Abstract
In recent years, bacteria have gained considerable attention as a promising drug carrier that is critical in improving the effectiveness and reducing the side effects of anti‐tumor drugs. Drug carriers can be utilised in various forms, including magnetotactic bacteria, bacterial biohybrids, minicells, bacterial ghosts and bacterial spores. Additionally, functionalised and engineered bacteria obtained through gene engineering and surface modification could provide enhanced capabilities for drug delivery. This review summarises the current studies on bacteria‐based drug delivery systems for anti‐tumor therapy and discusses the prospects and challenges of bacteria as drug carriers. Furthermore, our findings aim to provide new directions and guidance for the research on bacteria‐based drug systems.
Keywords: anti‐tumor, bacteria, drug delivery system, synthetic biology
In this study, we discuss the application of bacteria or bacterial secretions, including magnetotactic bacteria, bacterial biohybrids, minicells, bacterial ghosts, and bacterial outer membrane vesicles, as drug carriers in the delivery of anti‐tumor drugs. Synthetic biology, chemistry and nanotechnology are widely used in bacteria‐based drug delivery systems to enhance tumor‐targeting ability and therapeutic efficacy. However, achieving clinical translation of bacteria‐based drug delivery systems for anti‐tumor therapy is challenging because of biosafety concerns, regulatory hurdles and ethical concerns.
Introduction
As of 2020, there were approximately 19 292 789 newly reported cancer cases worldwide, with 9 958 133 deaths. 1 Certain types of cancer exhibit low 5‐year survival rates, including pancreatic cancer (11%), oesophageal cancer (20%) and ovarian cancer (49%). 2 Surgery, radiotherapy and chemotherapy are frequently employed in clinical treatment, but they possess certain limitations. 3 , 4 , 5 While surgical treatment can provide curative results for early‐stage tumors, more than 70% of patients receive their diagnosis during the intermediate and advanced stages, so they miss the optimal window for surgery. 6 Consequently, there is an imperative need to explore novel anti‐tumor strategies.
As an emerging method for tumor treatment, nano‐drug delivery system can precisely target tumors and reduce the drug's toxic side effects. Nano‐drugs usually accumulate at the tumor site for the enhanced permeability and retention (EPR) effect. 7 Common nano‐drug delivery systems (PLGA nanoparticles, liposomes and iron nanoparticles) have been utilised for targeted cancer therapy. 8 , 9 However, as a result of the capture of drug carriers by the mononuclear phagocyte system in the liver and spleen, the delivery efficiency of nano‐drugs to solid tumors is only approximately 0.7%. 10 Moreover, complex intratumor environment can impede drug delivery, thereby diminishing the efficacy of anti‐tumor drugs. 11 , 12 , 13 Therefore, it is imperative to develop a drug delivery carrier that possesses clinical accessibility and ensures high delivery efficiency.
Bacteria are acknowledged as highly promising biological nano‐drug carriers and have gained significant attention. First, bacteria and bacterial derivatives possess the inherent ability to target tumors. Several bacterial species (Salmonella Typhimurium, Listeria and Streptococcus) have demonstrated a propensity for selectively targeting solid tumors in preclinical trials. 14 , 15 , 16 , 17 Because of the hypoxic, immunosuppressive and eutrophic characteristics of tumor microenvironment, obligate and facultative anaerobes (Escherichia and Clostridium) can enrich and proliferate at the tumor site. 18 , 19 , 20 Compared with chemically synthesised drug delivery systems, bacteria are expected to overcome the high osmotic pressure of tumors that hinder drug penetration and reduce damage to healthy tissues. Moreover, synthetic biology could facilitate the application of engineered bacteria in drug delivery systems. Under the guidance of engineering ideas, synthetic biology uses multi‐disciplinary techniques, including biology, bioinformatics and computer science to construct controllable genetic, metabolic, or signalling networks to enable cells to synthesise substances that are required for humans. 21 Bacteria can be genetically modified using CRISPR/Cas9 technology to construct targeted drug delivery platforms. 22 Exploiting gene circuits further enables bacteria to deliver nano‐drugs precisely, continuously and synchronously to tumor cells. 23 Furthermore, the innate virulence of bacteria accelerates tumor regression. 24
Bacteria exhibit potential for delivering anti‐tumor drugs, aiding drug accumulation at the tumor site, preventing drug leakage, and ultimately enhancing therapeutic efficacy against tumors. Bacteria have emerged as a promising carrier for the targeted delivery of anti‐tumor drugs, allowing for enhanced accumulation at tumor sites while minimising drug leakage. In this review, the authors aim to promote the utilisation and advancement of bacteria‐based drug delivery systems in anti‐tumor therapy.
Bacteria‐based nano‐drug carriers for anti‐tumor therapy
Bacteria and bacterial secretions have been widely used as promising drug carriers in anti‐tumor therapy. As carriers, various forms of bacteria, including magnetotactic bacteria, bacterial biohybrid nanobots, minicells, bacterial ghosts, bacterial spores and bacterial outer membrane vesicles (OMV), are expected to improve the efficiency of drug delivery (Figure 1a).
Figure 1.
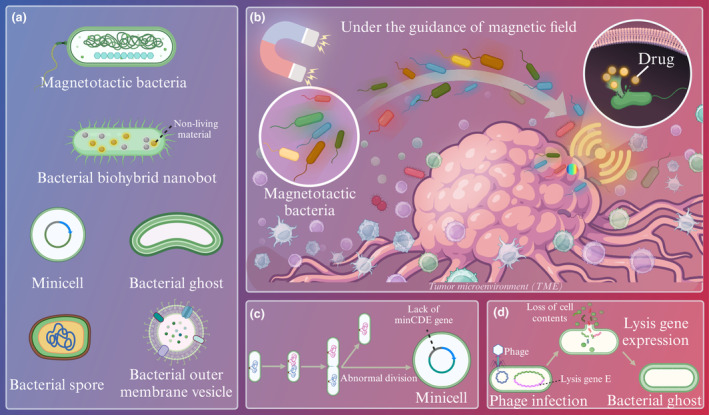
Bacteria‐based nanodrug carrier for anti‐tumor therapy. (a) Bacteria act as nanomedicine carriers in various forms, including magnetotactic bacteria, bacterial biohybrid nanobots, minicells, bacterial ghosts, bacterial spores and bacterial outer membrane vesicles, for drug delivery. (b) The magnetotactic bacteria can be manipulated to target the tumor site by magnetic field to achieve targeted drug delivery. (c) Formation of minicell. (d) Formation of bacterial ghost.
