ABSTRACT
Given that the skin is the largest tissue in the human body, performing external barrier functions with innate and adaptive immunity and undergoing substantial changes during aging, it is under investigation as a major target of various bioactive molecules. In the present study, we examined the biological activity of the senolytic piperlongumine by analyzing alterations in mRNA expression of notable skin genes using transformed aneuploid immortal epidermal keratinocytes, HaCaT cells. We observed that piperlongumine increased the mRNA expression of genes playing critical roles in skin barrier function. In addition, piperlongumine increased expression enzymes involved in the synthesis of ceramide, a major component of intercellular lipids. Furthermore, we measured the protein levels of various cytokines secreted by epidermal keratinocytes and found changes in the release of GRO-αβγ, CCL5, and MCP1. Additionally, we observed that piperlongumine treatment modulated the expression of keratinocyte-specific aging markers and influenced telomerase activity. Based on these findings, piperlongumine could regulate the physiological activity of epidermal keratinocytes to induce beneficial effects in human skin by regulating important skin-related genes.
KEYWORDS: Piperlongumine; keratinocyte, skin barrier gene, inflammation
Introduction
Aging causes diverse changes at the cellular level, resulting in physiological and psychological damage to organisms (López-Otín et al. 2013; Dziechciaż and Filip 2014; Ma et al. 2023). Aging can be attributed to DNA damage from environmental and metabolic factors, an increase in mutations due to incomplete DNA replication, and telomere attrition. In aging individuals, senescent cells produce a variety of secretory factors that trigger changes in surrounding normal cells, which are referred to as senescence-associated secretory phenotypes (Coppé et al. 2010). Recently accumulated data has revealed that senescent cells can be eliminated from an individual to trigger favorable physiological changes; these substances are called senolytic agents (Robbins et al. 2021; Chaib et al. 2022; Kim et al. 2022).
The efficacy of senolytic agents has been investigated by targeting various tissues, and clinical trials assessing these candidates are ongoing (Wissler Gerdes et al. 2020). For example, dasatinib and quercetin, which were the first to enter human trials, were shown to eliminate senescent cells from the adipose tissue of patients with diabetic kidney disease, thereby contributing to improvements in patient health (Wissler Gerdes et al. 2021). The skin is also a typical target tissue for senolytic activity and has been used as a model by several researchers owing to its capacity for rapid phenotypic changes with aging. The skin is a continuously dividing and differentiating regenerative tissue that forms the outermost barrier of the human body and performs various immunological regulatory functions. With aging, the skin exhibits graying, hyperpigmentation, wrinkles, and loss of elasticity. Certain dermatological diseases, such as psoriasis, have also been associated with aging (Blume-Peytavi et al. 2016; Iskandar et al. 2021).
Accordingly, the efficacy of senolytic agents in the skin has been examined. ABT-737, a small molecule targeting BCL-W and BCL-XL, was shown to improve keratinocyte function and proliferation of hair-follicle stem cells by targeting senescent keratinocytes and melanocytes (Yosef et al. 2016; Victorelli et al. 2019). Fisetin, a flavonoid found in large amounts in vegetables of the pomegranate family, reportedly possesses a variety of bioactivities, including senolytic, antioxidant, and anti-inflammatory activities. In a study by Wu et al., fisetin was shown to effectively protect against ultraviolet (UV) B-induced skin damage by regulating the NRF2 (Nuclear factor erythroid 2-related factor 2) pathway (Wu et al. 2017). The efficacy of quercetin, currently under active clinical investigation, has also been demonstrated in the skin. Treatment with quercetin could ameliorate radiation therapy-induced radiation ulcers in humans or radiation-induced senescence in animal models by eliminating senescent cells (Wang et al. 2020).
Piperlongumine is a senolytic amide alkaloid found in pepper plants. As a potential anticancer agent, piperlongumine was found to induce selective cell death by increasing the generation of reactive oxygen species (ROS) (Adams et al. 2012). Piperlongumine can target intracellular proteins, such as phosphatidylinositol-3-kinase, Akt (Protein kinase B), and mammalian target of rapamycin, and induce anti-angiogenic and anti-invasive effects in addition to cell cycle arrest. Notably, the anticancer activity of piperlongumine has recently been demonstrated in triple-negative breast cancer cells and non-small cell lung cancer (Ghassemi-Rad et al. 2020; Lu et al. 2021). Moreover, piperlongumine was shown to modulate various factors involved in the immune response. Piperlongumine can reportedly alter the expression of various cytokines in human umbilical vein endothelial cells, including monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-8 (Henrique et al. 2020), inhibit human polarization of primary human naïve CD4+ T cells into TH17 subsets, and promote regulatory T cell differentiation (Liang et al. 2020). However, the mechanisms by which piperlongumine acts in the physiological regulation of skin cells are still poorly understood, with recent few reports including its toxicity to melanocytes (Song et al. 2018) and its anti-inflammatory role in psoriasis (Thatikonda et al. 2020).
Taken together, senolytic substances may contribute to improvements in key skin functions with aging, with proof-of-concept evidence accumulated in recent studies. However, the physiological activity of piperlongumine in the skin has not been validated with respect to skin barrier functions and aging. Accordingly, in the present study, we aimed to provide preliminary evidence regarding the potential senolytic activity of piperlongumine in the skin. The findings of the present study may facilitate the use of piperlongumine in treatment development for various aging skin diseases in the future and may aid in developing functional materials to activate skin function.
Materials and methods
Cell culture
Transformed immortal epidermal keratinocyte HaCaT cells were cultivated in a CO2 incubator (BB15, Thermo Fisher Scientific, Waltham, MA, USA) with 5% CO2 at 37°C under humidified conditions. In addition, HaCaT cells were cultivated in a multigas chamber (JP/SMA-30D, Astec, Fukuoka, Japan) with 5% CO2 and 2% O2 adjusted by nitrogen injection. The cells were maintained in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The sample size for each experimental group was at least three for viability and gene expression assays and two for cytokine arrays. Cells were seeded at a 50% of visual confluency per well in polystyrene dishes for maintenance and analysis. Primary normal human epidermal keratinocytes from single juvenile donor were purchased from PromoCell (Cat#: C-12001, Heidelberg, Germany) and cultured in accordance with the manufacturer’s instructions using Keratinocyte Growth Medium 2 (Cat#: C-20011, PromoCell, Heidelberg, Germany).
Piperlongumine (Cat#: A4455, APExBIO, Houston, TX, USA) stock solution was prepared by adding 1.58 mL of dimethyl sulfoxide (DMSO) to 10 mg of piperlongumine to obtain a 20 mM stock, followed by dilution using culture medium to achieve indicated concentrations. Cell viability was automatically assessed using the Cellloger Mini Plus device (Curiosis, Seoul, Korea) for 48 h. Visual confluency at each time point was determined using the Celloger Mini Plus Analysis App (Curiosis, Seoul, Korea) without any manipulation from external input, according to the manufacturer’s instructions.
