Abstract
Introduction:
Endotracheal intubation is a common procedure to maintain an open airway with risks for traumatic injury. Pathological changes resulting from intubation can cause upper airway complications, including vocal fold scarring, laryngotracheal stenosis, and granulomas and present with symptoms such as dysphonia, dysphagia, and dyspnea. Current intubation-related laryngotracheal injury treatment approaches lack standardized guidelines, relying on individual clinician experience, and surgical and medical interventions have limitations and carry risks.
Areas covered:
The clinical and preclinical therapeutics for wound healing in the upper airway are described. This review discusses the current developments on local drug delivery systems in the upper airway utilizing particle-based delivery systems, including nanoparticles and microparticles, and bulk-based delivery systems, encompassing hydrogels and polymer-based approaches.
Expert opinion:
Complex laryngotracheal diseases pose challenges for effective treatment, struggling due to the intricate anatomy, limited access, and recurrence. Symptomatic management often requires invasive surgical procedures or medications that are unable to achieve lasting effects. Recent advances in nanotechnology and biocompatible materials provide potential solutions, enabling precise drug delivery, personalization, and extended treatment efficacy. Combining these technologies could lead to groundbreaking treatments for upper airways diseases, significantly improving patients’ quality of life. Research and innovation in this field are crucial for further advancements.
Keywords: drug delivery, endotracheal intubation, larynx, nanotechnology, biomaterials, trachea, vocal folds, wound healing
1. Introduction
The upper airway is a critical part of the respiratory system, serving as a crucial intersection for airflow. In cases where patients are unable to breathe adequately on their own or require mechanical ventilation, endotracheal intubation (ETI) is necessary. This procedure ensures the delivery of oxygen, reduces the risk of aspiration, and maintains airway patency. ETI is widely performed in various healthcare settings such as the emergency department, intensive care unit, and operating room [1,2]. In the United States, an estimated 13–20 million ETIs are conducted annually [3]. In 2020, over 390,000 intubations were performed in the emergency department alone [4]. During the peak of the COVID-19 pandemic, invasive mechanical ventilation was commonly employed to manage acute respiratory failure. The rates of intubation varied across hospitals, ranging from 11.4% to 35.3% of admitted patients between March and December 2020 [5]. While ETI is often a life-saving procedure and the standard for securing a safe airway, it carries the potential risk of injury to the delicate laryngotracheal structures and tissues that can result in long-term voice, airway, and swallow complications.
During ETI, the endotracheal tube (ETT) passes through the oral cavity and oropharynx and then enters the trachea by passing through the larynx. Depending on the size of the ETT used and the depth that the ETT is passed through the larynx, the vocal folds and the mucosal lining of the larynx and trachea are near the end of the ETT [Figure 1a]. In addition, as the ETT makes a curve from the oropharynx into the trachea, it creates pressure on the posterior portion of the larynx between the vocal folds; depending on an individual’s anatomy, this can put them at increased risk for laryngotracheal injury [6,7]. This region plays essential roles in respiration, swallowing, and phonation. The vocal folds are multi-layered vibratory structures, and air passing from the lungs through the glottis generates vocal fold vibration and sound production. The complex interplay of vibration and aerodynamics created by this region are the basis for voice production and control [8]. The other laryngeal muscles adjust tension and length that tighten the airway and supplement the mechanisms of swallowing alongside complementing phonation [9]. ETI can damage these structures and result in vocal fold (VF) scarring, laryngotracheal stenosis (LTS), and laryngeal granulomas, leading to voice, airway, and swallowing dysfunction.
Figure 1.
(a) Areas of potential injury along the upper airway during endotracheal intubation/extubation. Endoscopic view of (b) vocal fold scarring, (d) laryngotracheal stenosis, and (f) laryngeal granuloma. Histological images of (c) vocal fold scarring, (e) laryngotracheal stenosis, and (g) laryngeal granuloma, which are adapted from [26,32,34]. Copyright Elsevier, Wiley, and InTechOpen. Created with BioRender.com.
Symptoms stemming from upper airway trauma and prolonged intubation may include dysphonia, dysphagia, and dyspnea [10–13]. In most cases, these symptoms are generally mild and resolve spontaneously [14]. However, risks of developing severe or long-term complications still exist. ETT sizes with diameters greater than 8 cm, length of intubation, diabetes, and ischemic conditions are risk factors in developing fibrosis and dysfunction along the upper airway [6]. On average, ICU patients are intubated for more than 8.2 days. Patients who have been intubated for longer periods are more likely to experience laryngeal complications after the removal of the ETT [15]. Although surgical intubations are generally shorter in comparison to ICU-related intubations, severe laryngeal injury is still a possibility [16].
Intubation and especially prolonged use can lead to pathological changes in the injured tissues. For patients with moderate or severe upper airway symptoms, they may require active intervention as they are less likely to resolve spontaneously. Otolaryngologists consider multiple approaches including speech therapy, surgical and/or medical means for treatment. Currently, there are no standardized guidelines for the treatment of these upper airway conditions, with treatment decisions primarily based on the patient’s symptoms and preferences coupled with the otolaryngologist’s experience [13,17,18]. Surgical options may involve scar excision, endoscopic dilation, partial or full resection via laser, cryotherapy, or microsurgical techniques, or laryngotracheal reconstruction. All of these treatment approaches carry risks and may necessitate revision surgeries, recurrent laryngeal nerve palsy, airway restenosis, and tracheotomy [19,20]. The best available medical treatment consists of glucocorticoid administration, leveraging their immunomodulatory and anti-fibrotic effects to prevent and treat pathological changes along the upper airway. These drugs have proven effectiveness but often require subsequent repeat injections over months, increasing office visits and costs [21–23]. Overall, annual US healthcare costs for each patient with intubation-related injury exceed $5000 [24]. Thus, it is critical to develop practically innovative drug delivery systems for treatment of upper airway injuries, to reduce burden of the patients. Currently, there are no drug delivery systems available in clinics. In other words, drug is directly injected to the lesion via syringe needles, which is a bolus injection, with associated several side effects and inconvenience. This review will focus on introduce pathophysiology of upper airway diseases, and clinical testing to assess the disease processes, and the drugs currently used in clinics. Then, it will introduce current developments in drug delivery systems to overcome existing barriers to improve therapeutic outcomes. Animal models used for preclinical testing, including rabbit/rat/dog vocal fold injury model and rabbit/mice tracheal stenosis model, will also be summarized.
2. Pathophysiology
2.1. Vocal fold scarring
Injury to the vocal fold caused by the ETT can induce vocal fold scarring, leading to fibrosis along the mucosal surface of the vocal folds [Figure 1b] [25]. Immune cells and fibroblasts are recruited and mediate wound healing through phases of inflammation, proliferation, and remodeling. Histologically, the epithelial layer becomes deformed and collagen deposition increases at the site of injury, which increases structural support and strength in the extracellular matrix (ECM) of the wound [Figure 1c] [26]. Over time, this is degraded and replaced with ECM that is functionally appropriate in the vocal folds. Imbalance between deposition and breakdown of collagen in the ECM during remodeling will lead to scar formation [27]. This formation of scar tissue results in functional deficits due to increased stiffness of the vocal fold tissue [10,11]. The heightened rigidity impairs the vibrational function of the vocal fold during phonation.
2.2. Laryngotracheal stenosis
Complications of ETI can extend further down the respiratory tract, affecting the larynx and trachea. LTS, a term incorporating subglottic stenosis, tracheal stenosis, laryngeal stenosis, and posterior glottic stenosis, is the narrowing of the laryngotracheal tract and is frequently caused by ETI [28]. Prolonged intubation and a history of past intubation are risk factors for the development of narrowing in this region [29]. Trauma from contact and prolonged intubation may lead to stenosis formation, characterized by tissue necrosis, ulceration, and scarring along the subglottic space [Figure 1d]. The larynx has muscles that contribute to vocal fold function, while the trachea’s muscles control its expansion. Histopathological analysis of tracheal stenosis contains cicatricial and non-cicatricial tissues. Stenosis of either or both regions will exacerbate symptoms of dysphonia, dysphagia, and dyspnea to varying degrees. During wound healing, pro-inflammatory growth factors and chemokines are released by the epithelial lining to recruit immune cells and fibroblasts. Pathological findings in LTS reveal changes in tissue composition. This includes epithelial proliferation and increased collagen synthesis and decreased collagen and ECM degradation, resulting in a thickening of the laryngotracheal layers [Figure 1e] [30–32]. That pathophysiological response to intubation trauma ultimately leads to functional impairment and narrowing of the upper airway.
2.3. Laryngeal granuloma
Injury to the laryngeal wall can also result in a laryngeal granuloma, also known as a contact ulcer, which is another common complication observed after ETI [12,33]. Granulomas are benign inflammatory growths that occur in response to contact with an ETT. These granulomas occur most often where the ETT rubs against the mucosa of the mobile vocal process of the arytenoid cartilage and are frequently referred to as “vocal process granulomas.” They can continue to proliferate as round lesions along the laryngeal wall [Figure 1f] [13]. Histological examination of laryngeal granulomas shows ulceration, fibrotic necrosis, and granulation with a large presence of immune cells [Figure 1g] [34]. Like vocal fold scarring and LTS, laryngeal granulomas ultimately result in increased proliferation and hyperplasia of epithelial, mucosal and fibroblasts.
3. Assessments of disease processes
3.1. Clinical testing
Clinicians employ various tools to diagnose and monitor upper airway injuries such as vocal fold scarring, LTS, and laryngeal granuloma. However, there can be a significant discrepancy between objective clinical findings and their impact on patient disability and quality of life. To address this, clinicians and researchers often combine standardized surveys of patient symptoms, physical exams, videostrobolaryngoscopy, and objective functional metrics.
Standardized self-assessment tools enable capturing subjective patient experience related to laryngeal conditions. The Voice Handicap Index-30 (VHI-30) is a survey that evaluates physical aspects of the patient’s voice and its impact on functional performance and emotional state using a 30-question ordinal scale [35]. Despite competition, the Agency for Health Care Research and Quality’s 2002 review identified the VHI-30 as the only voice-related measure meeting its standards for internal and external validity at that time [36]. Subsequently, the Voice Handicap Index-10 (VHI-10) was derived from the VHI-30, offering a shorter version with the ten most robust questions that is currently the most frequently used version of this questionnaire [37]. Several derivatives of the VHI-10 cater to patients with different language backgrounds and pediatric patients.
In cases of vocal fold scarring, functional tests on phonation become challenging due to the highly active nature of the tissue. Clinical assessment of the upper airway relies on subjective evaluation by the clinician, focusing on overall voice characteristics like quality, pitch, and loudness. The GRBAS (Grade, Roughness, Breathiness, Asthenia, Strain) scale, developed by the Japan Society of Logopaedics and Phoniatrics, is commonly used for assessing patient voice [38]. Additionally, Maximum Phonation Time (MPT), or Maximum Phonation Duration, provides an indication of respiratory support and phonatory function for speech [39]. VHI-30 and MPT serve as broadly utilized clinical metrics with extensive normative data [40,41].
Objective measurement of airflow in the upper respiratory tract is achieved through pulmonary function tests (PFTs), offering valuable insights into detecting subclinical changes in subglottic stenosis [42]. PFTs also prove to be more accurate predictors of patient dyspnea in subglottic stenosis compared to clinician grading, making them useful diagnostic and monitoring tools [43].
