Summary
Background:
The addition of nivolumab to chemotherapy improves survival in patients with advanced esophagogastric (esophageal, gastric, or gastroesophageal junction) adenocarcinoma, though outcomes remain poor. We assessed the safety and efficacy of regorafenib in combination with nivolumab and chemotherapy in first-line, advanced esophagogastric adenocarcinoma.
Methods:
This was an investigator-initiated, open-label, single-arm, single-institution, phase 2 trial in patients 18 years or older with previously untreated, HER2-negative, metastatic esophagogastric adenocarcinoma. Eligible patients had measurable or evaluable, non-measurable disease (defined by Response Evaluation Criteria in Solid Tumors version 1·1 [RECIST v1·1]) and Easter Cooperative Oncology Group performance status of 0 or 1. Patients received 5-fluorouracil (400mg/m2 bolus and 2400mg/m2/day over 48 hours), leucovorin (400mg/m2), oxaliplatin (85mg/m2), and nivolumab (240mg) intravenously on days 1 and 15, and oral regorafenib (80mg) on days 1 through 21 of a 28-day cycle. Treatment was continued until disease progression (defined by RECISTv1·1), unacceptable toxicity, or withdrawal of consent. The primary endpoint was 6-month progression-free survival (PFS) in the per-protocol population, defined as the proportion of patients alive and free of progression at 6 months. The regimen would be considered worthy of further investigation if ≥24 of 35 patients were progression-free at 6 months. Safety was assessed in all patients who received at least one dose of treatment. This trial is registered with ClinicalTrials.gov (NCT04757363) and is now complete.
Findings:
Between February 11, 2021 and May 4, 2022, 39 patients were enrolled, received at least 1 dose of treatment, and were included in safety analyses. Thirty-five patients were evaluable for the primary endpoint of 6-month PFS. Median age was 57 years (IQR 52–66), 26 (74%) were men, 9 (26%) were women, 28 (80%) were White, and 7 (20%) were Asian. At time of data cut-off, median follow-up was 18·1 months (IQR 12·7–20·4). The primary endpoint was achieved; 25 (71%; 95% CI 54–85%) of 35 patients were progression-free at 6 months. Nine patients developed progression of disease and 1 patient died. Median PFS was 13·0 months (95% CI 7·6-NR). The most common adverse event (AE) of any grade was fatigue. The most common grade 3/4 AEs were decreased neutrophil count (18 [46%] of 39 patients), hypertension (6 [15%]), rash (5 [13%]), and anemia (4 [10%]). Serious treatment-related AEs occurred in 10 (26%) patients and included acute kidney injury, hepatotoxicity, sepsis, rash, nausea, and gastric perforation. Nivolumab was discontinued in 5 (13%) patients and regorafenib was discontinued in 7 (18%) patients. There were no treatment-related deaths.
Interpretation:
Regorafenib can be safely combined with nivolumab and chemotherapy and demonstrated promising activity in HER2-negative metastatic esophagogastric cancer. A randomized, phase 3 clinical trial is planned.
Funding:
Bristol Myers Squibb (BMS) and Bayer.
Introduction
With 1·3 million deaths annually, esophagogastric (esophageal, gastric, and gastroesophageal junction [GEJ]) cancer represents the second-leading cause of cancer-related death globally, and its incidence is rising among younger patients.1,2 Approximately half of patients present with metastatic disease at the time of diagnosis. CheckMate-649 changed practice for patients worldwide, demonstrating meaningful overall survival (OS) benefit with first-line nivolumab (anti-programmed death-1 [PD-1] antibody) plus chemotherapy compared to chemotherapy alone in patients with metastatic disease.3 While 3-year follow up has shown that a proportion of patients do derive long-term benefit, the majority develop therapeutic resistance.4
Resistance to immune checkpoint blockade (ICB) has been linked to inadequate immune response and self-tolerance as well as an immunosuppressive microenvironment resulting in insufficient T-cell trafficking.5 Low intratumoral T-cell infiltration in esophagogastric cancer may reflect the activity of myeloid-derived suppressor cells, regulatory T-cells (Tregs), tumor-associated macrophages (TAMs), tolerogenic dendritic cells, or transforming growth factor β, many of which are associated with immune-resistance.6,7 The efficacy of targeting each pathway in combination with PD-1 inhibitors is being explored in ongoing trials. Ipilimumab, an anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA4) antibody, has been reported to modulate Tregs, and although combination with nivolumab did not improve OS,8 exploratory biomarker analysis from CheckMate-649 revealed that patients with high Treg signature expression benefit from nivolumab and ipilimumab versus chemotherapy, regardless of PD-L1 combined positive score (CPS) status.9
Multi-targeted tyrosine kinase inhibitors (TKIs), such as regorafenib, activate and enhance the function of natural killer and CD8+ T-cells, while simultaneously inhibiting pathways critical to immunosuppressive TAMs and Tregs, a process that leads to increased immune cell infiltration.10,11 Augmentation of the tumor microenvironment is amplified when TKIs are administered in combination with ICB.12,13 Studies in refractory esophagogastric cancer have demonstrated promising activity with combined PD-1 and multi-targeted TKIs, including regorafenib.14,15 Regorafenib alone has also demonstrated improved survival compared to placebo in patients with refractory disease;16 however, in a previous phase 2 study, regorafenib with 5-fluorouracil and oxaliplatin (FOLFOX) was insufficient to improve outcomes in first-line esophagogastric cancer.17
This phase 2 trial was designed to determine whether regorafenib can be safely combined with nivolumab and FOLFOX, and can potentiate the anti-tumor immune response that would warrant future randomized studies. We incorporated tissue- and blood-based biospecimen analysis to develop predictors of durable benefit for esophagogastric cancer patients treated with this regimen.
Methods
Study design and participants
This study was an investigator-initiated, open-label, non-randomized, single-arm, single-center phase 2 trial performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, United States. The study protocol and all amendments were approved by the MSKCC institutional review board. The study was performed in accordance with the protocol, its amendments, and Good Clinical Practice guidelines, and was overseen by MSKCC’s data and safety monitoring committee. All patients provided written informed consent as per the Declaration of Helsinki principles.
Eligible patients were 18 years or older, with previously untreated, advanced unresectable or metastatic esophageal, gastric, or GEJ adenocarcinoma regardless of PD-L1 expression. Key inclusion criteria included disease that was measurable or evaluable per Response Evaluation Criteria in Solid Tumors (RECIST) v1·1 by investigator assessment, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, adequate organ function, and availability to provide a fresh or archival tumor sample to evaluate PD-L1 expression. Patients who received previous adjuvant or neoadjuvant chemotherapy or radiotherapy were eligible if >6 months had elapsed between the end of treatment and study enrollment.
