Abstract
Adjuvant bisphosphonates are often recommended in postmenopausal women with early breast cancer at intermediate-to-high risk of disease recurrence, but the magnitude and duration of their effects on bone mineral density (BMD) and bone turnover markers (BTMs) are not well described. We evaluated the impact of adjuvant zoledronate on areal BMD and BTMs in a sub-group of patients who had completed the large 5-yr randomized Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial. About 224 women (recurrence free) who had completed the AZURE trial within the previous 3 mo were recruited from 20 UK AZURE trial sites. One hundred twenty had previously been randomized to zoledronate (19 doses of 4 mg over 5 yr) and 104 to the control arm. BMD and BTMs were assessed at sub-study entry, 6 (BTMs only), 12, 24, and 60 mo following the completion of AZURE. As expected, mean BMD, T-scores, and Z-scores at sub-study entry were higher in the zoledronate vs the control arm. At the lumbar spine, the mean (SD) standardized BMD (sBMD) was 1123 (201) and 985 (182) mg/cm2 in the zoledronate and control arms, respectively (P < .0001). The baseline differences in sBMD persisted at all assessed skeletal sites and throughout the 5-yr follow-up period. In patients completing zoledronate treatment, BTMs were significantly lower than those in the control arm (α- and β-urinary C-telopeptide of type-I collagen, both P < .00001; serum intact pro-collagen I N-propeptide, P < .00001 and serum tartrate-resistant acid phosphatase 5b, P = .0001). Some offset of bone turnover inhibition occurred in the 12 mo following the completion of zoledronate treatment. Thereafter, during the 60 mo of follow-up, all BTMs remained suppressed in the zoledronate arm relative to the control arm. In conclusion, in addition to the known anti-cancer benefits of adjuvant zoledronate, there are likely to be positive, lasting benefits in BMD and bone turnover.
Keywords: breast cancer, adjuvant bisphosphonates, AZURE trial, bone mineral density, bone turnover markers
Although great progress has been made in breast cancer treatment, a proportion of patients still experience spread of their cancer to other organs, known as metastasis. In around 70% of these patients, the spread goes to the bones. Because this ‘bone metastasis’ is very difficult to cure, research has focused on how to prevent it happening. In the last 10 years or so, it has been shown that drugs called bisphosphonates (which are normally used to prevent loss in bone density and consequent osteoporosis), can also reduce the occurrence of metastasis, but only in patients who are past the menopause. This ‘adjuvant’ treatment, which improves patient’s survival, is usually given for 3 – 5 years, but further research was needed to study possible long term side effects and other possible benefits of the treatment. We therefore carried out a clinical trial in 224 women who we studied for a further 5 years. The first group of 120 women had already received zoledronate (a bisphosphonate drug) for 5 years and a second group of 104 women had been followed for 5 years, but had not received the drug. Reassuringly, we found that there were no new, unexpected detrimental side effects of having received zoledronate for 5 years. By comparing these two groups, we were able to study the effects of this drug on the patient’s bones, using a special X-ray approach which measures the bone density, as we know that bone density normally decreases after the menopause. Our results confirmed that the 5-years of zoledronate treatment not only improved survival, but also improved bone density and that this benefit lasted for at least another 5 years. This was also confirmed by special blood tests. Our main conclusion is that adjuvant zoledronate treatment has additional long-term beneficial effects on bone density.
Introduction
More than 90% of women who develop breast cancer present with disease that appears to be localized to the breast (stages I–III). These patients have an increasingly favorable prognosis due to early diagnosis through mammographic screening and increased patient awareness, coupled with appropriate loco-regional treatments and improvements in adjuvant systemic therapies. As a result, there are increasing numbers of long-term survivors following a diagnosis of breast cancer, with many of these individuals at an increased risk for osteoporosis and fragility fractures due to the adverse effects of cancer treatments, such as aromatase inhibitors and ovarian suppression therapy, on bone physiology.
Bisphosphonates are anti-resorptive drugs that alter osteoclast function, inhibit bone resorption, reduce fracture risk, advocating them as first-line treatments for osteoporosis.1 In breast cancer, bisphosphonates are frequently included in the adjuvant treatment program for postmenopausal women to reduce the risk of disease recurrence and to protect against cancer treatment-induced bone loss.2,3 The Early Breast Cancer Trialists’ Collaborative Group performed a meta-analysis on the individual data from nearly 19 000 patients participating in randomized trials of bisphosphonate use in the adjuvant setting of early breast cancer.4 This showed that in postmenopausal women (either due to a natural menopause or as a result of ovarian suppression therapy), adjuvant bisphosphonates stopped one in four patients from developing bone metastases within 10 yr of diagnosis and prevented one in six breast cancer deaths. Although bisphosphonates do not have regulatory approval for use as disease-modifying agents in the adjuvant setting, international guidelines in both Europe2 and North America5 recommend their use for this purpose in postmenopausal women at intermediate to high risk of recurrence, while in women at low risk for recurrence, bisphosphonates are also the first-line option for fracture prevention in women at an increased risk for fracture.2,3
The Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial was a prospective, randomized controlled phase III, open label multi-national and multi-centre clinical trial that examined whether adjuvant zoledronate could improve disease outcomes in patients with stage II/III breast cancer.6 The findings from the AZURE trial showed that 5 yr of adjuvant zoledronate therapy improved disease-free survival and reduced breast cancer mortality in postmenopausal women.6,7 In addition, skeletal morbidity was reduced with fewer fractures in the zoledronate arm.8
After completion of 5 yr of study treatment in the AZURE trial, patients remained on follow-up until 10 yr after breast cancer diagnosis. This post-treatment follow-up study (Bone Health in breast cancer survivors Following Adjuvant Bisphosphonate therapy [BoHFAB]) provides a unique opportunity to evaluate the long-term effects of adjuvant bisphosphonates on bone metabolism and BMD, improve our understanding of the offset of effects on bone with zoledronate, and evaluate the long-term consequences of adjuvant treatments in breast cancer survivors. Here we report on a subset of patients from the AZURE trial who participated in a detailed bone health sub-study during years 6–10 of the trial.