Videolaryngoscopy, involving recording visual inspection of the larynx, is another objective functional metric that allows for the detection of structural abnormalities, gross motor disturbances, and lesions. To study disorders affecting vocal fold vibratory function, specialized tools are essential due to the oscillation frequency during phonation. Widely used, videostroboscopy, or “stroboscopy,” illuminates the larynx with a strobe light synchronized to the patient’s voice fundamental frequency where the strobe rate is just off the pitch frequency to record snapshots of motion across the vibratory cycle, facilitating the detection of vocal fold lesions or scarring disrupting the mucosal wave [44]. Stroboscopic findings can be objectively quantified using image analysis software, providing metrics like glottal area waveform and its features. However, this technique has limitations when vocal fold vibrations are not periodic, as with vocal tremor, or when there is not one fundamental frequency, as in diplophonia. High-speed digital imaging offers an alternative with limitations and has frame capture rates from 4000 to 14000 frames per second [45]. Data storage and tedious playback and analysis are current clinical barriers to wider implementation of this technology. Videokymography, involving successive high-speed images of a single transverse section superimposed vertically on real-time video, provides another technique for assessing aperiodic glottic vibration [46].
3.2. Biomechanical testing
Phonation depends on vibration generated through interaction of vocal fold layers with distinct material properties like elasticity and stiffness. Techniques for quantifying these properties have provided key insights into how pathophysiologic processes affect voice production. Viscoelastic material properties, including storage (elastic), loss (viscous), and complex moduli, are pertinent metrics to understand normal and altered vocal fold function [47]. Biomechanical testing represents powerful pre-clinical validation for targeted vocal fold therapeutics.
Several techniques have been explored to measure vocal fold biomechanics in vivo, but none have proven sufficiently accurate or reproducible for clinical trials or living animal models [48–50]. While synthetic in vitro replicas have contributed to the study of voice production, they lack the biologic component necessary for studying the effects of targeted drug interventions [51]. Ex vivo human or animal larynges yield the most accurate tissue biomechanical data, with swine, canine, and leporine animal models showing excellent reproducibility and utility for pre-clinical testing [52]. Biomechanical testing methods applicable to ex vivo models include force-elongation, rheometry, and indentation mapping [53]. Micro and nano-indentation mapping techniques have the advantage of measuring displacement perpendicular to the vocal fold plane, which is representative of phonation physiology [52], and quantify the impact of disease processes [54]. These approaches allow for reproducible measurements of vocal fold properties in response to potential therapeutics in live animals or explant models.
4. Upper airway therapeutics targeting wound healing
Wound healing therapeutics aim to target and modulate the local inflammatory and immune response in upper airway injuries and pathophysiological changes. Effective therapeutics studied in human patients are summarized in Table 1. Preclinical therapeutics tested in vitro and with animals will also be discussed. Although these medications have different mechanisms of action, they similarly possess anti-fibrotic and anti-proliferative properties. The following therapeutics hold promise for modulating wound healing processes in upper airways. However, further studies, including preclinical and clinical trials, are necessary to assess their efficacy, safety, and optimize their application in clinical settings.
Table 1.
Current treatment and prevention of vocal fold scarring, laryngotracheal stenosis, and laryngeal granuloma.
| Drug | Indication | Administration Route | Drug Function | Side Effects | Sources |
|---|---|---|---|---|---|
| Glucocorticoids (Dexamethasone, Triamcinolone, Methylprednisolone, Betamethasone) |
Vocal fold scarring Laryngotracheal stenosis Laryngeal granuloma |
Oral Inhalation Intravenous Intralesional Injection |
• Decrease inflammation and collagen deposition • Increased collagen degradation |
Hyperemia, hematoma, epithelial thinning, and muscle atrophy; rare with intralesional injection |
[21,27,55,56] |
| Mitomycin C | Laryngotracheal stenosis | Intraoperative Topical | • Acts as an alkylating agent with anti-fibroblastic properties | No adverse effects reported | [60–62] |
| Paclitaxel | Tracheal Stenosis | Drug-Eluting Stent | • At low doses, inhibits fibroblast proliferation without inducing apoptosis • Beneficial effect on scar and granulation tissue |
Tissue necrosis | [64] |
| Methotrexate | Laryngotracheal Stenosis | Oral | • Immunomodulatory properties that has a favorable side effect profile at low doses | Mild hair thinning and onychomycosis | [65] |
| Basic Fibroblast Growth Factors | Vocal fold scarring | Intralesional Injection | • Modulates fibroblast function • Stimulates hyaluronic acid production • Inhibits collagen deposition |
Hyperemia | [67–69] |
| Mesenchymal Stem Cells | Vocal fold scarring | Intralesional Injection | • Improved vocal fold vibratory function | No adverse effects reported | [72] |
| Pirfenidone | Tracheal stenosis | Oral | • Inhibit TGF-beta-mediated fibroblast proliferation • Prevent and reverses formation of fibrosis and scar |
No adverse effects reported | [75] |
4.1. Clinical therapeutics
4.1.1. Corticosteroids
Corticosteroids, known for their anti-inflammatory and immunomodulatory properties, are extensively studied for treating upper airway injuries. They have shown efficacy in managing laryngeal pathologies by reducing collagen deposition, delaying fibrosis and wound healing, and improving voice quality [55–57]. Steroids also decrease the migration of inflammatory cells, reducing cytokine release that activate fibroblasts and inhibiting their mitotic activity [21]. Inhaled steroids are commonly used in treating laryngeal granulomas, but patient outcomes vary with intravenous, inhaled, and oral administration in vocal fold lesions [18,56,58]. Local intralesional administration through direct injection is preferred to minimize side effects and achieve direct application to the lesion site. Intralesional injections are generally well-tolerated by not harming the voice. Commonly used steroids for direct injection include dexamethasone and triamcinolone. However, the effects of a single injection are short-lived, often requiring follow-up injections after several weeks due to symptom recurrence [21,23]. Although largely considered safe, steroid injections do carry a risk of side effects such as hematoma, tissue atrophy, and injection-related complications [59]. Despite the considerations, corticosteroids are commonly used and effective in reducing scarring and fibrosis following upper airway injury.
4.1.2. Chemotherapeutics
Chemotherapeutic drugs, known for their ability to target highly proliferative cells like cancer cells, also exhibit immunomodulatory and anti-fibrotic functions at lower doses. The side effects associated with chemotherapy are minimized and more tolerable at this dosing range. Chemotherapeutics used in clinical settings for upper airway lesions include mitomycin C (MMC), paclitaxel, and methotrexate. MMC, an antibiotic acting as an alkylating agent, is commonly topically administered as an adjuvant therapy in airway stenosis. It inhibits fibroblastic activity and reduces scar formation [60–62]. Paclitaxel, an inhibitor of mitosis and tubulin synthesis, has shown inhibition of fibroblast proliferation and G1 cell cycle arrest. In vitro application of paclitaxel was shown to inhibit proliferation of human respiratory cells cultured from patients with tracheal stenosis [63]. At low doses, fibroblast proliferation can also be inhibited through G1 cell cycle arrest. A clinical study demonstrated remission in cases of benign cicatricial airway stenosis when paclitaxel is used as an adjuvant following balloon dilation [64]. Methotrexate, an immunomodulating chemotherapeutic, has shown a favorable adverse effect profile. Low-dose oral methotrexate increased surgery-free days in patients with LTS [65]. Larger-scale studies are needed to establish clinical efficacy and treatment standards for chemotherapeutic use in upper airway wound healing.
4.1.3. Fibroblast growth factor
Fibroblast growth factors are chemical messengers that regulate cell growth, proliferation, and differentiation. They bind to the surface receptors of fibroblasts, initiating a cascade of cellular responses. Basic fibroblast growth factor (bFGF) has regenerative capabilities and has been used to treat injured tissues. In the context of upper airway injuries, bFGF influences the local ECM composition by stimulating hyaluronic acid production and suppressing collagen production in fibroblasts. Studies on vocal fold scarring have shown reduced collagen density with bFGF treatment [66–68]. A study comparing bFGF and steroid injections demonstrated the effectiveness of both treatments in patients with vocal fold scarring, with each treatment showing different improvements in objective voice parameters, reflecting their different mechanisms of action [69]. However, bFGF has a short half-life, likely requiring multiple injections for a regenerative effect. Further clinical studies are needed to understand the long-term effects and establish treatment regimens for bFGF.
4.1.4. Mesenchymal stem cells
Cell-based therapy using mesenchymal stem cells (MSCs) show promise in their anti-fibrotic potential. MSCs, adult stem cells capable of differentiating into various mesodermal cell types, possess immunomodulatory, regenerative, and trophic properties. Preclinical animal studies on vocal fold scarring and LTS have shown decreased viscosity and elastic modulus, increased mucosal wave amplitude, and a more favorable inflammatory response and tissue regeneration in treated groups [70,71]. A phase I/II clinical study with direct MSC injection into patient vocal folds reported improvements in vibratory functions and voice quality without severe adverse effects [72]. Continued research on cell-based therapies utilizing MSCs for various upper airway applications and long-term effects is warranted.
4.1.5. Pirfenidone
Pirfenidone, commonly used to reduce fibrosis and inflammation in idiopathic pulmonary fibrosis, is believed to regulate fibroblast proliferation and transformation by decreasing the production of TGFβ (transforming growth factor beta) and TNFα (tumor necrosis factor alpha). In vitro studies on ferret fibroblast isolated from vocal fold scars have shown reduced collagen expression and gel contraction with pirfenidone treatment [73]. In vivo studies with rats have demonstrated a reduction in both fibrosis and tracheal narrowing following tracheotomy and intraperitoneal administration of pirfenidone [74]. A case report has also shown promising results in human translation in treating tracheal stenosis with oral pirfenidone [75]. Further studies are needed to understand the long-term effects and optimize the application of pirfenidone in upper airway injuries.
4.2. Preclinical therapeutics
4.2.1. Biologics and targeted therapies
Hepatocyte Growth Factor (HGF) is a chemical messenger involved in tissue regeneration, and it has shown potential as an anti-fibrotic agent. Animal studies have demonstrated its effectiveness in preventing or resolving fibrosis in various animal organs [76]. Topical injection of HGF in rabbit vocal folds has exhibited reduced collagen deposition, stiffness, and vocal fold contraction compared to control groups [77]. HGF has been shown to increase hyaluronic acid and elastin production while reducing collagen accumulation in cultured vocal fold fibroblasts [78,79]. Pharmacokinetics tests in rats contribute to a favorable safety profile [80]. However, HGF’s short half-life limits its effectiveness. Novel approaches using polymer-based nanoparticles and hydrogels are being explored to address this issue. Encapsulating HGF in nanoparticles has shown inhibition of TGFβ-induced procollagen mRNA expression [81]. Direct injection of HGF-loaded hydrogel in rabbit vocal folds has resulted in less fibrosis and improved viscoelastic properties [82]. Further research on HGF for wound healing treatment, including its delivery optimization, is necessary for clinical translation.
Neutralizing antibodies offer an alternative therapeutic approach. By targeting specific antigens and blocking their function, they can modulate disease processes. For fibrotic conditions, targeting TGFβ, a key mediator in scar tissue formation, has shown promise. In animal models, neutralizing antibodies to TGFβ1 have demonstrated a decrease in fibronectin and procollagen [83]. Combined intravenous and local injection of anti-TGFβ in a canine study reduced tracheal stenosis and increased survival time [84]. Research on neutralizing antibodies for wound healing applications in the upper airway should be continued to assess their potential and translation.
Nintedanib, a receptor tyrosine kinase inhibitor originally developed for idiopathic pulmonary fibrosis, has potential for treating fibrosis along the upper airway. Inhaled nintedanib in rabbits reduced collagen deposition, histone deacetylase 2 expression, and suppressed vascular endothelial growth factor and interleukin-8 [85]. Studies with rats and human lung fibroblasts have also demonstrated its ability to decrease collage deposition, inhibit fibrotic markers, and inhibit TGFβ-induced fibroblast differentiation [86]. Further investigation of nintedanib as a potential therapy is warranted.
