Keywords: GCaMP, lumbar splanchnic, mechanosensitive, pelvic, sex difference, visceral pain
Abstract
Sex differences in visceral nociception have been reported in clinical and preclinical studies, but the potential differences in sensory neural encoding of the colorectum between males and females are not well understood. In this study, we systematically assessed sex differences in colorectal neural encoding by conducting high-throughput optical recordings in intact dorsal root ganglia (DRGs) from control and visceral hypersensitive mice. We found an apparent sex difference in zymosan-induced behavioral visceral hypersensitivity: enhanced visceromotor responses to colorectal distension were observed only in male mice, not in female mice. In addition, a higher number of mechanosensitive colorectal afferents were identified per mouse in the zymosan-treated male group than in the saline-treated male group, whereas the mechanosensitive afferents identified per mouse were comparable between the zymosan- and saline-treated female groups. The increased number of identified afferents in zymosan-treated male mice was predominantly from thoracolumbar (TL) innervation, which agrees with the significant increase in the TL afferent proportion in the zymosan group as compared with the control group in male mice. In contrast, female mice showed no difference in the proportion of colorectal neurons between saline- and zymosan-treated groups. Our results revealed a significant sex difference in colorectal afferent innervation and sensitization in the context of behavioral visceral hypersensitivity, which could drive differential clinical symptoms in male and female patients.
NEW & NOTEWORTHY We used high-throughput GCaMP6f recordings to study 2,275 mechanosensitive colorectal afferents in mice. Our results revealed significant sex differences in the zymosan-induced behavioral visceral hypersensitivity, which were present in male but not female mice. Male mice also showed sensitization of colorectal afferents in the thoracolumbar pathway, whereas female mice did not. These findings highlight sex differences in sensory neural anatomy and function of the colorectum, with implications for sex-specific therapies for treating visceral pain.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder that affects a significant portion of the global population. According to Lovell and Ford (1), IBS affects ∼11.2% of individuals worldwide, and Hungin et al. (2) report a prevalence of 14.1% in the United States. Visceral pain is a prominent symptom in patients with IBS and substantially impacts their quality of life (3). A meta-analysis of demographic data from 55 studies reveals that IBS is more prevalent in women than in men, with a female-to-male ratio from 2:1 (questionnaire-based diagnostics) to 4:1 (practice-based diagnostics) (4). Moreover, Tang et al. (5) and Chang et al. (6) reported that women with IBS experience more abdominal pain, heightened pain perception, and discomfort during colorectal distension compared with men. This sex difference in pain perception is further supported by functional magnetic resonance imaging studies that reported distinct patterns of brain activation in male and female patients with IBS in response to noxious intestinal stimuli (7).
Numerous animal studies have investigated the underlying mechanisms of this sex difference in visceral pain perception, particularly using visceromotor responses (VMR) to colorectal distension as an objective measure. Several studies in rats and mice have reported a higher magnitude of VMR in females than in males (8–14). This sex difference in behavioral visceral hypersensitivity is largely attributed to ovarian hormones, particularly estrogen, which appears to be pronociceptive in rodent visceral pain models. Ji et al. (15) found that female rats during proestrus, with elevated levels of estrogen, exhibited increased VMR to colorectal distension compared with metestrus/diestrus when estrogen levels were lower. Moreover, in ovariectomized female rats, estradiol injection that normalizes plasma estrogen levels increased VMR to colorectal distension (16). Estradiol injection in control male rats also increased stress-induced visceral hypersensitivity, likely through modulatory effects on excitatory and inhibitory neural transmissions in the spinal cord (17, 18). Thus, sex hormones, especially estrogen, are likely key factors contributing to the sexual dimorphism of IBS-related visceral pain perception.
Visceral pain perception is predominantly initiated by sensory afferents innervating the large intestine, particularly those innervating the distal colon and rectum (colorectum), also known as colorectal afferents. Extensive research has been conducted on the neural encoding of these afferents and their sensitization, which has been correlated with the development of behavioral visceral hypersensitivity in various IBS-like mouse models (19, 20). Studies have shown that increased peripheral drive from colorectal afferent sensitization is necessary for the persistence of visceral pain in patients with IBS (21–24). However, previous studies have primarily focused on male animals, leaving a gap in our knowledge regarding sex differences in visceral afferent neural encoding and sensitization. This lack of understanding has likely impeded the development of sex-specific treatments for IBS pain.
In this study, we used a recently developed high-throughput optical recording system to systematically characterize afferent neural encoding of male and female mice in both thoracolumbar and lumbosacral pathways in a mouse IBS model (9, 25). Our approach involved intracolonic enema of zymosan, previously established in male mice (19, 26), and functional characterization of afferents by delivering two mechanical stimuli to the attached colorectum and recording GCaMP6f signals from individual dorsal root ganglia (DRG) neurons. Our high-throughput optical recording approach allowed us to unbiasedly characterize colorectal neural encoding from a large number of DRG neurons (2,275 in total) in both male and female mice, revealing sex differences in colorectal afferent encoding and sensitization.
MATERIALS AND METHODS
All experiments were reviewed and approved by the University of Connecticut Institutional Animal Care and Use Committee. All the mice used in the following experiments were aged 8–16 wk and weighed 20–30 g. They were housed in pathogen-free facilities, which are Public Health Service assured and American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited following the Guide for the Care and Use of Laboratory Animals (8th Edition). Mice resided in individual ventilated caging systems in polycarbonate cages (Animal Care System M.I.C.E.) and were provided with contact bedding (Envigo T7990 B.G. Irradiated Teklad Sani-Chips). Mice were fed ad lib with either 2918 irradiated Teklad Global 18% rodent diet or 7904 irradiated S2335 mouse breeder diet supplied by Envigo and supplied with reverse osmosis water chlorinated to 2 ppm using a water bottle. Nestlets and huts were supplied for enrichment. Rodent housing temperature was set for 73.5°F with a range from 70°F to 77°F. Humidity was set for 50% with a range of 35%–65%. Mice were housed with a maximum of 5 animals/cage but were individually housed following surgical procedures, such as fast blue injection or electrode implantation. All animals were housed on a 12:12 light-dark cycle. Animals were observed daily by the animal care services staff. Cages were changed every 2 wk.
Transgenic Mice
The Ai95 mice (C57BL/6 background) carrying heterozygous GCaMP6f gene (Strain No. 28865, The Jackson Laboratory, CT) and homozygous VGLUT2-Cre mice (Strain No. 28863, Jackson Laboratory, CT) were crossbred. The Ai95 mice carry the gene “CAG-GCaMP6f” in the Gt(ROSA)26Sor locus, which is preceded by a LoxP-flanked STOP cassette to prevent its expression. By crossing Ai95 mice with VGLUT2-Cre mice, the Cre-expressing cell population has the STOP cassette trimmed, resulting in the expression of GCaMP6f in glutamatergic neurons expressing type 2 vesicular glutamate transporter (VGLUT2), which made up the vast majority of sensory neurons innervating the colorectum (27, 28). Offspring of both sexes aged 10–20 wk with both heterozygous GCaMP6f and VGLUT2-Cre genes (i.e., VGLUT2/GCaMP6f) were used for optical GCaMP6f recordings.