Magnetotactic bacteria
In recent years, magnetic nanodrugs that are guided by external magnetic field have been considered having the potential to overcome the dense extracellular matrix and high tumor interstitial pressure to improve the efficiency of targeted drug delivery. 25 , 26 However, the larger the nanoparticles are, the stronger the magnetic properties are, thus forming aggregates among the magnetic particles and preventing the nanomaterials from penetrating the tumor tissue. Using magnetotactic bacteria as drug carriers can solve the problem that magnetic nano‐drugs penetrating tumor tissue is blocked, and it is expected to improve the efficiency of drug delivery.
Low‐hazard and non‐invasive magnetic fields (MFs) enable the navigation of drug‐carrying bacteria to achieve controlled manipulation of drug delivery 27 , 28 (Figure 1b). Bacteria with magnetic properties can independently migrate deep inside the tumor after entering it through the vessel wall. In addition to naturally occurring bacteria, magnetotactic bacteria can also be obtained by biochemical coupling and biomimetic mineralisation for application in MF‐mediated drug delivery systems. 29 , 30 Once the tumor is localised by MF, imaging techniques are no longer needed to track the bacteria. 31 Because of the bacterial magnetosome that contains iron oxide particles, magnetotactic bacteria can be manipulated by external MF, 32 and they also show high clinical safety, 27 so magnetotactic bacteria are widely used to enable steerable delivery of drugs.
For instance, without chemical modification, the doxorubicin (DOX)‐internalised MSR‐1 swimming bacteria (MSRD) was constructed by co‐incubation. 33 When the tumor mice were administered, MSRD achieved the best anti‐tumor effect, which showed the smallest volume and weight of the tumor as well as the highest number of apoptotic tumor cells. MSRD enhances the anti‐tumor effect of DOX, thus holding great promise for spatio‐manipulable drug delivery in precision medicine.
Bacterial biohybrid nanobots
Bacterial biohybrids, consisting of bacteria and non‐living materials, emerged as a promising approach for drug delivery in anti‐tumor therapy. 34 The structural composition of the bacterial surface enables the adhesion of non‐living materials through physical and chemical interactions. The teichoic acids found on the cell wall of gram‐positive bacteria create a negatively charged bacterial surface, which is essential for attachment through electrostatic interactions. 35 Gram‐negative bacteria possess surfaces abundant in hydrophobic lipids, which facilitates the reduction of surface energy by attaching to hydrophobic materials. For example, Salmonella enterica serovar Typhimurium (S. Typhimurium) rapidly attaches to hydrophobic microcubic surfaces within 5 s. 36 Next, the high‐affinity interaction between biotin and streptomycin is a common chemical strategy that may require the chemical biotinylation of bacteria via gene engineering. 37 , 38
Bacteria can specifically target tumors, allowing bacterial biohybrid nanobots to accumulate at tumor sites. Moreover, bacteria possess the capability to detect environmental stimuli such as pH and temperature, which is vital for the controlled release of drugs. 39 Consequently, bacteria‐based biohybrid nanorobots exhibit enhanced delivery efficiency and lower therapeutic costs in drug delivery systems compared with non‐living carriers, such as liposomes and nanoparticles. 40 For example, researchers developed an Escherichia coli (E. coli)‐based biohybrid nanobot that was loaded with anti‐tumor drugs and magnetic nanoparticles for applications in photothermal therapy. 30 In 3D CRC tumor spheroids, the combined treatment of near‐infrared (NIR) light irradiation and bacterial biohybrid nanobot treatment results in a remarkable seven‐fold increase in doxorubicin (DOX) uptake by the tumor, with outstanding performance.
Minicells
Minicells, about 400 nm, are enucleated cells formed by abnormal cell division 41 (Figure 1c). The absence of the minCDE gene in minicells prevents them from dividing cells and interfering with the normal growth and division of other cells. 42 Because of their high drug loading capacity, safety and low toxicity, minicells have emerged as a focal point in drug delivery systems. 43 The drugs released from minicells can effectively reach the hypoxic zone of the tumor, enhancing efficacy and overcoming drug resistance. 44
In a phase I clinical trial, paclitaxel was delivered using S. Typhimurium‐derived minicells, which showed moderate clinical efficacy and safety. 45 Among the 22 patients who completed at least one cycle of treatment, the minicell‐based delivery system resulted in stable disease in 45%, with only mild self‐limited increases in the inflammatory cytokines IL‐6, IL‐8, TNF‐α and anti‐inflammatory IL‐10 observed. 45 The minicells generated by E. coli Nissle 1917 (EcN) were developed to deliver DOX to breast tumor cells, thus resulting in a smaller tumor volume (494 mm3) compared with the control group in BALB/c mice with orthotopic 4T1 breast cancers. 44 Overall, minicell‐based drug delivery systems have a relatively high safety profile and enhanced anti‐tumor effects.
Bacterial ghosts
Gram‐negative bacteria produce bacterial ghosts (BGs) that are essentially porous empty bacterial shells with about 250 femtoliter per BG intracellular spaces for drug delivery. 46 The expression of lysis gene E on bacteriophages ΦX174 that is achieved via gene engineering can transform gram‐negative bacteria into BGs 47 , 48 (Figure 1d). The protein encoded by gene E leads to the formation of transmembrane tunnel structures that allow the excretion of cytoplasmic contents under osmotic pressure. 49
Bacterial ghosts are favored as a drug carrier for its advantages compared with the non‐living carriers. The safety of BG has been discovered through in vivo and in vitro uptake studies. 50 Afterwards, as a result of the mild production process of BGs, all structural antigens expressed by the bacteria are not destroyed, which allows BGs to retain immunogenicity to stimulate the host's immune system. 51 , 52 Compared with liposomes, as carriers loaded with tumor lysates, BGs have achieved better immunotherapy efficacy. 53 Furthermore, as a natural adjuvant, BGs enhance the immunogenic cell death of tumor cells induced by oxaliplatin. 54 Finally, BGs are highly stable and can be maintained at ambient temperatures for several months. 55
Bacterial spores
In the tumor microenvironment, bacterial spores, a form of survival for bacteria in extremely harsh environments, can adapt to hypoxic conditions. 56 In the gut, a significant number of bacteria can produce spores, accounting for about 50% of the gut bacteria, among which Bacillus subtilis and Clostridium difficile have been extensively studied. 56 , 57 , 58 , 59 Spore growth is highly specific to the tumor site, thus providing bacterial spores a powerful potential as drug carriers. 60 The strategies to exert the carrier potential of bacterial spores include recombinant and non‐recombinant methods. Recombinant methods rely on coating proteins on the surface of bacterial spores, such as CotB and CotC, which are widely used as anchor proteins in vaccine antigen delivery systems. 61 The non‐recombinant methods is to attach the desired protein drug to the spore surface by hydrophobic or electrostatic interactions, or by the use of cross‐linking agents, such as glutaraldehyde. 62
Because of the high stability and resistance of bacterial spores, probiotic spores have great application potential in oral drug delivery systems. 63 , 64 The use of spores as carriers can improve the oral efficacy of drugs with poor chemical stability and pharmacokinetics, because drug‐loaded spores can survive in the gut across the stomach. 64 , 65 A spore‐based oral drug delivery system (SPORE‐MGEM) was constructed, in which Clostridium butyricum is covalently coupled to gemcitabine‐loaded mesoporous silicon nanoparticles (MGEM). 66 The treatment with SPORE‐MGEM results in an approximately three‐fold increase in intratumoral drug accumulation. Moreover, SPORE‐MGEM treatment inhibits 80% of tumor growth in the orthotopic transplanted pancreatic ductal adenocarcinoma mice model. As a carrier, bacterial spores greatly improve the efficiency of oral drug delivery, which is conducive to reducing the toxic side effects of drugs.