Materials and methods
Study design
The BoHFAB study recruited 224 women at 20 study sites in the UK who had completed the 5-yr treatment phase of the AZURE trial. Patients in the AZURE trial had been randomized within an open-label multicenter, international phase III trial to either receive zoledronate in addition to standard loco-regional and adjuvant systemic treatments as per institutional practice (zoledronate arm) or to no adjuvant bisphosphonates (control arm). Patients with chronic kidney disease were excluded from the study. Zoledronate had been administered at a dose of 4 mg by intravenous infusion every 3–4 wk for 6 doses then every 3 mo for 8 doses and then every 6 mo for 5 doses to complete a 5-yr treatment program and a maximum of 19 treatments (cumulative dose of zoledronate = 76 mg).6 The trial was registered (16 November 2004) with ClinicalTrials.gov (International Standard Randomised Controlled Trial Number: ISRCTN79831382) and European Union Drug Regulating Authorities Clinical Trials (EudraCT Number: 2004-000608-42). The BoHFAB study was not planned at the same time as the main AZURE study; however, it was designed during the first 2 yr following the AZURE study commencement. In this sense, this was a pre-planned analysis as no patient had completed the AZURE study when it was planned.
The aims of the BoHFAB study were to determine the magnitude and duration of effects of the intensive 5 yr schedule of zoledronate utilized in the AZURE study on BMD and bone turnover markers (BTMs) compared with those in the control group.
Study population
Eligibility was restricted to women with stage II–III breast cancer who had participated in the AZURE trial and had completed the initial 5-yr “treatment phase” of the study within the previous 3 mo. Patients were not eligible to participate if (1) they had metastatic or recurrent breast cancer, (2) had taken bisphosphonates other than zoledronate as randomly allocated within the AZURE study, (3) had severe physical or psychological concomitant diseases that might have impaired compliance with the study protocol, (4) had pre-existing pathology or prior surgery that made it impossible to obtain reliable DXA scans of the spine and hip, or (5) were pregnant or breast feeding. To simplify trial logistics and quality assurance of BMD assessments, recruitment was limited to 20 high-recruiting AZURE centers within the UK.
The BoHFAB study was approved by West Midlands Research Ethics Committee and all participants gave informed written consent prior to their participation. All investigations were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines.
Study assessments
The BoHFAB study entry assessments included (1) medical history taking, (2) recording of concomitant medication, history of symptomatic fracture occurring on the study and disease recurrence, (3) completion of a skeletal health and a FRAX questionnaire, (4) a physical examination, (5) blood and urine sampling for BTMs measurement, and (6) measurement of areal lumbar spine BMD and proximal femur BMD.
Follow-up assessments consisted of (1) recording of concomitant medication, history of symptomatic fracture occurring on the study and disease recurrence (at 6, 12, 24, 36, 48, and 60 mo), (2) a physical examination (at 12, 24, 36, 48, and 60 mo), (3) blood and urine sampling for BTM measurement (at 6, 12, 24, 36, 48, and 60 mo), and (4) measurement of BMD (at 12, 24, and 60 mo).
Height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were measured at each DXA scanning visit and used to calculate BMI (to the nearest kg/m2) using the Quetelet equation. BMD (in g/cm2) of the lumbar spine (L1–L4) and proximal femur (total hip and femoral neck) was measured by DXA using Hologic (Hologic Inc, Marlborough, MA) and GE (GE Healthcare, Chicago, IL) devices. Scan acquisition was performed locally at the participating study sites following centrally devised standard operating procedures. A comprehensive quality assurance program, providing stringent monitoring of all study DXA devices, was implemented prior to any scan acquisition and ran for the entire duration of the study. Quality control data and charts, displaying the area, areal bone mineral content, and BMD of the manufacturer’s test objects, were reviewed centrally on a monthly and 6-monthly basis, respectively, to monitor the stability of all DXA devices. Cross-calibration consisted of local scanning of a single study-specific “travelling” European Spine Phantom. Universal scanner calibration was achieved by applying the standardization formulae and approaches described by Genant et al.9 and Hanson.10 These approaches allowed the standardization of femoral neck, total hip, and lumbar spine aBMD values. These values were expressed as standardized BMD (sBMD) in mg/cm2. All densitometry was centrally coordinated by the Mellanby Centre for Musculoskeletal Research (MCMR), at the University of Sheffield, Sheffield, UK. T and Z scores were done on both pre- and postmenopausal women. For Z-score and T-score of the lumbar spine, we used the results provided by the DXA manufacturer for white women. For T-score of the hip, we used the National Health and Nutrition Examination Survey (NHANES) data11 to calculate the baseline femoral neck and total hip, using the non-Hispanic white female reference data.
Fasting morning blood samples and second morning void urine samples were taken on the day of the study visit and stored locally (at −80°C) prior to their transfer to central storage (at −80°C) at the MCMR in Sheffield. Markers of bone resorption and formation were measured centrally in Sheffield within the MCMR laboratories. To assess bone resorption, urinary C-telopeptide of type I collagen (α- and β-CTX) was measured using manual ELISAs (Immunodiagnostic Systems (IDS), inter-assay CV = 6.5%). Intact serum pro-collagen I N-propeptide (P1NP), a marker of bone formation, was measured by automated immunoassay (Cobas e411, Roche Diagnostics, inter-assay CV = 3.4%). Serum tartrate-resistant acid phosphatase 5b (TRAP5b), a marker of osteoclast activity, was measured by manual ELISA (IDS, inter-assay CV = 4.2%).