To characterize the efficiency of VGLUT2-Cre mice in driving expression in colorectal sensory neurons, we crossed the VGLUT2-Cre mice with a tdTomato reporter line (Ai14, Strain No. 7914, The Jackson Laboratory, CT). Offspring with both heterozygous tdTomato (tdT) and VGLUT2-Cre genes, i.e., VGLUT2/tdT mice were used for anatomical tracing studies.
Intracolonic Treatment with Zymosan
To create an IBS-like mouse model of prolonged visceral hypersensitivity, we administered zymosan intracolonically, as described in previous studies (19). Briefly, under isoflurane-induced general anesthesia, we administered 0.1 mL of zymosan (30 mg/mL; Sigma Chemical Company, St. Louis, MO) transanally into the colorectum via a 22-gauge feeding needle with a round tip. After the feeding needle was retracted, the mouse was held in a slightly tilted position with the anus pointing up for 3 min to retain the infused zymosan in the colorectum. Zymosan was infused once per day for three consecutive days (days 0, 1, and 2). Mice receiving intracolonic saline infusion were used as a control throughout the study. Experimenters conducting the subsequent experiments are blinded to the intracolonic treatments.
Behavioral Visceromotor Responses to Colorectal Distension
The procedure for conducting the visceromotor responses (VMR) to colorectal distension (CRD) was reported in detail in prior studies (19, 29). Briefly, mice were anesthetized with 1%–3% isoflurane (Hospira, Inc., Lake Forest, IL). The lower abdomen and back neck of the mice were shaved and scrubbed. The left side of the abdominal muscle was exposed by a skin incision (∼1 cm). A pair of sterile stainless-steel wires (Cooner Wire Company, Chatsworth, CA) were sutured onto the muscle, tunneled subcutaneously, and externalized at the nape of the neck. For postoperative analgesia, meloxicam (2 mg/kg, Boehringer Ingelheim Vetmedica, Duluth, GA) was given three times in 72 h. Mice were ear-tagged and housed separately to avoid the damage of the exteriorized leads by cage mates.
One week after surgery, VMR baseline was recorded on day 0. Mice were anesthetized with isoflurane inhalation (1%–3%). A lubricated polyethylene balloon (1.5 cm long, Ø 0.9 cm) was inserted transanally. Mice were then placed in a three-dimensional (3-D)-printed dark plastic cylinder to limit their movement. Once the mice were recovered from the anesthesia, electromyographic (EMG) activity was recorded from the implanted muscle electrodes for 10 s before (resting) and during graded colorectal distension (15, 30, 45, or 60 mmHg pressure steps of 10 s duration). Each pressure step was tested three times within a 4-min period. At least 60-s intervals were given between successive distensions. Visceromotor response was quantified as the total area under the curve (AUC) of rectified EMG signal during colorectal distension minus the resting EMG signal.
After the baseline VMR response to CRD on day 0 was established, each mouse was treated with intracolonic saline (as control) or zymosan on days 0, 1, and 2, and then reassessed with VMR to CRD for behavioral visceral hypersensitivity at six additional time points (days 3, 6, 10, 13, 17, and 20). Experimenters conducting the VMR to CRD were blinded to the intracolonic treatments.
Vaginal Smear
To determine the estrous phase of the mouse, female mice were sedated with isoflurane inhalation (1%) between 10:00 AM and 12:00 PM on the day of VMR recordings. The vagina was flushed with saline five times using a pipette, and the vaginal smear was transferred onto a diamond white glass slide (Globe Scientific, Inc., Mahwah, NJ) and covered with a thin glass coverslip (Corning, Inc., Corning, NY). A binocular dark field microscope (AmScope) at ×10 magnification was used to view and photograph cells from the vaginal smear, which were used to identify the estrous phase of the mouse. The proestrus and estrus phases (Pro/Es) were characterized by the appearance of nucleated epithelial cells and cornified epithelial cells, respectively, whereas the metestrus and diestrus phases (Met/Di) were characterized by small leukocytes (30). For each female mouse, a vaginal smear was conducted on the same day of every behavioral CRD test to ensure that the tests were conducted exclusively during the Met/Di phases of the estrous cycle. Repeated CRD tests were scheduled every 3 days to align with the mouse estrous cycle, which ranges from 3 to 6 days (30). If a mouse was identified to be in the Pro/Es phase, the subsequent CRD test was delayed by 24 h, ensuring the mouse was in the Met/Di phases.
Anatomic Tracing of Colorectal DRG Neurons
Under anesthesia via isoflurane inhalation (2%), the colon of VGLUT2/tdT mice was exposed by laparotomy, and a fluorescent retrograde tracer fast blue (FB, No. NC0182483, Fisher Scientific, East Greenwich, RI) was injected into the colon wall (1 mg/100 µL in sterile saline, 10 µL total distributed into 2–4 sites) using a syringe with a 33-gauge needle (model 7643-01, Hamilton Company, Arlington, MA). The peritoneal cavity was rinsed with sterile saline to remove extra leaking before the muscle and skin were sutured in layers. To alleviate pain and distress, meloxicam (2 mg/kg, Boehringer Ingelheim Vetmedica, Duluth, GA) was given with intraperitoneal injection three times in 72 h for postoperative analgesia.
VGLUT2/tdT mice were euthanized via CO2 inhalation. Thoracolumbar (T12 to L2) and lumbosacral (L6 to S1) DRGs were harvested and fixed with 10% formalin solution (Sigma-Aldrich). After cryoprotection in 20% sucrose overnight, DRGs were embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned at 25-μm thick. The tissue section was imaged by a confocal fluorescent microscope (A1R, Nikon, Inc., Japan) for red (tdTomato) and blue (fast blue) fluorescent signals. Details on the quantification of positive fluorescent signals were reported previously (9). Briefly, fast blue-containing and tdTomato-expressing DRG neurons were identified and quantified from 8-bit grayscale staining images. The images were set with a threshold that excluded at least 99.99% of background intensity based on its Gaussian distribution using ImageJ, version 1.53t (31). The threshold value was calculated from the standard normal distribution (Z) equation: threshold = 3.72 SD + m, where 3.72 is the Z-score at P = 0.9999, SD and m are the standard deviation and mean of the background intensities in 8-bit grayscale (0–255), respectively.
Ex Vivo Preparation of Colorectum-Spinal Nerves-DRG in Continuity
Mice receiving intracolonic zymosan or saline (as control) treatment were euthanized between day 14 and 18 for ex vivo GCaMP6f recordings in an ex vivo preparation that includes the distal colorectum, lumbar splanchnic nerve (LSN), pelvic nerve (PN), and T12 to S1 DRGs in continuity shown in the schematic in Fig. 1A. Mice of the correct genotype (VGLUT2/GCaMP6f), between 10 and 20 wk of age, were deeply anesthetized by intraperitoneal injection of a 0.4-mL cocktail of ketamine (120 mg/kg) and xylazine (10 mg/kg). Mice were then euthanized by perfusion from the left ventricle with modified ice-cold Krebs solution replacing sodium chloride with an equal molar of sucrose (in mM: 236 sucrose, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, 20 acetate, 0.004 nifedipine, and 0.003 indomethacin) bubbled with carbogen (95% O2-5% CO2), which suppresses neural activities during dissection. A dorsal laminectomy was performed to expose the thoracic and lumbar spinal cord. The colorectum with attached T12, T13, L1, L2, S1, L6, and L5 spinal nerves and DRGs were then dissected via blunt dissection, as shown in Fig. 1B, and transferred to a tissue chamber that was perfused with 32°C–34°C Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, 20 acetate, 0.004 nifedipine, and 0.003 indomethacin), consistent with prior ex vivo studies on colorectal afferents (9, 25, 32, 33). The colorectum was cannulated and connected to a custom-built distending device, consisting of computer-controlled fluid valves and hydrostatic pressure columns of 15, 30, 45, and 60 mmHg, filled with PBS, as illustrated in Fig. 1A.