Bacterial outer membrane vesicles
Bacterial membrane vesicles are membranous bodies secreted by bacteria and contain thalli active substances. As a class of bacterial membrane vesicles, bacterial OMVs have been widely explored, and they are spherical bilayer nanolipids that are released by gram‐negative bacteria, ranging from 20 to 300 nm. 67 , 68
Firstly, the characteristics of the parental bacteria are inherited, so vesicles can target tumor cells. 69 Additionally, vesicles, containing lipopolysaccharides, pathogen‐related proteins and lipoproteins, are highly immunogenic, so they can stimulate the natural immune system. 70 At present, vesicles are expected to act as carriers and immune adjuvants and be widely used in vaccine platforms. 71 Secondly, vesicles can enter the host cell by directly fusing with the host cell membrane or by endocytosis. For instance, OMV can be taken up by infected cells and effectively inhibit the intracellular survival of Staphylococcus aureus. 72 Finally, they are less virulent than bacteria and can be absorbed by cells.
Outer membrane vesicles provide an alternative solution to the dilemma of poor drug delivery efficiency for refractory diseases. In the drug delivery system for non‐small cell lung cancer, OMV delivers the strongest intracellular DOX fluorescence intensity, which indicates that OMV significantly improves the efficiency of DOX delivery to tumor cells. 73 OMV has promoted the rapid development of tumor vaccines. Combining gene engineering and molecular glue technology, a personalised tumor mRNA vaccine using OMVs was constructed. 74 The OMV‐based vaccine induces a complete regression of 37.5% in the CRC model and produces a stronger immune response when compared to the liposome‐based vaccine. Besides, OMV facilitates the development of cancer vaccines with short treatment cycles, which is expected to reduce the financial burden on patients. 75 Basic fibroblast growth factor (BFGF), a tumor‐promoting factor, plays an important role in angiogenesis, proliferation and invasion of tumor cells, resistance to apoptosis, and tumor immunity. 76 , 77 , 78 In mouse models of lung tumors, BFGF‐loaded OMV treatment resulted in complete tumor suppression in 50% of the mice, with elevated levels of BFGF antibodies persisting for a period following three immunisations.
Applications of synthetic biology, chemistry and nanotechnology to functionalised and engineered bacteria
In bacteria‐based drug delivery systems, synthetic biology technologies provide an alternative way to customise bacteria with tumor‐targeting capabilities for efficient drug delivery. Besides, the applications of chemical and nanotechnology, including surface modification and electrostatic adsorption, have facilitated bacterial drug loading and protected the drug‐carrying bacteria before reaching the target site to avoid premature drug leakage and enhance delivery efficiency.
Synthetic biology
Genome editing and gene circuits that are representative of synthetic biology have been applied to bacteria‐based drug delivery systems. Traditional tumor therapies suffer from the lack of tumor targeting, low concentration of drugs at the tumor site, and resistance of tumor stem cells to various therapies. Engineered bacteria modified by synthetic biology are expected to overcome these problems. 79
Genome editing
Bacteria are genetically engineered to efficiently express exogenous genes by specifically adding, deleting and altering genetic material to become engineered bacteria (Figure 2a). Engineered bacteria can inhibit tumor growth, regulate gut bacteria, and alleviate chemically induced dysbiosis. 80 For instance, intracellular L‐arginine concentration directly affects the metabolic adaptability and viability of T cells, and it is essential for anti‐tumor response. 81 L‐arginine (L‐Arg) bacteria that direct ammonia for arginine synthesis were synthesised 82 (Figure 2b). The arginine repressor gene (ArgR) of E. coli was deleted and a resistant mutant ArgAfbr that was not repressed by L‐Arg was integrated into the bacterial genome. L‐Arg bacteria promoted the accumulation of arginine at a high concentration and had the best anti‐tumor effect when combined with PD‐L1 antibody. The engineered bacteria enhance the anti‐tumor immune response by regulating the tumor microenvironment. Additionally, gene engineering can also create a built‐in biological ‘kill switch’ to prevent the bacteria from surviving in vitro. 83
Figure 2.

Applications of synthetic biology to functionalized and engineered bacteria. (a) Common genome editing methods for engineered bacteria. (b) Construction of engineered bacterial L‐Arg with the ability to synthesise anticancer substances. (c) Programmable bacterial surface capsular polysaccharide (CAP) expression system.
Targeted gene insertion or replacement is an effective gene editing tool for gene engineering. Currently, many researchers have revealed that combining anti‐tumor drugs or acoustic sensitisers with focused ultrasound therapy can significantly improve the therapeutic effect. 84 An ultrasound‐responsive bacterium (URB) has been constructed by gene replacement and insertion plasmid recombination. In a 4T1 tumor mouse model, URB combined with ultrasound treatment inhibits tumor metastasis to distant areas and extends survival time (median survival time 60 days), which provides a new idea for bacteria‐mediated tumor therapy. 85 Moreover, the engineered bacteria that expressed tumor growth inhibiting nano antibody and temperature‐dependent genes was developed. 86 Combined with focused ultrasound technology, engineered bacteria can be applied for targeted treatment of deep‐seated tumors.