Small interfering ribonucleic acids (siRNAs) represent a developing field utilizing RNA interference mechanisms to regulate gene expression [87]. Targeting disease-causing genes, such as SMAD3 and SERPINH1, holds potential for modulating fibrosis and inflammation. SMAD3 (“small” mothers against decapentaplegic homolog 3) acts as a mediator of the signals initiated by the transforming growth factor beta (TGF-β) superfamily of cytokines, which regulate cell proliferation, differentiation and death. Preclinical studies have shown that SMAD3 siRNA limited TGFβ-mediated collagen production [88], while SERPINH1 knockdown in mouse fibroblasts resulted in misfolded and weaker collagen [89]. However, nucleic acid degradation and instability continue to be a challenge in the delivery of RNA-based therapeutics [87]. Nanoparticle delivery approaches using lipitoid oligomers and liposomes have shown promise in enhancing cellular uptake and stability [90–92]. Research on RNA-based therapeutics and their delivery approaches offer potential treatments to modulate wound healing processes.
4.2.2. Immunosuppressive agents
Tacrolimus, an immunosuppressive agent commonly used for organ transplantation, has shown promise in reducing tissue granulation. Studies with lung transplant recipients who received tacrolimus showed lower tissue granulation formation along the stent in comparison to nontransplant patients, and those patients also underwent fewer follow-up procedures [93]. Low-dose intramuscular injection of tacrolimus in rats has suggested its preventative effect on immune cells via the calcineurin/nuclear factor of activated T cell/interleukin 2 pathway [94]. Rapamycin, another immunosuppressant used for organ transplantation, has shown inhibition of fibroblast proliferation, metabolism, and collagen deposition in human fibroblasts isolated from patients with LTS [95]. In a mouse model, a rapamycin-eluting stent reduced lamina propria thickness and decreased expression of fibrotic and inflammatory markers [96]. Arsenic trioxide (ATO), a chemotherapeutic used to treat acute promyelocytic leukemia, has also demonstrated anti-inflammatory effects. ATO inhibits the differentiation of TGFβ-induced fibroblasts to myofibroblasts and induces fibroblast cell death [97,98]. In rabbit models, ATO prevented hypertrophic scar progression and reduced tissue granulation and collagen deposition in the upper airway, maintaining a safe profile within normal ranges [99,100]. Cisplatin, a chemotherapeutic agent inducing DNA damage and apoptosis, has shown antiproliferative and antifibrotic effects, increasing myofibroblast apoptosis and downregulating collagen in vocal fold fibroblasts [101,102]. Cisplatin-eluting stents in rabbit airways inhibited granulation tissue formation and collagen deposition [103]. These immunosuppressive agents show potential in modulating fibroblast activity, but careful monitoring of dosing is necessary to minimize adverse effects. Further studies on their application in upper airway injuries should be continued.
4.2.3. Other therapeutics
Doxycycline, a broad-spectrum antibiotic, has inhibitory effects on matrix metalloproteinase (MMP) activity, modulating immune cells and wound healing processes. MMP levels are elevated in inflamed, fibrotic, and stenotic tissues, and they regulate tissue remodeling by breaking down and depositing components of the ECM [104]. Doxycycline-coated tracheal stent cultured with fibrosarcoma cells has shown inhibition of cell growth and MMP mRNA expression [105]. Rabbit tracheas treated with doxycycline-coated tracheal stents showed less granulation compared to non-coated stents [106]. Further investigations are needed to explore the potential application of doxycycline in targeting the wound healing processes.
Eupatilin, a natural flavonoid isolated from Artemisia asiatica, exhibits anti-inflammatory effects on the TGFβ pathway. In vitro studies have shown eupatilin’s inhibition of myofibroblast differentiation by targeting the SMAD and p38 pathways [107,108]. More studies on eupatilin are warranted to evaluate its potential as a wound healing therapeutic.
5. Drug delivery systems
5.1. Current challenges and design criteria
Despite current efforts in developing drug delivery systems for treating laryngotracheal diseases, none of them has been translated into clinical settings yet. This is partly due to the complex three-dimensional anatomy of the larynx and limited access, second to its vital role in respiration, phonation, and cough. The current standard of care is still frequent injections of the therapeutic agents to the diseased lesions or repeated surgery. The emergence of nanotechnology and biomaterials may offer significant long-term solutions to these currently challenging medical conditions if the access and delivery challenges are solved. Nanotechnology utilizing nanomaterials, such as liposomes and nanoparticles, allows us to encapsulate drugs and deliver them precisely to the affected sites. These nanocarriers can protect the drugs from degradation, prolong their release, and facilitate their absorption, ensuring optimal therapeutic outcomes. These approaches build on the work outlined and can be readily adaptable to deliver a variety of therapeutics, creating a foundational technological delivery vessel. However, the efficacy when nanoparticles were used lasts up to several days to date. Biocompatible and biodegradable materials have also been widely used to develop coatings or scaffolds for drug delivery systems, such as stents or patches. Such materials significantly prolong the drug delivery efficacy, up to several weeks, and minimize the risk of tissue damage and adverse reactions, promoting the overall safety and effectiveness of the treatment. Therefore, when nanotechnology and the biocompatible polymeric materials are combined, targeted, personalized, and prolonged treatment methods are expected. In this review, current technology in particulate delivery systems and bulk delivery systems that represent nanotechnology and biomaterials, respectively, will be summarized. Although the drug delivery technology summarized below is promising to be practical in the near future, none of the technology is currently used in clinics. The barriers to overcome for each technology will also be summarized.
5.2. Particulate delivery systems
Several studies have shown promising results in the field of employing particulate-based drug delivery in the treatment of upper airway injuries while it is still in its early stages. The design and development of these particulate drug delivery systems, consisting of microparticles or nanoparticles, require careful consideration of factors like biocompatibility, stability, and release kinetics to ensure their ability to be utilized in medical applications. In addition, research is needed to better understand the safety, efficacy, and long-term effects of particulate-based therapies in wound healing treatment of the upper airway. Advancements in nanotechnology and ongoing research efforts will provide further insights into the potential of particulate delivery systems for personalized and targeted treatments with improved therapeutic outcomes.
It is worth noting that the term microparticle or nanoparticle is a broad concept that encompasses particles of various materials and sizes, and its significance may vary depending on the context in which it is used. Compared to the conventional therapeutic methods, particulate-mediated drug delivery has several advantages in terms of protection to the encapsulated drug from degradation, enzymatic breakdown, rapid clearance, enhance solubility, selective targeting, and drug accumulation in the region of interest that can further enhance the drug’s stability and therapeutic efficacy by minimizing their side effects [109]. As a result, researchers are exploring particulate constructs to deliver therapeutic agents directly to the site of injury such as scar tissue to promote tissue regeneration and mitigate the formation of excessive scar tissue [110,111]. The current developments for wound healing treatment in upper airway are listed in Table 2.
Table 2.
Recent developments in particulate delivery systems for upper airway injuries and their ability to enhance drug delivery.
| Drug Delivery Technology | Encapsulating Material Composition | Drug Payload | Study Type / Model (Administration Route) | Summary of Results | Reference | |
|---|---|---|---|---|---|---|
| Particulate Delivery System | Nanoparticles | PLGA Nanoparticles | HGF |
in vitro: mouse lung fibroblasts in vivo: mouse (direct vocal fold injection) |
Prolonged localization of dye encapsulated in nanoparticles within the mouse vocal folds. | [81] |
| Lipitoid Oligomer | SMAD3 siRNA |
in vitro: immortalized human vocal fold fibroblasts in vivo: rabbit with unilateral vocal fold injury (direct vocal fold injection 7 days after injury) |
Enhanced transfection efficiency of SMAD3 siRNA in vocal fold fibroblasts. | [90] | ||
| SMAD3 expression was acutely suppressed using the lipitoid-siRNA complex in vivo, but the effects were not sustained. | [91] | |||||
| Liposome | Serpinh1 siRNA |
in vitro: harvested rat vocal fold mucosa in vivo: rat with bilateral vocal fold injury (direct vocal fold injection 2 months after injury) |
Targeted Serpinh1 knockdown in vocal fold fibroblasts by siRNA was only observed when encapsulated in a liposome and not as a naked construct. | [92] | ||
| Microparticles | PLGA Microparticles | Dexamethasone | in vitro: immortalized human vocal fold fibroblasts | Gene expression of collagen subtypes COL1A2 and COL3A1 were most suppressed by the dexamethasone-encapsulated microparticles group. | [112] | |
Table Acronyms/Abbreviations: HGF: hepatocyte growth factor, PLGA: poly(lactic-co-glycolic acid), TGFβ: transforming growth factor-beta
5.2.1. Microparticles
Microparticles are small particles or tiny solid or liquid substances that have a size ranging between 1 to 1000 microns in diameter. They can be synthesized from natural or synthetic polymers and have several potential applications in medicine, especially with vocal fold scar treatment as drug carriers. A past study assessed the in vitro effectiveness for controlled dexamethasone release using poly-lactic-co-glycolic acid (PLGA) microparticles. They demonstrated how their microparticle composition using a lower PLGA molecular weight (6700 Da) released dexamethasone at a higher rate in comparison to higher PLGA molecular weight (~111.500 kDa). When cultured with vocal fold fibroblasts, the dexamethasone-loaded microparticles downregulated collagen subtypes COL1A2 and COL3A1 decrease interleukin-6 synthesis after TGFβ treatment [112]. However, no significant differences were seen in comparing the dexamethasone-loaded microparticles to free dexamethasone and non-loaded microparticles. Observing the drug release was also limited to 4 days, so the complete longevity of dexamethasone-loaded microparticles and their use in vivo continue to be in question. Nonetheless, this study considered how compositional content of a drug carrier impacts drug release.
5.2.2. Nanoparticles
More recently, owing to their physical, chemical, biological, and unique structural characteristics, nanoparticle (NP) applications are widely explored in the several field areas including drug delivery, imaging, biosensing, diagnosis, and therapy [113]. NPs are routinely defined as tiny particles with sizes range of 1 and 100 nm that have a unique property that are not found in bulk samples of the same material due to their small size and high surface area-to-volume ratio [114,115]. As the result, several researchers have tried to investigate the application of NPs in the vocal fold scar tissue as carriers to deliver anti-scarring drugs, anti-inflammatory drugs, cytokines, anti-fibrotic growth factors (such as basic fibroblast growth factor and hepatocyte growth factor) and RNA-based therapeutics. One of the earlier studies for laryngeal applications were tested with PLGA NPs for encapsulation and delivery of hepatocyte growth factor (HGF), Texas Red-dextran, and bovine serum albumin as controls, which were fabricated using a modified double emulsion method. Figure 2A and B shows that uptake and retention of Texas-red-encapsulated PLGA NPs and HGF-encapsulated PLGA NPs, respectively, in 3T3 fibroblast cells in vitro. Furthermore, injection studies with mice vocal folds showed the retention of encapsulated Texas Red-dextran for a prolonged period in comparison to free dye [Figure 2C] [81]. Overall, their results revealed the potential utility of PLGA NPs as an effective method for long-term delivery of therapeutics to the larynx.
Figure 2.

Localization of nanoparticle-encapsulated contents in 3T3 fibroblast cells. (a) Cellular uptake of Texas Red dye encapsulated in PLGA nanoparticles (b) Cellular uptake of FITC-HGF (c) Prolonged localization of Texas Red dye in a mouse vocal fold and table comparing the testing groups of sustained fluorescence. Adapted from [81]. Copyright Wiley.
Other NP compositions include a novel synthetic oligomer, known as lipitoid, that possesses cationic side-chains and a phospholipid moiety. Lipitoid was complexed with SMAD3 siRNA into 50–100 nm particles to improve the stability and cellular uptake for increased efficiency of siRNA-based therapeutics. Early studies reported the synthesized lipitoid did not confer additional toxicity compared to commercially available reagents, and an in vivo experiment revealed that the lipitoid-complexed SMAD3 siRNA significantly reduced increases in SMAD3 expression following vocal fold injury at 4 and 24 hours post-injection [90]. A follow-up study compared the transfection efficiency of the lipitoid by creating structural and chemical variants. They found no statistically significant differences with variants, with similar changes in SMAD3 knockdown and cell proliferation. An in vivo experiment with the lipitoid-complexed siRNA saw acute effects on SMAD3 mRNA expression, but it was not sustained after 1 day [91]. Moreover, a different study investigated liposome-mediated delivery using SERPINH1 siRNA. Downregulation of SERPINH1 expression was only observed when the siRNA was encapsulated in a liposome, with flow cytometry and fluorescent labeling confirming cellular uptake and intracellular distribution. In their in vivo studies, siRNA-complexed liposomes downregulated SERPINH1 expression for in day 2 following injection and returned to basal levels in day 6. Looking at the downstream effect on collagen synthesis, decreases in the levels of hydroxyproline abundance were not observed until days 4 and 6 [92]. The conclusions from these studies highlight the utility of other particle composites for successful protection of siRNA from extracellular degradation and improved delivery.