Patients with known HER2-positive status (defined as immunohistochemistry (IHC) 3+ or 2+ and fluorescence in situ hybridization HER2:CEP17 ratio ≥2), untreated central nervous system metastases, peripheral neuropathy (grade 1 or greater), uncontrolled hypertension despite optimal medical management, active or previously documented autoimmune disease or history of immunodeficiency were excluded. Women who were pregnant or breastfeeding and patients who had previously received an anti-PD-1, anti-PD-L1, or anti-CTLA-4 antibody at any time were also excluded.
Sex and ethnicity data were collected per the institutional guidelines of Memorial Sloan Kettering Cancer Center (New York, NY, USA). Sex and ethnicity were defined by electronic medical records.
Procedures
Patients were administered nivolumab (240mg flat dose) and FOLFOX chemotherapy (5-fluorouracil [400mg/m2 bolus and 2400mg/m2/day over 48 hours], leucovorin [400mg/m2], and oxaliplatin [85mg/m2]) intravenously on days 1 and 15 of a 28-day cycle with regorafenib (80mg) orally on days 1 through 21 of the 28-day cycle. Regorafenib 80mg was the recommended phase 2 dose.14 At the discretion of the treating investigator, patients received an induction cycle with regorafenib and nivolumab alone for one 28-day cycle, and FOLFOX was added during cycle 2. Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent.
All patients underwent a CT or MRI at baseline (within 28 days of beginning therapy) and those who started with an induction cycle of regorafenib and nivolumab had a repeat CT/MRI at week 4 to assess response to the induction phase. All patients, including those who began with an induction cycle, had imaging at week 8 and every 8 weeks thereafter. Toxicity and adverse events (AEs) were assessed according to the NCI Common Terminology Criteria for Adverse Events version 5·0 (CTCAEv5) and laboratory evaluations included hematology, blood chemistry, and magnesium, and were performed on days 1 and 15 of each cycle. Patients were monitored for adverse events (AEs) throughout follow-up and for 30 days after the last dose of study treatment.
Dose reductions of regorafenib, 5-fluorouracil, and oxaliplatin were permitted; dose modification of nivolumab was not. Discontinuation of individual treatment components was allowed, with patients permitted to continue the other components of the combination regimen. Treatment-related AEs leading to discontinuation were recorded in a cumulative manner throughout the duration of treatment and used to calculate the proportion of patients who discontinued treatment due to treatment-related AEs. Patients who received at least 1 dose of treatment were included in safety analyses.
Response and progression were evaluated per RECISTv1·1. Criteria for removal from the study included disease progression, loss of ability to participate, withdrawal of consent, substantial deviation from the protocol or eligibility criteria, non-compliance, treatment-related AEs, and repeated drug-related toxicity that did not resolve despite dose reduction or discontinuation. Patients who were removed from study continued to be followed for disease progression and death. Patients who started with an induction cycle and had progression on the week 4 CT scan prior to starting chemotherapy, were permitted to continue on study and initiate chemotherapy.
Pretreatment tumor and blood samples were collected for genomic analyses using the MSK-IMPACT assay, an FDA-approved capture-based next-generation sequencing (NGS) assay that detects mutations, copy-number alterations, and select rearrangements in up to 505 cancer-associated genes.18 PD-L1 IHC was performed using clone E1L3N (Cell Signaling, Danvers MA) per standard MSKCC practice. PD-L1 CPS was defined as the number of PD-L1-positive tumor cells, lymphocytes, or macrophages divided by the total number of viable tumor cells multiplied by 100. Whole blood was collected at baseline, at the time of all imaging studies, and at end of treatment for isolation of circulating tumor DNA (ctDNA) and peripheral blood mononuclear cells, which were analyzed using the MSK-Analysis of Circulating cfDNA to examine Somatic Status (MSK-ACCESS) assay, a high-depth, NGS assay with molecular barcoding technology for ultra-sensitive detection of somatic alterations in 129 genes.
Outcomes
The primary endpoint was 6-month progression-free survival (PFS) defined as the proportion of patients alive and free of progression at 6 months. Secondary endpoints included safety and tolerability, proportion of patients who achieved an objective response (complete or partial response per RECISTv1·1); disease control rate (DCR), defined as the proportion of patients with stable disease, partial response or complete response; median and 12-month OS, calculated as the time from start of treatment to the date of death; and median and 12-month PFS, calculated as the time from start of treatment (cycle 1, day 1) until documentation of clinical or radiological disease progression or death, whichever occurred first. Progression of disease was defined according to RECIST v1.1, including evidence of progression in non-measurable or measurable lesions, or the development of new lesions. Pre-specified exploratory outcomes included association between PD-L1 status, and PFS and objective response rate (ORR), as well as correlation of ctDNA clearance with survival.
Statistical analysis
Using an exact single-stage binomial design, the study sample size of 35 endpoint-evaluable patients provided 80% power to detect an improvement in the 6-month PFS from the CheckMate-649 historical control of 53%3,8 to 74% with a type I error of 5%. The regimen would be considered worthy of further investigation if ≥24 of 35 patients were progression-free at 6 months. Patients who received at least 1 dose of regorafenib and nivolumab were included in the safety analyses. Patients who received at least 1 dose of regorafenib, nivolumab, and FOLFOX were considered evaluable for the primary endpoint. Patients who came off study because of toxicity before 6 months without documented progression were continually assessed at regular intervals to obtain 6 months of data.
Demographic, disease, and treatment characteristics were summarized using frequency and percentage for categorical variables, and median and 95% confidence interval (CI) for continuous variables. Analyses of OS, PFS, ORR, and DCR included patients who received all 3 components of therapy. Duration of response (DOR) was analyzed post-hoc for all patients who had a best response of complete or partial response and defined as time of best response until date of progression. OS, PFS, and DOR were estimated using Kaplan-Meier methods. Subjects who were alive and progression-free at the time of analysis were censored at the date of the last evaluable tumor assessment. Responses after 4 weeks of therapy, PFS, and OS were also evaluated in the subgroup of patients who began treatment with an induction cycle. Safety was assessed in all patients who received at least 1 dose of study treatment and was reported using descriptive statistics.