Quantitative real-time PCR
HaCaT cells were seeded on culture plates with ∼70% of visual confluency and incubated with 1 μM piperlongumine for 48 h. Following the incubation period, the cells were washed with 1× phosphate-buffered saline and RNA was purified using TRIzol Reagent (Invitrogen, Waltham, MA, USA). Three distinct cDNA synthesis reactions were conducted using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) from the extracted RNA. All procedures were performed according to the manufacturer's instructions. Real-time PCR was performed on a Rotor-Gene Q machine (Qiagen, Hilden, Germany) using the amfiSure qGreen Q-PCR Master Mix (Gendepot, Katy, TX, USA). Real-time PCR was performed using the following protocol: 40 cycles of 10 s at 95°C, 30 s at 55°C, and 30 s at 72°C. The primer sequences used are listed below. All reactions were performed independently in triplicate.
| No | Primers | Sequence |
|---|---|---|
| 1 | CERS2-F | TTTGCCTTTGACTCCCTGAC |
| 2 | CERS2-R | GCCCACAGATGGGACAATAA |
| 3 | CERS3_F | GGAGGAGGTGGTGAAACAGG |
| 4 | CERS3_R | TCCAACCAGCTTCGTTCTCC |
| 5 | FLG-F | GGACTCTGAGAGGCGATCTG |
| 6 | FLG-R | TGCTCCCGAGAAGATCCAT |
| 7 | IVL-F | GGCCCTCAGATCGTCTCATA |
| 8 | IVL-R | CCTAGCGGACCCGAAATAA |
| 9 | SPTLC1-F | TTGTCCTCTTCCAGAATTGGTT |
| 10 | SPTLC1-R | GCTCTCCTAGGACTCCAAATGA |
| 11 | SPTLC2-F | CCTCTTTCAGCAGATCACATCA |
| 12 | SPTLC2-R | GGGCTTTTGACATCTCCTAGC |
| 13 | SPTLC3-F | GTTTTGGAGCTTCAGGAGGTT |
| 14 | SPTLC3-R | CCCGTAAATAATCCACGAGGT |
| 15 | H2AJ-F | AAAGTGACCATCGCTCAGGG |
| 16 | H2AJ-R | TTCAAAAGCATTGCGGGACG |
| 17 | ITGA6-F | AGTTGGTGGAGAGACTGAGC |
| 18 | ITGA6-R | CTGTATAGGAAACGCTGGTCA |
Human cytokine antibody array
After incubation with piperlongumine at indicated concentrations for 48 h, the culture media were centrifuged, and the supernatant was collected to analyze secreted cytokines and chemokines using a RayBio C-Series Human Cytokine Antibody Array C3 Kit (RayBiotech, Peachtree Corners, GA, USA). The array is designed to perform duplicates to detect the same antigen. Quantitative analysis was performed using ImageJ software with Protein Array Analysis (plugin by Gilles Carpentier). GraphPad Prism software version 9.5 (GraphPad Software, Inc., La Jolla, CA, USA) was used to prepare graphical representations and for statistical analysis, which was performed using one-way analysis of variance (ANOVA).
Droplet digital TRAP (telomerase repeated amplification protocol)
ddTRAP was performed as described previously (Ludlow et al. 2018). Briefly, cells were lysed with NP40 lysis buffer (10 mM Tris-HCl, pH 8.0; 1 mM MgCl2; 1 mM EDTA; 1% (vol/vol) NP-40; 0.25 mM sodium deoxycholate; 10% glycerol; 150 mM NaCl; 5 mM – mercaptoethanol; 0.1 mM AEBSF) and incubated on ice for 1 hour. The extension reaction was performed by incubating the lysates with TS primer (5’-AAT CCG TCG AGC AGA GTT-3’) for 40 minutes at 25°C, and the ddPCR mix was prepared and combined with the extension product. Droplets up to 20,000 were generated with EvaGreen oil, transferred to a sealed PCR plate and heated. PCR was then performed under specific conditions (40 cycles at 95oC-30 s, 54oC-30 s, 72oC-30 s). Droplets were evaluated using a QX200 droplet reader.
Results
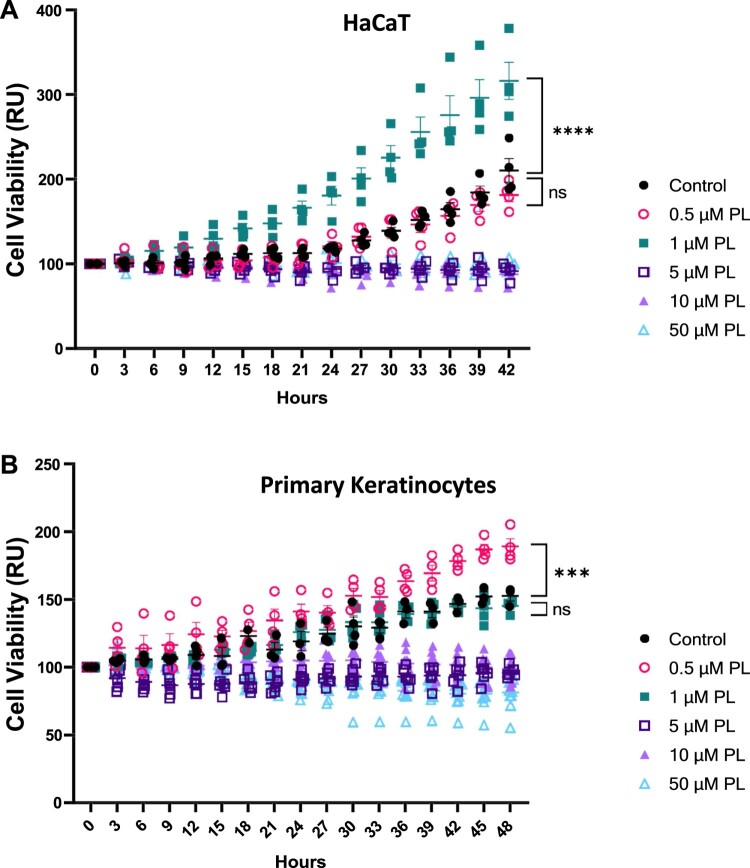
We first aimed to determine the survival of human keratinocytes in response to piperlongumine treatment. Accordingly, we treated HaCaT cells with piperlongumine and enumerated the number of cells based on microscopic confluency every 3 h for 42 h (Figure 1A). By employing live cell imaging techniques, we were able to capture and analyze cell images at multiple timepoints, thereby tracking the dynamic changes in viability. This enabled us to monitor in real-time how the cells responded to external stimuli over the course of the experiment. Piperlongumine exerted relatively low cytotoxicity; however, in the 5–50 μM range, piperlongumine interfered with normal cell division, maintaining the initial cell number even 40 h after piperlongumine treatment. Although treatment with 0.5 μM piperlongumine did not significantly alter cell growth when compared with that of control, treatment with 1 μM increased the cell number by two-fold. Moreover, we examined the effect of piperlongumine treatment on the viability and growth of human primary normal keratinocytes (Figure 1B). While the application of 1 μM piperlongumine did not induce enhanced cell proliferation, consistent with observations in HaCaT cells, it exhibited no discernible impact on cell viability. Based on these results, 1 μM was selected as the reference concentration for further investigation into the regulatory role of piperlongumine in keratinocytes.
Figure 1.