NPs hold considerable potential as a carrier for therapeutic delivery to the upper airway. Their ability to modulate the composition, size, drug load, and surface chemistry will affect their functionality. Biocompatibility, safety, and particle stability are also important factors that warrant evaluation for eventual translation in human applications around the larynx. Further studies should continue long-term observation for critical and thorough analysis of these delivery systems.
5.3. Bulk delivery systems
Particulate delivery systems utilize small particles to encapsulate therapeutics, enabling controlled release and targeted delivery. In contrast, bulk delivery systems evenly distribute the therapeutic within a larger carrier material, resulting in sustained release commonly through degradation. The following section, summarized in Table 3, will discuss various innovative approaches in bulk delivery systems for medical implants, with a focus on hydrogel and polymer-based systems, as well as their potential applications in treating upper airway injuries and laryngeal stenosis. Further research and clinical validation are necessary to fully validate the effectiveness and safety of these approaches for human patients with upper airway conditions.
Table 3.
Recent developments in bulk delivery systems for upper airway injuries and their release profiles.
| Drug Delivery Technology | Encapsulating Material Composition | Drug Payload | Study Type / Model (Administration Route) | Release Profile | Reference | |
|---|---|---|---|---|---|---|
| Bulk Delivery Sytems | Hydrogel | Hyaluronic Acid & Alginate Composite Hydrogel | Human Adipose-derived Mesenchymal Stem Cells | in vivo: rabbit with unilateral and bilateral vocal fold injury (direct vocal fold injection immediately after injury) | MSCs in hydrogel remained viable after 1 month, with some differentiating into fibroblasts. | [121] |
| Hyaluronic Acid & Alginate Composite Hydrogel | Hepatocyte Growth Factor | in vivo: rabbit with unilateral and bilateral vocal fold injury (direct vocal fold injection immediately after injury) | HGF release in hydrogel was sustained for up to 3 weeks. | [82] | ||
| Glutaraldehyde-Gelatin Hydrogel | Basic Fibroblast Growth Factor | in vivo: dog with unilateral vocal fold injury (direct vocal fold injection 1 month after injury) | bFGF release duration in hydrogel was not studied but described as a slow release. | [122] | ||
| Glutaraldehyde-Gelatin Hydrogel Microspheres | Basic Fibroblast Growth Factor | in vivo: rabbit with bilateral vocal fold injury (direct vocal fold injection immediately after surgery) | bFGF release duration in hydrogel was not studied but references 14-day hydrogel degradation time. | [26] | ||
| Polymer-Based Stent | PLLA-PCL | Mitomycin C |
in vitro: distilled water in vivo: rabbit (drug-eluting stent placed in trachea) |
33% of mitomycin C loaded into stent was released after 12 weeks in vitro in a diffusion-controlled manner. | [133] | |
| PCL & PLGA | Cisplatin |
in vitro: phosphate buffer in vivo: rabbit with tracheal stenosis (drug-eluting stent placed in trachea) |
Sustained release of cisplatin was observed for over 4 weeks in vitro and present cisplatin presence for over 5 weeks along rabbit trachea. | [134] | ||
| PDLLA Shell & PU Core | Doxycycline | in vivo: rabbit with tracheal stenosis (drug-eluting stent placed in trachea) | An initial burst release is observed in all compositions, with a delayed release to over 80% present at 24 hours. Increased PDLLA shell to PU core composition ratio slowed release of doxycycline. | [106] | ||
| in vitro: fibrosarcoma cell line | [105] | |||||
| PLLA-PCL Blend & PDLGA | Rapamycin |
in vitro: harvested human fibroblasts in vivo: mice with tracheal stenosis (drug-eluting stent placed in trachea) |
PLLA-PCL stent exhibited 30% rapamycin elution after 6 weeks. Also, PLLA-PCL composition had better mechanical strength in comparison to PDLGA over a 4-week period. | [96] | ||
| PLCL | Arsenic Trioxide |
in vitro: human embryonic pulmonary fibroblasts, bronchial epithelial cells, & airway smooth muscle cells in vivo: rabbit (drug-eluting stent placed in trachea) |
A burst release of arsenic trioxide was seen, with almost total release of ATO from the stent was observed by 24 hours in vitro. | [100] | ||
| PCL | Silver Nanoparticles & Cisplatin |
in vitro: PBS (pH 7.4), Tracheal Simulation Fluid, and human embryonic lung fibroblasts in vivo: rabbit (drug-eluting stent placed in trachea) |
Release behavior can be described as two phases: burst- and constant-release. Over 70% of cisplatin and over 50% of AgNPs were released in the first 8 days, and the coated stent lost at least 70% of its weight after 35 days after testing in vitro, either with PBS or Tracheal Simulation Fluid. | [103] | ||
| PCL, PLA, & PLGA | Low Molecular Weight Methylprednisolone | in vivo: rabbit (drug-eluting stent placed in trachea) | PCL and PLA are most resistant to mechanical damage. At 10 days with in vivo studies, PLGA-coating degraded the most, PLA-coating being partially preserved, and PCL-coating seen as the most stable. PLA and PLGA coatings showed therapeutic potential in prolonged and pronounced drug release. | [132] | ||
| Polymer-Coated Endotracheal Tube | PLGA | Mometasone Furoate |
in vitro: SDS Aqueous Solution in vivo: rat with trans-oral intubation for 3–6 hours |
Exhibited an initial burst release of 15% followed by sustained drug release over 2 weeks in vitro. Approximately 70% of mometasone furoate was released after 1 week. | [136] | |
| in vivo: rat with trans-cervical intubation for 1 week | SEM images of tracheal mucosa show relatively preserved surface structures with mild disorganization of cilia and mucus droplets. | [137] | ||||
| PCL | Dexamethasone | in vivo: pig with laryngotracheal burn injury and transglottic ETT segment for 3 and 7 days | Study was limited to 7 days, but PCL drug elution was referenced and validated in Miar et al. | [138] | ||
| Polymer-Coated Patch | PCL / PEG Composite | Dexamethasone | ex vivo: porcine trachea and cadaveric larynx | Dexamethasone-eluting patch exhibited a burst release within the first 2 hours and a sustained release over 28 days. | [141] | |
Table Acronyms/Abbreviations: AgNP: silver nanoparticles, bFGF: basic fibroblast growth factor, HGF: hepatocyte growth factor, MSC: mesenchymal stem cells, PBS: phosphate-buffered saline, PCL: polycaprolactone, PDLGA: poly(D,L-lactide-co-glycolide), PDLLA: poly(D,L-lactide), PEG: polyethylene glycol, PLCL: poly-L-lactide-caprolactone, PLGA: poly(lactic-co-glycolic acid), PLLA: poly-L-lactide, PU: polyurethane, SDS: Sodium Dodecyl Sulfate
5.3.1. Hydrogel approaches
Hydrogel implants are unique medical implants made from hydrogel, a polymeric material that swells in contact with water, maintaining a three-dimensional structure [116]. These hydrogels have been extensively used in pharmaceutical and biomedical applications since the 1960s due to their high-water content, which resembles tissues, excellent biocompatibility, and ability to effectively enclose hydrophilic drugs. Their solid-like characteristics and customizable porous structure make them promising for drug delivery applications [117–119]. In addition, hyaluronic acid hydrogel is completely degraded in the body by hyaluronidase and acid hydrolysis, which eliminates removal surgery [120].
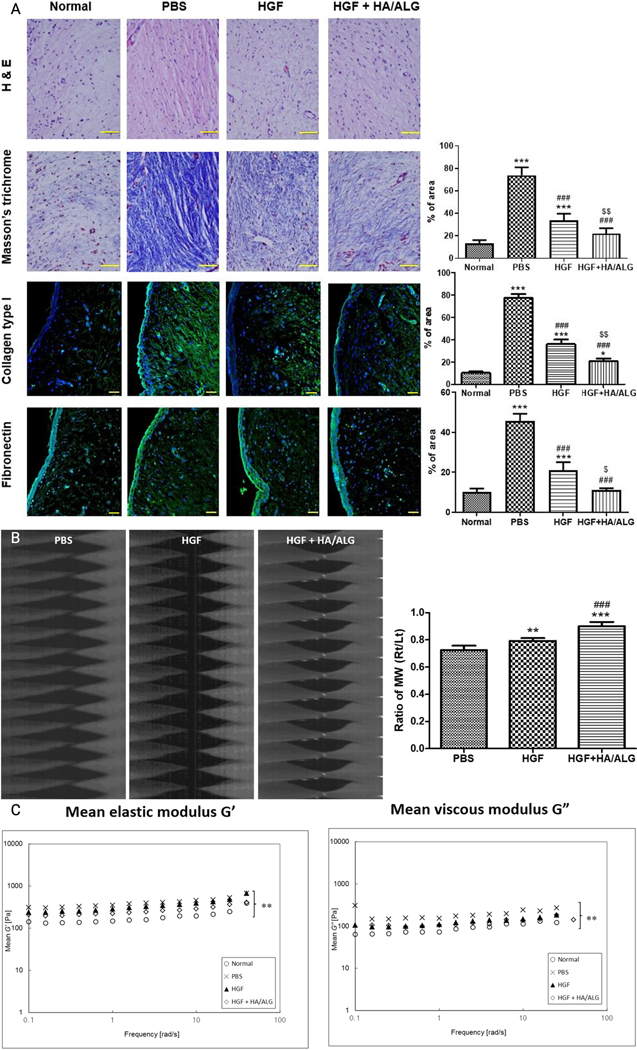
Research has explored the therapeutic applications of hydrogel implants incorporating diverse payloads for laryngeal applications. In one study, the authors investigated the regenerative potential of an injectable hyaluronic acid/mildly cross-linked alginate (HA/ALG) hydrogel containing human adipose-derived MSCs to heal rabbit vocal fold wounds. The findings revealed significant improvements in macroscopic morphologies 3 months after injury, with improved viscoelasticity and favorable ECM changes in regenerating the vocal folds [121]. They continued using these composite hydrogels with therapeutic delivery of HGF. HGF-loaded hydrogels exhibited a sustained drug release profile over 3 weeks, also displaying enhanced viscoelastic properties and antifibrotic changes to the ECM of HGF-treated rabbit vocal folds [Figure 3] [82]. Both studies showed continued long-term persistence of the therapeutic used in their hydrogel and suggest lasting effects in comparison to the therapeutic alone. Further examination regarding the optimal dosing for effective regeneration and the controlling factors in drug release should be in consideration. These compelling outcomes strongly indicate the promising utility of injectable HA/ALG hydrogel as a therapeutic carrier for stimulating vocal fold regeneration.
Figure 3.
(a) Immunohistochemical analysis demonstrating improved ECM remodeling in rabbit vocal folds using HGF-HA/ALG in comparison to normal, PBS-treated, and HGF-treated groups. Tissue samples stained with hematoxylin and eosin (H&E) are shown with stains using Masson’s trichrome, collagen type I and fibronectin, where the areas were quantified by pixel density in positively stained areas. Scale bars represent 30 µm. Biomechanical evaluation shows better vocal fold functionality in (b) mucosal wave oscillations using high-speed cameras and (c) viscoelasticity changes with elastic and viscous moduli following injury and subsequent treatment in HGF-HA/ALG over normal, PBS-treated, and HGF-treated groups. The immunohistochemical analysis and mucosal wave oscillations were compared using Kruska-Wallis test with Dunns’ post hoc test, while the viscoelastic properties were compared using two-way ANOVA with Bonferroni’s post hoc test. All results were evaluated at 3 months post-injury. Adapted from [82]. Copyright Springer.