Fisher’s exact test was used to compare PFS at 6 months, OS at 12 months, and ORR between PD-L1-negative and PD-L1-positive groups. Post-hoc analyses were performed using a PD-L1 CPS cutoff of 5 to compare outcomes between PD-L1-low (CPS<5) and PD-L1-high (CPS≥5) cohorts. To examine whether ctDNA clearance at any timepoint correlated with survival, the Cox regression model was used by including ctDNA clearance as a time-dependent covariate. Additionally, a landmark time of 8 weeks was chosen and the association of ctDNA clearance at 8 weeks with OS and PFS was evaluated using a Cox regression. ctDNA was identified using MSK-ACCESS with detection defined as the presence of a tumor-matched mutation. ctDNA clearance was defined as the conversion of detectable ctDNA at baseline to undetectable ctDNA following treatment.
All statistical analyses were performed using R version 4·2·2. This study is registered with ClinicalTrials.gov/NCT04757363.
Role of the funding source
Bristol Myers Squibb (BMS) funded this investigator-initiated study and provided nivolumab. Bayer provided regorafenib. BMS and Bayer had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. MSKCC was the study sponsor. The study was designed by the MSKCC authors. This study was supported in part by National Institutes of Health/NCI Cancer Center Support Grant P30 CA008748 to MSKCC, and a grant from MSKCC Cycle for Survival.
Results
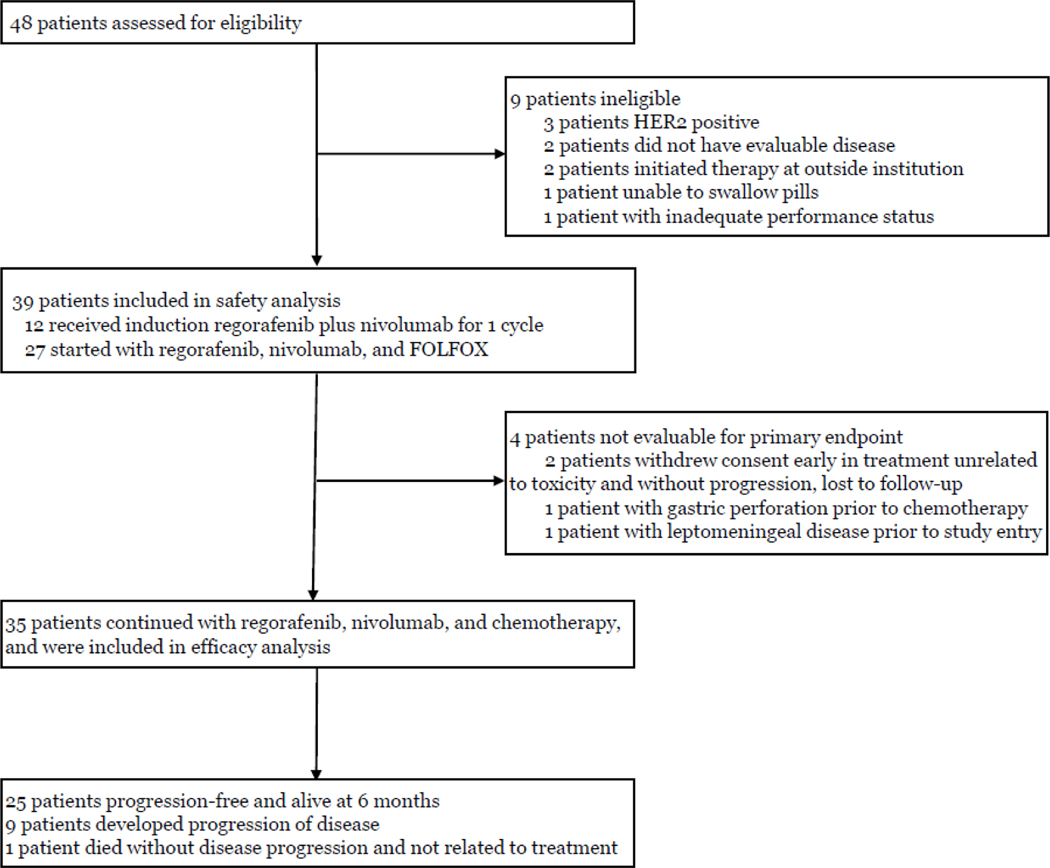
Between February 11, 2021 and May 4, 2022, 48 patients were consented, of whom 9 were ineligible and excluded (Figure 1). Four patients began therapy and were included in toxicity assessment, but were not eligible for efficacy assessments due to study withdrawal prior to the 6-month endpoint and were replaced per protocol. Two patients clinically deteriorated rapidly, one with a gastric perforation prior to receiving any chemotherapy and one with leptomeningeal disease whose symptoms were present prior to study entry. Brain imaging was not mandated per protocol and diagnostic MRI and lumbar puncture performed after treatment initiation confirmed leptomeningeal disease, which made the patient ineligible. Two patients withdrew consent at 0 and 5 months unrelated to toxicity and were lost to follow-up prior to progression of disease and 6-month imaging evaluation. As per the study design, these 4 patients were included in safety analysis, but not in efficacy analysis and were replaced. Thirty-five patients were included in efficacy analysis at the time of data lock on March 3, 2023 (Table 1). Median age was 57 years; 26 (74%) patients were male; 24 (69%) were ECOG performance status 0; 29 (83%) had measurable disease. Primary tumor location was esophageal in 11 (31%) patients, GEJ in 8 (23%), and gastric in 16 (46%). Thirty (86%) patients had 2 or more sites of metastatic disease, including 12 (34%) with liver metastasis and 11 (31%) with peritoneal disease. The majority of tumors (20 [57%]) were PD-L1 negative; 15 (43%) and 9 (26%) were PD-L1 CPS≥1 and CPS≥5, respectively.
Figure 1: Trial profile.
48 patients were screened. 39 patients were started on treatment and therefore included in the safety analysis. 35 patients were evaluable for the primary endpoint. 25 patients achieved the primary endpoint of 6-month progression-free survival.