Study endpoints
The primary endpoint of the BoHFAB study was to determine the difference in mean percentage change in lumbar spine sBMD at 24 mo after completing the treatment phase of AZURE between those patients who were randomized to zoledronate and those in the control arm.
Secondary endpoints were (1) to describe the differences in baseline values for mean sBMD (lumbar spine and total hip), serum PINP, TRAP5b, and urinary α- and β-CTX between the zoledronate treated and control groups; (2) the difference in mean percentage change in total hip sBMD at 24 mo between the zoledronate and control arms; (3) the mean percentage changes in lumbar spine sBMD and total hip sBMD at 12 and 60 mo; and (4) the mean percentage change in BTMs at, 6, 12, 24, 36, 48, and 60 mo between the zoledronate and control arms.
Sample size calculation and statistical analyses
We hypothesized that the use of zoledronate for 5 yr during the AZURE trial would result in positive effects on sBMD and reductions in BTMs and that these effects would persist for at least 2 yr after completing zoledronate. We used multivariate linear regression methods to compare the baseline sBMD and BTM measurements between the zoledronate and control groups to enable adjustment for prognostic factors including menopausal status, age, chemotherapy treatment, and aromatase inhibitor treatment. We evaluated any overall differences in sBMD and BTMs between the two arms. Exploratory analyses were also used to investigate whether BTM measurements were predictive of bone loss by including each of them as a factor in the statistical model and by simple summary measurements investigating associations.
At the 2-yr time-point, an expected difference in mean percentage change in lumbar spine sBMD between the zoledronate and control groups of 2.5% and a SD of 8.974 was assumed. To ensure a 95% CI of this difference from 1.2% to 3.8%, ie that the lower limit of the 95% CI was above the limit of detection for change in lumbar spine sBMD, 184 patients were required. It was estimated that 25% of the patients would withdraw or drop out of the study within 2 yr. We aimed to recruit a total of 244 patients.
Patients could be withdrawn from the BoHFAB study for bone-related reasons, including the development of bone metastases or recurrence of breast cancer at other sites, rapid bone loss, requiring therapeutic intervention and the use of bisphosphonate treatments or denosumab. If these individuals had been excluded from the analyses, it is possible that the mean sBMD may not have accurately reflected that of the total cohort. Therefore, for both primary and secondary endpoints, we adjusted for this by using the linear increment method, which assumed that data for patients who withdrew continued in a linear trend defined by the average change in the whole group between that time point and the next time point. The extrapolated values were used to calculate mean values, but not the SEs or the CIs.
Mean percentage changes in sBMD and BTMs over time in each of the zoledronate and control groups were plotted. Differences between the arms at different time points, including the primary time point of 2 yr were examined using t-tests. Similar plots were used to look at changes over time by the prognostic factors menopausal status, age, chemotherapy treatment, and aromatase inhibitor treatment. Only the primary and secondary endpoints were subjected to statistical significance testing. A 5% (2-sided) significance level was used. Where appropriate, the data were summarized descriptively.
Data were analysed using purpose-written Digital Visual Fortran (version 6.0A, Digital Equipment Corp) statistical software designed specifically to implement the linear increment method.
Results
Study population
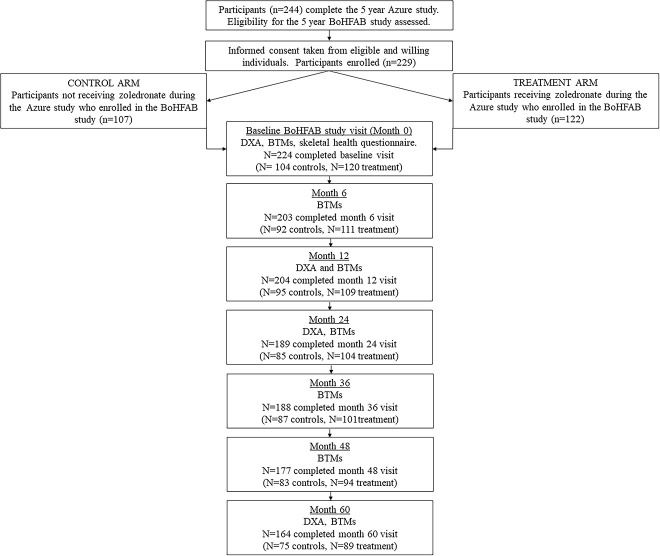
About 224 women were recruited from 20 AZURE study sites across the UK and completed the baseline visit of BoHFaB (n = 120 from the zoledronate arm and n = 104 from the control arm). All participants had completed 5 yr of the AZURE study within the previous 3 mo and were recurrence free. No patient received adjuvant chemotherapy and concomitant glutocorticoids on the BoHFAB study and there were no occurrences of osteonecrosis of the jaw. The CONSORT diagram for the study is shown in Figure 1.
Figure 1.
CONSORT diagram for the BoHFAB study. BoHFAB = bone health in breast cancer survivors following adjuvant bisphosphonate therapy.
Baseline characteristics are shown in Table 1 and were similar in the zoledronate and control arms. The mean age was 57.9 yr in the zoledronate arm and 55.9 yr in the control arm with a slightly higher proportion of patients in the zoledronate arm who were > 5 yr post-menopausal than in the control arm (n = 58 [25.8%] vs n = 41 [18.3%]). Only 37/224 (16.5%) of BoHFAB patients were continuing endocrine treatment after year 5 of the AZURE study (25 in the zoledronate arm and 12 in the control arm), which precluded meaningful sub-group analysis. It is recognized that when the trial was performed, extended endocrine treatment was not implemented in all centers.