Gene circuits
Recently, engineered bacteria have been used for anti‐tumor drug delivery through synthetic genetic circuits. In bacteria‐based drug delivery systems, the gene circuit can be divided into three basic parts, namely input, operation and output. 87 First, the drug‐carrying bacteria can sense and interpret the biological or abiotic signals of the environment or disease, such as pH, temperature and metabolites through sensing elements. Second, the expected gene expression results were generated by computing and processing appropriate bacterial behaviour based on molecular signals through genetic logic gates. Finally, the engineered bacteria were driven to perform the expected therapeutic behaviour, such as time, to release the drug. 88
The gene circuit controls the release of drugs delivered by bacteria, which is of great significance for the efficiency and toxicity of drug delivery. A Salmonella‐based intracellular delivery system was designed to deliver the central domain of nuclear inhibitor of protein phosphatase 1 (NIPP1‐CD) and constitutive two‐chain active caspase‐3 (CT Casp‐3) that are protein drugs that induce apoptosis for the treatment of solid tumors. 12 , 89 , 90 , 91 The system‐derived Salmonella invasion of tumor cells through the regulator flhDC and released the protein drug by triggering the promoter Salmonella pathogenicity island 2 to cleave the bacteria. The administration of NIPP1‐CD and CT Casp‐3 via Salmonella substantially suppresses tumor cell growth and metastasis in in vitro cultured cell model and 4T1 tumor mouse model. The Salmonella‐based delivery platform has increased the delivery of protein drugs by more than 500‐fold, with great potential for application in poorly treated tumors.
In recent years, gene circuits have acquired great potential to prevent premature degradation of drug‐carrying bacteria. EcN is often used in tumor treatment as a probiotic, with clinical therapeutic effects in live bacterial therapeutic. 92 The programmable bacterial surface capsular polysaccharide (CAP) expression system that sensed and responded to induce stimuli and regulate cell surface properties was constructed 93 (Figure 2c). CAP can accomplish bacterial immune escape, and the removal of CAP allows for the clearance of bacteria. CAP increased the maximum tolerated dose of EcN 10‐fold and significantly inhibited tumor growth in CT26 CRC and MMTV‐PyMT mouse models. This system provides a new direction to achieve microbial immune escape by controlling the expression of surface capsular polysaccharides outside the bacteria through gene circuits.
The Quorum sensing bacteria obtained by gene engineering release anti‐tumor drugs through genetic circuitry lysis, which provides systemic exposure to drugs and exerts powerful anti‐tumor effects. 94 , 95 The Salmonella‐based synchronous lytic circuit (SLC) achieves a high level of apoptosis and death of tumor cells in subcutaneous tumor models and extends survival time of mice with metastatic CRC. 23 The modified Salmonella produces and accumulates N‐lipoylhomoserine lactone to a threshold value through a positive feedback loop. Upon reaching a threshold, SLC can be triggered, which could activate the quorum‐sensing bacteria pathway and trigger bacterial lysis or rupture that releases the therapeutant.
Chemistry and nanotechnology
The rapid development of chemical and nanotechnology has provided great support for the customisation of efficient drug delivery bacteria. Surface modification changes the properties or functions of the surface of a material by chemical treatment, physical treatment, or coating. Here, surface modification is applied to adsorb anti‐tumor drugs to the bacteria and provide a coating to protect the drug‐carrying bacteria (Figure 3a). In addition to surface modification and synthetic biology techniques, positively charged nanomaterials can be used to load drugs to bacteria through electrostatic adsorption in a convenient manner (Figure 3b).
Figure 3.

Applications of chemistry and nanotechnology in bacteria‐based drug delivery systems. (a) Application of surface modification for drug loading and protection of drug‐carrying bacteria. (b) Application of electrostatic adsorption for drug loading.
Surface modification
Bacterial cell wall composition is complex and chemical groups, such as carboxyl and amine, offer the possibility to connect bacteria with multifunctional nanoparticles. As a non‐covalent strong effector, streptavidin‐biotin is widely used in drug delivery systems to connect bacteria with functional nanoparticles, such as photosensitisers and magnetic particles. 96 , 97
Bacterial surfaces are coated or modified by surface modifications to protect bacteria from drug leakage and enhance the ability of bacteria to target tumors. The use of synthetic materials, such as liposomes and hydrogels, to encapsulate bacteria can prevent the premature removal of drug‐carrying bacteria to some extent. For example, the survival rate of EcN with complete fibroin protein coating in simulated gastric juices was increased by nearly 52 times after four cycles of deposition. 98 A significant reduction in the expression level of the tumor necrosis factor TNF‐α with silk‐coated EcN treatment was obtained in a mouse model of intestinal mucositis, with the optimal therapeutic effect. The silk coating is expected to protect the drug‐loaded bacteria in the gastrointestinal tract from premature bacterial degradation and drug leakage.
Furthermore, nanopolymer can be removed in response to in vivo biological signals with the help of the gene circuit, thereby allowing the bacteria to function at specific sites. 99 , 100 However, it still activates the body's complement system, thus leading to allergic reactions. The blood cell membranes can be used to wrap the drug‐carrying bacteria. 101 In this way, the clearance capacity of the body's immune system can be significantly reduced to increase the enrichment of bacteria at the tumor site.
Electrostatic adsorption
The mechanism of electrostatic adsorption is that ions or groups with different charges attract each other under the guidance of electrostatic gravity, so that dissimilar charges or groups are combined. 102 Electrostatic adsorption is widely used in drug delivery systems to connect drugs and carriers for its simplicity of operation. Besides, electrostatic adsorption does not involve the use of organic solvents, thereby eliminating the generation of any residual organic solvents during the drug inclusion process. Therefore, the drug‐carrying preparations prepared by this method are relatively stable. Gene drugs, which are easily destroyed by enzymes and other substances in the body without a drug delivery system, are often connected to gene carriers by electrostatic adsorption.
As mentioned earlier, the surface of bacteria is usually negatively charged, which provides an opportunity to construct drug‐loaded bacteria by electrostatic adsorption. Wei et al. constructed a polarised nanoparticle‐loaded bacterial system based on E. coli. E. coli and PR848 (drug‐carrying nanoparticles) were connected with ethylene glycol chitosan to prepare PR848 nanoparticle‐load E. coli (Ec‐PR848) by electrostatic interaction. 103 It was discovered that the maximum area of the cell nuclear proliferation and cell necrosis, maximum area of apoptosis, and minimum area of proliferation were in Ec‐PR848‐treated mice cells. The drug delivery system constructed via electrostatic adsorption has the advantage of simple preparation, which could strongly support the promotion of highly effective anti‐tumor system.
Prospects and challenges
Conventional drug delivery methods, such as tablets and capsules, are widely used in clinical practice because of their simple preparation and high compliance, but there are many shortcomings in the delivery of anti‐tumor drugs. 104 , 105 While common small molecule anti‐tumor drugs exert anti‐tumor effects, they also have strong toxicity to normal tissues. However, traditional drug delivery systems lack specific targeting, thus resulting in non‐specific drug distribution. 106 After blood circulation, anti‐tumor drugs are dispersed in different tissues. In this way, not only the drug concentration at the tumor site is reduced, but also the toxic side effects are increased. In addition, the physicochemical properties of antineoplastic drugs, such as solubility and stability, also put forward higher requirements for drug delivery methods. For example, camptothecin antineoplastic drugs, such as topotecan and irinotecan, have strong anticancer effects. 107 Unfortunately, these drugs are poorly water‐soluble and easily decomposed in light, and the lactone ring, an essential group for anti‐tumor activity, is easily inactivated under physiological conditions. Therefore, conventional delivery methods lead to low drug delivery efficiency and marked toxic side effects.