The effect of piperlongumine on cell viability. (A) HaCaT cells and (B) primary human keratinocytes were incubated at up to 25% visual confluency and incubated with piperlongumine at indicated concentrations. Cell growth on 4 or more separate wells was determined by analyzing the confluence of images taken every 3 hours. GraphPad Prism was used to prepare the graph, and statistical significance was determined using one-way ANOVA. ns; non-significant, ***; p < 0.005, ****; p < 0.001
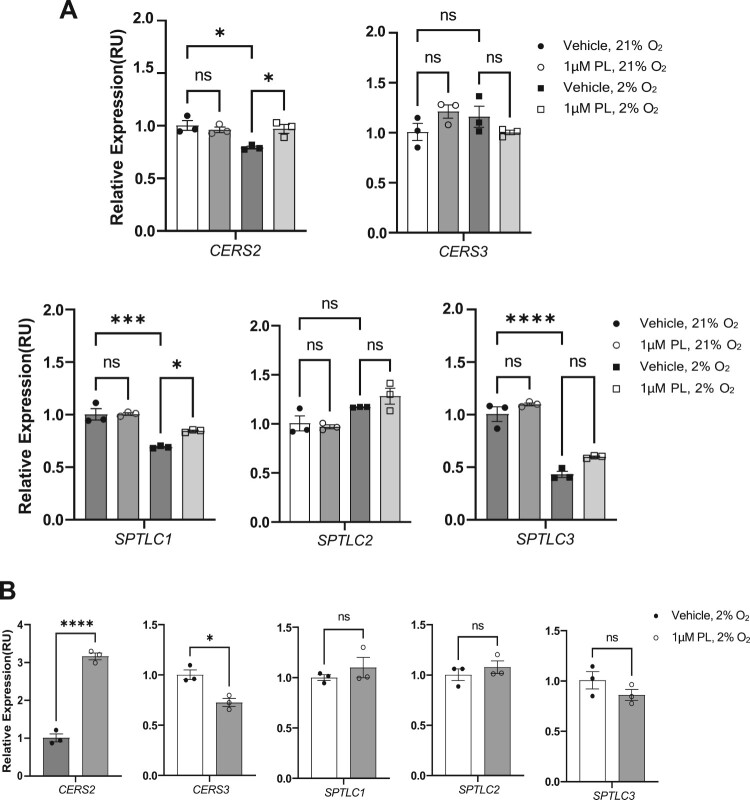
One of the primary functions of keratinocytes is to establish and maintain the skin barrier through ceramide synthesis (Mizutani et al. 2009). Ceramides synthesized and secreted from keratinocytes play a vital role in skin barrier function. Ceramides are the major components of the stratum corneum, the outermost layer of the skin, and form a protective layer that helps retain the natural moisture of the skin, prevents water loss, and provides a barrier against environmental damage and pathogens (Coderch et al. 2003). Ceramides are also involved in regulating skin cell growth and differentiation and are essential for the proper functioning of the epidermis (Li et al. 2020). A disrupted skin barrier associated with decreased ceramide levels can lead to several skin conditions, such as atopic dermatitis, psoriasis, and ichthyosis (Cho et al. 2004; Eckl et al. 2013; Noh et al. 2018; Fujii 2021). Thus we next analyzed the effect of piperlongumine on the expression of skin-related genes, CERS2 and CERS3, which participate in regulating skin ceramide synthesis (Figure 2A). Treatment of HaCaT cells with piperlongumine for 48 h increased the expression of CERS2, but did not impact CERS3 expression. Notably, piperlongumine treatment did not affect gene expression at normoxic oxygen concentrations (21%), but significantly altered the expression at physiological oxygen concentrations (2%). Since the oxygen concentration in human skin tissue remains below 2% (Lee et al. 2021), which is far from atmospheric oxygen concentration of 21%, these results support that piperlongumine may produce physiological changes when applied to human skin. Furthermore, we applied the same concentration of piperlongumine to human primary keratinocytes under physioxia to assess its impact on gene expression (Figure 2B). This revealed a three-fold increase in mRNA expression of CERS2, accompanied by a decrease in CERS3 expression following piperlongumine treatment.
Figure 2.
Treatment with piperlongumine increases the expression of ceramide synthesis-associated genes. (A) HaCaT cells were cultured with 1 μM piperlongumine at the indicated oxygen concentration (21% for normoxia and 2% for physioxia). After incubation for 48 h, real-time PCR was performed to determine the mRNA expression of ceramide synthesis-associated genes. (B) Human normal primary keratinocytes were cultured with 1 μM piperlongumine at a 2% oxygen concentration. After incubation for 48 h, real-time PCR was performed to determine the mRNA expression of ceramide synthesis-associated genes. ACTB was used as a normalization control. GraphPad Prism was used to prepare the graphs, and statistical significance was determined using one-way ANOVA. ns; non-significant, *; p < 0.05, ***; p < 0.005, ****; p < 0.001. CERS2; ceramide synthase 2, CERS3; ceramide synthase 3, SPTLC1; serine palmitoyltransferase long chain base subunit 1, SPTLC2; serine palmitoyltransferase long chain base subunit 2, SPTLC3; serine palmitoyltransferase long chain base subunit 3.
Ceramide synthase 2 (encoded by the CERS2 gene) and ceramide synthase 3 (CERS3) are enzymes responsible for ceramide synthesis (Levy and Futerman 2010). Ceramide synthases 2 and 3 are responsible for synthesizing ceramides with different carbon chain lengths, which play an important role in forming a lipid barrier that protects the skin from external factors. Downregulation of CERS2 reportedly disrupts ceramide synthesis, and improper ceramide synthase expression has been strongly associated with aging and metabolic diseases (Spassieva Stefka et al. 2009; Raichur 2020). Based on our findings, topical application of piperlongumine could increase the mRNA expression of CERS2, a major gene regulating the skin barrier, thereby improving skin function. The differential mRNA expression of CERS2 and CERS3 in human primary keratinocytes can provide insight into the complex regulation and roles of different ceramide synthases in cellular processes in response to piperlongumine, particularly in the skin.
Furthermore, piperlongumine treatment increased the mRNA expression of STPLC1 without altering the expression of SPTCL2 and SPTLC3 in HaCaT cells. SPTLC1 was significantly increased under 48 h treatment of piperlonumine in Figure 2A. Serine palmitoyl transferases (SPTLCs) are enzymes involved in the metabolism of sphingolipids (Boer et al. 2020), which are essential components of the skin’s natural barrier. SPTLCs participate in the production of ceramides, which are necessary for maintaining the integrity of the skin barrier and protecting it from environmental stress (Jungersted et al. 2008). Additionally, SPTLCs are produced in the endoplasmic reticulum and are involved in the production of sphingosine-1-phosphate, an important signaling molecule involved in cell proliferation, differentiation, migration, inflammation, and wound healing. SPTLCs play an essential role in maintaining the skin barrier and repairing processes associated with skin damage. Serine palmitoyl transferase 1 (SPTLC1) is pivotal in epidermal barrier formation in the skin. SPTLC1 is responsible for catalyzing the condensation of a fatty acid and sphingoid base, subsequently utilized to form ceramides, an essential component of the skin barrier (Holleran et al. 1991). SPTLC1 participates in the formation of other sphingolipids, including sphingomyelin and sphingosine, crucial for proper skin function (Hojjati et al. 2005; Herzinger et al. 2007). We also treated primary keratinocytes with piperlongumine under physioxia in Figure 2B, but there was no statistically significant change. We postulated that the disparate behavior observed in the treatment of piperlongumine in primary keratinocytes versus immortalized HaCaT cells may be attributed to the more confined and differentiated fate of the primary keratinocytes than HaCaT cells. Moreover, some reports have indicated that the HaCaT cells exhibit stem cell-like characteristics, expressing epidermal stem cell markers or replenishing damaged tissues (Boelsma et al. 1999; Schoop et al. 1999; Zhu et al. 2019). Therefore, we assume that piperlongumine treatment may indirectly benefit ceramide synthesis in epidermal keratinocytes through increased mRNA expression of SPTLC1.