Table 1.
Baseline characteristics of participants at entry to the BoHFAB study from the AZURE study.
| Characteristic | Total (n = 224) | Zoledronate (n = 120) | Control (n = 104) |
|---|---|---|---|
|
Demographics
Age (yr, mean ± SD) Height (cm, mean ± SD) Weight (kg, mean ± SD) BMI (kg/m2, mean ± SD) |
57.0 ± 9.1 162.0 ± 6.4 73.3 ± 14.1 27.8 ± 4.8 |
57.9 ± 8.7 162.2 ± 6.3 74.4 ± 13.2 28.2 ± 4.8 |
55.9 ± 9.5 162.0 ± 6.6 72.0 ± 14.6 27.3 ± 4.7 |
|
Baseline BMD T-score classification (n, %)
Normal Osteopenic Osteoporotic |
136 (60.7) 74 (33.0) 14 (6.3) |
92 (76.7) 24 (20.0) 4 (3.3) |
44 (42.3) 50 (48.1) 10 (9.6) |
|
Menopausal status at baseline (n, %)
Premenopausal Perimenopausal Postmenopausal ≤5 yr >5 yr Unknown Status unknown |
28 (12.5) 1 (0.4) 192 (85.7) 91 (40.6) 100 (44.6) 2 (0.9) 3 (1.3) |
11 (9.2) 1 (0.8) 105 (87.5) 46 (38.3) 58 (48.3) 1 (0.8) 3 (2.5) |
17 (16.3) 0 (0) 87 (83.7) 45 (43.2) 41 (39.4) 1 (1.0) 0 (0) |
|
Systemic anticancer therapy prior to study (n, %)
Chemotherapy alone Endocrine therapy alone Chemotherapy + endocrine therapy Radiotherapy Trastuzumab |
49 (21.9) 8 (3.6) 172 (76.8) 202 (90.2) 45 (20.1) |
27 (12.1) 3 (1.3) 92 (41.1) 107 (47.8) 20 (8.9) |
22 (9.8) 5 (2.2) 80 (35.7) 95 (42.4) 25 (11.2) |
|
Off study reason (n, %)
Completed study Bone metastases Recurrence or new primary tumor Death Rapid bone loss Use of prohibited concomitant treatment Use of open-label bisphosphonates Investigator recommendation Patient choice Consented but no longer eligible |
164 (73.2) 10 (4.5) 12 (5.4) 2 (0.9) 2 (0.9) 1 (0.4) 16 (7.1) 2 (0.9) 15 (6.7) 2 (0.9) |
75 (62.5) 5 (4.2) 6 (5.0) 2 (1.7) 0 (0) 1 (0.8) 6 (5.0) 2 (1.7) 8 (6.7) 1 (0.8) |
89 (85.6) 5 (4.8) 6 (5.8) 0 (0) 2 (1.9) 0 (0) 10 (9.6) 0 (0) 7 (6.7) 1 (1.0) |
| Years on BoHFAB study (mean ± SD) | 4.5 ± 1.5 | 4.5 ± 1.4 | 4.4 ± 1.6 |
Only 28 of the 224 patients recruited into the BoHFAB study were deemed premenopausal at study entry (having experienced at least one menstrual period in the preceding year). This is consistent with only 23/224 patients being aged less than 40 when they began the AZURE trial itself. Also, during the 5 yr of the BoHFAB study, many of these patients would have become postmenopausal. Because of the small number likely to remain pre-menopausal, and in order to use all the data, we included all patients in the analysis.
Use of open label bisphosphonates during the 5-yr duration of the BoHFAB study (leading to withdrawal from the study) was more frequent in the control arm (n = 10 [9.6%] than the zoledronate arm (n = 6 [5.0%]).
For the main AZURE study itself, calcium supplementation (calcium carbonate, 1250/1500 mg daily) and Vitamin D supplementation (400 IU/daily), were mandated and supplied to patients, but only during the intensive first 6 mo of the AZURE treatment regimen. Subsequently, patients were given advice regarding optionally continuing on these supplements for the rest of the AZURE study.6 During the BoHFAB study itself, patients were returned to the “real world” setting and subject to routine local follow-up. Neither serum vitamin D nor PTH were measured as part of the study. However, the BoHFAB information sheet gave advice regarding dietary requirements for calcium and vitamin-D and where this was not met, supplements were advised. From the BoHFAB medication history, we know that 26 patients took calcium carbonate (1250/1500 mg daily) and colecalciferol (400 IU/daily) supplements during the BoHFAB study (mean ± SD duration, 13.7 ± 14.3 months (range, 0.7–49.3 mo).
Detailed fracture data for the AZURE study itself has been reported elsewhere8 but, in summary, over 84.2 mo median follow-up, 244 out of 3359 patients experienced ≥1 fracture whilst enrolled on the AZURE study, with 104 patients (6.2%) reporting 120 fractures in the zoledronate arm and 140 patients (8.3%) reporting 171 fractures in the control arm. During the BoHFAB study, a total of 14 symptomatic fractures were reported by the 224 patients (5 in the zoledronate arm; 9 in the control arm. Sites of fractures were (zoledronate, control respectively) wrist2,3; lower leg/ankle1,3; ribs1,2; foot (0, 1); arm (0, 1). This small number of fractures were distributed evenly across the 5-yr BoHFAB observation period. No atypical femur fractures were reported.
