Abstract
Human parechovirus 1 (HPEV1) displays an arginine-glycine-aspartic acid (RGD) motif in the VP1 capsid protein, suggesting integrins as candidate receptors for HPEV1. A panel of monoclonal antibodies (MAbs) specific for integrins αvβ3, αvβ1, and αvβ5, which have the ability to recognize the RGD motif, and also a MAb specific for integrin α2β1, an integrin that does not recognize the RGD motif, were tested on A549 cells. Our results showed that integrin αv-specific MAb reduced infectivity by 85%. To specify which αv integrins the virus utilizes, we tested MAbs specific to integrins αvβ3 and αvβ1 which reduced infectivity significantly, while a MAb specific for integrin αvβ5, as well as the MAb specific for α2β1, showed no reduction. When a combination of MAbs specific for integrins αvβ3 and αvβ1 were used, virus infectivity was almost completely inhibited; this shows that integrins αvβ3 and αvβ1 are utilized by the virus. We therefore proceeded to test whether αv integrins' natural ligands fibronectin and vitronectin had an effect on HPEV1 infectivity. We found that vitronectin reduced significantly HPEV1 infectivity, whereas a combination of vitronectin and fibronectin abolished infection. To verify the use of integrins αvβ3 and αvβ1 as HPEV1 receptors, CHO cells transfected and expressing either integrin αvβ3 or integrin αvβ1 were used. It was shown that the virus could successfully infect these cells. However, in immunoprecipitation experiments using HPEV1 virions and allowing the virus to bind to solubilized A549 cell extract, we isolated and confirmed by Western blotting the αvβ3 heterodimer. In conclusion, we found that HPEV1 utilises both integrin αvβ3 and αvβ1 as receptors; however, in cells that express both integrins, HPEV1 may preferentially bind integrin αvβ3.
Human parechovirus 1 (HPEV1), a representative of an independent picornavirus genus (19, 24) previously classified as echovirus 22, is a small, nonenveloped, single-stranded RNA virus. Infection of humans, especially infants and young children, can induce respiratory symptoms, encephalitis, and flaccid paralysis (14, 16). HPEV1 carries a tripeptide arginine-glycine-aspartic acid (RGD) motif in its VP1 capsid protein (19, 35), a sequence recognized by αv integrins (18, 29). It has been found in previous studies using peptide libraries that HPEV1 possibly utilizes αv integrins and preferably αvβ1 as receptors in its infectious cycle (27).
Integrins are a large family of heterodimeric receptors, which appear to be major receptors by which cells attach to extracellular matrices; they also mediate important cell-cell adhesion events (18, 29). Integrins are also involved in a number of tissue remodeling events, such as wound repair and bone resorption (12, 15). Integrin-ligand interactions mediate the activation and regulation of intracellular signaling pathways within cells, which control transcriptional and ligand binding functions (31, 34). The RGD sequence which is present in many integrin natural ligands (vitronectin, fibronectin, fibrinogen, etc.) is recognized by specific cellular integrins such as αvβ3, αvβ5, αvβ1, αIIbβ3, and α5β1 (18, 29, 30).
Integrins have been also subverted by a number of bacterial pathogens such as Lyme disease spirochetes (9) and Bordetella pertussis (20), viral pathogens such as rotaviruses (10) and papillomaviruses (13), and also members of the Picornaviridae family. The latter include echoviruses 1, 8, and 9, which utilize integrins as receptors (4, 5, 38). Coxsackievirus A9 and foot and mouth disease virus, which both exhibit an RGD sequence found in the VP1 capsid protein (1, 7, 8), use integrin αvβ3 as a receptor molecule (6, 22, 23, 25, 26, 28, 37).
In this study, we investigated the requirements for HPEV1 attachment to cells and have shown that both integrin αvβ3 and integrin αvβ1 are directly involved in HPEV1 attachment by acting as the virus binding receptors in the viral infectious cycle.
MATERIALS AND METHODS
Cell lines.
The human lung carcinoma (A549) cell line was maintained in minimal essential medium containing 1% nonessential amino acids, 10% heat-inactivated fetal bovine serum, and 100 μg of gentamicin per ml.
Cell lines CHO-wt, CHO-αvβ3 (CHO transfected with αv and β3 cDNAs and expressing human integrin αvβ3), and CHO-αvβ1 (CHO transfected with αv and β1 cDNAs and expressing human integrin αvβ1) (36) were maintained in 1:1 Dulbecco's modified Eagle's medium–F-12 mix supplemented with 10% (vol/vol) non-heat-inactivated fetal bovine serum and 100 μg of G418 per ml. All cell lines were maintained at 37°C in a 7% CO2 atmosphere.
HPEV1 plaque assay.