Figure 1.
High-throughput functional characterization of colorectal afferents via ex vivo GCaMP6f optical recordings from intact DRG. A: schematic of the ex vivo preparation with the mouse distal colon and rectum (colorectum), nerve, and DRG in continuity. GCaMP6f signals from individual colorectal afferents were recorded from four thoracolumbar DRGs (T12, T13, L1, and L2) and three lumbosacral DRGs (L5, L6, and S1). Afferent endings were stimulated by stepped pressure distension and mucosal shear flow of the cannulated colorectum. B: photograph of the ex vivo preparation with colorectum, nerve, and DRG in continuity. C: representative GCaMP6f signals recorded from a colorectal afferent neuron in L2 DRG (indicated by the dark circle) that responded to both graded colorectal distension and mucosal shear flow. D: functional classification of colorectal afferents into four classes based upon their response profiles to colorectal distension and mucosal shear flow. DRG, dorsal root ganglia; IMG, inferior mesenteric ganglia; MPG, major pelvic ganglia; MS, mechanosensitive.
Optical Recording of Evoked Fluorescent GCaMP6f Signal
To capture evoked fluorescent GCaMP6f signal, we used an upright microscope platform (BX51WI, Olympus, Waltham, MA) equipped with a water immersion ×10 objective (UMPLFLN 10XW, 0.3 NA) and a regular halogen epi-illumination light source to image a single DRG entirely. The blue light delivered via the ×10 objective is focused on a ∼ϕ1.5 mm area sufficient to cover one mouse DRG while sparing DRGs in adjacent vertebral segments. A high-speed ultra-low noise sCMOS camera (Xyla-4.2P, 82% quantum efficiency, Andor Technology, South Windsor, CT) was used and controlled by Micro-Manager, an open-source microscopy software sponsored by the National Institutes of Health (34). Using this recording system, high-resolution images (1,920 × 1,920, 2 × 2 bin) were captured at 100 frames/s with a spatial resolution of 1.6 pixels/µm, which was sufficient to resolve individual DRG neurons, as shown in Fig. 1C.
An integrated routine was developed for automated detection of GCaMP6f signals. Image stacks were first aligned to remove the motion of DRG during the recording session using ImageJ plugin. We then used an image analysis algorithm, based on our previously reported procedure (9, 25), to automatically determine activated DRG neurons that had evoked APs (i.e., Ca2+ transients) by analyzing intensity fluctuations (over 3%) across different frames (200 ms) poststimulation. To locate different cells, we used marker-based watershed segmentation (35), which allowed us to identify GCaMP6f Ca2+ signals in multiple DRG neurons. With this approach, we were able to functionally characterize colorectal DRG neurons in intact T12 to S1 DRGs and locate them with high efficiency.
Afferent Identification and Classification
To identify and classify afferents, we followed a previously reported protocol (9, 25). First, we activated mouse colorectal afferents using two physiologically correlated stimuli at the colorectum: stepped luminal distension by a hydrostatic fluid column of phosphate-buffered saline (PBS) at 15, 30, 45, and 60 mmHg for 5-s steps, and luminal shear flow of PBS at 20–30 mL/min. For each mouse, we captured calcium images from all seven DRGs while applying either stepped distension or shear flow stimulation to the mouse colorectum. To minimize off-target stimulation, we alternated the recording sequence between the four TL and three lumbosacral (LS) DRGs. A minimum interval of at least 10 min was allowed between recordings from successive DRGs. Colorectal DRG neurons were functionally classified into four classes following our previously reported criteria (9, 25), as illustrated in Fig. 1D low-threshold muscular (LT-muscular), high-threshold muscular (HT-muscular), mucosal, and muscular-mucosal (mus-muc) classes. The afferent classification criteria are detailed in Ref. 25 and summarized as follows. LT-muscular afferents responded to all four distension pressure levels, whereas HT-muscular afferents only responded to noxious distension pressure (30, 45, or 60 mmHg). We considered colorectal intraluminal pressure beyond 20 mmHg noxious to mice based on previous studies (13, 36). Mucosal afferents did not respond to distension but responded to luminal shear flow, whereas mus-muc afferents responded to both luminal shear flow and colorectal distension at all four pressure levels.
Data Recording and Analysis
VMRs to CRD were quantified, following a published protocol (29). Briefly, electromyographic (EMG) activity was recorded from the peritoneal musculature, amplified, and rectified. The rectified EMG was quantified as area under the curve (AUC) using the modulus program in the Spike 2 software v7 [Cambridge Electronic Design (CED), Cambridge, UK]. Responses to CRD were taken as the difference in EMG during distension minus the resting EMG (recorded in the 10-s period before the four distension steps), normalized as a percentage of the baseline response to 60 mmHg CRD. Normalized VMR recorded at multiple time points posttreatment (saline or zymosan) were compared with the baseline VMR by two-way ANOVA with repeated measures and Bonferroni post hoc comparison. Comparisons were conducted between the VMR recorded before (baseline) and after treatment with either saline or zymosan. GCaMP6f signals were extracted in the form of pixel intensity (0–255) and calculated as the average intensity from individual DRG neurons. The responses of individual neurons were normalized by the prestimulus intensity. Responsive GCaMP6f transients were identified when the signal increased by 3% within 200 ms. Neurons with positive responses were classified into four subclasses following the criteria described in Fig. 1D earlier. A χ2 test was performed to compare the distributions of afferents between control and zymosan-treated mice or between sexes. Student’s t tests were applied to compare saline- and zymosan-treated groups. All statistical tests were performed using SigmaPlot v11.0 (Systat Software, Inc., San Jose, CA). P < 0.05 was considered significant.
RESULTS
Zymosan Induced Persistent Colorectal Hypersensitivity in Male but Not in Female Mice
A previous study demonstrated a reduced behavioral visceromotor response (VMR) to colorectal distension (CRD) during the metestrus/diestrus phases (Met/Di) with lower levels of estrogen as compared with the proestrus and estrus phases (Pro/Es) with higher estrogen levels (15). To eliminate the confounding factor of estrous cycle, we recorded VMR to CRD only in female mice during the Met/Di phases. The estrous cycle of each female mouse was determined by vaginal smear (Supplemental Fig. S1). The Pro/Es phases are characterized by the appearance of nucleated epithelial cells and cornified epithelial cells, respectively. The Met/Di phases are characterized by small leukocytes (30).