Bone mineral density
As expected, the mean sBMD, T scores, and Z scores at entry into the study were higher in zoledronate-treated patients than those in the control arm (Figure 2 and Table 2). At the lumbar spine, the mean (SD) sBMD was 1123 (201) mg/cm2 in the zoledronate arm compared with 985 (182) mg/cm2 in the control arm (P < .0001). Significant differences in baseline sBMD at the total hip (10% absolute difference) and femoral neck (8% absolute difference) were also observed between the two arms. At baseline, a slightly higher proportion of patients in the control arm (n = 50 [22.3%]) were classified as osteopenic or osteoporotic (n = 10 [4.5%]) than in the zoledronate arm (n = 24 [10.7%] and (n = 4 [1.8%] respectively), using the World Health Organization criteria12 and data from the NHANES III study.11
Figure 2.
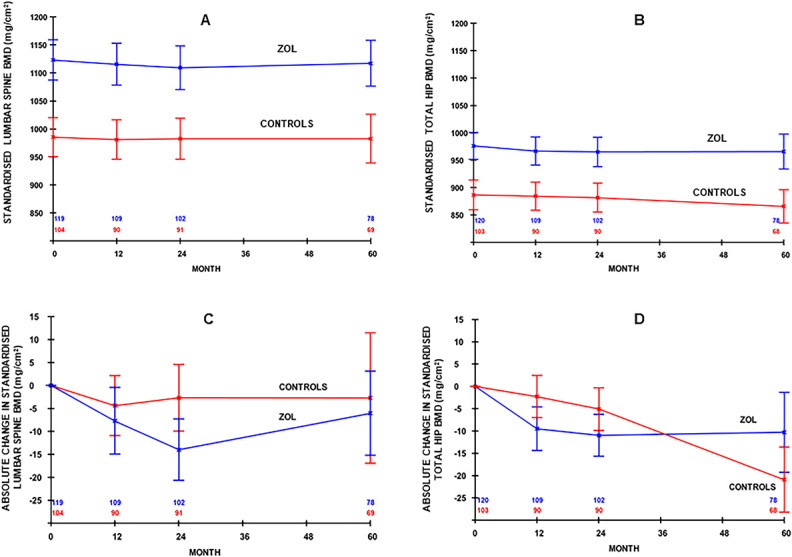
Standardized bone mineral density (sBMD) (mean, 95% CI) at the (A) lumbar spine and (B) total hip and absolute change (mean, 95% CI) in sBMD at the (C) lumbar spine and (D) total hip from baseline to 60 mo. (A change of −15 mg/cm2 is a reduction of approximately 1.5%. Data are adjusted for dropouts using the linear increment method and are shown by treatment group).
Table 2.
Bone mineral density (BMD) T-score and BMD Z-score at the femoral neck (FN), total hip (TH), and lumbar spine (LS) for the whole study population, those who received zoledronate and for those receiving no adjuvant therapy (controls) during the Azure study. Data are shown as mean ± SD at baseline, months 12, 24, and 60.
| Characteristic | Zoledronate | Control |
|---|---|---|
| Baseline (month 0) (n = 224) | (n = 120) | (n = 104) |
| BMD T-score FN | −0.11 ± 1.09 | −0.65 ± 1.01 |
| BMD T-score TH | 0.29 ± 1.02 | −0.35 ± 1.08 |
| BMD T-score LS | 0.39 ± 1.58 | −0.71 ± 1.43 |
| BMD Z-score FN | 0.94 ± 1.01 | 0.27 ± 1.02 |
| BMD Z-score TH | 1.08 ± 0.99 | 0.34 ± 1.12 |
| BMD Z-score LS | 1.51 ± 1.65 | 0.45 ± 1.64 |
| Month 12 (n = 200) | (n = 109) | (n = 91) |
| BMD T-score FN | −0.13 ± 1.08 | −0.64 ± 0.98 |
| BMD T-score TH | 0.24 ± 1.04 | −0.33 ± 0.94 |
| BMD T-score LS | 0.34 ± 1.58 | −0.66 ± 1.36 |
| BMD Z-score FN | 0.94 ± 1.06 | 0.37 ± 1.00 |
| BMD Z-score TH | 1.06 ± 1.08 | 0.43 ± 1.02 |
| BMD Z-score LS | 1.53 ± 1.75 | 0.42 ± 1.67 |
| Month 24 (n = 193) | (n = 102) | (n = 91) |
| BMD T-score FN | −0.14 ± 1.03 | −0.72 ± 0.98 |
| BMD T-score TH | 0.25 ± 1.04 | −0.34 ± 0.96 |
| BMD T-score LS | 0.33 ± 1.59 | −0.63 ± 1.41 |
| BMD Z-score FN | 0.98 ± 1.00 | 0.39 ± 1.04 |
| BMD Z-score TH | 1.13 ± 1.04 | 0.51 ± 1.09 |
| BMD Z-score LS | 1.61 ± 1.75 | 0.53 ± 1.72 |
| Month 60 (n = 113) | (n = 57) | (n = 56) |
| BMD T-score FN | −0.08 ± 0.98 | −0.59 ± 1.02 |
| BMD T-score TH | 0.32 ± 1.03 | −0.31 ± 1.01 |
| BMD T-score LS | 0.34 ± 1.50 | −0.31 ± 1.55 |
| BMD Z-score FN | 1.19 ± 1.02 | 0.54 ± 1.06 |
| BMD Z-score TH | 1.38 ± 1.10 | 0.65 ± 1.06 |
| BMD Z-score LS | 1.86 ± 1.59 | 0.92 ± 1.76 |
The baseline differences in sBMD persisted at all assessed skeletal sites and throughout the 5-yr follow-up period. There was very little change in mean sBMD in the zoledronate arm throughout the 5 yr follow-up period (Figure 2). As a result, the mean T- and Z-scores for the patients in the zoledronate arm remained significantly higher than those in the control arm throughout the 60 mo on study (Table 2).