For the production of virus plaques, the cells were infected with virus, and a plaquing overlay was used. The overlay consisted of the appropriate medium to which 0.5% (wt/vol) carboxymethyl cellulose was added. The HPEV1 plaque assays were also repeated without the presence of overlay. Plaques were visualized by staining with 0.2% (wt/vol) crystal violet in 1% (vol/vol) ethanol.
Antibodies and ligands.
Monoclonal antibodies (MAbs) LM609 (a function-blocking MAb specific for integrin αvβ3), 6S6 (a function-blocking MAb specific for integrin β1), B3B11 (specific for integrin β1), and P1F6 (a function-blocking MAb specific for integrin αvβ5) were obtained from Chemicon, as were VNR139 (αv-chain-specific MAb) and BHA2.1 (MAb specific for integrin α2β1). MAbs NK1-M9 (αv specific) and the Y2/51 (β3-chain specific) were obtained from Zymed Laboratories. Rabbit polyclonal sera specific for integrins α2 (AB1944), α5 (AB1928), β4 (AB1922), and β5 (AB1926) were obtained from Chemicon. HPEV1 neutralizing monkey polyclonal serum was obtained from the American Type Culture Collection. Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin (Ig) and HRP-conjugated goat anti-rabbit Ig were obtained from Kirkegaard & Perry Laboratories and Antibodies Incorporated, respectively. Normal monkey serum was obtained from Antibodies Incorporated. Vitronectin and fibronectin were obtained from Sigma.
Virus infectivity assays in the presence of integrin natural ligands.
Cell lines A549, CHO-αvβ3, CHO-αvβ1, and CHO-wt were grown as a monolayers in six-well plates (Nunc) and incubated with integrin natural ligands (10 to 80 μg/ml) in serum-free medium at room temperature for 50 min. Approximately 250 PFU of HPEV1 particles was added to each culture and incubated at room temperature for 50 min. The monolayer was washed with culture medium and overlaid with 0.5% (wt/vol) carboxymethyl cellulose in culture medium. The incubation was continued for 48 to 72 h in a 7% CO2 humidified incubator before plaque visualization with crystal violet. Control plates with isotype control IgG were similarly treated.
Virus blocking assays.
A549, CHO-αvβ3, CHO-αvβ1, and CHO-wt cells were grown as a monolayer in six-well plates (Nunc). MAbs (2.5, 5, 10, and 15 μg) were added in 1 ml of serum-free medium, the mixture was incubated at room temperature for 50 min, approximately 250 PFU of HPEV1 virus particles was added, and the mixture was incubated at room temperature for 50 min. The monolayer was washed with culture medium and overlaid with 0.5% (wt/vol) carboxymethyl cellulose in culture medium. Incubation was continued for 48 to 72 h in a 7% CO2 humidified incubator before plaque visualization with crystal violet. Control plates with isotype control IgG were similarly treated.
Labeling of cell surface with NHS-biotin.
A549, CHO-αvβ3, CHO-αvβ1, and CHO-wt cells were surface labeled with biotin, using 40 μl of 0.1 M membrane-impenetrable NHS (N-hydroxysuccinimide ester derivative)-biotin reagent (Amersham) in 2 ml of phosphate-buffered saline (PBS) per 108 cells. After 30 min, the reaction was stopped with 1 mM ethanolamine in PBS. Cells were washed three times with PBS and lysed in lysis buffer (1% digitonin, 15 mM NaCl, 1 mM MgCl2, 2 mM CaCl2, 2 mM phenylmethylsulfonyl fluoride).
Immunoprecipitation protocols.
A549, CHO-αvβ3, CHO-αvβ1, and CHO-wt cells were surface labeled with NHS-biotin and lysed in lysis buffer as described above. The lysate was precleared with normal monkey serum followed by the addition of 10% (wt/vol) protein A-Sepharose beads (Pharmacia Biotech, Uppsala Sweden) to remove nonspecific binding material. Virus receptor complexes were immunoprecipitated by the addition of 1.5 × 106 PFU of virus; after incubation for 1 h at room temperature, 2 μg of HPEV1-specific monkey serum was added for 1 h at 4°C. The resulting immune complexes were isolated with 10% protein A-Sepharose beads.
Immune complexes were eluted from protein A-Sepharose beads with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (125 mM Tris-HCl, 4% SDS, 20% glycerol, 1.4 M β-mercaptoethanol, 0.1% bromophenol blue). Eluates were electrophoresed in 4 to 20% gradient polyacrylamide gels (Ready Gel; Bio-Rad). Biotin-labeled proteins were transferred to nitrocellulose membranes; for the cell surface-labeled lysates, the gel was Western blotted with streptavidin-HRP conjugate as described below.
Western blotting.