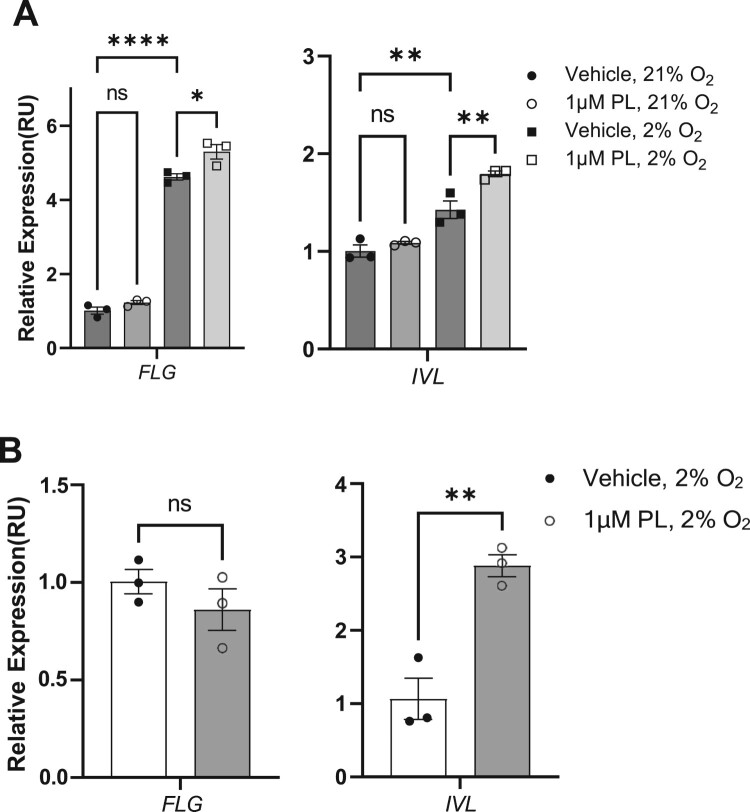
Next, we examined whether piperlongumine could enhance the protective function of the skin by increasing the expression of key proteins constituting the skin barrier, which, coupled with the previous increase in the expression of enzymes mediating ceramide synthesis, may provide data for developing piperlongumine as a skin barrier enhancer. Keratinocytes play a key role in the construction of a physical skin barrier by forming a robust network of structural proteins. Filaggrin aggregates keratin filaments in corneocytes for skin structural integrity and resilience. It maintains skin hydration, acidic pH for barrier homeostasis, and protection against microbial invasion. Its degradation products contribute to natural moisturizing factors, while mutations can cause skin barrier dysfunction linked to atopic dermatitis and ichthyosis vulgaris (Kezic and Jakasa 2016). Involucrin contributes to the formation of the cornified cell envelope (CCE) in the stratum corneum. It acts as a substrate for transglutaminases that cross-link proteins in the upper layers of the epidermis, creating a robust barrier against mechanical stresses and water loss (Yaffe et al. 1992). Accordingly, we analyzed the gene expression of FLG and IVL, genes involved in maintaining the skin barrier function, after 48 h of treatment with piperlongumine (Figure 3A). Following treatment with piperlongumine, the expression of both genes was unaltered at atmospheric oxygen level (21% O2), although a significant increase in expression was detected under physioxia (2% O2). Furthermore, we examined the mRNA expression of FLG and IVL in primary keratinocytes under physioxia following treatment with piperlongumine (Figure 3B). Our findings revealed that IVL mRNA expression was significantly elevated, with the extent of nearly three-fold. These results partially support that treatment with piperlongumine may promote the mRNA expression of filaggrin and involucrin, which play key structural roles in the skin barrier.
Figure 3.
Treatment with piperlongumine increases the expression of skin barrier-associated genes. (A) HaCaT cells were cultured under 1 μM piperlongumine at the indicated oxygen concentration (21% for normoxia and 2% for physioxia). After incubation for 48 h, real-time PCR was performed to determine the mRNA expression of genes associated with the skin barrier function. (B) Human normal primary keratinocytes were cultured under 1 μM piperlongumine at a 2% oxygen concentration. After incubation for 48 h, real-time PCR was performed to determine the mRNA expression of genes associated with the skin barrier function. ACTB was used as a normalization control. GraphPad Prism was used to prepare the graphs, and statistical significance was determined using one-way ANOVA. ns; non-significant, *; p < 0.05, **; p < 0.01, ****; p < 0.001. FLG; filaggrin, IVL; involucrin.
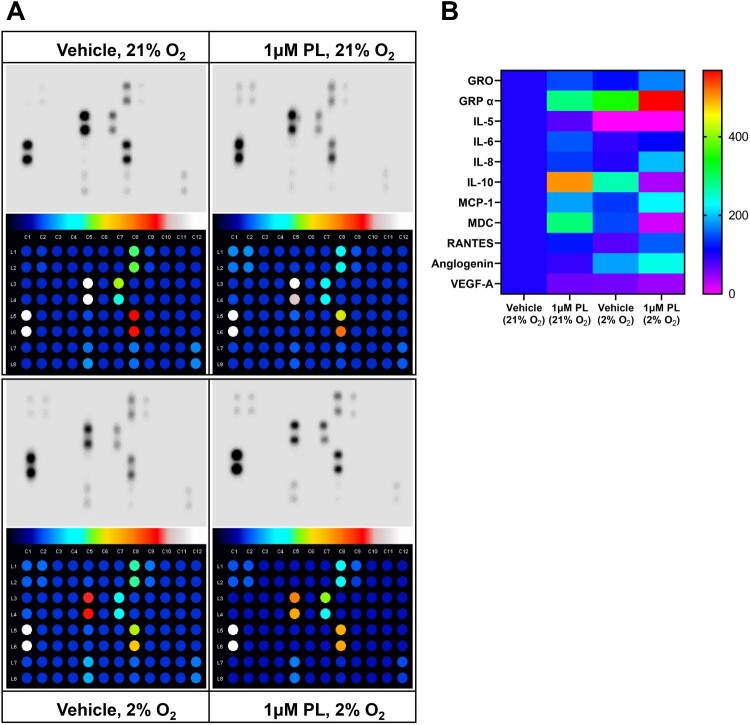
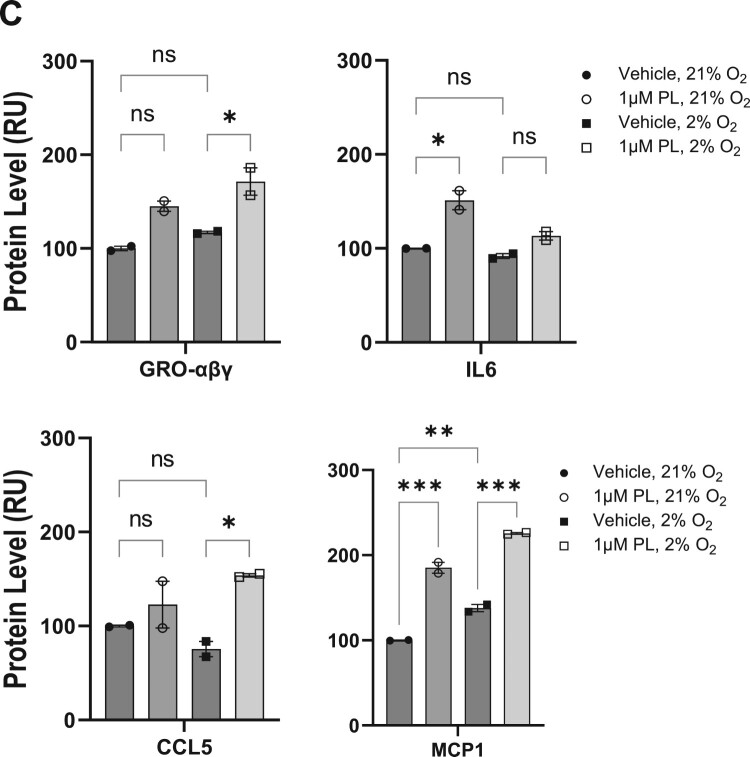
The subsequent analysis entailed a quantitative analysis of protein levels released by keratinocytes in response to piperlongumine treatment. This analysis was conducted to ascertain whether piperlongumine treatment influenced the secretion of pro-inflammatory cytokines and chemokines in terms of epidermal barrier regulation. (Figure 4A,B). The analysis was conducted on 42 human cytokines, yet a considerable number of proteins exhibited negligible secretion. Figure 4C presents a list of cytokines that were secreted in significant amounts following piperongumin administration, with levels exceeding those of the positive control on the array. However, of these, only GRO-αβγ, CCL5, and MCP1 demonstrated statistically significant alterations in secretion levels at physiological oxygen concentrations of 2% O2 (Figure 4C). The results demonstrated that GRO-αβγ, CCL5, and MCP1 exhibited a notable elevation following the administration of piperlongumine in a physiological oxygen concentration (Figure 4D).