Table 1:
Baseline demographic and clinical characteristics (n=35)
| Patients (n=35) | |
|---|---|
| Median age, years (interquartile range) | 57 (52, 66) |
| Sex | |
| Men | 26 (74%) |
| Women | 9 (26%) |
| Race | |
| White | 28 (80%) |
| Asian | 7 (20%) |
| Primary tumor location | |
| Esophageal | 11 (31%) |
| Gastroesophageal junction | 8 (23%) |
| Gastric | 16 (46%) |
| ECOG performance status | |
| 0 | 24 (69%) |
| 1 | 11 (31%) |
| Disease stage | |
| Metastatic | 29 (83%) |
| Recurrent disease | 6 (17%) |
| Locally advanced, unresectable | 0 (0%) |
| Organs with metastases | |
| 1 | 5 (14%) |
| ≥2 | 30 (86%) |
| Sites of metastases | |
| Lymph nodes | 30 (86%) |
| Liver | 12 (34%) |
| Peritoneum | 11 (31%) |
| Lungs | 11 (31%) |
| Bones | 6 (17%) |
| Pleura | 3 (9%) |
| Soft tissue | 3 (9%) |
| Adrenal glands | 2 (6%) |
| Ovaries | 2 (6%) |
| Kidneys | 2 (6%) |
| Bladder | 1 (3%) |
| Signet ring carcinoma | |
| Yes | 16 (46%) |
| No | 19 (54% |
| MMR or MSI status | |
| MMRp/MSS | 34 (97%) |
| MMRd/MSI-H | 0 (0%) |
| Unknown | 1 (3%) |
| Measurable disease | 29 (83%) |
| Non-measurable, evaluable disease | 6 (17%) |
| Pretreatment PD-L1 status | |
| CPS <1 (negative) | 20 (57%) |
| CPS ≥1 (positive) | 15 (43%) |
| CPS ≥5 (high) | 9 (26%) |
ECOG=Eastern Cooperative Oncology Group. MMRp/d=mismatch repair proficient/deficient. MSI=microsatellite instability. MSS=microsatellite stable. PD-L1=programmed death-ligand 1. CPS=combined positive score.
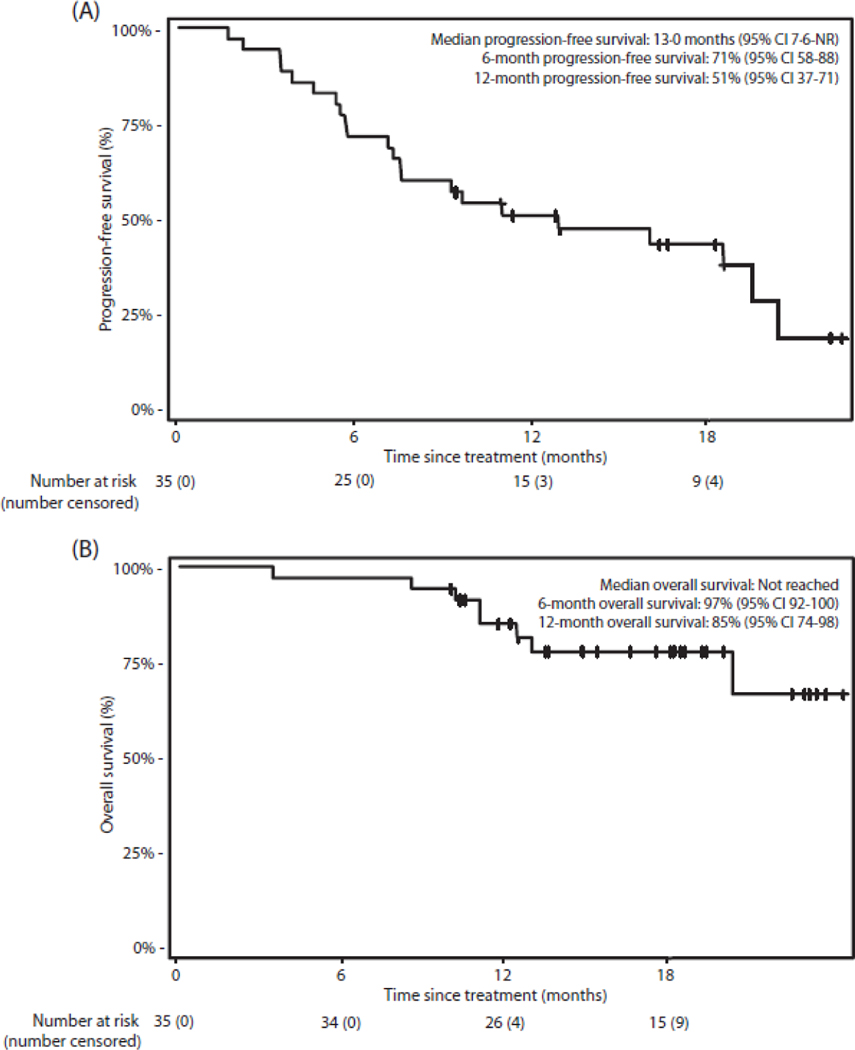
Median follow-up among surviving patients at time of data lock (n=27) was 18·1 months (IQR 12·7–20·4). Twenty-five (71%; 95% CI 54–85%) of 35 patients were alive and progression-free at 6 months, so the study met the decision rule for the primary endpoint (binary endpoint as per the statistical design). One (3%) of 35 patients died unrelated to either treatment or disease before 6 months; 9 (26%) of 35 had disease progression before 6 months. Median PFS was 13·0 months (95% CI 7·6-not reached [NR]) and the Kaplan-Meier estimates of 6- and 12-month PFS survival rates were 71% (95% CI 58–88%) and 51% (95% CI 37–71%), respectively (Figure 2A). Median OS was not reached, and 6- and 12-month OS rates were 97% (95% CI 92–100%) and 85% (95% CI 74–98%), respectively (Figure 2B).
Figure 2: Kaplan-Meier curves.