Immunoprecipitates were separated by SDS-PAGE and transferred onto a nitrocellulose filter (Schleicher & Schuell, Dassel, Germany) or Immobilon P membranes (Millipore). After transfer, the membrane was immersed for 1 h in blocking solution (5% low-fat dried milk dissolved in 0.1% PBS-Tween) and washed with 0.1% PBS-Tween (two rinses, a 15-min wash, and two 10-min washes). The membrane was then incubated with streptavidin-HRP conjugate or an appropriate dilution of MAbs, followed by 1 h of incubation with a dilution of HRP-conjugated goat anti-mouse Ig or HRP-conjugated goat anti-rabbit Ig. The optimum antibody concentration was determined by dot blot assay (data not shown). After extensive washing with 0.1% PBS-Tween, the antigen was visualized by the enhanced chemiluminescence procedure (Amersham) according to the manufacturer's instructions.
RESULTS
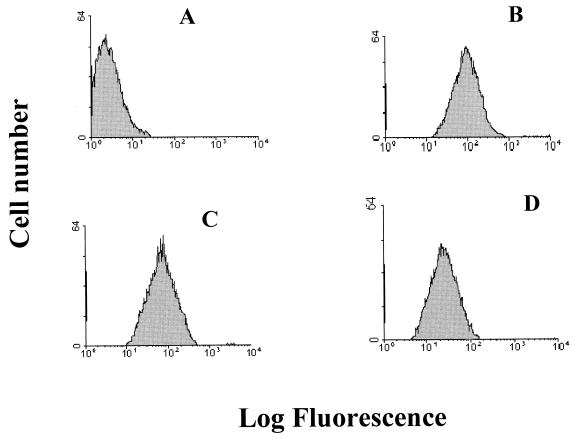
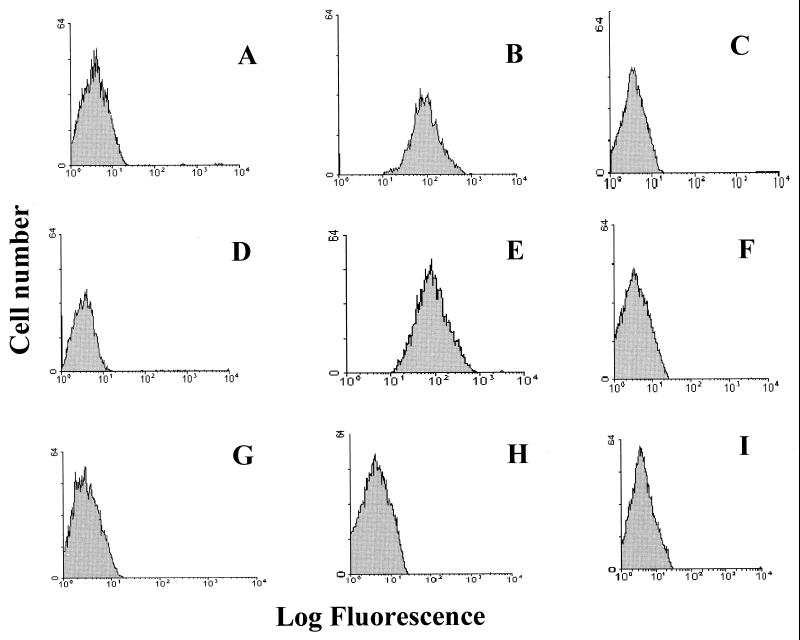
HPEV1 displays an RGD motif in the VP1 capsid protein (19, 35), suggesting integrins as candidate receptors for this virus. To analyze the involvement of integrins in HPEV1 attachment, we used A549 cells, which are susceptible to HPEV1 infection. To determine the presence of αv integrins on these cells and to obtain relative semiquantitive information about these integrins, flow cytometric analysis using fluorescein isothiocyanate (FITC)-conjugated antibodies was used. An integrin αvβ3-specific MAb (LM609), a β1-specific MAb (6S6), and an integrin αvβ5-specific MAb (P1F6), which were titrated on these cells to determine the optimum concentration of each antibody (data not shown), were used. Our results showed that these cells express integrins αvβ3, αvβ1, and αvβ5 (Fig. 1). However, integrins αvβ3 and αvβ1 (Fig. 1B and C) were more abundant than integrin αvβ5 (Fig. 1D).
FIG. 1.
Flow cytometric analysis of integrin αvβ3, αvβ1, and αvβ5 expression in A549 cells. Control A549 cells were incubated with FITC-conjugated rabbit anti-mouse IgG (A), integrin αvβ3-specific MAb LM609 (B), integrin β1-specific MAb 6S6 (C), and integrin αvβ5-specific MAb P1F6 (D). The histograms display relative cell numbers as a function of relative fluorescence intensities.