Another series of studies examined the regenerative impact of a hydrogel-based sustained drug delivery system composed of gelatin cross-linked with glutaraldehyde containing bFGF. Scarred canine vocal folds were treated with bFGF-loaded hydrogel, and they revealed better vibratory functions and histological restoration 5 months post-treatment compared to the control group. Notably, their drug delivery system demonstrated comparable or superior effects when compared to repeated injections of bFGF solution [122]. In a subsequent study, rabbits with vocal fold injuries were treated by injecting the same hydrogel composition into 10–70 µm microspheres with or without bFGF. Histological analysis two weeks after injection demonstrated that the vocal folds treated with bFGF-loaded hydrogel microspheres reduced scar formation compared to the control treatments [26]. Overall, these studies on hydrogel applications provide valuable insights into their utility and their ability to deliver therapeutic agents for promoting vocal fold regeneration and healing. Further research and clinical studies are necessary to validate these findings and explore their translation into effective wound healing treatments for human patients with upper airway conditions.
5.3.2. Polymer coatings and scaffolds
Polymer coatings offer significant advantages in various medical applications. They are biocompatible, which minimize the risk of adverse reactions. The ease of application allows for efficient manufacturing. Coatings reduce inflammation, promoting improved healing and outcomes. They can be modified for drug delivery or cell adhesion, adapting to specific medical needs. The enhanced durability ensures longevity and performance of medical devices. Polymer coating positively impacts patient care and treatment outcomes [123–125]. In treating upper airway injuries, the use of polymers in development will help avoid complications like granulation tissue overgrowth and restenosis.
5.3.2.1. Polymer-coated metallic stents and bioabsorbable stents
Stents have played a role in mechanically stabilizing the upper airway, but historically, these options were limited. Now, self-expanding metallic stents (SEMS) and silicone stents like the Dumon stent and Y-stent are commonly employed [126–130]. Nonetheless, stents can induce inflammation and complications, such as scar tissue formation and tracheal narrowing. To combat these concerns, researchers are investigating various approaches, including drug-coated stents using bioabsorbable materials, drug elution systems, nanofiber coatings, and even stents that are completely bioabsorbable. These endeavors aim to enhance stent biocompatibility and reduce complications [105,131] [6,7]. Both polymer-coated metallic stents and bioabsorbable stents present distinct advantages and challenges. The choice between them hinges on the specific medical condition, required support duration, potential complications, and overall treatment objectives.
Currently, the most tested procedure for treating vocal fold scarring and tracheal stenosis involves metallic stents with drug-eluting polymer coatings. To prevent tissue fibrosis and re-stenosis, researchers have explored various drugs embedded within different polymers in this field. Recently, this approach was investigated using a doxycycline-coated nitinol stent, where the coating was constructed with a poly(D,L-lactide) shell and a polyurethane core through coaxial electrospinning, resulting in reduced inflammation of the tracheal mucosa [106]. A subsequent study revealed that the release of doxycycline could be controlled by adjusting the compositional ratio between the shell and core polymers [105]. Another stent design, using an arsenic trioxide-eluting poly-L-lactide-caprolactone nanofiber, also inhibited granulation tissue cell growth in vitro. A notable burst release of ATO was observed, where almost complete drug release was achieved within one day [100]. These studies warrant additional investigation into the relationships between drug choice, the polymer used, and the fabrication methods for developing drug-eluting systems.
The potential of various biodegradable polymers in treating airway obstructions was explored. In vivo experiments conducted in rabbits by a specific study revealed that PLA and PLGA coatings effectively suppressed granulation tissue. PLGA-based coatings degraded within the trachea within 10 days, whereas PLA-based coatings partially retained their structure [Figure 4 (right)] [132]. The study emphasized the critical role of polymer choice in ensuring compatibility with drug loading and release. Moreover, efforts have been directed towards utilizing polymer-coated stents for co-delivering multiple therapies. An airway stent, designed to be dual-functional and coated with silver nanoparticles (AgNPs) and cisplatin (DDP) using electrospinning and a polycaprolactone fiber film as a drug carrier, demonstrated DDP’s inhibition of lung fibroblast proliferation, while AgNPs exhibited favorable biocompatibility without significant inhibition [Figure 4 (left)]. The authors harnessed AgNPs for their bactericidal properties and found no interference with cisplatin’s therapeutic effects when co-loaded together [103]. This dual-functional, surface-coated stent underscores the potential of delivering two therapeutic agents within a single drug delivery system.
Figure 4.
(left) General schematic of electrospinning DDP and AgNPs onto a polymer-coated metal stent, with the goal of preventing trachea stenosis. (right) (a) X-ray and (b,c) endoscopic images were taken following stent implantation in a rabbit model. (d) Comparison of stent-induced granulation among harvested rabbit tracheas 10 days after implantation without a stent, with an uncoated stent, and the experimental stent coated with 3 types of polymers eluting methylprednisolone. Adapted from [103] and [132]. Copyright Elsevier and MDPI.
On the other hand, bioabsorbable tracheal stents have emerged as an alternative to obviate the need for an additional procedure to remove metallic stents, as they gradually degrade within the body. A novel bioabsorbable tracheal stent composed of poly-L-lactide (PLLA) and polycaprolactone (PCL) with MMC drug elution, was developed and evaluated in a randomized animal. The bioabsorbable tubular stents with MMC exhibited optimal performance, demonstrating minimal mucus trapping and airway obstruction [133]. Another bioabsorbable PCL stent was designed with cisplatin eluting PLGA coating. They observed sustained drug release for at least four weeks in vitro, and cisplatin levels were present in the rabbit trachea for up to five weeks in vivo [134]. These findings highlight the comparable physical properties of these bioabsorbable stents to SEMS and their promising potential as a localized therapy option for the effective treatment of malignant airway obstruction. However, the actual response of the stent needs to be further tested on diseased rabbit trachea following the current clinical practice.
Two compositions of polymers for bioabsorbable drug-eluting stents were explored by Duvvuri et al. for LTS treatment: PLLA-PCL and PDLGA. The PLLA-PCL stent exhibited superior mechanical strength and reliable drug release (30%) of rapamycin over a 6-week period compared to the PDLGA stent, which only exhibited a release of less than 1% [Figure 5]. In vitro experiments showed that the rapamycin-eluting stent reduced collagen levels and fibroblast cell proliferation. In an LTS mouse model, the PLLA-PCL rapamycin-eluting stents effectively reduced lamina propria thickness and levels of collagen, TGFβ, and a-SMA [96]. Further experiments should increase the number of samples and other animal models should be considered as well.
Figure 5.
(a) Comparing the in vitro rapamycin release for 6 weeks between the PDLGA stent and the PLLA-PCL stent. (b) Exposure to physiological conditions for 21 days demonstrates that the PDLGA stent (bottom images) degrades more than the PLLA-PCL stent (top images), eventually losing its structural integrity. The stents’ compressive strength were measured using Young’s Modulus when applied with a 1-N force. (c) Initially, the PLLA-PCL stent elicits greater stiffness. (d) After 3 weeks, a reduction of Young’s Modulus was present in both groups, but the PLLA-PCL stent maintains its cylindrical stability while the PDLGA stent completely collapses. These measurements were taken at 37°C. Adapted from [96]. Copyright Royal Society of Chemistry.
In conclusion, innovative approaches in upper airway stenosis treatment show promise. Bioabsorbable stents with MMC and cisplatin prevent stenosis and minimize complications. Coated stents with doxycycline and nanofiber materials inhibit inflammation and tissue hyperplasia. Drug-eluting stents with rapamycin and arsenic trioxide reduce collagen deposition and promote tissue healing. Further research is needed for optimization and clinical validation. These advancements offer hope for improved respiratory health and better quality of life for patients.
5.3.2.2. Polymer-coated endotracheal tube
An ETT is a vital medical device used in critical care and surgery to maintain an open airway and assist breathing in patients unable to do so on their own [135]. There have been some developments in polymer-coated ETTs for drug elution to mitigate the initiation of the wound healing processes in the upper airway following intubation.
One ETT was coated with electrospun PLGA nanofibers and loaded with mometasone furoate (MF), a corticosteroid, and their design demonstrated a sustained drug release profile in vitro. The fiber coating remained intact during intubation and extubation, showing strong adhesion even after prolonged exposure to aqueous solution at body temperature. In an in vivo study with rats, MF-coated ETTs showed therapeutic benefits compared to bare ETTs, leading to reduced laryngeal mucosal thickness and submucosal laryngeal edema [136]. Their continued work evaluated the effectiveness of a self-designed, drug-eluting ETT in reducing complications caused by intubation, which confirmed reductions of tracheal fibrosis and thickness when comparing to blank ETTs. Scanning electron microscopy images showed severe mucosal surface structural damage in the intubated blank ETT rats, whereas the loaded-ETT group showed only mild damage after 1 week of intubation [137].
Another study explored the influence of localized dexamethasone delivery using an ETT coated with electrospun PCL polymer-mesh on the biomechanical and histological alterations in vocal folds following inhalational burns. After inducing inhalation burns in porcine models, their dexamethasone-loaded ETT coating decreased vocal fold rigidity with extended intubation at 7 days from 3 days following ETT implantation. Interestingly, vocal folds treated with dexamethasone exhibited heightened stiffness and inflammation, distinct from those lacking dexamethasone at the 7-day timepoint [138]. The authors acknowledged limitations in duration of intubation and follow-up, which would represent the ECM changes which occur over longer periods of time and may not be seen acutely. While histological indications displayed relative stability, this study provided insight into the nuanced repercussions of targeted dexamethasone release on early biomechanical and histological shifts within an inhalational burn model.
Further studies should investigate the long-term effects on fibrosis, inflammation, and granulation on the impacted regions of the larynx. These findings highlighted the potential of drug loaded ETTs to improve the standard of care for ETI and effective prophylaxis of associated complications following extubation.
5.3.2.3. Polymer-coated patches
The treatment of laryngeal stenosis with polymer-coated patches is relatively uncommon compared to other delivery systems. Laryngeal patches can be made from autologous tissue or synthetic materials [139,140]. Autologous tissue patches utilize the patient’s own tissue, while synthetic patches provide mechanical support. Although less commonly used, polymer-coated patches can still offer valuable support and aid in healing. The choice of patch material depends on the patient’s needs and the surgeon’s expertise.
For delivering drugs with patch system, an exploration study aimed to develop a novel mucoadhesive patch for laryngotracheal wound coverage that can be applied without sutures, mimicking the gecko’s ability to attach to any surface. They utilized electrospun PCL fibers and polyethylene glycol (PEG)-acrylate flocks to create a composite patch. The mucoadhesive properties of the patch were evaluated, and it was found that increased flocking density correlated with stronger mucoadhesion. The patch exhibited favorable handleability and demonstrated sustained drug release over a period of 21 days. The optimized patch, with a higher flocking density of PCL-PEG-2XFLK, showed promising results in terms of mucoadhesion strength, composite stiffness reduction, degradation rate, and drug release. This innovative mucoadhesive patch holds potential for laryngeal and tracheal wound coverage, providing structural support and the ability to deliver drugs for enhanced therapeutic outcomes [141]. Their results were limited to in vitro and ex vivo testing, so further research on the applicability of their drug delivery patch should continue testing with in vivo models and optimize their use in the management of upper airway complications. Overall, these drug-eluting patches offer a unique solution for addressing laryngeal stenosis and restoring laryngeal function. They contribute to the development of personalized treatment approaches that can improve the quality of life for individuals affected by this condition.