(A) Progression-free survival in all patients. (B) Overall survival in all patients. Crosses denote censored observations. CI=confidence interval. NR=not reached
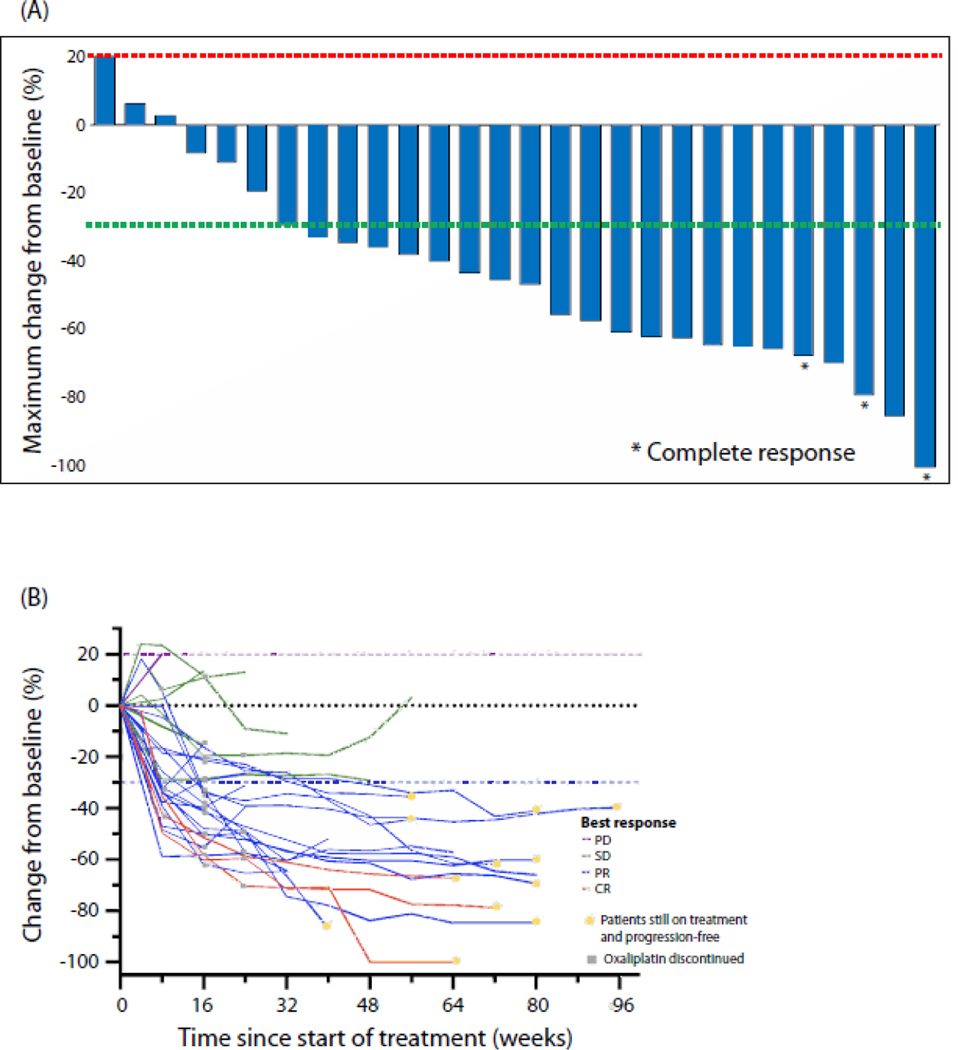
Disease control was achieved in 34 (97% [95% CI 85–99%]) of 35 patients, including 6 with evaluable disease by RECISTv1·1 criteria who had stable disease as best response. Of 29 patients with measurable disease, 22 (76%) patients achieved an objective response, including 3 (10%) achieving a complete response, 19 (66%) achieving a partial response, 6 (21%) with stable disease, and 1 (3%) with progressive disease as best response (Figure 3A). Among patients achieving a complete or partial response as best response (n=22), post-hoc analysis revealed that the median DOR was 17·0 months (95% CI 5·7-NR) and the median time to response was 2·1 months (95% CI 1·78–3·71%) (Figure 3B).
Figure 3: Changes in tumor burden in patients treated with regorafenib, nivolumab, and chemotherapy.
(A) Maximum percent change from baseline in size of tumors. Patients with evaluable, non-measurable lesions are not shown. (B) Percentage change from baseline over time. Growth during the induction cycle was not considered progression of disease. Dashed lines at 20% and –30% indicate the minimum change in tumor size for progressive disease and partial response, respectively, by RECISTv1·1. *Confirmed complete response. PD=progressive disease. SD=stable disease. PR=partial response. CR=complete response.
Patients were enrolled irrespective of PD-L1 status, and PD-L1 expression was not associated with a difference in either response or survival. Twenty (57%) patients had PD-L1 CPS<1, 15 (43%) PD-L1 CPS≥1, and 9 (26%) PD-L1 CPS≥5. ORR was 75% among patients with PD-L1 CPS<1 and 77% among patients with PD-L1 CPS≥1 (p>0·99). Six-month PFS was 75% among patients with PD-L1 CPS<1 and 67% among patients with PD-L1 CPS≥1 (p=0·71). Post-hoc analysis using a PD-L1 CPS cut-off of 5 also did not identify a significant difference in ORR (73% in PD-L1 CPS <5 cohort v 86% in PD-L1 CPS ≥5 cohort, p=0.65) or in 6-month PFS (81% v 44%, p=0.08) between the groups.
At time of data lock, 11 (31%) of 35 patients remained on study. Following disease progression, 17 (85%) of 20 patients received second-line therapy, 10 (59%) of whom received ramucirumab and paclitaxel. Currently, 13 patients remain alive following disease progression, 7 died of disease, 1 died from other causes, 1 remains on standard of care first-line therapy off-study, and 2 were lost to follow up.
Plasma for ctDNA analysis was collected at baseline and with each imaging time point. Thirty-one (89%) of 35 patients had detectable ctDNA at baseline. ctDNA analysis identified a mutational profile comparable to tissue-based tumor sequencing (Supplementary Figure 1A). After starting therapy, 31 (100%) patients had a decrease in their ctDNA levels and 15 (48%) achieved clearance of ctDNA at any timepoint. Median time to clearance was 8 weeks (range 8–40).
Clearance of ctDNA was not associated with a statistically significant reduction in all-cause mortality (HR 0·26, 95% CI 0·05–1·40, p=0·12) given the small sample size (Supplementary Figure 1B). Fifteen (48%) of 31 patients had a durable ctDNA response, of whom 73% remain progression-free. Sixteen (52%) of 31 patients eventually had a rise in ctDNA level following clearance or nadir, 88% of whom went on to develop radiographic progression of disease. Rise of ctDNA preceded radiographic progression by a median of 7·6 weeks (IQR 0–16), suggesting it may serve as an early indicator of therapeutic resistance.
Based on the efficacy seen with regorafenib and nivolumab in heavily pre-treated patients, and to explore the activity of this biologic-only, chemotherapy-free regimen, the study allowed, per investigator discretion, an initial induction cycle of regorafenib and nivolumab alone. Eleven patients received an induction cycle. This subgroup was enriched for patients with better performance status, though baseline patient and tumor characteristics were otherwise similar between the induction and non-induction cohorts (Supplementary Table 1). Ten patients had measurable disease, of whom 6 (60%) showed a reduction in at least some target lesions (range −2·2 to −19·9%) as assessed by repeat CT scan after 3 weeks of daily regorafenib and 2 doses of nivolumab, and before chemotherapy. Six-month PFS did not differ significantly between the induction and non-induction groups (82% v 67%, p=0·45).