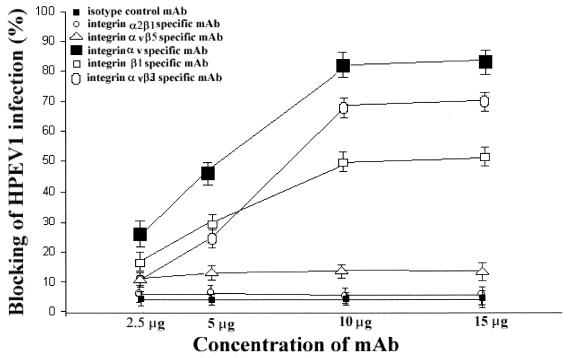
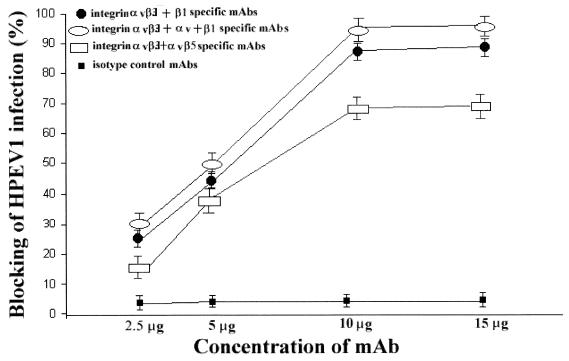
To investigate whether integrins are HPEV1 receptors, we performed blocking experiments using MAbs specific for αv integrins that are known to recognize the RGD motif in ligands and counterreceptors and a MAb specific for integrin α2β1 which recognizes the aspartic acid-glycine-glutamic acid-alanine sequence instead of the RGD motif (18, 29, 30). Therefore, an αvβ3-specific function-blocking MAb (LM609), an αv-specific MAb (NK1-M9), a β1-specific MAb (6S6), an αvβ5-specific MAb (P1F6), and an α2β1-specific MAb (BHA2.1) were used (Fig. 2 and 3). The results showed that at concentrations 10 μg and above, the αv-specific MAb (NK1-M9) inhibited infection by 80%, whereas the αvβ3-specific MAb (LM609) inhibited infection by 65% (Fig. 2). The β1-specific MAb (6S6) inhibited infection by 50%, while the integrin αvβ5-specific MAb (P1F6) inhibited infection by 10% (Fig. 2). The isotype control MAb had no effect on the virus infection (Fig. 2 and 3). A combination of MAbs LM609 and 6S6, used to saturate integrin αvβ3 and β1 receptors, inhibited virus infection by 85% (Fig. 3), whereas a combination of LM609 and P1F6, to saturate integrin αvβ3 and αvβ5 receptors, inhibited infection by 65%. A combination of LM609, 6S6, and NK1-M9 completely inhibited virus infection (Fig. 3). These results show that the αvβ5- and α2β1-specific MAbs had no significant effect on HPEV1 infectivity, thus leading us to believe that HPEV1 preferentially utilizes integrins αvβ3 and αvβ1.
FIG. 2.
Percent inhibition of HPEV1 infectivity to A549 cells in the presence of LM609 (αvβ3-specific MAb), NK1-M9 (αv-specific MAb), 6S6 (β1-specific MAb), P1F6 (αvβ5-specific MAb), BHA2.1 (α2β1-specific MAb), and isotype control MAb at concentrations of 2.5, 5, 10, and 15 μg. The error bars are calculated from the standard deviation over a number on independent experiments.
FIG. 3.
Percent inhibition of HPEV1 infectivity to A549 cells in the presence of combinations of MAbs at concentrations of 2.5, 5, 10, and 15 μg. For identities of the MAbs, see the legend to Fig. 2. The error bars are calculated from the standard deviation over a number on independent experiments.
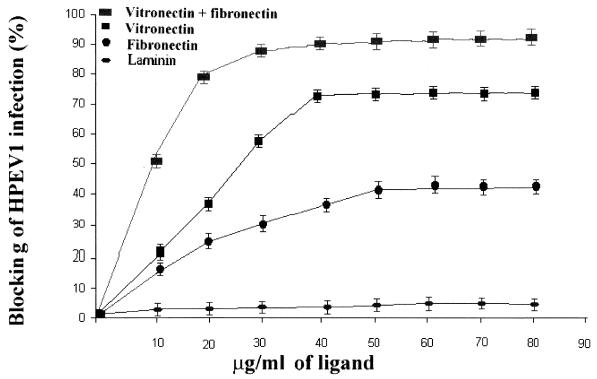
Vitronectin and fibronectin are cell matrix proteins and natural ligands for specific cell surface integrins including integrins αvβ1, αvβ3, and αvβ5 (18, 29, 30). Vitronectin and fibronectin, separately or in combination, were added to A549 cells before the addition of HPEV1 particles (Fig. 4) to determine whether they could block infectivity. Our results showed that infectivity was inhibited 70% by vitronectin (Fig. 4), 40% by fibronectin, and 90% by a combination of vitronectin and fibronectin. Since αv integrins are known to bind vitronectin, fibronectin, or both, these studies indicate that occupancy of αv integrins by cell matrix proteins significantly reduces the susceptibility to HPEV1 infection.