Figure 4.
Regulation of cytokines following treatment with piperlongumine. HaCaT cells were treated with 1 μM piperlongumine at the indicated oxygen concentration. After incubation for 48 h, the clear supernatant was analyzed to detect specific cytokines and chemokines using enzyme-linked immunosorbent assays. (A) Representative immunoblot images and quantified results from whole membranes. (B) Map of specific cytokines and chemokines corresponding to each spot. (C) Statistically significant levels of secreted proteins are depicted as a heatmap analysis. The arbitrary value of the protein quantitation was normalized to that of vehicle-treated supernatant in 21% oxygen. (D) The expression of selective secretory proteins was analyzed. Positive and negative blots supplied by the manufacturer were used as the internal normalization control. GraphPad Prism was used to prepare the graphs, and statistical significance was determined using one-way ANOVA. ns; non-significant, *; p < 0.05, **; p < 0.01, ***; p < 0.005
Considering proteins secreted by keratinocytes, CXCL1 (also known as growth-regulated protein alpha, GROα), CXCL2 (also known as growth-regulated protein beta, GROβ), and CXCL3 (also known as growth-regulated protein gamma, GROγ) are chemokines that reportedly play a role in skin function. CXCL1 is involved in recruiting immune cells to the skin (Sawant et al. 2016), while CXCL2 and CXCL3 help regulate inflammation (De Filippo et al. 2013). Additionally, these chemokines participate in wound healing and tissue regeneration (Zaja-Milatovic and Richmond 2008; Ridiandries et al. 2018), as well as play a role in the development of certain inflammatory skin diseases, including psoriasis (Furue et al. 2020). MCP1, derived from keratinocytes, regulates immune responses in association with dendritic cells and Langerhans cells in response to external stimuli (Nakamura et al. 1995). CCL5 has been shown to play an important role in skin function, including wound healing (Ishida et al. 2012), maintenance of immune responses, regulation of inflammation, and skin cancer metastasis (Payne and Cornelius 2002; Marques et al. 2013). CCL5 is also involved in regulating inflammation, given that it is upregulated in inflammatory skin diseases and is believed to play a role in the targeted destruction of melanin-producing cells in vitiligo (Marques et al. 2013; Rezk et al. 2017; Chang et al. 2021).
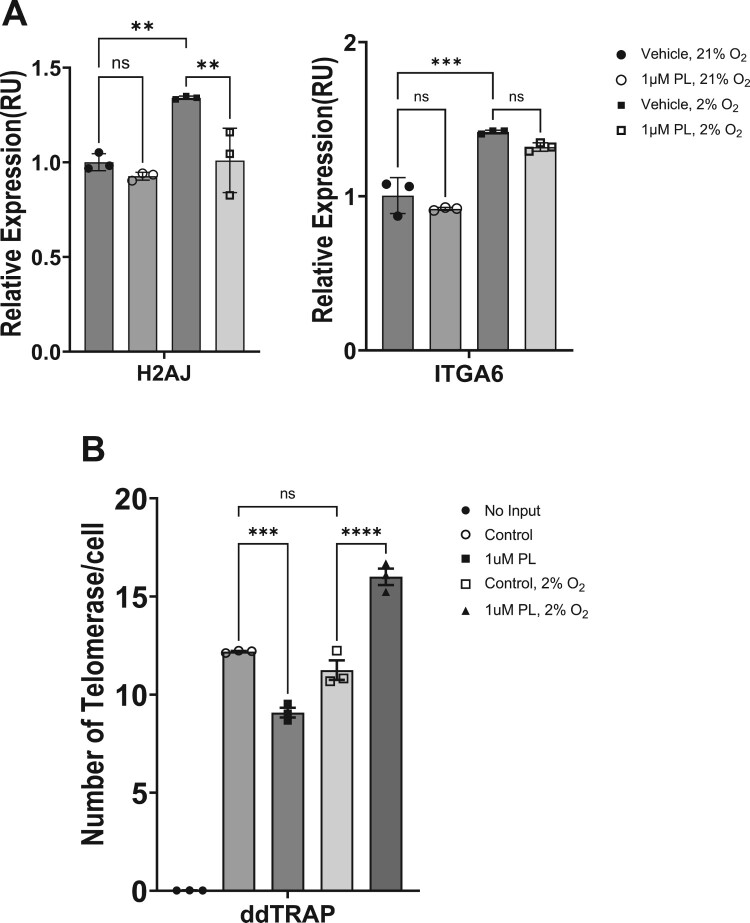
We next analyzed the effect of piperlongumine on the expression of key aging markers in skin cells (Figure 5A). In recent studies related to replicative senescence of skin cells, H2AJ and ITGA6 have been proposed as representative markers of senescence in human epidermal keratinocytes from different ages (18–90 years). H2AJ encodes the histone variant H2A.J and its putative role in cell cycle exit or differentiation of keratinocytes has been suggested. The percentage of H2AJ-positive cells in the epidermis increases linearly with age, and protein expression is mainly observed in the stratum basale and stratum spinosum. Integrin alpha-6, expressed by ITGA6, has been proposed as a marker for stem and progenitor keratinocyte in the human epidermis (Webb et al. 2004). However, in the present study, the gene was employed as an aging marker, rather than for the assessment of stemness in piperlongumine-treated HaCaT cells. Therefore, we analyzed the mRNA expression of H2AJ and ITGA6 genes to determine the effect of piperlongumine on the expression of senescence markers in human epidermal keratinocytes HaCaT cells. The expression of H2AJ was decreased by treatment with 1μM piperlongumine, while the expression of ITGA6 did not show a statistically significant difference. These results, in light of the previous study (Rübe et al. 2021), suggest that piperlongumine affects the differentiation of epidermal keratinocytes but is unlikely to affect the survival and proliferation of epidermal stem cells.