AEs occurred in 38 (97%) of 39 patients, with the most frequent events being fatigue, peripheral neuropathy, palmar-plantar erythrodysesthesia (PPE), constipation, and abdominal pain (Table 2). 31 (79%) patients experienced a grade 3 or higher AE; the most common (≥10%) were decreased neutrophil count, hypertension, rash, and anemia. Ten (26%) patients experienced a serious treatment-related AE, which included acute kidney injury (3 [8%]), hepatotoxicity (2 [5%]), sepsis (2 [5%]), rash (1 [3%]), nausea (1 [3%]), and gastric perforation (1 [3%]). Thirty-five (90%) patients required a dose reduction of at least one component of the regimen due to toxicity, primarily for fatigue and peripheral neuropathy. One (3%) patient discontinued 5-fluorouracil and 31 (79%) discontinued oxaliplatin due to toxicity, most commonly due to peripheral neuropathy, and after a median duration of 16 weeks. Eighteen (46%) patients experienced an immune-related AE due to nivolumab. The most common were arthralgias (6 [15%]), dermatitis (5 [13%]), acute interstitial nephritis (3 [8%]), hepatitis (3 [8%]), and hypothyroidism (2 [5%]). However, nivolumab was only discontinued in 5 (13%) patients for nephritis (3 [8%]), dermatitis (1 [3%]), and severe infusion reaction (1 [3%]). Seven (18%) patients discontinued regorafenib and 10 (26%) required dose reduction from 80mg to 40mg; the most common reasons being rash (5 [13%]), PPE (4 [10%]), and fatigue (3 [8%]). There were no treatment-related deaths.
Table 2:
Adverse events.
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Any adverse event | 7 (18%) | 23 (59%) | 8 (21%) |
| Treatment-related serious adverse event | 0 | 10 (26%) | 3 (8%) |
| Adverse events in >10% of patients | |||
| Fatigue | 34 (87%) | 2 (5%) | 0 |
| Paresthesia or peripheral neuropathy | 30 (77%) | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 24 (62%) | 2 (5%) | 0 |
| Constipation | 26 (67%) | 0 | 0 |
| Dry skin, pruritus, or rash | 20 (51%) | 5 (13%) | 0 |
| Abdominal pain | 24 (62%) | 1 (3%) | 0 |
| Anorexia or dysgeusia | 24 (62%) | 1 (3%) | 0 |
| Decreased neutrophil count | 3 (8%) | 14 (36%) | 4 (10%) |
| Nausea | 20 (51%) | 1 (3%) | 0 |
| Vomiting | 14 (36%) | 2 (5%) | 0 |
| Fever | 15 (38%) | 1 (3%) | 0 |
| Oral mucositis | 16 (41%) | 0 | 0 |
| Cough | 15 (38%) | 0 | 0 |
| Anemia | 10 (26%) | 4 (10%) | 0 |
| Diarrhea | 14 (36%) | 0 | 0 |
| Dyspnea | 13 (33%) | 1 (3%) | 0 |
| Hypersensitivity or infusion-related reaction | 12 (31%) | 0 | 0 |
| Dysphagia | 12 (31%) | 0 | 0 |
| Weight loss | 11 (28%) | 0 | 0 |
| Hypertension | 4 (10%) | 6 (15%) | 0 |
| Muscle cramps or myalgias | 9 (23%) | 0 | 0 |
| Increased AST or ALT | 6 (15%) | 2 (5%) | 1 (3%) |
| Increased creatinine | 5 (13%) | 3 (8%) | 0 |
| Headache | 6 (15%) | 1 (3%) | 0 |
| Arthralgias or joint pain | 7 (18%) | 0 | 0 |
| Decreased platelet count | 6 (15%) | 0 | 0 |
| Alopecia | 6 (15%) | 0 | 0 |
| Increased blood bilirubin | 6 (15%) | 0 | 0 |
| Thromboembolic event | 4 (10%) | 2 (5%) | 0 |
| Hypokalemia, hypomagnesemia, or hyponatremia | 3 (8%) | 2 (5%) | 0 |
| Limb edema | 4 (10%) | 0 | 0 |
| Decreased white blood cell count | 2 (5%) | 2 (5%) | 0 |
Data are n (%). AST=aspartate aminotransferase. ALT= alanine aminotransferase.
Discussion
In this single-arm phase 2 study, the combination of regorafenib and nivolumab with FOLFOX chemotherapy was tested in patients with untreated, advanced HER2-negative esophagogastric adenocarcinoma. The study reached its primary endpoint with 25 (71%) of 35 patients progression-free at 6 months. The median PFS of 13·0 months and the 12-month PFS and OS rates of 51% and 85%, respectively, were also numerically higher than the median PFS of 7·7 months, and the 12-month PFS and OS rates of 33% and 55% reported previously for chemotherapy plus nivolumab, the existing first-line standard.3,8
Outcomes were similar regardless of PD-L1 CPS status. These findings stand in contrast to phase 3 trials of chemotherapy with PD-1 inhibitors in which benefit was enriched in patients whose tumors had higher PD-L1 CPS.2,3,19 In this study only 26% had PD-L1 CPS≥5 tumors, in contrast to the 60% of patients PD-L1 CPS≥5 in Checkmate-649 and Orient-16. PD-L1 IHC antibodies may account for these differences, as E1L3N (Cell Signaling) was used in the MSKCC cohort, whereas IHC 28·8 (Agilent DAKO) and 22C3 (Agilent DAKO) were used in the phase 3 studies.
Patient referral may have also contributed to these differences, as patients with negative/low PD-L1 CPS may have been disproportionately referred for this trial, while PD-L1-positive/high tumors may have been directed toward standard nivolumab and chemotherapy.
AEs were observed in most patients, and the frequency of grade 3 or greater toxicities was higher than what has been reported for the combination of nivolumab and chemotherapy (79% v 60%). This was primarily due to higher rates of bone marrow suppression, namely, a relatively high rate of decreased neutrophil count (46% grade≥3 v 11% in CheckMate-649). Notably, an additional 15% of patients in CheckMate-649 had grade≥3 neutropenia, which was reported as a distinct AE from decreased neutrophil count. However, the increased toxicity could be related to pharmacokinetic effects of regorafenib and oxaliplatin, as a similarly higher than expected rate of neutropenia was observed in our previous phase 2 study of regorafenib with FOLFOX.17 In addition, 79% of patients discontinued oxaliplatin due to toxicity (primarily peripheral neuropathy) after a median of 4 months. The median time to best response was 2 months and 11% of patients experienced further reduction in the target lesions after oxaliplatin discontinuation, suggesting that a maintenance strategy is feasible.