FIG. 4.
Percent inhibition of HPEV1 infectivity to A549 cells in the presence of different concentrations (10 to 80 μg/ml) of vitronectin, fibronectin, laminin, and a combination of vitronectin and fibronectin. The error bars are calculated from the standard deviation over a number on independent experiments.
To verify the involvement of integrins αvβ1 and αvβ3 as HPEV1 receptors, CHO-αvβ1 (CHO cells transfected and expressing human integrin αvβ1) and CHO-αvβ3 (CHO cells transfected and expressing integrin αvβ3) and CHO-wt cells were used. The results showed that CHO-αvβ1 cells express integrin αvβ1 (Fig. 5E) but not integrin αvβ3 (Fig. 5F). CHO-αvβ3 cells expressed integrin αvβ3 (Fig. 5B) but not αvβ1 (Fig. 5C), whereas CHO-wt cells expressed neither integrin αvβ1 (Fig. 5H) nor integrin αvβ3 (Fig. 5I).
FIG. 5.
Flow cytometric analysis of integrin αvβ3 and αvβ1 expression on CHO-αvβ3, CHO-αvβ1, and CHO-wt cells. Control CHO-αvβ3 (A), CHO-αvβ1 (D), and CHO-wt (G) cells were incubated with FITC-conjugated rabbit anti-mouse IgG. To test integrin expression on CHO-αvβ3 cells, integrin αvβ3-specific MAb LM609 (B) or integrin β1-specific MAb 6S6 (C), followed by FITC-conjugated rabbit anti-mouse IgG, was added to the cells. To CHO-αvβ1 cells, integrin β1-specific MAb 6S6 (E) or integrin αvβ3-specific MAb LM609 (F), followed by FITC-conjugated rabbit anti-mouse IgG, was added. To test integrin expression on CHO-wt cells, specific MAb LM609 (I) or specific 6S6 (H) was added, followed by FITC-conjugated rabbit anti-mouse IgG. The histograms display relative cell numbers as a function of relative fluorescence intensities.
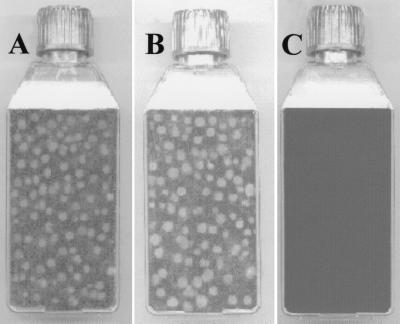
CHO-wt cells were tested and found not to be infected by HPEV1 (Fig. 6C). Our experiments with the CHO transfectants showed that the virus could successfully infect CHO-αvβ1 (Fig. 6A) and CHO-αvβ3 (Fig. 6B) cells. To exclude the possibility that CHO-wt cells were infected by the virus but no plaques were formed, HPEV1 particles (107 PFU) were added to CHO-wt (106) cells and also A549 (106) cells as a control. These cells were incubated at different time periods; for each time period, the cells were frozen and thawed to release HPEV1 particles that may have been produced. The cell lysate was added to A549 cells, which were then assayed for the presence of virus by plaque formation. The data showed no plaque formation on A549 cells when CHO-wt lysate had been added. In contrast, plaques formed on A549 cells when A549 lysate had been added (data not shown). The A549 cells were killed within 10 h, while the CHO-wt cells were incubated for up to 96 h without the formation of plaques.
FIG. 6.
Results of HPEV-1 plaque assay on CHO-αvβ1 (A), CHO-αvβ3 (B), and CHO-wt (C) cells. The plates are representative of a number of independent experiments.
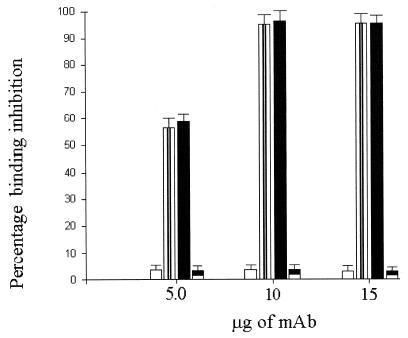
The results of blocking experiments performed with αv (NK1-M9)- and β1 (6S6)-specific MAbs showed that this combination of antibodies completely inhibited virus infection of CHO-αvβ1 cells. Also, the integrin αvβ3 MAb (LM609) completely inhibited virus infection of CHO-αvβ3 cells (Fig. 7). We found no effect on infectivity of CHO-αvβ1 and CHO-αvβ3 cells when isotype control MAbs were used (Fig. 7).