6. Conclusion
Upper airway injuries are commonly caused by ETI, which is a widely performed procedure for respiration and to maintain airway patency. The pathophysiology that follows highlights the wound healing processes of fibrosis, inflammation, and granulation, and these are the targets for treatment and discovery of potential alternatives. Current management of the subsequent symptoms do not follow a standard set of guidelines, relying on the otolaryngologist’s discretion. Many therapeutics have shown effectiveness in modulating fibrosis and inflammation at the cellular level. However, their utility is best approached with local application to the upper airway and will generally require multiple office visits as they are limited by short durations of action. Recent developments in drug delivery offer enticing solutions. Nanoparticles and microparticles possess capabilities of versatile modulation and improved cellular uptake, whereas hydrogels and polymer-based approaches can be employed alongside medical implants and yield extended-release profiles. Continued research on these novel approaches will explore the limitations and potential of improved delivery of wound healing therapeutics to the upper airway.
7. Expert opinion
Few ideal solutions currently exist for complex laryngotracheal diseases such as tracheal and subglottic stenosis and vocal fold scarring. The complex three-dimensional anatomy of the larynx and limited access second to its vital role in respiration, phonation, and cough prevents many traditional treatment approaches. Drug delivery technology for the treatment of vocal fold and upper airway diseases has undergone significant advancements in recent years, offering promising solutions for patients suffering from various conditions leveraging advances in manufacturing ability and technology. However, there are still several challenges that need to be addressed, one of which is the long-term delivery of medications to the affected areas. These regions are difficult to access, are exposed to aerodynamic stress, and have limited available real estate to accommodate therapeutics. For example, subglottic and tracheal stenosis, narrowing of the airway beneath the vocal folds, frequently involves employing a balloon and laser or cryotherapy in the operating room to dilate the trachea back towards its normal diameter to support airflow and best allow a patient to breath; nonetheless, the narrowing recurs, and drugs that can slow restenosis currently can only be delivered at the time of surgery or through post operative procedures in the office where a needle is passed through the neck to the site while the patient undergoes an awake bronchoscopy. Even with these procedures, drug delivery and timing are limited. Similarly, vocal fold scarring disrupts the delicate vocal fold epithelium resulting in aberrant vibratory patterns and poor voice quality. Although there are numerous high-quality studies illustrating how abnormal wound healing can result in vocal fold scarring, current technology is lacking to effectively deliver these therapeutics to the vocal folds that vibrate 100–300+ times per second. The studies outlined in this paper describe both some of the promising novel therapeutics to improve wound healing as well as approaches to optimize therapeutic delivery to leverage the benefits of these treatments more precisely.
The emergence of nanotechnology and biomaterials may offer significant long-term solutions to these currently challenging medical conditions if the access and delivery challenges are solved. Nanotechnology utilizing nanomaterials, such as liposomes and nanoparticles, allows us to encapsulate drugs and deliver them precisely to the affected sites. These nanocarriers can protect the drugs from degradation, prolong their release, and facilitate their absorption, ensuring optimal therapeutic outcomes. These approaches build on the work outlined and can be readily adaptable to deliver a variety of therapeutics, creating a foundational technological delivery vessel. Additionally, nanotechnology has potential for personalized medicine, when incorporated with genetic profiling and biomarker analysis, enabling the customization of treatments based on individual patient characteristics. However, the efficacy when nanoparticles were used lasts up to several days to date. Biocompatible and biodegradable materials have also been widely used to develop coatings or scaffolds for drug delivery systems, such as stents or patches. Such materials significantly prolong the drug delivery efficacy, up to several weeks, and minimize the risk of tissue damage and adverse reactions, promoting the overall safety and effectiveness of the treatment. Therefore, if nanotechnology and the biocompatible polymeric materials are combined, targeted, personalized, and prolonged treatment methods can be created. For example, an injectable polymeric capsule implant that encapsulates liposomal drug/siRNA can be safely and conveniently inserted to the affected area for a prolonged period for effective upper airway treatment [142]. In conclusion, experts in the field of drug delivery technology for vocal fold and upper airway diseases are optimistic about the transformative potential of nanotechnology, biomaterials, and personalized medicine. As research and innovation continue to progress, these technologies hold the promise of revolutionizing the treatment landscape for patients suffering from vocal fold and upper airway diseases, ultimately improving their quality of life and overall well-being. Continued advancement in this field is necessary so that patients can breathe and communicate better.
Article highlights.
Endotracheal intubation is a commonly performed procedure in several healthcare settings, but intubation trauma and prolonged use often lead to injury.
The unique and highly functional anatomy of the upper airway poses challenges for drug delivery, where wound healing therapies target the fibrotic and inflammatory processes.
This review covers the latest developments in laryngotracheal applications of particulate- and bulk-based drug delivery, where their potential, challenges, and utility are discussed.
Novel strategies may improve treatment through modulable characteristics, enhanced uptake, and long-term therapeutic delivery.
Funding
This work was supported by the National Institute of Deafness and Other Communication Disorders under Grant [1R21DC021038-01], the National Institute of Biomedical Imaging and Bioengineering under Grant [1R21EB034840], and Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2UL1TR001425.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- [1].Durbin CG Jr., Bell CT, Shilling AM. Elective intubation. Respir Care. 2014. Jun;59(6):825–46; discussion 847–9. [DOI] [PubMed] [Google Scholar]
- [2].Nadeem AUR, Gazmuri RJ, Waheed I, et al. Adherence to Evidence-Base Endotracheal Intubation Practice Patterns by Intensivists and Emergency Department Physicians. J Acute Med. 2017. Jun 1;7(2):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lovett PB, Flaxman A, Sturmann KM, et al. The insecure airway: a comparison of knots and commercial devices for securing endotracheal tubes. BMC Emerg Med. 2006. May 24;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cairns CK, Kai. National Hospital Ambulatory Medical Care Survey: 2020 Emergency Department Summary Tables. National Center for Health Statistic (U.S.); 2020. [Google Scholar]
- [5].Parish AJ, West JR, Caputo ND, et al. Early Intubation and Increased Coronavirus Disease 2019 Mortality: A Propensity Score-Matched Retrospective Cohort Study. Crit Care Explor. 2021. Jun;3(6):e0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hillel AT, Karatayli-Ozgursoy S, Samad I, et al. Predictors of Posterior Glottic Stenosis: A Multi-Institutional Case-Control Study. Ann Otol Rhinol Laryngol. 2016. Mar;125(3):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Katsantonis N-G, Kabagambe EK, Wootten CT, et al. Height is an independent risk factor for postintubation laryngeal injury. The Laryngoscope. 2018;128(12):2811–2814. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Z. Mechanics of human voice production and control. J Acoust Soc Am. 2016. Oct;140(4):2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiang J, Lin E, Hanson DG. Vocal fold physiology. Otolaryngol Clin North Am. 2000. Aug;33(4):699–718. [DOI] [PubMed] [Google Scholar]
- [10].Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996. Nov;115(5):474–82. [DOI] [PubMed] [Google Scholar]
- [11]. Pacheco-Lopez PC, Berkow LC, Hillel AT, et al. Complications of airway management. Respir Care. 2014. Jun;59(6):1006–19; discussion 1019–21. • This article provides an outline on the injury and pathophysiology related to endotracheal intubation.
- [12].Tsukamoto M, Taura S, Hitosugi T, et al. A Case of Laryngeal Granulomas After Oral and Maxillofacial Surgery With Prolonged Intubation. Anesth Prog. 2021. Jun 1;68(2):94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martins RHG, Dias NH, Soares CSP, et al. Treatment of Laryngeal Granulomas. Int Arch Otorhinolaryngol. 2019. Jul;23(3):e322–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tikka T, Hilmi OJ. Upper airway tract complications of endotracheal intubation. Br J Hosp Med (Lond). 2019. Aug 2;80(8):441–447. [DOI] [PubMed] [Google Scholar]
- [15].Brodsky MB, Levy MJ, Jedlanek E, et al. Laryngeal Injury and Upper Airway Symptoms After Oral Endotracheal Intubation With Mechanical Ventilation During Critical Care: A Systematic Review. Crit Care Med. 2018. Dec;46(12):2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brodsky MB, Akst LM, Jedlanek E, et al. Laryngeal Injury and Upper Airway Symptoms After Endotracheal Intubation During Surgery: A Systematic Review and Meta-analysis. Anesth Analg. 2021. Apr 1;132(4):1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rafii B, Sridharan S, Taliercio S, et al. Glucocorticoids in laryngology: a review. Laryngoscope. 2014. Jul;124(7):1668–73. [DOI] [PubMed] [Google Scholar]
- [18]. Govil N, Rafii BY, Paul BC, et al. Glucocorticoids for vocal fold disease: a survey of otolaryngologists. J Voice. 2014. Jan;28(1):82–7. • Describes the variability of steroid use between otolaryngologists for vocal fold treatment.