The core of bacteria‐based drug delivery systems is that bacteria, as living organisms, are used as carriers of anti‐tumor drugs because of their potential to target and enrich at the tumor site, providing an important prerequisite for the application of synthetic biology, chemistry and nanotechnology. Bacteria can be used as carriers in various forms, such as BGs, OMVs and minicells, which provides a broad platform for the application of technology. Unlike bacteria, other nano‐drug carriers, such as liposomes, must be tethered to technology to achieve tumor targeting. 104 In bacteria‐based drug delivery systems, although the application of various technologies plays an important role in optimising the bacteria‐based drug delivery systems, leading to the enhanced the anti‐tumor effect, bacteria are more critical as active anti‐tumor drug carriers. With the support of synthetic biology, chemistry and nanotechnology, bacteria‐based drug delivery systems have certain advantages over traditional drug delivery methods, because they can ultimately increase the accumulation of drugs at the tumor site and reduce the toxic side effects of drugs. First, bacteria can achieve targeted drug delivery to the tumor site, thus avoiding the non‐specific distribution of drugs. 108 Furthermore, bacteria are easy to culture and store, and the transformation into engineered bacteria is simple and feasible through genetic engineering. Scalable bacterial culture technology is expected to enable more cost‐effective mass production compared with other novel drug carriers. Additionally, synthetic biology techniques, such as gene circuits, allow the controlled release of drugs at tumor sites through the genetic modification of bacteria. 109 , 110 Fu and colleagues constructed a genetic circuit to program the bacterial lifestyle in response to NIR light to precisely control drug release based on the attenuated Pseudomonas aeruginosa (P. aeruginosa) via gene knockout. 111 After three cycles of tumor injection‐illumination treatment, drug release from the engineered bacteria induced 30% of tumor regression and significantly inhibited the remaining tumor growth in the A549 tumor‐bearing mouse model. 111 In this way, the early release of the drug is not only avoided but also expected to achieve a sustained slow release of the anti‐tumor drug, eventually leading to a significant enhancement of the anti‐tumor effect. Finally, in addition to their ability to efficiently deliver anti‐tumor drugs, some bacteria, such as magnetotactic bacteria AMB‐1, can also inhibit tumor growth by themselves, 112 thus achieving enhanced anti‐tumor effect in combination with drugs.
Nevertheless, the future of bacteria‐based drug delivery systems for clinical translation is challenging because of several limitations. First and foremost, potential biosafety hazards in bacteria‐based drug delivery systems are the greatest concern and have been a major impediment to regulatory approval. Except for a few bacteria (generally recognised as safe organisms ‘GRAS’) that can be directly used in tumor treatment among natural bacteria, most bacteria can cause bacterial infections and bring biosafety concerns. The accumulation of bacteria can cause adverse reactions, such as allergic and inflammatory responds. 113 Most patients with malignant tumors have low immunity, and even the residual virulence of bacteria after drug delivery may do harm to patients. Therefore, more studies on the bacterial biosafety need to be conducted to find the optimal bacterial loading concentration with high drug delivery efficiency and low bacterial toxicity. Attenuated engineered strains have been reported in previous studies and perform well in vivo. 12 However, there are still differences in pharmacokinetics and pharmacodynamics between human and mouse tumors. Therefore, the future evaluation of bacterial virulence is more suitable in the xenograft mouse model. Additionally, human microecological disorders may be induced by the involvement of drug‐carrying bacteria. In view of the close relationship between microecological dysbiosis and the occurrence of diseases, 114 it is necessary to consider the effect of drug‐carrying bacteria on human microecology in subsequent studies. Finally, genetic contamination should be given more attention because of the possibility of engineered bacteria invading the human genome, which raises serious ethical concerns. CRISPER/CAS9 that is commonly used in engineered bacteria has been reported to have the possibility of driving gene contamination. 115 Once genetic contamination occurs, it can proliferate and spread rapidly.
Conclusion
Bacteria are closely associated with cancer progression. Not only does bacteria target tumors, but it has a strong potential for gene engineering as an active organism. The traditional drug delivery method currently used in clinical practice leads to low efficiency of anti‐tumor drug delivery and high toxicity. Recently, bacteria or bacterial secretions as carriers for drug delivery made full use of the tumor‐targeting ability of bacteria. Synthetic biology, chemistry and nanotechnology techniques used in drug delivery systems provide a platform for loading drugs onto bacteria and preventing their premature degradation. Currently, most of the research remains in the preclinical stage, and it is challenging to achieve clinical translation. The potential biosafety hazards, regulatory hurdles and ethical considerations need to be addressed in bacteria‐based drug delivery systems. In conclusion, the involvement of bacteria into drug delivery systems holds the potential to enhance drug delivery efficiency and augment the therapeutic impacts of anti‐tumor drugs and deserves further investigation in the future.
Author contributions
Han Shuwen: Writing – original draft. Song Yifei: Writing – original draft. Wu Xinyue: Data curation. Qu Zhanbo: Visualization. Yu Xiang: Conceptualization. Yang Xi: Conceptualization.
Conflict of interest
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Zhejiang Provincial Natural Science Foundation (No. LTGY24H160001), China University Industry University Research Innovation Fund (No. 2023HT078), Public Welfare Technology Application Research Program of Huzhou (No. 2021GZB07) and Zhejiang Medical and Health Technology Project (No. 2024KY410).
Contributor Information
Yu Xiang, Email: 316835692@qq.com.
Yang Xi, Email: yangxi19900601@126.com.