Figure 5.
Piperlongumine affects keratinocyte aging markers and telomerase activity. HaCaT cells were treated with 1 μM piperlongumine at the indicated oxygen concentration. (A) After incubation for 48 h, real-time PCR was performed to determine the mRNA expression of genes associated with the skin barrier function. ACTB was used as a normalization control. H2AJ; H2A.J Histone, ITGA6; Integrin Subunit Alpha 6. (B) After incubation for 48 h, cell lysates were prepared to determine the activity of telomerase. The number of telomerases per cell represents the enzyme with activity after piperlongumine treatment. GraphPad Prism was used to prepare the graphs, and statistical significance was determined using one-way ANOVA. ns; non-significant, **; p < 0.01, ***; p < 0.005, ****; p < 0.001
Although various factors are involved in the aging of epidermal keratinocytes, replicative senescence induced by telomere attrition due to continuous division of epidermal stem cells has a major impact (Härle-Bachor and Boukamp 1996). Therefore, we also tested whether treatment with piperlongumine affects the activity of telomerases from human epidermal keratinocytes, HaCaT cells (Figure 5B). HaCaT cells cultured at atmospheric oxygen concentration (21%) showed decreased activity by piperlongumine treatment, but HaCaT cells cultured at physiological oxygen concentration (2%) showed significantly increased telomerase activity by piperlongumine treatment. Taken together, these results indicate that piperlongumine induced a variety of physiological changes in human epidermal keratinocytes. It decreased the expression of H2AJ, a marker of senescent skin cells, and increased the activity of telomerase, a key enzyme involved in regulation of replicative senescence, at physiological oxygen concentrations, suggesting that it may have some anti-aging properties.
While the immortalized HaCaT cell line is a widely used model for studying keratinocyte biology, it is important to acknowledge its limitations compared to primary human keratinocytes. As an immortalized cell line, HaCaT cells do not undergo replicative senescence, which is a normal process in primary cells. However, we found that piperlongumine treatment elevated telomerase activity in HaCaT cells. We assumed that piperlongumine could also regulate telomerase in normal keratinocytes, as there are no reports suggesting structural differences in the telomerase protein between immortalized and primary cell lines.
Discussion
Piperlongumine is a bioactive compound found in Piper species and has been shown to exert diverse pharmacological effects in various animal models (Salehi et al. 2019). Piperlongumine is well-known for its function as a senolytic substance, along with well-documented bioactivities, such as anti-inflammatory, antioxidant, and anticancer effects and anti-diabetic, anti-obesity, and neuroprotective properties (Go et al. 2018; Henrique et al. 2020; Dong et al. 2021). Piperlongumine has been shown to regulate various cellular processes, including cell proliferation, differentiation, apoptosis, and autophagy (Chen et al. 2019; Shi et al. 2023). In addition, piperlongumine reportedly participates in regulating various signaling pathways, such as the MAPK, PI3 K/Akt, and NF-κB pathways, suggesting its potential as a therapeutic agent for various diseases (Chen et al. 2015; Kumar and Agnihotri 2019; Kumar and Agnihotri 2021). However, despite the proposed benefits of piperlongumine in various tissues and diseases, its function in epidermal keratinocytes remains poorly explored. The epidermis is the outermost layer of the skin and performs important functions, such as building a physical barrier and regulating immunity. Therefore, determining the action of piperlongumine on epidermal keratinocytes may be critical, owing to its potential clinical applications in various fields.
Herein, piperlongumine increased the synthesis of ceramides and gene expression of key elements that constitute the physical barrier of the skin. Piperlongumine increased the secretion of specific proteins involved in the inflammatory response. For example, piperlongumine could significantly enhance the expression of GRO-αβγ, necessitating cautious application during inflammatory diseases. Moreover, it is important to assess the preexistence of inflammatory diseases before applying piperlongumine to the skin and establish any potential side effects associated with skin application. Elucidating the potential toxicity of piperlongumine in human skin cells would also aid in determining the appropriate concentration for skin application. Therefore, when applying piperlongumine to skin-related products, it is necessary to find the optimal concentration and conditions to maximize the skin barrier improvement effect while minimizing inflammation induction. Further detailed mechanistic studies are needed to more clearly elucidate the effects of piperlongumine. By understanding its mechanisms, piperlongumine's efficacy can be better leveraged for potential applications in skin care and other areas.
Considering genes that exhibited statistically significant differences in mRNA expression, changes were observed only under 2% oxygen levels but not under 21% oxygen levels. This finding suggests that piperlongumine could regulate gene expression in keratinocytes in vivo. The mRNA expression of FLG and IVL genes exhibited a substantial difference depending on the oxygen concentration, which is consistent with our previously reported findings (Lee et al. 2021). Notably, these results imply that establishing the physiological oxygen concentration during biological and pharmacological investigations involving skin cells is essential to obtain reliable results. It should be noted that piperlongumine altered the secretion of several proteins that play a key role in the proinflammatory response under physiological oxygen level (2% O2); hence, piperlongumine administration may increase the secretion of these proteins into the blood circulation in vivo. The senolytic function of piperlongumine has been long proposed and clinical trials are underway (de Lima Moreira et al. 2016). However, based on the observed findings, piperlongumine may synergistically increase the inflammatory response by acting on keratinocytes, thereby warranting careful application under certain situations.
One of the limitations of the present study would be the use of an immortalized cell line. HaCaT cells have undergone genetic drift and accumulated mutations over time due to their immortalized nature, which may impact their genetic profile and behavior compared to primary cells. The immortalization process itself could alter certain cellular pathways and responses compared to non-immortalized keratinocytes. HaCaT cells are a cell line adapted to grow in artificial culture conditions, which may not fully recapitulate the in vivo microenvironment and cell–cell/cell-matrix interactions present in human skin. Being a cell line, HaCaT cells lack the heterogeneity present in primary keratinocyte populations derived from different individuals. Therefore, while the HaCaT cell line is a useful model for initial studies, validation of key findings in primary human keratinocytes or reconstructed human skin models is important to account for these potential limitations.
Although additional investigations are needed, we revealed that piperlongumine could significantly impact the mRNA expression of skin barrier-related genes in epidermal keratinocytes. Therefore, piperlongumine could be developed as a potential drug for topical application and as an ingredient in cosmetics. In the future, the biological effects of piperlongumine need to be examined in various skin cells, which will further enhance our understanding of skin biology and lay the foundation for developing drugs that benefit humans.
Funding Statement
This work was supported by the research grant of the Gyeongsang National University in 2022. This research was also supported by the grant from lnstitute of Health Sciences of Gyeongsang National University [grant number HIS GNU-2021-01]; the National Research Foundation of Korea (NRF) funded by the Korea government, Ministry of Science and ICT [grant number 2021R1F1A1062392].