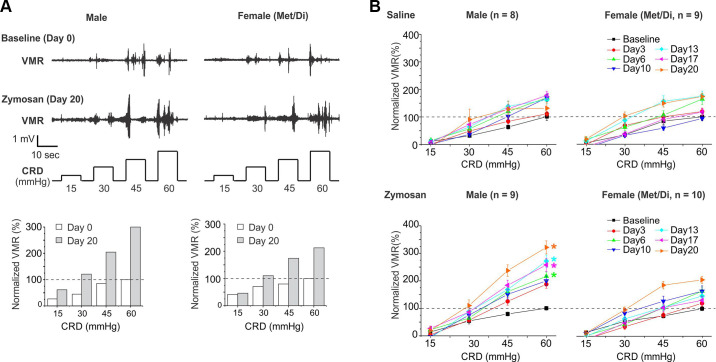
We assessed VMR to CRD at seven time points before (day 0) and after (days 3, 6, 10, 13, 17, and 20) intracolonic treatment for each of the following four groups of mice: saline-treated male (8 mice), zymosan-treated male (9 mice), saline-treated female (9 mice), and zymosan-treated female (10 mice). Displayed in Fig. 2A are typical EMG signals to CRD recorded from mice pre- (day 0) and postzymosan treatments (day 20). Displayed as bar graphs are quantified VMR by calculating the AUC of the rectified EMG signals minus the resting EMG. The baseline VMR to CRD recorded from 17 male and 19 female mice at day 0 were plotted in Supplemental Fig. S2, which showed no significant differences between sexes (two-way ANOVA, P = 0.43). To facilitate comparison of VMR recorded at multiple time points, the VMR is normalized by the baseline VMR at 60 mmHg on day 0, i.e., 100%. As shown in Fig. 2B, intracolonic saline treatment did not alter the VMR to CRD in either male (two-way ANOVA, F6,126 = 0.288, P = 0.936, male baseline vs. male postsaline) or female (two-way ANOVA, F6,144 = 1.132, P = 0.297, female baseline vs. female postsaline) groups, as compared with the baseline VMR recorded on day 0. In zymosan-treated male mice, VMR was significantly increased on days 6, 13, 17, and 20 posttreatment as compared with the baseline VMR (two-way ANOVA, F6,144 = 8.260, P < 0.05, Bonferroni post hoc comparison, P < 0.05 vs. male baseline). However, zymosan treatment in female mice showed no significant effect on the VMR to CRD (two-way ANOVA, F6,162 = 0.0344, P = 0.854, female day 0 vs. female postzymosan).
Figure 2.
Sex differences in zymosan-induced behavioral visceral hypersensitivity as measured by visceromotor responses (VMR) to colorectal distension (CRD). A: representative EMG signal traces that were recorded from mice of both sexes before (day 0) and after zymosan injection (day 20). The EMG were quantified, normalized by the response at 60 mmHg on day 0, and shown in the bar graphs. B: VMR to CRD recorded from mice receiving intracolonic saline (as control) and zymosan treatment of both sexes. To avoid the confounding effects of sex hormones, female mice were studied exclusively in Met/Di phases. Intracolonic zymosan produced long-lasting behavioral visceral hypersensitivity, as evidenced by enhanced VMR to CRD only in male mice but not in female mice. Intracolonic saline resulted in no apparent changes in VMR in either sex. *Significant difference (P < 0.05) from the baseline VMR recorded on day 0 (two-way ANOVA). EMG, electromyographic; Met/Di, metestrus and diestrus.
Validation of VGLUT2 Expression in Colorectal Sensory Afferents
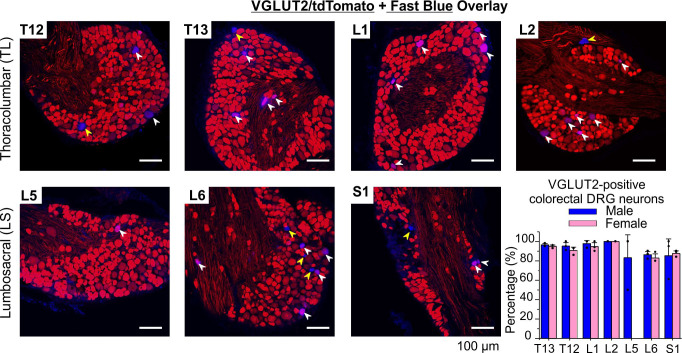
To assess the efficiency of Cre-driven expression of VGLUT2 in colorectal neurons, we conducted retrograde tracing by injecting the tracer FB into the colorectal wall of VGLUT2/tdT mice. We displayed typical images from thoracolumbar (TL, T12, to L2) and lumbosacral (LS, L5, to S1) DRGs in Fig. 3 (red for tdTomato, blue for FB), along with the percentage of VGLUT2-positive colorectal neurons summarized in the bar graph. All tissue sections in each DRG were analyzed (18 to 36 sections each), and special care was taken not to double count the FB-positive neurons from adjacent sections. In three male mice, FB-positive neurons were detected in 41, 60, 66, 4, 16, 88, and 44 sections of T12, T13, L1, L2, L5, L6, and S1 DRGs, respectively. A total of 1,149 FB-positive neurons were detected, of which 1,029 neurons were VGLUT2-positive. The fractions of VGLU2-positive neurons in FB-positive colorectal neurons in each DRG group (T12 to L1, total 1,029/1,149) are 114/118, 205/221, 214/226, 5/5, 28/29, 401/477, and 62/73. Similarly, in three female mice, FB-positive neurons were detected in 60, 62, 56, 9, 0, 58, and 35 sections of T12 to S1 DRGs. The fractions of VGLUT2-positive neurons in FB-positive colorectal neurons in each DRG group (T12 to L1, total 725/801) are 54/57, 135/150, 141/148, 35/35, 0/0, 220/251, and 140/160. As displayed in the bar graph in Fig. 3, our findings revealed no sex-associated differences in the proportion of VGLUT2-positive colorectal DRG neurons in each DRG (Fisher’s exact test). Specifically, the percentages were 96.6% versus 94.7% in T12 (P = 1), 92.8% versus 90.0% in T13 (P = 0.88), 94.7% versus 95.3% in L1 (P = 1), 100.00% versus 100.00% in L2, 84.1% versus 87.7% in L6 (P = 0.73), and 84.9% versus 87.5% in S1 (P = 0.92). A sex-related comparison was not conducted for L5 DRG, as no FB-positive neurons were observed in female mice in that region.
Figure 3.
Validation of VGLUT2 expression (labeled by tdTomato) in colorectal DRG neurons labeled by retrograde injection of a neural tracer fast blue (FB) into the colorectal wall. Merged images of blue (FB) and red channels (tdTomato) showed VGLUT2-positive (white arrowhead) and VGLUT2-negative (yellow arrowhead) colorectal DRG neurons. Bar graph displays the proportions of VGLUT2-positive neurons in FB-labeled colorectal neurons in four thoracolumbar (T12, T13, L1, and L2) and three lumbosacral DRGs (L5, L6, and S1). DRG, dorsal root ganglia.
Significant Sex Differences in the Distribution of Colorectal Mechanosensitive Afferents in TL and LS Innervations
To specifically identify mechanosensitive afferents, we used an ex vivo imaging method and have identified 619 and 633 mechanosensitive (MS) afferent neurons that were activated by either mechanical colorectal distension or shear flow or both in the DRG from 16 male and 14 female control mice, respectively, which were distributed in seven vertebral segments (Fig. 4A). Our results showed that in male colorectum innervations, there were 59.1% afferents identified in the L5 to S1 DRGs (LS pathway) and 40.9% afferents identified in the T12 to L2 DRGs (TL pathway), whereas a significantly higher proportion of LS afferents (69.9%) and a lower proportion of TL afferents (30.1%) (Fisher’s exact test, P < 0.01, control male vs. control female) were identified in female. In zymosan-treated visceral hypersensitive mice, we identified 538 MS afferents from 10 male colorectums and 485 afferents from 11 female colorectums, and the vertebral distribution was plotted in Fig. 4B. Similar to the control groups, female mice also showed a significantly higher proportion of LS afferent counts than the male counterparts in zymosan-treated groups (Fisher’s exact test, P < 0.01, zymosan male vs. zymosan female). Zymosan treatment resulted in a significant increase in the proportion of TL afferents and a complementary decrease in the proportion of LS afferents only in male mice (χ2 test, P < 0.01, control male vs. zymosan male), whereas in female mice, the proportion remained unchanged between the control and zymosan-treated groups (χ2 test, P = 0.1, control female vs. zymosan female).