FIG. 7.
Percent inhibition of HPEV1 binding to CHO-αvβ1 and CHO-αvβ3 cells in the presence of a combination of αv-specific MAb NK1-M9 and β1-specific MAb 6S6 (black bars), in the presence of an isotype control MAb (clear bars), in the presence of αvβ3-specific MAb LM609 (striped bars), and in the presence of an isotype control MAb (black and white bars). The error bars are calculated from the standard deviation over a number of independent experiments.
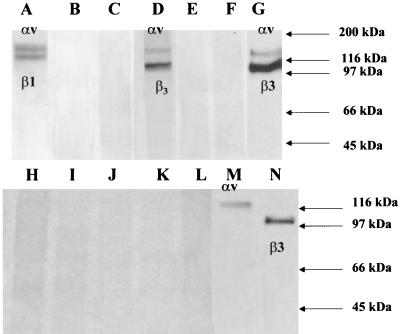
To test whether HPEV1 utilizes any cell surface molecules in its infectious cycle other than the integrins mentioned above, A549 cells, which are susceptible to HPEV1 infection, were used for immunoprecipitation experiments. A549 cell lysate was incubated with virus particles followed by the addition of HPEV1-specific neutralizing serum and protein A-Sepharose beads. SDS-PAGE analysis of the immunoprecipitated material revealed the presence of 120- and 100-kDa bands (Fig. 8G); a faint band of 20 kDa was also visible after extended exposure (data not shown). No proteins were detected in the absence of virus particles (Fig. 8E) or when an irrelevant antiserum was used (Fig. 8F). Western blotting was used to determine the identity of these bands; a panel of integrin α- and β-chain-specific antibodies, MAbs VNR139 (αv specific) and Y2/51 (β3 chain specific), revealed that these bands corresponded to the αv and β3 chains of integrin αvβ3 (Fig. 8M and N). When CHO-αvβ1, CHO-αvβ3, and CHO-wt biotinylated cell surface lysates were used for immunoprecipitation experiments with HPEV1 particles, Western blotting using a panel of integrin-chain-specific antibodies (data not shown) demonstrated that integrin αvβ1 was immunoprecipitated by the virus particles from CHO-αvβ1 cells (Fig. 8A), whereas when CHO-αvβ3 cell lysate was used, integrin αvβ3 was immunoprecipitated by HPEV1 particles (Fig. 8D). When CHO-wt cell lysate was used, no proteins were immunoprecipitated by HPEV1 particles (Fig. 8B).
FIG. 8.
SDS-PAGE of immunoprecipitated HPEV1 receptor complexes under reducing conditions. Cell surface-biotinylated A549 cells were solubilized in 1% digitonin and immunoprecipitated with HPEV1 virions followed by HPEV1-specific monkey neutralizing serum (G), or in the absence of HPEV1 virions, with HPEV1-specific monkey neutralizing serum alone (E), with an irrelevant antiserum (F), or with normal monkey serum (C). As controls, cell surface-biotinylated CHO-αvβ1 (A), CHO-αvβ3 (D), and CHO-wt (B) were solubilized in 1% digitonin and immunoprecipitated with HPEV1 virions followed by HPEV1-specific monkey neutralizing serum. The membrane from the A549 cell lysate immunoprecipitations was Western blotted with αv-chain-specific MAb VNR139 (M), with β3-chain-specific MAb Y2/51 (N), with β1-chain-specific MAb B3B11 (H), and with rabbit polyclonal sera specific for integrins α2 (I), β4 (J), β5 (K), and α5 (L), followed by either HRP-conjugated goat anti-mouse Ig or HRP-conjugated goat anti-rabbit Ig. The positions of molecular weight markers are shown to the right.
DISCUSSION
Worldwide HPEV1 infections are very common, causing mainly respiratory and gastrointestinal symptoms (16); in rare cases, HPEV1 is also responsible for the more serious and life-threatening disease encephalitis as well as flaccid paralysis (14, 16). Receptor-virus associations are the initial step of a viral infection. Previous studies using phage display peptide libraries to identify the HPEV1 receptor molecules have shown that the virus binds peptides containing amino acid motifs found in the αvβ1 integrin (27). In this study, we attempted to identify molecules involved in HPEV1 binding. To this end, we performed blocking experiments with a panel of MAbs specific for integrins αvβ3, αvβ1, and αvβ5; as a control, we used an integrin α2β1-specific MAb, since it does not recognize the RGD sequence displayed on natural ligands. Our results showed that the α2β1-specific MAb had no effect whereas the αvβ5-specific MAb had a very minor effect on virus infection. The αvβ3 MAb showed a 65% inhibition. A combination of αvβ5 and αvβ3 MAbs reduced infectivity by 65%, the same effect as for the αvβ3 MAb; a combination of β1 and αvβ3 MAbs could inhibit virus infection by 85%, and a mixture of β1, αv, and αvβ3 MAbs completely inhibited virus infection. Thus, these data suggest that whereas integrins αvβ1 and αvβ3 play an important role in HPEV1 infection, integrin αvβ5 is not involved in this virus infectious cycle.