Bone turnover markers
In patients completing treatment with zoledronate, BTMs were suppressed at study entry and were significantly lower than the corresponding values in the control arm ((α-CTX (t-test statistic = 9.9, P < .00001), β-CTX (t-test statistic = 13.5, P < .00001), PINP (t-test statistic = 11.7, P < .00001), and TRACP5b (t-test statistic = 4.73, P = .0001)).
Figure 3 shows that there was some offset of the inhibition of bone turnover during the first 12 mo after completing treatment with zoledronate. Throughout the 60 mo of follow-up, all BTMs remained suppressed at a relatively constant level in the zoledronate arm (Table 3). This was always significantly lower than the mean levels recorded in the control arm (Figure 3).
Figure 3.
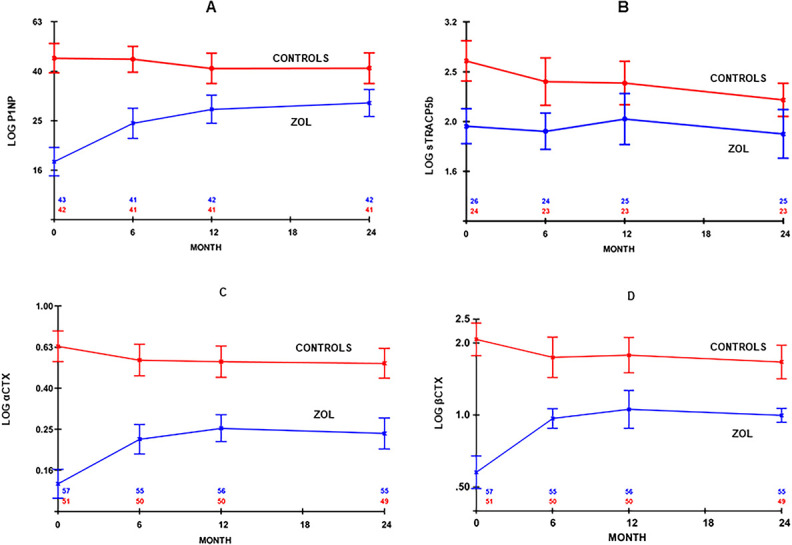
Change (mean, 95% CI) in (A) log serum P1NP, (B) log serum TRACP5b, (C) log urinary alpha CTX corrected for creatinine clearance (αCTX/Cr), and (D) log urinary beta CTX corrected for creatinine clearance (βCTX/Cr) from baseline to 24 mo of the BoHFAB study. (Data are adjusted for dropouts using the linear increment method and are shown by treatment group). P1NP = pro-collagen I N-propeptide.
Table 3.
Long-term impact of treatment with zoledronate on bone turnover markers.
| Characteristic | Zoledronate | Control |
|---|---|---|
| Baseline (month 0) | ||
| α-CTX/Cr (μg/mmol) | 0.16 (0.08–0.26) | 1.05 (0.62–1.41) |
| β-CTX/Cr (μg/mmol) | 0.36 (0.18–0.64) | 1.93 (1.19–2.72) |
| P1NP (ng/mL) | 20.4 (16.1–26.8) | 51.3 (36.5–64.5) |
| TRACP5b (U/L) | 2.1 (1.8–2.5) | 3.2 (2.4–3.4) |
| Month 36 | ||
| α-CTX/Cr (μg/mmol) | 0.30 (0.17–0.59) | 1.00 (0.61–1.38) |
| β-CTX/Cr (μg/mmol) | 0.70 (0.50–1.08) | 1.52 (0.87–2.60) |
| P1NP (ng/mL) | 27.6 (22.8–32.2) | 40.0 (33.7–59.4) |
| TRACP5b (U/L) | 2.2 (1.8–2.4) | 2.4 (1.9–2.7) |
| Month 60 | ||
| α-CTX/Cr (μg/mmol) | 0.41 (0.24–0.66) | 0.96 (0.53–1.35) |
| β-CTX/Cr (μg/mmol) | 0.97 (0.53–1.27) | 1.76 (0.85–2.25) |
| P1NP (ng/mL) | 25.3 (19.7–30.9) | 35.8 (29.9–46.4) |
| TRACP5b (U/L) | 2.0 (1.7–2.2) | 2.6 (1.9–3.0) |
Urinary α-CTX corrected for creatinine (α-CTX/Cr), urinary β-CTX corrected for creatinine (β-CTX/Cr), serum P1NP, and serum TRACP5b for those who received zoledronate and for those receiving no adjuvant therapy (controls) during the AZURE study. Data are shown as median and interquartile range (IQR) at baseline, 36, and 60 mo. Geometric mean (reference interval) data are: α-CTX/Cr, 0.32 (0.1–0.99) μg/mmol; β-CTX/Cr, 1.66 (0.83–3.32) μg/mmol; P1NP (31.4 (16.2–60.9) ng/mL; TRACP5b, 2.59 (1.03–4.15) U/L as provided by IDS, the kit manufacturers.11
For information, Table 3 also shows the geometric mean and reference intervals for BTMs in healthy, young premenopausal women, usually taken as the “normal” for comparison purposes.13
Discussion
This bone health sub-study of the large, randomized AZURE trial, designed to evaluate the effects of adjuvant zoledronate on disease outcomes in stage II/III breast cancer, provides an opportunity to evaluate the impact of adjuvant zoledronate on sBMD and BTMs and the duration of effect following completion of treatment. As expected, after 5 yr of treatment with zoledronate, patients had higher sBMD at all measured skeletal sites compared with those patients randomized to the control arm and markedly suppressed rates of bone turnover. The difference in sBMD between the randomized treatment groups was maintained throughout the 5 yr of follow-up. BTMs increased somewhat in the 12 mo following the end of study treatment with zoledronate but remained suppressed in comparison to the control arm with no evidence of any further offset of treatment effect. Our findings reflect the prolonged half-life of zoledronate in bone and the known long-term impact of treatment on bone cell function that is observed for years after just a single dose of 4–5 mg.14-16
The pattern of change in sBMD and BTMs in response to cessation of bisphosphonates is similar to that reported in prior studies in postmenopausal osteoporosis. For example, after stopping zoledronate in the HORIZON study, there was only a small decrease in hip BMD17 and, after stopping oral bisphosphonates in the TRIO study, there was some resolution of bone turnover suppression over the first 6 mo, but persistent partial suppression for a further 18 mo of the study.18
There is an overlap of the 95% CI for TRACP5b between the two groups up to 24 mo, but this is not observed for urinary α and β CTX. This would indicate that the effects of zoledronic acid on TRACP5b are smaller than those on urinary CTX. This has also been found in other studies19 and is presumably due to the biological differences between the markers (TRACPb is an enzyme that is expressed in high amounts by bone-resorbing osteoclasts and is used as a marker of osteoclast number and, by inference, bone resorption, whereas CTX is derived directly from collagen breakdown due to bone resorption).