Figure 4.
Sex differences in the distribution of mechanosensitive (MS) colorectal afferents identified from 2,275 neurons in thoracolumbar (TL, T12, to L2) and lumbosacral (LS, L5, to S1) DRGs. A: distribution of MS colorectal afferents in seven TL and LS DRGs in control male and female mice. Male colorectum exhibited a higher proportion of TL innervation compared with female colorectum. B: distribution of MS colorectal afferents in seven TL and LS DRGs in male and female mice receiving intracolonic zymosan treatment. In male mice, the proportion of TL innervation was further increased in the zymosan-treated group as compared with the control group. However, in female mice, there was no significant difference in the afferent distributions between control and zymosan groups. C: box-and-whisker plots showing the average number of colorectal afferents identified from each DRG in control and zymosan-treated groups of both sexes. +Outliers exceeding 1.5 times the interquartile range from the first and third quartiles. *P < 0.05 between control and zymosan groups; †P < 0.05 between male and female groups (χ2 test for A and B and t test for C). DRG, dorsal root ganglia; LS, lumbosacral; TL, thoracolumbar.
The average number of MS colorectal afferent neurons identified from each of the four groups of mice (control or zymosan-treated mice of both sexes) is displayed as box-and-whisker plots in Fig. 4C. In control groups, the number of afferents identified from each mouse showed no significant differences between sexes (blue and red, Fig. 4C; two-way ANOVA, F1,196 = 1.73, P > 0.05, control male vs. control female). In zymosan-treated groups, the number of afferents identified from each vertebral DRG differed significantly between male and female mice (green and magenta, Fig. 4C; two-way ANOVA, F1,133 = 5.74, P = 0.02, male vs. female). Specifically, more colorectal neurons were identified from male T13, L1, and L2 DRGs than from the female counterparts (t test, P < 0.05, zymosan male vs. zymosan female), whereas more neurons were identified from female S1 DRG than from the male counterpart (t test, P < 0.05, zymosan male vs. zymosan female). Zymosan treatment resulted in a significantly higher number of colorectal neurons identified from L1 and L2 DRGs and a lower number of neurons from S1 DRG in male mice (t test, P < 0.05, control male vs. zymosan male at L1, L2, and S1). In contrast, the number of colorectal neurons identified from female mice did not differ between the control and zymosan-treated groups (two-way ANOVA, F1,161 = 1.36, P > 0.05, control female vs. zymosan female).
Sex Differences in Zymosan-Induced Changes in Four Functionally Classified Colorectal Afferent Classes
As shown in Fig. 1D, we identified MS afferents from both TL (TL, T12, to L2) and LS (LS, L5, to S1) DRGs, and classified them into mucosal, mus-muc, LT-muscular, and HT-muscular subclasses based on their response profiles to graded colorectal distension and luminal shear flow. In control mice, mucosal afferents showed differential distributions between male and female groups (Fig. 5A1), with a higher proportion of TL innervation in male mice than in female mice (χ2 test, P < 0.05, control male vs. control female). Zymosan treatment increased the proportion of TL mucosal afferents only in male mice (χ2 test, P < 0.05, control male vs. zymosan male) but not in female mice (χ2 test, P = 0.317, control female vs. zymosan female). Figure 5A2 showed comparable distributions of mus-muc afferents between sexes in control groups (χ2 test, P = 0.107, control male vs. control female) but shifted toward a higher proportion of TL afferents in zymosan-treated groups of both sexes (χ2 test, P < 0.05 control vs. zymosan in male and P < 0.05 in female). As shown in Fig. 5A3, the proportions of LT-muscular afferents in control mice showed a higher proportion in the TL pathway in male mice than in female mice. In zymosan-treated groups, the TL proportion of LT-muscular afferents was significantly increased in male mice as compared with control male mice (χ2 test, P < 0.05, control male vs. zymosan male) but remained unchanged in female mice. The proportions of HT-muscular afferents in Fig. 5A4 displayed similar trends as of aforementioned LT-muscular afferents, also showing an increased proportion of TL afferents in zymosan-treated male mice (χ2 test, P < 0.05, control male vs. zymosan male) but not in zymosan-treated female mice.
Figure 5.
Distribution of four functionally classified colorectal afferent classes in control and zymosan-treated mice of both sexes. A: distribution of the four afferent classes in the seven TL and LS DRGs: mucosal (A1), mus-muc (A2), LT-muscular (A3), and HT-muscular (A4) afferents. The number of a particular afferent class identified per mouse are presented as box-and-whisker plots for mucosal (B), mus-muc (C), LT-muscular (D), and HT-muscular afferents (E). +Outliers exceeding 1.5 times the interquartile range from the first and third quartiles. *P < 0.05 between control and zymosan groups; †P < 0.05 between male and female groups (χ2 test for A and t test for B–E). ctl, control group; DRG, dorsal root ganglia; F, female mice; HT, high-threshold; LS, lumbosacral; M, male mice; TL, thoracolumbar; zy, zymosan group.
The box-and-whisker plot in Fig. 5B summarizes the number of mucosal afferents identified per animal, indicating a significantly higher number of mucosal afferents from T12, L1, and L2 DRGs in zymosan-treated male mice than in control male mice (t test, T12, P < 0.1; L1, P < 0.05; L2, P < 0.05, control male vs. zymosan male). Conversely, the number of mucosal afferents from S1 DRG in the zymosan group was significantly lower than in the control group (t test, P < 0.05, control male vs. zymosan male). However, there was no significant difference in the number of identified mucosal afferents per animal between control and zymosan-treated female mice. For the number of mus-muc, LT-, and HT-muscular afferents identified per animal in the box-and-whisker plots of Fig. 5, C, D, and E, there was a similar trend as in the mucosal afferents toward an increased number of afferents in TL DRGs and a decreased number in S1 DRG in zymosan-treated male mice, as compared with control male mice, but these differences were not significant.
Sex Differences in the Proportions of the Four Colorectal Afferent Classes
Figure 6 presents the proportions of the four functionally classified colorectal afferent classes in TL (Fig. 6A) and LS afferents (Fig. 6B). There were no significant sex differences in the proportions of afferent classes in the TL pathway between control male and female mice (Fig. 6A), but zymosan-treated male and female mice showed a significant difference (χ2 test, P < 0.05, zymosan male vs. zymosan female). In LS pathway in Fig. 6B, there were no significant differences in the proportions of afferent classes between sexes in either control or zymosan-treated groups.
Figure 6.

Proportions of four functionally classified colorectal afferent classes in TL and LS innervation pathways. The proportions of TL afferents and LS afferents from control and zymosan-treated groups are displayed in A and B, respectively. †P < 0.05 between male and female groups. LS, lumbosacral; TL, thoracolumbar.