References
- 1. Li J. Digestive cancer incidence and mortality among young adults worldwide in 2020: A population‐based study. World J Gastrointest Oncol 2022; 14: 278–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 3. Wang K, Eblan MJ, Deal AM et al. Cardiac toxicity after radiotherapy for stage III non‐small‐cell lung cancer: Pooled analysis of dose‐escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017; 35: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Z, Liu Y, Bao Y et al. Higher lung and heart doses decrease early and long‐term survival, respectively, in patients with non‐small cell lung cancer undergoing postoperative radiation. Adv Radiat Oncol 2023; 8: 101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beusterien K, Grinspan J, Kuchuk I et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 2014; 19: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 7. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–6392. [PubMed] [Google Scholar]
- 8. Kokate RA, Chaudhary P, Sun X et al. Rationalizing the use of functionalized poly‐lactic‐co‐glycolic acid nanoparticles for dendritic cell‐based targeted anticancer therapy. Nanomedicine (Lond) 2016; 11: 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xue T, Xu P, Padelford J et al. Actively targeted delivery of SN38 by ultrafine iron oxide nanoparticle for treating pancreatic cancer. Investig New Drugs 2022; 40: 546–555. [DOI] [PubMed] [Google Scholar]
- 10. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 2016; 1: 16014. [Google Scholar]
- 11. Zahednezhad F, Saadat M, Valizadeh H, Zakeri‐Milani P, Baradaran B. Liposome and immune system interplay: Challenges and potentials. J Control Release 2019; 305: 194–209. [DOI] [PubMed] [Google Scholar]
- 12. Raman V, Van Dessel N, Hall CL et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat Commun 2021; 12: 6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen R, Umeano AC, Kou Y, Xu J, Farooqi AA. Nanoparticle systems for cancer vaccine. Nanomedicine (Lond) 2019; 14: 627–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor‐targeting mutant of Salmonella Typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104: 10170–10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toso JF, Gill VJ, Hwu P et al. Phase I study of the intravenous administration of attenuated Salmonella Typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria‐based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 2009; 69: 5860–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut 2008; 57: 483–491. [DOI] [PubMed] [Google Scholar]
- 18. Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti‐tumour agents. Trends Microbiol 2006; 14: 190–196. [DOI] [PubMed] [Google Scholar]
- 19. Yu YA, Shabahang S, Timiryasova TM et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light‐emitting proteins. Nat Biotechnol 2004; 22: 313–320. [DOI] [PubMed] [Google Scholar]
- 20. Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res 1955; 15: 473–478. [PubMed] [Google Scholar]
- 21. Cameron DE, Bashor CJ, Collins JJ. A brief history of synthetic biology. Nat Rev Microbiol 2014; 12: 381–390. [DOI] [PubMed] [Google Scholar]
- 22. Chen W, Zhang Y, Yeo WS, Bae T, Ji Q. Rapid and efficient genome editing in Staphylococcus aureus by using an engineered CRISPR/Cas9 system. J Am Chem Soc 2017; 139: 3790–3795. [DOI] [PubMed] [Google Scholar]
- 23. Din MO, Danino T, Prindle A et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 2016; 536: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 2010; 10: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliveira H, Pérez‐Andrés E, Thevenot J, Sandre O, Berra E, Lecommandoux S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J Control Release 2013; 169: 165–170. [DOI] [PubMed] [Google Scholar]
- 26. Zhang B, Yu Q, Liu Y. Alternating magnetic field controlled targeted drug delivery based on graphene oxide‐grafted nanosupramolecules. Chemistry 2020; 26: 13698–13703. [DOI] [PubMed] [Google Scholar]
- 27. Felfoul O, Mohammadi M, Taherkhani S et al. Magneto‐aerotactic bacteria deliver drug‐containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol 2016; 11: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martel S, Mohammadi M, Felfoul O, Lu Z, Pouponneau P. Flagellated magnetotactic bacteria as controlled MRI‐trackable propulsion and steering systems for medical nanorobots operating in the human microvasculature. Int J Robot Res 2009; 28: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan AA, Khan S, Khan S, Rentschler S, Laufer S, Deigner HP. Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa . Sci Rep 2021; 11: 20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akolpoglu MB, Alapan Y, Dogan NO et al. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli‐responsive cargo delivery. Sci Adv 2022; 8: eabo6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gwisai T, Mirkhani N, Christiansen MG, Nguyen TT, Ling V, Schuerle S. Magnetic torque‐driven living microrobots for increased tumor infiltration. Sci Robot 2022; 7: eabo0665. [DOI] [PubMed] [Google Scholar]
- 32. Lower BH, Bazylinski DA. The bacterial magnetosome: A unique prokaryotic organelle. J Mol Microbiol Biotechnol 2013; 23: 63–80. [DOI] [PubMed] [Google Scholar]
- 33. Xiang Z, Jiang G, Fan D, Tian J, Hu Z, Fang Q. Drug‐internalized bacterial swimmers for magnetically manipulable tumor‐targeted drug delivery. Nanoscale 2020; 12: 13513–13522. [DOI] [PubMed] [Google Scholar]
- 34. Schwarz L, Medina‐Sánchez M, Schmidt OG. Hybrid BioMicromotors. Appl Phys Rev 2017; 4: 31301. [Google Scholar]
- 35. Neuhaus FC, Baddiley J. A continuum of anionic charge: Structures and functions of D‐alanyl‐teichoic acids in gram‐positive bacteria. Microbiol Mol Biol Rev 2003; 67: 686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huh K, Oh D, Yoo HJ et al. Bacteria‐based microrobot for chemotaxis delivery. In: International Conference on Control. Anchorage: IEEE; 2015. [Google Scholar]
- 37. Suganuma M, Kubo T, Ishiki K, et al. Mirror-image streptavidin with specific binding to L-biotin, the unnatural enantiomer. Sci Rep 2022; 12: 9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schauer O, Mostaghaci B, Colin R, et al. Motility and chemotaxis of bacteria-driven microswimmers fabricated using antigen 43-mediated biotin display. Sci Rep 2018; 8: 9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhuang J, Wright Carlsen R, Sitti M. pH‐taxis of biohybrid microsystems. Sci Rep 2015; 5: 11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mostaghaci B, Yasa O, Zhuang J, Sitti M. Bioadhesive bacterial microswimmers for targeted drug delivery in the urinary and gastrointestinal tracts. Adv Sci (Weinh) 2017; 4: 1700058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacDiarmid JA, Mugridge NB, Weiss JC et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell 2007; 11: 431–445. [DOI] [PubMed] [Google Scholar]