What is the likely effect of a maintained higher sBMD in the zoledronate-treated population and suppressed bone turnover during the follow-up period in our study? In the Horizon trial,20 Jacques et al. reported that change in total hip sBMD explained 40% of the reduction in vertebral fracture risk and 61% of the reduction in non-vertebral fracture risk over 3 yr. They also reported that change in P1NP explained 58% of the reduction in vertebral fracture risk (but not non-vertebral fracture risk). Thus, the higher sBMD and lower BTMs that result from prior zoledronate therapy are likely to be associated with a lower risk of fractures, and so are clinically important.
Although adjuvant bisphosphonates are included in the systemic treatment recommendations of patients with early breast cancer and recommended by international guidelines, 2,5 the intensive regimen tested in the AZURE trial (76 mg cumulative dose of zoledronate) is rarely, if ever, recommended, and less-intensive regimens are in use. This is based on the evidence of the long residence time of zoledronate in bone and more recent adjuvant trials that have demonstrated the efficacy of less intensive zoledronate scheduling. Current guidance for adjuvant zoledronate suggests that patients receive 6 monthly zoledronate for 3–5 yr (24–48 mg cumulative dose of zoledronate) or a loading schedule of 3 doses of 4 mg during adjuvant chemotherapy followed by 6 monthly zoledronate to complete 3–5 yr of treatment (32–56 mg cumulative dose of zoledronate).2,5 Alternatively, patients may be treated with oral clodronate 1600 mg daily or oral ibandronate 50 mg daily for 3–5 yr. Although we would reasonably expect BMD and bone turnover outcomes from these lower intensity regimens to be in the same direction as those we found in the BoHFAB study, our findings may not be directly or quantitatively applicable to these less intensive regimens.
It should be noted that our data are only applicable to adjuvant zoledronate use in early breast cancer and not denosumab. Although denosumab can be used to prevent bone loss due to endocrine treatments, it is important to be aware of the multiple vertebral fractures which can occur following its cessation. A single dose of zoledronate may be given to prevent this rebound effect.21
It is also recognized that, although only a small proportion of patients continued to receive endocrine therapy after entry into the BoHFAB study, the data refer to a period when extended adjuvant treatment with an aromatase inhibitor beyond 5 yr was only just beginning to be implemented for patients with high-risk estrogen receptor-positive disease. Nevertheless, our data suggest that patients completing a course of adjuvant zoledronate can expect sustained suppression of bone turnover and are unlikely to experience significant age-related or treatment-induced bone loss over the subsequent 5 yr. BMD monitoring in such patients and further use of bone targeted therapy to prevent further aromatase inhibitor-induced bone loss may not be necessary.
Acknowledgments
The authors wish to thank all the patients who participated in the BoHFAB study.
Contributor Information
Janet Brown, Division of Clinical Medicine, University of Sheffield, Sheffield, S10 2SJ, United Kingdom; Sheffield Teaching Hospitals NHS Foundation Trust, Glossop Rd, Sheffield, S10 2JF, United Kingdom.
Margaret A Paggiosi, Division of Clinical Medicine, University of Sheffield, Sheffield, S10 2SJ, United Kingdom.
Emma Rathbone, Huddersfield Royal Infirmary, Calderdale and Huddersfield NHS Foundation Trust, Huddersfield, HD3 3EA, United Kingdom.
Walter Gregory, Leeds Cancer Research UK Clinical Trials Unit, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds, LS2 9JT, United Kingdom.
Gian Bertelli, Sussex Cancer Centre, University Hospitals Sussex NHS Trust, Bristol Gate, Brighton, BN2 5BD, United Kingdom.
Omar Din, Sheffield Teaching Hospitals NHS Foundation Trust, Glossop Rd, Sheffield, S10 2JF, United Kingdom.
Eugene McCloskey, Division of Clinical Medicine, University of Sheffield, Sheffield, S10 2SJ, United Kingdom; Sheffield Teaching Hospitals NHS Foundation Trust, Glossop Rd, Sheffield, S10 2JF, United Kingdom.
David Dodwell, Leeds General Infirmary, LeedsTeaching Hospitals NHS Trust, Leeds, LS1 3EX, United Kingdom.
David Cameron, Cancer Research UK Edinburgh Centre, Institute of Genetics and Cancer, Crewe Road South, University of Edinburgh, Edinburgh, Edinburgh EH4 2XR, United Kingdom.