To verify the use of αv integrins by HPEV1, integrin αv natural ligands, such as fibronectin and vitronectin, were used. The results showed that vitronectin and fibronectin reduced virus infection, while a combination of the two ligands inhibited infection by 90%, thus verifying that HPEV1 utilizes αv integrins.
To confirm our finding that the virus could utilize αv integrins, specifically integrins αvβ3 and αvβ1, we tested whether HPEV1 could bind on cell surface integrin αvβ1 and also αvβ3. To achieve this, CHO-αvβ1 and CHO-αvβ3 cells expressing integrins αvβ1 and αvβ3, respectively, were used. The experiments showed that the cell lines could be successfully infected by HPEV1, thus confirming that the virus can bind on integrins αvβ3 and αvβ1.
Immunoprecipitation experiments using cell surface-labeled A549 cell lysate and HPEV1 particles were performed to see whether the virus utilizes receptor molecules other than integrins αvβ3 and αvβ1. The results showed that the virus could immunoprecipitate a 100/120-kDa heterodimer which was identified as integrin αvβ3 by Western blotting with a panel of integrin-chain-specific antibodies. The β3 chain seems to be more intensely labeled than the αv chain, possibly due to the labeling procedure. This chain may express more lysine residues than the α chain; since NHS-biotin (our labeling reagent) labels lysines, the β3 chain might be more heavily labeled. Results of immunoprecipitation experiments performed with cell surface-labeled cell lysates showed that HPEV1 could immunoprecipitate integrin αvβ3 from CHO-αvβ3 cell lysate, integrin αvβ1 from CHO-αvβ1 cell lysate, and no protein from CHO-wt lysate, leading us to believe that HPEV1 utilizes only integrins αvβ1 and αvβ3.
Overall, we found that HPEV1 can utilize efficiently both integrin αvβ3 and integrin αvβ1 as receptor molecules, making its infectious cycle more efficient by virtue of the ability to alternate receptors. In this respect HPEV1 is like the coxsackie B viruses, which can use either decay-accelerating factor (3, 33), a 100-kDa nucleolin-related protein (11), or coxsackievirus-adenovirus receptor protein (2) as receptor molecules, as well as measles virus, which can utilize both CD46 and moesin (32). Another example is encephalomyocarditis virus, which can use either the Ig vascular cell adhesion molecule (17) or a 70-kDa cell surface sialoglycoprotein (21).
Although it has been shown that HPEV1 can bind both integrins, we found that in A549 solubilized cell extract the virus binds integrin αvβ3; this could be explained by the fact that the virus interacts initially with αvβ3 and then with αvβ1, or preferentially in the presence of both integrins utilizes αvβ3. We therefore conclude that HPEV1 binds both integrins as receptor molecules, but in cells which express both αvβ3 and αvβ1, it may have a higher affinity for integrin αvβ3.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by the BBSRC and by National Institutes of Health grant GM47157 to Y.T.
We thank K. M. Wilson for helpful discussions.