- 42. Islam MT, Davis Z, Chen L et al. Minicell‐based fungal RNAi delivery for sustainable crop protection. Microb Biotechnol 2021; 14: 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ali MK, Liu Q, Liang K, Li P, Kong Q. Bacteria‐derived minicells for cancer therapy. Cancer Lett 2020; 491: 11–21. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Ji W, He L et al. E. coli Nissle 1917‐derived minicells for targeted delivery of chemotherapeutic drug to hypoxic regions for cancer therapy. Theranostics 2018; 8: 1690–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solomon BJ, Desai J, Rosenthal M et al. A first‐time‐in‐human phase I clinical trial of bispecific antibody‐targeted, paclitaxel‐packaged bacterial minicells. PLoS One 2015; 10: e0144559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: Production and applications. Bioeng Bugs 2010; 1: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henrich B, Lubitz W, Plapp R. Lysis of Escherichia coli by induction of cloned phi X174 genes. Mol Gen Genet 1982; 185: 493–497. [DOI] [PubMed] [Google Scholar]
- 48. Witte A, Bläsi U, Halfmann G, Szostak M, Wanner G, Lubitz W. Phi X174 protein E‐mediated lysis of Escherichia coli . Biochimie 1990; 72: 191–200. [DOI] [PubMed] [Google Scholar]
- 49. Witte A, Wanner G, Bläsi U, Halfmann G, Szostak M, Lubitz W. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J Bacteriol 1990; 172: 4109–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stein E, Inic‐Kanada A, Belij S et al. In vitro and in vivo uptake study of Escherichia coli Nissle 1917 bacterial ghosts: Cell‐based delivery system to target ocular surface diseases. Invest Ophthalmol Vis Sci 2013; 54: 6326–6333. [DOI] [PubMed] [Google Scholar]
- 51. Witte A, Wanner G, Sulzner M, Lubitz W. Dynamics of PhiX174 protein E‐mediated lysis of Escherichia coli . Arch Microbiol 1992; 157: 381–388. [DOI] [PubMed] [Google Scholar]
- 52. Sheweita SA, Batah AM, Ghazy AA, Hussein A, Amara AA. A new strain of Acinetobacter baumannii and characterization of its ghost as a candidate vaccine. J Infect Public Health 2019; 12: 831–842. [DOI] [PubMed] [Google Scholar]
- 53. Dobrovolskiene N, Pasukoniene V, Darinskas A et al. Tumor lysate‐loaded bacterial ghosts as a tool for optimized production of therapeutic dendritic cell‐based cancer vaccines. Vaccine 2018; 36: 4171–4180. [DOI] [PubMed] [Google Scholar]
- 54. Groza D, Gehrig S, Kudela P et al. Bacterial ghosts as adjuvant to oxaliplatin chemotherapy in colorectal carcinomatosis. Onco Targets Ther 2018; 7: e1424676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muhammad A, Kassmannhuber J, Rauscher M et al. Subcutaneous immunization of dogs with Bordetella bronchiseptica bacterial ghost vaccine. Front Immunol 2019; 10: 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Egan M, Dempsey E, Ryan CA, Ross RP, Stanton C. The sporobiota of the human gut. Gut Microbes 2021; 13: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tetz G, Tetz V. Introducing the sporobiota and sporobiome. Gut Pathog 2017; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng W, Feng YQ, Ren J, Jing D, Wang C. Anti‐tumor role of Bacillus subtilis fmbJ‐derived fengycin on human colon cancer HT29 cell line. Neoplasma 2016; 63: 215–222. [DOI] [PubMed] [Google Scholar]
- 59. Awad MM, Johanesen PA, Carter GP, Rose E, Lyras D. Clostridium difficile virulence factors: Insights into an anaerobic spore‐forming pathogen. Gut Microbes 2014; 5: 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lemmon MJ, van Zijl P, Fox ME et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther 1997; 4: 791–796. [DOI] [PubMed] [Google Scholar]
- 61. Iwanicki A, Piątek I, Stasiłojć M et al. A system of vectors for Bacillus subtilis spore surface display. Microb Cell Factories 2014; 13: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saggese A, Baccigalupi L, Donadio G, Ricca E, Isticato R. The bacterial spore as a mucosal vaccine delivery system. Int J Mol Sci 2023; 24: 10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang M, Zhu G, Korza G, Sun X, Setlow P, Li J. Engineering Bacillus subtilis as a versatile and stable platform for production of nanobodies. Appl Environ Microbiol 2020; 86: e02938‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yin L, Meng Z, Zhang Y et al. Bacillus spore‐based oral carriers loading curcumin for the therapy of colon cancer. J Control Release 2018; 271: 31–44. [DOI] [PubMed] [Google Scholar]
- 65. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem 2017; 60: 1620–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Han ZY, Chen QW, Fu ZJ, Cheng SX, Zhang XZ. Probiotic spore‐based oral drug delivery system for enhancing pancreatic cancer chemotherapy by gut‐pancreas‐Axis‐guided delivery. Nano Lett 2022; 22: 8608–8617. [DOI] [PubMed] [Google Scholar]
- 67. Guerrero‐Mandujano A, Hernández‐Cortez C, Ibarra JA, Castro‐Escarpulli G. The outer membrane vesicles: Secretion system type zero. Traffic 2017; 18: 425–432. [DOI] [PubMed] [Google Scholar]
- 68. Bishop DG, Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine‐limiting conditions. Biochem J 1965; 96: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: From discovery to applications. Ann Rev Microbiol 2021; 75: 609–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hendrix A, De Wever O. Systemically circulating bacterial extracellular vesicles: Origin, fate, and function. Trends Microbiol 2022; 30: 213–216. [DOI] [PubMed] [Google Scholar]
- 71. Gerritzen MJH, Salverda MLM, Martens DE, Wijffels RH, Stork M. Spontaneously released Neisseria meningitidis outer membrane vesicles as vaccine platform: Production and purification. Vaccine 2019; 37: 6978–6986. [DOI] [PubMed] [Google Scholar]
- 72. Goes A, Lapuhs P, Kuhn T et al. Myxobacteria‐derived outer membrane vesicles: Potential applicability against intracellular infections. Cells 2020; 9: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuerban K, Gao X, Zhang H et al. Doxorubicin‐loaded bacterial outer‐membrane vesicles exert enhanced anti‐tumor efficacy in non‐small‐cell lung cancer. Acta Pharm Sin B 2020; 10: 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li Y, Ma X, Yue Y et al. Rapid surface display of mRNA antigens by bacteria‐derived outer membrane vesicles for a personalized tumor vaccine. Adv Mater 2022; 34: e2109984. [DOI] [PubMed] [Google Scholar]
- 75. Huang W, Shu C, Hua L et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater 2020; 108: 300–312. [DOI] [PubMed] [Google Scholar]
- 76. Todorović‐Raković N, Radulovic M, Vujasinović T, Rabi ZA, Milovanović J, Nikolić‐Vukosavljević D. bFGF in tumor tissue independently prognosticates disease outcome of a natural course of invasive breast cancer. Cancer Biomark 2017; 20: 151–158. [DOI] [PubMed] [Google Scholar]
- 77. Dai H, Zhao S, Xu L, Chen A, Dai S. Expression of Efp, VEGF and bFGF in normal, hyperplastic and malignant endometrial tissue. Oncol Rep 2010; 23: 795–799. [PubMed] [Google Scholar]