- [19].Lorenz RR. Adult laryngotracheal stenosis: etiology and surgical management. Curr Opin Otolaryngol Head Neck Surg. 2003. Dec;11(6):467–72. [DOI] [PubMed] [Google Scholar]
- [20].Wright CD, Li S, Geller AD, et al. Postintubation Tracheal Stenosis: Management and Results 1993 to 2017. Ann Thorac Surg. 2019. Nov;108(5):1471–1477. [DOI] [PubMed] [Google Scholar]
- [21].Franco RA, Husain I, Reder L, et al. Awake serial intralesional steroid injections without surgery as a novel targeted treatment for idiopathic subglottic stenosis. The Laryngoscope. 2018;128(3):610–617. [DOI] [PubMed] [Google Scholar]
- [22].Cohen SM, Kim J, Roy N, et al. Direct health care costs of laryngeal diseases and disorders. Laryngoscope. 2012. Jul;122(7):1582–8. [DOI] [PubMed] [Google Scholar]
- [23].Wang CT, Lai MS, Cheng PW. Long-term Surveillance Following Intralesional Steroid Injection for Benign Vocal Fold Lesions. JAMA Otolaryngol Head Neck Surg. 2017. Jun 1;143(6):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yin LX, Padula WV, Gadkaree S, et al. Health Care Costs and Cost-effectiveness in Laryngotracheal Stenosis. Otolaryngol Head Neck Surg. 2019. Apr;160(4):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kitahara S, Masuda Y, Kitagawa Y. Vocal fold injury following endotracheal intubation. J Laryngol Otol. 2005. Oct;119(10):825–7. [DOI] [PubMed] [Google Scholar]
- [26].Imaizumi M, Nakamura R, Nakaegawa Y, et al. Regenerative potential of basic fibroblast growth factor contained in biodegradable gelatin hydrogel microspheres applied following vocal fold injury: Early effect on tissue repair in a rabbit model. Braz J Otorhinolaryngol. 2021. May-Jun;87(3):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Young WG, Hoffman MR, Koszewski IJ, et al. Voice Outcomes following a Single Office-Based Steroid Injection for Vocal Fold Scar. Otolaryngol Head Neck Surg. 2016. Nov;155(5):820–828. [DOI] [PubMed] [Google Scholar]
- [28].Pena J, Cicero R, Marin J, et al. Laryngotracheal reconstruction in subglottic stenosis: an ancient problem still present. Otolaryngol Head Neck Surg. 2001. Oct;125(4):397–400. [DOI] [PubMed] [Google Scholar]
- [29].Koshkareva Y, Gaughan JP, Soliman AM. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol. 2007. Mar;116(3):206–10. [DOI] [PubMed] [Google Scholar]
- [30].Davis RJ, Hillel AT. Inflammatory pathways in the pathogenesis of iatrogenic laryngotracheal stenosis: what do we know? Translational Cancer Research. 2020;9(3):2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dorris ER, Russell J, Murphy M. Post-intubation subglottic stenosis: aetiology at the cellular and molecular level. European Respiratory Review. 2021;30(159). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chang E, Wu L, Masters J, et al. Iatrogenic subglottic tracheal stenosis after tracheostomy and endotracheal intubation: A cohort observational study of more severity in keloid phenotype. Acta Anaesthesiol Scand. 2019. Aug;63(7):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aibara S, Okada M, Tanaka-Nishikubo K, et al. Laryngeal complications after endotracheal intubation and prone positioning in patients with coronavirus disease 2019. Laryngoscope Investig Otolaryngol. 2022. Dec;7(6):1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Çomunoğlu N, Batur Ş, Mine Önenerk A. Pathology of Nonneoplastic Lesions of the Vocal Folds. Voice and Swallowing Disorders; 2020. [Google Scholar]
- [35].Jacobson BH, Johnson A, Grywalski C, et al. The Voice Handicap Index (VHI). American Journal of Speech-Language Pathology. 1997;6(3):66–70. [Google Scholar]
- [36].Biddle AK, Watson LR, Hooper CR, et al. Criteria for determining disability in speech-language disorders. Evid Rep Technol Assess (Summ). 2002. Jan(52):1–4. [PMC free article] [PubMed] [Google Scholar]
- [37].Rosen CA, Lee AS, Osborne J, et al. Development and validation of the voice handicap index-10. Laryngoscope. 2004. Sep;114(9):1549–56. [DOI] [PubMed] [Google Scholar]
- [38].Hirano M. Clinical Examination of Voice. Springer-Verlag; 1981. [Google Scholar]
- [39].Solomon NP, Garlitz SJ, Milbrath RL. Respiratory and laryngeal contributions to maximum phonation duration. J Voice. 2000. Sep;14(3):331–40. [DOI] [PubMed] [Google Scholar]
- [40].Barsties VLB, Watts CR, Neumann K. The effectiveness of voice therapy on voice-related handicap: A network meta-analysis. Clin Otolaryngol. 2020. Sep;45(5):796–804. [DOI] [PubMed] [Google Scholar]
- [41].Barsties v. Latoszek B, Watts CR, Schwan K, et al. The maximum phonation time as marker for voice treatment efficacy: A network meta-analysis. Clinical Otolaryngology. 2022;48(2):130–138. [DOI] [PubMed] [Google Scholar]
- [42].Song SA, Sandhu G, Franco RA. Should We Routinely Use Pulmonary Function Testing in the Management of Subglottic Stenosis? The Laryngoscope. 2020;131(2):245–247. [DOI] [PubMed] [Google Scholar]
- [43].Bhatt NK, Huang VP, Bertelsen C, et al. Pulmonary Function Tests May Better Predict Dyspnea-Severity in Patients with Subglottic Stenosis Compared to Clinician-Reported Stenosis. Ann Otol Rhinol Laryngol. 2022. Jul;131(7):791–796. [DOI] [PubMed] [Google Scholar]
- [44].Woo P. Quantification of videostrobolaryngoscopic findings--measurements of the normal glottal cycle. Laryngoscope. 1996. Mar;106(3 Pt 2 Suppl 79):1–27. [DOI] [PubMed] [Google Scholar]
- [45].Woo P. Objective Measures of Laryngeal Imaging: What Have We Learned Since Dr. Paul Moore. Journal of Voice. 2014;28(1):69–81. [DOI] [PubMed] [Google Scholar]
- [46].Svec JG, Schutte HK. Videokymography: high-speed line scanning of vocal fold vibration. J Voice. 1996. Jun;10(2):201–5. [DOI] [PubMed] [Google Scholar]
- [47]. Dion GR, Guda T, Mukudai S, et al. Quantifying vocal fold wound-healing biomechanical property changes. Laryngoscope. 2020. Feb;130(2):454–459. • Investigation of biomechanical and histological changes in the vocal folds following injury.
- [48].Tran QT, Berke GS, Gerratt BR, et al. Measurement of Young’s modulus in the in vivo human vocal folds. Ann Otol Rhinol Laryngol. 1993. Aug;102(8 Pt 1):584–91. [DOI] [PubMed] [Google Scholar]
- [49].Tanaka S, Hirano M. Fiberscopic estimation of vocal fold stiffness in vivo using the sucking method. Arch Otolaryngol Head Neck Surg. 1990. Jun;116(6):721–4. [DOI] [PubMed] [Google Scholar]
- [50].Hsiao TY, Wang CL, Chen CN, et al. Elasticity of human vocal folds measured in vivo using color Doppler imaging. Ultrasound Med Biol. 2002. Sep;28(9):1145–52. [DOI] [PubMed] [Google Scholar]
- [51].Kniesburges S, Thomson SL, Barney A, et al. In vitro experimental investigation of voice production. Curr Bioinform. 2011. Sep 1;6(3):305–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dion GR, Lavoie JF, Coelho P, et al. Automated Indentation Mapping of Vocal Fold Structure and Cover Properties Across Species. Laryngoscope. 2019. Jan;129(1):E26–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dion GR, Jeswani S, Roof S, et al. Functional assessment of the ex vivo vocal folds through biomechanical testing: A review. Materials Science and Engineering: C. 2016;64:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dion GR, Coelho PG, Teng S, et al. Dynamic nanomechanical analysis of the vocal fold structure in excised larynges. Laryngoscope. 2017. Jul;127(7):E225–E230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mortensen M. Laryngeal steroid injection for vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010. Dec;18(6):487–91. [DOI] [PubMed] [Google Scholar]
- [56].Hillel AT, Lin LM, Samlan R, et al. Inhaled triamcinolone with proton pump inhibitor for treatment of vocal process granulomas: a series of 67 granulomas. Ann Otol Rhinol Laryngol. 2010. May;119(5):325–30. [DOI] [PubMed] [Google Scholar]
- [57].Campagnolo AM, Tsuji DH, Sennes LU, et al. Histologic study of acute vocal fold wound healing after corticosteroid injection in a rabbit model. Ann Otol Rhinol Laryngol. 2010. Feb;119(2):133–9. [DOI] [PubMed] [Google Scholar]
- [58].Amin MR, Achlatis S, Gherson S, et al. The Role of Oral Steroids in the Treatment of Phonotraumatic Vocal Fold Lesions in Women. Otolaryngol Head Neck Surg. 2019. Mar;160(3):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang CT, Lai MS, Hsiao TY. Comprehensive Outcome Researches of Intralesional Steroid Injection on Benign Vocal Fold Lesions. J Voice. 2015. Sep;29(5):578–87. [DOI] [PubMed] [Google Scholar]
- [60].Rahbar R, Shapshay SM, Healy GB. Mitomycin: effects on laryngeal and tracheal stenosis, benefits, and complications. Ann Otol Rhinol Laryngol. 2001. Jan;110(1):1–6. [DOI] [PubMed] [Google Scholar]
- [61].Fawaz SA, Sabri SM, Sweed AS, et al. Use of local mitomycin C in enhancing laryngeal healing after laser cordotomy: a prospective controlled study. Head Neck. 2014. Sep;36(9):1248–52. [DOI] [PubMed] [Google Scholar]
- [62].Feinstein AJ, Goel A, Raghavan G, et al. Endoscopic Management of Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg. 2017. May 1;143(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Montes-Worboys A, Gilabert-Porres J, Lopez-Lisbona R, et al. Effect of paclitaxel delivered nanoparticles to treat tracheal stenosis. 1.4 Interventional Pulmonology 2015. [Google Scholar]
- [64].Qiu X-j, Zhang J, Wang J, et al. Application of paclitaxel as adjuvant treatment for benign cicatricial airway stenosis. Journal of Huazhong University of Science and Technology [Medical Sciences]. 2016;36(6):817–822. [DOI] [PubMed] [Google Scholar]
- [65].Rosow DE, Ahmed J. Initial Experience With Low-Dose Methotrexate as an Adjuvant Treatment for Rapidly Recurrent Nonvasculitic Laryngotracheal Stenosis. JAMA Otolaryngology–Head & Neck Surgery. 2017;143(2). [DOI] [PubMed] [Google Scholar]
- [66].Kanazawa T, Komazawa D, Indo K, et al. Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope. 2015. Oct;125(10):E338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ban MJ, Park JH, Kim JW, et al. The Efficacy of Fibroblast Growth Factor for the Treatment of Chronic Vocal Fold Scarring: From Animal Model to Clinical Application. Clin Exp Otorhinolaryngol. 2017. Dec;10(4):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hirano S, Sugiyama Y, Kaneko M, et al. Intracordal Injection of Basic Fibroblast Growth Factor in 100 Cases of Vocal Fold Atrophy and Scar. Laryngoscope. 2021. Sep;131(9):2059–2064. [DOI] [PubMed] [Google Scholar]
- [69].Nozawa M, Takahashi S, Kanazawa T, et al. Intracordal injection therapy for vocal fold scarring: Steroid versus basic fibroblast growth factor. Laryngoscope Investig Otolaryngol. 2022. Oct;7(5):1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wingstrand VL, Gronhoj Larsen C, Jensen DH, et al. Mesenchymal Stem Cell Therapy for the Treatment of Vocal Fold Scarring: A Systematic Review of Preclinical Studies. PLoS One. 2016;11(9):e0162349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jakobsen KK, Gronhoj C, Jensen DH, et al. Mesenchymal stem cell therapy for laryngotracheal stenosis: A systematic review of preclinical studies. PLoS One. 2017;12(9):e0185283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hertegard S, Nagubothu SR, Malmstrom E, et al. Treatment of vocal fold scarring with autologous bone marrow-derived human mesenchymal stromal cells-first phase I/II human clinical study. Stem Cell Res Ther. 2020. Mar 20;11(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kodama H, Kumai Y, Nishimoto K, et al. Potential treatment for vocal fold scar with pirfenidone. Laryngoscope. 2018. May;128(5):E171–E177. [DOI] [PubMed] [Google Scholar]
- [74].Turkmen E, Pata YS. Prevention of tracheal stenosis with pirfenidone after tracheotomy: An experimental study. Laryngoscope. 2019. May;129(5):E178–E186. [DOI] [PubMed] [Google Scholar]
- [75].Li X, Pan J, Qian H, et al. Treatment of scarring central airway stenosis with pirfenidone: Case report. Medicine (Baltimore). 2022. Oct 28;101(43):e31354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Matsumoto K, Nakamura T. Hepatocyte Growth Factor (HGF) as a Tissue Organizer for Organogenesis and Regeneration. Biochemical and Biophysical Research Communications. 1997;239(3):639–644. [DOI] [PubMed] [Google Scholar]
- [77].Hirano S, Bless DM, Rousseau B, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004. Mar;114(3):548–56. [DOI] [PubMed] [Google Scholar]
- [78].Luo Y, Kobler JB, Zeitels SM, et al. Effects of Growth Factors on Extracellular Matrix Production by Vocal Fold Fibroblasts in 3-Dimensional Culture. Tissue Engineering. 2006;12(12):3365–3374. [DOI] [PubMed] [Google Scholar]
- [79].Kishimoto Y, Hirano S, Suehiro A, et al. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2009. Aug;118(8):606–11. [DOI] [PubMed] [Google Scholar]
- [80].Mizuta M, Hirano S, Kishimoto Y, et al. Pharmacokinetics and safety of human recombinant hepatocyte growth factor administered to vocal folds. Laryngoscope. 2014. Sep;124(9):2131–5. [DOI] [PubMed] [Google Scholar]
- [81]. Kolachala VL, Henriquez OA, Shams S, et al. Slow-release nanoparticle-encapsulated delivery system for laryngeal injection. Laryngoscope. 2010. May;120(5):988–94. •• Prolonged release of Texas Red dye in the vocal folds when encapsulated in nanoparticles.