REFERENCES
- 1.Acharya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F. The three dimensional structure of foot and mouth disease virus at 2.9 Å resolution. Nature. 1989;337:709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1322. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Mohantry J G, Crowell R L, St. John N F, Lublin D M, Finberg R W. Coxsackievirus B3 adapted to growth on RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1909. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Shepley M P, Chan B M C, Hemler M E, Finberg R W. Identification of integrin VLA-2 as a receptor for echovirus 1. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, St. John N F, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berinstein A, Roivainen M, Hovi T, Mason P W, Baxt B. Antibodies to the vitronectin receptor (integrin αvβ3 inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69:2664–2666. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K H, Auvinen P, Hyypiä T, Stanway G. The nucleotide sequence of coxsackievirus A9: implications for receptor binding and enterovirus classification. J Gen Virol. 1989;70:3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- 8.Chang K H, Day C, Walker J, Hyypiä T, Stanway G. The nucleotide sequences of wild type coxsackievirus A9 strains imply that the RGD motif in VP1 is functionally significant. J Gen Virol. 1992;73:621–626. doi: 10.1099/0022-1317-73-3-621. [DOI] [PubMed] [Google Scholar]
- 9.Coburn J, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin αIIβ3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulson B, Lodrigan S L, Lee D J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus cell entry into cells. Proc Natl Acad Sci USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vertugo U R, Selinka H-C, Huber M, Kramer B, Kellermann J, Hofscheider P H, Kandolf R. Characterization of a 100-kilodalton binding protein for the six serotypes of coxsackie B viruses. J Virol. 1995;69:6751–6757. doi: 10.1128/jvi.69.11.6751-6757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duband J L, Rocher S, Chen W T, Yamada K M, Thiery J P. Cell-adhesion and migration in the early vertebrate embryo—location and possible role of the putative fibronectin receptor complex. J Cell Biol. 1986;102:160–168. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evander M, Frazer I, Payne E, Qi Y, Hengst K, McMillan N. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figurea J P, Ashley D, King D, Hull B. An outbreak of accute flaccid paralysis in Jamaica associated with echovirus type 22. J Med Virol. 1989;29:315–319. doi: 10.1002/jmv.1890290418. [DOI] [PubMed] [Google Scholar]
- 15.Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992;101:1–9. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Grist N R, Bell E J, Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- 17.Huber S A. VCAM-1 is a receptor for encephalomyocarditis virus on murine vascular endothelial cells. J Virol. 1994;68:3453–3458. doi: 10.1128/jvi.68.6.3453-3458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes R O. Integrins: versality, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 19.Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA. 1992;89:8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi J, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y-M, Pardoe I U, Burness A T H, Michalak T I. Identification and characterization of a 70-kDa sialoglycoprotein as a candidate receptor for encephalomyocarditis virus on human nucleated cells. J Virol. 1994;68:7308–7319. doi: 10.1128/jvi.68.11.7308-7319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason P W, Rieder E, Baxt B. RGD sequence of foot and mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement mechanism. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateu M G, Valero M L, Andreu D, Domingo E. Systematic replacement of aminoacid residues within the Arg-Gly-Asp, containing loop of foot and mouth disease virus and effect on cell recognition. J Biol Chem. 1996;271:12814–12819. doi: 10.1074/jbc.271.22.12814. [DOI] [PubMed] [Google Scholar]
- 24.Mayo M A, Pringle C R. Virus taxonomy 1997. J Gen Virol. 1998;79:649–657. doi: 10.1099/0022-1317-79-4-649. [DOI] [PubMed] [Google Scholar]
- 25.McKenna T St C, Lubroth J, Rieder E, Baxt B, Mason P W. Receptor binding site-deleted foot and mouth (FMD) virus protects cattle from FMD. J Virol. 1995;69:5787–5790. doi: 10.1128/jvi.69.9.5787-5790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neff S, Sa-Carvalho D, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot and mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulli T, Koivunen E, Hyypiä T. Cell surface interactions of echovirus 22. J Biol Chem. 1997;272:21176–21180. doi: 10.1074/jbc.272.34.21176. [DOI] [PubMed] [Google Scholar]
- 28.Roivainen M, Piirainen L, Hovi T, Virtanen I, Riikonen T, Heino J, Hyypiä T. Entry of coxsackievirus A9 into host cells: specific interactions with αvβ3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 29.Ruoslahti E. Integrins. J Clin Investig. 1991;87:1–7. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–496. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 31.Schartz M, Shaller M, Ginsberg M. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 32.Schneider-Schaulies J, Dunster L M, Schwartz R A, Krohne G, Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackievirus B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3880. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shattil S J, Ginsberg M H, Brugge J S. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6:695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 35.Stanway G, Hyypiä T. Parechoviruses. J Virol. 1999;73:5249–5254. doi: 10.1128/jvi.73.7.5249-5254.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takagi J, Kamata T, Meredith J, Puzon-McLaughlin W, Takada Y. Changing ligand specificities of αvβ1 and αvβ3 integrins by swapping a short diverse sequence of the β subunit. J Biol Chem. 1997;272:19794–19800. doi: 10.1074/jbc.272.32.19794. [DOI] [PubMed] [Google Scholar]
- 37.Triantafilou M, Triantafilou K, Wilson K M, Takada Y, Fernandez N, Stanway G. Involvement of β2-microglobulin and integrin αvβ3 molecules in the coxsackievirus A9 virus infectious cycle. J Gen Virol. 1999;80:2591–2600. doi: 10.1099/0022-1317-80-10-2591. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman H, Eggers H J, Nelsen-Salz B. Cell attachment of mouse virulence of echovirus 9 correlate with an RGD motif in the capsid protein VP1. Virology. 1997;233:149–156. doi: 10.1006/viro.1997.8601. [DOI] [PubMed] [Google Scholar]