Data availability statement
The datasets generated and/or analyzed in the present study are available from the corresponding author upon reasonable request.
Author contributions
Kyung-Ha Lee conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored and reviewed drafts of the paper, and approved the final draft. Deok Gyeong Kang conceived and designed the experiments, performed the experiments, analyzed the data, and approved the final draft. Dae-Wook Kim designed the experiments, analyzed the data, and approved the final draft. Hwan-Kwon Do authored and reviewed drafts of the paper, and approved the final draft. Do-Yeon Kim conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft. Wanil Kim conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft. All authors have reviewed and finalized the manuscript, and have accepted full responsibility for all aspects of the study, ensuring that any questions regarding the accuracy or integrity of the work have been thoroughly investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adams DJ, Dai M, Pellegrino G, Wagner BK, Stern AM, Shamji AF, Schreiber SL.. 2012. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc Natl Acad Sci USA. 109(38):15115–15120. doi: 10.1073/pnas.1212802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Peytavi U, Kottner J, Sterry W.. 2016. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 56(Suppl_2):S230–S242. doi: 10.1093/geront/gnw003. [DOI] [PubMed] [Google Scholar]
- Boelsma E, Verhoeven MC, Ponec M.. 1999. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). J Invest Dermatol. 112(4):489–498. doi: 10.1046/j.1523-1747.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- Boer DEC, van Smeden J, Bouwstra JA, Aerts JMFG.. 2020. Glucocerebrosidase: functions in and beyond the lysosome. J Clin Med. 9(3):736. doi: 10.3390/jcm9030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaib S, Tchkonia T, Kirkland JL.. 2022. Cellular senescence and senolytics: the path to the clinic. Nat Med. 28(8):1556–1568. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WL, Lee WR, Kuo YC, Huang YH.. 2021. Vitiligo: an autoimmune skin disease and its immunomodulatory therapeutic intervention. Front Cell Dev Biol. 9:797026. doi: 10.3389/fcell.2021.797026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Huang HY, Lin HP, Fang CY.. 2019. Piperlongumine induces autophagy in biliary cancer cells via reactive oxygen species-activated Erk signaling pathway. Int J Mol Med. 44(5):1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu JM, Xiong XX, Qiu XY, Pan F, Liu D, Lan SJ, Jin S, Yu SB, Chen XQ.. 2015. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget. 6(8):6406–6421. doi: 10.18632/oncotarget.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Lew BL, Seong K, Kim NI.. 2004. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 19(6):859–863. doi: 10.3346/jkms.2004.19.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderch L, Lopez O, de la Maza A, Parra JL.. 2003. Ceramides and skin function. Am J Clin Dermatol. 4(2):107–129. doi: 10.2165/00128071-200304020-00004. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J.. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N.. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- de Lima Moreira F, Habenschus MD, Barth T, Marques LMM, Pilon AC, da Silva Bolzani V, Vessecchi R, Lopes NP, de Oliveira ARM.. 2016. Metabolic profile and safety of piperlongumine. Sci Rep. 6(1):33646. doi: 10.1038/srep33646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XH, Peng C, Zhang YY, Jiang Y, Yang L-J, He J-B, Tao X, Zhang C, Chen AF, Xie H-H.. 2021. Low-dose piperlongumine rescues impaired function of endothelial progenitor cells and reduces cerebral ischemic injury in high-fat diet-fed mice. Front Pharmacol. 12:689880. doi: 10.3389/fphar.2021.689880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziechciaż M, Filip R.. 2014. Biological psychological and social determinants of old age: bio-psycho-social aspects of human aging. Ann Agric Environ Med. 21(4):835–838. doi: 10.5604/12321966.1129943. [DOI] [PubMed] [Google Scholar]
- Eckl KM, Tidhar R, Thiele H, Oji V, Hausser I, Brodesser S, Preil M-L, Önal-Akan A, Stock F, Müller D, et al. 2013. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J Invest Dermatol. 133(9):2202–2211. doi: 10.1038/jid.2013.153. [DOI] [PubMed] [Google Scholar]
- Fujii M. 2021. The pathogenic and therapeutic implications of ceramide abnormalities in atopic dermatitis. Cells. 10(9):2386. doi: 10.3390/cells10092386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue M, Furue K, Tsuji G, Nakahara T.. 2020. Interleukin-17A and keratinocytes in psoriasis. Int J Mol Sci. 21(4):1275. doi: 10.3390/ijms21041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi-Rad J, Fernando W, Hoskin D.. 2020. Piperlongumine-loaded nanoparticles inhibit the growth, migration and invasion and epithelial-to-mesenchymal transition of triple-negative breast cancer cells. Int J Funct Nutr. 2(1):1–1. doi: 10.3892/ijfn.2020.11. [DOI] [Google Scholar]
- Go J, Park TS, Han GH, Park HY, Ryu YK, Kim YH, Hwang JH, Choi DH, Noh JR, Hwang DY, et al. 2018. Piperlongumine decreases cognitive impairment and improves hippocampal function in aged mice. Int J Mol Med. 42(4):1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härle-Bachor C, Boukamp P.. 1996. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 93(13):6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique T, CdF Z, Girol AP, Stefanini ACB, Contessoto NSA., da Silveira NJF, Bezerra DP, Silveira ER, Barbosa-Filho JM, Cornélio ML, et al. 2020. Biological and physical approaches on the role of piplartine (piperlongumine) in cancer [OriginalPaper]. Sci Rep. 10(1):1–14. doi: 10.1038/s41598-020-78220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzinger T, Kleuser B, Schäfer-Korting M, Korting HC.. 2007. Sphingosine-1-phosphate signaling and the skin. Am J Clin Dermatol. 8(6):329–336. doi: 10.2165/00128071-200708060-00002. [DOI] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Jiang XC.. 2005. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 1737(1):44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Man MQ, Gao WN, Menon GK, Elias PM, Feingold KR.. 1991. Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J Clin Invest. 88(4):1338–1345. doi: 10.1172/JCI115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kimura A, Kuninaka Y, Inui M, Matsushima K, Mukaida N, Kondo T.. 2012. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest. 122(2):711–721. doi: 10.1172/JCI43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM.. 2021. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender*. Br J Dermatol. 184(2):243–258. doi: 10.1111/bjd.19169. [DOI] [PubMed] [Google Scholar]
- Jungersted JM, Hellgren LI, Jemec GBE, Agner T.. 2008. Lipids and skin barrier function – a clinical perspective. Contact Dermatitis. 58(5):255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- Kezic S, Jakasa I.. 2016. Filaggrin and skin barrier function. Curr Probl Dermatol. 49:1–7. doi: 10.1159/000441539. [DOI] [PubMed] [Google Scholar]
- Kim E-J, Woo J, Shin S, Choi H, Kim Y, Kim J, Kang C.. 2022. A focused natural compound screen reveals senolytic and senostatic effects of Isatis tinctoria. Animal Cells Syst. 26(6):310–317. doi: 10.1080/19768354.2022.2143895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Agnihotri N.. 2019. Piperlongumine, a piper alkaloid targets Ras/PI3 K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomed Pharmacother. 109:1462–1477. doi: 10.1016/j.biopha.2018.10.182. [DOI] [PubMed] [Google Scholar]