In this study, ctDNA was collected in patients at baseline and longitudinally during treatment. Plasma-based ctDNA sequencing identified a similar mutational profile compared to tissue-based sequencing, but has the advantage of dynamic monitoring and the potential to address genomic heterogeneity among metastatic sites not assessable using a single tumor biopsy. Almost all (89%) patients had detectable ctDNA at baseline and nearly half (48%) achieved clearance of ctDNA. Moreover, given that the median time to ctDNA clearance is 8 weeks, if ctDNA monitoring is planned, our data suggest it is best to collect plasma for ctDNA evaluation before initiating therapy. Clearance of ctDNA was not found to be associated with a statistically significant improvement in OS given the small sample size. Rise of ctDNA levels following clearance or nadir preceded radiographic disease progression by 8 weeks. Taken together, these data add to the growing body of evidence indicating that ctDNA may be a useful disease monitoring tool,20–22 though larger, randomized trials to validate these findings are warranted.
The first-line treatment landscape for esophagogastric adenocarcinoma is rapidly evolving, with phase 3 trials demonstrating OS improvement with anti-claudin (CLDN) 18·2 monoclonal antibody, zolbetuximab, and chemotherapy compared to chemotherapy alone in CLDN18·2-positive patients, which has established CLDN18·2 as another therapeutic target.23,24 Despite these advances, there is still a dire need for improved therapeutic options for patients with HER2-negative, PD-L1-negative, and CLDN18·2-negative tumors. LEAP-015 is an ongoing randomized phase 3 trial evaluating lenvatinib in combination with nivolumab and chemotherapy; however, the comparator arm is chemotherapy alone without nivolumab.25
In sum, these results suggest that regorafenib with nivolumab and chemotherapy is safe and active in patients with advanced esophagogastric cancer. Additional biomarker work is underway to dissect the relationships among neoantigen immunogenicity, immune suppression, and response to ICB. Limitations of the study include its modest sample size and single-arm design in which our population was younger than the average age of patients with this disease and a high proportion received second-line therapy. However, 34% and 31% of patients had liver and peritoneal metastases, respectively, and 86% of patients had at least 2 sites of metastatic disease, demonstrating a large disease burden in this cohort. This study was also limited by the fact, that while the referenced historical control is based on a large multicenter study, recruitment in our trial was limited to a single institution. Therefore, a confirmatory phase 3 study is necessary before this regimen could be adopted into clinical practice.
We believe that the responses and survival observed in this study support the development of regorafenib-based combinations in future clinical trials. Given the burden of disease and symptoms experienced by many patients at the time of diagnosis, we would not recommend initiating treatment with the chemotherapy-free induction cycle. However, due to the relatively high toxicity of the quadruplet, and given that oxaliplatin’s maximum benefit is typically achieved within the first several months of therapy, regorafenib may be best utilized as an addition to maintenance therapy in combination with fluoropyrimidine and nivolumab, following discontinuation of oxaliplatin.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for published studies from database inception until April 1, 2023, using the search terms [(“gastric cancer” OR “gastroesophageal cancer” OR “esophageal cancer”) AND (“regorafenib” OR “PD-1 inhibitor” OR “PD-L1 inhibitor”)]. The search was restricted to clinical trials with no language restrictions. We found several trials evaluating PD-1 or PD-L1 inhibitors in combination with chemotherapy for patients with previously untreated metastatic esophagogastric adenocarcinoma. The CheckMate 649 trial demonstrated significantly improved overall survival with nivolumab plus chemotherapy compared to chemotherapy alone in patients with a PD-L1 combined positive score ≥5 and in all randomized patients. Following the results of the CheckMate 649 trial in 2021, nivolumab plus fluoropyrimidine and platinum chemotherapy became standard of care for HER2-negative advanced esophagogastric cancer. The multi-targeted tyrosine kinase inhibitor, regorafenib, has improved outcomes in refractory, advanced esophagogastric cancer and when administered in combination with nivolumab in the phase 1b REGONIVO trial, was found to be safe and lead to significant anti-tumor activity in heavily pre-treated patients.
Added value of this study
To our knowledge, this is the first study to evaluate the efficacy of regorafenib, nivolumab, and chemotherapy in patients with previously untreated metastatic esophagogastric cancer. We also explored potential molecular determinants of response to inform future studies and identify subsets of patients most likely to benefit from this combination.
Implications of all the available evidence
The results of this study suggest that the addition of regorafenib to nivolumab and chemotherapy is safe and active in treating metastatic esophagogastric cancer. A randomized phase 3 clinical trial of regorafenib in combination with first-line nivolumab and chemotherapy is planned.
Acknowledgments
Bristol Myers Squibb (BMS) funded this investigator-initiated study and provided nivolumab. Bayer provided regorafenib. BMS and Bayer were not involved in data collection, analysis, or interpretation, or writing of the report. MSKCC was the study sponsor. The study was designed by the MSKCC authors. This study was supported in part by National Institutes of Health/NCI Cancer Center Support Grant P30 CA008748 to MSKCC, and a grant from MSKCC Cycle for Survival. This study was presented in poster format at the European Society of Medical Oncology Annual Congress 2022, September 9-13, 2022, Paris, France.
SLC reports stock ownership in Pfizer, Moderna, and BioNTech.
RHM reports consulting with PureTech Health, advisory board with IDEAYA Biosciences, and research funding from Nimbus Therapeutics.
JFC reports an investigator role on a research study sponsored by Paige.AI.
GYK reports consulting fees from AstraZeneca, Bristol-Myers Squibb, Merck, Pieris, and Zymeworks; and grants or contracts from AstraZeneca, Bristol-Myers Squibb, CARsgen, Zymeworks, Daiichi Sankyo, Oncolys, Pieris, and Adaptimmune
SBM reports honoraria from Natera, Bicara, Novartis, Basilea, Elevation Oncology, Purple Oncology, Pinetree Therapeutics, and Daiichi Sankyo; research support from Epic Sciences, grant funding from the Conquer Cancer Foundation, and research travel support from AstraZeneca outside the submitted work.
MAS reports consulting for Boston Scientific and Novo Nordisk.