Richard Eastell, Division of Clinical Medicine, University of Sheffield, Sheffield, S10 2SJ, United Kingdom; Sheffield Teaching Hospitals NHS Foundation Trust, Glossop Rd, Sheffield, S10 2JF, United Kingdom.
Robert Coleman, Division of Clinical Medicine, University of Sheffield, Sheffield, S10 2SJ, United Kingdom; Sheffield Teaching Hospitals NHS Foundation Trust, Glossop Rd, Sheffield, S10 2JF, United Kingdom.
Author contributions
Janet Brown (trial design, conceptualization, acquisition of funding, administration of the study, methodology, clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Margaret Paggiosi (trial design, methodology, clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Emma Rathbone (clinical investigation, data collection, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Walter Gregory (methodology, sample size calculation and statistical analysis, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Gian Bertelli (clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Omar Din (clinical investigation, data collection, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Eugene McCloskey (methodology, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), David Dodwell (conceptualization, acquisition of funding, clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), David Cameron (conceptualization, acquisition of funding, clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Richard Eastell (data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work), Robert Coleman (trial design, conceptualization, acquisition of funding, administration of the study, methodology, clinical investigation, data collection, data interpretation, drafting of manuscript and editing for important intellectual content, approval of final manuscript, agreement to act as guarantor of the work).
Funding
Funding support was provided by Novartis Pharmaceuticals, the National Cancer Research Network (NCRN), and the National Institute for Health and Care Research (NIHR). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest
JB declares participation in consultancy/advisory boards/speakers bureaux for Novartis, Ipsen, Amgen, MSD, Bristol, Myers Squibb and Bayer and receipt of research funding paid to institution from NIHR and travel expenses from Ipsen. RE receives consultancy funding from 5 IDS, Sandoz, Samsung, Haoma Medica, CL Bio, Biocon, Takeda, meeting presentations for Pharmacosmos, Alexion and Amgen, and grant funding from Roche, Pharmacosmos and Alexion. RC has received consultancy fees from Sanofi and Astra Zeneca, speaker fees from Amgen and Beigene and has stock options with Inbiomotion. Other authors have no disclosures.
Data availability
Data supporting this work are available on reasonable request. All requests will be reviewed by relevant stakeholders, based on the principles of a controlled access approach. Requests to access data should be made to CTRU-DataAccess@leeds.ac.uk
References
- 1.Eastell R, O’Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2(Sep 29):1–16. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R, Hadji P, Body JJ, et al. On behalf of the ESMO Guidelines Committee. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1650–1663. [DOI] [PubMed] [Google Scholar]
- 3.Hadji P, Aapro MS, Body J-J, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;23(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman R, Powles T, Paterson A, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. [DOI] [PubMed] [Google Scholar]
- 5.Eisen A, Somerfield MR, Accordino MK, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: ASCO-OH (CCO) Guideline Update. J Clin Oncol. 2022;40(7):787–800. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE, Marshall H, Cameron D, et al. Breast cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Collinson M, Gregory W, et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol. 2018;13(Sept 27):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson C, Bell R, Hinsley S, et al. Adjuvant zoledronic acid reduces fractures in breast cancer patients; AZURE (BIG 01/04) study. Eur J Cancer. 2018;94(May):70–78. [DOI] [PubMed] [Google Scholar]
- 9.Genant HK, Gramp S, Gluer C-C, et al. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1994;9(10):1503–1514. [DOI] [PubMed] [Google Scholar]
- 10.Hanson J. Standardization of femur BMD. J Bone Miner Res. 1997;12(8):1316–1317. [DOI] [PubMed] [Google Scholar]
- 11.Looker AC, Johnston CC Jr, Wahner HW, et al. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10(5):796–802. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, McCloskey EV, Johansson H, Oden A, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. [DOI] [PubMed] [Google Scholar]
- 13.Immunodiagnostic Systems Holdings Ltd .
- 14.Brown JE, Ellis SP, Lester JE, et al. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res. 2007;13(18):5406–5410. [DOI] [PubMed] [Google Scholar]
- 15.Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR. Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Min Res. 2010;25(10):2251–2255. [DOI] [PubMed] [Google Scholar]
- 16.Grey A, Bolland MJ, Horne A, Mihov B, Gamble G, Reid IR. Bone mineral density and bone turnover 10 years after a single 5 mg dose or two 5-yearly lower doses of zoledronate in osteopenic older women: an open-label extension of a randomized controlled trial. J Bone Min Res. 2022;37(1):3–11. [DOI] [PubMed] [Google Scholar]
- 17.Kim TY, Bauer DC, McNabb BL, et al. Comparison of BMD changes and bone formation marker levels 3 years after bisphosphonate discontinuation: FLEX and HORIZON-PFT extension I trials. J Bone Miner Res. 2019;34(5):810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naylor KE, Bradburn M, Paggiosi MA, et al. Effects of discontinuing oral bisphosphonate treatments for postmenopausal osteoporosis on bone turnover markers and bone density. Osteoporos Int. 2018;29(6):1407–1417. [DOI] [PubMed] [Google Scholar]
- 19.Eastell R, Nagase S, Small M, et al. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN Study. J Bone Miner Res. 2014;29(2):458–466. [DOI] [PubMed] [Google Scholar]
- 20.Jacques RM, Boonen S, Cosman F, et al. Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(8):1627–1634. [DOI] [PubMed] [Google Scholar]
- 21.Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this work are available on reasonable request. All requests will be reviewed by relevant stakeholders, based on the principles of a controlled access approach. Requests to access data should be made to CTRU-DataAccess@leeds.ac.uk