- [82].Choi JS, Heang Oh S, Kim YM, et al. Hyaluronic Acid/Alginate Hydrogel Containing Hepatocyte Growth Factor and Promotion of Vocal Fold Wound Healing. Tissue Eng Regen Med. 2020. Oct;17(5):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dillard DG, Gal AA, Roman-Rodriguez J, et al. Transforming growth factor and neutralizing antibodies in subglottic stenosis. Ann Otol Rhinol Laryngol. 2001. May;110(5 Pt 1):393–400. [DOI] [PubMed] [Google Scholar]
- [84].Simpson CB, White S, McGuff HS. Anti-transforming growth factor beta as a treatment for laryngotracheal stenosis in a canine model. Laryngoscope. 2008. Mar;118(3):546–51. [DOI] [PubMed] [Google Scholar]
- [85].Wei P, Huang Z, Gan L, et al. Nintedanib ameliorates tracheal stenosis by activating HDAC2 and suppressing IL-8 and VEGF in rabbit. Am J Transl Res. 2020;12(8):4739–4748. [PMC free article] [PubMed] [Google Scholar]
- [86].Fan Y, Li X, Fang X, et al. Antifibrotic Role of Nintedanib in Tracheal Stenosis After a Tracheal Wound. Laryngoscope. 2021. Sep;131(9):E2496-E2505. [DOI] [PubMed] [Google Scholar]
- [87].Friedrich M, Aigner A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs. 2022. Sep;36(5):549–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Paul BC, Rafii BY, Gandonu S, et al. Smad3: an emerging target for vocal fold fibrosis. Laryngoscope. 2014. Oct;124(10):2327–31. [DOI] [PubMed] [Google Scholar]
- [89].Ito S, Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol. 2017. Feb;62:142–151. [DOI] [PubMed] [Google Scholar]
- [90].Hiwatashi N, Kraja I, Benedict PA, et al. Nanoparticle delivery of RNA-based therapeutics to alter the vocal fold tissue response to injury. Laryngoscope. 2018. May;128(5):E178–E183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mukudai S, Kraja I, Bing R, et al. Implementing Efficient Peptoid-Mediated Delivery of RNA-Based Therapeutics to the Vocal Folds. Laryngoscope Investig Otolaryngol. 2019. Dec;4(6):640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Kishimoto Y, Yamashita M, Wei A, et al. Reversal of Vocal Fold Mucosal Fibrosis Using siRNA against the Collagen-Specific Chaperone Serpinh1. Mol Ther Nucleic Acids. 2019. Jun 7;16:616–625. •• Liposomal delivery of siRNA protected against degradation and downregulated SERPINH1 expression.
- [93].Shlomi D, Peled N, Shitrit D, et al. Protective effect of immunosuppression on granulation tissue formation in metallic airway stents. Laryngoscope. 2008. Aug;118(8):1383–8. [DOI] [PubMed] [Google Scholar]
- [94].Mizokami D, Araki K, Tanaka N, et al. Tacrolimus prevents laryngotracheal stenosis in an acute-injury rat model. Laryngoscope. 2015. Jun;125(6):E210–5. [DOI] [PubMed] [Google Scholar]
- [95].Namba DR, Ma G, Samad I, et al. Rapamycin inhibits human laryngotracheal stenosis-derived fibroblast proliferation, metabolism, and function in vitro. Otolaryngol Head Neck Surg. 2015. May;152(5):881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Duvvuri M, Motz K, Murphy M, et al. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater Sci. 2019. Apr 23;7(5):1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].You BR, Park WH. Arsenic trioxide induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion. Oncol Rep. 2012. Aug;28(2):749–57. [DOI] [PubMed] [Google Scholar]
- [98].Luo F, Zhuang Y, Sides MD, et al. Arsenic trioxide inhibits transforming growth factor-beta1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res. 2014. Apr 24;15(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhong L, Hao H, Chen D, et al. Arsenic trioxide inhibits the differentiation of fibroblasts to myofibroblasts through nuclear factor erythroid 2-like 2 (NFE2L2) protein and the Smad2/3 pathway. Journal of Cellular Physiology. 2018;234(3):2606–2617. [DOI] [PubMed] [Google Scholar]
- [100].Li Y, Li M, Wang X, et al. Arsenic trioxide-eluting electrospun nanofiber-covered self-expandable metallic stent reduces granulation tissue hyperplasia in rabbit trachea. Biomed Mater. 2020. Dec 16;16(1):015013. [DOI] [PubMed] [Google Scholar]
- [101].Nakazono-Kusaba A, Takahashi-Yanaga F, Morimoto S, et al. Staurosporine-induced cleavage of alpha-smooth muscle actin during myofibroblast apoptosis. J Invest Dermatol. 2002. Nov;119(5):1008–13. [DOI] [PubMed] [Google Scholar]
- [102].Xu H, Wang Q, Fan GK. The Antiproliferative and Antifibrotic Effects of Cisplatin on Primary Human Vocal Fold Fibroblasts. ORL J Otorhinolaryngol Relat Spec. 2020;82(4):188–200. [DOI] [PubMed] [Google Scholar]
- [103].Li Z, Tian C, Jiao D, et al. Synergistic effects of silver nanoparticles and cisplatin in combating inflammation and hyperplasia of airway stents. Bioact Mater. 2022. Mar;9:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lamparter S, Slight SH, Weber KT. Doxycycline and tissue repair in rats. J Lab Clin Med. 2002. May;139(5):295–302. [DOI] [PubMed] [Google Scholar]
- [105].Baskaran R, Ko UJ, Davaa E, et al. Doxycycline-Eluting Core-Shell Type Nanofiber-Covered Trachea Stent for Inhibition of Cellular Metalloproteinase and Its Related Fibrotic Stenosis. Pharmaceutics. 2019. Aug 19;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Choi JS, Kim JM, Kim JW, et al. Prevention of tracheal inflammation and fibrosis using nitinol stent coated with doxycycline. Laryngoscope. 2018. Jul;128(7):1558–1563. [DOI] [PubMed] [Google Scholar]
- [107].Kim HS, Yoon YM, Meang MK, et al. Reversion of in vivo fibrogenesis by novel chromone scaffolds. EBioMedicine. 2019. Jan;39:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Park SJ, Choi H, Kim JH, et al. Antifibrotic effects of eupatilin on TGF-beta1-treated human vocal fold fibroblasts. PLoS One. 2021;16(3):e0249041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yao Y, Zhou Y, Liu L, et al. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci. 2020;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ohno T, Hirano S, Kanemaru S, et al. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2007. Oct;116(10):762–9. [DOI] [PubMed] [Google Scholar]
- [111].Erndt-Marino JD, Jimenez-Vergara AC, Diaz-Rodriguez P, et al. In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment. J Biomed Mater Res B Appl Biomater. 2018. Apr;106(3):1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kosinski AM, Pothen JM, Panitch A, et al. Dexamethasone Controlled Release on TGF-beta1 Treated Vocal Fold Fibroblasts. Ann Otol Rhinol Laryngol. 2015. Jul;124(7):572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry. 2019;12(7):908–931. [Google Scholar]
- [114].Auffan M, Rose J, Bottero JY, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009. Oct;4(10):634–41. [DOI] [PubMed] [Google Scholar]
- [115].Baig N, Kammakakam I, Falath W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Materials Advances. 2021;2(6):1821–1871. [Google Scholar]
- [116].Kopecek J. Hydrogels from Soft Contact Lenses and Implants to Self-Assembled Nanomaterials. J Polym Sci A Polym Chem. 2009. Nov 15;47(22):5929–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008. Dec 14;60(15):1638–49. [DOI] [PubMed] [Google Scholar]
- [118].Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016. Dec;1(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- [120].Gerecht S, Burdick JA, Ferreira LS, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007. Jul 3;104(27):11298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim YM, Oh SH, Choi JS, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 2014. Mar;124(3):E64–72. [DOI] [PubMed] [Google Scholar]
- [122].Kobayashi T, Mizuta M, Hiwatashi N, et al. Drug delivery system of basic fibroblast growth factor using gelatin hydrogel for restoration of acute vocal fold scar. Auris Nasus Larynx. 2017. Feb;44(1):86–92. [DOI] [PubMed] [Google Scholar]
- [123].Murata H, Chang BJ, Prucker O, et al. Polymeric coatings for biomedical devices. Surface Science. 2004;570(1–2):111–118. [Google Scholar]
- [124].Qiang Wei RH. Universal polymer coatings and their representative biomedical applications. Materials Horizons. 2015;2:567–577. [Google Scholar]
- [125].Oh AJNaTH. Biopolymer Coatings for Biomedical Applications. Polymers. 2020;12(12):3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Johnson CM, Luke AS, Jacobsen C, et al. State of the Science in Tracheal Stents: A Scoping Review. Laryngoscope. 2022. Nov;132(11):2111–2123. [DOI] [PubMed] [Google Scholar]
- [127].Wahidi MM, Ernst A. The Montgomery T-tube tracheal stent. Clin Chest Med. 2003. Sep;24(3):437–43. [DOI] [PubMed] [Google Scholar]
- [128].Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration. 2017;93(6):430–435. [DOI] [PubMed] [Google Scholar]
- [129].Chen DF, Chen Y, Zhong CH, et al. Long-term efficacy and safety of the Dumon stent for benign tracheal stenosis: a meta-analysis. J Thorac Dis. 2021. Jan;13(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gompelmann D, Eberhardt R, Schuhmann M, et al. Self-expanding Y stents in the treatment of central airway stenosis: a retrospective analysis. Ther Adv Respir Dis. 2013. Oct;7(5):255–63. [DOI] [PubMed] [Google Scholar]
- [131]. Terrier B, Dechartres A, Girard C, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicentre experience. Rheumatology (Oxford). 2015. Oct;54(10):1852–7. •• Liposomal delivery of siRNA protected against degradation and downregulated SERPINH1 expression.
- [132].Sindeeva OA, Prikhozhdenko ES, Schurov I, et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics. 2021. Sep 9;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zhu GH, Ng AH, Venkatraman SS, et al. A novel bioabsorbable drug-eluting tracheal stent. Laryngoscope. 2011. Oct;121(10):2234–9. [DOI] [PubMed] [Google Scholar]
- [134].Chao YK, Liu KS, Wang YC, et al. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: in vivo and in vitro studies. Chest. 2013. Jul;144(1):193–199. [DOI] [PubMed] [Google Scholar]
- [135].Haas CF, Eakin RM, Konkle MA, et al. Endotracheal tubes: old and new. Respir Care. 2014. Jun;59(6):933–52; discussion 952–5. [DOI] [PubMed] [Google Scholar]
- [136].Abu Ammar A, Gruber M, Martin P, et al. Local delivery of mometasone furoate from an eluting endotracheal tube. J Control Release. 2018. Feb 28;272:54–61. [DOI] [PubMed] [Google Scholar]
- [137].Jahshan F, Abu Ammar A, Ertracht O, et al. Local Delivery of Mometasone Furoate from an Eluting Endotracheal Tube Reduces Airway Morbidity Following Long-Term Animal Intubation. ACS Appl Bio Mater. 2021. May 17;4(5):4131–4139. [DOI] [PubMed] [Google Scholar]
- [138].Malka R, Gonzales G, Detar W, et al. Effect of continuous local dexamethasone on tissue biomechanics and histology after inhalational burn in a preclinical model. Laryngoscope Investigative Otolaryngology. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hazekamp MG, Nijdam N. Use of autologous arterial patches for tracheal reconstruction in young infants. Ann Thorac Surg. 2004. Jun;77(6):2262–3; author reply 2263. [DOI] [PubMed] [Google Scholar]
- [140].Anton-Pacheco JL, Morante R. Operative or non-operative treatment of congenital tracheal stenosis: is there something new? J Thorac Dis. 2017. Dec;9(12):4878–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Miar S, Dion GR, Montelongo S, et al. Development of a Bioinspired, Self-Adhering, and Drug-Eluting Laryngotracheal Patch. Laryngoscope. 2021. Sep;131(9):1958–1966. [DOI] [PubMed] [Google Scholar]
- [142].Zheng A, Waterkotte T, Debele T, et al. Biodegradable dexamethasone polymer capsule for long-term release. Korean Journal of Chemical Engineering. 2023;40(2):452–460. [Google Scholar]