Topological Distribution of Colorectal MS Afferent Neurons in TL and LS DRGs
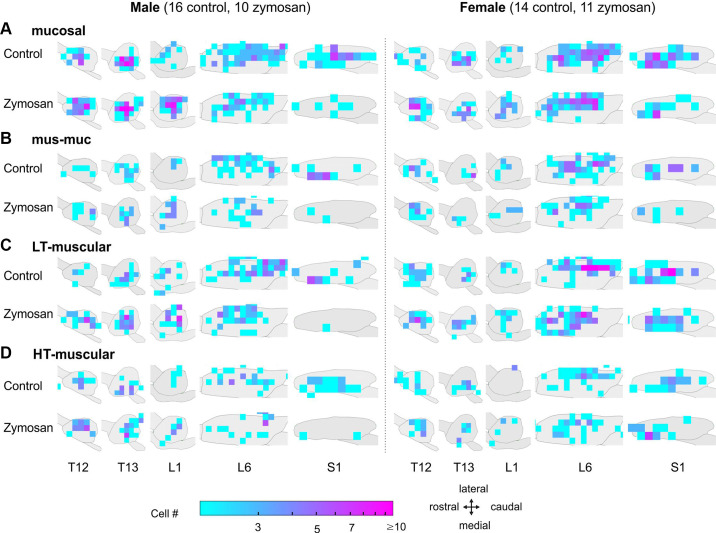
The topological distribution of colorectal MS afferent neurons in TL and LS DRGs is presented in Fig. 7. Our optical approach, which extracts both the spatial locations of each colorectal afferent somata in the DRG and their activity-evoked calcium transients, allows for a topological distribution assessment as previously reported (9). We excluded L2 and L5 DRGs from the analysis due to the small number of neurons identified. The contours of each DRG were consistent with the optical recordings of DRGs on the right side of the animal from a dorsal orientation. We used logarithmic heatmaps to indicate the densities of the colorectal DRG neurons, with red indicating a dense area and blue indicating a scattered area. In TL DRGs, colorectal DRG neurons were distributed throughout all regions and did not concentrate in specific areas. In zymosan-treated male mice, there was an increased number of TL neurons compared with control male mice, especially for the HT-muscular, LT-muscular, and mucosal afferents. In LS DRGs, colorectal neurons clustered toward the caudal region in the L6 DRG and toward the rostral region in the S1 DRG, particularly for LT-muscular afferents in both sexes. The topological distribution of colorectal afferents in S1 DRG showed an apparent sex difference with more dense areas in female S1 DRG than in male S1 DRG in the LT-muscular, HT-muscular, and mucosal afferent classes. However, the small number of neurons in each grid in Fig. 7 precludes a thorough statistical comparison between groups.
Figure 7.
Topological distributions of four classes of colorectal afferent somata in TL (T12, T13, and L1) and LS (L6, S1) DRGs: mucosal afferents (A), mus-muc afferents (B), LT-muscular afferents (C), and HT-muscular afferents (D). DRG, dorsal root ganglia; HT, high-threshold; LS, lumbosacral; TL, thoracolumbar.
DISCUSSION
In this study, we used high-throughput optical recordings to functionally characterize a large number of afferent neurons (2,275) innervating the mouse distal colon and rectum (colorectum), which is relatively less innervated by extrinsic afferents compared with cutaneous tissues and is difficult to study by conventional electrophysiological recordings from axons or somata in the DRG. Afferent innervation of the colorectum was previously studied mostly via ex vivo single-fiber recordings from the dissected colon with attached nerves, and these results form our current fundamental understanding of the functional role of colorectal afferents in normal gastrointestinal physiology and pathophysiological conditions, such as visceral hypersensitivity (19, 20, 32, 33, 37–39). Because of the sparse presence of colorectal neurons in the DRG, functional recording of colorectal afferents by perforating the intact DRG with glass pipette electrodes is technically challenging and thus were reported only by a handful of studies (40, 41). Alternatively, colorectal afferents in the DRG were more widely studied by patch-clamp electrophysiological recordings from dissociated DRG neurons retrogradely labeled with injected tracers in the colorectum, e.g., see Refs. 42, 43, and 44. These studies provide valuable information on the membrane ionic properties at afferent somata, but the membrane properties measured in dissociated somata do not directly translate to afferent neural encoding at the sensory nerve endings, where the membrane ionic channel densities differ greatly from the somata (45). In the current study, we implemented GCaMP6f recordings from intact ganglia in an ex vivo preparation with excised colorectum, nerve, and DRG in continuity. This high-throughput approach, which we established recently, enables focused functional characterization of a large number of colorectal afferents in the absence of confounding factors from in vivo recordings (9, 25).
We acknowledge that the fluorescent optical signals recorded from genetically encoded calcium indicators, such as GCaMP, are not reliable predictors of sensory neural spiking, as indicated by a recent study combining GCaMP and patch-clamp techniques on dissociated sensory ganglion neurons (46). Therefore, we did not use the recorded GCaMP6 signals to quantify colorectal afferent spiking frequency in our study. Instead, we used them as a salient indicator of whether or not the afferent responded to specific stimuli applied to the attached colorectum, namely four levels of stepped colorectal distension ranging from innocuous (15 mmHg) to noxious intraluminal pressure (45–60 mmHg) and mucosal shear flow. The use of these two mechanical stimuli limited the characterization of only mechanosensitive colorectal afferents in this study. Mechanically insensitive afferents that also innervate the colorectum were systematically characterized in a previous study using a nonbiased electrical search strategy with the colorectum cut open and pinned flat (32). Since mechanical stimuli, particularly distension of hollow visceral organs, are the most reliable modalities to evoke noxious perception from the viscera (see Ref. 47 for a review), we focused this study on characterizing the mechanosensitive afferents in both control and visceral hypersensitive mice.
In previous studies by us and others, mice receiving intracolonic zymosan treatment were considered a model of IBS, featuring weeks-long behavioral visceral hypersensitivity with increased VMR to CRD in the absence of apparent tissue damage or inflammation (19, 26, 48). It is worth noting that all the above studies were conducted on male mice. The current study is the first to report an apparent sex difference in zymosan-induced behavioral visceral hypersensitivity, with a robust increase in VMR observed in male mice, whereas no significant increase was found in female mice following zymosan treatment. To avoid the confounding effect of varying levels of ovarian hormones, VMR to CRD was recorded in the Met/Di phases of the female mice when estrogen levels were less variable, and the magnitude of VMR to CRD was lower than in the proestrus/estrus phase, based on a prior study on rats (15). Despite the low baseline level of VMR recorded in the Met/Di phases, sensitization of VMR following zymosan treatment was still absent in female mice. We speculate that the absence of enhanced VMR to CRD in female mice is likely due to the greater level of the ovarian hormone estrogen in females than in males. Indeed, estrogen decreases colonic permeability by upregulating the expression of the tight junction proteins occluding and junctional adhesion molecule (JAM)-A in epithelial cells via the ERβ-mediated signaling pathway (49, 50). In further support, a study in rats showed that the female small intestine was more resistant to gut injury and inflammation than the male small intestine (51). Thus, peripheral irritants alone may not be a reliable means of evoking visceral hypersensitivity in female mice. IBS is a multifactorial syndrome that involves centrally mediated factors like stress, anxiety, and depression (52), which women are more vulnerable to than men (53–56). Therefore, developing IBS models with female rodents should incorporate stress components like material separation stress or water avoidance stress, which reportedly induce robust behavioral visceral hypersensitivity (57, 58). Furthermore, this study highlights the limitations of current rodent models in accurately reproducing the clinical sex differences observed in patients with IBS. Thus, caution must be exercised when extrapolating findings from rodent studies to human IBS conditions.
