023/a:1008021402908. [DOI] [PubMed] [Google Scholar]
- 52.Seed B, Sheen J. A simple phase-extraction assay for chloramphenicol acetyl transferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Asplund T, Yamashita H, Heldin C H, Heldin P. Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J. 1995;307:817–821. doi: 10.1042/bj3070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzushima H, Assou N, Fujimoto T, Nishimura S, Okubo T, Yamasaki H, Osato M, Matsuoka M, Tsukamoto A, Takai K, et al. Lack of the expression of EBNA-2 and LMP-1 in T-cell neoplasmas possessing Epstein-Barr virus. Blood. 1995;85:480–486. [PubMed] [Google Scholar]
- 55.Terajima J, Tsutsumi A, Freire-Moar J, Cherwinski H M, Ransom J T. Evidence for clonal heterogeneity of the expression of six protein kinase C isoforms in murine B and T lymphocytes. Cell Immunol. 1992;142:197–206. doi: 10.1016/0008-8749(92)90280-3. [DOI] [PubMed] [Google Scholar]
- 56.Tovey M, Lenoir G, Lours-Begon J. Activation of latent Epstein-Barr virus by antibody to human IgM. Nature. 1978;272:373–375. doi: 10.1038/276270a0. [DOI] [PubMed] [Google Scholar]
- 57.Tsutsumi A, Kubo M, Fuji H, Freire-Moar J, Turck C W, Ransom J T. Regulation of protein kinase C isoform proteins in phorbol ester-stimulated Jurkat T cells. J Immunol. 1993;150:1746–1754. [PubMed] [Google Scholar]
- 58.Urier G, Buisson M, Chambar P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from differents responsive elements including AP-1 binding sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vindevoghel L, Lechleider R J, Kon A, de Caestcker M P, Uitto J, Roberts A, Mauviel A. SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor β. Proc Natl Acad Sci USA. 1998;95:14769–14774. doi: 10.1073/pnas.95.25.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W, Zhou G, Hu M C T, Yao Z, Tan T H. Activation of the hematopoietic progenitor kinase-1-dependent, stress-activated c-jun N-terminal kinase (JUN K) pathway by transforming growth factor beta-activated kinase, a kinase mediator of TGF beta signal transduction. J Biol Chem. 1997;272:22771–22775. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 62.Yao Q Y, Rickinson A B, Gaston J S H, Epstein M A. In vitro analysis of the Epstein-Barr virus-host balance in long term renal allograft recipients. Int J Cancer. 1985;35:43–49. doi: 10.1002/ijc.2910350108. [DOI] [PubMed] [Google Scholar]
- 63.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 64.zur Hausen H, O'Neill F J, Freese U K, Hecher E. Persisting oncogenic herpesvirus induced by the tumor promoter TPA. Nature. 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]