- 78. Takase N, Koma Y, Urakawa N et al. NCAM‐ and FGF‐2‐mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor‐associated macrophages and cancer cells. Cancer Lett 2016; 380: 47–58. [DOI] [PubMed] [Google Scholar]
- 79. Riglar DT, Silver PA. Engineering bacteria for diagnostic and therapeutic applications. Nat Rev Microbiol 2018; 16: 214–225. [DOI] [PubMed] [Google Scholar]
- 80. Chung Y, Ryu Y, An BC et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome 2021; 9: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Geiger R, Rieckmann JC, Wolf T et al. L‐arginine modulates T cell metabolism and enhances survival and anti‐tumor activity. Cell 2016; 167: 829–842.e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Canale FP, Basso C, Antonini G et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021; 598: 662–666. [DOI] [PubMed] [Google Scholar]
- 83. Reardon S. Genetically modified bacteria enlisted in fight against disease. Nature 2018; 558: 497–498. [DOI] [PubMed] [Google Scholar]
- 84. Choi W, Kim C. Synergistic agents for tumor‐specific therapy mediated by focused ultrasound treatment. Biomater Sci 2021; 9: 422–436. [DOI] [PubMed] [Google Scholar]
- 85. Chen Y, Du M, Yuan Z, Chen Z, Yan F. Spatiotemporal control of engineered bacteria to express interferon‐gamma by focused ultrasound for tumor immunotherapy. Nat Commun 2022; 13: 4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abedi MH, Yao MS, Mittelstein DR et al. Ultrasound‐controllable engineered bacteria for cancer immunotherapy. Nat Commun 2022; 13: 1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kobayashi H, Kaern M, Araki M et al. Programmable cells: Interfacing natural and engineered gene networks. Proc Natl Acad Sci USA 2004; 101: 8414–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med 2019; 25: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chatterjee J, Beullens M, Sukackaite R et al. Development of a peptide that selectively activates protein phosphatase‐1 in living cells. Angew Chem Int Ed Engl 2012; 51: 10054–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase‐3 and caspase‐7 are functionally distinct proteases. Proc Natl Acad Sci USA 2008; 105: 12815–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Slee EA, Adrain C, Martin SJ. Executioner caspase‐3, ‐6, and ‐7 perform distinct, non‐redundant roles during the demolition phase of apoptosis. J Biol Chem 2001; 276: 7320–7326. [DOI] [PubMed] [Google Scholar]
- 92. Lynch JP, Goers L, Lesser CF. Emerging strategies for engineering Escherichia coli Nissle 1917‐based therapeutics. Trends Pharmacol Sci 2022; 43: 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Harimoto T, Hahn J, Chen YY et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nat Biotechnol 2022; 40: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shong J, Collins CH. Engineering the esaR promoter for tunable quorum sensing‐ dependent gene expression. ACS Synth Biol 2013; 2: 568–575. [DOI] [PubMed] [Google Scholar]
- 95. Kalia VC, Patel SKS, Cho BK, Wood TK, Lee JK. Emerging applications of bacteria as antitumor agents. Semin Cancer Biol 2022; 86: 1014–1025. [DOI] [PubMed] [Google Scholar]
- 96. Suh S, Jo A, Traore MA et al. Nanoscale bacteria‐enabled autonomous drug delivery system (NanoBEADS) enhances Intratumoral transport of nanomedicine. Adv Sci (Weinh) 2019; 6: 1801309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li J, Xia Q, Guo H et al. Decorating bacteria with triple immune nanoactivators generates tumor‐resident living Immunotherapeutics. Angew Chem Int Ed Engl 2022; 61: e202202409. [DOI] [PubMed] [Google Scholar]
- 98. Hou W, Li J, Cao Z et al. Decorating bacteria with a therapeutic nanocoating for synergistically enhanced biotherapy. Small 2021; 17: e2101810. [DOI] [PubMed] [Google Scholar]
- 99. Feng P, Cao Z, Wang X, Li J, Liu J. On‐demand bacterial reactivation by restraining within a triggerable nanocoating. Adv Mater 2020; 32: e2002406. [DOI] [PubMed] [Google Scholar]
- 100. Han C, Zhang X, Pang G et al. Hydrogel microcapsules containing engineered bacteria for sustained production and release of protein drugs. Biomaterials 2022; 287: 121619. [DOI] [PubMed] [Google Scholar]
- 101. Cao Z, Cheng S, Wang X, Pang Y, Liu J. Camouflaging bacteria by wrapping with cell membranes. Nat Commun 2019; 10: 3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shen X, Zhang Y, He H et al. Electrostatic adsorption behaviors of charged polymer‐tethered nanoparticles on oppositely charged surfaces. Macromol Rapid Commun 2022; 43: e2200171. [DOI] [PubMed] [Google Scholar]
- 103. Wei B, Pan J, Yuan R et al. Polarization of tumor‐associated macrophages by nanoparticle‐loaded Escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett 2021; 21: 4231–4240. [DOI] [PubMed] [Google Scholar]
- 104. Park H, Otte A, Park K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J Control Release 2022; 342: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jyoti A, Gabriela H, Mahesh N. Enhancing the delivery of chemotherapeutics: Role of biodegradable polymeric nanoparticles. Molecules 2018; 23: 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang L, Yang Y, Chen Y, Xu Y, Peng J. Cell‐based drug delivery systems and their in vivo fate. Adv Drug Deliv Rev 2022; 187: 114394. [DOI] [PubMed] [Google Scholar]
- 107. Khaiwa N, Maarouf NR, Darwish MH, Alhamad DWM, Al‐Tel TH. Camptothecin's journey from discovery to WHO essential medicine: Fifty years of promise. Eur J Med Chem 2021; 223: 113639. [DOI] [PubMed] [Google Scholar]
- 108. Morrissey D, O'Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther 2010; 10: 3–14. [DOI] [PubMed] [Google Scholar]
- 109. Zhou S. Synthetic biology: Bacteria synchronized for drug delivery. Nature 2016; 536: 33–34. [DOI] [PubMed] [Google Scholar]
- 110. Chien T, Doshi A, Danino T. Advances in bacterial cancer therapies using synthetic biology. Curr Opin Syst Biol 2017; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fu S, Zhang R, Gao Y et al. Programming the lifestyles of engineered bacteria for cancer therapy. Natl Sci Rev 2023; 10: nwad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang B, Qin Y, Liu J et al. Magnetotactic bacteria‐based drug‐loaded micromotors for highly efficient magnetic and biological double‐targeted tumor therapy. ACS Appl Mater Interfaces 2023; 15: 2747–2759. [DOI] [PubMed] [Google Scholar]
- 113. Hosseinidoust Z, Mostaghaci B, Yasa O, Park BW, Singh AV, Sitti M. Bioengineered and biohybrid bacteria‐based systems for drug delivery. Adv Drug Deliv Rev 2016; 106: 27–44. [DOI] [PubMed] [Google Scholar]
- 114. Wang X, Yang S, Li S et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020; 69: 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Courtier‐Orgogozo V, Danchin A, Gouyon PH, Boëte C. Evaluating the probability of CRISPR‐based gene drive contaminating another species. Evol Appl 2020; 13: 1888–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]