In this study, 2,275 MS colorectal afferent somata were functionally characterized with distribution across seven thoracolumbar (T12 to L2) and lumbosacral DRGs (L5 to S1), which is consistent with prior anatomic tracing studies (59, 60). The LS innervation was predominantly concentrated in the L6 DRG in both control and zymosan-treated groups and in both sexes. Previous reports using sharp glass electrodes to conduct intracellular recordings from colorectal DRG have also focused on L6 DRG (40, 41). The MS colorectal afferents were more evenly distributed in the T12, T13, and L1 DRGs in the TL innervation pathway, which is consistent with prior anatomic studies (60). Interestingly, female mice had a significantly higher proportion of LS colorectal afferents compared with male mice (70% vs. 59.1%). This sex difference has not been reported previously. The sex difference was more apparent in mouse groups receiving zymosan treatment, where male mice receiving zymosan showed a significantly higher proportion of TL afferent innervation compared with control male mice. In contrast, female mice had comparable proportions of innervation between control and zymosan groups. The increased TL proportion in the male zymosan group was mainly due to the increased number of TL afferents identified from T13, L1, and L2 DRG, whereas the afferent number in L6 DRG remained unchanged between control and zymosan groups. This indicates that zymosan treatment led to the recruitment of previously unresponsive afferents in male mice to mechanical colorectal distension or luminal shear flow, i.e., the sensitization of colorectal afferents in the TL pathway. This finding is consistent with a previous study indicating significant sensitization of TL afferents in male rats following intracolonic treatment of trinitrobenzene sulphonic acid (TNBS) (61). In contrast, the comparable proportions of TL afferents between the control and zymosan groups in female mice do not support an apparent sensitization of TL afferents. This sex difference in afferent sensitization is also consistent with the zymosan-induced increase in VMR to CRD only in male mice but not in female mice (Fig. 2B). Therefore, peripheral afferent sensitization appears to closely correlate with the behavioral visceral hypersensitivity in rodent IBS models, which is consistent with prior reports of sensitized stretch-sensitive afferents and recruitment of mechanically insensitive afferents in visceral hypersensitive mice induced by intracolonic treatment of zymosan (19), TNBS (20), and acid hypertonic solution (62).
The MS colorectal afferents were functionally classified based on their response profiles to two physiologically correlated stimuli, as previously reported: colorectal distension and mucosal shear flow (9, 25). Colorectal distension at 15 mmHg intraluminal pressure and mucosal shear are considered innocuous stimuli in mice, whereas distension beyond 20 mmHg (30, 45, and 60 mmHg) are nociceptive and sufficient to evoke nocifensive responses (13). HT-muscular afferents that respond exclusively to nociceptive stimuli are thus the putative colorectal nociceptors, which make up 13% to 19% of all MS afferents characterized in the current study in TL and LS innervations of both sexes. This proportion is consistent with prior reports of 14% to 25% HT stretch-sensitive afferents in visceral organ innervations (36, 63). In addition, the LT-muscular afferents and mus-muc afferents encode the noxious distension range and are also likely to participate in visceral nociception. Compared with prior reports from single-fiber recordings (32, 39), we reported a higher proportion of mucosal and mus-muc afferents, especially in the TL innervation pathway in the current study. This difference is likely due to different experimental conditions. In the prior single-fiber recordings, the colorectum was cut open and pinned flat, and mucosal shear was delivered by fine stroking with a 10-mg force brush, while in the current study, mucosal shearing was delivered more physiologically by saline perfusion through the tubular colorectum. Zymosan treatment resulted in a significantly higher proportion of TL afferents in all four afferent classes in male mice. Interestingly, in the male zymosan group, the number of mucosal afferents that detect innocuous mucosal shearing was significantly increased in the TL innervation pathway compared with the control group. The exact role of the increased number of nonnociceptive mucosal afferents following zymosan treatment in male mice awaits further investigation.
The topological distribution of colorectal afferent somata identified in the current study spreads throughout all recorded DRG without distinctive clustering in specific regions, consistent with prior anatomic tracing studies (28, 64). In the male zymosan group, compared with the control group, an increased number of mucosal, LT-muscular, and HT-muscular afferents is apparent in the three TL DRGs, whereas the number and distribution of TL colorectal afferents are comparable between female control and zymosan groups. Mucosal, LT-muscular, and HT-muscular afferents slightly concentrate toward the caudal region of the L6 DRG in control mice of both sexes, and the distribution appears to shift toward the rostral regions in zymosan-treated groups. Another sex difference is the more densely distributed colorectal afferents in female S1 DRG than in the male counterpart for mucosal, LT-muscular, and HT-muscular afferents, particularly in the zymosan-treated groups. The overall topological distribution reported by the current study strongly suggests that targeting afferent innervations at the DRG for treating visceral pain should consider potential sex differences, such as focusing on the TL DRG in men and the LS DRG, especially the distal sacral DRG, in women.
Conclusions
In this study, we conducted functional characterization of 2,275 mechanosensitive colorectal afferents from VGLUT2/GCaMP6f mice using high-throughput GCaMP6f recordings from intact thoracolumbar and lumbosacral DRGs in an ex vivo preparation of colorectum, nerves, and DRGs in continuity. Our findings showed significant sex differences in zymosan-induced behavioral visceral hypersensitivity, with increased visceromotor responses to colorectal distension following zymosan treatment observed in male but not female mice. Consistently, sensitization of colorectal afferents in the thoracolumbar pathway was apparent in male mice but absent in female mice. In addition, four functionally classified colorectal afferents in the thoracolumbar innervation pathway were differentially sensitized between male and female mice. Apparent sex differences were also observed in the topological distribution of colorectal afferent somata in the DRG in zymosan-treated groups, with concentrated neurons in the thoracolumbar DRGs in male mice and the sacral S1 DRG in female mice. These results reveal fundamental neural anatomic and functional differences between sexes in sensory innervations of the distal colon and rectum, which have implications for the future development of personalized therapies that target colorectal sensory afferents for treating visceral pain.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.23948757.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant U01 NS113873 awarded to B. Feng and G. Zheng and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK120824 awarded to B. Feng.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.G., G.Z., and B.F. conceived and designed research; T.G., J.L., L.C., and Z.B. performed experiments; T.G., J.L., L.C., Z.B., and B.F. analyzed data; T.G., J.L., Z.B., G.Z., and B.F. interpreted results of experiments; T.G., J.L., L.C., and Z.B. prepared figures; T.G. and B.F. drafted manuscript; T.G., J.L., L.C., Z.B., G.Z., and B.F. edited and revised manuscript; T.G., J.L., L.C., Z.B., G.Z., and B.F. approved final version of manuscript.