MEL reports ownership/equity interests with Apricity Health; intellectual property rights with John Wiley & Sons, Inc. and Taylor & Francis Group; uncompensated provision of services for Oncoderm LLC; and provision of services for Adgero Biopharmaceuticals Inc., the American Academy of Dermatology, the American Society of Pediatric Hematology/Oncology (ASPHO), Apricity Health, AstraZeneca, Atlantic Canada Oncology Group, BGB Communications LLC, Bicara Therapeutics, Inc., Deciphera, DelMar Pharmaceuticals, Inc., EMD Serono, Inc., GCO Global, Hoth Therapeutics, Inc., Incyte, Innovaderm Research Inc., Johnson & Johnson, La Fonderie Ressources, La Roche-Posay, Loxo Oncology, Lutris Pharma Ltd., MJH Life Sciences, the Michigan Dermatological Society, NKMax America, Inc., NanOlogy LLC, Novartis, Novartis Pharmaceuticals Corporation, Novocure, OnQuality Pharmaceuticals Ltd., Patient Resource LLC, QED Therapeutics, Inc., RBC Consulting, RMEI Medical Education, LLC, Society for Immunotherapy of Cancer, Takeda Millennium, The Lynx Group, LLC, Tyra Biosciences, Inc., Varsona Pharmaceuticals, LLC, WebMD, Wolters Kluwer, and eSquared Communication Consulting.
JS reports consulting for Paige.AI.
MFB reports consulting for Eli Lilly and AstraZeneca (not related to this work).
YYJ reports research funding from Bayer, Bristol-Myers Squibb, Memorial Sloan Kettering Cancer Center Cycle for Survival, the United States Department of Defense, Eli Lilly, Fred’s Team, Genentech/Roche, Merck, the National Cancer Institute, and RGENIX; advisory board/consulting with Abbvie, Amerisource Bergen, Ask-Gene Pharma, Inc., Arcus Biosciences, Astellas, Astra Zeneca, Basilea Pharmaceutica, Bayer, Bristol-Myers Squibb, Clinical Care Options, Daiichi-Sankyo, Eli Lilly, Geneos Therapeutics, GlaxoSmithKline, Guardant Health, Inc., Imedex, Imugene, Lynx Health, Merck, Merck Serono, Mersana Therapeutics, Michael J. Hennessy Associates, Paradigm Medical Communications, PeerView Institute, Pfizer, Research to Practice, RGENIX, Seagen, Silverback Therapeutics, and Zymeworks Inc.; and stock options in RGENIX.
Footnotes
Declaration of interests
All other authors declare no competing interests to report.
Supplementary material
A supplemental figure is included as Supplementary Figure 1. A supplemental table is included as Supplementary Table 1. The study protocol and statistical analysis plan are included in Supplementary Appendix 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
Data collected for the study including individual participant data, de-identified participant data, participant data with identifiers, and a data dictionary may be requested by qualified researchers and will be assessed by a scientific review board. The study protocol and statistical analysis plan are included in Supplementary Appendix 1.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72(1): 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021; 398(10302): 759–71. [DOI] [PubMed] [Google Scholar]
- 3.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398(10294): 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janjigian YY, Shitara K, Moehler MH, et al. Nivolumab plus chemotherapy vs chemo as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: 3-year follow up from CheckMate 649 (Abstract No. LBA291, presented in oral format at the 2023 American Society of Clinical Oncology [ASCO] GI Cancers Symposium, January 19, 2023, San Francisco, CA). J Clin Oncol 2023; 41(Supplement 4, Abstract 291). [Google Scholar]
- 5.Vesely MD, Zhang T, Chen L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu Rev Immunol 2022; 40: 45–74. [DOI] [PubMed] [Google Scholar]
- 6.Derks S, de Klerk LK, Xu X, et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol 2020; 31(8): 1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci 2020; 111(8): 2696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022; 603(7903): 942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janjigian YY, Shitara K, Ajani J, et al. Nivolumab plus ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: CheckMate 649 biomarker analyses. Abstract No. CT037. Presented at the 2023 American Association for Cancer Research (AACR) Annual Meeting, April 18, 2023, Orlando. FL. [Google Scholar]
- 10.Liu J, Tao H, Yuan T, et al. Immunomodulatory effects of regorafenib: Enhancing the efficacy of anti-PD-1/PD-L1 therapy. Front Immunol 2022; 13: 992611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou DL, Chen CW, Hsu CL, et al. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer 2021; 9(3): e001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doleschel D, Hoff S, Koletnik S, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res 2021; 40(1): 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer 2020; 8(2): e001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol 2020; 38(18): 2053–61. [DOI] [PubMed] [Google Scholar]
- 15.Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol 2020; 21(8): 1057–65. [DOI] [PubMed] [Google Scholar]
- 16.Pavlakis N, Shitara K, Sjoquist KM, et al. INTEGRATE IIa: A randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—A study led by the Australasian Gastro-intestinal Trials Group (AGITG). ASCO GI Symposium (Presented January 19, 2023). J Clin Oncol 2023; 41(no. 4_suppl): LBA294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moy RH, Dos Santos Fernandes G, Jonsson P, et al. Regorafenib in Combination with First-Line Chemotherapy for Metastatic Esophagogastric Cancer. Oncologist 2020; 25(1): e68–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zehir A, Benayed R, Shah RH et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017; 23(6): 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Jiang H, Pan Y, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study. Ann Oncol 2021; 21(Supplement 5): S1331 10.016/j.annonc.2021.08.133 (Accessed May 8, 2023). [DOI] [Google Scholar]
- 20.Huffman BM, Aushev VN, Budde GL, et al. Analysis of Circulating Tumor DNA to Predict Risk of Recurrence in Patients With Esophageal and Gastric Cancers. JCO Precis Oncol 2022; 6: e2200420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron SB, Chase LM, Lomnicki S, et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin Cancer Res 2019; 25(23): 7098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ococks E, Frankell AM, Masque Soler N, et al. Longitudinal tracking of 97 esophageal adenocarcinomas using liquid biopsy sampling. Ann Oncol 2021; 32(4): 522–32. [DOI] [PubMed] [Google Scholar]
- 23.Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18·2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023. April 14 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 24.Xu R, Shitara K, Ajani JA, et al. Zolbetuximab + CAPOX in 1L claudin-18·2+ (CLDN18·2+)/HER2−locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW (2023 American Society of Clinical Oncology [ASCO] Monthly Plenary Series abstract). J Clin Oncol 2023; 41(No. 35 Supplement, April 20, 2023): 405736. https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.36_suppl.?role=tab (Accessed May 8, 2023). [Google Scholar]
- 25.National Library of Medicine (U.S.). Efficacy and safety of Lenvatinib (E7080/MK-7902) plus pembrolizumab (MK-3475) plus chemotherapy in participants with advanced/metastatic gastroesophageal adenocarcinoma (LEAP-015). Identifier NCT04662710. (2020, December-). https://clinicaltrials.gov/ct2/show/NCT04662710 (Accessed May 11, 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study including individual participant data, de-identified participant data, participant data with identifiers, and a data dictionary may be requested by qualified researchers and will be assessed by a scientific review board. The study protocol and statistical analysis plan are included in Supplementary Appendix 1.