- Kumar S, Agnihotri N.. 2021. Piperlongumine targets NF-κB and its downstream signaling pathways to suppress tumor growth and metastatic potential in experimental colon cancer. Mol Cell Biochem. 476(4):1765–1781. doi: 10.1007/s11010-020-04044-7. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim DY, Kim W.. 2021. Cultivation of human skin cells under physiological oxygen concentration modulates expression of skin significant genes and response to hydroxy acids. Biochem Biophys Res Commun. 551:161–167. doi: 10.1016/j.bbrc.2021.02.113. [DOI] [PubMed] [Google Scholar]
- Levy M, Futerman AH.. 2010. Mammalian ceramide synthases. IUBMB Life. 62(5):347–356. doi: 10.1002/iub.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fang H, Dang E, Wang G.. 2020. The role of ceramides in skin homeostasis and inflammatory skin diseases. J Dermatol Sci. 97(1):2–8. doi: 10.1016/j.jdermsci.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Liang J, Ziegler JD, Jahraus B, Orlik C, Blatnik R, Blank N, Niesler B, Wabnitz G, Ruppert T, Hubner K, et al. 2020. Piperlongumine acts as an immunosuppressant by exerting prooxidative effects in human T cells resulting in diminished TH17 but enhanced treg differentiation [Original Research]. Front Immunol. 11:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L.. 2013. The hallmarks of aging. Cell. 153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Xu C, Xu Z, Lu C, Yang R, Zhang F, Zhang G, . 2021. Piperlongumine inhibits the growth of non-small cell lung cancer cells via the miR-34b-3p/TGFBR1 pathway [OriginalPaper]. BMC Complement Med Ther. 21(1):1–10. doi: 10.1186/s12906-020-03162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Shelton D, Wright WE, Shay JW.. 2018. Ddtrap: A Method for Sensitive and Precise Quantification of Telomerase Activity. Methods Mol Biol. 1768:513–529. doi: 10.1007/978-1-4939-7778-9_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Zhang Y, Li H, Kang HR, Kim Y, Han K.. 2023. Cell-autonomous reduction of CYFIP2 is insufficient to induce Alzheimer's disease-like pathologies in the hippocampal CA1 pyramidal neurons of aged mice. Animal Cells Syst (Seoul). 27(1):94–102. doi: 10.1080/19768354.2023.2192263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques RE, Guabiraba R, Russo RC, Teixeira MM.. 2013. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 17(12):1439–1460. doi: 10.1517/14728222.2013.837886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y.. 2009. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 91(6):784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Williams IR, Kupper TS.. 1995. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol. 105(5):635–643. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- Noh Y-H, Lee J, Seo SJ, Myung SC.. 2018. Promoter DNA methylation contributes to human β-defensin-1 deficiency in atopic dermatitis. Animal Cells Syst (Seoul). 22(3):172–177. doi: 10.1080/19768354.2018.1458652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA.. 2002. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 118(6):915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Raichur S. 2020. Ceramide synthases are attractive drug targets for treating metabolic diseases [mini review]. Front Endocrinol (Lausanne). 11. doi: 10.3389/fendo.2020.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk AF, Kemp DM, El-Domyati M, El-Din WH, Lee JB, Uitto J, Igoucheva O, Alexeev V.. 2017. Misbalanced CXCL12 and CCL5 chemotactic signals in vitiligo onset and progression. J Invest Dermatol. 137(5):1126–1134. doi: 10.1016/j.jid.2016.12.028. [DOI] [PubMed] [Google Scholar]
- Ridiandries A, Tan JTM, Bursill CA.. 2018. The role of chemokines in wound healing. Int J Mol Sci. 19(10):3217. doi: 10.3390/ijms19103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, Passos JF, Pignolo RJ, Tchkonia T, Niedernhofer LJ.. 2021. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 61(1):779–803. doi: 10.1146/annurev-pharmtox-050120-105018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübe CE, Bäumert C, Schuler N, Isermann A, Schmal Z, Glanemann M, Mann C, Scherthan H.. 2021. Human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis. NPJ Aging Mech Dis. 7(1):7. doi: 10.1038/s41514-021-00060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, Khan T, Sharifi-Rad J, Ozleyen A, Turkdonmez E, et al. 2019. Piper species: a comprehensive review on their phytochemistry, biological activities and applications. Molecules. 24(7):1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, Rajarathnam K.. 2016. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. 6(1):33123. doi: 10.1038/srep33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoop VM, Mirancea N, Fusenig NE.. 1999. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol. 112(3):343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Shi C, Huang K, Soto J, Sankaran R, Kalia V, Onwumere O, Young M, Einbond L, Redenti S.. 2023. Piperlongumine inhibits proliferation and oncogenic MYCN expression in chemoresistant metastatic retinoblastoma cells directly and through extracellular vesicles. Biomed Pharmacother. 161:114554. doi: 10.1016/j.biopha.2023.114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Gao T, Lei Q, Zhang L, Yao Y, Xiong J.. 2018. Piperlongumine induces apoptosis in human melanoma cells via reactive oxygen species mediated mitochondria disruption. Nutr Cancer. 70(3):502–511. doi: 10.1080/01635581.2018.1445769. [DOI] [PubMed] [Google Scholar]
- Spassieva Stefka D, Mullen Thomas D, Townsend Danyelle M, Obeid Lina M.. 2009. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem J. 424(2):273–283. doi: 10.1042/BJ20090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatikonda S, Pooladanda V, Sigalapalli DK, Godugu C.. 2020. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis. 11(1):21. doi: 10.1038/s41419-019-2212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A, et al. 2019. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 38(23):e101982. doi: 10.15252/embj.2019101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang Z, Huang Y, Zhou Y, Sheng X, Jiang Q, Wang Y, Luo P, Luo M, Shi C, . 2020. Senolytics (DQ) mitigates radiation ulcers by removing senescent cells [original research]. Front Oncol. 9:1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A, Li A, Kaur P.. 2004. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 72(8):387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- Wissler Gerdes EO, Misra A, Netto JME, Tchkonia T, Kirkland JL, . 2021. Strategies for late phase preclinical and early clinical trials of senolytics. Mech Ageing Dev. 200:111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL.. 2020. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. 287(12):2418–2427. doi: 10.1111/febs.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P-Y, Lyu J-L, Liu Y-J, Chien T-Y, Hsu H-C, Wen K-C, Chiang H-M.. 2017. Fisetin regulates Nrf2 expression and the inflammation-related signaling pathway to prevent UVB-induced skin damage in hairless mice [article]. Int J Mol Sci. 18(10):2118. doi: 10.3390/ijms18102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MB, Beegen H, Eckert RL.. 1992. Biophysical characterization of involucrin reveals a molecule ideally suited to function as an intermolecular cross-bridge of the keratinocyte cornified envelope. J Biol Chem. 267(17):12233–8. doi: 10.1016/S0021-9258(19)49829-3. [DOI] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 7(1):11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Richmond A.. 2008. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 23(11):1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Liu J, He B, Qu X, Peng D.. 2019. The role of human immortal skin keratinocytes-acellular dermal matrix scaffold in skin repair and regeneration. J Cell Biochem. 120(8):12182–12191. doi: 10.1002/jcb.28588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in the present study are available from the corresponding author upon reasonable request.