REFERENCES
- 1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10: 712–721.e4, 2012. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2. Hungin APS, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 21: 1365–1375, 2005. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 3. Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol 94: 1320–1326, 1999. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 4. Chial HJ, Camilleri M. Gender differences in irritable bowel syndrome. J Gend Specif Med 5: 37–45, 2002. [PubMed] [Google Scholar]
- 5. Tang Y-R, Yang W-W, Wang Y-L, Lin L. Sex differences in the symptoms and psychological factors that influence quality of life in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 24: 702–707, 2012. doi: 10.1097/MEG.0b013e328351b2c2. [DOI] [PubMed] [Google Scholar]
- 6. Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol 291: R277–R284, 2006. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 7. Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain 4: 157–172, 2000. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 8. Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain 14: 270–280, 2013. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 9. Guo T, Bian Z, Trocki K, Chen L, Zheng G, Feng B. Optical recording reveals topological distribution of functionally classified colorectal afferent neurons in intact lumbosacral DRG. Physiol Rep 7: e14097, 2019. doi: 10.14814/phy2.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holdcroft A, Sapsed‐Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distension in male and female rats anaesthetized with halothane. Br J Anaesth 85: 907–910, 2000. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 11. Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol 291: R307–R314, 2006. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 12. Ji Y, Tang B, Cao DY, Wang G, Traub RJ. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. Pain 153: 1965–1973, 2012. doi: 10.1016/j.pain.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamp EH, Jones RC 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- 14. López-Gómez L, López-Tofiño Y, Abalo R. Dependency on sex and stimulus quality of nociceptive behavior in a conscious visceral pain rat model. Neurosci Lett 746: 135667, 2021. doi: 10.1016/j.neulet.2021.135667. [DOI] [PubMed] [Google Scholar]
- 15. Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 154: 1562–1567, 2008. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain 117: 433–442, 2005. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 17. Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut 50: 471–474, 2002. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ji Y, Hu B, Li J, Traub RJ. Opposing roles of estradiol and testosterone on stress-induced visceral hypersensitivity in rats. J Pain 19: 764–776, 2018. doi: 10.1016/j.jpain.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012. doi: 10.1152/ajpgi.00490.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303: G817–G824, 2012. doi: 10.1152/ajpgi.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. NeuroImage 47: 995–1001, 2009. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 23. Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J Pain 6: 493–496, 2005. doi: 10.1016/j.jpain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139: 218–224, 2008. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bian Z, Guo T, Jiang S, Chen L, Liu J, Zheng G, Feng B. High-throughput functional characterization of visceral afferents by optical recordings from thoracolumbar and lumbosacral dorsal root ganglia. Front Neurosci 15: 657361, 2021. doi: 10.3389/fnins.2021.657361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brumovsky PR, Robinson DR, La JH, Seroogy KB, Lundgren KH, Albers KM, Kiyatkin ME, Seal RP, Edwards RH, Watanabe M, Hökfelt T, Gebhart GF. Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum. J Comp Neurol 519: 3346–3366, 2011. doi: 10.1002/cne.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo T, Patel S, Shah D, Chi L, Emadi S, Pierce DM, Han M, Brumovsky PR, Feng B. Optical clearing reveals TNBS-induced morphological changes of VGLUT2-positive nerve fibers in mouse colorectum. Am J Physiol Gastrointest Liver Physiol 320: G644–G657, 2021. doi: 10.1152/ajpgi.00363.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- 30. Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6: 5, 2020. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng B, Joyce SC, Gebhart GF. Optogenetic activation of mechanically insensitive afferents in mouse colorectum reveals chemosensitivity. Am J Physiol Gastrointest Liver Physiol 310: G790–G798, 2016. doi: 10.1152/ajpgi.00430.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using μManager software. J Biol Methods 1: e10, 2014. doi: 10.14440/jbm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang X, Li H, Zhou X. Nuclei segmentation using marker-controlled watershed, tracking using mean-shift, and Kalman filter in time-lapse microscopy. IEEE Trans Circuits Syst I 53: 2405–2414, 2006. doi: 10.1109/TCSI.2006.884469. [DOI] [Google Scholar]
- 36. Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010. doi: 10.1152/ajpgi.00487.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brierley SM, Carter R, Jones W 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 39. Feng B, Gebhart GF. In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp 95: 52310, 2015. doi: 10.3791/52310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hibberd TJ, Kestell GR, Kyloh MA, Brookes SJH, Wattchow DA, Spencer NJ. Identification of different functional types of spinal afferent neurons innervating the mouse large intestine using a novel CGRPα transgenic reporter mouse. Am J Physiol Gastrointest Liver Physiol 310: G561–G573, 2016. doi: 10.1152/ajpgi.00462.2015. [DOI] [PubMed] [Google Scholar]
- 41. Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci 29: 743–752, 2009. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- 43. La JH, Gebhart GF. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am J Physiol Gastrointest Liver Physiol 301: G165–G174, 2011. doi: 10.1152/ajpgi.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu Y, Villalobos-Hernandez EC, Pradhananga S, Baker CC, Keating C, Grundy D, Lomax AE, Reed DE. Deoxycholic acid activates colonic afferent nerves via 5-HT3 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 317: G275–G284, 2019. doi: 10.1152/ajpgi.00016.2019. [DOI] [PubMed] [Google Scholar]
- 45. Feng B, Zhu Y, La JH, Wills ZP, Gebhart GF. Experimental and computational evidence for an essential role of NaV1.6 in spike initiation at stretch-sensitive colorectal afferent endings. J Neurophysiol 113: 2618–2634, 2015. doi: 10.1152/jn.00717.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hartung JE, Gold MS. GCaMP as an indirect measure of electrical activity in rat trigeminal ganglion neurons. Cell Calcium 89: 102225, 2020. doi: 10.1016/j.ceca.2020.102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng B, Guo T. Visceral pain from colon and rectum: the mechanotransduction and biomechanics. J Neural Transm (Vienna) 127: 415–429, 2020. doi: 10.1007/s00702-019-02088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones RC 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart G. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133: 184–194, 2007. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 49. Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, Martin PG, Theodorou V, Fioramonti J, Houdeau E. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci USA 107: 448–453, 2010. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol 587: 3317–3328, 2009. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Homma H, Hoy E, Xu D-Z, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol 288: G466–G472, 2005. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- 52. Drossman DA. Irritable bowel syndrome: a multifactorial disorder. Hosp Pract (Off Ed) 23: 119–133, 1988. doi: 10.1080/21548331.1988.11703538. [DOI] [PubMed] [Google Scholar]
- 53. Kendler K. Gender differences in the genetic epidemiology of major depression. J Gend Specif Med 1: 28–31, 1998. [PubMed] [Google Scholar]
- 54. Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry 157: 1243–1251, 2000. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 55. Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry 58, Suppl 15: 12–18, 1997. [PubMed] [Google Scholar]
- 56. Zender R, Olshansky E. Women's mental health: depression and anxiety. Nurs Clin North Am 44: 355–364, 2009. doi: 10.1016/j.cnur.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 57. Bradesi S, Schwetz I, Ennes HS, Lamy CMR, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289: G42–G53, 2005. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 58. Moloney RD, O'Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early-life stress induces visceral hypersensitivity in mice. Neurosci Lett 512: 99–102, 2012. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 59. Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience 140: 247–257, 2006. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 60. Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol 494: 246–259, 2006. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 61. Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11: 2113–2116, 2000. doi: 10.1097/00001756-200007140-00011. [DOI] [PubMed] [Google Scholar]
- 62. La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 303: G802–G809, 2012. doi: 10.1152/ajpgi.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71: 2046–2060, 1994. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- 64. Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 16: 113–124, 2004. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.23948757.
Data Availability Statement
Data will be made available upon reasonable request.